Abstract
Background
A cancer diagnosis can cause severe emotional distress and affect quality of life as well as social relationships. The transition from inpatient to outpatient treatment is burdened by stressful uncertainties and a gap of psycho-oncological care. In addition, further barriers, such as information deficits or fear of stigmatization, might hinder cancer patients to use psycho-oncological face-to-face interventions. Online interventions can be a low-threshold adjunct to existing face-to-face services. This study aims to evaluate the effect of the online self-help program epos (emotion-based psycho-oncological online self-help) on improving symptoms of anxiety and depression in German-speaking cancer patients.
Methods
A randomized controlled trial (RCT) is carried out in a parallel group design. N = 325 patients will be enrolled in the trial, randomly assigned to an intervention and a control group. While the intervention group has access to nine modules of epos, the control group gets access to an informational website. Participants will complete online questionnaires at baseline (T0), after the intervention (T1) and three-month follow-up (T2). Primary outcome is a combined measure of depression and anxiety. Secondary outcomes include psychological distress, anxiety, depression, quality of life, emotional control, posttraumatic growth, and satisfaction with epos. Participants are at least 18 years old, have a cancer diagnosis, currently receive cancer treatment or aftercare, have sufficient German language competence, and have access to the Internet. Exclusion criteria are severe mental comorbidities (i.e. severe depression, suicidality) or somatic comorbidities (i.e. visual disabilities).
Discussion
The results of this study will provide information about acceptability, feasibility, and efficacy of epos in improving symptoms of depression and anxiety in cancer patients and thus contribute to the research on web-based interventions. If found efficacious, epos will improve psycho-oncological care in cancer patients in transition from inpatient to outpatient care and in those who struggle to find adequate psycho-oncological support due to other (perceived) barriers.
Keywords: Cancer, Psycho-oncology, Online intervention, Psychological distress, RCT, E-mental health
1. Background
In Germany, the lifetime risk of developing cancer is between 42.6% and 47.5%, which means that almost every second person is diagnosed with cancer during their lifetime (Robert Koch-Institut, 2019). Advances in cancer research, prevention, treatment, and care have led to an increasing number of people surviving cancer (Barnes et al., 2016). Yet, cancer is associated with considerable psychological burden and a decrease of quality of life (QoL). Fifty-two percent of cancer patients experience psychological distress at some point (Mehnert et al., 2018). In a current analysis of a representative sample in Germany, 39.4% of cancer patients suffered from mental illnesses, with a 12-month prevalence for anxiety disorders of 15.8% and for depressive disorders of 12.5% (Kuhnt et al., 2016). These rates are comparable to a meta-analysis in which 12-month prevalences for anxiety disorders of 19.3% and 17.9% for affective disorders were reported. Furthermore, the pooled adjusted 4-week prevalence of affective disorders was 11.1% (resp. 10.8% based on German studies only) and of anxiety disorders 10.2% (resp. 13.5% in German studies; Vehling et al., 2012). Psycho-social burdens often persist after cancer is diagnosed, compromising the mental health of patients. Forty-two percent of cancer patients report experiencing psycho-social burden 6 months post hospital admission (Singer et al., 2011). A large representative study found male cancer patients to be twice as likely to report a lifetime diagnosis of depression as men without cancer (Ernst et al., 2019).
The increasing proportion of cancer survivors underlines the need for supportive measures to help patients improve their QoL. Psycho-oncology has been recognized as an effective strategy for coping with psychological challenges associated with cancer and has become part of standard cancer care in Germany (Deutsche Krebsgesellschaft, 2014; Nationaler Krebsplan, 2017). Psycho-oncological interventions aim to support coping with psycho-social and physical problems caused by cancer. Methods in psycho-oncology include psycho-education, group therapy, or self-help programs (Deutsche Krebsgesellschaft, 2014). However, there is a treatment gap between inpatient and outpatient psycho-oncological care. While patients usually have good access to psycho-oncological care during inpatient treatment in Germany, the usage of such offers is low. In a large German survey including N = 6.143 cancer survivors, 9% reported having used psycho-oncological care in the hospital (Zeissig et al., 2015). In certified cancer centers, on average 37.3% of all patients received psycho-oncological consultations (Singer et al., 2013). After discharge from hospital, cancer patients can find support in cancer counseling centers or at psychotherapists. However, the use of psycho-oncological support is lower in the outpatient setting, with percentages of self-reported usage ranging from 3 to 24.2% for cancer counseling (Zeissig et al., 2015; Faller et al., 2017) to 13.4% for psychotherapy (Faller et al., 2017). The barriers to taking up psycho-oncological offers are manifold. Although public awareness of mental health is slowly growing, there are still concerns and fears of stigmatization in the use or helpfulness of psychological and psychotherapeutic support (Neumann et al., 2010). The availability of cancer-related counseling for patients may be limited (Haun et al., 2018), e.g. by lack of transportation, living in a rural area, suffering from physical disabilities, or information deficits, which prevent cancer patients from taking advantage of face-to-face support (Zimmermann-Schlegel et al., 2017; Sakellariou et al., 2019; Neumann et al., 2010). In addition, barriers on the side of psychotherapists refer to uncertainties in the treatment of somatically ill patients, organizational problems, or lack of expertise, which are reflected in low proportions of patients in psychotherapeutic practices (Schwarz et al., 2006).
E-mental health has the potential to reduce this treatment gap in psycho-oncological care, by overcoming previous limitations. Online interventions enable patients to use psychological support independent of time and location. Furthermore, they provide low-threshold treatment options complementing existing stepped care programs (Nicholas et al., 2019; National Institute for Clinical Excellence, 2009) and can reach patients rejecting psychotherapeutic help because of perceived barriers, such as reservations about psychotherapy or the fear of being stigmatized. A large body of psychological research demonstrates that web-based online interventions can be effective in improving symptoms of depression and anxiety disorders (Karyotaki et al., 2017; Olthuis et al., 2016). Web-based interventions are also increasingly used in supportive cancer care. In recent years, several online interventions have been developed in psycho-oncology to reduce cancer-related stress and improve QoL (Beatty et al., 2019; van den Berg et al., 2015; Ringwald et al., 2019; Beatty et al., 2016; Cockle-Hearne et al., 2018; Russell et al., 2018; Urech et al., 2018). Several randomized controlled trials (RCT) have revealed promising results and suggest that cancer survivors can benefit from online interventions, with effect sizes ranging from small to medium (e.g. Beatty et al., 2019; van den Berg et al., 2015; Beatty et al., 2016; Willems et al., 2017; Urech et al., 2018).
