Abstract
Management of trauma-related liver injury has undergone a paradigm shift over the past four decades. In hemodynamically stable patients, the standard of care in the majority of level-one trauma centers has shifted to nonoperative management with high success rates, especially with low-grade liver injuries (i.e., grade I and II liver injuries). Advances in critical care medicine, cross-sectional imaging, and transarterial embolization techniques have led to the improvement of patient outcomes and decreased mortality rates in patients with arterial injuries. Currently, no consensus guidelines on appropriate patient selection criteria have been published by the Society of Interventional Radiology (SIR) or the American Association for the surgery of Trauma (AAST). Based off the current literature, nonoperative management with hepatic angiography and transarterial embolization (TAE) should be the treatment of choice in hemodynamically stable patients with clinical suspicion of arterial injury. TAE has been shown to improve success rates of nonoperative management and is well tolerated by most patients with low complication rates. Hepatic necrosis is the most common and concerning reported complication but can be reduced with selective approach and choice of embolic agent. The majority of literature supporting the use of TAE for trauma-related liver injury consists of retrospective case series and additional larger scale studies are needed to determine the efficacy of TAE in this setting. However, it is clear from the current literature that hepatic TAE is an effective and safer option to operative management in treating arterial hemorrhage in the setting of traumatic hepatic injury.
Keywords: Transarterial embolization (TAE), hepatic trauma, emergency interventions
Introduction
The liver is one of the most commonly injured organs in blunt and penetrating abdominal trauma (1). Motor vehicle collision is the most common mechanism of trauma in blunt injuries, typically resulting in injury within the left hepatic lobe (2). Penetrating liver injuries can range from simple parenchymal to major vascular insults in a random pattern depending on the path of the penetrating object.
The management of trauma-related liver injury has evolved since the early 1990s, transitioning from a predominantly surgical approach to a nonoperative multidisciplinary approach where surgeons, intensivists and interventional radiologists work together to manage the patient. Advances in critical care and minimally invasive procedures have facilitated a paradigm shift, with nonoperative management serving as the current standard of care for the hemodynamically stable patient, even in the most severe form with venous injury (3,4). The results have led to a decrease in abdominal infections, decreased transfusions, and decreased length of hospital stay (5,6). In addition, advances in imaging have allowed the treatment teams to quickly identify hepatic injury and formulate an approach to the management of the patient. The rapid identification of injury grade, arterial hemorrhage and/or juxtahepatic venous injury is critical in the management algorithm.
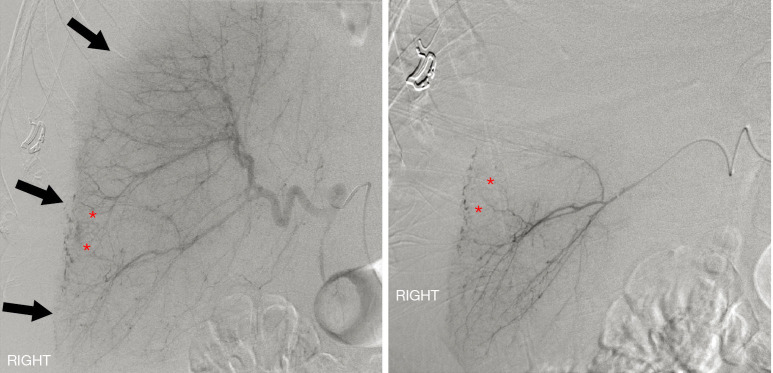
Transarterial embolization (TAE) has emerged as an invaluable adjunct to the successful nonoperative management of hepatic trauma patients with reported success rates as high as 93% in stopping arterial hemorrhage (6).Hepatic TAE can also be used to treat patients who have failed observational management or patients that have ongoing bleeding or rebleeding after surgical management (7,8). Generally, hepatic TAE is well-tolerated with low rates of associated complications (6,9). Major hepatic necrosis is the most commonly reported and concerning complication occurring in approximately 16% of patients (6,10). Although the exact mechanism is unknown, it appears the combination of hepatic injury and ischemia induced by TAE may predispose to hepatic necrosis. Prior studies have shown increased rates of major hepatic necrosis with higher liver injury grade and nonselective embolization (3,6,7,10-12). Decreased rates of major hepatic necrosis have been reported through the use of microcatheter systems with superselective embolization (10,13) (Figure 1).
