Abstract
Rationale & Objective
Patient awareness of disease is the first step toward effective management and disease control. Awareness of chronic kidney disease (CKD) has consistently been shown to be low, but studies estimating patient awareness of CKD have used different methods. We sought to determine whether the estimated prevalence of CKD awareness differed by the wording used to ascertain awareness or by setting characteristics.
Study Design
Systematic review and meta-analysis.
Setting & Study Populations
Adults with CKD not receiving dialysis.
Selection Criteria for Studies
We included studies that estimated CKD awareness, determined CKD status by laboratory criteria, and provided the exact question wording used to ascertain awareness.
Data Extraction
2 reviewers independently extracted data for each study; discordance was resolved by a third independent reviewer.
Analytical Approach
Mixed-effects models were used to calculate pooled CKD awareness estimates and 95% CIs.
Results
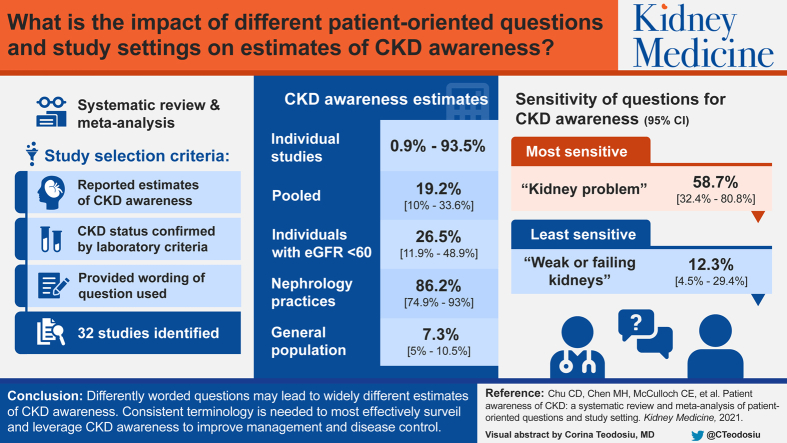
32 studies were included. Publication year ranged from 2004 to 2017, with study populations ranging from 107 to 28,923 individuals. CKD awareness in individual studies ranged from 0.9% to 94.0%. Pooled CKD awareness was 19.2% (95% CI, 10.0%-33.6%) overall and was 26.5% (95% CI, 11.9%-48.9%) among individuals with an estimated glomerular filtration rate < 60 mL/min/1.73 m2. “Kidney problem” was the most sensitive question for CKD awareness (58.7%; 95% CI, 32.4%-80.8%); “weak or failing kidneys” was the least sensitive (12.3%; 95% CI, 4.5%-29.4%). CKD awareness was highest among patients from nephrology practices (86.2%; 95% CI, 74.9%-93.0%) and lowest in the general population (7.3%; 95% CI, 5.0%-10.5%).
Limitations
Significant heterogeneity across studies overall and among examined subgroups of wording and study setting.
Conclusions
Differently worded questions may lead to widely different estimates of CKD awareness. Consistent terminology is likely needed to most effectively surveil and leverage CKD awareness to improve management and disease control.
Index Words: Chronic kidney disease, CKD awareness, knowledge, review, meta-analysis
Graphical abstract
Plain-Language Summary.
Most individuals with chronic kidney disease (CKD) are unaware of having it, but studies of CKD awareness have used variable methodologies. To examine the current state of CKD awareness and how question wording or study setting may affect ascertainment of awareness, we systematically reviewed 32 studies of CKD awareness. Studies were conducted across hospital, primary care, nephrology clinic, and general population settings, and they used varying terminology to ascertain awareness of CKD, including “weak or failing kidneys,” “kidney problem,” and “kidney disease.” We found significant heterogeneity across studies, even after accounting for setting and wording. Apart from studies based in nephrology clinics, most studies reported low (<50%) CKD awareness, underscoring a need for greater public awareness efforts.