Psycho-oncological online interventions differ in various aspects, including patient population (e.g. specific vs. different types of cancer, patients' sex), method (e.g. psycho-educative vs. therapeutic, guided vs. unguided), and underlying theoretical concept. With regard to the latter, existing interventions in cancer care predominantly follow a cognitive-behavioral or mindfulness-based rationale (Beatty et al., 2016; Cockle-Hearne et al., 2018; Beatty et al., 2019; Urech et al., 2018; Chambers et al., 2017; Russell et al., 2018). Although there is evidence that psychodynamic online interventions are feasible and effective (Zwerenz et al., 2017a, Zwerenz et al., 2017b; Lindqvist et al., 2020; Johansson et al., 2013a, Johansson et al., 2017), there is still a lack of psychodynamic online interventions compared to the large number of cognitive behavioral based online interventions (Johansson et al., 2013b). Different reasons for the low number of psychodynamic online interventions can be assumed. First, structured and manualized procedures, such as cognitive-behavioral therapy, are presumably easier to be transferred into the digital context. Secondly, CBT predominance in psychotherapy research has led to a low representation of psychodynamic research projects. However, like any psychotherapeutic procedure, psychodynamic interventions have important advantages. For instance, one central component of psychodynamic interventions is to support the perception and expression of emotions. When emotions are perceived, it is not primarily about regulating them. Rather, expressed emotions should be used to recognize underlying wishes and needs. This in turn helps patients to deal with strong feelings, to communicate with important others, and in this way to experience emotional relief. The concept of mentalization supports patients in the perception of such internal processes. Further, by exploring defense mechanisms, patients can gain a deeper understanding of their own emotional reactions and those of important others. With epos (emotion-based psycho-oncological online self-help), we have developed a comprehensive digital self-help program which is based on key elements of psychodynamic psychotherapy and also integrates theoretical concepts of emotional mindfulness and positive psychology.
1.1. Objective and research questions
The aim of the study is to examine the efficacy of the self-guided internet-based intervention epos compared to treatment as usual (TAU) in reducing symptoms of anxiety and depression in cancer patients. We hypothesize that patients in the intervention group (IG; online self-help program epos + TAU) will report significantly lower levels of depression and anxiety at the end of the intervention compared to the control group (CG; TAU) as primary outcome. Further, we expect that patients in the IG will report higher levels of QoL, social support, resilience, emotional control, and post-traumatic growth, compared to the CG. In addition to the primary and secondary endpoints, the study examines how participants of the IG evaluate individual components of epos (user satisfaction) and which factors influence adherence. Analyzing user satisfaction and adherence will allow drawing conclusions on acceptance of the online self-help in the light of participant's socio-demographic and medical characteristics. Conclusions about the feasibility of the intervention will be drawn from how the uptake of the online self-help varies with recruitment channel, medical characteristics (e.g., treatment type and status), and socio-demographic characteristics (e.g., sex). Besides usage behavior (e.g. number of modules completed) and compliance, the association between the self-reported use of psycho-oncological face-to-face treatment and the use of the online self-help will be analyzed. By doing this, conclusions can be drawn as to whether epos is a suitable, low-threshold offer for people with cancer, who might experience potential barriers towards the use of psycho-oncological face-to-face offers.
2. Methods
2.1. Study design
This two-arm RCT compares the efficacy of the self-guided web-based intervention epos to a control group receiving TAU. The monocentric trial is registered in the German Clinical Trials Register (registration number DRKS00021144). Participants are recruited from May 2020 until June 2021. Ethical approval for the study was obtained from the Ethics Committee of the Federal State of Rhineland-Palatinate (registration number 2019–14460).
2.2. Recruitment
Different recruitment strategies are combined in this study. Cancer patients in hospitals will be informed about the study by oncological nurses, physicians, or psycho-oncologists and receive a flyer with further information including the link to the study homepage. This will take place in particular at the University Medical Center of the Johannes Gutenberg University Mainz, but also in psycho-oncological departments of cooperating hospitals. In addition, the study will be advertised via classical media (e.g. newspapers) and social media campaigns (e.g. Instagram, Facebook, cancer-related websites). Patients who are interested in the study are directed to the study website where they can find all information (e.g. inclusion criteria) and register for the study.
2.3. Procedure
Registration, diagnostic self-assessment, and intervention are conducted online and take place in several steps (Fig. 1). In a first step, interested persons enter their email address and consent to the secure processing of personal data in a contact form which is offered on the publicly accessible study website. Persons who have expressed their interest via the contact form automatically receive a link to a non-public registration website. On this non-public registration page, participants are again provided the inclusion and exclusion criteria, the online version of detailed patient information and informed consent in plain language, which include information about the procedure, evaluation, pseudonymization, legal data security regulations as well as data analysis, storage and deletion procedures after completion of the study. Participants agree to the study conditions by click-to-agree and register for the study by setting their individual password and thereby creating a user account. Participants complete their registration by clicking on the confirmation link in a subsequent email and thus enter the password protected area of the epos website.
Fig. 1.
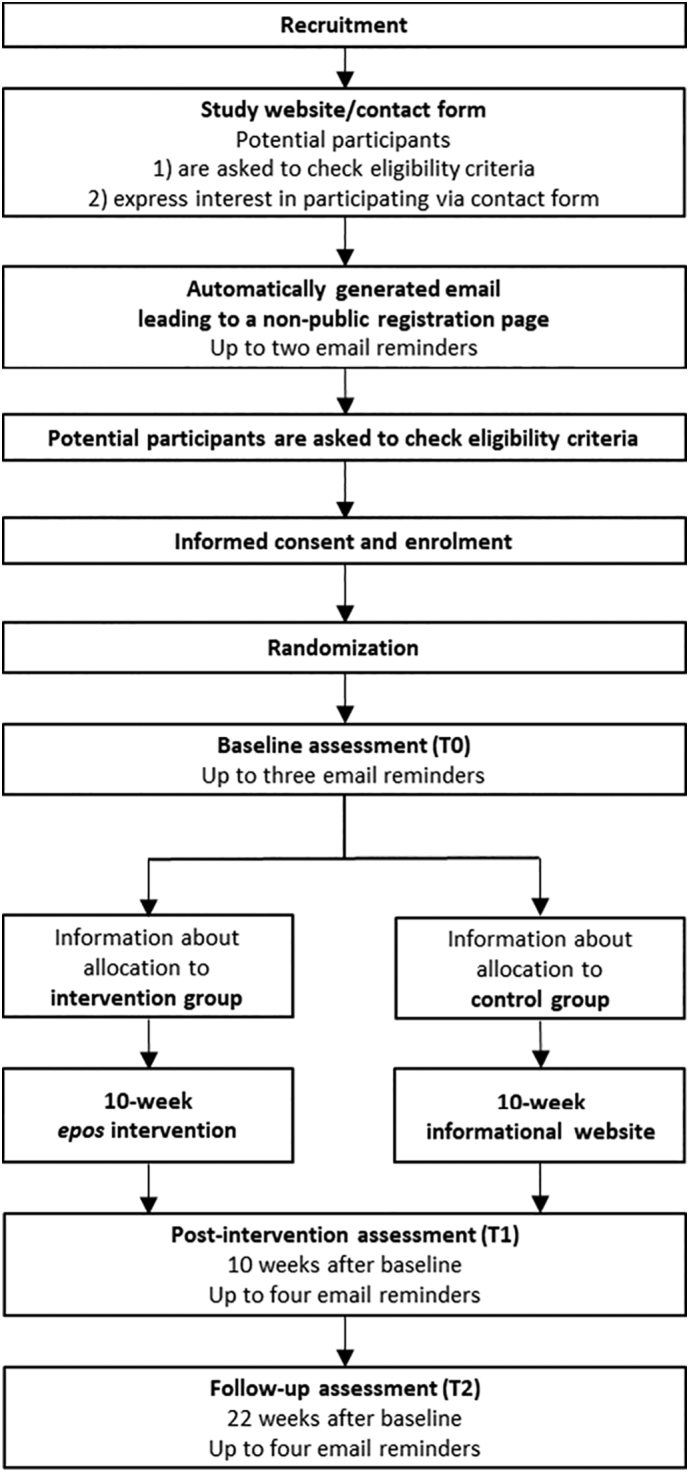
Study design.
