Abstract
Rationale & Objective
Acid retention may occur in the absence of overt metabolic acidosis; thus it is important to identify populations at risk. Because obesity may alter renal acid-base handling, we sought to determine whether overweight and obesity are associated with increased risk for low serum bicarbonate levels, suggesting metabolic acidosis.
Study Design
Retrospective cohort study.
Setting & Participants
Adult patients (n = 96,147) visiting outpatient clinics in the Bronx, NY, between January 1, 2010, and December 31, 2015.
Predictor
Body mass index (BMI).
Outcome
Low serum bicarbonate level (≤23 mEq/L).
Analytical Approach
Longitudinal analyses were conducted using mixed-effects models to examine associations of BMI with serum bicarbonate levels over time and Cox proportional hazards models to examine associations of BMI with incident low bicarbonate levels.
Results
During a median follow-up of 4.4 (interquartile range, 2.3-6.3) years, patients had a median of 8 serum bicarbonate measurements and 34,539 patients developed low bicarbonate levels. Higher BMI was associated with progressively lower serum bicarbonate levels, with attenuation of the association in the highest BMI groups, suggesting a J-shaped relationship. Compared with the reference group (BMI, 18.5 to <25 kg.m2), patients with BMIs of 25 to <30, 30 to <35, 35 to <40, and ≥40 kg/m2 had HRs for incident low bicarbonate levels of 1.10 (95% CI, 1.05-1.14), 1.16 (95% CI, 1.11-1.21), 1.20 (95% CI, 1.14-1.26), and 1.15 (95% CI, 1.09-1.22). Results were similar after adjustment for serum urea nitrogen level and exclusion of patients with diabetes, hypertension, or estimated glomerular filtration rates < 60 mL/min/1.73 m2.
Limitations
Arterial pH measurements were unavailable.
Conclusions
Higher BMI is independently associated with progressively greater risk for developing low serum bicarbonate levels, indicating likely metabolic acidosis. Further research should explore the causes of low bicarbonate levels in patients with overweight and obesity.
Index Words: Obesity, overweight, metabolic acidosis, bicarbonate, body mass index, insulin resistance, acid-base
Graphical abstract
Plain-Language Summary.
Overweight and obesity are risk factors for chronic kidney disease (CKD) and have been associated with lower urinary pH, suggesting possible abnormalities in acid-base status. It has not been determined whether obesity is associated with developing low serum bicarbonate levels, an indicator of acid-base abnormality and a risk factor for CKD. We analyzed a large cohort of patients using an observational study design to determine whether greater body mass index (BMI) is associated with greater risk for developing low serum bicarbonate levels. Our results showed that greater BMI is associated with progressively greater risk for developing low serum bicarbonate levels. These findings reveal a potentially treatable abnormality to help prevent CKD and other morbidity in those with overweight and obesity.
Metabolic acidosis is a common complication of chronic kidney disease (CKD)1 that is implicated in higher rates of skeletal muscle protein breakdown,2 muscle weakness,3 functional limitation,4 and bone disease.5 Population studies of adults with and without CKD6, 7, 8 reveal that metabolic acidosis, defined by a low serum bicarbonate level, is also associated with increased risk for kidney function decline and all-cause mortality. Randomized trials have shown that treatment of metabolic acidosis in patients with CKD slows kidney functional decline9, 10, 11, 12 and improves physical functioning.9
Adverse clinical consequences have been linked to acid-base changes even in the absence of overt metabolic acidosis. Lower serum bicarbonate levels within the normal range are associated with kidney function decline,13 in addition to low cardiorespiratory fitness,14 diabetes,15 and low bone mineral density.16 Furthermore, alkali treatment of patients with CKD without metabolic acidosis reduces levels of markers of kidney damage and slows glomerular filtration rate (GFR) decline,17,18 whereas in adults without CKD who do not have overt metabolic acidosis, alkali treatment reduces levels of markers of muscle and bone loss and preserves bone mineral density.19, 20, 21 Therefore, treatment of acid-mediated organ damage may be indicated before metabolic acidosis is evident. Thus, it is important to identify populations at risk for this process.
Intriguing data suggest that one such risk factor may be obesity. In cross-sectional analyses, greater waist circumference has been associated with lower serum bicarbonate levels in patients with CKD,22 and greater body mass index (BMI) has been associated with lower serum bicarbonate levels in patients without CKD.13 Additionally, higher BMI is associated with lower urinary pH,23,24 higher net acid excretion,24 and lower urinary citrate excretion.23,24 Therefore, obesity and its associated metabolic consequences may predispose patients to metabolic acid-base disturbances, and these may be detectable before the development of overt metabolic acidosis.
However, to date, the relationship of BMI with acid-base status has been examined to only a limited extent.13,22 Given the independent risk for CKD associated with obesity and metabolic syndrome,25,26 it is important to clarify the extent to which obesity predisposes patients to developing low bicarbonate levels. This information may suggest appropriate clinical interventions to reduce the already high burden of disease among overweight and obese individuals.27
We hypothesized that higher BMI would be associated with lower serum bicarbonate levels and increased risk for developing low serum bicarbonate levels, even in adults without baseline kidney disease.