Figure 1.
Digital subtraction angiography in a patient status post rollover all-terrain vehicle accident. Selective common hepatic (left) and superselective right hepatic (right) angiography demonstrate multifocal areas of arterial extravasation from the liver (asterisks). Note the displacement of the liver capsule from the chest and abdominal wall, indicating the presence of a large perihepatic hematoma (arrows). Images courtesy of Dr. Keith Quencer.
The majority of literature supporting the use of TAE for trauma-related liver injury consists of retrospective case series (6,10). Additional, larger scale studies are needed to determine the efficacy of TAE in the setting of hepatic hemorrhage secondary to trauma and well as the complication rates following embolization of the liver. Based on the current published data, the Society of Interventional Radiology (SIR) has released some general parameters and recommendations for practicing interventionalists in the use of TAE for hepatic trauma (10). In this review, we present the contemporary approach to liver trauma evaluation and management, with an emphasis on transarterial embolization therapies.
Trauma evaluation and diagnosis
Most trauma centers have standardized protocols for the initial resuscitation, diagnostic evaluation, and management of the trauma patient. Trauma protocols are predominantly based upon the Advanced Trauma Life Support (ATLS) program, established by the American College of Surgeons Committee on Trauma.
Hemodynamically unstable patients should immediately undergo surgical evaluation. If the patient is clinically stable, a Focused Assessment with Sonography for Trauma (FAST) exam or computed tomography (CT) scan should be performed to evaluate for injury. Signs of liver injury on FAST examination include subcapsular fluid, perihepatic fluid or fluid within the hepatorenal space. A negative FAST examination, however, does not completely exclude liver injury.
CT is now widely used in the management of the acute trauma patient, proving to be invaluable in the evaluation of patients as it allows for complete imaging of multiple regions of the body and organ systems in a single examination (2). Contrast-enhanced CT of the abdomen can not only detect the presence and extent of hepatic parenchymal injury with high accuracy, it can also be used to monitor the course of healing and detect vascular or biliary complications that may go undetected clinically (2). The introduction of multiphasic imaging has added additional value in classifying injuries. Arterial phase imaging is obviously the most important phase for accurate detection of arterial injury, however, venous and delayed phase imaging plays an important role in the detection of vascular shunts and pseudoaneurysms (14,15).
Hepatic injury grading
The American Association for the Surgery of Trauma (AAST) classification system is the most widely accepted and used injury grading scale, Table 1 (1,16). This was most revised in 2018 to include updates to the CT diagnosis of vascular injury, defined as either a pseudoaneurysm or arteriovenous fistula. The National Trauma Data Bank reports the majority of hepatic injuries are low grade, with Grades I–III making up 67% of all hepatic injuries (1). The AAST grading system is an important and useful tool for predicting the likelihood of success with nonoperative management, which is higher for Grade I-III injuries as opposed to Grade IV–V injuries.
Table 1. Classification according to the American Association for the Surgery of Trauma (AAST) Liver Injury Scale-2018 Revision (16).
| AAST grade | CT imaging findings |
|---|---|
| I | Subcapsular hematoma <10% surface area |
| Parenchymal laceration <1 cm in depth | |
| II | Subscapular hematoma 10–50% surface area; intraparenchymal hematoma <10 cm in diameter |
| Laceration 1–3 cm in depth and ≤10 cm length | |
| III | Subcapsular hematoma >50% surface area; ruptured subcapsular or parenchymal hematoma |
| Intraparenchymal hematoma >10 cm | |
| Laceration >3 cm depth | |
| Any injury in the presence of a liver vascular injury or active bleeding contained within the liver parenchyma | |
| IV | Parenchymal disruption involving 25–75% of a hepatic lobe |
| Active bleeding extending beyond the liver parenchyma into the peritoneum | |
| V | Parenchymal disruption >75% of hepatic lobe |
| Juxtahepatic venous injury to include retrohepatic vena cava and central major hepatic veins |
Vascular injury defined as a pseudoaneurysm or arteriovenous fistula and appears as a focal collection of vascular contrast that decreases in attenuation with delayed imaging.
Approach to management
Advancements in the speed and sensitivity of computed tomography (CT) scanning along with advances in critical care monitoring have resulted in a paradigm shift from operative to nonoperative management for most hemodynamically stable patients with hepatic injury.
