Abstract
Background
Currently, three chimeric antigen receptor (CAR)-T cell products axicabtagene ciloleucel, tisagenlecleucel, and lisocabtagene maraleucel have been approved by the U.S. Food and Drug Administration for the treatment of large B cell lymphoma, which provide a novel and promising choice for patients with relapsed or refractory to traditional anti-tumor treatments. Thus, it is pertinent to describe the efficacy and safety profile of the three products available by summarizing the current evidence.
Methods
Two reviewers independently searched the Embase, PubMed, Web of Science, and Cochrane Library, to identify studies related to the use of the three CAR-T cell products for treating hematologic malignancies published up to October 5, 2020. We pooled the overall response rate, complete response rate, cytokine release syndrome, and immune effector cell-associated neurotoxicity syndrome of three products, and then performed subgroup analysis based on the type of product and type of tumor.
Results
Thirty-three studies involving 2,172 patients were included in the analysis. All three products showed promising results in patients with different pathological subtypes and clinical characteristics that included those who did not meet the eligibility criteria of licensing trials, with overall response rates of nearly 70% or above and complete response rates of more than 50%. However, high rates of severe immune effector cell-associated neurotoxicity syndrome in patients undergoing axicabtagene ciloleucel treatment and life-threatening cytokine release syndrome in patients with leukemia undergoing tisagenlecleucel treatment required special attention in practice (31%; 95% CI: 0.27–0.35 and 55%; 95% CI: 0.45–0.64, respectively). Moreover, lisocabtagene maraleucel that showed a favorable efficacy and safety in the licensing trial lacked corresponding real-world data.
Conclusion
Both axicabtagene ciloleucel and tisagenlecleucel showed considerable efficacy in practice, but need special attention with respect to life-threatening toxicity that can occur in certain situations. Lisocabtagene maraleucel demonstrated excellent efficacy and safety profiles in the licensing trial, but lacked corresponding real-world data. Additional data on the three products are needed in rare histological subtypes to benefit a broader patient population.
Keywords: chimeric antigen receptor T-cell product, CAR-T cell therapy, immunotherapy, lymphoma, leukemia, hematologic malignancy, efficacy, safety
Introduction
First conceptualized in the late 1980s, chimeric antigen receptor (CAR)-T cell therapy has developed rapidly over the decade and is considered one of the most promising treatments for hematologic malignancies (1). CAR-T cell therapy involves injecting of genetically modified autologous or allogeneic T cells into the patient to specifically target patient’s tumor cells (2). The efficacy of CAR T-cell therapy appears considerably better than that of traditional chemotherapy and autologous/allogeneic stem cell transplant in the setting of relapsed/refractory disease, however, it is associated with potentially fatal side effects such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) (3).
A CAR consists of antigen-binding domains (most commonly, a single-chain variable fragment), transmembrane domains, signaling domains, and additional co-stimulatory domains (2, 4). To date, CAR-T cells have progressed from the first generation to the fourth generation. The main difference between the first- and second-generation CAR-T cells is the incorporation of co-stimulatory endodomain. The anti-tumor activity of the first-generation CARs is disappointing because it only contains CD3ζ signaling domain, while the second-generation CARs possess one co-stimulatory endodomain (CD28, 4-1BB, or OX40) incorporated with CD3ζ, which can effectively promote T cell activation and prevent apoptosis. The third-generation CAR-T cells contain multiple co-stimulatory domains, and the fourth-generation CAR-T cells, also called TRUCKs, need additional clinical data to demonstrate their safety and efficacy (4, 5). Currently, five second-generation CAR-T cell products, axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisa-cel), lisocabtagene maraleucel (liso-cel), brexucabtagene autoleucel, and idecabtagene vicleucel have been approved by the U.S. Food and Drug Administration due to their prominent efficacy 1 ; axi-cel, tisa-cel, and liso-cel have been approved for similar indications of large B cell lymphoma. All three products use anti-CD19 single-chain variable fragment to recognize and target tumor antigens. Both tisa-cel and liso-cel utilize a 4-1BB co-stimulatory domain fused to CD3ζ signaling domain, and axi-cel utilizes a CD28 co-stimulatory domain fused to CD3ζ signaling domain for full T-cell activation. In addition, liso-cel is administered as a sequential infusion of two components (CD8⁺ and CD4⁺ CAR⁺ T cells) at equal target doses, whereas both axi-cel and tisa-cel are generated from bulk T cells; however, the proportion of these cells differ among different patients. Differences in these key elements lead to different expansion, persistence, and cytotoxicity in vivo of the three products, and it is still not concluded about which structure is better (6) ( Table 1 ).
Table 1.
CAR T-cell product composition comparisons.
| Axicabtagene ciloleucel | Tisagenlecleucel | Lisocabtagene maraleucel | |
|---|---|---|---|
| Target Antigen (scFv) | CD19 | CD19 | CD19 |
| Transmembrane domain | CD28 | CD8-α | CD28 |
| Co-stimulation domain | CD28 | 4-1BB | 4-1BB |
| T-cell manufacturing | Unspecified | Unspecified | 1:1 CD4:CD8 |
| Signaling domain | CD3ζ | CD3ζ | CD3ζ |
| Indications | Adult patients with relapsed or refractory (r/r) large B-cell lymphoma after two or more lines of systemic therapy. | 1. Patients up to 25 years of age with r/r B-cell ALL 2. Adult patients with r/r large B-cell lymphoma after two or more lines of systemic therapy. | Adult patients with relapsed or refractory (r/r) large B-cell lymphoma after two or more lines of systemic therapy. |
Meta-analysis and systematic reviews on the safety and efficacy of CAR-T cell therapy for hematologic and solid malignancies have been published, but a comprehensive evaluation of the three currently marketed CAR-T cell products is lacking (7–15). In real-world clinical setting, most patients receive only these approved products rather than the experimental CAR-T cells that were mainly focused in the previous studies. In addition, the pivotal trials that supported the approval of the three CAR-T cell products were limited by the strict inclusion and exclusion criteria, and therefore the characteristics of the study patients were different from those in the real-world. For example, more than half of the patients with new diffuse large B cell lymphoma (DLBCL) are older than 65 years and show a worse prognosis than younger patients; however, most patients included in the pivotal trials were younger than 65 years; thus, it may limit the generalizability of trial findings (16–19). Therefore, it is necessary to conduct a systematic study including more data to establish the performance and differences of the three products.
Our study aims to analyze the risks and benefits associated with the three CAR-T cell products in the treatment of malignant tumors through systematically summarizing the existing relevant literature and data, which will further assist clinicians in choosing more appropriate products and preventing side effects in patients.
Methods
The present systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement, and the protocol was registered in the International Prospective Register of Systematic Reviews (CRD42020197902) (20).
Eligibility Criteria
The types of study involved all phases of clinical trials, including randomized or non-randomized controlled trials and single-arm studies. The study participants were all patients with hematologic malignancies treated with either of the three CAR-T cell products (axi-cel, tisa-cel, and liso-cel). The efficacy outcomes of interest were complete response rate (CRR) and overall response rate (ORR) defined by the combined rate of complete and partial responses. Safety outcomes of interest were severe CRS and ICANS defined by grade 3 or higher. Case series involving less than four patients, conference abstracts, reviews, editorials, commentary, animal experiments, unpublished gray literature, and other literature with unavailable study data were excluded.
Search Strategies
We systematically searched the Embase, PubMed, Web of Science, and Cochrane Library to identify relevant articles by subject words combined with free words. Databases were searched on October 5, 2020. Additionally, we reviewed the reference lists of related reviews and included articles, and there was no language limit: (1) Subject words: Neoplasms; Malignant Neoplasm; Free words: Neoplasia; Neoplasias; Neoplasm; Tumors; Tumor; Cancer; Cancers; Malignancy; Malignancies; Malignant Neoplasms; Malignant Neoplasm; Neoplasm, Malignant; Neoplasms, Malignant; Benign Neoplasm; Neoplasms, Benign; Malignant Tumor; Neoplasm, Benign; (2) Subject word: Axicabtagene ciloleucel; Free words: Yescarta; KTE-C19; (3) Subject word: Tisagenlecleucel; Free words: KYMRIAH; CTL019; (4) Subject word: Lisocabtagene maraleucel; Free words: jcar 017; jcar 17; The complete search strategy for each database is available in the Appendix.
Data Extraction and Quality Assessment
According to the eligibility criteria, two reviewers independently reviewed and screened the literature and cross-checked the included literature. The reasons for exclusion were recorded, and any discrepancies were resolved by consensus of all reviewers. We collected data of eligible literature including first author, year of publication, number of patients, age, gender, type of CAR-T cell, type of tumor, scales of toxicity and response, CRS, ICANS, and response outcomes. The Newcastle-Ottawa scale (NOS) was used to assess the quality of the included literature, including selection, comparability, and outcome.
Statistical Analysis
In this study, data analysis was performed using the Stata 14.0 software. Dichotomous data (ORR, CRR, CRS, and ICANS) with 95% confidence intervals (95% CIs) were analyzed to estimate the efficacy and adverse effects of the three CAR-T cells; P ≤0.05 was considered statistically significant. Due to rapid deterioration of the patients with lymphoma, majority of CAR-T cell therapy clinical trials are single-arm studies. Considering the lack of a control group to balance the differences in eligibility criteria and subsequent intervention methods of each study, we used a random-effects model for all data synthesis to better reflect real-world conditions, and fixed-effects model was used simultaneously for sensitivity analysis. Moreover, we assessed the heterogeneity of the included studies by Cochran’s Q-test and Higgins’ I2 statistic. The heterogeneity was considered significant at P <0.1 and I2 >75%; the sources of heterogeneity were identified by reviewing the patient characteristics, type of CAR-T product, or endpoint assessment scale of the included studies. Only when heterogeneity remained following the above steps, we used the method of excluding one study at a time (21). Subgroup analyses were performed to investigate the efficacy and safety according to the type of product and type of tumor. We assessed publication bias by funnel plots and confirmed by Egger’s and Begg’s tests. When arising a symmetrical inverted funnel shape, and Egger’s and Begg’s test yielded P-values greater than 0.5, we consider no publication bias.