Chronic kidney disease (CKD) affects more than 30 million adults in the United States,1 yet most population-based studies indicate that a minority are aware of having kidney disease. Despite broad public health efforts,2, 3, 4, 5 low patient awareness of CKD has persisted over nearly 2 decades without substantive improvement.6 Underscoring the importance of CKD awareness, the National Kidney Foundation, American Society of Nephrology, and US Department of Health and Human Services jointly announced a Public Awareness Initiative in 2019 as part of the Advancing American Kidney Health executive order.7
To assess the impact of this initiative and facilitate reliable national and local surveillance of CKD awareness, determining a baseline on which to gauge improvement and an understanding of how different methods can affect estimates of CKD awareness are necessary. Prior studies of CKD awareness have used differently worded questions to describe kidney disease when ascertaining awareness, including “chronic kidney disease,” “weak or failing kidneys,” and “kidney problem,” among others. These differences in wording may contribute to variable estimates of CKD awareness; few studies have simultaneously tested more than 1 question. A single-center study in an urban safety net primary care setting showed that estimates of CKD awareness can vary based on the wording of the question asked, with “kidney problem” associated with the highest level of awareness (thus being the most sensitive) and “kidney damage” being the least sensitive.8 Another study comparing question wordings for ascertaining awareness in a community-based population likewise found “kidney problem” to be most sensitive.9
To examine the current state of CKD awareness and how question wording or patient setting may affect ascertainment of awareness, we conducted a systematic review and meta-analysis.
Methods
Search Strategy
We searched PubMed, EMBASE, Web of Science, PsycINFO, and Sociological Extracts to identify research reporting patient awareness of CKD status. Search terms were developed in collaboration with an academic research librarian to optimize search inclusivity. Details of the search strategy are provided in Item S1. The search strategy did not include searching reference lists of included studies. All databases were searched on June 24, 2019.
Study Selection
Studies were included if they: (1) were peer-reviewed original research, (2) were published in English, (3) were published in 2000 or later, (4) had a study population of at least 100 participants, (5) provided the exact wording of the question used to ascertain CKD awareness, and (6) defined CKD using laboratory-based criteria (estimated glomerular filtration rate [eGFR] at a minimum). Conference abstracts, narrative reviews, editorials, and commentaries were excluded. Given the focus on adult awareness of CKD, we excluded studies of pediatric populations and studies limited exclusively to individuals with end-stage kidney disease.
Based on these criteria, each study abstract was screened for eligibility by 2 of 3 independent reviewers (CDC, MHC, and DST) using a standardized data abstraction form. In cases of discordance regarding a study’s eligibility between 2 reviewers, eligibility was determined by the third reviewer. If no exclusion criteria were apparent from an abstract, it was included for manuscript review.
Data Extraction
Full-text manuscript reviews and data extraction were performed by 2 of 3 independent reviewers each (from CDC, MHC, and DST) using a standardized data extraction form, with discrepancies reconciled by the third reviewer. Studies that did not meet eligibility criteria based on review of the manuscript were excluded at this stage. Of the remaining articles, data extraction included the study setting (general population, hospital, primary care, or nephrology clinic), years of data collection, criteria to define CKD, question(s) used to define CKD awareness, estimate of CKD awareness prevalence, size of the study population, and characteristics associated with greater CKD awareness. If a study reported CKD awareness within subgroups of CKD stage, the corresponding prevalence estimates and subgroup sizes were also recorded. If a study compared multiple questions for ascertaining CKD awareness, awareness estimates corresponding to each different question were recorded. For each study, we assessed the risk of bias using the Joanna Briggs Institute (JBI) critical appraisal checklist for descriptive cross-sectional studies reporting prevalence data.10 The JBI checklist assesses studies across 9 domains, including considerations for appropriate sampling methods, identification of the condition under study, and statistical analysis.
Statistical Analysis
Point estimates for CKD awareness were obtained from each study, and 95% CIs were calculated for each prevalence estimate. Because multiple studies examined the same study population, we retained only the largest study in cases of overlapping study populations for our primary analysis. To account for the fact that some studies reported more than 1 estimate of CKD awareness (using differently worded questions), we used mixed effects model to estimate the pooled prevalence of CKD awareness, accounting for the individual study as a random effect.11 Between-study heterogeneity was assessed using Cochran Q test for heterogeneity and I2 statistics. Prespecified subgroup analyses were performed to explore heterogeneity explained by subgroup effects and obtain pooled estimates (subgroup summary effects) and 95% CIs of CKD awareness by question wording and by study setting (general population, hospital, primary care, and nephrology clinic). For analyses of question wording, we excluded studies of populations in non–English-speaking countries and wordings used in fewer than 2 studies. Not all studies used the same definition of CKD; thus, we conducted a sensitivity analysis including only CKD awareness estimates among individuals with eGFRs < 60 mL/min/1.73 m2. All analyses were performed using Stata/MP, version 16.1 (StataCorp), and R, version 3.6.1 (R Foundation for Statistical Computing). We followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement guidelines for the conduct and reporting of this systematic review and meta-analysis.12 Review methods were established before conduct of the literature search with no deviations from the protocol apart from the addition of more robust procedures for assessing risk of bias and between-study heterogeneity.