After initial clinical evaluation, the management strategy of the patient depends most importantly on the hemodynamic status, followed by grade of liver injury and presence of other associated injuries or medical comorbidities. Once all these factors have been determined the decision can then be made to proceed with nonoperative or operative management.
Nonoperative management
Currently, nonoperative management of blunt hepatic injuries is the treatment modality of choice in hemodynamically stable patients, irrespective of the grade of injury or patient age (17). Nonoperative management consists of observation with supportive care and if clinically indicated with the adjunctive use of arteriography and hepatic embolization.
Contraindications to nonoperative management of liver injury include hemodynamic instability after initial resuscitation, other indication for abdominal surgery (e.g., peritonitis), gunshot injury (relative contraindication if extrahepatic injury is suspected), and absence of an appropriate clinical environment to provide serial monitoring or availability of facilities and personnel for hepatic embolization or urgent surgery (5,18,19). Nonoperative management of penetrating injuries, such as, gunshot wounds remains controversial (20). Nonoperative management fails in up to one-third of patients due to ongoing bleeding, missed injuries to the gastrointestinal tract or the development of abdominal compartment syndrome (20-22).
Indications for embolization and patient selection
Despite the current lack of consensus guidelines on patient selection and indications for embolization, hepatic TAE can be utilized to improve success rates of nonoperative management. Successful management with embolization varies depending upon institution, embolization technique, arterial accessibility, operator skill, and the type of embolization material used (18,23,24).
In one systematic review, the overall efficacy in controlling arterial hemorrhage with the use of TAE in hepatic trauma was 93% (6). Hepatic TAE appears to be most successful when used preemptively in hemodynamic stable patients with a suspected arterial liver injury based on imaging and/or mechanism. A recent retrospective study evaluated 746 patients over a 7 year period and found a reduced failure rate of nonoperative management when hepatic TAE was used in patients with grade III or IV injuries and concomitant contrast extravasation visualized on CT scan (25).
Unfortunately, the literature has yet to answer the ideal timing of TAE in the setting of hepatic trauma. There is currently no standardization for patient selection or reporting, which results in heterogeneity in the published data (6). General recommendations based on the current published literature were recently released by the Society for Interventional Radiology (SIR) to help guide practicing clinicians when determining to intervene with embolization. SIR recommends that nonoperative management should be the treatment of choice in hemodynamically stable patients, embolization can be considered in patients with clinical evidence of ongoing bleeding, identification of an arterial source of bleeding on imaging, or suspicion of arterial bleeding despite operative intervention (10).
Embolization techniques
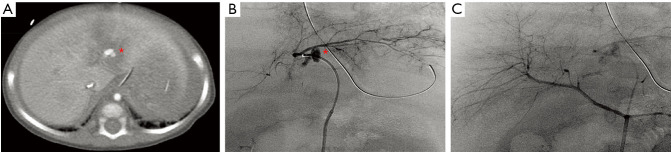
Hepatic TAE requires access to imaging facilities with the equipment necessary for rapid catheterization, staff experienced in the management of trauma patients and a vascular interventionalist that is skilled with mesenteric catheterization and embolization techniques. Comprehensive diagnostic angiography typically includes selective mesenteric angiography of the celiac, hepatic, and superior mesenteric arteries. Operators must be aware of variant hepatic arterial anatomy and intrahepatic collaterals (Figure 2). Multiple selective and superselective catheter injections and projections may be required to optimally demonstrate the source of bleeding and plan for the optimal embolization approach (26).
Figure 2.
TAE for non-penetrating trauma. (A) Contrast enhanced CT done in an 8 month old victim of non-accidental trauma showed arterial extravasation/pseudoaneurysm formation in the left lobe of the liver (asterisk). (B) Common hepatic angiogram failed to show the extravasation/pseudoaneurysm. (C) Selective left hepatic artery injection via cannulation of an accessory left hepatic artery arising from the left gastric artery showed the site of extravasation(asterisk). This was treated with gelatin sponge slurry embolization to good angiographic effect (not shown). Care was withdrawn due to significant intracranial injuries and the patient unfortunately passed shortly thereafter. Images courtesy of Dr. Keith Quencer. TAE, transarterial embolization.