Results
Study Characteristics
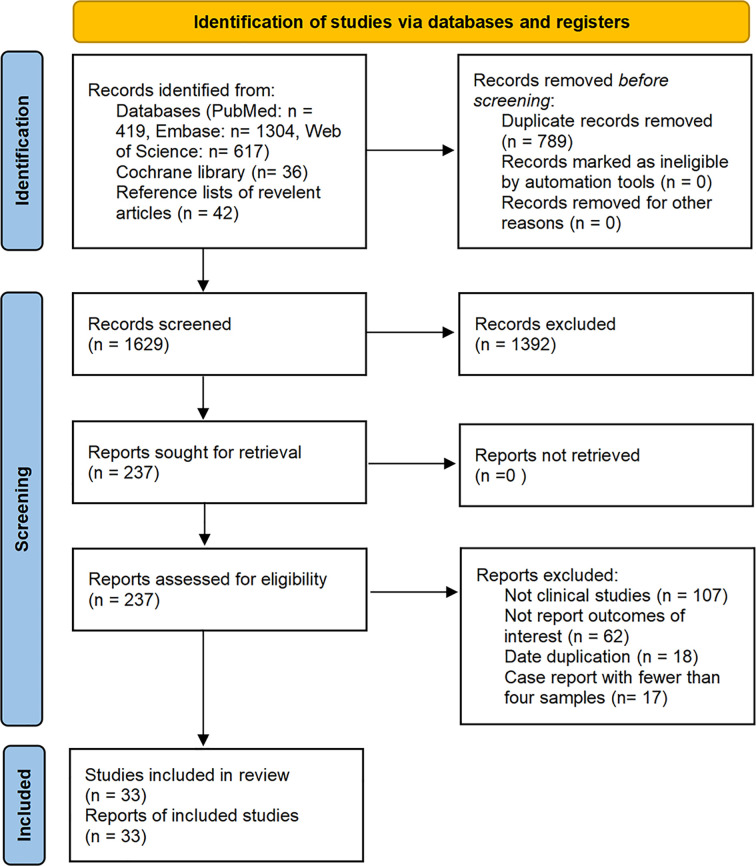
The database search resulted in identification of a total of 2,418 articles published on studies related to the treatment of malignant tumors with axi-cel, tisa-cel, or liso-cel, of which 33 studies met our eligibility criteria and were included in the analysis after de-duplication and screening title, abstract, and full-text. These studies included eighteen axi-cel, nine tisa-cel, one liso-cel, and five studies with both axi-cel and tisa-cel ( Figure 1 ).
Figure 1.
Flow diagram of the study select process.
A total of 2,172 patients were analyzed from the selected studies, including 1,352 (62.2%) patients with DLBCL, 192 (8.8%) patients with follicular lymphoma (FL) or transformed follicular lymphoma (tFL), 95 (4.3%) patients with primary mediastinal B-cell lymphoma (PMBCL), 70 (3.2%) patients with high-grade B cell lymphoma (HGBCL), 140 (6.4%) patients with acute lymphoblastic leukemia (ALL), 14 (0.6%) patients with chronic lymphocytic leukemia (CLL), 10 (0.5%) patients with multiple myeloma (MM), 6 patients with transformed marginal zone lymphoma (TMZL), 11 patients with Richter’s syndrome (RS), and 282 (13.0%) patients with unidentified tumor types. Among all patients, 1,718 (79.1%) were evaluated for response, 1,860 (85.6%) were evaluated for cytokine release syndrome (CRS), and 2,079 (95.7%) were evaluated for ICANS.
There were 14 studies with median age of the patients <60 years, 15 studies with median age ≥60 years, 1 study with mean age ≥60, and 3 studies did not report age of the patients. The studies by Sermer et al. and Wudhikarn et al. included patients treated in the same institution for a similar period, so the data of the patient had a large overlap; however, Sermer et al. reported only efficacy data, while Wudhikarn et al. reported only adverse effects, so data synthesis was not affected (22, 23). Moreover, the studies by Frey et al. (30 samples) and by Maude et al. (35 samples) included five patients with ALL from the same trial (ClinicalTrials.gov number: NCT02030847); however, considering the proportion of overlapping patients was small, no study was omitted (24, 25). Sensitivity analysis was performed to test for stability in subgroup analysis of patients with ALL. Notably, all patients included in the pooled analysis actually received CAR-T cell infusion, and those patients were excluded who were intent to receive CAR-T cell administration but finally discontinued. The detailed characteristics of the included studies are shown in Table 2 .
Table 2.
Characteristics of included studies.
| First Author | Year | No. | Median age (range)-year | Histological type | CAR-T type | Efficacy evaluation | Scale | Toxicity evaluation (grade ≥3) | Scale (CRS/ICANS) | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Schuster | 2018 | 111 | 56 (22–76) | DLBCL | tisa-cel | CR: 37/93 | Lugano | CRS: 24/111 | Penn/CTCAE 4.03, MDRA 20.1 | (17) |
| PR: 11/93 | ICANS: 13/111 | |||||||||
| Schubert | 2020 | 21 | 52 (20–68) | 16 DLBCL, 3 PMBCL, 1 DHL, 1 tFL | axi-cel | CR: 9/21 | Lugano | CRS: 0/21 | ASTCT/ASTCT | (26) |
| PR: 10/21 | ICANS: 6/21 | |||||||||
| Pinnix | 2020 | 124 | 60 (18–85) | 95 DLBCL, 20 tFL, 9 PMBCL | axi-cel | CR: 60/124 | Lugano | CRS: 11/124 | ASTCT, CARTOX/ASTCT, CARTOX | (27) |
| PR: 36/124 | ICANS: 49/124 | |||||||||
| Nastoupil | 2020 | 298 | 60 (21–83) | 203 DLBCL, 76 tFL, 19 PMBCL | axi-cel | CR: 175/275 | Lugano | CRS: 19/275 | CARTOX, Lee/CARTOX, CTCAE 4.03 | (28) |
| PR: 50/175 | ICANS: 85/275 | |||||||||
| Neelapu | 2017 | 101 | 58 (23–76) | 77 DLBCL, 16 tFL, 8 PMBCL | axi-cel | CR: 55/101 | IWGRC | CRS: 13/101 | Lee/CTCAE 4.03 | (19) |
| PR: 28/101 | ICANS: 28/101 | |||||||||
| Locke | 2017 | 7 | 46 (29–69) | DLBCL | axi-cel | CR: 4/7 | IWGRC | CRS: 1/7 | Lee/CTCAE 4.03 | (29) |
| PR: 1/7 | ICANS: 4/7 | |||||||||
| Jain | 2019 | 4 | 56 (38–66) | DLBCL | axi-cel | CR: 2/4 | NP | CRS: 0/4 | NP/NP | (30) |
| PR: 1/4 | ICANS: 0/4 | |||||||||
| Abbasi | 2020 | 10 | 66 (55–77) | DLBCL | axi-cel | CR: 8/10 | NP | CRS: 1/10 | ASTCT/ASTCT | (31) |
| PR: 0/10 | ICANS: 3/10 | |||||||||
| Garfall | 2018 | 10 | 61 (48–68) | MM | tisa-cel | CR: 6/10 | IMWGRC | CRS: 0/10 | NP/NP | (32) |
| PR: 2/10 | ICANS: 0/10 | |||||||||
| Maude | 2018 | 75 | 11 (3–23) | ALL | tisa-cel | CR: 61/75 | Independent scale | CRS: 35/75 | Penn/CTCAE 4.03 | (33) |
| PR: 0/75 | ICANS: 10/75 | |||||||||
| Maude | 2014 | 30 | 14 (5–60) | ALL | tisa-cel | CR: 27/30 | Independent scale | CRS: 8/30 | Independent scale/NP | (24) |
| PR: 0/30 | ICANS: NP | |||||||||
| Schuster | 2017 | 28 | 58 (25–77) | 14 DLBCL | tisa-cel | CR: 16/28 | 1999 IWGRC | CRS: 5/28 | Penn/NP | (34) |
| 14 FL | PR: 2/28 | ICANS: 3/28 | ||||||||
| Frigault | 2019 | 8 | 50 (17–79) | 5 DLBCL, 2 HGBCL, 1 PMBCL | tisa-cel | CR: 2/8 | NP | CRS: 0/8 | Lee, ASTCT/Lee, ASTCT | (35) |
| PR: 2/8 | ICANS: 0/8 | |||||||||
| Sim | 2019 | 11 | NP | 8 DLBCL, 3 tFL, | axi-cel | CR: 5/11 | Lugano | CRS: 1/11 | CTCAE 5.0/CTCAE 5.0 | (36) |
| PR: 4/11 | ICANS: 3/11 | |||||||||
| Porter | 2015 | 14 | 66 (51–78) | CLL | tisa-cel | CR: 4/14 | IWG on CLL RC | CRS: 7/14 | Penn/CTCAE 3.0 | (37) |
| PR: 4/14 | ICANS: 1/14 | |||||||||
| Shah | 2018 | 7 | NP | 3 DLBCL, 4 FL | tisa-cel | CR: 3/7 | Lugano | CRS: NP | NP/NP | (38) |
| PR: 2/7 | ICANS: NP | |||||||||
| Wright | 2020 | 31 | NP | 26 DLBCL, 5 tFL | 18 axi-cel, 13 tisa-cel | CR: 11/27 | Lugano | CRS: 6/31 | Penn/NP | (39) |
| PR: 3/27 | ICANS: 4/31 | |||||||||
| Jacobson | 2020 | 122 | 62 (21–79) | 57 DLBCL, 33 tFL, 17 HGBCL, 8 PMBCL, 5 TMZL, 2 RS | axi-cel | CR: 61/122 | Lugano | CRS: 19/122 | Lee/CTCAE 4.03 | (40) |
| PR: 24/122 | ICANS: 43/122 | |||||||||
| Abramson | 2019 | 268 | 63 (18–86) | 206 DLBCL, 33 HGBCL, 14 PMBCL, 2FL3B | liso-cel | CR: 135/255 | Lugano | CRS: 6/268 | Lee/CTCAE 4.03 | (16) |
| PR: 51/255 | ICANS: 27/268 | |||||||||