Methods
Study Population
We assembled a cohort of patients who visited outpatient clinics within the Montefiore Medical Center (MMC) health system between January 1, 2010, and December 31, 2015. Inclusion criteria were age 18 years or older, complete demographic data, 2 or more outpatient clinic visits during the study period with a basic metabolic panel checked within 24 hours of the visit, and BMI or weight value recorded at the office visit or within 90 days before or after each visit. The index date was defined as the first basic metabolic panel date. The index BMI was calculated as the average of BMI values obtained during the 90 days before and after the index date. To ensure adequate collection of comorbid condition data, all patients were additionally required to have been seen in an MMC clinic within 1 year before the index date.
Patients were excluded if they had, within 10 years before or 90 days after the index date, any International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for a kidney transplant, congestive heart failure, pulmonary hypertension, asthma, chronic obstructive pulmonary disease or other diffuse parenchymal lung disease, obesity hypoventilation syndrome or obstructive sleep apnea syndrome, hyperaldosteronism, inflammatory bowel disease or chronic diarrhea, ascites, cirrhosis, liver disease, connective tissue disease, HIV infection, AIDS, or any malignancy or if they had filled within 90 days before or after the index date any outpatient prescription for diuretics, sodium citrate, sodium bicarbonate, potassium chloride, or antiretroviral medications. Patients with baseline bicarbonate levels < 15 or >40 mEq/L or baseline BMI < 15 or >60 kg/m2 were excluded as outliers (n = 636). The final cohort consisted of 96,147 patients (Fig 1).
Figure 1.
Study flow diagram. Abbreviation: BMI, body mass index.
The study was approved by the Institutional Review Board for MMC/Albert Einstein College of Medicine (Institutional Review Board number: 2018-9100). The need for informed consent was waived due to the use of deidentified data.
Data Collection
Age, sex, race/ethnicity, insurance status, zip codes, comorbid medical conditions, and laboratory data were obtained from the electronic health record of MMC. Available body metric and laboratory data for each patient were collected from the index date through July 1, 2018. BMI was calculated as weight in kilograms divided by height in meters squared. BMI values > 10 kg/m2 higher or lower than those temporally adjacent for a given patient were excluded as erroneous. Patients who identified as Black Hispanic were included as Hispanic. Patients who identified as Asian were pooled with patients who did not identify as Black, Hispanic, or White because of the small sample size.
Baseline diabetes was defined by any occurrence within 5 years before or 90 days after the index date, an ICD-9 code related to type 2 diabetes, hemoglobin A1c value 6.5%, or any prescription for diabetes medications. Baseline hypertension and coronary artery disease were defined by ICD-9 codes within 5 years before or 90 days after the index date. Income was defined as the median income within each patient’s home zip code using the 2013 to 2017 American Community 5-year estimates from the Census Bureau.28
To qualify for inclusion, laboratory data had to have been collected within 24 hours of the index date and any subsequent office visits. Data were excluded if additional basic metabolic panels occurred within 7 days after this so as to avoid inclusion of probable hospital or emergency department blood samples or those drawn during acute illness or medication changes in the outpatient setting. Incident low bicarbonate level was defined as the presence of at least 2 bicarbonate values ≤ 23 mEq/L after the index date; time to incident low bicarbonate level was defined by the date of the first of these.
All outpatient blood samples were processed centrally at the same MMC hospital laboratory and analyzed using a standardized process. Samples were drawn by venipuncture and delivered immediately to the laboratory or refrigerated at 4 to 8 °C until analyzed. Serum creatinine was measured using a modified kinetic Jaffé reaction, and bicarbonate levels, by a phosphoenolpyruvate carboxylase method on the Hitachi Modular System (Roche Diagnostics). Estimated GFR (eGFR) was calculated using the 4-variable CKD Epidemiology Collaboration (CKI-EPI) formula.29
Statistical Analyses
Patients were stratified by 6 categories of BMI based on World Health Organization classifications. Baseline characteristics by BMI category were analyzed using analysis of variance for continuous variables and χ2 tests for categorical variables. Linear regression models were created to examine baseline associations of BMI with serum bicarbonate levels. Effect modification was examined between BMI and key covariates by including multiplicative interaction terms in the models. The association of BMI with serum bicarbonate levels over time was examined using mixed-effects models specifying random intercepts to account for within-person correlation and incorporating time-updated eGFR, BMI, and serum bicarbonate values. Values for BMI and bicarbonate were averaged over every 6-month period to facilitate the use of data from visits in which only BMI or only bicarbonate was measured. The risk for incident low bicarbonate levels by BMI category among patients who did not have low bicarbonate levels at baseline was examined using Cox proportional hazards models. Time zero was the date of the first serum bicarbonate value. Patients were censored at the last available bicarbonate value. The proportional hazards assumption was verified by visual inspection of log-log plots.
For all models, covariates selected a priori as potential confounders included age, sex, race/ethnicity, eGFR, income, insurance status, baseline hypertension, baseline diabetes, and baseline coronary artery disease. The continuous association of BMI with risk for incident low bicarbonate levels was also examined by creating a restricted cubic spline model. P < 0.05 was considered statistically significant. All analyses were performed using Stata, version 13.1 (StataCorp).