After completion of the informed consent and registration on the platform, participants have direct access to the baseline questionnaire, which is available on the epos website once they have logged in. After completing the baseline questionnaire, participants are informed about which of the two study groups they have been assigned to. Blinding of the group assignment is not possible due to the study design. Participants in the CG get access to an informational website for 10 weeks, while participants in the IG can use the online self-help epos for 10 weeks. In order to motivate and not to withhold treatment for participants of the CG, participants are informed at the beginning of the study that every participant, regardless of group allocation, will have access to epos after completing the final questionnaire 22 weeks after baseline assessment.
2.4. Eligibility
Participants included are (1) adult patients (at least 18 years old), (2) diagnosed with any type of cancer and (3) currently undergoing cancer treatment or aftercare. They are required to (4) be able to read and write German and (5) have Internet access. Exclusion criteria are severe mental or somatic comorbidities (e.g. severe depression, suicidal tendencies, psychosis), which hinder participation in the study. Eligibility screening is conducted via self-assessment of the participants. At two points in the registration process, participants are asked to check whether inclusion criteria apply to them before proceeding with their registration. Participants are strongly encouraged to contact the study team if there is any uncertainty regarding eligibility.
2.5. Randomization
Participants are randomly assigned to either the intervention group or the control group. A computer-generated randomization list is prepared by the Interdisciplinary Center for Clinical Trials of the University Medical Center of the Johannes Gutenberg University Mainz. The randomization list is generated with permuted blocks with fixed and concealed block length and without further stratification factors. Once the registration has been confirmed by following the link in the confirmation email, the newly registered participant is assigned to the next unused randomization number from the randomization list and is thus randomly assigned to one of the two study groups. Each participant is informed about his treatment assignment automatically after filling out the baseline online questionnaire (T0). The study procedure is presented in Fig. 1.
2.6. Intervention
Participants of the IG have access to the emotion-based psycho-oncological online self-help program epos, which was developed based on a qualitative pre-study that assessed cancer patients' needs from different perspectives (Mayer et al., 2021). The pre-study was conducted with focus groups and patient interviews. Two focus groups were held with N = 14 healthcare professionals (HCP) of the University Medical Center of the Johannes Gutenberg University Mainz. The HCP were oncological physicians, psycho-oncologists, nurses, social workers, and physiotherapists. Furthermore, ten interviews were conducted with cancer patients of different entities. Based on this data and comprehensive research of literature, the online self-help epos was developed. Ultimately, the prototype of epos was piloted and revised based on the feedback of eight cancer patients.
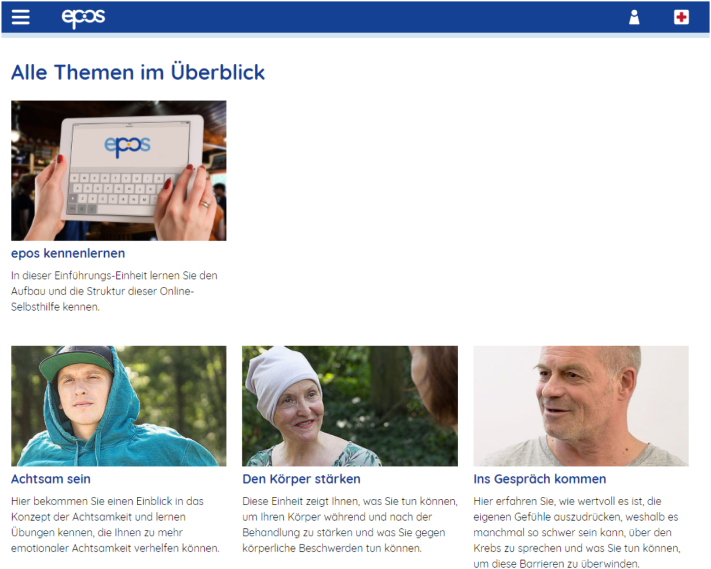
Epos consists of nine modules covering relevant topics (Table 1). In addition to these content related modules, there is a mandatory introductory unit in which the participants learn how epos is structured and how to use it (Fig. 2). Fundamental concepts of the intervention are psychodynamic psychotherapy, emotional mindfulness, and positive psychology. The psychodynamic orientation is mainly implemented by the following components: perceiving inner processes, such as feelings and underlying needs (of oneself and important others, e.g. through mentalization), developing an empathic connection to the own wishes and needs, focusing on social relationships, dealing with defense mechanisms.
Table 1.
Content of the epos intervention.
| Module | Chapter | |
|---|---|---|
| 0 | Introduction | Guided Tour Introduction of patients and experts Recommendations for using epos |
| 1 | Being mindful | Getting to know mindfulness Accepting the unchangeable Perceiving feelings mindfully |
| 2 | Strengthening the body | Staying active Restful sleeping Enjoying mindfully Dealing with physical complaints Rewarding body and soul |
| 3 | Talking about cancer | Expressing feelings One sentence – many messages Overcoming communicational barriers |
| 4 | Managing cancer together | Understanding change Accepting social support My social network Ways out of loneliness |
| 5 | Strengthening the soul | Understanding feelings Anxiety Sadness Pleasant feelings |
| 6 | Cancer and me | Who I am Focusing on positive things Strengthening body awareness Living personal values |
| 7 | Living with cancer | Back to everyday life Regaining control Discovering happy moments Gaining strength from gratitude Using sources of power |
| 8 | Family and partnership | My roles in life Sticking together as a family Strengthening the partnership |
| 9 | Practical advice | Linking to and giving information on further counseling services (e.g. psychological/psycho-oncological counseling, social service, self-help organizations) |
Fig. 2.
Excerpt from the module overview.
It is recommended to cover one module weekly, while the time required for one module is about 45–60 min. If necessary, participants can interrupt a module and proceed later. After finishing the mandatory introduction unit, participants can decide in which order they would like to complete the remaining modules. There are no time limits for working through a module, as participants should be given the opportunity to work through the units according to their current situation and needs. At the same time, the free choice is assumed to strengthen participants' sense of self-determination, which is usually severely limited by the disease and treatment.
Every module starts with assessing the current level of distress over the last week (Mehnert et al., 2006), and a mindfulness exercise. Two experts and four fictional patients guide the participants through the program. The modules are presented using a selection of different strategies, i.e. psycho-educational texts, interactive exercises for self-reflection as well as videos that enable the user to identify with the fictional patients and to visualize psychological states like fears and worries about cancer in a comprehensible and personal manner. Expert videos with psycho-oncologists provide psycho-educational information and encourage participants to reflect on the presented topics. Every module ends with an interactive review of the module and a gratitude exercise as well as a short evaluation of the presented content. In addition to the basic part, at numerous points there is the possibility of obtaining in-depth information on specific topics, so that the participants can obtain the amount of information they require from the online self-help. The online self-help is designed for use on computer, laptop and tablet. Smartphone use is possible, but not recommended due to the small screen size.
Epos is not designed to provide help for suicidal patients in acute crisis. An emergency button is presented in the program that provides contact numbers in case of psychological emergencies.
