Abstract
Background
With the comprehensive implementation of the second child policy in China, the proportion of multiparous women has increased dramatically in the past few years. As nearly half of them have a history of previous cesarean delivery, there is widespread concern regarding complications of their pregnancy.
Methods
The aim of this study was to evaluate the effect of the previous delivery mode on subsequent pregnancy outcomes in the real world based on data from a Chinese birth registry. Birth registry data from July 1, 2016 to June 30, 2017 among a Chinese population were collected and analyzed retrospectively. This study included 14 representative hospitals from 10 provinces of China. All delivery data were collected by an internet-based system using a birth registration platform. The study enrolled 36,355 multiparas. Information extracted for analysis included basic demographic characteristics, previous delivery mode, current delivery mode, major maternal complications, and neonatal outcomes. Pregnancy outcomes of women with previous cesarean delivery (PCS group, n=14,774) were compared with the outcomes of women with previous vaginal delivery (PVD group, n=21,581).
Results
There were statistically significant differences in the major pregnancy outcomes between the PCS group and the PVD group. The PCS group had a higher incidence of cesarean section (CS), placenta previa, postpartum hemorrhage, uterus rupture, hysterectomy, gestational diabetes, gestational hypertension, delivery before 37 weeks of gestation, low birth weight, and Apgar Score at 5 min ≤3.
Conclusions
Women with previous cesarean delivery had poorer pregnancy outcomes than women with previous vaginal delivery. Avoiding unnecessary CS, especially in primiparas is essential to improving maternal and neonatal outcomes in later pregnancies.
Keywords: Cesarean delivery, multipara, pregnancy outcomes, scarred uterus
Introduction
According to an investigation by the World Health Organization (WHO) in 2012, the rate of cesarean section (CS) in China is 46% and is much higher than that in other countries (1). As China has a large population, the number of multiparas has been increasing dramatically following the comprehensive implementation of the second child policy in 2016. The complications in a subsequent pregnancy associated with a scarred uterus are a serious concern. Scar pregnancy, placenta implantation, uterus rupture, and related consequences have become health problems in China (2). However, there is a lack of detailed clinical information following a large-scale investigation on the outcomes of pregnancy associated with a scarred uterus in China. Here we have evaluated the pregnancy outcomes of multiparas with a history of CS, based on data from a nationwide birth registration program in China.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/atm-20-8127).
Methods
Resources
This retrospective nationwide multicenter childbirth registration study assessed the birth registration information from 14 medical centers (including 3 second-level hospitals, 11 third-level hospitals, 7 general hospitals, and 7 maternal and child health care centers) in 4 economic divisions of China (including Beijing, Hunan, Guangdong, Zhejiang, Henan, Heilongjiang, Shandong, Sichuan, Shanghai and Shanxi provinces). The basic demographic information, major pregnancy complications, delivery, and newborn information of pregnant women were collected and recorded in the central database through the web-based data collection system. From the data of 99,977 pregnant women from July 1, 2016 to June 30, 2017, a total of 38,438 (36.4%) multiparas with complete basic information and those with gestational week was greater than or equal to 20 weeks were selected for evaluation. The diagnosis standard for each complication was defined based on the relevant guidelines (3). Postpartum hemorrhage was defined as blood loss greater than or equal to 500 mL in a vaginal delivery or greater than or equal to 1,000 mL in a CS in the 24 h postpartum period (4).
Ethical statement
Our study was performed in compliance with the Declaration of Helsinki (as revised in 2013). Our study method was approved by the Peking Union Medical College Hospital Review Board (reference number: JS-1151). The need for written informed consent was waived because of the retrospective nature of the study, and the dataset was deidentified in order to protect patient privacy.
Methods
All the multiparous women were divided into 2 groups according to the mode of previous delivery: the previous CS group (PCS group) and the previous vaginal delivery group (PVD group). The PCS group had 14,296 patients (37.50%) and the PVD group had 21,810 patients (62.50%). The exclusion criteria include multiple pregnancies ≥5, history of premature labor or miscarriage, history of stillbirth, history of preeclampsia, history of placenta abruption, history of other complications, age ≥45, incomplete data.
Basic information, pregnancy complications (placenta previa, placental abruption, postpartum hemorrhage, uterus rupture, hysterectomy, gestational hypertension, and gestational diabetes) and neonatal outcomes (gestational age at delivery, birth weight, Apgar score at 5 min, stillbirth, and neonatal death) were extracted for further analysis (Figure 1).