Sensitivity Analyses
To account for the effects of dietary protein, we included serum urea nitrogen (SUN) level in additional linear regression and Cox proportional hazards model analyses. To determine whether our findings were driven by participants with CKD, we analyzed a subgroup comprising patients with eGFRs ≥ 60 mL/min/1.73 m2 and by additional eGFR subgroups. In light of the significant influence of angiotensin-converting enzyme inhibitors on bicarbonate levels,22 we analyzed a subgroup of patients without hypertension at baseline. To test the robustness of our definition of incident low bicarbonate level, we conducted a second analysis in which 2 bicarbonate levels ≤ 23 mEq/L were required with an interval of 365 days or less between them.
Results
Baseline Characteristics
Mean age of the study population was 50.3 ± 17 years, with 61,864 (64%) women, 34,335 (36%) Hispanic, 32,700 (34%) Black, and 11,044 (12%) White participants (Table 1). Most patients were overweight (BMI, 25-<30 kg/m2), followed by obese class 1 (BMI, 30-<35 kg/m2), and normal weight (BMI, 18-<25 kg/m2). Higher BMI was associated with a greater prevalence of hypertension, diabetes, and coronary artery disease (Table 1). The prevalence of Black and Hispanic race/ethnicity tended to increase with higher BMI, whereas the prevalence of Asian/other and White race/ethnicity tended to decrease. Higher BMI was associated with lower median income but with a higher prevalence of commercial insurance (Table 1). Small but statistically significant differences in serum bicarbonate, SUN, and eGFR values were seen across BMI categories.
Table 1.
Baseline Characteristics by BMI Sextiles (World Health Organization Classification)
| Characteristic | BMI, kg/m2 |
P | |||||
|---|---|---|---|---|---|---|---|
| <18.5 | 18.5-<25 | 25-<30 | 30-<35 | 35-<40 | ≥40 | ||
| Patients | 1,059 (1.1%) | 20,357 (21.2%) | 33,212 (34.5%) | 22,996 (23.9%) | 10,945 (11.4%) | 7,578 (7.9%) | <0.001 |
| Age, y | 43.3 (22.8) | 48.7 (20.0) | 52.0 (16.5) | 51.4 (15.2) | 49.2 (14.6) | 45.5 (14.1) | <0.001 |
| Women | 773 (73.0%) | 13,231 (65.0%) | 19,842 (59.7%) | 14,698 (63.9%) | 7,671 (70.1%) | 5,649 (74.5%) | <0.001 |
| Race/ethnicity | |||||||
| Black | 332 (31.3%) | 5,879 (28.9%) | 10,670 (32.1%) | 8,369 (36.4%) | 4,264 (39.0%) | 3,186 (42.0%) | <0.001 |
| Hispanic | 320 (30.2%) | 6,687 (32.9%) | 12,106 (36.5%) | 8,535 (37.1%) | 3,965 (36.2%) | 2,722 (35.9%) | |
| Asian/other race | 249 (23.5%) | 4,668 (22.9%) | 6,623 (19.9%) | 3,818 (16.6%) | 1,679 (15.3%) | 1,031 (13.6%) | |
| White | 158 (14.9%) | 3,123 (15.3%) | 3,813 (11.5%) | 2,274 (9.9%) | 1,037 (9.5%) | 639 (8.4%) | |
| Insurance | |||||||
| Commercial | 586 (55.3%) | 12,112 (59.5%) | 20,570 (61.9%) | 14,445 (62.8%) | 6,877 (62.8%) | 4,695 (62.0%) | <0.001 |
| Medicare | 180 (17.0%) | 3,418 (16.8%) | 5,264 (15.8%) | 3,255 (14.2%) | 1,345 (12.3%) | 749 (9.9%) | |
| Medicaid | 237 (22.4%) | 3,795 (18.6%) | 5,763 (17.4%) | 4,217 (18.3%) | 2,197 (20.1%) | 1,799 (23.7%) | |
| Self-pay | 56 (5.3%) | 1,032 (5.1%) | 1,615 (4.9%) | 1,079 (4.7%) | 526 (4.8%) | 335 (4.4%) | |
| Median income by zip code | |||||||
| <$30,000 | 193 (18.2%) | 3,585 (17.6%) | 6,528 (19.7%) | 4,844 (21.1%) | 2,463 (22.5%) | 2,000 (26.4%) | <0.001 |
| $30,000-$49,999 | 476 (45.0%) | 8,965 (44.0%) | 15,345 (46.2%) | 10,897 (47.4%) | 5,187 (47.4%) | 3,452 (45.6%) | |
| $50,000-$69,999 | 164 (15.5%) | 3,436 (16.9%) | 5,333 (16.1%) | 3,468 (15.1%) | 1,598 (14.6%) | 949 (12.5%) | |
| $70,000 | 84 (7.9%) | 1,710 (8.4%) | 2,344 (7.1%) | 1,416 (6.2%) | 635 (5.8%) | 374 (4.9%) | |
| Not specified | 142 (13.4%) | 2,661 (13.1%) | 3,662 (11.0%) | 2,371 (10.3%) | 1,062 (9.7%) | 803 (10.6%) | |
| Baseline diagnoses | |||||||
| Hypertension | 261 (24.6%) | 7,084 (34.8%) | 15,715 (47.3%) | 12,430 (54.1%) | 6,253 (57.1%) | 4,306 (56.8%) | <0.001 |
| Diabetes | 90 (8.5%) | 3,236 (15.9%) | 7,872 (23.7%) | 6,989 (30.4%) | 3,761 (34.4%) | 2,745 (36.2%) | <0.001 |
| Coronary artery disease | 64 (6.0%) | 1,382 (6.8%) | 2,755 (8.3%) | 1,892 (8.2%) | 842 (7.7%) | 495 (6.5%) | <0.001 |
| Baseline laboratory values | |||||||
| eGFR,a mL/min/1.73 m2 | 101.3 (29.5) | 92.5 (25.6) | 88.4 (23.3) | 88.5 (23.3) | 90.9 (23.7) | 95.6 (23.9) | <0.001 |
| eGFR < 60 mL/min/1.73 m2 | 98 (9.3%) | 2,106 (10.4%) | 3,642 (11.0%) | 2,476 (10.8%) | 1,030 (9.4%) | 552 (7.3%) | <0.001 |
| Serum bicarbonate,b mEq/L | 25.4 (2.6) | 25.6 (2.6) | 25.6 (2.5) | 25.5 (2.6) | 25.3 (2.6) | 25.4 (2.6) | <0.001 |
| Serum urea nitrogen,c mg/dL (n = 86,673) | 14.2 (6.0) | 14.8 (6.1) | 15.0 (5.6) | 14.9 (5.7) | 14.7 (5.8) | 14.2 (5.6) | <0.001 |
Note: Values expressed as number (percent) or continuous variables as mean (standard deviation).