| Fehse | 2019 | 10 | 56 (24–79) | 7 DLBCL, 3 PMBCL | axi-cel | CR: 2/10 | NP | CRS: 2/10 | ASTCT/ASTCT | (41) |
| PR: 5/10 | ICANS: 1/10 | |||||||||
| Gupta | 2019 | 78 | 60+–13※ | DLBCL | 69 axi-cel, 9 tisa-cel | CR+PR: 43/78* | NP | CRS: 10/78 | CTCAE 5.0, Lee/CTCAE 5.0 | (42) |
| ICANS: 22/78 | ||||||||||
| Korell | 2020 | 25 | 54 (20–68) | 24 DLBCL, 1 PMBCL | axi-cel | CR: 9/25 | Lugano | CRS: NP | NP/NP | (43) |
| PR: 10/25 | ICANS: NP | |||||||||
| Frey | 2019 | 35 | 34 (21–70) | ALL | tisa-cel | CR: 24/35 | Independent scale | CRS: 25/35 | Penn/CTCAE 4.03 | (25) |
| PR: 0/35 | ICANS: 2/35 | |||||||||
| Sesques | 2020 | 61 | 59 (27–75) | 38 DLBCL, 18 PMBCL, 4 tFL, 1 TMZL | 28 axi-cel, 33 tisa-cel | CR: 28/61 | Lugano | CRS: 5/61 | ASTCT/ASTCT | (44) |
| PR: 9/61 | ICANS: 6/61 | |||||||||
| Holtzman | 2020 | 45 | 60 (26–75) | 35 DLBCL, 3 PMBCL, 7 tFL | axi-cel | CR: 22/45 | NP | CRS: NP | NP/CTCAE 4.03 | (45) |
| PR: NP | ICANS: 18/45 | |||||||||
| Strati | 2020 | 100 | 60 (18–85) | LBCL (Including 77 DLBCL) | axi-cel | CR: NP | Lugano | CRS: 9/100 | CARTOX/CARTOX | (46) |
| PR: NP | ICANS:41/100 | |||||||||
| Faramand | 2020 | 75 | 63 (23–79 | 50 DLBCL, 25 Transformed Indolent lymphomas | axi-cel | CR: 36/68 | Lugano | CRS: 12/75 | ASTCT/CARTOX, ASTCT, CTCAE v4.03 | (47) |
| PR: 29/68 | ICANS: 23/75 | |||||||||
| Kittai | 2020 | 9 | 64 (40–77) | RS | axi-cel | CR: 8/8 | Lugano | CRS: 1/9 | ASTCT/ASTCT | (48) |
| PR: 5/8 | ICANS: 3/9 | |||||||||
| Deng | 2020 | 24 | 58 (24–74) | 16 DLBCL, 6 tFL, 2 PMBCL | axi-cel | CR: NP | NP | CRS: 4/24 | NP/NP | (49) |
| PR: NP | ICANS: 12/24 | |||||||||
| Dean | 2020 | 96 | 64 (19–79) | 47 DLBCL, 15 HGBCL, 5 PMBCL, 29 NP | axi-cel | CR: 74/96 | NP | CRS: 9/96 | Lee/CTCAE 4.03 | (50) |
| PR: 63/96 | ICANS: 28/96 | |||||||||
| Sermer | 2020 | 69 | 63 (19–85) | DLBCL | 47 axi-cel, 22 tisa-cel | CR: 50/69 | Lugano | CRS: NP | NP/NP | (22) |
| PR: 36/69 | ICANS: NP | |||||||||
| Wudhikarn | 2020 | 60 | 63 (20–86) | DLBCL | 43 axi-cel, 17 tisa-cel | CR: NP | NP | CRS: 7/60 | NP/NP | (23) |
| PR: NP | ICANS: 13/60 | |||||||||
| Rubin | 2020 | 204 | 60+–12※ | Inexact# | axi-cel | CR: NP | NP | CRS: NP | NP/CTCAE 4.03 | (51) |
| PR: NP | ICANS: 51/204 |
CR, complete response; PR, partial response; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; DLBCL, diffuse large B cell lymphoma; FL/tFL, follicular lymphoma or transformed follicular; PMBCL, primary mediastinal B-cell lymphoma; HGBCL, high-grade B cell lymphoma; ALL, acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; CAR-T, chimeric antigen receptor T; TMZL, transformed marginal zone lymphoma; MM, multiple myeloma; NP, not provided; ref, reference; MDRA, Medical Dictionary for Regulatory Activities, version; CTCAE, Common Terminology Criteria for Adverse Events; RS, Richter’ s syndrome; ASTCT, American Society for Transplantation and Cellular Therapy criteria; CARTOX, CAR-T-cell-therapy-associated TOXicity; IWGRC, International Working Group Response Criteria; IMWGRC, International Myeloma Working Group response criteria; IWG on CLL RC, International Workshop Group on CLL response criteria; Independent scale, the institution used their own criteria instead of international criteria, which can be found in original text.
*No separate CR and PR numbers were provided
※Mean ± standard deviation.
#Patients with aggressive (e.g., diffuse large B-cell lymphoma, primary mediastinal B-cell lymphoma) or indolent (e.g., follicular lymphoma, marginal zone lymphoma) histologic subtype.
All studies were independently assessed for quality using NOS (cohort studies). Since all included studies were single-arm studies, the selection of the non-exposed cohort of NOS was not applicable. Overall study quality was rated as moderate to high as shown in Table S1 .
Meta-Analysis of Overall Efficacy of the CAR-T Cell Products
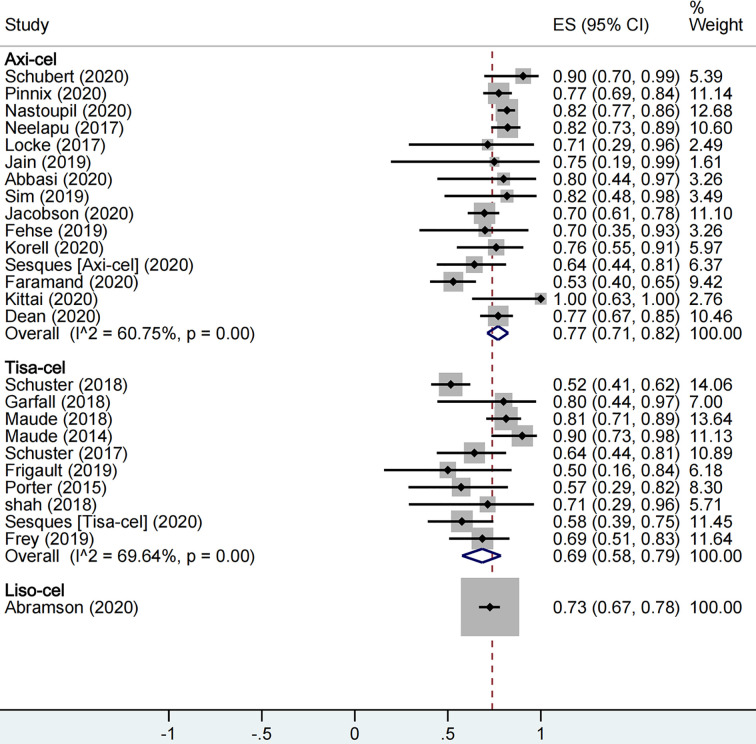
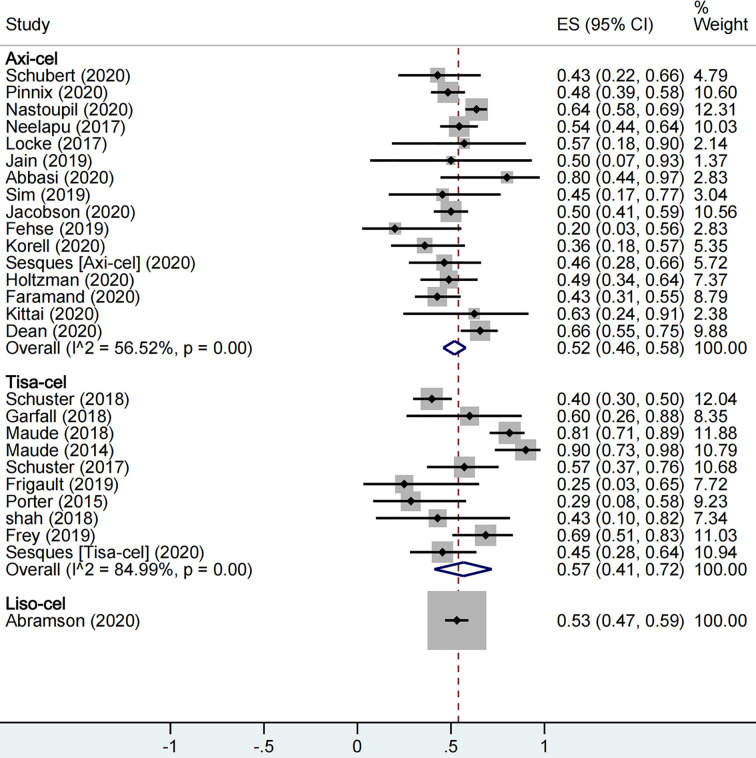
A total of 1,673 patients were included for ORR evaluation. ORR was calculated as 73% (95% CI: 0.68–0.77; I2 = 70.9%, P < 0.01) for all patients, and axi-cel, tisa-cel, and liso-cel groups showed individual response rates of 77% (95% CI: 0.71–0.82; I2 = 60.8%, P < 0.01), 69% (95% CI: 0.58–0.79; I2 = 69.6%, P < 0.01), and 73% (95% CI: 0.67–0.78), respectively ( Figure 2 ). A total of 1,640 patients were included for CRR evaluation. CRR was calculated as 54% (95% CI: 0.48–0.59; I2 = 73.0%, P < 0.01) for all patients, and it was estimated as 52% (95% CI: 0.46–0.58; I2 = 56.5%, P < 0.01), 57% (95% CI: 0.41–0.72; I2 = 85.0%, P < 0.01), and 53% (95% CI: 0.47–0.59) in axi-cel, tisa-cel, and liso-cel groups, respectively ( Figure 3 ).
Figure 2.
The forest plot of total overall response rate of each product.
Figure 3.
The forest plot of total complete response rate of each product.
Among all, tisa-cel group showed significant heterogeneity. Therefore, we divided the group into lymphoma group and ALL group for subgroup analysis considering that the indications of tisa-cel included lymphoma and ALL. The respective ORR and CRR were estimated as 57% (95% CI: 0.50–0.65; I2 = 0.0%, P = 0.50) and 44% (95% CI: 0.36–0.52; I2 = 0.0%, P = 0.47) in the lymphoma group, whereas CRR was estimated as 81% (95% CI: 0.69–0.90; I2 = 55.7%, P = 0.10) in ALL group, suggesting this indication as the source of heterogeneity in tisa-cel study group.
Meta-Analysis of Overall Safety of the CAR-T Cell Products
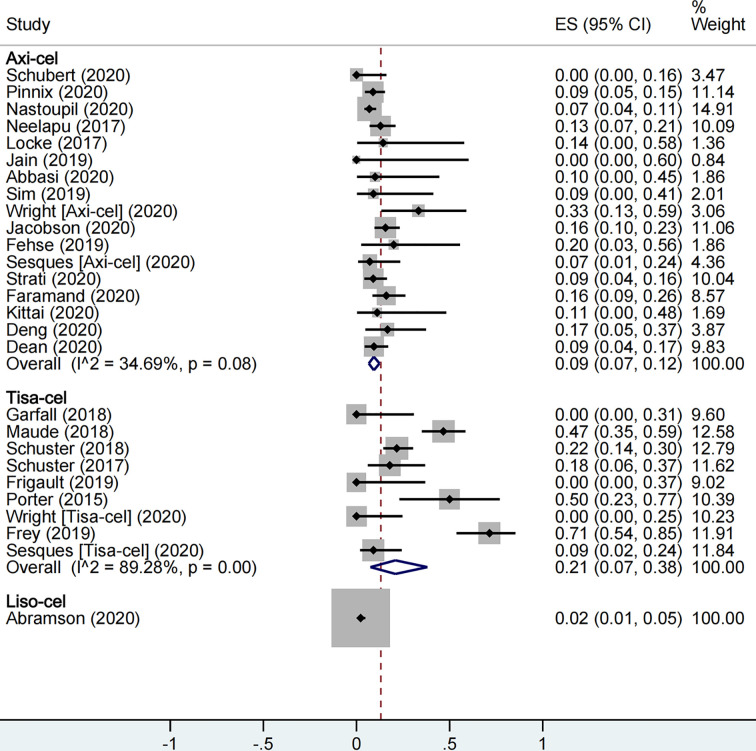
With respect to safety, a total of 1,860 patients were included for CRS rate evaluation. The proportion of patients with severe CRS among all patients was 13% (95% CI: 0.09–0.19; I2 = 86.7%, P < 0.01), and the proportion was 9% (95% CI: 0.07–0.12; I2 = 34.7%, P = 0.08), 21% (95% CI: 0.07–0.38; I2 = 89.3%, P < 0.01), and 2% (95% CI: 0.01–0.05) in axi-cel, tisa-cel, and liso-cel groups, respectively ( Figure 4 ). It could be observed that the tisa-cel group showed significant heterogeneity.