2.7. Control condition
Control group participants have access to an informational website with contact addresses of counseling offers and informational websites with evaluated cancer-related content (e.g. German Cancer Information Service, German Cancer Aid, or the Psycho-social Cancer Counseling Center at the Tumor-Center Rhineland-Palatinate). After completing the final questionnaire (T2), epos is automatically activated for participants of the CG.
2.8. Data collection
Data collection takes place exclusively online and does not involve any personal contact with the study staff. The questionnaires are offered in the same online system as the informed consent, the online self-help epos (IG) and the informational website of the CG. Assessment takes place at baseline (T0), post-intervention (T1; 10 weeks after baseline), and after a follow-up period (T2; 12 weeks after T1 or 22 weeks after baseline if T1 was not completed). The baseline questionnaire (T0) is presented automatically (after informed consent and randomization). Questionnaires T1 and T2 are announced via email. Participants are reminded up to four times by email to complete the questionnaire if they have not already done so.
2.9. Outcomes
Table 2 provides an overview of the data collected at the three measuring points.
Table 2.
Schedule of assessments.
| Construct | Questionnaire | T0 | T1 | T2 |
|---|---|---|---|---|
| Primary endpoint | ||||
| Combined measure of anxiety and depression | PHQ-ADS | x | x | x |
| Secondary endpoints | ||||
| Psychological distress | Distress Thermometera | x | x | x |
| Quality of life | EORTC-QLQ-C30 | x | x | x |
| Social support | SSUK | x | x | x |
| Resilience | RS-5 | x | x | x |
| Loneliness | Self-constructed item | x | x | x |
| Emotion regulation | ERQ | x | x | x |
| Post-traumatic growth | PTG | x | x | x |
| Other measures | ||||
| Demographics | Socio-demographic data | x | xb | xb |
| Medical history | Self-reported medical data | x | xc | xc |
| Health behavior | Godin leisure-time exercise questionnaire (physical activity) Self-constructed items (nutrition, smoking status, utilization of relaxation techniques) |
x | x | x |
| E-health literacy | eHEALS | x | ||
| Internet utilization/knowledge | Self-constructed items | x | ||
| Attitudes towards psychological online interventions | APOI | x | x | x |
| Satisfaction with epos | UEQd ZUF-8d Self-constructed itemsd, e |
x | ||
| Adherence | Frequency (number of sessions)f Duration (total time logged in)f Total progress per modulef |
n.a. | n.a. | n.a. |
This item is additionally provided at the beginning of every module in epos and thus regularly answered by participants of the IG during the intervention phase
Short version: marital status, children, employment status, weight, and sickness absences because of cancer.
Short version: metastasis, cancer recurrence, current and planned cancer treatments, utilization of psycho-social counseling or treatment offers, and somatic or mental comorbidities.
Only participants of the IG.
Short questionnaire querying comprehensibility and satisfaction provided at the end of every module during the intervention phase.
Objective data collected within the software.
2.9.1. Primary outcome
The primary outcome are symptoms of anxiety and depression, assessed with the Patient Health Questionnaire Anxiety and Depression scale (PHQ-ADS; Kroenke et al., 2016), which is a composite measure of depression and anxiety using the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder Scale (GAD-7). The German version of the PHQ-ADS is the sum of a total of 16 items comprising 9 items on depression and 7 items on anxiety. Participants are asked how much each of the described problems, e.g. “Feeling down, depressed, or hopeless”, has bothered them over the past two weeks and respond on a 4-point scale ranging from 0 = “not at all” to 3 = “nearly every day”. The scale demonstrates good psychometric properties. PHQ-ADS cut-off points of 10, 20, and 30 indicate mild, moderate, and severe levels of depression/anxiety, respectively.
2.9.2. Secondary outcomes
In addition to the combined assessment of anxiety and depression as primary outcome, anxiety and depression are also analyzed separately using the two subscales of the above described PHQ-ADS. The two subscales reflect the items of the Patient Health Questionnaire-9 (PHQ-9; Kroenke et al., 2001) and the Generalized Anxiety Disorder Scale (GAD-7; Spitzer et al., 2006). Analyzing anxiety and depression separately allows drawing conclusions on effects of epos on the individual scales. This procedure, as proposed by the European Medicines Agency (2002), provides supportive information on whether one of the two scales has a stronger influence on the overall score.
Psychological distress is assessed with the German adaptation of the NCCN Distress-Thermometer (Mehnert et al., 2006). This 1-item-screening instrument assesses psycho-social burden in oncological patients on a scale from 0 = “no distress” to 10 = “extreme distress” by asking “Please circle the number (0-10) that best describes how much distress you have been experiencing in the past week including today”. A cut-off-score of 5 is recommended. The Distress-Thermometer has high values in acceptance, brevity and practice orientation. The corresponding problem list is not used. Participants in the IG are additionally presented with this item at the beginning of every module during intervention phase.
Quality of Life is assessed through the EORTC Quality of Life questionnaire (EORTC-QLQ-C30; Aaronson et al., 1993). Participants are asked to rate health-related questions, e.g. “During the past week: Have you had trouble sleeping?”. The questionnaire consists of 28 items on a 4-point scale ranging from 0 = “not at all” to 3 = “very much” and two additional questions querying overall health and QoL during the past week on a scale ranging from 1 = “very poor” to 7 = ”excellent”. The questionnaire shows good values in reliability and validity, especially in cancer patients.
The eight-item short version of the scales of social support in illnesses (SSUK) is used to assess social support (Ullrich and Mehnert, 2010). On a 5-point scale ranging from 0 = “never” to 4 = “always”, participants have to rate if they have a supportive social environment (e.g. “Among the people who are close to you, is there someone who is there for you when you need him?”). The consistency of the SSUK has been proven to be good to very good and the criterion and construct validity is also good.
Resilience is assessed with the RS-5 Resilience Scale (von Eisenhart Rothe et al., 2013). Using five items, e.g. “Keeping interested in things is important to me”, and a 7-point scale from 1 = “strongly disagree” to 7 = “strongly agree”, the RS-5 is a reliable and valid instrument to provide information on successful coping.
Loneliness is assessed by a single item (Beutel et al., 2017): “I am frequently alone, resp. have few contacts” rated as 0 = “no, does not apply/yes, does apply, but I do not suffer from it”, 1 = “yes, does apply, and I suffer slightly”, 2 = “yes, does apply, and I suffer moderately”, 3 = “yes, does apply, and I suffer strongly” (Beutel et al., 2017).
Emotional control is assessed with the 10-item Emotion Regulation Questionnaire (ERQ), which investigates two common emotion regulation strategies (suppression, reappraisal) on two scales. A 7-point-scale ranging from 1 = ”strongly agree” to 7 = ”strongly disagree” is used to express agreement to the statements (e.g. “I control my emotions by not expressing them”). The German translation of the ERQ demonstrated sufficient psychometric properties: reliability, factor structure and indicators for construct validity (Wiltink et al., 2011).