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate.
Calculated using Chronic Kidney Disease Epidemiology Collaboration creatinine equation.
Conversion to SI units (mmol/L): 1 mEq/L = 1 mmol/L.
Conversion to SI units (mmol/L): 1 mg/dL = 0.357 mmol/L.
Association of BMI With Serum Bicarbonate
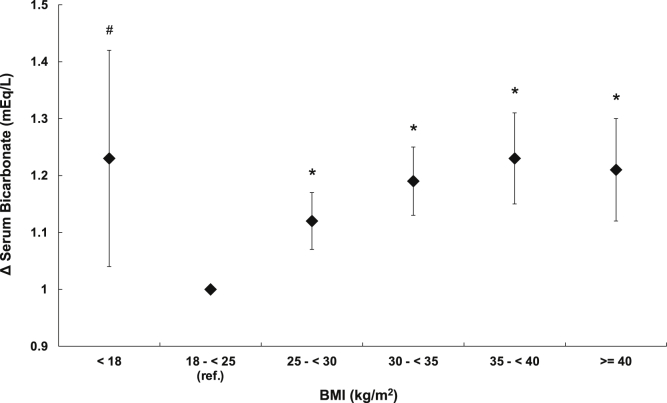
After multivariable adjustment, there was a J-shaped association of BMI with serum bicarbonate levels. Higher BMI above the normal range was associated with a graded decrease in serum bicarbonate levels, which was attenuated somewhat but remained significant with BMI ≥ 40 kg/m2 (Fig 2A). Analyses using smaller BMI categories confirmed a graded association and suggested that higher BMI was associated with lower serum bicarbonate levels beginning with BMI ≥ 22.5 kg/m2 and confirmed that at the highest BMI values, the association between BMI and serum bicarbonate levels begins to invert (Fig 2B).
Figure 2.
Association of body mass index (BMI) with serum bicarbonate levels. (A, B) Differences in baseline serum bicarbonate levels according to (A) World Health Organization (WHO) BMI groups and (B) smaller BMI categories. (C, D) Association of time-updated BMI with serum bicarbonate levels over time according to (C) WHO BMI groups and (D) smaller BMI categories. Models adjusted for age, sex, race/ethnicity, and baseline income, insurance status, estimated glomerular filtration rate, hypertension, diabetes, and coronary artery disease status. Bars denote 95% CI. n = 96,147; ∗P < 0.001; #P < 0.01. Abbreviation: ref, reference.
Association of BMI With Serum Bicarbonate Over Time and With Incident Low Bicarbonate
During a median follow-up of 4.4 (interquartile range, 2.3-6.3) years, patients had a median of 8 (interquartile range, 5-13) serum bicarbonate measurements, and 34,539 (36%) developed low bicarbonate levels. Using time-updated BMI, eGFR, and serum bicarbonate values, the same J-shaped association was observed (Fig 2C and D), whereas the effect of time was significantly positive (0.01 mEq/L per 6-month period; P < 0.001). Higher BMI was associated with a progressively greater hazard of incident low bicarbonate levels through BMI of 40 kg/m2 (Fig 3A). Compared with patients with normal BMI, those with BMI of 35 to <40 kg/m2 had a 20% (95% CI, 14%-26%) higher hazard of developing low bicarbonate levels. This association was attenuated at higher BMI values but remained significant. When examined within smaller BMI categories, the same significant J-shaped relationship was observed (Fig 3B). Analysis using a restricted cubic spline model also revealed a J-shaped relationship (Fig S1).
Figure 3.
Association of body mass index (BMI) with incident low bicarbonate levels. (A, B) Hazard ratios according to (A) World Health Organization (WHO) BMI groups and (B) smaller BMI categories among participants without low bicarbonate levels at baseline. Models adjusted for age, sex, race/ethnicity, and baseline income, insurance status, estimated glomerular filtration rate, hypertension, diabetes, and coronary artery disease status. Bars denote 95% CIl. n = 81,400; ∗P < 0.001; #P < 0.05. Abbreviation: ref, reference.
