Abstract
Less than 40% of depressed older adults treated with an antidepressant achieve remission. Incomplete response to treatment is common. Current augmentation strategies have limited efficacy, and many have side effects that restrict their utilization in older adults. We conducted the first open pilot trial of minocycline augmentation in older adults who had failed to achieve remission after adequate psychopharmacologic treatment. Subjects older than 55 years of age with major depression and failure to achieve substantial improvement of depressive symptoms after at least 6 weeks of antidepressant treatment were given augmentation with minocycline 100 mg twice daily over an 8-week period. At the end of 8 weeks of augmentation with minocycline, 31% (4/13) patients achieved remission. Remitters had higher baseline ratings of hopelessness and apathy. Minocycline was well tolerated with no reported adverse events or discontinuation due to intolerance. Larger placebo-controlled studies are needed to evaluate the effects of minocycline augmentation in older adults who had failed to achieve remission after adequate treatment with antidepressants.
Keywords: antidepressant, bipolar disorder, inflammation, major depressive disorder, anti-inflammatory
Introduction
Less than 40 percent of depressed older adults treated with an antidepressant achieve remission, and incomplete response to treatment is common (Alexopoulos, 2019). Augmentation strategies have limited efficacy and side effects often restrict their utilization in older adults. The limited effectiveness of antidepressants underscores the need for augmenting agents with novel mechanisms of action.
A few studies suggest that minocycline has antidepressant properties in depression of younger adults (Rosenblat et al., 2018). The rationale for using minocycline in depression was based on findings of inflammatory biomarker elevation during depressive episodes, and an association of inflammatory indices with decreased functional connectivity within the corticostriatal reward circuitry in depression (Felger, 2012; Alexopoulos et al., 2011). Minocycline has anti-inflammatory properties, suppressing microglia activation and proliferation, and inhibiting the subsequent release of inflammatory cytokines, chemokines, and matrix metalloproteases, which disrupt the blood–brain barrier (Cankaya, 2019). Minocycline exerts neuroprotective effects in various experimental illness models, including cerebral ischemia, traumatic brain injury, amyotrophic lateral sclerosis, Parkinson’s disease, Huntingtons’ disease, and multiple sclerosis (Cankaya, 2019).
Aging-related pro-inflammatory changes in the brain and the periphery make depression of older adults an appealing target for minocycline treatment. Aging disrupts periphery-brain communication and leads to a pro-inflammatory shift in the brain. This shift is characterized by increased numbers of both activated and primed microglia, continuous production of pro-inflammatory cytokines, and decreases in anti-inflammatory molecules (Sparkman and Johnson, 2008).
The pro-inflammatory changes in the aging brain are mediated in part by a shift of microglia from the neuroprotective M2-polarized microglia phenotype to the M1-polarized phenotype, which is characterized by reduced motility of microglia processes, aberrant morphology, decreased phagocytic ability, and increased production of pro-inflammatory cytokines (Daria, 2017). The end results of aging-related microglia changes are neuron loss, inefficient clearance of neurotoxic molecules, and reduction of neurogenesis (Lucin et al., 2009). Aging-related pro-inflammatory states may decrease the response of depressed older adults to traditional pharmacological interventions. We conducted an 8-week open pilot augmentation trial with the anti-inflammatory agent minocycline in older adults who had failed to achieve remission after adequate antidepressant treatment.
Methods
Participants
Middle-aged and older participants with depression who remained symptomatic after at least 6 weeks of treatment with a therapeutic dose of either an antidepressant or mood stabilizer were consecutively recruited from the community. All participants signed informed consent approved by the Weill Cornell Institutional Review Board.
The inclusion criteria were (1) age of 55 years and older; (2) major depression, either unipolar or bipolar without psychotic features (by Diagnostic and Statistical Manual of Mental Disorders, fourth edition [DSM-IV] criteria and assessed on the Structured Clinical Interview-Revised [SCID]); (3) failure to achieve substantial improvement of depressive symptoms after at least 6 weeks of treatment with an antidepressant or a mood stabilizer; and (4) severity of depression: 17-item Hamilton Depression Rating Scale > 14.
