Abstract
Adenosine triphosphate–binding cassette subfamily B member 1 (ABCB1), also known as permeability glycoprotein, multidrug-resistant protein 1, or cluster of differentiation 243 (CD243), is a crucial protein for purging foreign substances from cells. The functions of ABCB1 have been investigated extensively for their roles in cancer, stem cells, and drug resistance. Abundant pharmacogenetic studies have been conducted on ABCB1 and its association with treatment responsiveness to various agents, particularly chemotherapeutic and immunomodulatory agents. However, its functions in the skin and implications on dermatotherapeutics are far less reported. In this article, we reviewed the roles of ABCB1 in dermatology. ABCB1 is expressed in the skin and its appendages during drug delivery and transport. It is associated with treatment responsiveness to various agents, including topical steroids, methotrexate, cyclosporine, azathioprine, antihistamines, antifungal agents, colchicine, tacrolimus, ivermectin, tetracycline, retinoid acids, and biologic agents. Moreover, genetic variation in ABCB1 is associated with the pathogenesis of several dermatoses, including psoriasis, atopic dermatitis, melanoma, bullous pemphigoid, Behçet disease, and lichen planus. Further investigation is warranted to elucidate the roles of ABCB1 in dermatology and the possibility of enhancing therapeutic efficacy through ABCB1 manipulation.
Keywords: ABCB1, Permeability glycoprotein, Pharmacogenetics
Introduction
Adenosine triphosphate (ATP)-binding cassette subfamily B member 1 (ABCB1), also known as permeability glycoprotein (p-gp), multidrug-resistant protein 1 (MDR1), or cluster of differentiation 243 (CD243), is an ATP-dependent drug transporter that purges foreign substances from cells. It belongs to a transporter superfamily, the ATP-binding cassette (ABC) family, which consists of seven subfamilies (ABCA to ABCG), with 49 transporters identified in humans [1]. Each gene within the ABC superfamily is an ATP-dependent transporter; however, the tissue expression profiles, substrates, and physiological functions of ABC genes exhibit considerable variability. The ABCB subfamily consists of 11 members, each with diverse distribution in cell membranes, mitochondria, and lysosomes and with functions related to metabolism, trafficking, adaptive immunity, and excretion [2]. Among them, ABCB1 is the most investigated transporter, first identified in 1970 and cloned in 1985 [3, 4].
Gene and protein characteristics of ABCB1
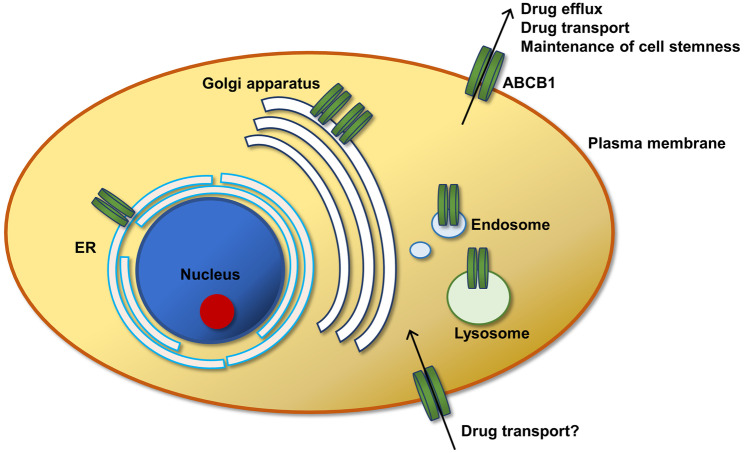
ABCB1 is a 170-kD protein consisting of 1280 amino acids with two 6-transmembrane domains, each separated by a linker polypeptide chain, forming a central pore for drug efflux. The cytoplasmic regions contain nucleotide-binding domains, which confer conformational changes on binding to ATP. Notably, ABCB1 is well conserved across various kingdoms, including animals of both mammalian and nonmammalian classes, fungi, and bacteria [5]. ABCB1 is mostly expressed in tissues with barrier functions, such as the brain, liver, kidney, intestine, testis, ovary, placenta, pancreas, endometrium, adrenal glands, and skin [6]. At the cellular level, ABCB1 is expressed abundantly in the plasma membrane, particularly at the apices of cells with excretion functions (i.e., the luminal side of the capillary endothelium of the blood–brain barrier) [7]. Moreover, ABCB1 is found in the endoplasmic reticulum (ER), Golgi apparatus, endosome, and lysosomes, which partly reflects the process of its trafficking to the plasma membrane (Fig. 1) [8].
Fig. 1.
Cellular distribution of ABCB1 and its proposed functions. ABCB1, or p-gp, is mainly expressed in the plasma membrane for effluxing substrates as well as maintaining stem cell properties. Furthermore, some studies have suggested that ABCB1 is responsible for substrate influx, resulting in transepidermal drug delivery. ABCB1 is also found in other intracellular sites, including ER, Golgi apparatus, endosomes, and lysosomes, mainly as a result of protein trafficking
Functions of ABCB1
The functions of ABCB1 have been extensively investigated for their roles in cancer, stem cells, and drug metabolism and resistance. The physiological functions of ABCB1 in healthy organs involve drug metabolism (Fig. 1). For example, ABCB1 eliminates drugs absorbed from the gut with high expression in the intestinal endothelium as part of the first-pass effect. Moreover, its expression in renal cells is crucial for drug elimination. Abundant pharmacogenetic studies have been conducted on ABCB1 and its association with treatment responsiveness to various agents, particularly chemotherapeutic and immunomodulatory agents. For instance, polymorphism in ABCB1 has been suggested to be related to treatment responses and toxicity of chemotherapeutic agents in breast cancer, osteosarcoma, colorectal cancer, and lung cancer, although studies have reported varied results [9–12]. Approximately 50 single-nucleotide polymorphisms (SNPs) have been identified in ABCB1, with C3435T being the most investigated site. Some SNPs in specific loci of ABCB1 are reported to be associated with either increased or decreased expression, whereas some of the SNPs do not affect its expression levels [13]. For example, 3435C > T, 2677G > TA, and − 129 T > C polymorphisms are associated with increased ABCB1 expression. Thus, ABCB1 polymorphisms may determine the pharmacokinetics, treatment responses, drug toxicity, and even risks of certain diseases. Furthermore, ABCB1 is involved in substrate sequestration in lysosomes as part of drug resistance [14]. However, its functions in dermatology and associated therapeutics are far less reported. In the skin, the functions of ABCB1 are mainly thought to involve drug efflux. However, current evidence also suggests that ABCB1 is crucial for transepidermal drug delivery and affects the risk of developing several dermatoses.
ABCB1 in skin disorders
ABCB1 is involved in the pathogenesis of various skin disorders. Furthermore, its polymorphism is related to the risk of certain dermatoses, ranging from malignancy to inflammatory dermatoses (Table 1).
Table. 1.
Dermatoses associated with ABCB1
| Associated with ABCB1 | No association | Unknown |
|---|---|---|
| Psoriasis | SJS-TEN [109, 110] | DRESS [111] (probably) |
| Atopic dermatitis | Pemphigus | |
| Lichen planus | Rosacea | |
| Melanoma | Acne (probably) | |
| Bullous pemphigoid | Other skin tumors | |
| Behcet disease | Sarcoidosis | |
| Systemic lupus erythematosus | Neutrophilic dermatoses | |
| Urticaria | ||
| Ichthyosis | ||
| Dermatitis herpetiformis | ||
| Dermatomyositis | ||
| Alopecia [30] (probably) |
SJS-TEN Stevens–Johnson syndrome and toxic epidermal necrolysis, DRESS drug reaction with eosinophilia and systemic symptoms
Skin cancer
ABCB1 is the putative efflux pump for chemotherapeutic agents in various skin malignancies, particularly melanoma. ABCB1 expression patterns and levels vary greatly in different melanoma cell lines and primary and metastatic cancer cells. Some studies have revealed that its overall expression in primary and metastatic melanoma is low [15, 16]. By contrast, some early studies have indicated high levels of ABCB1 through immunohistochemical staining or reverse transcription polymerase chain reaction in certain melanoma cell lines [17]. However, ABCB1 expression is high in its side population, which is a cell population distinct from the main population of specific markers in flow cytometry and shares similar characteristics to cancer stem cells [18]. The enriched expression of ABCB1 in the side population was found to be related to metastasis ability, clonogenicity, and tumorigenicity [19].
Psoriasis and psoriatic arthritis
Few studies have demonstrated the roles of ABCB1 in the pathogenesis of psoriasis and psoriatic arthritis. One study revealed that mRNA levels decreased in human skin with psoriasis [20]. Nevertheless, ABCB1 remains a crucial factor in the treatment of psoriasis and psoriatic arthritis, as ABCB1 is associated with the responsiveness of various therapeutic agents. Such agents include cyclosporine, topical steroids, methotrexate, and etanercept.