Effect Modification by Race/Ethnicity
In prospective analyses, there was significant effect modification of the association of BMI with incident low bicarbonate levels by race/ethnicity (P < 0.01). At every BMI level, the hazard was greatest among White patients while nonsignificant among Black patients (Table 2). Among Hispanic and Asian/other patients, the hazard was significant but lower in magnitude compared with White patients (Table 2). These differences by race/ethnicity prompted further subgroup analysis to explore potential explanations for the effect modification. Given the association of diabetes with acid-base disturbances30 and the known differences in diabetes prevalence by race/ethnicity,31 we repeated analyses in the subgroup of patients without baseline diabetes mellitus. In comparison to the whole cohort (Fig 3A and B), the association of higher BMI with higher hazard of low bicarbonate levels was similar (Fig 4). Among all subgroups analyzed, the largest magnitude of effect was seen among White patients without baseline diabetes (Fig S2a). As a comparison, among Black patients without baseline diabetes, the association of BMI with incident low bicarbonate levels was nonsignificant (Fig S2b).
Table 2.
The Hazard of Incident Low Bicarbonate by BMI Group, Subgroups by Race/Ethnicity
| BMI Group, kg/m2 | Black |
Hispanic |
||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
| <18.5 | 1.15 | 0.88 - 1.49 | 0.31 | 1.22 | 0.95 - 1.58 | 0.12 |
| 18.5-< 25 | Reference | 0 | – | Reference | 0 | – |
| 25-< 30 | 1.02 | 0.94-1.09 | 0.66 | 1.10 | 1.03-1.18 | 0.01 |
| 30-< 35 | 1.06 | 0.98-1.14 | 0.14 | 1.17 | 1.09-1.26 | <0.001 |
| 35-< 40 | 1.08 | 0.99-1.18 | 0.08 | 1.22 | 1.12-1.33 | <0.001 |
| >40 | 1.04 | 0.94-1.14 | 0.49 | 1.21 | 1.09-1.34 | <0.001 |
| BMI Group, kg/m2 | Asian/Other | White | ||||
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
| <18.5 | 0.93 | 0.66-1.30 | 0.66 | 1.32 | 0.90-1.93 | 0.16 |
| 18.5-<25 | Reference | 0 | – | Reference | 0 | – |
| 25-<30 | 1.15 | 1.05-1.26 | <0.01 | 1.21 | 1.08-1.36 | <0.01 |
| 30-<35 | 1.18 | 1.06 - 1.30 | <0.01 | 1.38 | 1.21 - 1.57 | <0.001 |
| 35-<40 | 1.26 | 1.11 - 1.43 | <0.001 | 1.41 | 1.20 - 1.66 | <0.001 |
| >40 | 1.15 | 0.98 - 1.34 | 0.08 | 1.38 | 1.14 - 1.67 | <0.01 |
Abbreviations: BMI, body mass index.
Figure 4.
Association of body mass index (BMI) with serum bicarbonate levels among participants without diabetes. Differences in baseline serum bicarbonate levels according to World Health Organization (WHO) BMI groups among patients without baseline diabetes mellitus. Model adjusted for age, sex, race/ethnicity, and baseline income, insurance status, estimated glomerular filtration rate, hypertension, and coronary artery disease status. Bars denote 95% CI. n = 71,454; ∗P < 0.001.
Sensitivity Analyses
Including SUN level in our model attenuated slightly the degree to which higher BMI was associated with incident low bicarbonate levels but did not meaningfully change our results (Fig S3). When restricted to patients with eGFRs ≥ 60 mL/min/1.73 m2 (Fig S4), the increase in hazard of incident low bicarbonate level was greater in magnitude compared with the combined cohort. The same occurred when analysis was limited to those without baseline hypertension (Fig S5). Analyses by eGFR subgroups revealed that associations of higher BMI with lower baseline serum bicarbonate levels and higher hazard of incident low bicarbonate levels were of greatest magnitude among patients with relatively preserved eGFRs (Fig S6; Table S1). Restricting the definition of incident low bicarbonate level to 2 low bicarbonate levels within 365 days or less of each other did not materially change the point estimates of hazard or the significance of the results (Table S2).
Discussion
In a large diverse multiethnic cohort, we found that obesity is associated with lower serum bicarbonate levels and increased risk for developing low bicarbonate levels, an indicator of likely metabolic acidosis. Our results demonstrate graded associations of higher BMI with lower serum bicarbonate levels, even after adjustment for multiple potential confounders. Furthermore, among individuals without low bicarbonate levels at baseline, higher BMI was associated with a progressively greater risk for developing low bicarbonate levels. These associations increased in magnitude up to BMI of 40 kg/m2, after which they were attenuated but remained significant. These findings persisted even after excluding individuals with diabetes or CKD. Overall, our results indicate that obesity is a previously unrecognized risk factor for the development of low bicarbonate levels in a non-CKD population.
Given that greater BMI and waist circumference have been associated with higher Pco2 levels,32 it is unlikely that lower serum bicarbonate levels among obese individuals on a population level is a result of renal compensation for hyperventilation-induced respiratory alkalosis. Therefore, these results likely represent metabolic acidosis. Prior research demonstrates an association of greater obesity with impaired renal acid excretion, including lower urinary pH.23,24,33 More metabolic syndrome features, and specifically greater insulin resistance, are also associated with progressively lower urinary pH,34,35 implicating a causal role for obesity-related metabolic dysfunction.