Figure 4.
The forest plot of total severe cytokine release syndrome rate of each product.
Previous studies have shown that the Penn scale tends to upgrade toxicity relative to other systems (52–54); thus, we conducted a subgroup analysis based on whether the Penn scale was used considering that majority of tisa-cel studies used the Penn scale. The proportion of patients with severe CRS in tisa-cel group with Penn scale and non-Penn scale were 32% (95% CI: 0.14–0.53; I2 = 90.3%, P < 0.01) and 4% (95% CI: 0.00–0.13; I2 = 0.0%, P = 0.51), and significant heterogeneity still appeared in groups using the Penn scale ( Figure S1 ). Furthermore, we observed that groups using the Penn scale included all ALL studies and part of lymphoma studies; accordingly, we conducted a further subgroup analysis and observed that severe CRS rate was 55% (95% CI: 0.45–0.64; I2 = 0.0%) in the ALL group and 19% (95% CI: 0.06–0.36; I2 = 74.9%, P = 0.01) in the lymphoma group ( Figure S2 ).
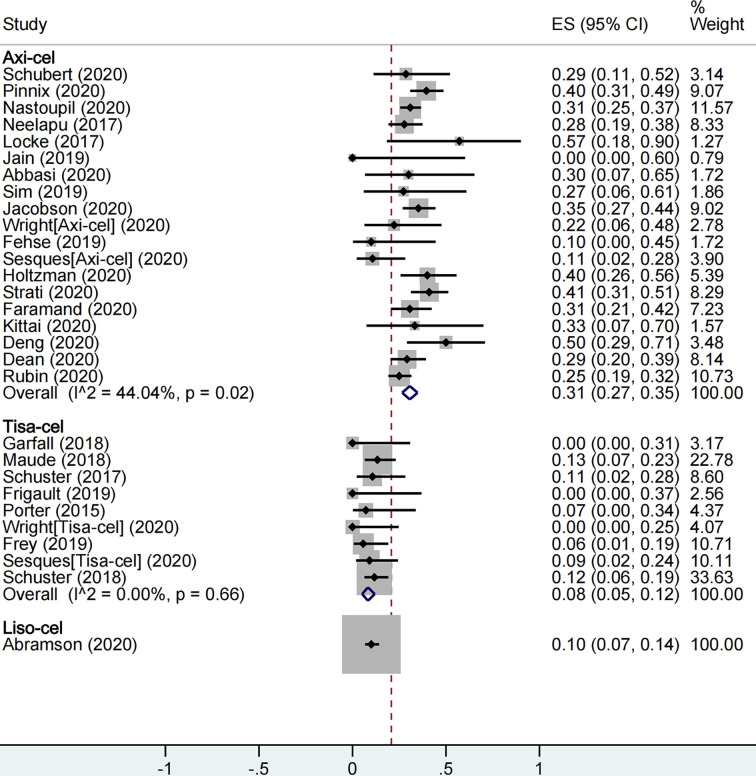
A total of 2,079 patients were included for ICANS evaluation. The overall proportion of patients with severe ICANS among all was 22% (95% CI: 0.17–0.27; I2 = 81.9%, P < 0.01), and the proportion was 31% (95% CI: 0.27–0.35; I2 = 44.0%, P = 0.02), 8% (95% CI: 0.05–0.12; I2 = 0.0%, P = 0.66), and 10% (95% CI: 0.07–0.14) in axi-cel, tisa-cel, and liso-cel groups, respectively ( Figure 5 ).
Figure 5.
The forest plot of total severe immune effector cell-associated neurotoxicity syndrome rate of each product.
Subgroup Analysis Based on the Type of Tumor
Since many of the included studies simultaneously reported more than one tumor type as listed in Table 2 , therefore, we divided them into different groups based on the type of tumor for analysis. For studies that did not report efficacy and safety results by type of tumor, we grouped them by their major tumor type (19, 26, 27, 43–47, 50). Case series that involved fewer than four patients after regrouping were excluded from analysis ( Table 3 ).
Table 3.
The results of performed meta-analysis in subgroups of product and tumor.
| Subgroup | ORR, (95% CI) | CRR, (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| tisa-cel | axi-cel | liso-cel | Overall | tisa-cel | axi-cel | liso-cel | Overall | |
| All patients | 69% (.58–.79) | 77% (.71–.82) | 73% (.67–.78) | 73% (.68–.77) | 57% (.41–.72) | 52% (.46–.58) | 53% (.47–.59) | 54% (.48–.59) |
| DLBCL | 53% (.44–.61) | 75% (.67–.83) | 72% (.65–.78) | 70% (.63–.76) | 40% (.32–.49) | 52% (.44–.60) | 52% (.45–.59) | 50% (.45–.56) |
| FL/tFL | 86% (.64–1.00) | 81% (.69–.90) | NA | 83% (.73–.92) | 73% (.48–.93) | 64% (.54–.74) | NA | 66% (.56–.76) |
| ALL | 81% (.69–.90) | NA | NA | 81% (.69–.90) | 81% (.69–.90) | NA | NA | 81% (.69–.90) |
| Subgroup | CRS, (95% CI) | ICANS, (95% CI) | ||||||
| tisa-cel | axi-cel | liso-cel | Overall | tisa-cel | axi-cel | liso-cel | Overall | |
| All patients | 21% (.07–.38) | 9% (.07–.12) | 2% (.01–.05) | 13% (.09–.19) | 8% (.05–.12) | 31% (.27–.35) | 10% (.07–.14) | 22% (.17–.27) |
| DLBCL | 8% (.01–.21) | 8% (.05–.12) | NA | 9% (.07–.13) | 8% (.03–.13) | 32% (.26–.38) | NA | 25% (.19–.31) |
| FL/tFL | NA | 2% (.00–.08) | NA | 2% (.00–.08) | NA | 31% (.18–.46) | NA | 31% (.18–.46) |
| ALL | 55% (.45–.64) | NA | NA | 55% (.45–.64) | 11% (.05–.17) | NA | NA | 11% (.05–.17) |
ORR, overall response rate; CRR, complete response rate; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; DLBCL, diffuse large B cell lymphoma; FL/tFL, follicular lymphoma or transformed follicular lymphoma; ALL, acute lymphoblastic leukemia; NA, not applicable.
Diffuse Large B Cell Lymphoma
A total of 26 studies reported the efficacy and/or safety of the three products in the treatment of DLBCL (16, 17, 19, 22, 23, 26–31, 34–36, 38–47, 49, 50). The ORR of the three products for DLBCL was 70% (95% CI: 0.63–0.76; I2 = 71.7%, P < 0.01), and response rate was 75% (95% CI: 0.67–0.83; I2 = 66.0%, P < 0.01), 53% (95% CI: 0.44–0.61; I2 = 0.0%, P = 0.88), and 72% (95% CI: 0.65–0.78) in axi-cel, tisa-cel, and liso-cel groups, respectively ( Figure 6A ). The CRR was 50% (95% CI: 0.45–0.56; I2 = 57.1%, P < 0.01) in all patients, whereas individual complete response rate was 52% (95% CI: 0.44–0.60; I2 = 62.3%, P < 0.01), 40% (95% CI: 0.32–0.49; I2 = 0.00%, P = 0.79), and 52% (95% CI: 0.45–0.59) in axi-cel, tisa-cel, and liso-cel groups, respectively ( Figure 6B ).
Figure 6.
The forest plots of pooled results in patients with diffuse large B-cell lymphoma. (A) The forest plot of overall response rate of each product. (B) The forest plot of complete response rate of each product. (C) The forest plot of severe cytokine release syndrome rate of each product. (D) The forest plot of severe immune effector cell-associated neurotoxicity syndrome rate of each product.
The proportion of patients with severe CRS among all was 9% (95% CI: 0.07–0.13; I2 = 40.2%, P = 0.04), and the respective rates of severe CRS for the patients with axi-cel and tisa-cel were 8% (95% CI: 0.05–0.12; I2 = 31.9%, P = 0.13) and 8% (95% CI: 0.01–0.21; I2 = 60.4%, P = 0.06) ( Figure 6C ). The study on liso-cel did not report the safety outcomes according to disease type.
Subgroup analysis was performed in tisa-cel group according to whether the Penn scale was used; the findings revealed that rates of severe CRS were 18% (95% CI: 0.11–0.26; I2 = 0.0%) in the group using the Penn scale and 5% (95% CI: 0.00–0.17; I2 = 0.0%) in the group using non-Penn scale ( Figure S3 ).
The proportion of patients with severe ICANS among all was 25% (95% CI: 0.19–0.31; I2 = 73.4%, P < 0.01), and the respective ICANS rates for the patients with axi-cel and tisa-cel were 32% (95% CI: 0.26–0.38; I2 = 44.5%, P = 0.04) and 8% (95% CI: 0.03–0.13; I2 = 0.0%, P = 0.54) ( Figure 6D ).
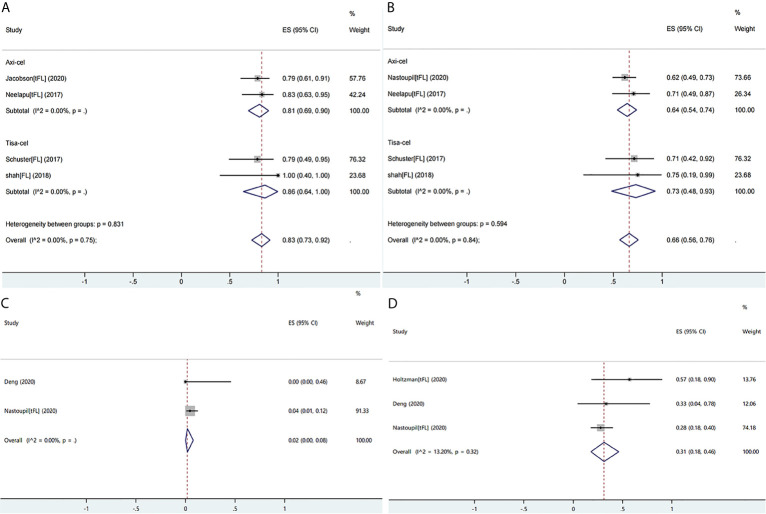
Follicular Lymphoma or Transformed Follicular Lymphoma
Ten studies reported the efficacy or safety of the three products in the treatment of FL/tFL (16, 19, 28, 34, 36, 38–40, 45, 49). The ORR of the patients with FL/tFL was 83% (95% CI: 0.73–0.92; I2 = 0.0%, P = 0.75) and CRRs of the patients undergoing treatment with axi-cel and tisa-cel were 81% (95% CI: 0.69–0.90 I2 = 0.0%) and 86% (95% CI: 0.64–1.00 I2 = 0.0%), respectively ( Figure 7A ). The CRR of the patients with FL/tFL was 66% (95% CI: 0.56–0.76; I2 = 0.0%, P = 0.84), and CRRs of the patients undergoing treatment with axi-cel and tisa-cel were 64% (95% CI: 0.54–0.74; I2 = 0.0%) and 73% (95% CI: 0.48–0.93, I2 = 0.0%), respectively ( Figure 7B ). The study on liso-cel included three patients with grade 3B FL, two patients among them achieved CR and maintained it for more than one year, but the study did not report the safety events.
