Abstract
The presence of circulating microbiome in blood has been reported in both physiological and pathological conditions, although its origins, identities and function remain to be elucidated. This study aimed to investigate the presence of blood microbiome by quantitative real-time PCRs targeting the 16S rRNA gene. To our knowledge, this is the first study in which the circulating microbiome has been analyzed in such a large sample of individuals since the study was carried out on 1285 Randomly recruited Age-Stratified Individuals from the General population (RASIG). The samples came from several different European countries recruited within the EU Project MARK-AGE in which a series of clinical biochemical parameters were determined. The results obtained reveal an association between microbial DNA copy number and geographic origin. By contrast, no gender and age-related difference emerged, thus demonstrating the role of the environment in influencing the above levels independent of age and gender at least until the age of 75. In addition, a significant positive association was found with Free Fatty Acids (FFA) levels, leukocyte count, insulin, and glucose levels. Since these factors play an essential role in both health and disease conditions, their association with the extent of the blood microbiome leads us to consider the blood microbiome as a potential biomarker of human health.
Keywords: blood microbiome, 16S rRNA gene, geographic origin, aging, free fatty acids, leukocytes, insulin, glucose
Introduction
The human body is considered an ecosystem, consisting of trillions of bacterial, fungal and viral taxa that inhabit the skin, gastrointestinal, respiratory and urogenital tracts.
For years, researchers have been interested in the characterization of gut microbiome, which appears taxonomically and functionally peculiar in each part of the gastrointestinal tract and displays high inter-individual heterogeneity according to age, gender, and environmental factors, including dietary regime and antibiotic use (Yatsunenko et al., 2012; Tidjani Alou et al., 2016; Rinninella et al., 2019; D’Aquila et al., 2020). More recently, some studies revealed the presence of a “dormant,” not-immediately cultivatable blood microbiome in both physiological and pathological conditions (Castillo et al., 2019). The first evidence date back to the late 1960s, with the observation of bacterial forms in erythrocytes of healthy individuals (Tedeschi et al., 1969). Afterward, the presence of bacterial DNA and bacteria-like structures was evaluated in blood specimens of healthy human subjects as well as various other species (McLaughlin et al., 2002; Sze et al., 2014; Jeon et al., 2017; Vientós-Plotts et al., 2017). More particularly, Nikkari et al. (2001) detected members of seven phylogenetic groups and five bacterial divisions or subdivisions in the blood specimen of healthy individuals, with the majority of the 16S rDNA sequences highly similar to that of Pseudomonas fluorescens. An independent work has subsequently identified a variety of bacteria, namely those belonging to the Aquabacterium, Stenotrophomonas, Budvicia, Serratia, Bacillus, and Flavobacterium subgroups (Moriyama et al., 2008). Besides, transmission electron and dark-field microscopy as well as flow cytometry assays revealed the existence of pleomorphic bacteria exhibiting limited growth and susceptibility to antibiotics in the serum of healthy human subjects (McLaughlin et al., 2002). Viable bacteria, including Propionibacterium acnes, Staphylococcus epidermidis, Staphylococcus caprae, Micrococcus luteus, and Acinetobacter lwoffii were isolated in 53% of the plasma and 35% of the RBC-suspension samples from blood donors, suggesting the inability of conventional tests employed by blood banks to detect bacteria in standard blood-pack units (Damgaard et al., 2015). Païssé et al. (2016) implemented the above-reported evidence by characterizing the distribution of taxa both in whole blood and in the different blood fractions. Data revealed that the bacterial DNA mostly localizes to the buffy coat fraction (about 94%) and little in the plasma (0.03%) and that Proteobacteria represents the most frequent phylum in each fraction. In addition, the different blood fractions appeared characterized by different taxa, with the Sphingobacteria highly represented in buffy coat and plasma and Clostridia found mostly in plasma and the red blood fraction. The high similarity of results across independent studies revealed the existence of a core blood microbiome, in which Proteobacteria predominate, and Actinobacteria, Firmicutes, and Bacteroidetes are present to a lesser extent (Amar et al., 2013; Lelouvier et al., 2016; Païssé et al., 2016; Olde Loohuis et al., 2018; Whittle et al., 2019).
Similarly, pathological conditions have been associated with blood microbiota dysbiosis and disease-specific changes in its composition, thus suggesting its potential role as a biomarker for diseases risk (Potgieter et al., 2015; Traykova et al., 2017; Li et al., 2018; Dutta et al., 2019).
A longitudinal study carried out by Amar et al. (2011) revealed that the 16S rDNA concentration in blood appeared to be a good predictor of the onset of diabetes and abdominal adiposity in a general population. More recent evidence reported that, although no significant difference in the relative abundance of blood microbiome between type 2 diabetes mellitus T2DM patients and not-T2DM controls at both phylum and class levels, the genera Sediminibacterium and Bacteroides are significantly associated with an increased and decreased risk of T2DM, respectively (Sato et al., 2014; Qiu et al., 2019; Anhê et al., 2020). An increased proportion of Proteobacteria phylum in blood was also demonstrated to predict the occurrence of cardiovascular events, meanwhile an increased Actinobacteria: Proteobacteria ratio is a distinctive feature of cardiovascular disease patients (Amar et al., 2013; Dinakaran et al., 2014). Lastly, the microbial reactivation of dormant non-replicating blood bacteria was demonstrated to increase the risk of developing Alzheimer disease in response to iron dysregulation, by shedding potent inflammagens such as lipopolysaccharide (LPS) and lipoteichoic acid (LTA) that promote systemic inflammation, fibrin amyloidogenesis, blood brain barrier permeability and affect the hematological system (Kell and Pretorius, 2018; Pretorius et al., 2018).