A recent study has also shown a positive association of BMI and net acid excretion that is independent of diet, and that obese individuals excrete a lower proportion of total urinary acid as ammonium.24 Thus, obese individuals may have elevated acid generation rates in addition to an impaired capacity to excrete the daily acid load. Over time, resultant net acid retention would be expected to deplete body buffers, with a reduction in serum bicarbonate levels a relatively late effect.36 Our results now support the idea that this acid retention is proportional to excess weight and also incompletely compensated. Although our study was not designed to examine whether metabolic acidosis contributes to CKD development in the obese, our results now link obesity to a well-known risk factor for CKD development.
We suspect that the J-shaped relationship in our results likely reflects the presence of undiagnosed obesity hypoventilation syndrome at higher BMI values. The inflection points observed in our analyses occurred at BMI values between 40 and 45 kg/m2, consistent with the average BMI among patients with diagnosed obesity hypoventilation syndrome.37 However, prior research has shown that obesity-related carbon dioxide retention is not limited to morbid obesity but rather occurs in lesser stages of obesity.38 For this reason, our results likely underestimate the true magnitude of acid-base disturbance among individuals with greater degrees of obesity.
The near absence of a relationship between BMI and bicarbonate levels in Black patients was especially striking. Prior research has shown that postmenopausal Black women compared with White women have significantly higher urinary pH that is independent of dietary intake and other urinary markers.39 Skin fibroblasts in Black compared with White patients have higher sodium-hydrogen antiporter activity,40 suggesting possible differences in the kinetics of acid-base homeostatic mechanisms. Alternatively, BMI compared with other markers has been shown to be a poor predictor of metabolic risk in Black women,41 which may limit its utility as a predictor of outcomes in our study.
The near absence of an association of BMI with serum bicarbonate levels or incident low bicarbonate levels among those with lower eGFRs (Fig S6; Table S2) may be due to undocumented use among this subgroup of diuretics or alkali. Additionally, because declining GFR is a significant risk factor for metabolic acidosis,1 the risk for low bicarbonate levels attributable to BMI at lower GFRs may be more difficult to detect. Although the wide CIs make it difficult to draw conclusions about the association of BMI and baseline bicarbonate levels among patients with eGFRs of 15 to <30 mL/min/1.73 m2, these results suggest a positive rather than negative association in this subgroup (Fig S6). Discordant with this is the suggestion of a still elevated risk for incident low bicarbonate levels among obese class II patients in this subgroup in the survival analysis (Table S1). These results again may be due to alkali and diuretic treatment, which may raise the average bicarbonate levels among these groups, but not completely mitigate the risk for incident low bicarbonate levels. Further investigation is warranted in larger CKD cohorts.
Limitations of our study relate to the observational study design. In this large cohort, we were unable to confirm acid-base status because of the lack of arterial blood gases. Future studies should confirm our findings with arterial pH measurements. Measurements of bicarbonate are subject to daily fluctuations from diet42 and variations due to sample handling,43 effects that we would expect to bias our results toward the null hypothesis. There is also the possibility that differences in dietary alkali or protein intake related to obesity could partly explain our findings; we were also unable to control for the effect of dietary net endogenous acid production on serum bicarbonate levels44 and were limited to using SUN level as a surrogate for dietary protein. Finally, we were unable to definitively exclude from our analysis patients with undocumented obesity hypoventilation syndrome, whose inclusion may have confounded our results toward an underestimation of the degree of low bicarbonate levels in those with obesity.
If confirmed by future studies, our findings suggest that weight loss and measures to reduce acid production, such as increased consumption of fruits and vegetables,18,45 may be important population-level interventions to reduce risk factors for CKD development and mortality in those with overweight and obesity.
Article Information
Authors’ Full Names and Academic Degrees
Douglas C. Lambert, MD, and Matthew K. Abramowitz, MD, MS.
Authors’ Contributions
Research idea and study design: DCL, MKA; data acquisition: DCL; data analysis and interpretation: DCL, MKA; statistical analysis: DCL, MKA: supervision or mentorship: MKA. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
Dr Abramowitz was supported by K23DK099438 from the National Institutes of Health.
Financial Disclosure
Dr Abramowitz has received consulting fees from Tricida, Inc. Dr Lambert declares that he has no relevant financial interests.
Prior Presentation
Data from this project were presented as an online poster abstract at the Society of General Internal Medicine 2020, May 6-9, 2020, virtual meeting.
Peer Review
Received July 10, 2020. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form February 15, 2021.
Footnotes
Complete author and article information provided before references.
Figure S1: Restricted Cubic Spline Model of Association of BMI With Incident Low Bicarbonate.
Figure S2: Association of BMI With Incident Low Bicarbonate Among Patients Without Diabetes.
Figure S3: Association of BMI With Incident Low Bicarbonate After Adjustment for Serum Urea Nitrogen.
Figure S4: Association of BMI With Incident Low Bicarbonate Among Patients With an Estimated Glomerular Filtration Rate (eGFR) 60 mL/min/1.73 m2.
Figure S5: Association of BMI With Incident Low Bicarbonate Among Patients Without Hypertension.