Personal growth after cancer diagnosis is assessed with the Posttraumatic Growth Inventory which comprises five subscales: new possibilities, relating to others, personal strength, spiritual change, and appreciation of life (Tedeschi and Calhoun, 1996). Participants are asked to state their agreement to 21 statements, e.g. “I developed new interests”. The response scale used in this study is the 3-point scale as proposed and validated in the German translation with the values 0 = “not at all”, 1 = “something”, and 2 = “strongly”. In the German translation, reliability and factor structure of the Posttraumatic Growth Inventory were largely confirmed (Maercker and Langner, 2001).
2.9.3. Other measures
Socio-demographic data included in the study are sex, age, domicile, nationality (plus nationality of parents), time living in Germany, first language, marital status, children, education, qualification, current qualification status, employment status, sickness absences because of cancer, height, and weight.
Medical history is assessed by asking questions about diagnosis, time of diagnosis, metastasis, cancer recurrence, past cancer diagnosis, current and planned cancer treatments, somatic or mental comorbidities, utilization of psycho-oncological counseling or treatment offers, and reasons against the utilization of psycho-oncological treatment offers.
Health behavior includes self-constructed items assessing nutrition, smoking status, and utilization of relaxation techniques (e.g. yoga, meditation). Additionally, the Godin Leisure-Time Exercise Questionnaire (Godin and Shephard, 1985) is used to assess physical activity behavior by reporting physical activity (in minutes) in the last two weeks in three categories (intensive, moderate or light activity). The questionnaire has shown high validity in past research (Amireault and Godin, 2015).
eHealth literacy, i.e. the ability to read, use computers, search for information, understand health information and put it into context, is assessed with the German adaptation of the eHealth Literacy Scale (eHEALS; Soellner et al., 2014). It consists of eight items (e.g. “I know how to use the Internet to answer my health questions”). The eHEALS uses a 5-point Likert scale ranging from 1 = “strongly disagree” to 5 = “strongly agree”. Previous research showed good internal consistency of the questionnaire (Norman and Skinner, 2006), also for older adult populations (Chung and Nahm, 2015).
General attitude towards psychological online self-help programs is measured by the Attitudes towards Psychological Online Interventions questionnaire (APOI; Schröder et al., 2015), which assesses acceptance of internet interventions with 16 items. Answers are provided on a 5-point Likert scale (1 = ”strongly disagree”, 5 = ”strongly agree”). The questionnaire shows acceptable to good internal consistency (Schröder et al., 2015).
Utilization of the Internet is assessed with self-constructed items that query internet utilization (e.g. online time per day, type of mobile devices used).
Satisfaction with epos is assessed by providing a short questionnaire at the end of each module. Participants can indicate how they liked the texts, exercises, and videos. In addition, participants can enter suggestions and comments in an open text box. The T1 questionnaire also assesses satisfaction and use of the online self-help (IG) or the informational website (CG). Participants of the IG fill out the User Experience Questionnaire (UEQ) which contains the scales Attractiveness (six items), Perspicuity, Dependability, Efficiency, Novelty, and Stimulation (four items each; Laugwitz et al., 2008). Users rate the online-intervention in regard to 26 adjective pairs on a seven stage semantic differential (e.g. confusing vs. clear). The questionnaire shows sufficient psychometric properties (Laugwitz et al., 2008). Further, the one-dimensional ZUF-8, which is the German adaptation of the Client Satisfaction Questionnaire (CSQ-8; Attkisson and Zwick, 1982), is used as a short and economic scale to assess overall satisfaction with the online self-help program (Schmidt et al., 1989). The instrument measures patient satisfaction with 8 items (e.g. “How would you rate the quality of epos?”) on a 4-point-scale, whereby the total score over all items is interpreted. Six self-constructed open format questions provide the opportunity to give qualitative feedback on epos.
Adherence is assessed with objective data collected within the software and includes frequency of logins, duration of time logged in, and total progress per module.
Finally, participants are asked to indicate how they became aware of the study (e.g. in the hospital, through the Internet) and whether they have received a recommendation of an HCP to register for the study.
2.10. Sample size estimation
According to Kroenke et al. (2016), who validated the PHQ-ADS in three clinical populations including people with cancer, the minimum clinically relevant effect (MCID) was determined as MCID = 3.81 in the group of people with cancer, the standard deviation was SD = 11.0. The effect size d = 3.81/11.0 = 0.35 thus served as the basis for the sample size calculation. The calculation of sample size was performed with SAS Version 9.4, based on t-tests with two independent samples and a two-sided significance level of 5%: To achieve a power of 80%, data from 260 study participants (130 study participants per group) would be necessary for the statistical analyses. Regarding the expected dropout rate, a study with a comparable online intervention was considered, which reported a dropout rate of approximately 20% (Urech et al., 2018). Based on a dropout rate of 20%, we plan to randomize a total of 325 study participants.
2.11. Statistical analyses
Statistical analyses are carried out by the Interdisciplinary Center for Clinical Trials of the University Medical Center of the Johannes Gutenberg University Mainz in order to guarantee independence from the study team and high quality of the analyses. Efficacy of epos regarding the self-reported symptoms of anxiety and depression (primary endpoint, T1 minus T0) between IG and CG will be examined using analysis of covariance (ANCOVA). In the model, group (IG vs. CG) is considered as fixed effect. The baseline PHQ-ADS value serves as a covariate. This analysis allows statements about the effect of epos on the reduction of anxiety and depression after completion of the online self-help. Missing values will not be replaced for the primary analysis. However, for sensitivity, missing values will be replaced by multiple imputation. Additional sensitivity analyses that consider a potential impact of the recruitment strategy (i.e., recruitment location, received recommendation by health care professional versus participated on own initiative) are determined before the start of the analysis. Further sensitivity analyses including other potential influencing variables will also be determined before the start of the evaluation. The efficacy at T2 will be assessed with a linear model with mixed model repeated measurements (MMRM) analysis. In the model, group and time point of measurement will be considered as fixed effects, and the baseline PHQ-ADS as covariate. The interaction term of group and time of measurement will also be included in the model. The significance level is 5% (two-sided). Treatment group differences of the PHQ-ADS will be assessed by model estimates of the treatment difference and 95% confidence intervals and additionally be presented with descriptive methods and with graphics. Since all secondary endpoints are continuous variables, these items will be analyzed analogously to the primary endpoint. Descriptive analyses will be conducted for acceptance and feasibility, followed by regression models with variable selections.
3. Discussion
Psycho-oncological support is currently lacking continuity between inpatient and outpatient care (Nationaler Krebsplan, 2017), although symptoms of anxiety and depression are high in cancer patients and often manifest themselves especially after discharge from the clinic (Mehnert et al., 2018). Digital offers can help to close these gaps. This paper introduces the emotion-based psycho-oncological online self-help program epos and presents the study protocol for the RCT evaluating its efficacy, feasibility, and acceptance to reduce cancer-related anxiety and depression.
By using the online self-help epos, patients with any type of cancer are given support in coping with cancer-related burdens, especially in times when psycho-oncological care may not be available or when patients struggle to find adequate psycho-social support. While patients in inpatient care have access to psycho-oncological support, uptake rates of psycho-oncological offers in the outpatient setting are low (Zeissig et al., 2015). By investigating feasibility, especially in light of the recruiting channel, treatment status (e.g. current vs. completed treatment), and time since diagnosis, conclusions can be drawn about the stage of treatment at which epos might be an appropriate supportive measure. If found feasible for patients transitioning from inpatient to outpatient care, epos may be considered as a potential intervention to help bridge the gap in psycho-oncology care during this phase of treatment. In terms of a “blended-care” approach (linking regular therapy to web-based interventions), future psycho-oncological services can be improved sustainably.