Whether it has been widely reported that the gut microbiome evolves during the lifespan, differs between populations, and responds to changing lifestyles, there is still no evidence of this about the blood microbiome (Yatsunenko et al., 2012; Wilmanski et al., 2021). To address these gaps, we carried out a preliminary investigation of the abundance of blood microbiome in 1285 Randomly recruited Age-Stratified Individuals from the General population (RASIG) from several different geographical regions of Europe recruited within the EU Project MARK-AGE (HEALTH-F4-2008–200880 MARK-AGE).
MARK-AGE is a Europe-wide cross-sectional population study aimed at the identification of biomarkers of aging in subjects within the age range 35–75 (Bürkle et al., 2015; Capri et al., 2015; Moreno-Villanueva et al., 2015a).
Considering the availability of several biochemical and clinical data from the MARK-AGE project, another purpose of the present study was to evaluate the association between the circulating bacterial DNA and several laboratories and clinical parameters.
Materials and Methods
Study Population and Blood Sample Collection
The present study included 1285 RASIG (Randomly recruited Age-Stratified Individuals from the General population) participants recruited in the MARK-AGE cross-sectional study from 2008 to 2012 (Bürkle et al., 2015). Subjects (503 males and 782 females) were in the age range of 35–75 years and came from several European countries: Germany, Belgium, Poland, Greece, Austria, Italy and Finland (Capri et al., 2015). RASIG, representing the “normal” aging, were included in the project if they met the following criteria:-inclusion criteria: randomly recruited age-stratified individuals from the general population (both sexes) aged 35–75 and able to give informed consent.
-exclusion criteria: (i). self-reported seropositivity for HIV, HBV (except seropositivity by vaccination) and HCV (HBV and HCV seropositivity assessed after blood collection). (ii). presence of actual cancer and current use of anti-cancer drugs or glucocorticoids; (iii). less than 50% of a lifetime spent in the country of residence. iv. inability to give informed consent or (vi) any acute illness (e.g., common cold) within 7 days preceding blood collection (Bürkle et al., 2015; Capri et al., 2015). Participants were asked to classify their self-rated health (SRH) using a standard 5-point scale with the responses excellent, very good, good, fair, or poor (Assari et al., 2016; Osibogun et al., 2018). In order to obtain a comparable group size, those answering excellent and very good and those answering fair and poor were grouped, respectively (Piacenza et al., 2021). Ethical approval was given by the ethics committee of each of the recruiting center. Written informed consent was obtained from all participants (Moreno-Villanueva et al., 2015a). Other details of the recruitment procedures and the collection of anthropometric, clinical and demographic data have been published elsewhere (Moreno-Villanueva et al., 2015a, b; Jansen et al., 2015). This study utilized biobanked samples stored at –80°C as prescribed by the standard operating procedures (Moreno-Villanueva et al., 2015a). In brief, all biological samples were processed within 2 h and 10 min following a master protocol elaborated for MARK-AGE purposes. Anticoagulated whole blood, obtained by phlebotomy after overnight fasting, was collected shipped from the various recruitment centers to the MARK-AGE Biobank located at the University of Hohenheim (Stuttgart, Germany). Whole blood, serum and EDTA/LiHep plasma aliquots were prepared from monovette blood tubes and stored at −80°C immediately after cryotubes were filled. From the biobank, coded samples were subsequently shipped to the Scientific and Technological Pole of INRCA of Ancona, Italy, on dry ice, where they were stored at –80°C until use. For each proband, sample collection time, sample preparation starting time and sample freezing time were documented by the recruiting staff. Abnormalities like erythrocyte contamination in any samples were also recorded. In the recruiter centers of the MARK-AGE project, the sampling was performed according to a very strict protocol (see Capri et al., 2015; Moreno-Villanueva et al., 2015a). As a result, the quality of the serum and plasma samples was very good (Jansen et al., 2015).
Determination of Clinical Biochemical and Laboratory Parameters
Clinical, laboratory and biochemical parameters were measured as previously described (Hosaka et al., 1981; Bürkle et al., 2015; Moreno-Villanueva et al., 2015b; Jansen et al., 2015). In particular, glucose, insulin and Free Fatty Acid (FFA) were measured by the LX20 autoanalyzer. Glucose and insulin assays were obtained from Beckman–Coulter (Woerden, Netherlands), whereas FFA ones from Wako Diagnostics (Neuss, Germany). All the assays on the LX20 were enzymatic with a colorimetric endpoint. FFA and glucose were measured in serum samples, whereas insulin in Li/Hep EDTA plasma aliquots (Jansen et al., 2015).
16S rRNA Quantification by Real-Time qPCR
DNA was extracted from 300 μl of whole blood biobanked samples using QIAamp DNA Blood mini kit (Qiagen GmbH, Germany) according to the manufacturer’s instructions.
Highly sensitive and specific universal primers targeting the V3-V4 hypervariable region of the bacterial 16S rDNA were used in real-time qPCR reactions to quantify the 16S rRNA gene copy number in DNA samples (Nadkarni et al., 2002; Qian et al., 2018). The PCR mixture (20 μL) consisted of 20 ng of DNA, SensiFAST SYBR Hi-ROX Mix 1X (Bioline, London, United Kingdom) and 0.4 μM of the following primers: For 5′-TCCTACGGGAGGCAGCAGT-3′ and Rev 5′-GGACTACCAGGGTATCTAATCCTGTT-3′.
The thermal profile used for the reaction included a heat activation of the enzyme at 95°C for 2 min, followed by 40 cycles of denaturation at 95°C for 15 s and annealing/extension at 60°C for 60 s, followed by melt analysis ramping at 60–95°C. All measurements were taken in the log phase of amplification. Standard curves obtained using a 10-fold dilution series of bacterial DNA standards (Femto bacterial DNA quantification kit, Zymo research) ranging from 2 ng to 200 fg were routinely run with each sample set and compared with previous standard curves to check for consistency between runs. Amplicon quality was ascertained by melting curves. Amplifications of samples and standard dilutions were performed in triplicate on the StepOne Real-Time PCR System (Applied Biosystems by Life Technologies). Bacterial DNA levels were expressed as ng per ml of whole blood.