Atopic dermatitis
Although strong evidence is lacking regarding to the association of atopic dermatitis with ABCB1 in humans, data from mouse models suggest that ABCB1 may be protective for pruritus and inflammatory burdens in atopic dermatitis. In the mice atopic dermatitis model, scratch bouts increased in Mdr1a/1b/Bcrp-deficient mice with decreased ABCB1 expression. Moreover, ABCB1 expression levels decreased in the skin of patients with atopic dermatitis [20].
Bullous pemphigoid
ABCB1 is associated with the risk of developing bullous pemphigoid. In a Polish patient group, the risk for bullous pemphigoid was five times higher in patients carrying the 2677TA genotype and two times higher in those carrying the 2677TT genotype in ABCB1 [21]. Furthermore, the same group of researchers revealed that the 1236 T-2677G-3435 T haplotype in ABCB1 was less frequent in patients with bullous pemphigoid than in healthy controls. These SNPs are associated with the levels of cytokine and interleukin secretions as cellular responses to methotrexate and steroids [22].
Behçet disease
Some studies have reported that ABCB1 may be associated with Behçet disease. In a Turkish cohort, a combined CC–GG binary genotype for the C1236T–G2677T/A loci couple in ABCB1 was identified in the Behçet patient group, although the researcher did not observe a significant difference in ABCB1 SNPs within individual loci [23]. In Iranian–Azeri Turks, no association was observed between Behçet disease risk and SNPs in ABCB1. However, researchers discovered that C3435T polymorphism is associated with the risk of related symptoms, including erythema nodosum, pseudofolliculitis, and skin lesions [24].
Lichen planus
In an Indian patient group, ABCB1 expression was significantly increased in oral lichen planus, particularly after corticosteroid treatment [25]. Its degree of increase correlates with its severity because erosive oral lichen planus has higher ABCB1 levels than does reticular lichen planus. The change in ABCB1 expression may be part of the pathogenesis of malignant transformation and multidrug resistance [25].
ABCB1 and dermatological therapeutic agents
ABCB1 is expressed in the epidermis, dermis, and skin appendages (Table 2). Studies of normal human skin have revealed that ABCB1 expression is more abundant in the dermis than in the epidermis. At the protein level, immunostaining revealed ABCB1 signals on the basal keratinocytes, sweat glands, vessels, adipocytes, nerve sheaths, and musculature [26]. The staining pattern is largely consistent with the murine model of ABCB1 expression and is mainly restricted to the dermis [27]. Moreover, ABCB1 is expressed in suprabasal keratinocytes in healthy individuals and patients with psoriasis [28].
Table. 2.
Expression of ABCB1 on the skin
| Skin compartment | Relative expression* | Functions |
|---|---|---|
| Epidermis | ||
| Keratinocytes | Intermediate | ABCB1 mostly detected in the suprabasal keratinocytes [28] |
| Dermis | ||
| Vasculature | High | Presumed functions for drug transport to the bloodstream and barriers of xenobiotics [26] |
| Adipocyte | Intermediate | Functions unknown [26] |
| Nerve sheath | High | Functions unknown [26] |
| Musculature | High | Functions unknown [26] |
| Appendages | ||
| Sebaceous glands | Undetermined in human | ABCB1 expressed in the murine sebocytes; may be involved in the pathogenesis of acne [29] |
| Hair follicles | High | Highest expression on the inner root sheath, outer root sheath, connective tissue sheath, and arrector pili muscle; also expressed on the epithelium and dermal papillae. May be involved in stem cell features [27, 30] |
| Sweat glands | High | Presumed functions for drug transport to the sweat ducts [26] |
In the dermis, sebocytes are enriched in ABCB1 and involved in sebum secretion [29]. Cutibacterium acnes upregulates ABCB1 in sebocytes along with increased triglyceride secretion [29]. Moreover, ABCB1 is expressed in the epithelium of hair follicles, such as the bulb, the bulge, suprabulbar regions, and associated vasculatures, in the connective tissue sheath [30]. The proposed roles of ABCB1 in hair follicles include effluxing drugs and maintaining stem cell properties.
ABCB1 and transepidermal drug delivery
In the skin, although the functions of ABCB1 are mainly centered on drug efflux, ABCB1 is also crucial for drug delivery through the transepidermal route [27]. Evidence from ABCB1 knockout (Mdr1a/1b−/−) mice has suggested that ABCB1 is also crucial in transepidermal delivery of selective topical agents on the skin [27, 30–32]. ABCB1-deficient (Mdr1a/1b−/−) mice exhibited reduced transepidermal delivery of rhodamine 123 to the dermis and plasma, and this effect was even more pronounced in (Mdr1a/1b/bcrp[breast cancer resistance protein]−/−) triple knockout mice [32]. The authors observed a similar phenomenon in topically applied prednisolone and dexamethasone, and in vitro studies have demonstrated that ABCB1 rather than breast cancer resistance protein is mainly responsible for the phenotype [31].
ABCB1 and metabolism of dermatologic therapeutic agents
Various dermatologic agents, both topical and systemic, are associated with ABCB1. The agents can be the substrates, inducers, or inhibitors of ABCB1 (Table 3). Many dermatologic therapeutic agents share overlapping or even contradictory features. For example, ivermectin, cyclosporine, tacrolimus, and erythromycin are both inducers and inhibitors according to different investigations. When other drugs are administered along with those dermatologic therapeutic agents, considering their effects on drug metabolism and interaction is crucial because the activities of ABCB1 are altered.
Table. 3.
Common dermatologic agents associated with ABCB1
| P-gp modifiers | Diseases |
|---|---|
| P-gp substrates | |
| Colchicine | Gout, urticarial vasculitis |
| Cyclosporine | Psoriasis, atopic dermatitis, urticaria, pyoderma gangrenosum, Behçet’s disease, etc. |
| Dexamethasone | Widely used immunosuppressant |
| Doxycycline | Widely used antibiotic and anti-inflammatory agent |
| Erythromycin | Acne, rosacea, erythrasma, pityriasis lichenoides |
| Fexofenadine | Widely used antihistamine |
| Itraconazole | Fungal infection, eczema, seborrheic dermatitis |
| Ivermectin | Parasitic infection, demodicosis |
| Ketoconazole | Fungal infection, seborrheic dermatitis |
| Methotrexate | Psoriasis, psoriatic arthritis, atopic dermatitis, pityriasis lichenoides, PLEVA (Pityriasis lichenoides et varioliformis acuta), morphea, pompholyx, cutaneous lupus erythematosus, lichen planus |
| Methylprednisolone | Widely used immunosuppressant |
| Sirolimus | Renal transplant |
| Tacrolimus | Atopic dermatitis |
| Terfenadine | Widely used antihistamine |
| Tetracycline | Widely used antibiotic and anti-inflammatory agent |
| P-gp inhibitors | |
| Cetirizine | Widely used antihistamine |
| Cyclosporine* | Psoriasis, atopic dermatitis, urticaria, pyoderma gangrenosum, Behçet’s disease, etc. |
| Erythromycin* | Acne, rosacea, erythrasma, pityriasis lichenoides |
| Itraconazole | Fungal infection, eczema, seborrheic dermatitis |
| Ivermectin* | Parasitic infection, demodicosis |
| Ketoconazole | Fungal infection, seborrheic dermatitis |
| Retinol | Acne, psoriasis, palmoplantar keratoderma, pityriasis rubra pilaris, Darier disease, lichen planus, etc. |
| Sirolimus | Renal transplant |
| Tacrolimus* | Atopic dermatitis |
| Terfenadine | Widely used antihistamine |
| P-gp inducers | |
| Clotrimazole | Fungal infection |
| Colchicine | Gout, urticarial vasculitis |
| Cyclosporine* | Psoriasis, atopic dermatitis, urticaria, pyoderma gangrenosum, Behçet’s disease, etc. |
| Dexamethasone | Widely used immunosuppressant |
| Doxycycline | Widely used antibiotic and anti-inflammatory agent |
| Erythromycin* | Acne, rosacea, erythrasma, pityriasis lichenoides |
| Ivermectin* | Parasitic infection, demodicosis |
| Methotrexate | Psoriasis, psoriatic arthritis, atopic dermatitis, pityriasis lichenoides, PLEVA, morphea, pompholyx, cutaneous lupus erythematosus, lichen planus |
| Retinoid acid (i.e., tretinoin, bexarotene) | Acne, psoriasis, palmoplantar keratoderma, pityriasis rubra pilaris, Darier disease, lichen planus, etc. |
| Tacrolimus* | Atopic dermatitis |
*Contradictory results from different studies are listed
Diets and herbs modulating ABCB1 activity
Diet and herbs modulate ABCB1 activities (Table 4). Isoflavone-rich foods, such as miso and soymilk, are ABCB1 activators and may reduce drug concentrations [33]. For example, some soy-derived food items, including soymilk and miso, induce ABCB1 expression and thus reduce intracellular cyclosporine levels. In those consuming soymilk and miso, the maximal concentration of ABCB1 decreased by 64.5% and 78.3%, and its area under the curve decreased by 64.9% and 78.3%, respectively [33].