In addition to providing information on the efficacy of epos in reducing symptoms of anxiety and depression, by recording the general attitudes towards psychological online interventions (APOI; Schröder et al., 2015) as well as the use and adherence of the online self-help epos, statements can be made about the acceptance. Furthermore, it can be investigated, whether potential barriers of conventional offers can be reduced by providing an online self-help-program. It can be assumed that the increasing distribution and use of digital devices (e.g. tablets and smartphones), which is also observed in higher age groups, has a positive effect on the willingness to use online services. Comparisons with earlier studies on psycho-oncological online interventions regarding adherence, acceptance, and recruitment rate may allow conclusions about such an assumed trend. Moreover, acceptance of online self-help programs can be improved and patients rejecting face-to-face psycho-oncological support offers (though in need of psychological help) may be more open towards an online intervention due to less fear of double stigmatization associated with mental health issues. Furthermore, by engaging in epos, the continuity of psycho-oncological care can be improved by providing patients with a flexible and independent opportunity to improve psychological functioning and prevent comorbid psychological disorders.
3.1. Strengths and limitations
Different strengths characterize this study. The intensive development phase, which considers the views of both patients and HCP, ensures that patient needs are met in terms of content. The pilot phase ensured that the technical implementation is user-friendly. Patient feedback was used to finally optimize the online self-help program. Epos exceeds topics of psycho-oncological acute interventions and offers the opportunity of transferring contents (e.g. partnership, dealing with emotions) into everyday life through exercises (e.g. mentalization exercises, reflecting upon individual values). The psychodynamic orientation of epos has the potential to provide innovative results and information that go beyond the findings of previous psycho-oncological online interventions that are primarily CBT- or mindfulness-based. Thus, the important variety of therapeutic orientations is being transferred to the online context. Further, epos can be combined with psycho-oncological care, but also be used as a low-threshold intervention without additional psycho-social care. Although conceived to reach a wide range of people with cancer (e.g. different cancer entities and age groups), individual interests, needs and desires for more in-depth content can still be met. Great efforts have been made to balance prototypical patients regarding tumor entity, sex, age, and social status, in order to foster identification of the participants with the patients shown.
However, there are several limitations that need to be considered. The sample of the RCT is heterogeneous. All types of cancer, male and female patients and a wide age range are included. This implies that specific problems of certain cancer entities may not be met due to the universality of epos. By reaching out to all cancer entities, very particular issues of individuals may be overlooked.
As an online intervention study, a high dropout rate must also be expected, especially in participants of the CG. By providing epos for members of the CG, in the form of a wait list control group, we want to enhance adherence and prevent dropouts in this study group. Furthermore, dropouts are considered in the statistical analyses.
When using epos, participants may prefer to work through the modules differently than recommended. In order to restrict participants as little as possible, all modules are available after completion of the obligatory module. This means that participants could complete all modules at once, contrary to the recommendation of one module per week. To prevent this, patients receive a comprehensible recommendation of how epos is used in an optimal way.
Authors' contributions
AT, AM, CR, NL, JW, MB, and RZ designed the study. AT, AM, and CN wrote the first draft of the manuscript. CR, NL, JW, MB, and RZ revised the manuscript critically. All authors contributed feedback, read, and approved the final manuscript.
Trial status
Trial start date: 06th May 2020; currently recruiting (Ncurrent = 273 as of May 27, 2021). Recruitment will continue through June 2021. Follow-up assessment is expected to be completed by December 2021.
Ethical statement
The study protocol and informed consent have been approved by the Ethics Committee of the Federal State of Rhineland-Palatinate (registration number 2019–14460) on 26.07.2019 and 19.05.2020. This trial is conducted in compliance with the approved study protocol and the Declaration of Helsinki. The RCT was registered at the German Clinical Trials Registry (Deutsches Register Klinischer Studien: DRKS; Registry number: DRKS00021144). Informed consent will be obtained from every participant.
Funding
This study is funded by the Innovation Fund of the Federal Joint Committee (G-BA), grant number 01VSF17018.
Declaration of competing interest
No conflicts of interest to disclose.
Acknowledgements
We thank Tamara Schwinn, Zoe Bünning, and Theresa Schorch for contributing to the development of epos. We thank the Center for Audiovisual Production of the Johannes Gutenberg University Mainz for the creative and audiovisual realization of epos. We thank the AOK Federal Association and the company H6-Communications Berlin for the technical implementation of epos. We thank Katharina Nora Hoffers for proofreading the manuscript.
References
- Aaronson N.K., Ahmedzai S., Bergman B., Bullinger M., Cull A., Duez N.J., Filiberti A., Flechtner H., Fleishman S.B., DE Haes J. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- Amireault S., Godin G. The Godin-Shephard leisure-time physical activity questionnaire: validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept Mot Skills. 2015;120:604–622. doi: 10.2466/03.27.PMS.120v19x7. [DOI] [PubMed] [Google Scholar]
- Attkisson C.C., Zwick R. The client satisfaction questionnaire: psychometric properties and correlations with service utilization and psychotherapy outcome. Eval Program Plann. 1982;5:233–237. doi: 10.1016/0149-7189(82)90074-x. [DOI] [PubMed] [Google Scholar]
- Barnes B., Kraywinkel K., Nowossadeck E., Schönfeld I., Starker A., Wienecke A., Wolf U. Robert Koch-Institut; 2016. Bericht zum Krebsgeschehen in Deutschland 2016. [Google Scholar]
- Beatty L., Koczwara B., Wade T. Evaluating the efficacy of a self-guided web-based CBT intervention for reducing cancer-distress: a randomised controlled trial. Support Care Cancer. 2016;24:1043–1051. doi: 10.1007/s00520-015-2867-6. [DOI] [PubMed] [Google Scholar]
- Beatty L., Kemp E., Coll J.R., Turner J., Butow P., Milne D., Yates P., Lambert S., Wootten A., Yip D. Finding my way: results of a multicentre RCT evaluating a web-based self-guided psychosocial intervention for newly diagnosed cancer survivors. Support Care Cancer. 2019;27:2533–2544. doi: 10.1007/s00520-018-4526-1. [DOI] [PubMed] [Google Scholar]
- Beutel M.E., Klein E.M., Brähler E., Reiner I., Jünger C., Michal M., Wiltink J., Wild P.S., Münzel T., Lackner K.J. Loneliness in the general population: prevalence, determinants and relations to mental health. BMC Psychiatry. 2017;17:97. doi: 10.1186/s12888-017-1262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S.K., Ritterband L., Thorndike F., Nielsen L., Aitken J.F., Clutton S., Scuffham P., Youl P., Morris B., Baade P. A study protocol for a randomised controlled trial of an interactive web-based intervention: CancerCope. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S.Y., Nahm E.S. Testing reliability and validity of the eHealth literacy scale (eHEALS) for older adults recruited online. Comput Inform Nurs. 2015;33:150–156. doi: 10.1097/CIN.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockle-Hearne J., Barnett D., Hicks J., Simpson M., White I., Faithfull S. A web-based intervention to reduce distress after prostate Cancer treatment: development and feasibility of the getting down to coping program in two different clinical settings. JMIR Cancer. 2018;4 doi: 10.2196/cancer.8918. (e8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF. 2014. Leitlinienprogramm Onkologie. S3-Leitlinie Psychoonkologische Diagnostik, Beratung und Behandlung von erwachsenen Krebspatienten, Langversion 1.1. (Accessed 09.10.2020).