Figure 7.
The forest plots of pooled results in patients with follicular lymphoma or transformed follicular lymphoma. (A) The forest plot of overall response rate of each product. (B) The forest plot of complete response rate of each product. (C) The forest plot of severe cytokine release syndrome rate of axi-cel. (D) The forest plot of severe immune effector cell-associated neurotoxicity syndrome rate of axi-cel.
Three studies on axi-cel that reported ICANS or CRS events were eligible for meta-analysis, and the rates of severe CRS and ICANS were 2% (95% CI: 0.00–0.08; I2 = 0.0%) and 31% (95% CI: 0.18–0.46; I2 = 13.2%, P = 0.32), respectively ( Figures 7C, D ). The severe ICANS rate in patients with tisa-cel was also acceptable, and no associated death was reported, but the available data did not support a meta-analysis.
Primary Mediastinal B Cell Lymphoma
Seven studies reported efficacy and safety of the three products in the patients with PMBCL, but the sample size was insufficient for their inclusion in a meta-analysis (16, 28, 35, 40, 41, 45, 49). Two real-world studies on axi-cel reported that the ORR, CRR, and rates of CRS and ICANS of the patients with PMBCL were 62% (95% CI: 0.24–0.91), 58% (95% CI: 0.33–0.80), 5% (95% CI: 0.00–0.26), and 37% (95% CI: 0.16–0.62), respectively (28, 40). A study on tisa-cel that included one patient with PMBCL with central nervous system (CNS) involvement indicated that the patient was showing ongoing response at day 90 and developed only grade 1 CRS and no ICANS (35). The study on liso-cel indicated that the ORR and CRR of the patients with PMBCL were 79% (95% CI: 0.49–0.95) and 50% (95% CI: 0.23–0.77), respectively; however, the study did not report the safety events (16).
High Grade B Cell Lymphoma
Three studies (each on axi-cel, tisa-cel, and liso-cel) were included to evaluate the efficacy and safety of the patients with HGBCL (16, 35, 40). The ORR and CRR of patients undergoing treatment with liso-cel were 76% (95% CI: 0.58–0.89) and 61% (95% CI: 0.42–0.77), respectively (16). The ORR was 88% (95% CI: 0.64–0.99) in the study on axi-cel, and the CRR was not reported (40). The study on tisa-cel included two patients with HGBCL with CNS involvement, one of them achieved CR, and disease progression was observed in the other patient who later experienced grade 1 CRS (35). Both axi-cel and liso-cel studies did not report the safety events.
Acute Lymphoblastic Leukemia and Chronic Lymphocytic Leukemia
Three studies involving 135 patients reported ALL, and one study involving 14 patients reported CLL; all patients received only tisa-cel because the other two products were not approved for ALL. The CRR of the patients with ALL was 81% (95% CI: 0.69–0.90; I2 = 55.7%, P = 0.10). Moreover, pediatric patients showed higher CRR than that of adult patients (84 vs. 69%, respectively) ( Figure 8A ) (24, 25, 33).
Figure 8.
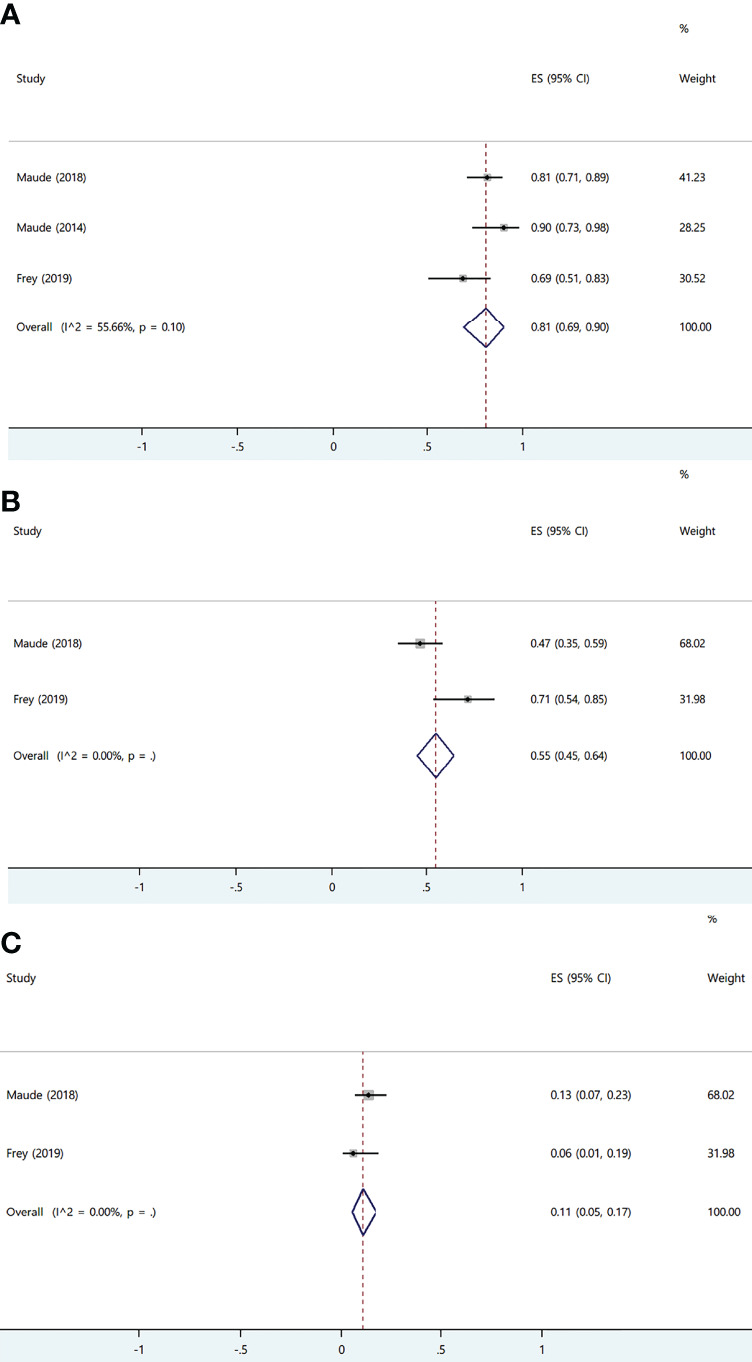
The forest plots of pooled results in patients with acute lymphoblastic leukemia. (A) The forest plot of complete response rate of tisa-cel. (B) The forest plot of severe cytokine release syndrome rate of tisa-cel. (C) The forest plot of severe immune effector cell-associated neurotoxicity syndrome rate of tisa-cel.
The ORR and CRR of the patients with CLL were 57% (95% CI: 0.29–0.82) and 29% (95% CI: 0.08–0.58), respectively, and no relapse occurred in patients achieving CR with median duration of response of 40 months (range: 21–53 months) (37).
With respect to safety, the patients with ALL and CLL showed a high rate of severe CRS (ALL: 55%; 95% CI: 0.45–0.64; I2 = 0.0%; CLL: 50%; 95% CI: 0.23–0.77) ( Figure 8B ). High rate of CRS may only be partly attributable to the Penn scale because CRS had been observed as the most prominent and serious adverse effect for all studies on ALL, and respectively 27 and 47% of total patients in two ALL trials which were mainly pediatric patients and 29% of the patients with CLL were admitted to the intensive care unit; moreover, 9% of total patients developed grade 5 CRS in the ALL trial on adult patients (24, 25, 33, 37). Adult patients with ALL were more likely to develop grade ≥3 CRS than pediatric patients (71 vs 47%, respectively). The rate of severe ICANS in patients with ALL was 11% (95% CI: 0.05–0.17; I2 = 0.0%) and was similar in pediatric and adult cohorts ( Figure 8C ), and it was 7% (95% CI: 0.00–0.34) in patients with CLL. Neurological events typically occurred during occurrence of CRS or shortly after its resolution and were self-limited (24, 33, 37).
One study involving adult patients investigated the effects of dose levels and schedule of tisa-cel on its efficacy and safety; the results demonstrated that the high-dose fractionated cohort had superior safety and efficacy profiles compared to the low-dose cohort and the high-dose single infusion cohort; however, the other two studies did not observe apparent relationship between the dose and efficacy and/or toxicity of tisa-cel (24, 25, 33). Because the latter two studies did not set up a split dosing cohort and the patients were mainly pediatric and young adults, the doses of cells were not directly comparable to that of the adult trial. Therefore, the real impact of dosage and schedule of administration on unselected patients is not clear and deserve further investigation.
Other Tumor Types
A study involving 10 patients with MM revealed that patients receiving tisa-cel following autologous stem cell transplantation and high-dose melphalan experienced clinical benefit; eight patients among them achieved a partial response or better without occurrence of severe CRS and ICANS (32). A real-world study on axi-cel reported two patients with RS and five patients with TMZL discovered an overall response of one and four cases, respectively, but did not report the complete response and toxicity (40). In another study on axi-cel involving nine patients with RS, all patients were evaluated for an overall response and five patients among them achieved complete response; moreover, no fatal adverse effect was observed related to the treatment (48).