Figure 1.
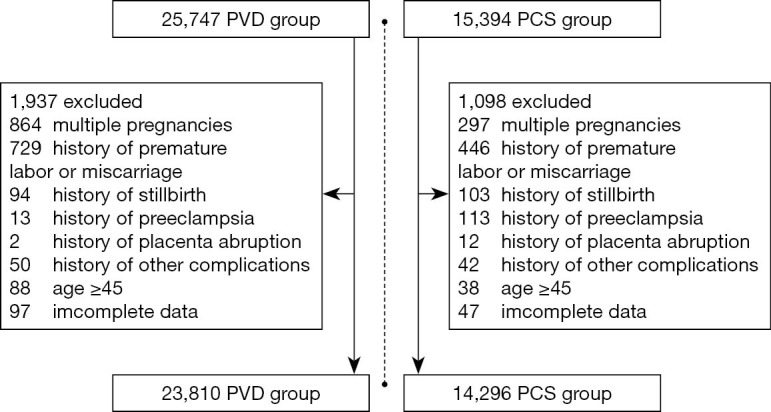
Screening process & result. All of the above pregnancy complications refer to complications during the last pregnancy.
Statistical analysis
Statistical analysis was performed by using SPSS 21.0 software. The measurement data were represented by , and the t-test was used for inter-group comparisons. Counting data have been represented by rate, and the χ2 test was performed for comparison between the groups. The difference was said to be statistically significant when P<0.05. Multivariate Logistic regression analysis was performed to control for possible confounders (OR is adjusted by multi-factors).
Results
Population description (Table 1)
Table 1. (PCS vs. PVD) basic information.
| Characteristics | PCS group (n=14,296) | PVD group (n=23,810) | P |
|---|---|---|---|
| Age | 33.51±8.17 | 32.11±8.93 | <0.01 |
| ≥35 years old | 6,067 (42.44%) | 7,369 (30.94%) | <0.01 |
| Pregnancy times | 3.20±2.27 | 3.09±2.10 | <0.01 |
| BMI | 27.16±6.83 | 26.44±6.50 | <0.01 |
| BMI ≥28 | 5,142 (35.97%) | 6,680 (28.06%) | <0.01 |
| IVF-ET | 91 (0.63%) | 349 (1.47%) | <0.01 |
IVF-ET, in vitro fertilization and embryo transfer.
The PCS group had 14,296 women (37.50%) and the PVD group had 23,810 women (62.50%). The average age of women with previous CS (33.51±8.17) was slightly higher than that of the women in the vaginal delivery group (32.11±8.93, P<0.01). What’s more, the proportion of women aged 35 years or older was higher in PCS group than in the PCD group (42.44% vs. 30.94%, P<0.01). The average number of women with previous CS (3.20±2.27, P<0.01) was slightly higher than that of the vaginal delivery group (3.09±2.10, P<0.01). The mean BMI of women with previous CS (27.16±6.83) was slightly higher than that of the vaginal delivery group (26.44±6.50), and the proportion of women with BMI ≥28 was higher than that of the vaginal delivery group (35.97% vs. 28.06%, P<0.01).
Mode of present delivery
The CS rate in our cohort was 32.90% in primiparas and 52.50% in multiparas. There were 23,810 cases of vaginal delivery (62.50%) and 14,296 cases of cesarean delivery (37.50%) among the 38,106 multiparas. In the PCS group, the rate of cesarean delivery was 95.85%, and only 620 (4.15%) women had a successful vaginal delivery. However, the rate of cesarean delivery and vaginal delivery was only 28.12% and 71.88%, respectively, in the PCD group. The cesarean delivery rate was much higher in the PCS group than in the PVD group (OR: 62.78, 95% CI: 54.14–64.42).
The indications for the CS in these multiparas were scarred uterus (82%), CS on patients’ request (8%), placenta previa (3%), gestational hypertension (2%), fetal position such as breech presentation (1%), macrosomia (1%), fetal distress (1%), and multiple pregnancies (1%).
Indications for previous CS were as follows: CS on patients’ request (50%), fetal distress (10%), fetal position (7%), dystocia (6%), scarred uterus (history of uterine surgery, except CS) (4%), cephalopelvic disproportion (3%), gestational hypertension (2%), and placenta previa (1%).