- Ernst M., Wiltink J., Tibubos A.N., Brähler E., Schulz A., Wild P.S., Burghardt J., Münzel T., König J., Lackner K., Pfeiffer N., Michal M., Beutel M.E. Linking cancer and mental health in men and women in a representative community sample. J Psychosom Res. 2019;124:109760. doi: 10.1016/j.jpsychores.2019.109760. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency 2002. Points to consider on multiplicity issues in clinical trials. Doc. Ref.: CPMP/EWP/908/99.
- Faller H., Weis J., Koch U., Brähler E., Härter M., Keller M., Schulz H., Wegscheider K., Boehncke A., Hund B. Utilization of professional psychological care in a large German sample of cancer patients. Psychooncology. 2017;26:537–543. doi: 10.1002/pon.4197. [DOI] [PubMed] [Google Scholar]
- Godin G., Shephard R.J. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- Haun M.W., Sklenarova H., Zimmermann-Schlegel V., Herzog W., Hartmann M. Psycho-oncology care in rural areas : results from a cross-sectional survey on the utilisation of community-based psychosocial support services. Bundesgesundheitsbl. Gesundheitsforsch. Gesundheitsschutz. 2018;61:89–97. doi: 10.1007/s00103-017-2656-0. [DOI] [PubMed] [Google Scholar]
- Johansson R., Bjorklund M., Hornborg C., Karlsson S., Hesser H., Ljotsson B., Rousseau A., Frederick R.J., Andersson G. Affect-focused psychodynamic psychotherapy for depression and anxiety through the Internet: a randomized controlled trial. PeerJ. 2013;1 doi: 10.7717/peerj.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson R., Frederick R.J., Andersson G. Using the internet to provide psychodynamic psychotherapy. Psychodyn Psychiatry. 2013;41:513–540. doi: 10.1521/pdps.2013.41.4.513. [DOI] [PubMed] [Google Scholar]
- Johansson R., Hesslow T., Ljotsson B., Jansson A., Jonsson L., Fardig S., Karlsson J., Hesser H., Frederick R.J., Lilliengren P., Carlbring P., Andersson G. Internet-based affect-focused psychodynamic therapy for social anxiety disorder: a randomized controlled trial with 2-year follow-up. Psychother (Chic) 2017;54:351–360. doi: 10.1037/pst0000147. [DOI] [PubMed] [Google Scholar]
- Karyotaki E., Riper H., Twisk J., Hoogendoorn A., Kleiboer A., Mira A., Mackinnon A., Meyer B., Botella C., Littlewood E., Andersson G., Christensen H., Klein J.P., Schröder J., Bretón-López J., Scheider J., Griffiths K., Farrer L., Huibers M.J., Phillips R., Gilbody S., Moritz S., Berger T., Pop V., Spek V., Cuijpers P. Efficacy of self-guided internet-based cognitive behavioral therapy in the treatment of depressive symptoms: A meta-analysis of individual participant data. JAMA Psychiatry. 2017;74:351–359. doi: 10.1001/jamapsychiatry.2017.0044. [DOI] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Wu J., Yu Z., Bair M.J., Kean J., Stump T., Monahan P.O. The patient health questionnaire anxiety and depression scale (PHQ-ADS): initial validation in three clinical trials. Psychosom Med. 2016;78:716. doi: 10.1097/PSY.0000000000000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnt S., Brähler E., Faller H., Harter M., Keller M., Schulz H., Wegscheider K., Weis J., Boehncke A., Hund B., Reuter K., Richard M., Sehner S., Wittchen H.U., Koch U., Mehnert A. Twelve-month and lifetime prevalence of mental disorders in Cancer patients. Psychother Psychosom. 2016;85:289–296. doi: 10.1159/000446991. [DOI] [PubMed] [Google Scholar]
- Laugwitz B., Held T., Schrepp M. Construction and evaluation of a user experience questionnaire. In: Holzinger A., editor. Symposium of the Austrian HCI and Usability Engineering Group, 2008 Graz. Springer; Austria: 2008. pp. 63–76. [Google Scholar]
- Lindqvist K., Mechler J., Carlbring P., Lilliengren P., Falkenström F., Andersson G., Johansson R., Edbrooke-Childs J., Dahl H.-S.J., Lindert Bergsten K., Midgley N., Sandell R., Thorén A., Topooco N., Ulberg R., Philips B. Affect-focused psychodynamic internet-based therapy for adolescent depression: randomized controlled trial. J Med Internet Res. 2020;22(3) doi: 10.2196/18047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maercker A., Langner R. Persönliche Reifung (personal growth) durch Belastungen und Traumata: Validierung zweier deutschsprachiger Fragebogenversionen. Diagnostica. 2001;47:153–162. [Google Scholar]
- Mayer A., Tsiouris A., Nölke C., Schwinn T., Wiltink J., Beutel M.E., Zwerenz R. Development of a psychosocial online self-help program for Cancer patients: A patient-oriented approach. Rehabilitation (Stuttg) 2021;60:132–141. doi: 10.1055/a-1361-4993. [DOI] [PubMed] [Google Scholar]
- Mehnert A., Müller D., Lehmann C., Koch U. Die deutsche version des NCCN distress-thermometers: empirische Prüfung eines screening-instruments zur erfassung psychosozialer belastung bei krebspatienten. Z Psychiatr Psychol Psychother. 2006;54:213–223. [Google Scholar]
- Mehnert A., Hartung T.J., Friedrich M., Vehling S., Brähler E., Härter M., Keller M., Schulz H., Wegscheider K., Weis J., Koch U., Faller H. One in two Cancer patients is significantly distressed: prevalence and indicators of distress. Psychooncology. 2018;27:75–82. doi: 10.1002/pon.4464. [DOI] [PubMed] [Google Scholar]
- National Institute For Clinical Excellence 2009. Depression in adults: recognition and management. NICE guideline (CG 90). Retreived from https://www.nice.org.uk/guidance/cg90.
