To the Editor:
Disease manifestations of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis are diverse and prediction of disease course remains challenging. This includes its kidney involvement, with wide variability in clinical and histologic presentation. Up to 64% to 85% of patients with ANCA-associated vasculitis develop a crescentic glomerulonephritis (GN) with a histologic spectrum from early fibrinoid necrosis to global sclerosis.1,2 Significant strides have been made in the diagnosis and treatment of ANCA-associated vasculitis. However, a significant proportion of patients progress to end-stage kidney disease (ESKD) and often experience treatment-related adverse effects, including infection. Disease prognostication in ANCA-associated vasculitis remains elusive and previous attempts of histopathologic classifications are not devoid of limitations.3
Although kidney biopsy has diagnostic and prognostic value in ANCA-associated GN, the predictive value is enhanced when histologic parameters are combined with clinical parameters. The recently developed ANCA renal risk score (ARRS) incorporates not only histologic features but also estimated glomerular filtration rate (eGFR) at the time of diagnosis to predict kidney survival.2 The ARRS was validated first in a cohort of 115 patients in Germany and then with similar results in England and Turkey.4 This risk score is now applied in a cohort of patients with ANCA-associated vasculitis for the first time in the United States to ascertain its validity.
Patients with biopsy-proven ANCA-associated GN and categorized according to the Chapel Hill consensus nomenclature were included in this single-center retrospective study, after approval by the institutional review board (IRB00090103) and obtaining informed consent from patients. Information on demographics and clinical variables were extracted from review of electronic patient records. Kidney biopsies were reviewed to calculate ARRS. Three parameters were used in the risk prediction score: (1) percentage of normal glomeruli (N0, >25%; N1, 10%-25%; N2, <10%), (2) tubular atrophy and interstitial fibrosis (T0, <25%; T1, >25%), and (3) eGFR at the time of diagnosis (G0, >15; G1, <15). A weighted assignment of points to each parameter was as follows: N1 [4], N2 [6], T1 [2], and G1 [3], and the resulting aggregate risk score used to classify predicted ESKD risk was low (0), intermediate (2-7), or high (8-11 points). Kidney survival was defined as time from diagnosis to the development of ESKD, defined as the need for kidney replacement therapy.
In a cohort of 119 patients, median age was 63 (interquartile range [IQR], 46-69) years, 53% were women, and median eGFR at diagnosis was 22.5 (IQR, 12-34) mL/min/1.73 m2. Sixty-four patients were positive for myeloperoxidase, 47 were proteinase-3 positive, and 8 patients were ANCA negative. Clinical characteristics and histologic findings are shown in Table 1.
Table 1.
Patient Clinical Characteristics and Histologic Findings
| Characteristics and Histologic Features | Overall (119) | Low-Risk Group (34) | Medium-Risk Group (59) | High-Risk Group (26) | P |
|---|---|---|---|---|---|
| Age, y | 63 (46-69) | 63 (44-67) | 65 (57-73) | 59 (51-71) | 0.16a |
| Female sex | 63 (53%) | 18 (53%) | 32 (54%) | 13 (50%) | 0.94b |
| eGFR at diagnosis, mL/min/1.73 m2 | 22.5 (12-34) | 43 (29-61) | 21 (11-29) | 14 (10-23) | <0.001a |
| ANCA antibody type | 0.62b | ||||
| MPO, n (%) | 64 (54%) | 18 (53%) | 29 (49%) | 17 (65%) | |
| PR3, n (%) | 47 (40%) | 16 (47%) | 26 (44%) | 5 (19%) | |
| Negative | 8 (6%) | 0 (0%) | 4 (7%) | 4 (15%) | |
| Normal glomeruli (N) | 27 (10-56.5) | 60 (50-71) | 28 (20-50) | 6 (0-10) | <0.001a |
| IFTA > 25% | 47 (40%) | 0 (0%) | 28 (47%) | 19 (73%) | <0.001b |
| Risk score | 3 (0-7) | 0 (0-0) | 3 (2-5) | 9 (8-9) | <0.001a |
Note: Values expressed as median (interquartile range) or number (percent).