Figure S6: Association of BMI With Serum Bicarbonate Levels Among Estimated Glomerular Filtration Rate (eGFR) Subgroups.
Table S1: Association of BMI With Incident Low Bicarbonate Among Estimated Glomerular Filtration Rate (eGFR) Subgroups.
Table S2: Effect of Low Bicarbonate Definition on Association of BMI With Incident Low Bicarbonate.
Supplementary Material
Figures S1-S6, Tables S1-S2.
References
- 1.Moranne O., Froissart M., Rossert J. Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol. 2009;20(1):164–171. doi: 10.1681/ASN.2008020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garibotto G., Russo R., Sofia A. Muscle protein turnover in chronic renal failure patients with metabolic acidosis or normal acid-base balance. Miner Electrolyte Metab. 1996;22(1-3):58–61. [PubMed] [Google Scholar]
- 3.Abramowitz M.K., Hostetter T.H., Melamed M.L. Association of serum bicarbonate levels with gait speed and quadriceps strength in older adults. Am J Kidney Dis. 2011;58(1):29–38. doi: 10.1053/j.ajkd.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yenchek R., Ix J.H., Rifkin D.E. Association of serum bicarbonate with incident functional limitation in older adults. Clin J Am Soc Nephrol. 2014;9(12):2111–2116. doi: 10.2215/CJN.05480614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraut J.A., Mishler D.R., Singer F.R., Goodman W.G. The effects of metabolic acidosis on bone formation and bone resorption in the rat. Kidney Int. 1986;30(5):694–700. doi: 10.1038/ki.1986.242. [DOI] [PubMed] [Google Scholar]
- 6.Raphael K.L., Wei G., Baird B.C., Greene T., Beddhu S. Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int. 2011;79(3):356–362. doi: 10.1038/ki.2010.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovesdy C.P., Anderson J.E., Kalantar-Zadeh K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant. 2009;24(4):1232–1237. doi: 10.1093/ndt/gfn633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raphael K.L., Murphy R.A., Shlipak M.G. Bicarbonate concentration, acid-base status, and mortality in the Health, Aging, and Body Composition Study. Clin J Am Soc Nephrol. 2016;11(2):308–316. doi: 10.2215/CJN.06200615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wesson D.E., Mathur V., Tangri N. Long-term safety and efficacy of veverimer in patients with metabolic acidosis in chronic kidney disease: a multicentre, randomised, blinded, placebo-controlled, 40-week extension. Lancet. 2019;394(10196):396–406. doi: 10.1016/S0140-6736(19)31388-1. [DOI] [PubMed] [Google Scholar]
- 10.Mahajan A., Simoni J., Sheather S.J., Broglio K.R., Rajab M.H., Wesson D.E. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010;78(3):303–309. doi: 10.1038/ki.2010.129. [DOI] [PubMed] [Google Scholar]
- 11.Di Iorio B.R., Bellasi A., Raphael K.L. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J Nephrol. 2019;32(6):989–1001. doi: 10.1007/s40620-019-00656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Brito-Ashurst I., Varagunam M., Raftery M.J., Yaqoob M.M. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20(9):2075–2084. doi: 10.1681/ASN.2008111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Driver T.H., Shlipak M.G., Katz R. Low serum bicarbonate and kidney function decline: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2014;64(4):534–541. doi: 10.1053/j.ajkd.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abramowitz M.K., Hostetter T.H., Melamed M.L. Lower serum bicarbonate and a higher anion gap are associated with lower cardiorespiratory fitness in young adults. Kidney Int. 2012;81(10):1033–1042. doi: 10.1038/ki.2011.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandel E.I., Curhan G.C., Hu F.B., Taylor E.N. Plasma bicarbonate and risk of type 2 diabetes mellitus. CMAJ. 2012;184(13):E719–E725. doi: 10.1503/cmaj.120438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W., Melamed M.L., Abramowitz M.K. Serum bicarbonate and bone mineral density in US adults. Am J Kidney Dis. 2015;65(2):240–248. doi: 10.1053/j.ajkd.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goraya N., Simoni J., Jo C.H., Wesson D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014;86(5):1031–1038. doi: 10.1038/ki.2014.83. [DOI] [PubMed] [Google Scholar]
- 18.Goraya N., Simoni J., Jo C., Wesson D.E. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. 2012;81(1):86–93. doi: 10.1038/ki.2011.313. [DOI] [PubMed] [Google Scholar]
- 19.Frassetto L., Morris R.C., Jr., Sebastian A. Potassium bicarbonate reduces urinary nitrogen excretion in postmenopausal women. J Clin Endocrinol Metab. 1997;82(1):254–259. doi: 10.1210/jcem.82.1.3663. [DOI] [PubMed] [Google Scholar]
- 20.Sebastian A., Harris S.T., Ottaway J.H., Todd K.M., Morris R.C., Jr. Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. N Engl J Med. 1994;330(25):1776–1781. doi: 10.1056/NEJM199406233302502. [DOI] [PubMed] [Google Scholar]
- 21.Jehle S., Hulter H.N., Krapf R. Effect of potassium citrate on bone density, microarchitecture, and fracture risk in healthy older adults without osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2013;98(1):207–217. doi: 10.1210/jc.2012-3099. [DOI] [PubMed] [Google Scholar]