Parenchymal liver injuries tend to involve the small and/or medium sized arteries. Extensive collateral circulation of the liver can provide distal circulation to areas of vascular injury resulting in continuous bleeding. Operators must be aware of this phenomenon and make every effort to embolize distal and proximal to the lesion. This technique requires crossing the area of injury, which is possible with pseudoaneurysms, but may not be in cases of vessel transection. If this approach is not possible, the general recommendation is to perform Gelfoam embolization to achieve distal control followed by proximal coil embolization (27,28).
The reported use of liquid agents in trauma is limited. Absolute alcohol or other sclerosing agents are not generally used in trauma due to the potential for tissue necrosis. N-butyl cyanoacrylate (Trufull NBCA, Cordis Neurovascular) and ethylene vinyl alcohol copolymer (Onyx, Mico Therapeutics, Inc.) have however been used successfully in the treatment of selected trauma patients with coagulopathy and may play a role in successful embolization independent of the patient’s coagulation status. The disadvantages include the high cost of these agents and the need for extensive experience by the operator to prevent serious complications (27,28).
Intrahepatic vascular fistulas are another complication encountered in traumatic hepatic injury. These fistulas are abnormal communications that can form between intrahepatic vasculature and can involve the arterial, portal, or hepatic venous systems. Traumatic hepatic arterioportal fistulas are much more common than hepatic arterial to hepatic venous fistulas, which is likely due to the larger distance between the hepatic arteries and hepatic veins (29-31). Arterioportal vascular fistulas are generally managed with coil or microcoil embolization. Similarly, two case reports detailed the treatment of the rarer hepatic arterial to hepatic venous fistula with microcoil embolization of the arterial feeding vessel with good angiographic result (29,31). Although the data is currently limited on the indications for treatment of intrahepatic shunts. Dessouky et al. (30), proposed a strategy for management by categorizing patients based on both imaging and clinical findings. Group I included asymptomatic patients with small non-neoplastic fistulas (3–6 mm) and with shunt ratios (total blood volume in the shunt compared to inflow of the vessel) <30%. Patients that fell into this group were monitored conservatively with follow up Doppler and clinical exam. Patients in group II were recommended to undergo angiographic intervention for management. Patients who fell into this group were identified by development of either a symptomatic large fistula (15–23 mm), aneurysmal fistula (28–45 mm) or a shunt ratio >30%. Group III patients had diffuse fistulas or a shunt ratio >60% and were recommended for surgical treatment.
Similar to the treatment of intrahepatic fistulas, pseudoaneurysms can usually be managed with coil embolization of both the distal and the proximal portions of the injured vessel. Packing the pseudoaneurysm sac with coils is not advisable given the risk of rupture secondary to increased pressure. Coil embolization is generally acceptable in the management of small and/or medium sized vessels, however, pseudoaneurysms that have developed in larger more proximal vessels such as the right hepatic, proper, or common hepatic arteries can be treated with stent graft placement with high success rates (32-34).
Occasionally angiography fails to show a discrete bleeding site in spite of evidence of contrast extravasation on the initial contrast-enhanced abdominal CT scan. A previous study found that out of 143 patients with blunt abdominal injuries and concern for vascular injury on contrast-enhanced abdominal CT, 24 of those patients showed no evidence of arterial extravasation or contrast blush on angiography. Approximately, 7 of the 24 patients in that study developed rebleeding (35). The results of this study pose a significant clinical dilemma, given the significant risk for recurrent hemorrhage. Although empiric embolization can be considered such as in cases with splenic trauma, however, more studies are needed to validate this consideration as empiric embolization of the liver is ostensibly more difficult and poses a much greater risk for major hepatic necrosis (35).
Morbidity and mortality
Mortality rates are generally low for grade I and II hepatic injuries with nonoperative management. The greatest reduction in mortality has occurred for higher grade liver injuries (Grades III–V) (7,11,36). Due to the advancements in critical care and embolization techniques many of the higher-grade liver injuries can be successfully managed nonoperatively with overall low mortality rates. Higher mortality rates are seen in those patients with high-grade liver injuries who require surgical management either upon presentation or with failed nonoperative management (4). A systematic review investigating higher grade injuries (Grades III–V), found mortality rates as low as 5% (range, 0–8%) with nonoperative management and 51% (range, 30–68%) after surgery (4) and a separate review found a mortality rate of 9.6% among patients undergoing embolization (range, 0–27%) (6).