Abbreviations: ANCA, antineutrophil cytoplasmic antibody; eGFR, estimated glomerular filtration rate by the Modification of Diet in Renal Disease Study equation; IFTA, interstitial fibrosis and tubular atrophy; MPO, myeloperoxidase; PR3, proteinase 3.
Analysis of variance test
χ2.
With regard to risk stratification, 34 patients were in the low-risk category; 59, in the medium-risk category; and 26, in the high-risk category. Median percentage of normal glomeruli was 27% (IQR, 10%-56.5%). A total of 47 patients (40%) had a degree of interstitial fibrosis and tubular atrophy > 25%.
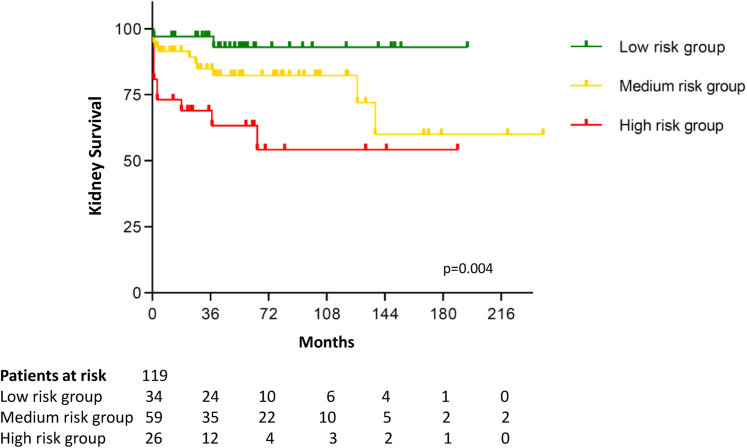
During a median follow-up of 58 (IQR, 28-97) months, 23 patients (19.3%) progressed to ESKD. In the low-risk category (n = 34), 2 patients developed ESKD (5.9%). Of 59 patients in the medium-risk group, 11 transitioned to ESKD (18.6%). With regard to the high-risk group, 10 of 26 patients developed ESKD (38.5%). The Kaplan-Meier survival curve demonstrates the kidney survival of the respective risk groups (Fig 1; P = 0.004). Renal survival was 97.1%, 91.5%, and 73.1% according to the risk groups low, medium, and high after 1 year of follow-up (Table 2; P = 0.008). After 36 months' follow-up, 94.1% in the low-risk group, 86.4% in the medium-risk group, and 69.2% in the high-risk group remained dialysis independent (P = 0.009). After a follow-up of 60 months, kidney survival was 94.1%, 84.7%, and 65.4% according to the risk groups (P = 0.01).
Figure 1.
Kaplan-Meier curve shows kidney survival in the low-, medium-, and high-risk groups (P=0.004).
Table 2.
Development of ESKD at 12 and 36 Months' Follow-up
| Risk Group | Low Risk (n = 34) | Medium Risk (n = 59) | High Risk (n = 26) |
|---|---|---|---|
| ESKD at 12 mo | 1 (2.9%) | 5 (8.5%) | 7 (26.9%) |
| ESKD at 36 mo | 2 (5.9%) | 8 (13.6%) | 8 (30.8%) |
| ESKD at 60 mo | 2 (5.9%) | 9 (15.3%) | 9 (34.6%) |
Abbreviation: ESKD, end-stage kidney disease.
The evolution of treatment regimens in ANCA-associated vasculitis has improved outcomes, but treatment-related adverse effects remain a significant problem.5,6 In addition, the great variability in disease course and relapses add more uncertainty to outcome prediction. Prediction scores could aid tailoring therapy, assisting clinicians on decisions of treatment intensity and duration, thereby providing effective treatment and preventing its untoward effects.