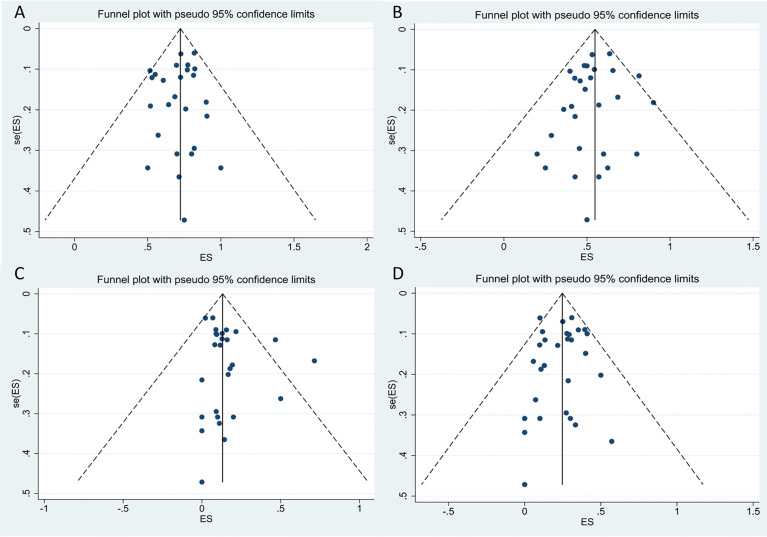
Assessment of Publication Bias
We performed a funnel-plot analysis for the ORR, CRR, and rates of severe CRS and ICANS of all studies, which showed good symmetry ( Figure 9 ). Egger’s and Begg’s tests were also performed, and all P-values obtained were greater than 0.5 ( Table 4 ), suggesting absence of significant publication bias.
Figure 9.
The funnel plots for included studies. (A) The funnel plot of total overall response rate. (B) The funnel plot of total complete response rate. (C) The funnel plot of total cytokine release syndrome rate. (D) The funnel plot of total immune effector cell-associated neurotoxicity syndrome rate.
Table 4.
Begg’s and Egger’s tests of ORR, CRR, CRS and ICANS in all included studies.
| ORR | CRR | CRS | ICAMS | |
|---|---|---|---|---|
| Begg’s test | 0.693 | 0.721 | 0.593 | 0.441 |
| Egger’s test | 0.437 | 0.290 | 0.151 | 0.713 |
ORR, overall response rate; CRR, complete response rate; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome.
Sensitivity Analysis
We used random-effects model and fixed-effects model to analyze the stability of the results in all analysis, and the two results showed a good stability in all cohorts. In the cohort with more than 75% heterogeneity, we performed subgroup analysis based on the possible causes of heterogeneity, which had been shown in the Results section.
Real-World Performance of the Products
A real-world study involving 122 patients with DLBCL as the main tumor type along with tFL, HGBCL, and PMBCL observed that axi-cel had comparable overall efficacy and safety between clinical and trial settings (ORR: 70 vs. 68%, P = 0.25; CRR: 63 vs. 42%, P = 0.016; severe CRS rate: 15 vs. 16%; P = 0.83; severe ICANS rate: 35 vs. 36%; P = 0.81) in ZUMA-1 eligible and ineligible groups, respectively, although the CRR and duration of response were more favorable in patients of ZUMA-1 eligible group (40). In addition, another real-world study including 298 patients undergoing standard-of-care axi-cel treatment showed consistent results (ORR: not provided; CRR: 69 vs. 56%, P = 0.02; severe CRS rate: 5 vs. 10%; P = 0.10; severe ICANS rate: 28 vs. 36%; P = 0.18) in patients without vs. with comorbidities indicated in ZUMA-1 exclusion criteria, respectively, while the latter had shorter progression-free survival (PFS) and overall survival (OS) (28).
Of note, regarding the concerns for the inconsistency of bridging therapy in the pivotal trials, there have been two studies reported on axi-cel and one study on both axi-cel and tisa-cel that evaluated the impact of bridging therapy on the efficacy and safety of patients with R/R LBCL. Although bridging therapy is needed in patients who tended to have a higher tumor burden, the results were not significantly different between the patients with bridging therapy and non-bridging therapy; radiation therapy had been safely administered as a bridging therapy and resulted in a superior efficacy outcome (27, 36, 39).
In addition, a study which included patients with DLBCL involving CNS disease, HIV, and active HBV also provided the evidence of efficacy and safety of axi-cel in the real-world setting since these patients with severe comorbidities were excluded from the pivotal clinical trial (31). Another study that included eight patients with secondary CNS involvement LBCL receiving tisa-cel did not report severe ICANS; the findings are suggestive of potential of CAR T-cell product treatment for the patients with CNS involvement (35).
Discussion
Our study demonstrated that the three CAR-T cell products were mainly used to treat patients with DLBCL, FL/tFL, ALL/CLL, PMBCL, and HGBCL along with other B-cell lymphomas such as MM, RS, and TMZL. Overall, the results showed promising efficacy and safety of all three products in all histological types; however, efficacy of liso-cel requires further validation from real-world data.
According to our pooled result, axi-cel showed an increased response rate in patients with lymphoma except tFL/FL as compared to tisa-cel. However, comparative efficacy of different products should be determined by randomized controlled trials in the future owing to the presence of significant heterogeneity between patients of different studies. For example, compared with JULIET trial, bridging therapy was not allowed in ZUMA-1 trial, resulting in likely exclusion of a group of patients with high tumor burden or those who are in need of emergent therapy. The ORR and CRR (77 and 52%, respectively) in patients treated with axi-cel were similar to those in the ZUMA-1 trial (ORR: 82%; CR: 54%); moreover, the ORR and CRR (57 and 44%, respectively) in patients with large B cell lymphoma and undergoing treatment with tisa-cel were similar to those in the JULIET trial (ORR: 52%; CR: 40%). In addition, we observed a favorable efficacy and manageable safety in patients with pathological subtypes that were not included in pivotal trials, such as HGBCL, MM, and RS (32, 48). However, due to the absence of large sample studies, treatment of patients with these subtypes should be monitored carefully. Another two CAR-T cell products recently approved by the FDA, brexucabtagene autoleucel and idecabtagene vicleucel, have shown excellent efficacy in R/R mantle cell lymphoma (ORR: 93%, CR: 67%) and multiple myeloma (ORR: 73%, CR: 33%), respectively. Therefore, these two CAR-T cell products should be the preferred treatment alternative in these two subtypes (55, 56).
The differences observed between safety profiles of axi-cel and tisa-cel in pivotal trials remained consistent in this analysis. Axi-cel tended to be associated with a higher rate of severe ICANS than tisa-cel (31 vs. 8%, respectively), possibly due to its CD28 co-stimulation domain. Therefore, patients receiving axi-cel who are at high risk of neurotoxicity, which may include preexisting endothelial activation, such as a high angiopoietin 2:angiopoietin 1 ratio and von Willebrand factor concentration in serum or other characteristics that are considered to increase the risk of neurotoxicity after CAR-T cell infusion, should be certainly monitored for serum biomarkers including interleukin (IL)-2, IL-6, IL-15, and IL-2Rα, ferritin, granulocyte–macrophage colony-stimulating factor, and other markers significantly associated with severe ICANS (19, 57). When grade 1–2 neurotoxicity occurs, corticosteroids are the preferred treatment to prevent exacerbation, and anti-IL-6 therapy is recommended in patients with concurrent CRS. In addition, the use of levetiracetam and phenobarbital to prevent seizures is important (3).
Notably, subgroup analysis indicated that higher rate of severe CRS of tisa-cel in patients with LBCL may be attributed to using the Penn scale (19% in the Penn scale group vs. 4% in non-Penn scale group). However, patients with ALL or CLL were at a high risk of developing severe CRS and requiring intensive care; therefore, medical staff need to closely monitor body temperature of the patient and levels of IL-6, C-reactive protein, interferon-gamma, and ferritin, and other predictive cytokines after tisa-cel infusion, especially for patients with high tumor burden. Tocilizumab can be considered as an early treatment immediately after appearance of elevation of temperature or biomarkers or low-grade CRS symptoms to prevent further exacerbation (37). The use of corticosteroids and anti-IL-6 therapy did not appear to affect the efficacy of CAR-T cells, and supportive care is also important (3, 19, 40).
In addition, CAR-T cell therapy, unlike conventional treatment, uses genetically modified cells; hence the success rate and manufacturing speed of the three products also need to be considered. In the JULIET trial, most patients discontinued participation due to disease progression; 7% of the enrolled patients discontinued due to manufacturing failure of tisa-cel and 10% due to death. In the TRANSCEND NHL 001 trial, 7% of patients received non-conforming products, two patients experienced manufacturing failure of liso-cel, and 10% of patients died before receiving liso-cel. Manufacturing time of axi-cel was approximately one week shorter than those of tisa-cel and liso-cel. In the phase 2 of ZUMA-1 trial, only 1% of patients discontinued due to manufacture failure of axi-cel and 2% of patients died before receiving axi-cel (16, 17, 19). These results reflected that the studied population is at high-risk; therefore, properties of the product manufacturing speed and success rate are crucial for patients with rapidly progressing disease.
In order to ensure presentation of high-quality and appropriate information is available, we excluded studies published in the form of conference abstracts when we conducted the study selection. Of note, conference abstracts published by several European registries indicated that patients with axi-cel and tisa-cel administration in the real-world setting showed poor responses, PFS, or OS, compared to the pivotal trial, while the adverse events were manageable and of similar intensity. Real-world data from institutions in Spain, Germany, France, and the United Kingdom showed that the ORR of patients receiving axi-cel and tisa-cel did not exceed 70%, and the CRR of patients except from France was approximately 30% (58–62). Similarly, the French and British institutions observed that patients were likely to have a higher frequency of rapid relapses and a shorter survival period than expected (58, 59, 62), Poorer outcomes may be related to greater proportion of patients with advanced stage, refractory to previous treatments, or multiple number of previous lines of treatment. In addition, although the eligible studies included a large proportion of real-world studies, including those by Nastoupil et al. and Jacobson et al., however, the intention-to-treat (ITT) analysis was not strictly implemented. They performed a modified ITT analysis (included all patients who had received CAR-T cells) or used patients with T cells collected as an ITT set, which might have overestimated effectiveness because some patients with worse conditions were excluded. In the JULIET trial, patients achieved an ORR of only 34% when all the enrolled patients were included in the ITT set (17). The most common factors related to patient discontinuation of CAR-T infusions are disease progression and death; in particular, the median time from leukapheresis to CAR T-cell infusion is longer in the real-world practice. Progression of the disease during this interval may impact the efficacy of subsequent CAR-T treatment. Bridging patients with intention to treat from leukapheresis to CAR-T cell infusion in the real world remains a challenge.
Our study has some limitations. First, the CRS and ICANS grading scales used in the included studies were not absolutely consistent. Although we analyzed the Penn scale, due to the lack of data to further analyze the non-Penn scales, it is unknown whether other scales could still confound the result. It is necessary to use a unified toxicity assessment scale in future studies. Second, all patients included in the study actually received CAR-T infusion, and no ITT analysis was performed. Therefore, the pooled results may overestimate the effectiveness of CAR-T treatment. Third, considering the quality of the research and the completeness of the information, we excluded conference abstracts without full text. Nonetheless, the data reported in these abstracts may be important to understand the performance of commercial CAR-T cells in the real world; therefore, future systematic reviews focusing on the real-world performance may need to include them. Fourth, we evaluated only two most common side effects of CAR-T cell treatment, but many patients also experienced other serious side effects such as white-cell count decreased, neutropenia, thrombocytopenia, and infection, which should be included in risk assessment in clinical setting. Fifth, we used only the ORR and CRR of the three products as efficacy endpoints, but the duration of response and median PFS of responders are of great significance as well. Furthermore, none of the included studies was a randomized controlled trial, although the risk factors that may influence the efficacy and safety outcomes of different products could be partly balanced in the real-world setting. Considering inevitable differences in the level of care and the patient baseline and lack of post-approval data on liso-cel, so our study could only provide the safety and efficacy profiles and characteristics of use for each product rather than providing a comparative result.