Table. 4.
Diets and herbs modulating ABCB1 activities
| Diet and herbs | Usage |
|---|---|
| ABCB1 inducers | |
| Aloe | Laxatives, flavoring |
| Camellia sinensis | Beverages |
| Coptidis rhizoma | Various usage in traditional medicine |
| Eruca vesicaria | Culinary usage |
| Ginkgo biloba | Culinary usage; traditional medicine; dietary supplement |
| Hypericum perforatum | Traditional medicine |
| Miso | Culinary usage |
| Rhubarb | Culinary usage; traditional medicine |
| Soymilk | Beverages |
| ABCB1 inhibitors | |
| Curcumin | Culinary usage |
| Folium Sennae | Laxatives |
| Piperine | Culinary usage |
| Quercetin | Found in dietary supplements, beverages, and foods |
| Rutin | Found in vegetables and fruits |
| Silymarin | Treatment of toxic liver damage |
Similarly, common ingredients of beverages and herbal medicines, such as aloe, Camellia sinensis, Hypericum perforatum, Ginkgo biloba, Coptidis rhizoma, Eruca vesicaria, and rhubarb, are also ABCB1 activators [34–38]. Conversely, some common condiments and herbal medicines, such as Folium Sennae, silymarin, curcumin, and piperine, are ABCB1 inhibitors [35, 39]. Furthermore, dietary flavonoids, such as quercetin and rutin, inhibit ABCB1 [40]. Therefore, considering dietary factors in the real-word clinical setting is crucial when patients use these agents concomitantly.
Topical corticosteroids
ABCB1 and transepidermal delivery of selective topical steroids
Various steroids are ABCB1 substrates. The association between ABCB1 and systemic corticosteroids has been extensively studied. However, relatively few reports have shown how ABCB1 in the skin affects the treatment effects of topical corticosteroids. Hashimoto et al. revealed that ABCB1 is involved in the transepidermal delivery of prednisolone and dexamethasone to the bloodstream in a mouse model [31]. Furthermore, several key amino acids were identified in the nucleotide-binding domain of ABCB1 for its interaction with various corticosteroids, including dexamethasone and triamcinolone acetonide. The interaction sites varied across different steroid types because of variations in their structures [41].
ABCB1 for treatment responsiveness to topical steroids
Studies have indicated that ABCB1 in the skin may be involved in the treatment resistance to corticosteroids in addition to drug absorption. ABCB1 is more upregulated in psoriasis skin resistant to topical corticosteroids than in psoriasis skin responsive to topical corticosteroids and normal skin [42]. Moreover, ABCB1 upregulates in human skin after topical corticosteroid application. These results are consistent with evidence from in vitro studies of dexamethasone in cell lines [43, 44]. Notably, SNP G1199A in ABCB1 increases the resistance, intracellular drug accumulation, and efflux of several specific steroids. These include aldosterone, dexamethasone, cortisol, and corticosterone [45]. This evidence suggests a regulatory mechanism for ABCB1 in the resistance to topical corticosteroids.
Systemic agents
Antifungal agents
Both itraconazole and ketoconazole are ABCB1 inhibitors (Table 3). In vitro studies have indicated that itraconazole and ketoconazole, being ABCB1 inhibitors, hampered ABCB1-mediated drug efflux in mammalian cell lines, whereas such activities were not observed with fluconazole [46, 47]. Notably, itraconazole-mediated ABCB1 inhibition is substrate dependent, as varied inhibitory effects were observed with the administration of different substrates [47].
Itraconazole and ketoconazole are also ABCB1 substrates responsible for drug efflux and transepidermal drug delivery. In an experimental model, Ito et al. determined that transepidermal transport was hindered with topical itraconazole in ABCB1-deficient mice. Consistent with this finding, when itraconazole was administered intravenously, the itraconazole level was higher on the skin of knockout mice than on the skin of normal mice [27]. The aforementioned evidence suggests that the interaction among ABCB1 and various antifungal agents is relatively complex and even reciprocal.
Antihistamines
Selective antihistamines as substrates or inhibitors of ABCB1
Various antihistamine classes have been proposed as substrates or inhibitors of ABCB1, which is associated with the adverse effects of and treatment responsiveness to antihistamines. As substrates, the association of drug efflux with ABCB1 among different individual antihistamines varies greatly and has been extensively investigated. ABCB1-mediated efflux is a crucial mechanism in antihistamine transport across the blood–brain barrier to exert its effect on the central nervous system (CNS). This phenomenon has been reported in various H1 antihistamines, including acrivastine, astemizole, levocetirizine, terfenadine, desloratadine, azelastine, misolastine, and epinastine [48]. Nevertheless, some contradictory results exist because desloratadine was reported to be a nonsubstrate for ABCB1 in a related study [49].
Most antihistamines are ABCB1 substrates, but some antihistamine classes are ABCB1 inhibitors. For example, cetirizine downregulates ABCB1 in vitro [50]. Similarly, terfenadine demonstrated strong inhibitory effects on ABCB1 in vitro [51]. However, in vivo evidence for the regulatory effects of antihistamines in ABCB1 expression on the skin is scant.
Fexofenadine as a complex model of ABCB1 interaction with antihistamines
Notably, fexofenadine, a second-generation antihistamine widely prescribed worldwide, has been extensively investigated. The results have indicated that ABCB1 is crucial for its transport and metabolism and plays a role in its side effects. ABCB1 is expressed on the apical aspect of the intestinal endothelium and expels fexofenadine from the intracellular space back to the gastrointestinal lumen [52]. Early studies in canines indicated that mutant ABCB1 resulted in modestly increased serum concentrations of fexofenadine, which is suggestive of its role in intestinal absorption [53]. Animal studies have revealed that ABCB1-deficient mice had a five- and nine-time increase in its levels in the plasma and brain [54]. Moreover, ABCB1 preferentially transports (S)-fexofenadine over (R)-fexofenadine according to studies with canine models [55]. However, human studies have yielded varied results. In Caucasian people, ABCB1 G2677A polymorphism has been reported to be only partly associated with lower serum maximal concentration and slightly increased clearance but not other pharmacokinetic parameters [56, 57]. Efflux activities for fexofenadine are associated with ABCB1 C3435T polymorphism [58]. By contrast, another study found no difference in fexofenadine pharmacokinetics with ABCB1 genetic polymorphism [59]. In Jordanian patients, ABCB1 C1236T was associated with decreased treatment responses for men with fexofenadine-induced allergic symptoms [60].
Similarly, polymorphism in ABCB1 is also associated with the pharmacokinetics of other antihistamines. In a Chinese population, ABCB1 C3435T was associated with rupatadine metabolism because healthy individuals carrying homozygous T/T alleles in this locus revealed shortened time of maximal concentration (Tmax) as well as decreased area under the curve [61]. Furthermore, for ebastine, polymorphism in ABCB1 C3435T is associated with its urine excretion in humans. Participants carrying homozygous C/C alleles in this locus exhibited higher urine excretion than those with C/T and T/T alleles [62].
Azathioprine
The literature has no direct evidence indicating the association of ABCB1 polymorphism with treatment responses to azathioprine in skin disorders. However, several studies have revealed that ABCB1 polymorphism affects azathioprine toxicity and metabolism. In a Korean cohort of pediatric patients with inflammatory bowel disease, rs2032582 in ABCB1 was associated with the ratio of 6-thioguanine nucleotide to azathioprine, which is related to its toxicity [63].
Biological agents
Most of the evidence regarding the interaction of ABCB1 and biological agents arises from rheumatology patients, but this interaction may indicate possible interactions in dermatology. Etanercept downregulated ABCB1 on peripheral CD4 + and CD19 + lymphocytes in patients with rheumatoid arthritis and thus reverses the elimination of intracellular dexamethasone through ABCB1 [64]. Moreover, Yan et al. found an association of rs2032582 and rs1128503 polymorphisms of ABCB1 with treatment responsiveness to etanercept in a Chinese Han population with ankylosing spondylitis [65].
Colchicine
Colchicine is both a substrate and an inducer of ABCB1. ABCB1 was initially identified in colchicine-resistant Chinese hamster ovary cells [66]. Cellular studies have indicated that colchicine efflux, as an ABCB1 substrate, is pH-sensitive and ATPase-dependent. Lowering pH levels may result in diminished efflux and thus greater colchicine absorption [67]. Moreover, colchicine is an ABCB1 inducer. In vitro assays revealed that colchicine increased ABCB1 expression [68].