The incidence of complications increases with the gradeof liver injury (3,21). Among those reported, biliary tree disruption with persistent bile leak was found to have a incidence ranging from 0.5% to 21% and was predominantly managed with endoscopic retrograde cholangiopancreatography (ERCP) and stent placement (37,38). Perihepatic abscesses was another common complication reported with an incidence of 7.5% (6). Management with antibiotics and percutaneous drainage is usually sufficient, however, surgery may be required if interventional techniques fail to provide adequate drainage (3).
The most commonly reported complication following TAE is major hepatic necrosis (6,7,11,12). Prior studies have shown that hepatic necrosis developed more commonly in cases with nonselective proper hepatic artery embolization in the setting of patient decompensation or in cases with multiples sites of bleeding requiring numerous embolizations (12). Higher liver injury grading, not surprisingly has been shown to be directly related to the increased complication rates, in particular hepatic necrosis (3,6,12). Despite the liver’s protective dual arterial and portal venous blood supply, it appears the combination of major liver devascularization through traumatic insult and ischemia induced by therapeutic embolization may predispose to major hepatic necrosis (6,12). The use of microcatheter systems to obtain superselective positioning prior to embolization is critical in the prevention or mitigation of hepatic necrosis. Although at times superselective approach is nonfeasible, such as with massive bleeds, multiples sites of extravasation and/or patient instability, superselective technique has been linked to decreased rates of hepatic necrosis (10,13). In cases of severe hepatic necrosis, patients may require laparotomy with debridement of necrotic tissue which may lead to prolonged hospital course. Although rare, non-target embolization of the cystic artery has been reported, most commonly in cases where rapid embolization is needed and a superselective approach cannot be obtained (9).
Discussion
Over the past four decades there has been a significant paradigm shift in the management of trauma-related liver injury. In hemodynamically stable patients, the standard of care in the majority of level-one trauma centers has shifted to nonoperative management with high success rates and significant decreases in mortality rates, especially with low-grade liver injuries (i.e., grade I and II) (17,19). Operative management is reserved for patients that are hemodynamically unstable on presentation or do not respond to resuscitation efforts. Blunt hepatic injury makes up the majority of liver trauma with motor vehicle accidents serving as the most common injury mechanism.
Appropriate management of blunt hepatic injury is critical due to the high mortality rate associated with uncontrolled bleeding (10). Initial assessment by trauma teams and access to computed tomography (CT) imaging in hemodynamically stable patients allows for a rapid determination of clinical status and liver injury grade. AAST grade I-III injuries can usually be managed nonoperatively with serial monitoring in an intensive care unit (4,6). Patients with grade III-V liver injuries with evidence of contrast extravasation on CT or hemodynamic instability require the rapid efforts of a multidisciplinary team in management.
Interventionalists trained to manage traumatic arterial bleeding have become integral in the management of these patients. A recent systematic review reported a 93% effective rate in stopping arterial hemorrhage with hepatic TAE (6). Patients that had failed nonoperative management despite successful embolization were found to have significant juxtahepatic venous injuries (4,6). Venous injuries can be difficult to identify during angiography but should be suspected in patients with high-grade liver lacerations that require continued fluid resuscitation despites successful TAE (6,10).
Hepatic TAE is generally well tolerated by patients, even among those that are critically ill the overall mortality rate for embolized patients has been reported in the range of 10% (1,4,6). Complication rates related to TAE are generally low, with hepatic necrosis remaining the most common and concerning. However, this complication is not unique to TAE and can develop following laparotomy and hepatorrhaphy (12). Hepatic necrosis can be associated with longer hospital stays, increased transfusion requirements, and the possibility for multiple additional operations (6). Most studies report a complication rate in the range of 16% of patients for developing hepatic necrosis. Hepatic necrosis following TAE can be decreased through appropriate choice of embolic agent, microcatheter systems and selective embolization (6,9,10,12). Abscess formation and bile leak are among the most commonly reported complications in high grade liver injuries (3,39). These complications if identified early can usually be managed by minimally invasive techniques without a significant impact on the hospital course of the patient.