A series of controls both in silico and in vitro was performed to exclude artifacts from sample manipulation, reagent contamination and non–specific amplifications. The primers were checked for possible cross–hybridization with genes from eukaryotic and mitochondrial genomes using the database similarity search program BLAST. The BLAST search results showed no hits thus confirming the specificity of primers for the bacterial 16S rRNA as previously reported (Nadkarni et al., 2002). Separate working areas for real–time PCR mix preparation, template addition, and performing the PCR reactions were used and all experimental procedures were performed under a laminar flow hood by using dedicated pipettes, filter–sealed tips and plasticware DNA–free guaranteed. In addition, samples were processed in random order and replicated by using different reagent batches. Negative controls, in which ultrapure water instead of DNA was added, were also run in each plate. Compared with bacterial DNA detected in the blood, the levels of negative template controls were missing or very low (Supplementary Figure 1). In particular, when amplification of these controls resulted in a value about 0.05 pg, the run was discarded and the samples re–analyzed, meanwhile when values were less than 0.05 pg, they were subtracted as background from all the analyzed samples.
Statistical Analysis
To assure the MARK–AGE data quality the following cleaning strategy was performed (1) clearing of missing values, (2) removal of outliers and (3) detection of batch effects. If detected, any batch effect for each biomarker was corrected (Baur et al., 2015).
Continuous variables were described using means and SD or SEM. Due to positively skewed values, several variables (bacterial DNA levels, whole blood leukocytes, neutrophils, lymphocytes, monocytes and platelet counts, red blood cells, C–Reactive Protein (CRP), homocysteine, fibrinogen hemoglobin, ferritin, iron and transferrin serum levels, insulin, glucose, creatinine, FFA, total cholesterol, High–Density Lipoprotein (HDL), Low–Density Lipoprotein (LDL), triglycerides, albumin and Body Mass Index (BMI) failed to pass the Kolmogorov–Smirnov test and Shapiro–Wilk test for the normal distribution. ANOVA (after correction for confounding factors) was used to evaluate differences in bacterial DNA levels among age classes (35–44; 45–54; 55–64; 65–75 years), countries and gender or differences in FFA, glucose, insulin levels, and leukocyte counts among bacterial DNA quartiles. Linear regression analysis using a stepwise method was carried out to explore the main predictors of bacterial DNA levels. The variables inserted were: circulating bacterial DNA, age, gender, country, BMI, whole blood leukocytes, neutrophil, lymphocyte, monocyte and platelet counts, red blood cells, systemic inflammation parameters (CRP, homocysteine, fibrinogen), hemoglobin (Hb) levels, ferritin, iron and transferrin serum levels, insulin, glucose, creatinine, FFA, total cholesterol, HDL, LDL, triglycerides and albumin.
All the analyses were performed using the SPSS/Win program (SPSS Statistics V22.0, Chicago, IL).
Results
Characteristics of Participants and Identification of Bacterial DNA in Blood RASIG Subjects From Different European Regions
The main characteristics of the study population, subdivided by gender, were reported in Supplementary Table 1. 88% of females and 90.3% of males declared a good or very good/excellent health status.
The abundance of blood microbiome in 1285 RASIG subjects from several different geographical regions of Europe was determined by quantification of 16S rRNA gene copies through quantitative real-time PCR reactions. Negative control samples, routinely run with each sample set, confirmed the lack of contamination of each step of the experimental procedure, since their detected fluorescence signal was very low compared with that of blood samples or even absent (see section “Materials and Methods”). The performance of each qPCR assay was carefully assessed so that only runs in which calibration curved displayed a correlation coefficient (R2) of 0.99, a mean slope of about 3.3 and a PCR efficiency > 95% with no significant intra-plate variation, were accepted. The distribution of bacterial DNA levels concerning gender and geographical origin was broad, with Germany and Poland showing significantly higher values than Belgium and Austria (p < 0.05), while other countries, such as Italy, Greece and Finland showed intermediate levels (Table 1). The main differences among countries were observed in male subjects.
TABLE 1.
Bacterial DNA levels in sex stratified healthy human subjects from different European countries.
| Countries | Gender | Mean | Standard error | N |
| Germany | Females | 180.25§ | 23.66 | 138 |
| Males | 143.21 | 20.49 | 114 | |
| Total | 163.49**/§ | 15.94 | 252 | |
| Belgium | Females | 82.14 | 10.61 | 68 |
| Males | 78.08 | 10.82 | 68 | |
| Total | 80.11 | 7.55 | 136 | |
| Poland | Females | 158.28 | 21.47 | 88 |
| Males | 111.96* | 16.82 | 99 | |
| Total | 133.76* | 13.54 | 187 | |
| Greece | Females | 108.81 | 13.33 | 117 |
| Males | 149.76** | 18.27 | 102 | |
| Total | 127.88* | 11.16 | 219 | |
| Austria | Females | 88.763 | 8.28 | 100 |
| Males | 81.30 | 11.18 | 97 | |
| Total | 85.08 | 6.92 | 197 | |
| Italy | Females | 121.27 | 15.26 | 113 |
| Males | 114.67* | 13.93 | 120 | |
| Total | 117.87* | 10.29 | 233 | |
| Finland | Females | 102.72 | 12.79 | 42 |
| Males | 139.99* | 38.78 | 19 | |
| Total | 114.33*/§ | 14.91 | 61 |
§ p < 0.01 vs. Belgium. *p < 0.05 vs. Austria. **p < 0.01 vs. Austria. ANCOVA analysis corrected for age.