ABCB1 polymorphism has been studied in relation to treatment responsiveness to colchicine. In a Turkish cohort of patients with familial Mediterranean fever, C3435T polymorphism in ABCB1 was associated with colchicine unresponsiveness because increased T allele frequency was noted among the unresponsive group [69, 70]. Similarly, in a Turkish cohort of patients with Behçet disease, the C3435T and G2677T/A polymorphisms were associated with responsiveness to colchicine. Higher frequencies of T alleles and TT genotypes were identified in C3435T, and higher frequencies of T alleles were identified in G2677T [23]. By contrast, another Turkish group found no association between ABCB1 C3435T polymorphism and colchicine responsiveness in patients with Behçet disease [71].
Cyclosporine
Cyclosporine is regarded as a substrate, inducer, and inhibitor of ABCB1. Current evidence indicates that ABCB1 is crucial in cyclosporine metabolism and determines its treatment responsiveness. As a substrate, human studies have revealed that ABCB1 in the intestines is crucial for oral absorption of cyclosporine through the first-pass effect [72]. Moreover, cyclosporine levels in tissues, particularly the brain, was significantly higher in MDR1a-deficient mice following intravenous cyclosporine administration, which is suggestive of its roles in drug excretion [73]. Cyclosporine also inhibits ABCB1 expression in the peripheral blood monocytes of patients with psoriatic arthritis and reverses the resistance to methotrexate [74]. In practice, the coadministration of cyclosporine and colchicine was reported to cause colchicine toxicity due to cyclosporine-related ABCB1 inhibition [75]. By contrast, an early study demonstrated that cyclosporine increased the mRNA levels of ABCB1 in a colon carcinoma cell line [76]. The cell machinery, condition, and substrates may contribute to the diverse roles of cyclosporine in ABCB1 regulation.
A meta-analysis revealed that ABCB1 C3435T polymorphism is crucial for cyclosporine pharmacokinetics in kidney-transplant patients [77]. In a study of patients with Greek psoriasis, ABCB1 C3435T polymorphism was found to be associated with unresponsiveness to cyclosporine. Patients carrying allele 3435T exhibited lower ABCB1 activity compared with those carrying allele 3435C [78].
Ivermectin
ABCB1 and ivermectin in animal models
Most of the reviewed studies for ABCB1 and ivermectin were derived from investigations in canines and felines, with proposed functions as an ABCB1 substrate, inducer, and inhibitor. Ivermectin is used extensively in dermatology for the treatment of demodicosis, scabies, and various parasites and is now a candidate drug for coronavirus disease 2019 (COVID-19) in some studies [79–81]. ABCB1 is associated with resistance to ivermectin in animals because ivermectin is a substrate of ABCB1 [82]. In an early study with mouse models, Schinkel et al. observed that the neurotoxicity of systemic ivermectin was higher in mice deficient in the MDR1a gene than in normal mice. Furthermore, the ivermectin concentration was 27–87 times higher in the brains of knockout mice than in normal mice, which might be attributed to the disruption of its functions in the blood–brain barrier [7, 83, 84]. In MRD1ab-deficient mice, oral ivermectin hindered intestinal clearance and increased plasma ivermectin concentration [84].
Notably, several lines of evidence also support the notion that ivermectin is either an inhibitor or inducer of ABCB1. One study suggested that ivermectin inhibits ABCB1. The drug decreased ABCB1 expression indirectly by inhibiting EGFR and its downstream extracellular signal-regulated kinase (ERK)/Akt/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling [85]. Furthermore, ex vivo studies on rat intestines have suggested that ivermectin interferes with ABCB1-mediated efflux of the intestine lumen and reduces its ATPase activity [86–89]. However, several cell line studies have demonstrated that ivermectin induces ABCB1 expression, including the cells derived from murine and Drosophilas [90, 91].
ABCB1 and ivermectin in humans
A few investigations on human have reported that ABCB1 influences the treatment effects and adverse events of ivermectin. In a Ghanaian population with onchocerciasis, the percentage of C3435T in ABCB1 was found to be almost two times higher in suboptimal responders to ivermectin than in nonresponders [92]. Notably, in a small group of Cameroonian patients, those with ivermectin-induced severe CNS adverse events tended to exhibit a higher frequency of ABCB1 1236 T/2677G/3435 T haplotype [93]. Furthermore, one study reported that nonsense mutations of c.2380C → T and c.3053_3056delITTGA in ABCB1 resulted in ivermectin intoxication with the usual dosage in humans [94].
Methotrexate
Methotrexate is an inducer and possibly also a substrate of ABCB1. Highlighting its role as an inducer, an in vitro study revealed that the addition of methotrexate increased ABCB1 protein level in synoviocytes [95]. Furthermore, some authors proposed that methotrexate may also be a substrate of ABCB1 on the basis of the pharmacokinetics in ABCB1 polymorphism studies [96].
In dermatology, ABCB1 polymorphism is related to methotrexate responsiveness in psoriasis. In Chinese patients with psoriasis, ABCB1 rs1045642 TT genotype is associated with poor responsiveness to methotrexate, particularly in patients with moderate to severe psoriasis [97]. Other investigations involving methotrexate and ABCB1 have mainly focused on rheumatoid arthritis and malignancy. Surprisingly, in patients with systemic lupus erythematosus, relative to methotrexate nonresponders, those that responded to methotrexate exhibited a higher ABCB1 expression level in polymorphous neutrophils and mononuclear cells [98]. By contrast, in a series of studies with subcutaneous rheumatoid nodules, no correlation was observed between ABCB1 expression and methotrexate treatment responses [99].
Retinoid acids
Of various classes of retinoid acids, tretinoin was reported to induce ABCB1 expression in in vitro assays [100, 101]. Moreover, bexarotene induces ABCB1 in vitro [102]. By contrast, a cellular study revealed that retinol exerts inhibitory effects on ABCB1 expression [103].
Tacrolimus
Tacrolimus is a substrate and inhibitor of ABCB1. A meta-analysis revealed that ABCB1 C3435 polymorphism is crucial for tacrolimus pharmacokinetics in renal transplant patients [104]. For patients with rheumatoid arthritis, ABCB1 expression level correlates with treatment responsiveness to systemic corticosteroids. This phenomenon is explained by the inhibitory effects of tacrolimus on ABCB1, which then extrudes intracellular corticosteroids [105].
Tetracyclines
Cellular studies have indicated that tetracycline resistance is mediated by ABCB1 [106, 107]. Furthermore, in a study with cancer cell lines, doxycycline upregulated ABCB1 [108].
Conclusions
Growing evidence suggests that ABCB1 manipulation may be a strategy to optimize treatment effects in dermatology. First, variations in ABCB1 are associated with the risk of adverse effects of ivermectin, azathioprine, and various antihistamines. Second, variations in ABCB1 are associated with treatment responsiveness to many crucial dermatologic agents, including colchicine, biologic agents, cyclosporine, antihistamine, methotrexate, and topical steroids. Third, ABCB1 is crucial for transepidermal delivery of topical agents, notably topical corticosteroids. ABCB1 expression increases in erosive lichen planus, which may contribute to therapeutic refractoriness to topical corticosteroids. Finally, food may also play a considerable role in ABCB1 expression, and food may affect the efficacy and safety of some medications, such as cyclosporine. However, we must interpret the data carefully because many of the studies were performed in cell lines instead of primary culture and in vivo studies. The regulatory mechanism may differ among these processes, and the data do not reflect the processes in vivo. Because the role of ABCB1 in malignancy has been studied extensively, further investigation is warranted to elucidate the roles of ABCB1 in nonneoplastic dermatologic diseases.
Abbreviations
- ABCB1
ATP-binding cassette subfamily B member 1
- ATP
Adenosine triphosphate
- MDR1
Multidrug-resistant protein 1
- p-gp
Permeability glycoprotein
- SNP
Single-nucleotide polymorphism
Author contribution
TF Tsai and HJ Weng contributed equally to the conceptualization, drafting, literature search, and writing of this article.
Funding
This article was partly funded by Research Grants TMU109-AE1-B16 for Newly Hired Faculty in Taipei Medical University to HJ Weng.
Declarations
Conflict of interest
Dr. Tsen-Fang Tsai conducted clinical trials and received honoraria for serving as a consultant for Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli-Lilly, Galderma, Janssen-Cilag, Merck Sharp & Dohme, Novartis International AG, Pfizer Inc., and UCB Pharma. Dr. Hao-Jui Weng has no conflict of interest to declare.