- Nationaler Krebsplan . Bundesministerium für Gesundheit; Berlin: 2017. Handlungsfelder, Ziele, Umsetzungsempfehlungen und Ergebnisse. [Google Scholar]
- Neumann M., Galushko M., Karbach U., Goldblatt H., Visser A., Wirtz M., Ernstmann N., Ommen O., Pfaff H. Barriers to using psycho-oncology services: a qualitative research into the perspectives of users, their relatives, non-users, physicians, and nurses. Support Care Cancer. 2010;18:1147–1156. doi: 10.1007/s00520-009-0731-2. [DOI] [PubMed] [Google Scholar]
- Nicholas J., Ringland K.E., Graham A.K., Knapp A.A., Lattie E.G., Kwasny M.J., Mohr D.C. Stepping up: predictors of ‘Stepping’ within an iCBT stepped-care intervention for depression. Int J Environ Res Public Health. 2019;16(23):4689 doi: 10.3390/ijerph16234689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman C.D., Skinner H.A. eHEALS: the eHealth literacy scale. J Med Internet Res. 2006;8 doi: 10.2196/jmir.8.4.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olthuis J.V., Watt M.C., Bailey K., Hayden J.A., Stewart S.H. Therapist-supported internet cognitive behavioural therapy for anxiety disorders in adults. Cochrane Database Syst Rev. 2016;3 doi: 10.1002/14651858.CD011565.pub2. (Cd011565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringwald J., Gerstner L., Junne F., Ziser K., Schäffeler N., Wallwiener M., Hautzinger M., Bäuerle A., Brucker S., Zipfel S. Mindfulness and skills based distress reduction in oncology: das Webbasierte Psychoonkologische make it training. Psychother Psychosom Med Psychol. 2019;69:407–412. doi: 10.1055/a-0835-6905. [DOI] [PubMed] [Google Scholar]
- Robert Koch-Institut . Robert-Koch-Institut; Ausgabe. Berlin: 2019. Krebs in Deutschland für 2015/2016. 12. [Google Scholar]
- Russell L., Ugalde A., Milne D., Krishnasamy M.E.O.S.C., Austin D.W., Chambers R., Orellana L., Livingston P.M. Feasibility of an online mindfulness-based program for patients with melanoma: study protocol for a randomised controlled trial. Trials. 2018;19:223. doi: 10.1186/s13063-018-2575-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakellariou D., Anstey S., Gaze S., Girt E., Kelly D., Moore B., Polack S., Pratt R., Tyrer G., Warren N., Wilkinson W., Courtenay M. Barriers to accessing cancer services for adults with physical disabilities in England and Wales: an interview-based study. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-027555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J., Lamprecht F., Wittmann W. Zufriedenheit mit der stationären Versorgung. Entwicklung eines Fragebogens und erste Validitätsuntersuchungen [Satisfaction with inpatient care: development of a questionnaire and first validity assessments] Psychother Psychosom Med Psychol. 1989;39(7):248–255. [PubMed] [Google Scholar]
- Schröder J., Sautier L., Kriston L., Berger T., Meyer B., Späth C., Köther U., Nestoriuc Y., Klein J.P., Moritz S. Development of a questionnaire measuring attitudes towards psychological online interventions–the APOI. J Affect Disord. 2015;187:136–141. doi: 10.1016/j.jad.2015.08.044. [DOI] [PubMed] [Google Scholar]
- Schwarz R., Rucki N., Singer S. Onkologisch Kranke als Patienten der psychotherapeutischen Praxis. Psychotherapeut. 2006;51:369–375. [Google Scholar]
- Singer S., Hohlfeld S., Müller-Briel D., Dietz A., Brähler E., Schröter K., Lehmann-Laue A. Psychosoziale Versorgung von Krebspatienten. Psychotherapeut. 2011;56:386–393. [Google Scholar]
- Singer S., Dieng S., Wesselmann S. Psycho-oncological care in certified cancer centres—a nationwide analysis in Germany. Psychooncology. 2013;22:1435–1437. doi: 10.1002/pon.3145. [DOI] [PubMed] [Google Scholar]
- Soellner R., Huber S., Reder M. The concept of eHealth literacy and its measurement: German translation of the eHEALS. J Media Psychol. 2014;26:29. [Google Scholar]
- Spitzer R.L., Kroenke K., Williams J.B., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Tedeschi R.G., Calhoun L.G. The posttraumatic growth inventory: measuring the positive legacy of trauma. J Trauma Stress. 1996;9:455–471. doi: 10.1007/BF02103658. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Mehnert A. Psychometrische Evaluation and Validierung einer 8-Item Kurzversion der Skalen zur Sozialen Unterstützung bei Krankheit (SSUK) bei Krebspatienten. Klinische Diagnostik und Evaluation. 2010;3:359–381. [Google Scholar]
- Urech C., Grossert A., Alder J., Scherer S., Handschin B., Kasenda B., Borislavova B., Degen S., Erb J., Faessler A. Web-based stress management for newly diagnosed patients with cancer (STREAM): a randomized, wait-list controlled intervention study. J Clin Oncol. 2018;36:780–788. doi: 10.1200/JCO.2017.74.8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg S.W., Gielissen M.F., Custers J.A., van der Graaf W.T., Ottevanger P.B., Prins J.B. BREATH: web-based self-management for psychological adjustment after primary breast cancer--results of a multicenter randomized controlled trial. J Clin Oncol. 2015;33:2763–2771. doi: 10.1200/JCO.2013.54.9386. [DOI] [PubMed] [Google Scholar]
- Vehling S., Koch U., Ladehoff N., Schön G., Wegscheider K., Heckl U., Weis J., Mehnert A. Prevalence of affective and anxiety disorders in cancer: systematic literature review and meta-analysis. Psychother Psychosom Med Psychol. 2012;62:249–258. doi: 10.1055/s-0032-1309032. [DOI] [PubMed] [Google Scholar]
- Von Eisenhart Rothe A., Zenger M., Lacruz M.E., Emeny R., Baumert J., Haefner S., Ladwig K.-H. Validation and development of a shorter version of the resilience scale RS-11: results from the population-based KORA–age study. BMC Psychol. 2013;1:25. doi: 10.1186/2050-7283-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems R.A., Bolman C.A., Mesters I., Kanera I.M., Beaulen A.A., Lechner L. Short-term effectiveness of a web-based tailored intervention for cancer survivors on quality of life, anxiety, depression, and fatigue: randomized controlled trial. Psychooncology. 2017;26:222–230. doi: 10.1002/pon.4113. [DOI] [PubMed] [Google Scholar]
- Wiltink J., Glaesmer H., Canterino M., Wölfling K., Knebel A., Kessler H., Brähler E., Beutel M.E. Regulation of emotions in the community: suppression and reappraisal strategies and its psychometric properties. GMS Psychosoc Med. 2011;8 doi: 10.3205/psm000078. URN: urn:nbn:de:0183-psm0000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeissig S.R., Singer S., Koch L., Blettner M., Arndt V. Inanspruchnahme psychoonkologischer Versorgung im Krankenhaus und in Krebsberatungsstellen durch Brust-, Darm-und Prostatakrebsüberlebende. Psychother Psychosom Med Psychol. 2015;65:177–182. doi: 10.1055/s-0034-1395627. [DOI] [PubMed] [Google Scholar]
- Zimmermann-Schlegel V., Hartmann M., Sklenarova H., Herzog W., Haun M.W. Accessibility, availability, and potential benefits of psycho-oncology services: the perspective of community-based physicians providing cancer survivorship care. Oncologist. 2017;22:719–727. doi: 10.1634/theoncologist.2016-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwerenz R., Becker J., Gerzymisch K., Siepmann M., Holme M., Kiwus U., Spörl-Dönch S., Beutel M.E. Evaluation of a transdiagnostic psychodynamic online intervention to support return to work: A randomized controlled trial. PLoS One. 2017;12 doi: 10.1371/journal.pone.0176513. (e0176513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwerenz R., Becker J., Johansson R., Frederick R.J., Andersson G., Beutel M.E. Transdiagnostic, psychodynamic web-based self-help intervention following inpatient psychotherapy: results of a feasibility study and randomized controlled trial. JMIR Mental Health. 2017;4(4) doi: 10.2196/mental.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]