Exclusion criteria were (1) intent or plan to attempt suicide in near future; (2) presence of any current axis I psychiatric disorder (other than unipolar or bipolar major depression or generalized anxiety disorder), including substance abuse; (3) antisocial personality disorder, schizotypal or severe borderline personality, mental retardation, and pervasive developmental disorder (DSM-IV); (4) dementia of more than mild severity (Mini-Mental State Examination [MMSE]<20); (5) history of psychotic depression or another psychotic disorder; (6) acute or severe medical illness; (7) presence of a significant neurological disease; (8) history of intolerance of tetracyclines; (9) use of concomitant drugs that may provide reason to believe that minocycline is contraindicated; (10) patients on anticoagulants (except low-dose aspirin), ergot alkaloids, and monoamine oxidase inhibitors; (11) patients’ unwillingness or inability to gradually withdraw all other psychotropic medications (except antidepressants, mood stabilizers, and low and stable doses of opiates and nonbenzodiazepine hypnotics); and (12) inability to perform any of the activities of daily livings (ADLs) (Multilevel Assessment Instrument: ADL subscale).
Assessment
Diagnostic evaluation of major depression consisted of the SCID-R and review of symptoms by the study psychiatrist. We assessed depression severity using the Montgomery Åsberg Depression Rating Scale (MADRS), weekly and also measured apathy (Apathy Evaluation Scale; AES) and hopelessness (Snaith-Hamilton Pleasure Scale; SHAPS). Change in depression severity (MADRS) and medication side effects (Udvalg for Kliniske Undersogelser) were assessed at weeks 1, 2, 3, 4, 6, and 8.
Treatment
Participants received minocycline 100 mg twice daily over 8 weeks. Their antidepressant/mood stabilizer regimens remained stable during the trial.
Statistical analysis
Because of the small sample size, analysis of data consisted of descriptive statistics and graphic representation, and we did not attempt to test statistical significance. Remission was defined as a Hamilton total score ≤ 10 at any time point during the follow-up period. We compared baseline demographic and clinical characteristics between remitters and non-remitters using non-parametric tests.
Results
Thirteen non-demented participants (MMSE total > 25) with major depression signed consent and received minocycline 100 mg twice daily as an augmentation to their existing treatment regimen. Of the 13 participants, 7 were women and all were Caucasian. They were aged 73.1 years (SD: 11.2, range: 55.6–89.1) and were well educated (mean: 15.7 years of education; SD: 2.4, range: 12–19 years of education). Their severity of depression ranged from mild to moderate (MADRS total: 24.3; SD: 4.4, range: 18–34).
Prior to receiving minocycline, participants had been treated for at least 6 weeks with stable dosages of: desvenlafaxine 50 mg daily (two participants), duloxetine 120 mg daily (one participant), sertraline 150 mg daily(one participant),trazodone150–200mg (two participants), mirtazapine 45 mg daily (one participant), citalopram 20 mg daily (two participants), vilazodone 40 mg daily (one participant), lamotrigine 200 mg daily (one participant), and escitalopram 20 mg daily (five participants).