References
- 1.Liu X (2019) ABC family transporters. In: Liu X, Pan G (eds) Drug transporters in drug disposition, effects and toxicity. Springer, Singapore, pp 13–100
- 2.Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42:1007–1017. doi: 10.1016/S0022-2275(20)31588-1. [DOI] [PubMed] [Google Scholar]
- 3.Biedler JL, Riehm H. Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Can Res. 1970;30:1174–1184. [PubMed] [Google Scholar]
- 4.Riordan JR, Deuchars K, Kartner N, Alon N, Trent J, Ling V (1985) Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. Nature 316:817–819 [DOI] [PubMed]
- 5.Dermauw W, Van Leeuwen T. The ABC gene family in arthropods: comparative genomics and role in insecticide transport and resistance. Insect Biochem Mol Biol. 2014;45:89–110. doi: 10.1016/j.ibmb.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Silva R, Vilas-Boas V, Carmo H, Dinis-Oliveira RJ, Carvalho F, de Lourdes BM, Remião F. Modulation of P-glycoprotein efflux pump: induction and activation as a therapeutic strategy. Pharmacol Ther. 2015;149:1–123. doi: 10.1016/j.pharmthera.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Schinkel A, Smit J, van Tellingen M, Beijnen J, Wagenaar E, Van Deemter L, Mol C, Van der Valk M, Robanus-Maandag E, Te Riele H. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 8.Fu D, Arias IM. Intracellular trafficking of P-glycoprotein. Int J Biochem Cell Biol. 2012;44:461–464. doi: 10.1016/j.biocel.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tulsyan S, Mittal RD, Mittal B. The effect of ABCB1 polymorphisms on the outcome of breast cancer treatment. Pharmacogenomics Person Med. 2016;9:47. doi: 10.2147/PGPM.S86672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong J, Guo Z, Fan L, Zhao X, Zhao B, Cao Z, Cheng L, Shi Y, Li X, Zhang Y. ABCB1 polymorphism predicts the toxicity and clinical outcome of lung cancer patients with taxane-based chemotherapy. Thorac Cancer. 2019;10:2088–2095. doi: 10.1111/1759-7714.13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panczyk M, Balcerczak E, Piaskowski S, Jamroziak K, Pasz-Walczak G, Mirowski M. ABCB1 gene polymorphisms and haplotype analysis in colorectal cancer. Int J Colorectal Dis. 2009;24:895–905. doi: 10.1007/s00384-009-0724-0. [DOI] [PubMed] [Google Scholar]
- 12.Caronia D, Patino-Garcia A, Peréz-Martínez A, Pita G, Moreno LT, Zalacain-Díez M, Molina B, Colmenero I, Sierrasesúmaga L, Benítez J (2011) Effect of ABCB1 and ABCC3 polymorphisms on osteosarcoma survival after chemotherapy: a pharmacogenetic study. PLoS One 6:e26091 [DOI] [PMC free article] [PubMed]
- 13.Leschziner G, Andrew T, Pirmohamed M, Johnson M. ABCB1 genotype and PGP expression, function and therapeutic drug response: a critical review and recommendations for future research. Pharmacogenomics J. 2007;7:154–179. doi: 10.1038/sj.tpj.6500413. [DOI] [PubMed] [Google Scholar]
- 14.Yamagishi T, Sahni S, Sharp DM, Arvind A, Jansson PJ, Richardson DR. P-glycoprotein mediates drug resistance via a novel mechanism involving lysosomal sequestration. J Biol Chem. 2013;288:31761–31771. doi: 10.1074/jbc.M113.514091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schadendorf D, Herfordt R, Czarnetzki B. P-glycoprotein expression in primary and metastatic malignant melanoma. Br J Dermatol. 1995;132:551–555. doi: 10.1111/j.1365-2133.1995.tb08710.x. [DOI] [PubMed] [Google Scholar]
- 16.Heimerl S, Bosserhoff AK, Langmann T, Ecker J, Schmitz G. Mapping ATP-binding cassette transporter gene expression profiles in melanocytes and melanoma cells. Melanoma Res. 2007;17:265–273. doi: 10.1097/CMR.0b013e3282a7e0b9. [DOI] [PubMed] [Google Scholar]
- 17.Berger W, Elbling L, Minai-Pour M, Vetterlein M, Pirker R, Kokoschka EM, Micksche M. Intrinsic MDR-1 gene and P-glycoprotein expression in human melanoma cell lines. Int J Cancer. 1994;59:717–723. doi: 10.1002/ijc.2910590522. [DOI] [PubMed] [Google Scholar]
- 18.Wouters J, Stas M, Gremeaux L, Govaere O, Maes H, Agostinis P, Roskams T, van den Oord JJ, Vankelecom H (2013) The human melanoma side population displays molecular and functional characteristics of enriched chemoresistance and tumorigenesis. PLoS One 8:e76550 [DOI] [PMC free article] [PubMed]
- 19.Landreville S, Agapova OA, Kneass ZT, Salesse C, William Harbour J. ABCB1 identifies a subpopulation of uveal melanoma cells with high metastatic propensity. Pigment Cell Melanoma Res. 2011;24:430–437. doi: 10.1111/j.1755-148X.2011.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto N, Nakamichi N, Nanmo H, Kimura K-i, Masuo Y, Sakai Y, Schinkel AH, Sato S, Soga T, Kato Y. Metabolome analysis reveals dermal histamine accumulation in murine dermatitis provoked by genetic deletion of P-glycoprotein and breast cancer resistance protein. Pharm Res. 2019;36:158. doi: 10.1007/s11095-019-2695-3. [DOI] [PubMed] [Google Scholar]
- 21.Rychlik‐Sych M, Barańska M, Dudarewicz M, Skrętkowicz J, Żebrowska A, Owczarek J, Waszczykowska E (2017) ABCB1 gene is associated with the risk of bullous pemphigoid in a polish population. JDDG: Journal der Deutschen Dermatologischen Gesellschaft 15:499–505 [DOI] [PubMed]
- 22.Rychlik-Sych M, Barańska M, Dudarewicz M, Skrętkowicz J, Żebrowska A, Woźniacka A, Owczarek J, Orszulak-Michalak D, Waszczykowska E. Haplotypes of ABCB1 1236C> T (rs1128503), 2677G> T/A (rs2032582), and 3435C> T (rs1045642) in patients with bullous pemphigoid. Arch Dermatol Res. 2018;310:515–522. doi: 10.1007/s00403-018-1842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rustemoglu A, Gül Ü, Gümüş-Akay G, Gönül M, Yiğit S, Bozkurt N, Karadağ A, Pişkin E, Sunguroğlu A, Kadıkıran A. MDR1 gene polymorphisms may be associated with Behçet's disease and its colchicum treatment response. Gene. 2012;505:333–339. doi: 10.1016/j.gene.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 24.Bonyadi M, Gholizadeh M, Soltan-Ali M. MDR 1 C3435T polymorphism associated with the development of clinical features in Behçet's disease in Iranian Azeri Turkish patients. Int J Dermatol. 2014;53:1235–1240. doi: 10.1111/ijd.12540. [DOI] [PubMed] [Google Scholar]
- 25.Jana A, Thomas J, Ghosh P (2017) P-glycoprotein expression in oral lichen planus. Braz Oral Res 31 [DOI] [PubMed]
- 26.Skazik C, Wenzel J, Marquardt Y, Kim A, Merk HF, Bickers DR, Baron JM. P-Glycoprotein (ABCB1) expression in human skin is mainly restricted to dermal components. Exp Dermatol. 2011;20:450–452. doi: 10.1111/j.1600-0625.2010.01237.x. [DOI] [PubMed] [Google Scholar]
- 27.Ito K, Nguyen HT, Kato Y, Wakayama T, Kubo Y, Iseki S, Tsuji A. P-glycoprotein (Abcb1) is involved in absorptive drug transport in skin. J Control Release. 2008;131:198–204. doi: 10.1016/j.jconrel.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Syrovets T, Kess D, Büchele B, Hainzl H, Lunov O, Weiss JM, Scharffetter-Kochanek K, Simmet T. Targeting NF-κB with a natural triterpenoid alleviates skin inflammation in a mouse model of psoriasis. J Immunol. 2009;183:4755–4763. doi: 10.4049/jimmunol.0900521. [DOI] [PubMed] [Google Scholar]
- 29.Mizuno K, Akimoto N, Kawamura M, Nakase K, Noguchi N, Sato T. Involvement of adenosine triphosphate-binding cassette subfamily B member 1 in the augmentation of triacylglycerol excretion by Propionibacterium acnes in differentiated hamster sebocytes. J Dermatol. 2017;44:1404–1407. doi: 10.1111/1346-8138.13963. [DOI] [PubMed] [Google Scholar]