Conclusion
The present meta-analysis showed promising response rate of axi-cel and tisa-cel for various B-cell malignancies; however, the high rate of severe CRS associated with tisa-cel for the treatment of leukemia and rate of ICANS with axi-cel require special attention in the clinical setting. Although liso-cel showed excellent efficacy and safety profiles, its real-world performance needs further validation. The pooled results validated consistency in efficacy and safety profiles of axi-cel and tisa-cel in real-world and clinical trial. However, in the future, it is necessary to conduct a review focusing on real-world data in the context of ITT set to further explore the factors that can affect their efficacy. In addition, some patients who did not meet eligibility criteria of pivotal trials had shown favorable results that warrant further investigation, to benefit a broader patient population.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author Contributions
JH, ZS contributed to the conception and design of the study. RX and RH searched the database. ML and XW extracted data. JM, ZS and RX conducted data analysis. XW, JM, ZS and ML wrote the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.698607/full#supplementary-material
The quality assessment of included studies
The forest plot of severe cytokine release syndrome rate of tisa-cel by Penn and non-Penn scales. (A) The forest plot of severe cytokine release syndrome rate according to non-Penn scale. (B) The forest plot of severe cytokine release syndrome rate according to Penn scale.
The forest plot of severe cytokine release syndrome rate of tisa-cel with Penn scale by pathologic subtype. (A) The forest plot of severe cytokine release syndrome rate in lymphoma patients. (B) The forest plot of severe cytokine release syndrome rate in patients with acute lymphoblastic leukemia.
The forest plot of severe cytokine release syndrome rate in patients with diffuse large B-cell lymphoma receiving tisa-cel infusion by Penn and non-Penn scales. (A) The forest plot of severe cytokine release syndrome rate according to Penn scale. (B) The forest plot of severe cytokine release syndrome rate according to non-Penn scale.
References
- 1. Rosenberg SA, Restifo NP. Adoptive Cell Transfer as Personalized Immunotherapy for Human Cancer. Science (2015) 348:62–8. doi: 10.1126/science.aaa4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T Cell Immunotherapy for Human Cancer. Science (2018) 359:1361–5. doi: 10.1126/science.aar6711 [DOI] [PubMed] [Google Scholar]
- 3. Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric Antigen Receptor T-Cell Therapy-Assessment and Management of Toxicities. Nat Rev Clin Oncol (2018) 15:47–62. doi: 10.1038/nrclinonc.2017.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boyiadzis MM, Dhodapkar MV, Brentjens RJ, Kochenderfer JN, Neelapu SS, Maus MV, et al. Chimeric Antigen Receptor (CAR) T Therapies for the Treatment of Hematologic Malignancies: Clinical Perspective and Significance. J Immunother Cancer (2018) 6(1):137. doi: 10.1186/s40425-018-0460-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chmielewski M, Abken H. TRUCKs: The Fourth Generation of CARs. Expert Opin Biol Ther (2015) 15(8):1145–54. doi: 10.1517/14712598.2015.1046430 [DOI] [PubMed] [Google Scholar]
- 6. Chow VA, Shadman M, Gopal AK. Translating Anti-CD19 CAR T-Cell Therapy Into Clinical Practice for Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Blood (2018) 132(8):777–81. doi: 10.1182/blood-2018-04-839217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu WL, Hua ZC. Chimeric Antigen Receptor T-Cell (CAR T) Therapy for Hematologic and Solid Malignancies: Efficacy and Safety-A Systematic Review With Meta-Analysis. Cancers (Basel) (2019) 11(1):47. doi: 10.3390/cancers11010047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li J, Wu Z, Zhao N. Individual Patient Data Meta-Analysis From 16 Trials for Safety Factors in Cytokine Release Syndrome After CAR-T Therapy in Patients With Non-Hodgkin Lymphoma (NHL) and Acute Lymphoblastic Leukemia. Adv Ther (2019) 36:2881–94. doi: 10.1007/s12325-019-01056-8 [DOI] [PubMed] [Google Scholar]
- 9. Hou B, Tang Y, Li W, Zeng Q, Chang D. Efficiency of CAR-T Therapy for Treatment of Solid Tumor in Clinical Trials: A Meta-Analysis. Dis Markers (2019) 2019:3425291. doi: 10.1155/2019/3425291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grigor EJM, Fergusson D, Kekre N, Montroy J, Atkins H, Seftel MD, et al. Risks and Benefits of Chimeric Antigen Receptor T-Cell (CAR-T) Therapy in Cancer: A Systematic Review and Meta-Analysis. Transfus Med Rev (2019) 33:98–110. doi: 10.1016/j.tmrv.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 11. Gagelmann N, Ayuk F, Atanackovic D, Kröger N. B Cell Maturation Antigen-Specific Chimeric Antigen Receptor T Cells for Relapsed or Refractory Multiple Myeloma: A Meta-Analysis. Eur J Haematol (2020) 104:318–27. doi: 10.1111/ejh.13380 [DOI] [PubMed] [Google Scholar]
- 12. Drokow EK, Ahmed HAW, Amponsem-Boateng C, Akpabla GS, Song J, Shi M, et al. Survival Outcomes and Efficacy of Autologous CD19 Chimeric Antigen Receptor-T Cell Therapy in the Patient With Diagnosed Hematological Malignancies: A Systematic Review and Meta-Analysis. Ther Clin Risk Manag (2019) 15:637–46. doi: 10.2147/TCRM.S203822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cao JX, Gao WJ, You J, Wu LH, Liu JL, Wang ZX. The Efficacy of Anti-CD19 Chimeric Antigen Receptor T Cells for B-Cell Malignancies. Cytotherapy (2019) 21:769–81. doi: 10.1016/j.jcyt.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 14. Cao G, Lei L, Zhu X. Efficiency and Safety of Autologous Chimeric Antigen Receptor T-Cells Therapy Used for Patients With Lymphoma: A Systematic Review and Meta-Analysis. Med (Baltimore) (2019) 98:e17506. doi: 10.1097/MD.0000000000017506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anagnostou T, Riaz IB, Hashmi SK, Murad MH, Kenderian SS. Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy in Acute Lymphocytic Leukaemia: A Systematic Review and Meta-Analysis. Lancet Haematol (2020) 7:e816–26. doi: 10.1016/S2352-3026(20)30277-5 [DOI] [PubMed] [Google Scholar]
- 16. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene Maraleucel for Patients With Relapsed or Refractory Large B-Cell Lymphomas (TRANSCEND NHL 001): A Multicentre Seamless Design Study. Lancet (2020) 396:839–52. doi: 10.1016/S0140-6736(20)31366-0 [DOI] [PubMed] [Google Scholar]
- 17. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med (2019) 380:45–56. doi: 10.1056/nejmoa1804980 [DOI] [PubMed] [Google Scholar]
- 18. Johnson PC, Abramson JS. Patient Selection for Chimeric Antigen Receptor (CAR) T-Cell Therapy for Aggressive B-Cell Non-Hodgkin Lymphomas. Leuk Lymphoma (2020) 61:2561–7. doi: 10.1080/10428194.2020.1786563 [DOI] [PubMed] [Google Scholar]
- 19. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med (2017) 377:2531–44. doi: 10.1056/nejmoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst Rev (2021) 10:89. doi: 10.1186/s13643-021-01626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JPT. Commentary: Heterogeneity in Meta-Analysis Should be Expected and Appropriately Quantified. Int J Epidemiol (2008) 37:1158–60. doi: 10.1093/ije/dyn204 [DOI] [PubMed] [Google Scholar]
- 22. Sermer D, Batlevi C, Lia Palomba M, Shah G, Lin RJ, Perales MA, et al. Outcomes in Patients With DLBCL Treated With Commercial CAR T Cells Compared With Alternate Therapies. Blood Adv (2020) 4:4669–78. doi: 10.1182/bloodadvances.2020002118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wudhikarn K, Palomba ML, Pennisi M, Garcia-Recio M, Flynn JR, Devlin SM, et al. Infection During the First Year in Patients Treated With CD19 CAR T Cells for Diffuse Large B Cell Lymphoma. Blood Cancer J (2020) 10:79. doi: 10.1038/s41408-020-00346-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N Engl J Med (2014) 371:1507–17. doi: 10.1056/nejmoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frey NV, Shaw PA, Hexner EO, Pequignot E, Gill S, Luger SM, et al. Optimizing Chimeric Antigen Receptor T-Cell Therapy for Adults With Acute Lymphoblastic Leukemia. J Clin Oncol (2020) 38:415–22. doi: 10.1200/JCO.19.01892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schubert ML, Dietrich S, Stilgenbauer S, Schmitt A, Pavel P, Kunz A, et al. Feasibility and Safety of CD19 Chimeric Antigen Receptor T Cell Treatment for B Cell Lymphoma Relapse After Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2020) 26:1575–80. doi: 10.1016/j.bbmt.2020.04.025 [DOI] [PubMed] [Google Scholar]