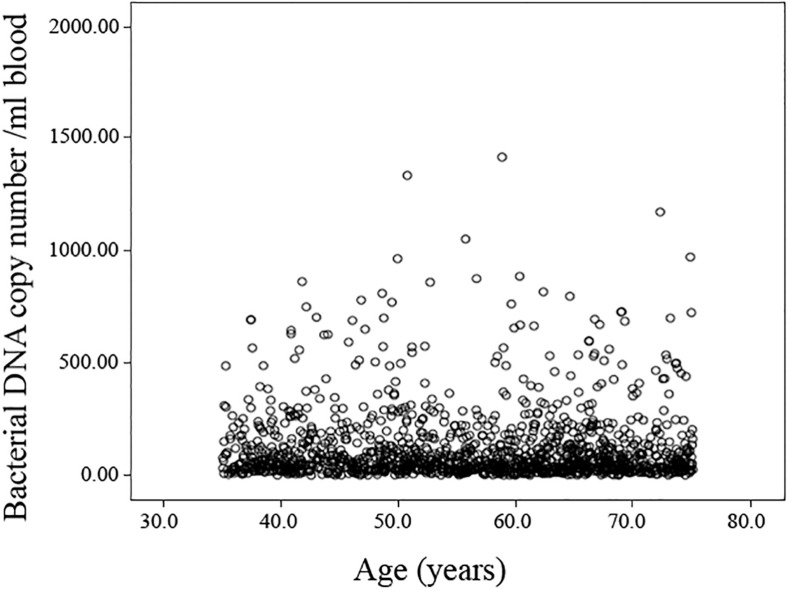
The presence of bacterial DNA was detected in all samples analyzed (121.64 ± 4.85 mean and standard error), but no significant association between 16S bacterial rDNA abundance and age (Figure 1 and Supplementary Table 2) or gender (Supplementary Table 3) emerged. Likewise, no age-related difference in the bacterial DNA amount was observed even when stratifying the sample by geographical origin (data not shown). The comparison of bacterial DNA abundance was also carried out in relation to SRH, but no difference was observed.
FIGURE 1.
Scatterplot of the bacterial DNA levels against the age of the analyzed population. Linear regression between bacterial DNA levels (log-transformed data) and age after adjusting for gender, country and BMI, smoke habits and Self-rated health status shows no significant association (Standardized Beta coefficient = –0.039, p = 0.264).
Bacterial DNA Levels and Hematological and Clinical-Chemical Parameters
Linear regression analysis with a stepwise approach was run in the population sample including bacterial DNA levels as the dependent variable and as independent variables several demographic, biochemical and laboratory parameters (see section “Statistical Analysis”). A significant positive association was found with leukocyte count (Standardized Beta coefficient = 0.110, p < 0.01) and FFA levels (Standardized Beta coefficient = 0.076, p < 0.05) (Table 2).
TABLE 2.
Multivariate stepwise linear regression analysis for variables independently associated with bacterial DNA levels in the population sample.
| Unstandardized coefficients |
Standardized coefficients |
P-value | ||
| B | Standard error | Beta | ||
| Leukocyte count | 0.298 | 0.099 | 0.110 | 0.003 |
| FFA | 0.206 | 0.080 | 0.076 | 0.010 |
Blood Parameters in Relation to Quartiles of Bacterial DNA Levels
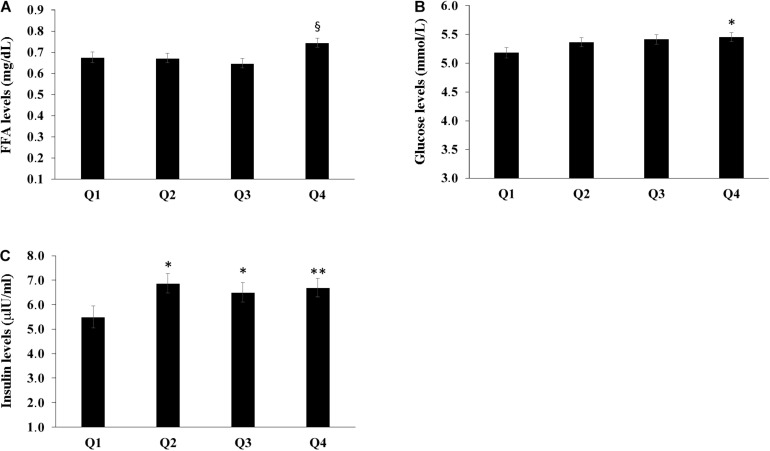
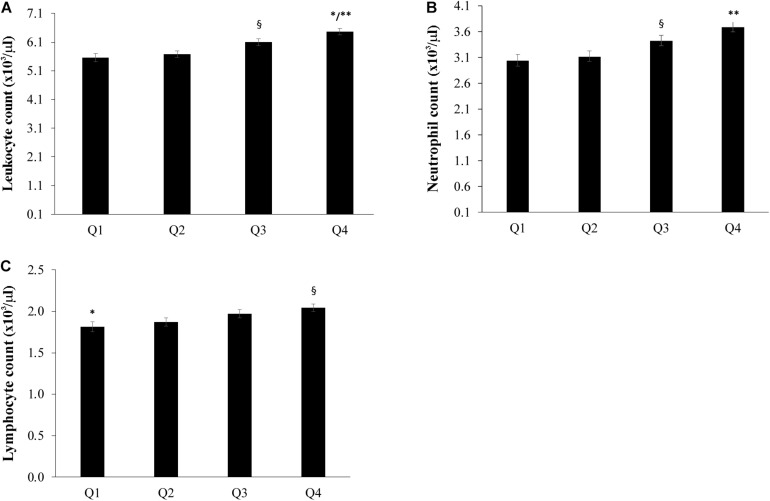
The population sample was divided into four groups according to the quartiles of bacterial DNA levels, and the relationship with FFA, glucose and insulin levels was evaluated. After adjusting for age, countries, gender, BMI, smoke habits and Self-rated health status we found that the group in the fourth quartile (Q4) appeared to have higher levels of FFA when compared with the first and the second quartiles (P < 0.05) (Figure 2A). Furthermore, subjects in the first quartile showed lower insulin levels for the fourth quartile (P < 0.05) (Figure 2B) and decreased glucose levels when compared with the fourth quartile (P < 0.05) (Figure 2C). The number of total leukocytes, lymphocytes and neutrophils according to the quartiles of bacterial DNA levels was also analyzed. The fourth quartile (Q4) showed increased leukocyte, lymphocyte and neutrophil counts as compared to the lowest quartiles (Q1, Q2) (Figure 3). The same analyzes were carried out stratifying by gender and showed similar results (Supplementary Tables 4–9).