- 30.Haslam IS, El-Chami C, Faruqi H, Shahmalak A, O'Neill C, Paus R. Differential expression and functionality of ATP-binding cassette transporters in the human hair follicle. Br J Dermatol. 2015;172:1562–1572. doi: 10.1111/bjd.13549. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto N, Nakamichi N, Yamazaki E, Oikawa M, Masuo Y, Schinkel AH, Kato Y. P-Glycoprotein in skin contributes to transdermal absorption of topical corticosteroids. Int J Pharm. 2017;521:365–373. doi: 10.1016/j.ijpharm.2017.02.064. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto N, Nakamichi N, Uwafuji S, Yoshida K, Sugiura T, Tsuji A, Kato Y. ATP binding cassette transporters in two distinct compartments of the skin contribute to transdermal absorption of a typical substrate. J Control Release. 2013;165:54–61. doi: 10.1016/j.jconrel.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Yu C, Hsieh Y, Lin S, Chi Y, Hariharan P, Chao P, Hou Y. Potential modulation on P-glycoprotein and CYP3A by soymilk and miso: in vivo and ex-vivo studies. Food Chem. 2014;149:25–30. doi: 10.1016/j.foodchem.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 34.Yang M-S, Yu C-P, Huang C-Y, Chao P-DL, Lin S-P, Hou Y-C. Aloe activated P-glycoprotein and CYP 3A: a study on the serum kinetics of aloe and its interaction with cyclosporine in rats. Food Funct. 2017;8:315–322. doi: 10.1039/C6FO00938G. [DOI] [PubMed] [Google Scholar]
- 35.Bogacz A, Deka-Pawlik D, Bartkowiak-Wieczorek J, Karasiewicz M, Kujawski R, Kowalska A, Chałas A, Czerny B, Grześkowiak E, Mrozikiewicz PM. The effect of herbal materials on the P-glycoprotein activity and function. Herba Polonica. 2013;59:129–141. doi: 10.2478/hepo-2013-0029. [DOI] [Google Scholar]
- 36.Yang F, Dong X, Yin X, Wang W, You L, Ni J (2017) Radix Bupleuri: a review of traditional uses, botany, phytochemistry, pharmacology, and toxicology. Biomed Res Int 2017 [DOI] [PMC free article] [PubMed]
- 37.Yu C-P, Lin H-J, Lin S-P, Shia C-S, Chang P-H, Hou Y-C, Hsieh Y-W. Rhubarb decreased the systemic exposure of cyclosporine, a probe substrate of P-glycoprotein and CYP 3A. Xenobiotica. 2016;46:677–682. doi: 10.3109/00498254.2015.1117159. [DOI] [PubMed] [Google Scholar]
- 38.Roma MI, Lampropulos VES, Ayllón-Cabrera I, Sanabria ANS, Nigro MML, Peroni RN, Carballo MA (2019) Modulation of hepatic ABC transporters by Eruca vesicaria intake: potential diet-drug interactions. Food Chem Toxicol 133:110797 [DOI] [PubMed]
- 39.Peng Y-H, Lin S-P, Yu C-P, Tsai S-Y, Chen M-Y, Hou Y-C, Chao P-DL. Serum concentrations of anthraquinones after intake of Folium Sennae and potential modulation on P-glycoprotein. Planta Med. 2014;80:1291–1297. doi: 10.1055/s-0034-1383040. [DOI] [PubMed] [Google Scholar]
- 40.Mohana S, Ganesan M, Agilan B, Karthikeyan R, Srithar G, Mary RB, Ananthakrishnan D, Velmurugan D, Prasad NR, Ambudkar SV. Screening dietary flavonoids for the reversal of P-glycoprotein-mediated multidrug resistance in cancer. Mol BioSyst. 2016;12:2458–2470. doi: 10.1039/C6MB00187D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mares-Sámano S, Badhan R, Penny J. Identification of putative steroid-binding sites in human ABCB1 and ABCG2. Eur J Med Chem. 2009;44:3601–3611. doi: 10.1016/j.ejmech.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 42.Abe Y, Shimizu K, Katayama I. Significance of MDR1-Gene and P-glycoprotein (P-gp) expressions in the lesional skin of psoriasis vulgaris. Acta Med Nagasaki. 2001;46:19–24. [Google Scholar]
- 43.Osman-Ponchet H, Boulai A, Kouidhi M, Sevin K, Alriquet M, Gaborit A, Bertino B, Comby P, Ruty B. Characterization of ABC transporters in human skin. Drug Metab Drug Interact. 2014;29:91–100. doi: 10.1515/dmdi-2013-0042. [DOI] [PubMed] [Google Scholar]
- 44.Manceau S, Giraud C, Declèves X, Batteux F, Chéreau C, Chouzenoux S, Scherrmann J-M, Weill B, Perrot J-Y, Tréluyer J-M. Expression and induction by dexamethasone of ABC transporters and nuclear receptors in a human T-lymphocyte cell line. J Chemother. 2012;24:48–55. doi: 10.1179/1120009X12Z.00000000010. [DOI] [PubMed] [Google Scholar]
- 45.Peng R, Zhang H, Zhang Y, Wei D-Y. Impacts of ABCB1 (G1199A) polymorphism on resistance, uptake, and efflux to steroid drugs. Xenobiotica. 2016;46:948–952. doi: 10.3109/00498254.2016.1138249. [DOI] [PubMed] [Google Scholar]
- 46.Wang E-j, Lew K, Casciano CN, Clement RP, Johnson WW. Interaction of common azole antifungals with P glycoprotein. Antimicrob Agents Chemother. 2002;46:160–165. doi: 10.1128/AAC.46.1.160-165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zolnerciks JK, Booth-Genthe CL, Gupta A, Harris J, Unadkat JD. Substrate-and species-dependent inhibition of P-glycoprotein-mediated transport: implications for predicting in vivo drug interactions. J Pharm Sci. 2011;100:3055–3061. doi: 10.1002/jps.22566. [DOI] [PubMed] [Google Scholar]
- 48.Broccatelli F, Carosati E, Cruciani G, Oprea TI. Transporter-mediated efflux influences CNS side effects: ABCB1, from antitarget to target. Mol Inf. 2010;29:16–26. doi: 10.1002/minf.200900075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agrawal DK. Pharmacology and clinical efficacy of desloratadine as an anti-allergic and anti-inflammatory drug. Expert Opin Investig Drugs. 2001;10:547–560. doi: 10.1517/13543784.10.3.547. [DOI] [PubMed] [Google Scholar]
- 50.Abbasi MM, Valizadeh H, Hamishekar H, Mohammadnejad L, Zakeri-Milani P. The effects of cetirizine on P-glycoprotein expression and function in vitro and in situ. Adv Pharm Bull. 2016;6:111. doi: 10.15171/apb.2016.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perloff MD, Störmer E, von Moltke LL, Greenblatt DJ. Rapid assessment of P-glycoprotein inhibition and induction in vitro. Pharm Res. 2003;20:1177–1183. doi: 10.1023/A:1025092829696. [DOI] [PubMed] [Google Scholar]
- 52.Ming X, Knight BM, Thakker DR. Vectorial transport of fexofenadine across Caco-2 cells: involvement of apical uptake and basolateral efflux transporters. Mol Pharm. 2011;8:1677–1686. doi: 10.1021/mp200026v. [DOI] [PubMed] [Google Scholar]
- 53.Kitamura Y, Koto H, Matsuura S, Kawabata T, Tsuchiya H, Kusuhara H, Tsujimoto H, Sugiyama Y. Modest effect of impaired P-glycoprotein on the plasma concentrations of fexofenadine, quinidine, and loperamide following oral administration in collies. Drug Metab Dispos. 2008;36:807–810. doi: 10.1124/dmd.107.017624. [DOI] [PubMed] [Google Scholar]
- 54.Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27:866–871. [PubMed] [Google Scholar]
- 55.Li F, Howard KD, Myers MJ. Influence of P-glycoprotein on the disposition of fexofenadine and its enantiomers. J Pharm Pharmacol. 2017;69:274–284. doi: 10.1111/jphp.12687. [DOI] [PubMed] [Google Scholar]
- 56.Drescher S, Schaeffeler E, Hitzl M, Hofmann U, Schwab M, Brinkmann U, Eichelbaum M, Fromm MF. MDR1 gene polymorphisms and disposition of the P-glycoprotein substrate fexofenadine. Br J Clin Pharmacol. 2002;53:526–534. doi: 10.1046/j.1365-2125.2002.01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yi SY, Hong KS, Lim HS, Chung JY, Oh DS, Kim JR, Jung HR, Cho JY, Yu KS, Jang IJ. A variant 2677A allele of the MDR1 gene affects fexofenadine disposition. Clin Pharmacol Ther. 2004;76:418–427. doi: 10.1016/j.clpt.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Liu S, Beringer PM, Hidayat L, Rao AP, Louie S, Burckart GJ, Shapiro B. Probenecid, but not cystic fibrosis, alters the total and renal clearance of fexofenadine. J Clin Pharmacol. 2008;48:957–965. doi: 10.1177/0091270008319707. [DOI] [PubMed] [Google Scholar]