The scared uterus indicates history of myomectomy, endometrial polypectomy and CS.
Maternal complications (Table 2)
Table 2. (PCS vs. PVD) pregnancy outcomes.
| Characteristics | PCS group (n=14296) | PVD group (n=23,810) | OR | 95% CI | P |
|---|---|---|---|---|---|
| CS delivery | 13,703 (95.85%) | 6,696 (28.12%) | 62.78 | 54.14–64.42 | <0.01 |
| Placenta previa | 698 (4.88%) | 600 (2.52%) | 1.95 | 1.78–2.22 | <0.01 |
| Placenta implatation | 783 (5.48%) | 575 (2.41%) | 2.48 | 2.10–2.61 | <0.01 |
| Placental abruption | 84 (0.59%) | 145 (0.61%) | 0.95 | 0.74–1.26 | 0.79 |
| Uterine rupture | 95 (0.66%) | 9 (0.04%) | 17.87 | 8.93–35.06 | <0.01 |
| Gestational hypertation | 595 (4.16%) | 831 (3.49%) | 0.990 | 1.08–1.34 | <0.01 |
| GDM | 2,428 (16.98%) | 2,995 (12.58%) | 1.296 | 1.34–1.51 | <0.01 |
| Postpartum hemorrhage | |||||
| ≤1,000 mL | 347 (2.43%) | 587 (2.47%) | 0.86–1.13 | 0.82 | |
| 1,000–2,000 mL | 105 (0.73%) | 71 (0.30%) | 1.83–3.35 | <0.01 | |
| ≥2,000 mL | 103 (0.72%) | 30 (0.13%) | 3.93–8.64 | <0.01 | |
| Causes of hemorrhage | |||||
| Postpartum hemorrhage | |||||
| Uterine inertia | 446 (3.12%) | 587 (2.47%) | 1.21 | 1.12–1.44 | <0.01 |
| Birth canal injury | 9 (0.06%) | 34 (0.14%) | 0.52 | 0.21–0.92 | <0.05 |
| Placenta factor | 132 (0.92%) | 155 (0.65%) | 1.51 | 1.13–1.80 | <0.01 |
| Coagulation disorders | 4 (0.03%) | 13 (0.05%) | 0.50 | 0.17–1.57 | <0.23 |
GDM, gestational diabetes mellitus.
Pregnancy complications were compared between the two groups. The incidence of placenta previa was 4.88% versus 2.52% (aOR: 1.95, 95% CI: 1.78–2.22), while that of postpartum hemorrhage was 0.73% versus 0.30% (aOR: 2.47, 95% CI: 1.83–3.35) in the PCS and PVD groups, respectively. The PCS group had a higher incidence of uterus rupture (0.66% vs. 0.04%, aOR 17.87, 95% CI, 8.93–35.06) and gestational diabetes (16.98% vs. 12.58%, aOR 1.296, 95% CI, 1.342–1.507). There was no significant difference in the probability of placental abruption between the two groups (0.59% vs. 0.61%, P=0.95) and gestational hypertension (4.16% vs. 3.49%, P=0.99).
Neonatal outcomes (Table 3)
Table 3. (PCS vs. PVD) neonatal outcome.
| PCS group (n=14,296) | PVD group (n=23,810) | OR | 95% CI | P | |
|---|---|---|---|---|---|
| Delivery weeks | |||||
| ≥37 weeks | 12,772 (89.34%) | 21,610 (90.76%) | |||
| <37 weeks | 15,241 (0.66%) | 2,200 (9.24%) | |||
| <34 weeks | 448 (3.13%) | 776 (3.25%) | 1.08 | 0.96–1.21 | 0.23 |
| Birth weeks | |||||
| ≥2,500 g | 13,413 (93.8%) | 22,262 (93.50%) | |||
| <2,500 g | 883 (6.18%) | 1,548 (6.50%) | 1.06 | 0.98–1.16 | 0.16 |
| <1,500 g | 206 (1.44%) | 345 (1.45%) | 1.11 | 0.94–1.32 | 0.23 |
| Apgar score | |||||
| 5 min score <7 | 70 (0.49%) | 123 (0.52%) | 1.06 | 0.79–1.42 | 0.70 |
| 5 min score ≥7 | 14,226 (99.51%) | 23,687 (99.48%) |
The mean gestational age at delivery in the PCS group and the PVD group was 38.2±1.9 and 38.6±2.1 weeks, respectively. Preterm birth rate before 37 weeks was 10.66% versus 9.24% (aOR: 1.17, 95% CI: 1.06–1.26, P<0.01), while the preterm birth rate before 34 weeks had no significance (aOR: 1.08, 95% CI, 0.96–1.21, P=0.23).