FIGURE 2.
Relationship between bacterial DNA quartiles and (A) FFA levels, (B) glucose levels, and (C) insulin levels. The fourth quartile (Q4) of bacterial DNA levels showed the highest levels of FFA as compared with the other quartiles. The first quartile had lower insulin levels than the other quartiles and decreased glucose levels when compared with the fourth quartile. ANCOVA analysis correcting for age, countries, gender, BMI, smoke habits and Self-rated health status was applied; data are reported from the model adjusted mean ± Standard Error of the Mean (SEM); *p < 0.05 as compared to Q1; **p < 0.01 as compared to Q1; §p < 0.05 as compared to Q1, Q2, Q3.
FIGURE 3.
Relationship between bacterial DNA quartiles and (A) whole blood leukocytes, (B) neutrophil count, and (C) lymphocyte count. The fourth quartile (Q4) of bacterial DNA levels displayed the highest levels of leukocytes, lymphocytes and neutrophils than the other quartiles. ANCOVA analysis correcting for age, countries, gender, BMI, smoke habits and Self-rated health status was applied; data are reported from the model adjusted mean ± Standard Error of the Mean (SEM); ∗∗p < 0.001 as compared to Q1 and Q2; §p < 0.05 as compared to Q1 and Q2; ∗p < 0.05 as compared to Q3.
Discussion
Several studies stated the presence of a microbial community in the blood of healthy individuals. Here, we report the results of a pilot study that, to our knowledge, represents the first quantitative description of the above population in 1285 subjects aged between 35 and 75 years recruited within the EU Project MARK-AGE with a different geographical origin, in whom a series of clinical biochemical parameters was determined. The presence of this microbiome in the blood is not surprising, since a number of reports from the literature is revealing its presence in both physiological and pathological conditions. Its origin, however, remains undefined although several data suggest a translocation into the circulatory system from different body sites, namely the gastrointestinal tract, skin, oral cavity, or translocation driven by cells of the immune system.
Our study allows assessing inter-individual variations in the extent of blood microbiome significantly associated with geographical origin rather than the age of the subject analyzed. This observation, together with the genetic peculiarities of each geographical area, could be the reflection of cultural/behavioral features like diet, environmental exposure, climate, and social habits. This suggests that the environment has a greater contribution than chronological age, at least up to 75 years old, and biological age (data not shown) (Mobeen et al., 2018). Nonetheless, an association between blood microbiome and more advanced age cannot be excluded, since dysbiosis and intestinal malabsorption as well as dysregulation of the immune response leading to a chronic systemic inflammatory state are common features of oldest-old subjects.
In our population, we observed a positive association between bacterial DNA levels and serum FFA levels by multivariate stepwise linear regression analysis. These results are in agreement with literature data reporting that gut microbiota composition is related to various lipoprotein particles and gut microbiota dysbiosis is associated with altered lipid metabolism and an increased expression of key genes involved in FFA synthesis, transport and triglyceride synthesis (TG) in the liver (Jin et al., 2016; Vojinovic et al., 2019). Likewise, a role played by FFA as mediators between metabolic conditions and the immune system has been described (Sindhu et al., 2016; Olmo et al., 2019). All in all, we may speculate that FFA in association with blood microbiome levels may represent active mediators of some biological processes. Furthermore, we observed that circulating bacterial DNA was also positively associated with total leukocytes, and the highest quartile of bacterial DNA showed an increased number of lymphocytes and neutrophils both in the whole population and after gender stratification. This finding is consistent with the well-established role played by these specialized cells in killing invading microorganisms. In addition, the strong correlation between circulating bacterial DNA and leukocyte populations may be explained by a cross-talk between the intestinal microbiota and bone marrow during bacterial infection, which induces increased myelopoiesis (Balmer et al., 2014). Moreover, circulating lipopolysaccharide is associated with activation and proliferation of lymphocyte subsets as observed for NK cells and B-cells (Ramendra et al., 2019; Sakaguchi et al., 2020). Interestingly, high levels of blood bacterial DNA were found associated with increased insulin and glucose levels. Increased insulin, a marker for insulin resistance, has been implicated in gut permeability, and impaired fasting glucose subjects show higher markers of gut permeability than controls (Carnevale et al., 2017; Mkumbuzi et al., 2020). On the other hand, gut and oral microbiota dysbiosis has been found in type 2 diabetes (DM2) subjects and different microbiome compartmentalization has been observed in DM2 (Brown et al., 2020; Shi et al., 2020). Therefore, increased circulating DNA in healthy subjects could also represent a predictor of pre-diabetes or diabetes conditions. Since our results did show differences in bacterial DNA levels among SRH categories, future longitudinal studies would be useful to clarify if persistent high levels of bacterial DNA levels in blood may represent a risk factor to develop DM2 or other age-associated diseases.
We are confident that the study was adequately controlled to avoid DNA contamination in extraction kits and laboratory reagents, which could lead to erroneous conclusions and thus significantly influence the results. In addition, the low or absent levels of DNA detected in negative template controls make us rest assured of the reliability of the results.