- 59.Vanhove T, Bouillon T, de Loor H, Annaert P, Kuypers D. Fexofenadine, a putative in vivo P-glycoprotein probe, fails to predict clearance of the substrate tacrolimus in renal recipients. Clin Pharmacol Ther. 2017;102:989–996. doi: 10.1002/cpt.718. [DOI] [PubMed] [Google Scholar]
- 60.Alzoubi KH, KhabourQ OF, Sayer I, Mayyasl F. The role of multidrug resistance-1 (MDR1) variants in response to fexofenadine among. Int J Clin Pharmacol Ther. 2013;51:880–887. doi: 10.5414/CP201968. [DOI] [PubMed] [Google Scholar]
- 61.Xiong Y, Yuan Z, Yang J, Xia C, Li X, Huang S, Zhang H, Liu M. CYP3A5* 3 and MDR1 C3435T are influencing factors of inter-subject variability in rupatadine pharmacokinetics in healthy Chinese volunteers. Eur J Drug Metab Pharmacokinet. 2016;41:117–124. doi: 10.1007/s13318-014-0236-3. [DOI] [PubMed] [Google Scholar]
- 62.Gervasini G, Vizcaino S, Carrillo JA, Caballero MJ, Benitez J. The effect of CYP2J2, CYP3A4, CYP3A5 and the MDR1 C3435T polymorphisms and gender on the urinary excretion of the metabolites of the H1-receptor antihistamine ebastine: a pilot study. Br J Clin Pharmacol. 2006;62:177–186. doi: 10.1111/j.1365-2125.2006.02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee M-N, Kang B, Choi SY, Kim MJ, Woo SY, Kim J-W, Choe YH, Lee S-Y. Impact of genetic polymorphisms on 6-thioguanine nucleotide levels and toxicity in pediatric patients with IBD treated with azathioprine. Inflamm Bowel Dis. 2015;21:2897–2908. doi: 10.1097/MIB.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 64.Tsujimura S, Saito K, Nakayamada S, Tanaka Y. Etanercept overcomes P-glycoprotein-induced drug resistance in lymphocytes of patients with intractable rheumatoid arthritis. Mod Rheumatol. 2010;20:139–146. doi: 10.3109/s10165-009-0247-0. [DOI] [PubMed] [Google Scholar]
- 65.Yan R-J, Lou T-T, Wu Y-F, Chen W-S (2017) Single nucleotide polymorphisms of ABCB1 gene and response to etanercept treatment in patients with ankylosing spondylitis in a Chinese Han population. Medicine 96 [DOI] [PMC free article] [PubMed]
- 66.Juliano RL, Ling V (1976) A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochimica et Biophysica Acta (BBA)-Biomembranes 455:152–162 [DOI] [PubMed]
- 67.Mitra P, Audus K, Williams G, Yazdanian M, Galinis D. A comprehensive study demonstrating that p-glycoprotein function is directly affected by changes in pH: Implications for intestinal pH and effects on drug absorption. J Pharm Sci. 2011;100:4258–4268. doi: 10.1002/jps.22596. [DOI] [PubMed] [Google Scholar]
- 68.Silva R, Carmo H, Vilas-Boas V, Barbosa DJ, Palmeira A, Sousa E, Carvalho F, de Lourdes BM, Remião F. Colchicine effect on P-glycoprotein expression and activity: In silico and in vitro studies. Chem Biol Interact. 2014;218:50–62. doi: 10.1016/j.cbi.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 69.Ozen F, Silan C, Uludag A, Candan F, Silan F, Ozdemir S, Atik S, Ozdemir O. Association between ABCB1 (MDR1) gene 3435 C> T polymorphism and colchicine unresponsiveness of FMF patients. Ren Fail. 2011;33:899–903. doi: 10.3109/0886022X.2011.605980. [DOI] [PubMed] [Google Scholar]
- 70.Uludag A, Silan C, Atik S, Akurut C, Uludag A, Silan F, Ozdemir O. Relationship between response to colchicine treatment and MDR1 polymorphism in familial Mediterranean fever patients. Genet Test Mol Biomarkers. 2014;18:73–76. doi: 10.1089/gtmb.2013.0293. [DOI] [PubMed] [Google Scholar]
- 71.Saricaoglu H, Yilmaz M, Karkucak M, Ozturk H, Yakut T, Gulten T, Baskan E, Aydogan K, Dilek K. Investigation of ABCB 1 gene polymorphism with coichicine response in Behcet's disease. Genet Mol Res. 2011;10:1–6. doi: 10.4238/vol10-1gmr824. [DOI] [PubMed] [Google Scholar]
- 72.Lown KS, Mayo RR, Leichtman AB, Hl H, Turgeon DK, Schmiedlin-Ren P, Brown MB, Guo W, Rossi SJ, Benet LZ. Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine. Clin Pharmacol Ther. 1997;62:248–260. doi: 10.1016/S0009-9236(97)90027-8. [DOI] [PubMed] [Google Scholar]
- 73.Schinkel AH, Wagenaar E, van Deemter L, Mol C, Borst P. Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Investig. 1995;96:1698–1705. doi: 10.1172/JCI118214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diamanti AP, Rosado M, Germano V, Scarsella M, Giorda E, Podestà E, D'Amelio R, Carsetti R, Laganà B. Reversion of resistance to immunosuppressive agents in three patients with psoriatic arthritis by cyclosporine A: Modulation of P-glycoprotein function. Clin Immunol. 2011;138:9–13. doi: 10.1016/j.clim.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Eleftheriou G, Bacis G, Fiocchi R, Sebastiano R. Colchicine-induced toxicity in a heart transplant patient with chronic renal failure. Clin Toxicol. 2008;46:827–830. doi: 10.1080/15563650701779703. [DOI] [PubMed] [Google Scholar]
- 76.Herzog CE, Tsokos M, Bates S, Fojo A. Increased mdr-1/P-glycoprotein expression after treatment of human colon carcinoma cells with P-glycoprotein antagonists. J Biol Chem. 1993;268:2946–2952. doi: 10.1016/S0021-9258(18)53865-5. [DOI] [PubMed] [Google Scholar]
- 77.Lee J, Wang R, Yang Y, Lu X, Zhang X, Wang L, Lou Y. The Effect of ABCB 1 C 3435 T Polymorphism on Cyclosporine Dose Requirements in Kidney Transplant Recipients: A Meta-Analysis. Basic Clin Pharmacol Toxicol. 2015;117:117–125. doi: 10.1111/bcpt.12371. [DOI] [PubMed] [Google Scholar]
- 78.Vasilopoulos Y, Sarri C, Zafiriou E, Patsatsi A, Stamatis C, Ntoumou E, Fassos I, Tsalta A, Karra A, Roussaki-Schulze A. A pharmacogenetic study of ABCB1 polymorphisms and cyclosporine treatment response in patients with psoriasis in the Greek population. Pharmacogenomics J. 2014;14:523. doi: 10.1038/tpj.2014.23. [DOI] [PubMed] [Google Scholar]
- 79.Padhy BM, Mohanty RR, Das S, Meher BR. Therapeutic potential of ivermectin as add on treatment in COVID 19: A systematic review and meta-analysis: Ivermectin in COVID-19: A meta-analysis. J Pharm Pharm Sci. 2020;23:462–469. doi: 10.18433/jpps31457. [DOI] [PubMed] [Google Scholar]
- 80.Martin RJ, Robertson AP, Choudhary S (2020) Ivermectin: An Anthelmintic, an Insecticide, and Much More. Trends Parasitol [DOI] [PMC free article] [PubMed]
- 81.Chang Y-W, Tseng H-C. The gift of honeymoon: An interesting case of furuncular myiasis caused by Dermatobia Hominis in Taiwan and review of the literature. Dermatol Sin. 2019;37:93. doi: 10.4103/ds.ds_27_18. [DOI] [Google Scholar]
- 82.Burkhart CN. Ivermectin: an assessment of its pharmacology, microbiology and safety. Vet Hum Toxicol. 2000;42:30–35. [PubMed] [Google Scholar]
- 83.Geyer J, Gavrilova O, Petzinger E. Brain penetration of ivermectin and selamectin in mdr1a, b P-glycoprotein-and bcrp-deficient knockout mice. J Vet Pharmacol Ther. 2009;32:87–96. doi: 10.1111/j.1365-2885.2008.01007.x. [DOI] [PubMed] [Google Scholar]
- 84.Kiki-Mvouaka S, Menez C, Borin C, Lyazrhi F, Foucaud-Vignault M, Dupuy J, Collet X, Alvinerie M, Lespine A. Role of P-glycoprotein in the disposition of macrocyclic lactones: a comparison between ivermectin, eprinomectin, and moxidectin in mice. Drug Metab Dispos. 2010;38:573–580. doi: 10.1124/dmd.109.030700. [DOI] [PubMed] [Google Scholar]
- 85.Jiang L, Wang P, Sun Y-J, Wu Y-J. Ivermectin reverses the drug resistance in cancer cells through EGFR/ERK/Akt/NF-κB pathway. J Exp Clin Cancer Res. 2019;38:265. doi: 10.1186/s13046-019-1251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ballent M, Maté L, Virkel G, Sallovitz J, Viviani P, Lanusse C, Lifschitz A. Intestinal drug transport: ex vivo evaluation of the interactions between ABC transporters and anthelmintic molecules. J Vet Pharmacol Ther. 2014;37:332–337. doi: 10.1111/jvp.12112. [DOI] [PubMed] [Google Scholar]