- 27. Pinnix CC, Gunther JR, Dabaja BS, Strati P, Fang P, Hawkins MC, et al. Bridging Therapy Prior to Axicabtagene Ciloleucel for Relapsed/Refractory Large B-Cell Lymphoma. Blood Adv (2020) 4:2871–83. doi: 10.1182/bloodadvances.2020001837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standard-Of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results From the US Lymphoma CAR T Consortium. J Clin Oncol (2020) 38:3119–28. doi: 10.1200/JCO.19.02104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Locke FL, Neelapu SS, Bartlett NL, Siddiqi T, Chavez JC, Hosing CM, et al. Phase 1 Results of ZUMA-1: A Multicenter Study of KTE-C19 Anti-CD19 CAR T Cell Therapy in Refractory Aggressive Lymphoma. Mol Ther (2017) 25:285–95. doi: 10.1016/j.ymthe.2016.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jain T, Sauter CS, Shah GL, Maloy MA, Chan J, Scordo M, et al. Safety and Feasibility of Chimeric Antigen Receptor T Cell Therapy After Allogeneic Hematopoietic Cell Transplantation in Relapsed/Refractory B Cell Non-Hodgkin Lymphoma. Leukemia (2019) 33:2540–4. doi: 10.1038/s41375-019-0476-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abbasi A, Peeke S, Shah N, Mustafa J, Khatun F, Lombardo A, et al. Axicabtagene Ciloleucel CD19 CAR-T Cell Therapy Results in High Rates of Systemic and Neurologic Remissions in Ten Patients With Refractory Large B Cell Lymphoma Including Two With HIV and Viral Hepatitis. J Hematol Oncol (2020) 13:1. doi: 10.1186/s13045-019-0838-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garfall AL, Stadtmauer EA, Hwang WT, Lacey SF, Melenhorst JJ, Krevvata M, et al. Anti-CD19 CAR T Cells With High-Dose Melphalan and Autologous Stem Cell Transplantation for Refractory Multiple Myeloma. JCI Insight (2019) 4:1–14. doi: 10.1172/jci.insight.127684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults With B-Cell Lymphoblastic Leukemia. N Engl J Med (2018) 378:439–48. doi: 10.1056/nejmoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N Engl J Med (2017) 377:2545–54. doi: 10.1056/nejmoa1708566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Frigault MJ, Maus MV, Dietrich J, Martinez-Lage M, Leick M, Choi BD, et al. Tisagenlecleucel CAR T-Cell Therapy in Secondary CNS Lymphoma. Blood (2019) 134:860–6. doi: 10.1182/blood.2019001694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sim AJ, Jain MD, Figura NB, Chavez JC, Shah BD, Khimani F, et al. Radiation Therapy as a Bridging Strategy for CAR T Cell Therapy With Axicabtagene Ciloleucel in Diffuse Large B-Cell Lymphoma. Int J Radiat Oncol Biol Phys (2019) 105:1012–21. doi: 10.1016/j.ijrobp.2019.05.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric Antigen Receptor T Cells Persist and Induce Sustained Remissions in Relapsed Refractory Chronic Lymphocytic Leukemia. Sci Transl Med (2015) 7:303ra139. doi: 10.1126/scitranslmed.aac5415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shah NN, Nagle SJ, Torigian DA, Farwell MD, Hwang WT, Frey N, et al. Early Positron Emission Tomography/Computed Tomography as a Predictor of Response After CTL019 Chimeric Antigen Receptor –T-Cell Therapy in B-Cell Non-Hodgkin Lymphomas. Cytotherapy (2018) 20:1415–8. doi: 10.1016/j.jcyt.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 39. Wright CM, LaRiviere MJ, Baron JA, Uche C, Xiao Y, Arscott WT, et al. Bridging Radiation Therapy Before Commercial Chimeric Antigen Receptor T-Cell Therapy for Relapsed or Refractory Aggressive B-Cell Lymphoma. Int J Radiat Oncol Biol Phys (2020) 108:178–88. doi: 10.1016/j.ijrobp.2020.05.014 [DOI] [PubMed] [Google Scholar]
- 40. Jacobson CA, Hunter BD, Redd R, Rodig SJ, Chen PH, Wright K, et al. Axicabtagene Ciloleucel in the Non-Trial Setting: Outcomes and Correlates of Response, Resistance, and Toxicity. J Clin Oncol (2020) 38:3095–106. doi: 10.1200/JCO.19.02103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fehse B, Badbaran A, Berger C, Sonntag T, Riecken K, Geffken M, et al. Digital PCR Assays for Precise Quantification of CD19-CAR-T Cells After Treatment With Axicabtagene Ciloleucel. Mol Ther - Methods Clin Dev (2020) 16:172–8. doi: 10.1016/j.omtm.2019.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gupta S, Seethapathy H, Strohbehn IA, Frigault MJ, O’Donnell EK, Jacobson CA, et al. Acute Kidney Injury and Electrolyte Abnormalities After Chimeric Antigen Receptor T-Cell (CAR-T) Therapy for Diffuse Large B-Cell Lymphoma. Am J Kidney Dis (2020) 76:63–71. doi: 10.1053/j.ajkd.2019.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Korell F, Laier S, Sauer S, Veelken K, Hennemann H, Schubert ML, et al. Current Challenges in Providing Good Leukapheresis Products for Manufacturing of CAR-T Cells for Patients With Relapsed/Refractory NHL or ALL. Cells (2020) 9:1225. doi: 10.3390/cells9051225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sesques P, Ferrant E, Safar V, Wallet F, Tordo J, Dhomps A, et al. Commercial Anti-CD19 CAR T Cell Therapy for Patients With Relapsed/Refractory Aggressive B Cell Lymphoma in a European Center. Am J Hematol (2020) 95:1324–33. doi: 10.1002/ajh.25951 [DOI] [PubMed] [Google Scholar]
- 45. Holtzman NG, Xie H, Bentzen S, Kesari V, Bukhari A, El Chaer F, et al. Immune Effector Cell–Associated Neurotoxicity Syndrome After Chimeric Antigen Receptor T-Cell Therapy for Lymphoma: Predictive Biomarkers and Clinical Outcomes. Neuro Oncol (2020) 23:112–21. doi: 10.1093/neuonc/noaa183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Strati P, Nastoupil LJ, Westin J, Fayad LE, Ahmed S, Fowler NH, et al. Clinical and Radiologic Correlates of Neurotoxicity After Axicabtagene Ciloleucel in Large B-Cell Lymphoma. Blood Adv (2020) 4:3943–51. doi: 10.1182/bloodadvances.2020002228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Faramand R, Jain M, Staedtke V, Kotani H, Bai R, Reid K, et al. Tumor Microenvironment Composition and Severe Cytokine Release Syndrome (CRS) Influence Toxicity in Patients With Large B-Cell Lymphoma Treated With Axicabtagene Ciloleucel. Clin Cancer Res (2020) 26:4823–31. doi: 10.1158/1078-0432.ccr-20-1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kittai AS, Bond DA, William B, Saad A, Penza S, Efebera Y, et al. Clinical Activity of Axicabtagene Ciloleucel in Adult Patients With Richter Syndrome. Blood Adv (2020) 4:4648–52. doi: 10.1182/bloodadvances.2020002783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Deng Q, Han G, Puebla-Osorio N, Ma MCJ, Strati P, Chasen B, et al. Characteristics of Anti-CD19 CAR T Cell Infusion Products Associated With Efficacy and Toxicity in Patients With Large B Cell Lymphomas. Nat Med (2020) 26:1878–87. doi: 10.1038/s41591-020-1061-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dean EA, Mhaskar RS, Lu H, Mousa MS, Krivenko GS, Lazaryan A, et al. High Metabolic Tumor Volume Is Associated With Decreased Efficacy of Axicabtagene Ciloleucel in Large B-Cell Lymphoma. Blood Adv (2020) 4:3268–76. doi: 10.1182/bloodadvances.2020001900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rubin DB, Al Jarrah A, Li K, Larose S, Monk AD, Ali AB, et al. Clinical Predictors of Neurotoxicity After Chimeric Antigen Receptor T-Cell Therapy. JAMA Neurol (2020) 02114:4–10. doi: 10.1001/jamaneurol.2020.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chavez JC, Bachmeier C, Kharfan-Dabaja MA. CAR T-Cell Therapy for B-Cell Lymphomas: Clinical Trial Results of Available Products. Ther Adv Hematol (2019) 10:204062071984158. doi: 10.1177/2040620719841581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pennisi M, Jain T, Santomasso BD, Mead E, Wudhikarn K, Silverberg ML, et al. Comparing CAR T-Cell Toxicity Grading Systems: Application of the ASTCT Grading System and Implications for Management. Blood Adv (2020) 4:676–86. doi: 10.1182/bloodadvances.2019000952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Porter D, Frey N, Wood PA, Weng Y, Grupp SA. Grading of Cytokine Release Syndrome Associated With the CAR T Cell Therapy Tisagenlecleucel. J Hematol Oncol (2018) 11:35. doi: 10.1186/s13045-018-0571-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med (2020) 382(14):1331–42. doi: 10.1056/NEJMoa1914347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Munshi NC, Anderson LD, Jr, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N Engl J Med (2021) 384(8):705–16. doi: 10.1056/NEJMoa2024850 [DOI] [PubMed] [Google Scholar]
- 57. Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, et al. Endothelial Activation and Blood–Brain Barrier Disruption in Neurotoxicity After Adoptive Immunotherapy With CD19 CAR-T Cells. Cancer Discov (2017) 7:1404–19. doi: 10.1158/2159-8290.CD-17-0698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kuhnl A, Roddie C, Tholouli E, Menne T, Linton K, Lugthart S, et al. Real-World Data of High-Grade Lymphoma Patients Treated With CD19 CAR-T in the UK. Br J Haematol (2020) 189(Supplement_1):30–1. doi: 10.1111/bjh.16638 [DOI] [Google Scholar]
- 59. Sanderson R, Benjamin R, Patten P, Potter V, Yallup D, Cuthill K, et al. Axicabtagene Ciloleucel CD19 CAR T-Cells for Relapsed/Refractory Large B-Cell Lymphoma: Realworld Outcomes, Toxicity and Predictors of Response From a Prospective UK Cohort. Bone Marrow Transplant (2020) 55:234–5. doi: 10.1038/s41409-020-01120-w [DOI] [Google Scholar]
- 60. Iacoboni G, Villacampa G, Martinez-Cibrian N, Bailén R, Lopez Corral L, Sanchez JM, et al. Real-World Evidence of Tisagenlecleucel for the Treatment of Relapsed or Refractory Large B-Cell Lymphoma. Cancer Med (2021) 10(10):3214–23. doi: 10.1002/cam4.3881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bücklein V, Blumenberg V, Schmidt C, Rejeski K, Ruzicka M, Müller N, et al. CD19 CAR T-Cells for Relapsed/Refractory Diffuse Large B-Cell Lymphoma: Real-World Data From LMU Munich. J ImmunoTher Cancer (2020) 8(Supplement_2):A48–9. doi: 10.1136/jitc-2020-ITOC7.95 [DOI] [Google Scholar]
- 62. Sesques P, Ferrant E, Safar V, Wallet F, Tordo J, Karlin L, et al. Real-World Results of Anti-CD19 CAR T Cells Use for Patients With Relapsed/Refractory Large B-Cell Lymphoma in Lyon Sud Hospital. HemaSphere (2020) 4(Supplement_4):565–6. doi: 10.1097/HS9.0000000000000404 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The quality assessment of included studies
The forest plot of severe cytokine release syndrome rate of tisa-cel by Penn and non-Penn scales. (A) The forest plot of severe cytokine release syndrome rate according to non-Penn scale. (B) The forest plot of severe cytokine release syndrome rate according to Penn scale.
The forest plot of severe cytokine release syndrome rate of tisa-cel with Penn scale by pathologic subtype. (A) The forest plot of severe cytokine release syndrome rate in lymphoma patients. (B) The forest plot of severe cytokine release syndrome rate in patients with acute lymphoblastic leukemia.
The forest plot of severe cytokine release syndrome rate in patients with diffuse large B-cell lymphoma receiving tisa-cel infusion by Penn and non-Penn scales. (A) The forest plot of severe cytokine release syndrome rate according to Penn scale. (B) The forest plot of severe cytokine release syndrome rate according to non-Penn scale.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.