The mean birth weight of newborns in the two groups was 3,230±576 and 3,226±594 g (P=0.53). The proportion of newborns with low birth weight (<2,500 g) were 6.18% in the PCS group and 6.5% in the PVD group (aOR: 0.84, 95% CI: 0.98–1.16, P=0.16). There was no difference in the proportion of very low birth weight children (<1,500 g) (2.0% vs. 1.9%, respectively, P=0.62).
The rate of 5-min Apgar score less than or equal to 7 in the PCS group was little lower than that in the PVD group (0.49% vs. 0.52%, aOR: 1.06, 95% CI: 0.79–1.42, P=0.70). There was no significant difference in perinatal mortality between the two groups (both showed 0.5%).
Discussion
Principal findings of the study
The risks faced by a pregnant women with previous uterine scarring include various complications during pregnancy as well as the delivery period (5,6). According to an analysis on the ways and outcomes of childbirth in Asia published in 2010 (7), cesarean delivery increases the risk of maternal death, admission to the ICU, blood transfusion, hysterectomy, and iliac artery ligation. Pregnant women with a history of cesarean delivery, irrespective of whether they choose a cesarean or vaginal delivery at the present, are at a higher risk of developing complications than those who had a normal vaginal delivery the first time, including serious adverse events such as uterine rupture and maternal and neonatal death (8,9).
Complications related to the placenta are among the more serious consequences of previous CS (10). Women with previous CS are more likely to have placenta previa and placental implantation, leading to severe postpartum hemorrhage and even hysterectomy (11,12). The reason that placenta previa occurs easily in a scarred uterus is unclear (13). It is assumed that the chronic inflammation resulting from abnormal deciduation in the lower segment of the uterus affects fertilized egg implantation and placental attachment under the catalysis of inflammatory factors (14,15). The risk of postpartum hemorrhage is also increased due to atonic uterine lower segment, which is more common in women with previous CS (16). In our present study, we confirmed that the rate of placenta previa, postpartum hemorrhage, blood transfusion, preterm birth, and hysterectomy are all higher in women with previous CS than that in women without previous CS.
Besides placental problems, uterine rupture is another severe complication of a scarred uterus. The uterus rupture rate in our cohort was 0.7% and would have been higher as most of the patients received selected CS without any attempt at a vaginal delivery.
Neonatal outcomes were also seen to be worse in the PCS group compared to that in the PVD group, and they correlated with the maternal complications (17). The weight of placenta, low perfusion, and damage of villi are all direct factors leading to adverse outcomes in the fetus (18). The greater number of low-birth-weight neonates observed in the PCS group may be the result of increased placental problems in this group. However, there was no difference in the neonatal mortality between the two groups. It has been suggested that the mode of delivery may influence the future health of the newborn (19). Observational studies have shown that children born through a cesarean delivery had an increased risk of developing immune-related diseases such as asthma, allergic conditions, type I diabetes, obesity, and malignancies. The underlying mechanism is a hot area of research and is probably related to delayed gut flora establishment and altered epigenetic modification (20-23). As a consequence, the high cesarean delivery rate may have a profound influence on the Chinese health care system in the future.
Clinical implication
Through our research, we advocate that vaginal birth should be chosen when health conditions permit. It can reduce the risk of placenta previa, postpartum hemorrhage, blood transfusion, preterm birth and hysterectomy. Currently, the rate of CS in China is much higher than that in other countries. Although trial of labor after CS (TOLAC) is suggested as a safe option in selected patients and in institutions capable of conducting emergency CS, up to 95% of the patients received repeat CS in our cohort, which we think is the reality in most areas of China (24). We believe that a lack of conditions required for the implementation of TOLAC and the fear of legal prosecution are the two main obstacles for TOLAC in China.
Strengths and limitations
It is reported that a history CS is an independent risk factor for pre-eclampsia (25,26). In our present cohort, complications such as gestational hypertension disease and gestational diabetes were also more frequent in women with previous CS, but selection bias cannot be ruled out.