A prognostication using the histologic classification was initially validated in a cohort of patients enrolled in European vasculitis trials. Subsequent studies and meta-analyses demonstrated no difference in outcome between crescentic and mixed classes.3,7,8 The ARRS, combining eGFR and histopathologic features, has been able to predict the development of ESKD in patients with ANCA-associated vasculitis.2 Histologic features include percentage of normal glomeruli and degree of tubular atrophy and interstitial fibrosis on the kidney biopsy. This has been revalidated with similar results in UK and Turkish cohorts.4 Furthermore, the ARRS has been validated in patients with advanced kidney damage in Mexico.9
Here, we applied the ARRS for the first time in the United States. The score was reliably able to predict the development of ESKD. The limitations of the study are its retrospective design, and an interobserver reliability was not used. Further analyses revalidating cutoffs and risk score points would potentially enable refinement of the score, improving its prediction accuracyeven further.
Article Information
Authors’ Contributions
Research idea and study design: SK, SB, DG; data acquisition: FC, PF, AR; data analysis/interpretation: SK, SB; statistical analysis: SB; supervision or mentorship: DG. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work is appropriately investigated and resolved.
Support
None.
Financial Disclosure
Dr Geetha reports receiving consultant fees from ChemoCentryx and Aurinia. The remaining authors declare that they have no relevant financial interests.
Peer Review
Received November 30, 2020. Evaluated by 1 external peer reviewer, with direct editorial input by the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form April 4, 2021.
References
- 1.Casal Moura M., Irazabal M.V., Eirin A. Efficacy of rituximab and plasma exchange in antineutrophil cytoplasmic antibody–associated vasculitis with severe renal disease. J Am Soc Nephrol. 2020;31(11):2688–2704. doi: 10.1681/ASN.2019111197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brix S.R., Noriega M., Tennstedt P. Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney Int. 2018;94(6):1177–1188. doi: 10.1016/j.kint.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y., Xu J., Pan X. Histopathological classification and renal outcome in patients with antineutrophil cytoplasmic antibodies-associated renal vasculitis: A study of 186 patients and metaanalysis. J Rheumatol. 2017;44(3):304–313. doi: 10.3899/jrheum.160866. [DOI] [PubMed] [Google Scholar]
- 4.Li A.S., Saleh C., Denley H., Patel M., Brix S.R. ANCA renal risk score predicts outcome in the Manchester cohort. Kidney Int. 2019;96(1):246–247. doi: 10.1016/j.kint.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Little M.A., Nightingale P., Verburgh C.A. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis. 2010;69(6):1036–1043. doi: 10.1136/ard.2009.109389. [DOI] [PubMed] [Google Scholar]
- 6.Robson J., Doll H., Suppiah R. Damage in the ANCA-associated vasculitides: long-term data from the European Vasculitis Study Group (EUVAS) therapeutic trials. Ann Rheum Dis. 2015;74(1):177–184. doi: 10.1136/annrheumdis-2013-203927. [DOI] [PubMed] [Google Scholar]
- 7.Tanna A., Guarino L., Tam F.W.K. Long-term outcome of anti-neutrophil cytoplasm antibody-associated glomerulonephritis: evaluation of the international histological classification and other prognostic factors. Nephrol Dial Transplant. 2015;30(7):1185–1192. doi: 10.1093/ndt/gfu237. [DOI] [PubMed] [Google Scholar]
- 8.Predictors of renal survival in ANCA-associated vasculitis Validation of a histopathological classification schema and review of the literature. Clin Exp Rheumatol. 2015;33(2 Suppl 89):S-56–S-63. [PubMed] [Google Scholar]
- 9.Mejía-Vilet J.M., Martín-Nares E., Cano-Verduzco M.L., Pérez-Arias A.A., Sedano-Montoya M.A., Hinojosa-Azaola A. Validation of a renal risk score in a cohort of ANCA-associated vasculitis patients with severe kidney damage. Clin Rheumatol. 2020;39(6):1935–1943. doi: 10.1007/s10067-020-04936-5. [DOI] [PubMed] [Google Scholar]