- 87.Ballent M, Lifschitz A, Virkel G, Sallovitz J, Lanusse C. Modulation of the P-glycoprotein-mediated intestinal secretion of ivermectin: in vitro and in vivo assessments. Drug Metab Dispos. 2006;34:457–463. doi: 10.1124/dmd.105.007757. [DOI] [PubMed] [Google Scholar]
- 88.Lespine A, Dupuy J, Orlowski S, Nagy T, Glavinas H, Krajcsi P, Alvinerie M. Interaction of ivermectin with multidrug resistance proteins (MRP1, 2 and 3) Chem Biol Interact. 2006;159:169–179. doi: 10.1016/j.cbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 89.Dupuy J, Alvinerie M, Ménez C, Lespine A. Interaction of anthelmintic drugs with P-glycoprotein in recombinant LLC-PK1-mdr1a cells. Chem Biol Interact. 2010;186:280–286. doi: 10.1016/j.cbi.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 90.Ménez C, Mselli-Lakhal L, Foucaud-Vignault M, Balaguer P, Alvinerie M, Lespine A. Ivermectin induces P-glycoprotein expression and function through mRNA stabilization in murine hepatocyte cell line. Biochem Pharmacol. 2012;83:269–278. doi: 10.1016/j.bcp.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 91.Luo L, Sun Y-J, Yang L, Huang S, Wu Y-J. Avermectin induces P-glycoprotein expression in S2 cells via the calcium/calmodulin/NF-κB pathway. Chem Biol Interact. 2013;203:430–439. doi: 10.1016/j.cbi.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 92.Kudzi W, Dodoo AN, Mills JJ. Genetic polymorphisms in MDR1, CYP3A4 and CYP3A5 genes in a Ghanaian population: a plausible explanation for altered metabolism of ivermectin in humans? BMC Med Genet. 2010;11:111. doi: 10.1186/1471-2350-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bourguinat C, Kamgno J, Boussinesq M, Mackenzie CD, Prichard RK, Geary TG. Analysis of the mdr-1 gene in patients co-infected with Onchocerca volvulus and Loa loa who experienced a post-ivermectin serious adverse event. Am J Trop Med Hyg. 2010;83:28–32. doi: 10.4269/ajtmh.2010.09-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baudou E, Lespine A, Durrieu G, André F, Gandia P, Durand C, Cunat S. Serious ivermectin toxicity and human ABCB1 nonsense mutations. N Engl J Med. 2020;383:787–789. doi: 10.1056/NEJMc1917344. [DOI] [PubMed] [Google Scholar]
- 95.Qin K, Chen K, Zhao W, Zhao X, Luo J, Wang Q, Gao C, Li X, Wang C (2018) Methotrexate combined with 4-hydroperoxycyclophosphamide downregulates multidrug-resistance P-glycoprotein expression induced by methotrexate in rheumatoid arthritis fibroblast-like synoviocytes via the JAK2/STAT3 pathway. J Immunol 2018 [DOI] [PMC free article] [PubMed]
- 96.Gregers J, Green H, Christensen I, Dalhoff K, Schroeder H, Carlsen N, Rosthoej S, Lausen B, Schmiegelow K, Peterson C. Polymorphisms in the ABCB1 gene and effect on outcome and toxicity in childhood acute lymphoblastic leukemia. Pharmacogenomics J. 2015;15:372–379. doi: 10.1038/tpj.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen M, Chen W, Liu P, Yan K, Lv C, Zhang M, Lu Y, Qin Q, Kuang Y, Zhu W. The impacts of gene polymorphisms on methotrexate in Chinese psoriatic patients. J Eur Acad Dermatol Venereol. 2020;34:2059–2065. doi: 10.1111/jdv.16440. [DOI] [PubMed] [Google Scholar]
- 98.García-Carrasco M, Mendoza-Pinto C, Macías-Díaz S, Etchegaray-Morales I, Méndez-Martínez S, Soto-Santillán P, Pérez-Romano B, Jiménez-Herrera EA, Guzmán-Ruiz O, Ruiz-Argüelles A. Clinical relevance of P-glycoprotein activity on peripheral blood mononuclear cells and polymorphonuclear neutrophils to methotrexate in systemic lupus erythematosus patients. Clin Rheumatol. 2017;36:2267–2272. doi: 10.1007/s10067-017-3728-0. [DOI] [PubMed] [Google Scholar]
- 99.Houlder E, Millier M, Highton J, Gwynne-Jones D, Stamp L, Hessian P. Expression of the genes facilitating methotrexate action within subcutaneous rheumatoid nodules. Clin Exp Rheumatol. 2017;35:943–947. [PubMed] [Google Scholar]
- 100.Inami K, Sasaki T, Kumagai T, Nagata K. Simultaneous evaluation of human CYP3A4 and ABCB1 induction by reporter assay in LS174T cells, stably expressing their reporter genes. Biopharm Drug Dispos. 2015;36:139–147. doi: 10.1002/bdd.1927. [DOI] [PubMed] [Google Scholar]
- 101.Zhang L, Yan Y, Zhu D, Yang W, Wang W, Hu Y, Yang B, He Q. Nutlin-1 strengthened anti-proliferation and differentiation-inducing activity of ATRA in ATRA-treated p-glycoprotein deregulated human myelocytic leukemia cells. Invest New Drugs. 2012;30:37–47. doi: 10.1007/s10637-010-9512-5. [DOI] [PubMed] [Google Scholar]
- 102.Kuntz M, Candela P, Saint-Pol J, Lamartiniere Y, Boucau M-C, Sevin E, Fenart L, Gosselet F. Bexarotene promotes cholesterol efflux and restricts apical-to-basolateral transport of amyloid-β peptides in an in vitro model of the human blood-brain barrier. J Alzheimers Dis. 2015;48:849–862. doi: 10.3233/JAD-150469. [DOI] [PubMed] [Google Scholar]
- 103.Klamt F, Passos DT, Castro MAA, Gelain DP, Grivicich I, Moreira JCF. Inhibition of MDR1 expression by retinol treatment increases sensitivity to etoposide (VP16) in human neoplasic cell line. Toxicol In Vitro. 2008;22:873–878. doi: 10.1016/j.tiv.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 104.Li Y, Hu X, Cai B, Chen J, Bai Y, Tang J, Liao Y, Wang L. Meta-analysis of the effect of MDR1 C3435 polymorphism on tacrolimus pharmacokinetics in renal transplant recipients. Transpl Immunol. 2012;27:12–18. doi: 10.1016/j.trim.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 105.Suzuki K, Saito K, Tsujimura S, Nakayamada S, Yamaoka K, Sawamukai N, Iwata S, Nawata M, Nakano K, Tanaka Y. Tacrolimus, a calcineurin inhibitor, overcomes treatment unresponsiveness mediated by P-glycoprotein on lymphocytes in refractory rheumatoid arthritis. J Rheumatol. 2010;37:512–520. doi: 10.3899/jrheum.090048. [DOI] [PubMed] [Google Scholar]
- 106.Kavallaris M, Madafiglio J, Norris M, Haber M. Resistance to tetracycline, a hydrophilic antibiotic, is mediated by P-glycoprotein in human multidrug-resistant cells. Biochem Biophys Res Commun. 1993;190:79–85. doi: 10.1006/bbrc.1993.1013. [DOI] [PubMed] [Google Scholar]
- 107.George AM, Davey MW, Mir AA. Functional expression of the human MDR1 gene in Escherichia coli. Arch Biochem Biophys. 1996;333:66–74. doi: 10.1006/abbi.1996.0365. [DOI] [PubMed] [Google Scholar]
- 108.Mealey KL, Barhoumi R, Burghardt RC, Safe S, Kochevar DT. Doxycycline induces expression of P glycoprotein in MCF-7 breast carcinoma cells. Antimicrob Agents Chemother. 2002;46:755–761. doi: 10.1128/AAC.46.3.755-761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He XJ, Jian LY, He XL, Tang M, Wu Y, Xu YY, Sun XJ, Zhao LM. Association of ABCB1, CYP3A4, EPHX1, FAS, SCN1A, MICA, and BAG6 polymorphisms with the risk of carbamazepine-induced S tevens-J ohnson syndrome/toxic epidermal necrolysis in C hinese H an patients with epilepsy. Epilepsia. 2014;55:1301–1306. doi: 10.1111/epi.12655. [DOI] [PubMed] [Google Scholar]
- 110.Ciccacci C, Di Fusco D, Marazzi MC, Zimba I, Erba F, Novelli G, Palombi L, Borgiani P, Liotta G. Association between CYP2B6 polymorphisms and Nevirapine-induced SJS/TEN: a pharmacogenetics study. Eur J Clin Pharmacol. 2013;69:1909–1916. doi: 10.1007/s00228-013-1549-x. [DOI] [PubMed] [Google Scholar]
- 111.Yaşar Ü. The role of pharmacogenetics of cytochrome P450s in phenytoin-induced DRESS syndrome. Cent Eur J Immunol. 2018;43:220. doi: 10.5114/ceji.2018.77393. [DOI] [PMC free article] [PubMed] [Google Scholar]