Being a retrospective study, the registration information was not comprehensive enough, the baseline characteristics of the two groups may not have been parallel, and multivariate analysis or stratification analysis could not be performed to rule out the effects of other factors on pregnancy and delivery outcomes.
Research implications
In the past two decades, the rate of cesarean delivery in China has increased dramatically, mostly attributed to CS on patient’s request without any medical indications (1). The reasons for this increase are complex with various medical, social, and economic factors playing a role. The CS rate is as high as 46% in China according to a WHO report. Presently, the Chinese are facing a ‘post-CS era’ since the comprehensive implementation of the second-child policy in 2016. The increase in the rate of CS has caused previously rare medical conditions such as scar pregnancy, uterus rupture, placental previa and placental implantation, and repeat CS and its associated complications to become more prevalent, and this has led to an increase in the maternal and neonatal mortality and morbidity. In our study, 40.6% of the multiparas had previous CS; they accounted for 14.8% of all pregnant women and contributed to 27.9% of all cesarean deliveries.
Conclusions
Our study reveals the seriousness of the “post-CS era” in China. Pregnant women who had previous cesarean deliveries had a higher risk of various pregnancy-related complications, including placenta previa, uterus rupture, postpartum hemorrhage, hysterectomy, preterm birth before 37 weeks of gestation, fetal distress, and gestational hypertension disease as well as gestational diabetes. Concerted efforts should be carried out to reduce the CS rate, and thereby, the related complications.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank the following hospitals, including Beijing Haidian Maternal and Child Health Hospital, Sichuan University West China Second Hospital, Southern Medical University Nanfang Hospital, Northwest Women and Children’s Hospital, Ruijin Maternal and Child Health Hospital, Hunan Maternal and Child Health Care Hospital, and Shengjing Hospital Affiliated to China Medical University, where the register work was conducted for this program.
Funding: National Science and Technology Support Program 13th Five-Year (No. 2015BAI13B04). CAMS Innovation Fund for Medical Science (CIFMS) (No. 2017-I2M-3-007).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Our study was performed in compliance with the Declaration of Helsinki (as revised in 2013). Our study method was approved by the Peking Union Medical College Hospital Review Board (reference number: JS-1151). The need for written informed consent was waived because of the retrospective nature of the study, and the dataset was deidentified in order to protect patient privacy.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/atm-20-8127
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-20-8127
Peer Review File: Available at https://dx.doi.org/10.21037/atm-20-8127
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-20-8127). The authors have no conflicts of interest to declare.
References
- 1.Hou L, Li G, Zou L, et al. Cesarean delivery rate and indications in mainland China: a cross sectional study in 2011. Zhonghua Fu Chan Ke Za Zhi 2014;49:728-35. [PubMed] [Google Scholar]
- 2.Ashwal E, Hiersch L, Melamed N, et al. Pregnancy outcome after induction of labor in women with previous cesarean section. J Matern Fetal Neonatal Med 2015;28:386-91. 10.3109/14767058.2014.916685 [DOI] [PubMed] [Google Scholar]
- 3.Matthys LA, Coppage KH, Lambers DS, et al. Delayed postpartum preeclampsia: an experience of 151 cases. Am J Obstet Gynecol 2004;190:1464-6. 10.1016/j.ajog.2004.02.037 [DOI] [PubMed] [Google Scholar]
- 4.Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum haemorrhage. Cochrane Database Syst Rev 2012;(4):CD005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martucci JL. Cesarean Section: An American History of Risk, Technology, and Consequence, by Jacqueline H. Wolf. Nurs Hist Rev 2019;28:235-7. 10.1891/1062-8061.28.235 [DOI] [PubMed] [Google Scholar]