Finally, we believe that this explorative study is useful in providing the groundwork in the future qualitative characterization of the blood microbiome composition and a potential correlation of the microbial diversity with different phenotypes thus providing useful knowledge for both basic and clinical research.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by European Commission (Project Full Name: European Study to Establish Biomarkers of Human Ageing; Project Acronym: MARK-AGE; Project No: 200880. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
DB and MP: conceptualization. PD’A and FP: methodology. RG, MM, and MV: software. TG and NB: biobank. BG-L, OT, FD-C, CS, JB, EG, CF, MC, ES, and AH: recruitment of subjects. PD’A and RG: validation and investigation. DB, MP, GP, AB, and MV: formal analysis. AB and DB: resources. RG, MM, PD’A, EJ, MD, and ES: data curation. DB and PD’A: writing original draft preparation. PD’A, RG, DB, MP, AB, MV, and MM: writing, review, and editing. DB, MP, and GP: supervision. AB: project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
CS and JB were employed by the company BioTeSys GmbH. The authors declare that this study received funding from the European Commission, the Italian Health Ministry and the nursing homes of SADEL S.p.A. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all study subjects for their willingness to participate. The work has been made possible by the collaboration with the nursing homes of SADEL S.p.A (San Teodoro, San Raffaele, Villa del Rosario, A.G.I srl, SAVELLI HOSPITAL., Casa di Cura Madonna dello Scoglio) in the frame of the agreement “SOLUZIONI INNOVATIVE PER L’INNALZAMENTO DELLA SALUTE E DELLA SICUREZZA DELLA POPOLAZIONE” with the University of Calabria.
Footnotes
Funding. This work was supported by the European Commission (Project Full Name: European Study to Establish Biomarkers of Human Aging; Project Acronym: MARK-AGE; Project No: 200880) and in part by the Project: SInergie di RIcerca della Rete Aging (SIRI) by Italian Health Ministry.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.707515/full#supplementary-material
References
- Amar J., Serino M., Lange C., Chabo C., Iacovoni J., Mondot S., et al. (2013). Blood microbiota dysbiosis is associated with the onset of cardiovascular events in a large general population: The D.E.S.I.R. study. PLoS One 8:e54461. 10.1371/journal.pone.0054461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amar J., Serino M., Lange C., Chabo C., Iacovoni J., Mondot S., et al. (2011). Involvement of tissue bacteria in the onset of diabetes in humans: evidence for a concept. Diabetologia 54 3055–3061. 10.1007/s00125-011-2329-8 [DOI] [PubMed] [Google Scholar]
- Anhê F. F., Jensen B. A. H., Varin T. V., Servant F., Van Blerk S., Richard D., et al. (2020). Type 2 diabetes influences bacterial tissue compartmentalisation in human obesity. Nat. Metab. 2 233–242. 10.1038/s42255-020-0178-9 [DOI] [PubMed] [Google Scholar]
- Assari S., Lankarani M. M., Burgard S. (2016). Black-white difference in long-term predictive power of self-rated health on all-cause mortality in United States. Ann. Epidemiol. 26 106–114. 10.1016/j.annepidem.2015.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer M. L., Schürch C. M., Saito Y., Geuking M. B., Li H., Cuenca M., et al. (2014). Microbiota-derived compounds drive steady-state granulopoiesis via MyD88/TICAM signaling. J. Immunol. 193 5273–5283. 10.4049/jimmunol.1400762 [DOI] [PubMed] [Google Scholar]
- Baur J., Moreno-Villanueva M., Kötter T., Sindlinger T., Bürkle A., Berthold M. R. (2015). MARK-AGE data management: Cleaning, exploration and visualization of data. Mech. Ageing Dev. 151 38–44. 10.1016/j.mad.2015.05.007 [DOI] [PubMed] [Google Scholar]
- Brown E. L., Essigmann H. T., Hoffman K. L., Palm N. W., Gunter S. M., Sederstrom J. M., et al. (2020). Impact of diabetes on the gut and salivary IgA microbiomes. Infect. Immun. 88 e301–e320. 10.1128/IAI.00301-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürkle A., Moreno-Villanueva M., Bernhard J., Blasco M., Zondag G., Hoeijmakers J. H., et al. (2015). MARK-AGE biomarkers of ageing. Mech. Ageing Dev. 51 2–12. 10.1016/j.mad.2015.03.006 [DOI] [PubMed] [Google Scholar]
- Capri M., Moreno-Villanueva M., Cevenini E., Pini E., Scurti M., Borelli V. (2015). MARK-AGE population: From the human model to new insights. Mech. Ageing Dev. 151 13–17. 10.1016/j.mad.2015.03.010 [DOI] [PubMed] [Google Scholar]
- Carnevale R., Pastori D., Nocella C., Cammisotto V., Baratta F., Del Ben M., et al. (2017). Low-grade endotoxemia, gut permeability and platelet activation in patients with impaired fasting glucose. Nutr. Metab. Cardiovasc. Dis. 27 890–895. 10.1016/j.numecd.2017.06.007 [DOI] [PubMed] [Google Scholar]