- 22.Raphael K.L., Zhang Y., Ying J., Greene T. Prevalence of and risk factors for reduced serum bicarbonate in chronic kidney disease. Nephrology (Carlton) 2014;19(10):648–654. doi: 10.1111/nep.12315. [DOI] [PubMed] [Google Scholar]
- 23.Taylor E.N., Curhan G.C. Body size and 24-hour urine composition. Am J Kidney Dis. 2006;48(6):905–915. doi: 10.1053/j.ajkd.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Bobulescu I.A., Park S.K., Xu L.H.R. Net acid excretion and urinary organic anions in idiopathic uric acid nephrolithiasis. Clin J Am Soc Nephrol. 2019;14(3):411–420. doi: 10.2215/CJN.10420818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H., Kuja-Halkola R., Chen X., Magnusson P.K.E., Svensson P., Carrero J.J. Higher body mass index is associated with incident diabetes and chronic kidney disease independent of genetic confounding. Kidney Int. 2019;95(5):1225–1233. doi: 10.1016/j.kint.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Thomas G., Sehgal A.R., Kashyap S.R., Srinivas T.R., Kirwan J.P., Navaneethan S.D. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2011;6(10):2364–2373. doi: 10.2215/CJN.02180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Must A., Spadano J., Coakley E.H., Field A.E., Colditz G., Dietz W.H. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 28.United States Census Bureau. 2013-2017 ACS 5-year estimates. Vol 2018. Accessed December 12, 2019. https://www.census.gov/topics/income-poverty/income/data/tables/acs.html
- 29.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reaven G.M., Hollenbeck C., Jeng C.Y., Wu M.S., Chen Y.D. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37(8):1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 31.Golden S.H., Brown A., Cauley J.A. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors--an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2012;97(9):E1579–E1639. doi: 10.1210/jc.2012-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabrielsen A.M., Lund M.B., Kongerud J., Viken K.E., Roislien J., Hjelmesaeth J. The relationship between anthropometric measures, blood gases, and lung function in morbidly obese white subjects. Obes Surg. 2011;21(4):485–491. doi: 10.1007/s11695-010-0306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maalouf N.M., Sakhaee K., Parks J.H., Coe F.L., Adams-Huet B., Pak C.Y. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65(4):1422–1425. doi: 10.1111/j.1523-1755.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 34.Abate N., Chandalia M., Cabo-Chan A.V., Jr., Moe O.W., Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65(2):386–392. doi: 10.1111/j.1523-1755.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 35.Maalouf N.M., Cameron M.A., Moe O.W., Adams-Huet B., Sakhaee K. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2(5):883–888. doi: 10.2215/CJN.00670207. [DOI] [PubMed] [Google Scholar]
- 36.Wesson D.E., Simoni J., Broglio K., Sheather S. Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am J Physiol Renal Physiol. 2011;300(4):F830–F837. doi: 10.1152/ajprenal.00587.2010. [DOI] [PubMed] [Google Scholar]
- 37.Mokhlesi B., Tulaimat A., Faibussowitsch I., Wang Y., Evans A.T. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath. 2007;11(2):117–124. doi: 10.1007/s11325-006-0092-8. [DOI] [PubMed] [Google Scholar]
- 38.Macavei V.M., Spurling K.J., Loft J., Makker H.K. Diagnostic predictors of obesity-hypoventilation syndrome in patients suspected of having sleep disordered breathing. J Clin Sleep Med. 2013;9(9):879–884. doi: 10.5664/jcsm.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor E.N., Curhan G.C. Differences in 24-hour urine composition between black and white women. J Am Soc Nephrol. 2007;18(2):654–659. doi: 10.1681/ASN.2006080854. [DOI] [PubMed] [Google Scholar]
- 40.Hatori N., Gardner J.P., Tomonari H., Fine B.P., Aviv A. Na(+)-H+ antiport activity in skin fibroblasts from blacks and whites. Hypertension. 1990;15(2):140–145. doi: 10.1161/01.hyp.15.2.140. [DOI] [PubMed] [Google Scholar]
- 41.Allister-Price C., Craig C.M., Spielman D., Cushman S.S., McLaughlin T.L. Metabolic markers, regional adiposity, and adipose cell size: relationship to insulin resistance in African-American as compared with Caucasian women. Int J Obes (Lond) 2019;43(6):1164–1173. doi: 10.1038/s41366-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rune S.J. Comparison of the rates of gastric acid secretion in man after ingestion of food and after maximal stimulation with histamine. Gut. 1966;7(4):344–350. doi: 10.1136/gut.7.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laski M.E. Penny wise and bicarbonate foolish. Am J Kidney Dis. 2000;35(6):1224–1225. doi: 10.1016/s0272-6386(00)70063-1. [DOI] [PubMed] [Google Scholar]
- 44.Amodu A., Abramowitz M.K. Dietary acid, age, and serum bicarbonate levels among adults in the United States. Clin J Am Soc Nephrol. 2013;8(12):2034–2042. doi: 10.2215/CJN.03600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goraya N., Munoz-Maldonado Y., Simoni J., Wesson D.E. Fruit and vegetable treatment of chronic kidney disease-related metabolic acidosis reduces cardiovascular risk better than sodium bicarbonate. Am J Nephrol. 2019;49(6):438–448. doi: 10.1159/000500042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1-S6, Tables S1-S2.