A strict criteria for when to perform hepatic angiography has yet to be implemented. Several articles have suggested early angiography and embolization improve outcomes in patients with high grade injuries (7,28,40,41). Although, the data is limited with small and heterogenous patient samples, a recent study found a trend towards reduced transfusion requirements for those patients undergoing early TAE. However, the authors admit higher transfusion requirements in the late TAE group may have been confounded by greater severity of injury in this group (6).
Currently, retrospective case series constitute the majority of investigations on the use of nonoperative management in hepatic injury. The Society of Interventional Radiology (SIR) recently released the 2020 position statement on endovascular intervention for trauma which provides guidance for practicing clinicians to promote high quality outcomes and patient safety. Based on the current published data, the recommendations state nonoperative management should be the treatment of choice in patients with blunt hepatic injury who are in hemodynamically stable condition. Embolization should be considered in cases of ongoing bleeding, identification of an arterial source of bleeding on imaging, or suspicion of a persistent source of arterial bleeding despite operate intervention (6,10). Although no consensus guidelines on appropriate patient selection criteria for those who would benefit from angiography and embolization have been determined by the AAST and SIR, it is clear from the literature that hepatic TAE is an effective and safer option to operative management in stopping arterial hemorrhage in the setting of traumatic hepatic injury.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dr. Keith Bertram Quencer) for the series “Endovascular interventions in trauma” published in Annals of Translational Medicine. The article has undergone external peer review.
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-4580
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4580). The series “Endovascular interventions in trauma” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
- 1.Tinkoff G, Esposito TJ, Reed J, et al. American Association for the Surgery of Trauma Organ Injury Scale I: spleen, liver, and kidney, validation based on the National Trauma Data Bank. J Am Coll Surg 2008;207:646-55. 10.1016/j.jamcollsurg.2008.06.342 [DOI] [PubMed] [Google Scholar]
- 2.Becker CD, Mentha G, Terrier F. Blunt abdominal trauma in adults: role of CT in the diagnosis and management of visceral injuries. Eur Radiol 1998;8:553-62. 10.1007/s003300050433 [DOI] [PubMed] [Google Scholar]
- 3.Kozar RA, Moore JB, Niles SE, et al. Complications of nonoperative management of high-grade blunt hepatic injuries. J Trauma 2005;59:1066-71. 10.1097/01.ta.0000188937.75879.ab [DOI] [PubMed] [Google Scholar]
- 4.Melloul E, Denys A, Demartines N. Management of severe blunt hepatic injury in the era of computed tomography and transarterial embolization. J Trauma Acute Care Surg 2015;79:468-74. 10.1097/TA.0000000000000724 [DOI] [PubMed] [Google Scholar]
- 5.Croce MA, Fabian TC, Menke PG, et al. Nonoperative management of blunt hepatic trauma is the treatment of choice for hemodynamically stable patients. Results of a prospective trial. Ann Surg 1995;221:744-53; discussion 753-5. 10.1097/00000658-199506000-00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green CS, Bulger EM, Kwan SW. Outcomes and complications of angioembolization for hepatic trauma. J Trauma Acute Care Surg 2016;80:529-37. 10.1097/TA.0000000000000942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asensio JA, Roldán G, Petrone P, et al. Operative management and outcomes in 103 AAST-OIS grades IV and V complex hepatic injuries: trauma surgeons still need to operate, but angioembolization helps. J Trauma 2003;54:647-53; discussion 653-4. 10.1097/01.TA.0000054647.59217.BB [DOI] [PubMed] [Google Scholar]