- Castillo D. J., Rifkin R. F., Cowan D. A., Potgieter M. (2019). The Healthy Human Blood Microbiome: Fact or Fiction? Front. Cell Infect. Microbiol. 9:148. 10.3389/fcimb.2019.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damgaard C., Magnussen K., Enevold C., Nilsson M., Tolker-Nielsen T., Holmstrup P., et al. (2015). Viable bacteria associated with red blood cells and plasma in freshly drawn blood donations. PLoS One 10:e0120826. 10.1371/journal.pone.0120826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aquila P., Carelli L. L., De Rango F., Passarino G., Bellizzi D. (2020). Gut Microbiota as Important Mediator Between Diet and DNA Methylation and Histone Modifications in the Host. Nutrients 12:597. 10.3390/nu12030597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinakaran V., Rathinavel A., Pushpanathan M., Sivakumar R., Gunasekaran P., Rajendhran J. (2014). Elevated levels of circulating DNA in cardiovascular disease patients: metagenomic profiling of microbiome in the circulation. PLoS One 9:e105221. 10.1371/journal.pone.0105221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D., Methe B., Amar S., Morris A., Lim S. H. (2019). Intestinal injury and gut permeability in sickle cell disease. J. Transl. Med. 17:183. 10.1186/s12967-019-1938-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka K., Kikuchi T., Mitsuhida N., Kawaguchi A. (1981). A new colorimetric method for the determination of free fatty acids with acyl-CoA synthetase and acyl-CoA oxidase. J. Biochem. 89 1799–1803. 10.1093/oxfordjournals.jbchem.a133380 [DOI] [PubMed] [Google Scholar]
- Jansen E., Beekhof P., Cremers J., Weinberger B., Fiegl S., Toussaint O., et al. (2015). Quality control data of physiological and immunological biomarkers measured in serum and plasma. Mech. Ageing Dev. 151 54–59. 10.1016/j.mad.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Jeon S. J., Cunha F., Vieira-Neto A., Bicalho R. C., Lima S., Bicalho M. L., et al. (2017). Blood as a route of transmission of uterine pathogens from the gut to the uterus in cows. Microbiome 5:109. 10.1186/s40168-017-0328-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Wu Y., Zeng Z., Jin C., Wu S., Wang Y., et al. (2016). From the Cover: Exposure to Oral Antibiotics Induces Gut Microbiota Dysbiosis Associated with Lipid Metabolism Dysfunction and Low-Grade Inflammation in Mice. Toxicol. Sci. 154 140–152. 10.1093/toxsci/kfw150 [DOI] [PubMed] [Google Scholar]
- Kell D. B., Pretorius E. (2018). No effects without causes: the iron dysregulation and dormant microbes hypothesis for chronic, inflammatory diseases. Biol. Rev. Camb. Philos. Soc. 93 1518–1557. 10.1111/brv.12407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelouvier B., Servant F., Païssé S., Brunet A. C., Benyahya S., Serino M., et al. (2016). Changes in blood microbiota profiles associated with liver fibrosis in obese patients: A pilot analysis. Hepatology 64 2015–2027. 10.1002/hep.28829 [DOI] [PubMed] [Google Scholar]
- Li Q., Wang C., Tang C., Zhao X., He Q., Li J. (2018). Identification and Characterization of Blood and Neutrophil-Associated Microbiomes in Patients with Severe Acute Pancreatitis Using Next-Generation Sequencing. Front. Cell Infect. Microbiol. 8:5. 10.3389/fcimb.2018.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin R. W., Vali H., Lau P. C., Palfree R. G., De Ciccio A., Sirois M., et al. (2002). Are there naturally occurring pleomorphic bacteria in the blood of healthy humans? J. Clin. Microbiol. 40 4771–4775. 10.1128/jcm.40.12.4771-4775.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkumbuzi L., Mfengu M. M. O., Engwa G. A., Sewani-Rusike C. R. (2020). Insulin Resistance is Associated with Gut Permeability Without the Direct Influence of Obesity in Young Adults. Diabet. Metab. Syndr. Obes. 13 2997–3008. 10.2147/DMSO.S256864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobeen F., Sharma V., Tulika P. (2018). Enterotype variations of the healthy human gut microbiome in different geographical regions. Bioinformation 14 560–573. 10.6026/97320630014560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Villanueva M., Capri M., Breusing N., Siepelmeyer A., Sevini F., Ghezzo A., et al. (2015a). MARK-AGE standard operating procedures (SOPs): a successful effort. Mech. Ageing Dev. 151 18–25. 10.1016/j.mad.2015.03.007 [DOI] [PubMed] [Google Scholar]
- Moreno-Villanueva M., Kötter T., Sindlinger T., Baur J., Oehlke S., Bürkle A., et al. (2015b). The MARK-AGE phenotypic database: structure and strategy. Mech. Ageing Dev. 151 26–30. 10.1016/j.mad.2015.03.005 [DOI] [PubMed] [Google Scholar]
- Moriyama K., Ando C., Tashiro K., Kuhara S., Okamura S., Nakano S., et al. (2008). Polymerase chain reaction detection of bacterial 16S rRNA gene in human blood. Microbiol. Immunol. 52 375–382. 10.1111/j.1348-0421.2008.00048.x [DOI] [PubMed] [Google Scholar]
- Nadkarni M. A., Martin F. E., Jacques N. A., Hunter N. (2002). Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148(Pt 1), 257–266. 10.1099/00221287-148-1-257 [DOI] [PubMed] [Google Scholar]
- Nikkari S., McLaughlin I. J., Bi W., Dodge D. E., Relman D. A. (2001). Does blood of healthy subjects contain bacterial ribosomal DNA? J. Clin. Microbiol. 39 1956–1959. 10.1128/JCM.39.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olde Loohuis L. M., Mangul S., Ori A. P. S., Jospin G., Koslicki D., Yang H. T., et al. (2018). Transcriptome analysis in whole blood reveals increased microbial diversity in schizophrenia. Transl. Psychiatry 8:96. 10.1038/s41398-018-0107-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmo I., Teuber S., Larrazabal C., Alarcon P., Raipane F., Burgos R. A., et al. (2019). Docosahexaenoic acid and TUG-891 activate free fatty acid-4 receptor in bovine neutrophils. Vet. Immunol. Immunopathol. 209 53–60. 10.1016/j.vetimm.2019.02.008 [DOI] [PubMed] [Google Scholar]
- Osibogun O., Ogunmoroti O., Spatz E. S., Burke G. L., Michos E. D. (2018). Self-rated health associated with ideal cardiovascular health? The Multi-Ethnic Study of Atherosclerosis. Clin. Cardiol. 41 1154–1163. 10.1002/clc.22995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Païssé S., Valle C., Servant F., Courtney M., Burcelin R., Amar J., et al. (2016). Comprehensive description of blood microbiome from healthy donors assessed by 16S targeted metagenomic sequencing. Transfusion 56 1138–1147. 10.1111/trf.13477 [DOI] [PubMed] [Google Scholar]