- 6.Betran AP, Ye J, Moller AB, et al. The Increasing Trend in Caesarean Section Rates: Global, Regional and National Estimates: 1990-2014. PLoS One 2016;11:e0148343. 10.1371/journal.pone.0148343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sentilhes L, Vayssiere C, Beucher G, et al. Delivery for women with a previous cesarean: guidelines for clinical practice from the French College of Gynecologists and Obstetricians (CNGOF). Eur J Obstet Gynecol Reprod Biol 2013;170:25-32. 10.1016/j.ejogrb.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 8.Curtin SC, Gregory KD, Korst LM, et al. Maternal Morbidity for Vaginal and Cesarean Deliveries, According to Previous Cesarean History: New Data From the Birth Certificate, 2013. Natl Vital Stat Rep 2015;64:1-13, back cover. [PubMed] [Google Scholar]
- 9.Uno K, Mayama M, Yoshihara M, et al. Reasons for previous Cesarean deliveries impact a woman's independent decision of delivery mode and the success of trial of labor after Cesarean. BMC Pregnancy Childbirth 2020;20:170. 10.1186/s12884-020-2833-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill JM, Yadava S, Patrick HS, et al. Pregnancy complications among women diagnosed with placenta previa and placental abruption. Am J Obstet Gynecol 2020;222:S292. 10.1016/j.ajog.2019.11.462 [DOI] [Google Scholar]
- 11.Naji O, Wynants L, Smith A, et al. Does the presence of a Caesarean section scar affect implantation site and early pregnancy outcome in women attending an early pregnancy assessment unit? Hum Reprod 2013;28:1489-96. 10.1093/humrep/det110 [DOI] [PubMed] [Google Scholar]
- 12.Jauniaux E, Moffett A, Burton GJ. Placental Implantation Disorders. Obstet Gynecol Clin North Am 2020;47:117-32. 10.1016/j.ogc.2019.10.002 [DOI] [PubMed] [Google Scholar]
- 13.Karagiozova J, Ivanov S, Masseva A, et al. Location of the placenta in pregnancy with previous caesarean section. Akush Ginekol (Sofiia) 2014;53 Suppl 2:26-8. [PubMed] [Google Scholar]
- 14.Palacios-Jaraquemada JM. Caesarean section in cases of placenta praevia and accreta. Best Pract Res Clin Obstet Gynaecol 2013;27:221-32. 10.1016/j.bpobgyn.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 15.Palova E, Redecha M, Malova A, et al. Placenta accreta as a cause of peripartum hysterectomy. Bratisl Lek Listy 2016;117:212-6. 10.4149/BLL_2016_040 [DOI] [PubMed] [Google Scholar]
- 16.Rombauts L, Motteram C, Berkowitz E, et al. Risk of placenta praevia is linked to endometrial thickness in a retrospective cohort study of 4537 singleton assisted reproduction technology births. Hum Reprod 2014;29:2787-93. 10.1093/humrep/deu240 [DOI] [PubMed] [Google Scholar]
- 17.Abenhaim HA, Benjamin A. Effect of prior cesarean delivery on neonatal outcomes. J Perinat Med 2011;39:241-4. 10.1515/jpm.2011.050 [DOI] [PubMed] [Google Scholar]
- 18.Weiner E, Miremberg H, Grinstein E, et al. Placental histopathology lesions and pregnancy outcome in pregnancies complicated with symptomatic vs. non-symptomatic placenta previa. Early Hum Dev 2016;101:85-9. 10.1016/j.earlhumdev.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 19.Sandall J, Tribe RM, Avery L, et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet 2018;392:1349-57. 10.1016/S0140-6736(18)31930-5 [DOI] [PubMed] [Google Scholar]
- 20.Kolokotroni O, Middleton N, Gavatha M, et al. Asthma and atopy in children born by caesarean section: effect modification by family history of allergies - a population based cross-sectional study. BMC Pediatr 2012;12:179. 10.1186/1471-2431-12-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardwell CR, Stene LC, Joner G, et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia 2008;51:726-35. 10.1007/s00125-008-0941-z [DOI] [PubMed] [Google Scholar]
- 22.Blustein J, Attina T, Liu M, et al. Association of caesarean delivery with child adiposity from age 6 weeks to 15 years. Int J Obes (Lond) 2013;37:900-6. 10.1038/ijo.2013.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho CE, Norman M. Cesarean section and development of the immune system in the offspring. Am J Obstet Gynecol 2013;208:249-54. 10.1016/j.ajog.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 24.Mittal S, Pardeshi S, Mayadeo N, et al. Trends in cesarean delivery: rate and indications. J Obstet Gynaecol India 2014;64:251-4. 10.1007/s13224-013-0491-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Carbajal MJ, Manriquez-Moreno ME, Galvez-Camargo D, et al. Risk factors associated to preclampsia. Rev Med Inst Mex Seguro Soc 2012;50:471-6. [PubMed] [Google Scholar]
- 26.Chen P, Wang S, Ji J, et al. Risk factors and management of gestational diabetes. Cell Biochem Biophys 2015;71:689-94. 10.1007/s12013-014-0248-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