Results
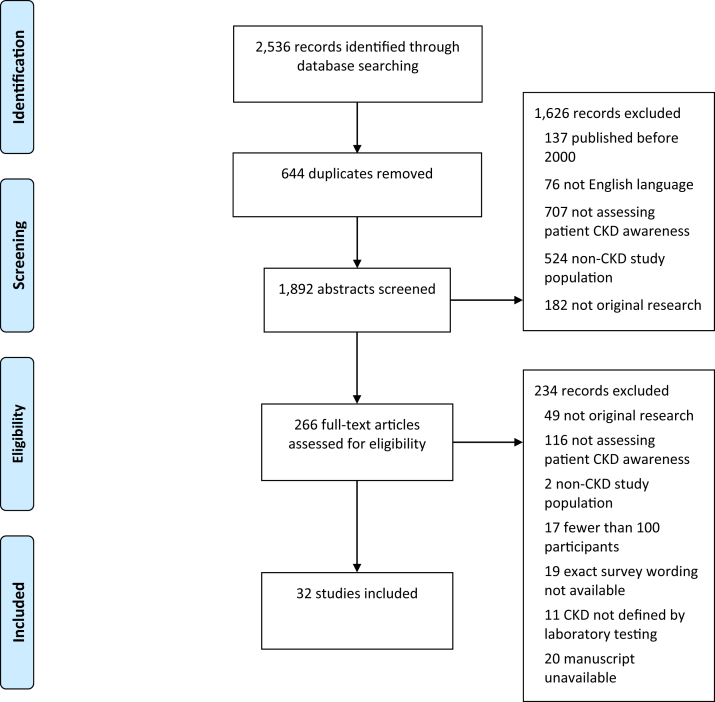
The search yielded 1,892 articles after duplicates were removed (Fig 1). Based on screening of abstracts, 266 studies were retained for full-text manuscript review. Following manuscript review, 32 articles were identified that satisfied inclusion criteria for data abstraction.8,13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 The full data abstraction table is available in Table S1.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram. Abbreviation: CKD, chronic kidney disease.
Populations Studied
Overall, studies examined 16 distinct source populations; several data sources provided the study population for multiple studies. Nine studies used the National Health and Nutrition Examination Survey (NHANES), a continuously conducted, nationally representative, cross-sectional survey of noninstitutionalized US citizens.14,21,23,24,32, 33, 34,41,42 Years of NHANES examined in these studies ranged from 1999 to 2012. Six studies analyzed data collected by the Kidney Early Evaluation Program (KEEP) study, a national health screening program targeted toward adults with hypertension, diabetes, or a family history of kidney disease.30,31,35,38,39,43 Four articles reported from the same study cohort based within 1 academic nephrology practice.17, 18, 19,29
Most studies (n = 21) were surveys of the general population.14,21,23,24,26, 27, 28,30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 Four studies were based in primary care clinics,8,13,16,25 and 5 were based in nephrology clinics.17, 18, 19, 20,29 Two studies examined CKD awareness among hospitalized patients.15,22
Questions Used to Ascertain CKD Awareness
Among the 32 articles, multiple different question wordings were used to ascertain CKD awareness. The most common question used for ascertaining CKD awareness was, “Have you ever been told by a health care professional that you have kidney disease?” This question was used by 6 studies of the KEEP cohort and 7 smaller studies. The second most common question was, “Has a doctor, nurse, or other health professional ever told you have weak or failing kidneys? Do not include kidney stones, bladder infection, or incontinence.” This question was used by the 9 studies analyzing NHANES and by 2 additional studies.8,40 Other question wordings included “kidney problem,” “kidney damage,” “chronic kidney disease,” “impaired kidney function,” and “protein in the urine.”