- Piacenza F., Giacconi R., Costarelli L., Basso A., Bürkle A., Moreno-Villanueva M., et al. (2021). Age, sex and BMI influence on copper, zinc and their major serum carrier proteins in a large European population including Nonagenarian Offspring from MARK-AGE study. J. Gerontol. Biol. Sci. Med. Sci. 13:glab134. 10.1093/gerona/glab134 [DOI] [PubMed] [Google Scholar]
- Potgieter M., Bester J., Kell D. B., Pretorius E. (2015). The dormant blood microbiome in chronic, inflammatory diseases. FEMS Microbiol. Rev. 39 567–591. 10.1093/femsre/fuv013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius L., Kell D. B., Pretorius E. (2018). Iron Dysregulation and Dormant Microbes as Causative Agents for Impaired Blood Rheology and Pathological Clotting in Alzheimer’s Type Dementia. Front. Neurosci. 12:851. 10.3389/fnins.2018.00851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Yang X., Xu S., Wu C., Qin N., Chen S. D., et al. (2018). Detection of microbial 16s rRNA gene in the blood of patients with Parkinson’s Disease. Front. Aging Neurosci. 24:156. 10.3389/fnagi.2018.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Zhou H., Jing Y., Dong C. (2019). Association between blood microbiome and type 2 diabetes mellitus: A nested case-control study. J. Clin. Lab. Anal. 33:e22842. 10.1002/jcla.22842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramendra R., Isnard S., Mehraj V., Chen J., Zhang Y., Finkelman M., et al. (2019). Circulating LPS and (1→3)-β-d-glucan: a folie à deux contributing to HIV-associated immune activation. Front. Immunol. 10:465. 10.3389/fimmu.2019.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinninella E., Raoul P., Cintoni M., Franceschi F., Miggiano G. A. D., Gasbarrini A., et al. (2019). What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 7:14. 10.3390/microorganisms7010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T., Okumura R., Ono C., Okuzaki D., Kawai T., Okochi Y., et al. (2020). TRPM5 negatively regulates calcium-dependent responses in lipopolysaccharide-stimulated B lymphocytes. Cell Rep. 31:107755. 10.1016/j.celrep.2020.107755 [DOI] [PubMed] [Google Scholar]
- Sato J., Kanazawa A., Ikeda F., Yoshihara T., Goto H., Abe H., et al. (2014). Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabet. Care 37 2343–2350. 10.2337/dc13-2817 [DOI] [PubMed] [Google Scholar]
- Shi B., Lux R., Klokkevold P., Chang M., Barnard E., Haake S., et al. (2020). The subgingival microbiome associated with periodontitis in type 2 diabetes mellitus. ISME J. 14 519–530. 10.1038/s41396-019-0544-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhu S., Al-Roub A., Koshy M., Thomas R., Ahmad R. (2016). Palmitate-induced MMP-9 expression in the human monocytic cells is mediated through the TLR4-MyD88 dependent mechanism. Cell Physiol. Biochem. 39 889–900. 10.1159/000447798 [DOI] [PubMed] [Google Scholar]
- Sze M. A., Tsuruta M., Yang S. W., Oh Y., Man S. F., Hogg J. C., et al. (2014). Changes in the bacterial microbiota in gut, blood, and lungs following acute LPS instillation into mice lungs. PLoS One 9:e111228. 10.1371/journal.pone.0111228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi G. G., Amici D., Paparelli M. (1969). Incorporation of nucleosides and amino-acids in human erythrocyte suspensions: possible relation with a diffuse infection of mycoplasms or bacteria in the L form. Nature 222 1285–1286. 10.1038/2221285a0 [DOI] [PubMed] [Google Scholar]
- Tidjani Alou M., Lagier J. C., Raoult D. (2016). Diet influence on the gut microbiota and dysbiosis related to nutritional disorders. Hum. Microbiome J. 1 3–11. 10.1016/j.humic.2016.09.001 [DOI] [Google Scholar]
- Traykova D., Schneider B., Chojkier M., Buck M. (2017). Blood microbiome quantity and the hyperdynamic circulation in decompensated cirrhotic patients. PLoS One 12:e0169310. 10.1371/journal.pone.0169310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vientós-Plotts A. I., Ericsson A. C., Rindt H., Grobman M. E., Graham A., Bishop K., et al. (2017). Dynamic changes of the respiratory microbiota and its relationship to fecal and blood microbiota in healthy young cats. PLoS One 12:e0173818. 10.1371/journal.pone.0173818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojinovic D., Radjabzadeh D., Kurilshikov A., Amin N., Wijmenga C., Franke L., et al. (2019). Relationship between gut microbiota and circulating metabolites in population-based cohorts. Nat. Commun. 10:5813. 10.1038/s41467-019-13721-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle E., Leonard M. O., Harrison R., Gant T. W., Tonge D. P. (2019). Multi-method characterization of the human circulating microbiome. Front. Microbiol. 9:3266. 10.3389/fmicb.2018.03266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmanski T., Diener C., Rappaport N., Patwardhan S., Wiedrick J., Lapidus J., et al. (2021). Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 3 274–286. 10.1038/s42255-021-00348-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T., Rey F. E., Manary M. J., Trehan I., Dominguez-Bello M. G., Contreras M., et al. (2012). Human gut microbiome viewed across age and geography. Nature 486 222–227. 10.1038/nature11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.