- 8.David Richardson J, Franklin GA, Lukan JK, et al. Evolution in the management of hepatic trauma: a 25-year perspective. Ann Surg 2000;232:324-30. 10.1097/00000658-200009000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monnin V, Sengel C, Thony F, et al. Place of arterial embolization in severe blunt hepatic trauma: a multidisciplinary approach. Cardiovasc Intervent Radiol 2008;31:875-82. 10.1007/s00270-007-9277-1 [DOI] [PubMed] [Google Scholar]
- 10.Padia SA, Ingraham CR, Moriarty JM, et al. Society of Interventional Radiology Position Statement on Endovascular Intervention for Trauma. J Vasc Interv Radiol 2020;31:363-9.e2. 10.1016/j.jvir.2019.11.012 [DOI] [PubMed] [Google Scholar]
- 11.Kozar RA, Moore FA, Cothren CC, et al. Risk factors for hepatic morbidity following nonoperative management: multicenter study. Arch Surg 2006;141:451-8; discussion 458-9. 10.1001/archsurg.141.5.451 [DOI] [PubMed] [Google Scholar]
- 12.Dabbs DN, Stein DM, Scalea TM. Major hepatic necrosis: a common complication after angioembolization for treatment of high-grade liver injuries. J Trauma 2009;66:621-7; discussion 627-9. 10.1097/TA.0b013e31819919f2 [DOI] [PubMed] [Google Scholar]
- 13.Kong YL, Zhang HY, He XJ, et al. Angiographic embolization in the treatment of intrahepatic arterial bleeding in patients with blunt abdominal trauma. Hepatobiliary Pancreat Dis Int 2014;13:173-8. 10.1016/S1499-3872(14)60027-8 [DOI] [PubMed] [Google Scholar]
- 14.Fang JF, Chen RJ, Wong YC, et al. Classification and Treatment of Pooling of Contrast Material on Computed Tomographic Scan of Blunt Hepatic Trauma. J Trauma 2000;49:1083-8. 10.1097/00005373-200012000-00018 [DOI] [PubMed] [Google Scholar]
- 15.Fang JF, Wong YC, Lin BC, et al. The CT risk factors for the need of operative treatment in initially hemodynamically stable patients after blunt hepatic trauma. J Trauma 2006;61:547-53; discussion 553-4. 10.1097/01.ta.0000196571.12389.ee [DOI] [PubMed] [Google Scholar]
- 16.Kozar RA, Crandall M, Shanmuganathan K, et al. Organ injury scaling 2018 update. J Trauma Acute Care Surg 2018;85:1119-22. 10.1097/TA.0000000000002058 [DOI] [PubMed] [Google Scholar]
- 17.Stassen NA, Bhullar I, Cheng JD, et al. Nonoperative management of blunt hepatic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg 2012;73:S288-93. 10.1097/TA.0b013e318270160d [DOI] [PubMed] [Google Scholar]
- 18.Badger SA, Barclay R, Campbell P, et al. Management of Liver Trauma. World J Surg 2009;33:2522-37. 10.1007/s00268-009-0215-z [DOI] [PubMed] [Google Scholar]
- 19.Richardson JD. Changes in the management of injuries to the liver and spleen. J Am Coll Surg 2005;200:648-69. 10.1016/j.jamcollsurg.2004.11.005 [DOI] [PubMed] [Google Scholar]
- 20.Navsaria P, Nicol A, Krige J, et al. Selective nonoperative management of liver gunshot injuries. Eur J Trauma Emerg Surg 2019;45:323-8. 10.1007/s00068-018-0913-z [DOI] [PubMed] [Google Scholar]
- 21.Zafar SN, Rushing A, Haut ER, et al. Outcome of selective non-operative management of penetrating abdominal injuries from the North American National Trauma Database. Br J Surg 2012;99 Suppl 1:155-64. 10.1002/bjs.7735 [DOI] [PubMed] [Google Scholar]
- 22.Demetriades D, Gomez H, Chahwan S, et al. Gunshot injuries to the liver: the role of selective nonoperative management11No competing interests declared. J Am Coll Surg 1999;188:343-8. 10.1016/S1072-7515(98)00315-9 [DOI] [PubMed] [Google Scholar]
- 23.Hoffer EK, Borsa JJ, Bloch RD, et al. Endovascular techniques in the damage control setting. Radiographics 1999;19:1340-8. 10.1148/radiographics.19.5.g99se051340 [DOI] [PubMed] [Google Scholar]
- 24.Misselbeck TS, Teicher EJ, Cipolle MD, et al. Hepatic Angioembolization in Trauma Patients: Indications and Complications. J Trauma 2009;67:769-73. 10.1097/TA.0b013e3181b5ce7f [DOI] [PubMed] [Google Scholar]