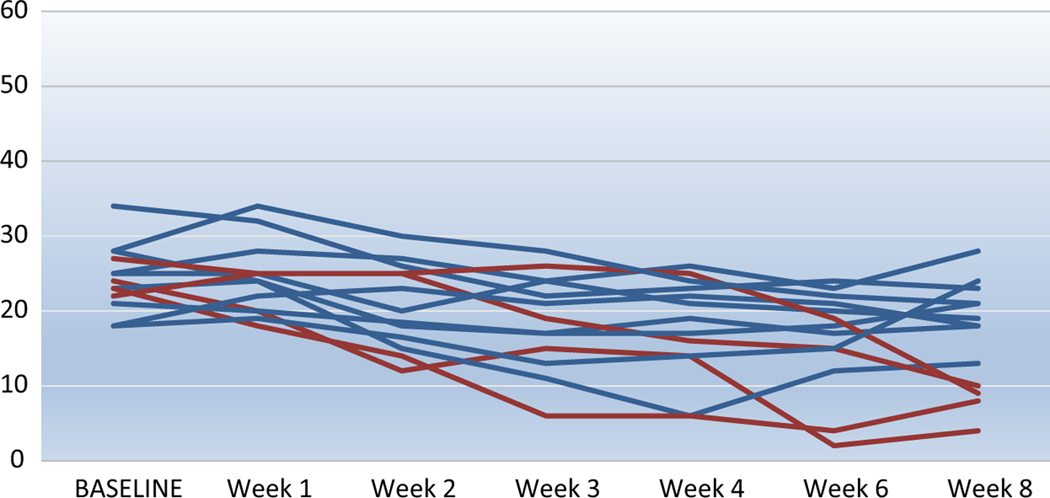
At the end of 8 weeks of treatment with minocycline, 31% (4/13) patients achieved remission (MADRS total ≤ 10; Figure 1). At baseline, the mean MADRS was 24.31 (SD = 4.37) and at week 8 (end of study) was 6.67 (SD = 7.19). The effect size of the difference in depression severity (MADRS) between the baseline and week 8 was large (Cohen’s D = 0.96; 95% confidence interval 0.14–1.77). Exploratory non-parametric analysis (Mann–Whitney U test) of differences in baseline variables showed that compared to non-remitters, remitters experienced greater degrees of apathy (AES: remitters mean: 43, SD: 5.2 vs. non-remitters mean:31, SD:6.2; Mann–Whitney U = 26, p = 0.024) and hopelessness (SHAPS: remitters: 6.8, SD:1.9 vs. non-remitter 2.3, SD:2.6 Mann–Whitney U = 33, p = 0.02). Of the four remitters, three were on a stable dosage of an selective serotonin reuptake inhibitor (citalopram 20 mg daily, escitalopram 20 mg daily, vilazodone 20 mg daily) during the study trial, and one was on an serotonin-norepinephrine reuptake inhibitor (desvenlafaxine 50 mg daily).
Figure 1.
Depression Severity (MADRS total score) in 13 older depressed adults over 8 weeks of open treatment with minocycline.
Discussion
This open, proof of concept study is the first to examine whether elderly depressed patients with treatment-resistant depressive symptoms benefit from minocycline augmentation. We observed that minocycline augmentation led to remission in 31% of older adults who had remained symptomatic after being on stable therapeutic dosages of antidepressants for at least 8 weeks. Minocycline was well tolerated with no reported adverse events or discontinuation due to intolerance.
Our observation is consistent with limited literature suggesting that minocycline is effective in the treatment of depression of younger adults (Rosenblat et al., 2018). A recent meta-analysis of minocycline treatment of unipolar depression (mean participant ages range: 35–51 years) included three randomized clinical trials. Two studies assessed minocycline as an adjunctive therapy, while one study assessed minocycline monotherapy for participants with mild to moderate depression with comorbid HIV. The authors reported a statistically significant, large antidepressant effect size (d = −0.78) of minocycline compared to placebo. The observed adverse effects were similar to placebo, and minocycline was deemed to be well tolerated (Rosenblat et al., 2018).
A finding of our study was that remitters to augmentation of minocycline exhibit higher symptoms of hopelessness and apathy at baseline. Exaggerated and prolonged immune responses of the central nervous system can influence the function of some of the emotional and cognitive networks pertinent to geriatric depression. Increased c-reactive protein levels are associated with higher apathy symptoms (Eurelings, 2015) suggesting that apathy in depression and could be targeted by minocycline. Elevations in hopelessness are associated with higher levels of pro-inflammatory tumor necrosis factor (TNF)-α receptors (i.e., TNFR1 and TNFR2) (Kupper et al., 2012). Minocycline inhibits pro-inflammatory cytokine output by attenuating TNF-α (Gong, 2015). The relationship between cytokine markers and specific symptoms of depression should be addressed in future studies.