- 25.Xu H, Jie L, Kejian S, et al. Selective Angiographic Embolization of Blunt Hepatic Trauma Reduces Failure Rate of Nonoperative Therapy and Incidence of Post-Traumatic Complications. Med Sci Monit 2017;23:5522-33. 10.12659/MSM.905115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salazar GM, Walker TG. Evaluation and management of acute vascular trauma. Tech Vasc Interv Radiol 2009;12:102-16. 10.1053/j.tvir.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 27.Lopera JE. Embolization in trauma: principles and techniques. Semin Intervent Radiol 2010;27:14-28. 10.1055/s-0030-1247885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrillo EH, Spain DA, Wohltmann CD, et al. Interventional techniques are useful adjuncts in nonoperative management of hepatic injuries. J Trauma 1999;46:619-22; discussion 622-4. 10.1097/00005373-199904000-00010 [DOI] [PubMed] [Google Scholar]
- 29.Chandrasekharan R, Kp S, Moorthy S, et al. Traumatic hepatic arteriohepatic venous fistula managed with selective coil embolization: a case report. BJR Case Rep 2017;3:20150512. 10.1259/bjrcr.20150512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dessouky BAM, El Abd OL, Abdel Aal ESM. Intrahepatic vascular shunts: Strategy for early diagnosis, evaluation and management. The Egyptian Journal of Radiology and Nuclear Medicine 2011;42:19-34. 10.1016/j.ejrnm.2011.02.005 [DOI] [Google Scholar]
- 31.Mzoughi Z, Djebbi A, Bayar R, et al. Open liver trauma causing hepatico caval fistula successfully treated by embolization. Trauma Case Rep 2017;7:3-6. 10.1016/j.tcr.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellemann N, Sommer CM, Mokry T, et al. Hepatic artery stent-grafts for the emergency treatment of acute bleeding. Eur J Radiol 2014;83:1799-803. 10.1016/j.ejrad.2014.06.030 [DOI] [PubMed] [Google Scholar]
- 33.Cui L, Kong L, Bai YH, et al. Covered stent placement for hepatic artery pseudoaneurysm. Abdom Radiol (NY) 2020;45:3337-41. 10.1007/s00261-020-02452-3 [DOI] [PubMed] [Google Scholar]
- 34.Venturini M, Marra P, Colombo M, et al. Endovascular Repair of 40 Visceral Artery Aneurysms and Pseudoaneurysms with the Viabahn Stent-Graft: Technical Aspects, Clinical Outcome and Mid-Term Patency. Cardiovasc Intervent Radiol 2018;41:385-97. 10.1007/s00270-017-1844-5 [DOI] [PubMed] [Google Scholar]
- 35.Alarhayem AQ, Myers JG, Dent D, et al. "Blush at first sight": significance of computed tomographic and angiographic discrepancy in patients with blunt abdominal trauma. Am J Surg 2015;210:1104-10; discussion 1110-1. 10.1016/j.amjsurg.2015.08.009 [DOI] [PubMed] [Google Scholar]
- 36.Sivrikoz E, Teixeira PG, Resnick S, et al. Angiointervention: an independent predictor of survival in high-grade blunt liver injuries. Am J Surg 2015;209:742-6. 10.1016/j.amjsurg.2014.06.024 [DOI] [PubMed] [Google Scholar]
- 37.Wahl WL, Brandt MM, Hemmila MR, et al. Diagnosis and management of bile leaks after blunt liver injury. Surgery 2005;138:742-7; discussion 747-8. 10.1016/j.surg.2005.07.021 [DOI] [PubMed] [Google Scholar]
- 38.Hommes M, Nicol AJ, Navsaria PH, et al. Management of biliary complications in 412 patients with liver injuries. J Trauma Acute Care Surg 2014;77:448-51. 10.1097/TA.0000000000000335 [DOI] [PubMed] [Google Scholar]
- 39.Bala M, Gazalla SA, Faroja M, et al. Complications of high grade liver injuries: management and outcomewith focus on bile leaks. Scand J Trauma Resusc Emerg Med 2012;20:20. 10.1186/1757-7241-20-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciraulo DL, Luk S, Palter M, et al. Selective hepatic arterial embolization of grade IV and V blunt hepatic injuries: an extension of resuscitation in the nonoperative management of traumatic hepatic injuries. J Trauma 1998;45:353-8; discussion 358-9. 10.1097/00005373-199808000-00025 [DOI] [PubMed] [Google Scholar]
- 41.Johnson JW, Gracias VH, Gupta R, et al. Hepatic angiography in patients undergoing damage control laparotomy. J Trauma 2002;52:1102-6. 10.1097/00005373-200206000-00013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as