The results of our pilot are tempered by its limitations including its small sample size, the lack of blinding procedures, the absence of a placebo control group, and of inflammatory markers. However, observing that one-third of depressed older participants attained remission suggests the need for a placebo-controlled study that can ascertain the efficacy of minocycline augmentation. Appropriately designed and powered should examine the role of minocycline augmentation in the treatment of mid- and late-life depression. Further, investigations of the biological mechanisms of action of minocycline, although not addressed by the current study, could provide meaningful information regarding the role of inflammation processes in late-life depression. Multi-modal imaging with high-field magnetic resonance imaging and positron emission tomography using ligands for the 1 kDA translocator protein, which is mainly expressed by activated microglia, may be used to clarify the mechanism of action of minocycline. Assessment of peripheral inflammation markers offers information on how to select patients for anti-inflammatory treatment. If minocycline is found efficacious in depression of older adults, a series of studies may investigate the efficacy of agents that enhance the neuroprotective M2 and suppress the M1 neurotoxic microglia (Cosenza-Nashat et al., 2009).
Our preliminary observations suggest a potentially novel treatment approach. Larger studies need to evaluate the effects of minocycline augmentation in older adults who had failed to achieve remission after adequate psychopharmacologic treatment.
Acknowledgments
Source of funding
Please credit P50 MH113838 and T32 MH019132.
Footnotes
Description of authors’ roles
Jimmy N. Avari - Study Physician, manuscript preparation.
Dora Kanellopoulos - Statistics, manuscript preparation.
Nili Solomonov - Manuscript preparation.
Lauren Oberlin - Manuscript preparation.
George S. Alexopoulos - Theory development, study design, manuscript preparation.
Conflict of interest
In the last 3 years, Dr. Alexopoulos has been a consultant to Allergan, Otsuka, and Takeda-Lundbeck. For the remaining authors, no conflicts of interest were declared.
References
- Alexopoulos GS (2019). Mechanisms and treatment of late-life depression. Translational Psychiatry, 9, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS and Morimoto SS (2011). The inflammation hypothesis in geriatric depression. International Journal of Geriatric Psychiatry, 26, 1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cankaya S. et al. (2019). The therapeutic role of minocycline in Parkinson’s disease. Drugs in Context, 8, 212553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza-Nashat M. et al. (2009). Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathology and Applied Neurobiology, 35, 306–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daria A. et al. (2017). Young microglia restore amyloid plaque clearance of aged microglia. EMBO Journal, 36, 583–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eurelings LS et al. (2015). Low-grade inflammation differentiates between symptoms of apathy and depression in community-dwelling older individuals. International Psychogeriatric, 27, 639–647. [DOI] [PubMed] [Google Scholar]
- Felger JC et al. (2012). Molecular signatures of peripheral blood mononuclear cells during chronic interferon-alpha treatment: relationship with depression and fatigue. Psychological Medicine, 42, 1591–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong K. et al. (2015). Minocycline inhibits neurogenic inflammation by blocking the effects of tumor necrosis factor-alpha. Clinical and Experimental Pharmacology and Physiology, 42, 940–949. [DOI] [PubMed] [Google Scholar]
- Kupper N, Widdershoven JW and Pedersen SS (2012). Cognitive/affective and somatic/affective symptom dimensions of depression are associated with current and future inflammation in heart failure patients. Journal of Affective Disorders, 136, 567–576. [DOI] [PubMed] [Google Scholar]
- Lucin KM and Wyss-Coray T. (2009). Immune activation in brain aging and neurodegeneration: too much or too little? Neuron, 64, 110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblat JD and McIntyre RS (2018). Efficacy and tolerability of minocycline for depression: a systematic review and meta-analysis of clinical trials. Journal of Affective Disorders, 227, 219–225. [DOI] [PubMed] [Google Scholar]
- Sparkman NL and Johnson RW (2008). Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation, 15, 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]