Prevalence of CKD Awareness
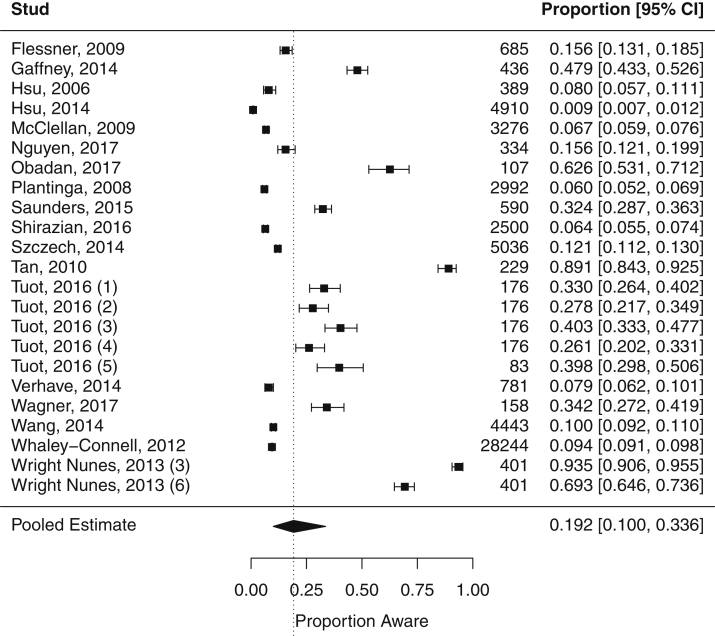
We restricted meta-analyses to studies examining nonoverlapping study populations, yielding a total of 18 studies (23 total estimates of CKD awareness because some studies reported >1 estimate). In the overall meta-analysis (Fig 2), the pooled summary estimate for CKD awareness was 19.2% (95% CI, 10.0%-33.6%). However, there was significant heterogeneity among the estimates of CKD awareness between studies (Cochran Q P < 0.001; I2 = 99.8%). Reported estimates of CKD awareness among all stages of disease severity ranged from 0.9% to 93.5%. The highest estimates (69.3%-93.5%) were from a study of prevalent outpatients in a nephrology practice19; the highest estimate from a non-nephrology practice–based study was 62.6% in a study of primary care patients with diabetes.13 The lowest estimate of CKD awareness (0.9%) was from a study of a community-based health screening survey in rural Taiwan.28
Figure 2.
Meta-analysis of chronic kidney disease awareness prevalence. An individual study may be represented by more than 1 marker if it reported multiple estimates of awareness using different survey questions.
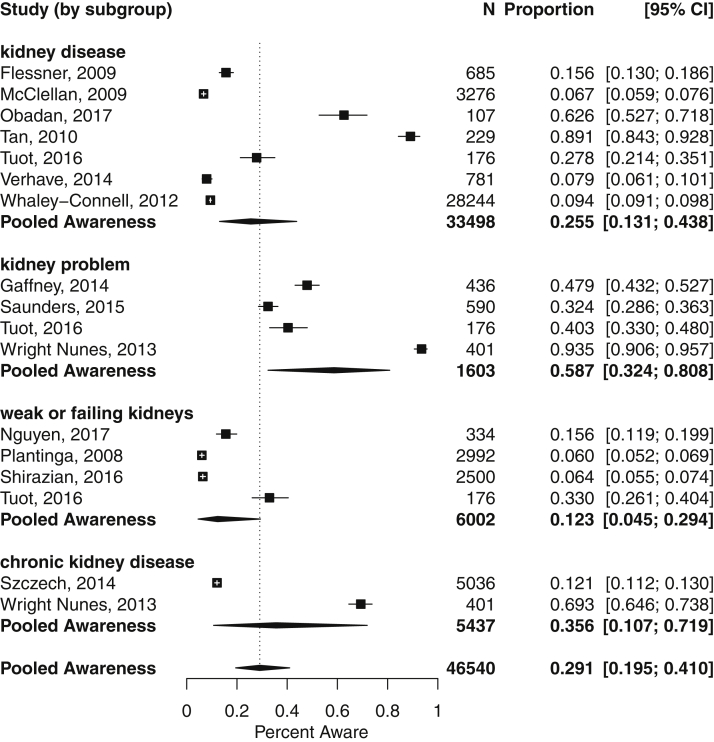
In a subgroup analysis by question wording (Fig 3), a total of 17 CKD awareness estimates were included using 4 different English question wordings: “kidney disease,” “weak or failing kidneys,” “kidney problem,” and “chronic kidney disease.” “Kidney problem” yielded the highest pooled estimate for CKD awareness (58.7%; 95% CI, 32.4%-80.8%), whereas “weak or failing kidneys” yielded the lowest (12.3%; 95% CI, 4.5%-29.4%). Although differences in the summary estimates by wording subgroup were significant (P = 0.03), there remained substantial residual heterogeneity not explained by wording (P < 0.001; I2 = 99.2%).
Figure 3.
Meta-analysis of chronic kidney disease awareness prevalence, grouped by terminology used to ascertain awareness. Heterogeneity by wording subgroup: “kidney disease” (P < 0.001; I2 = 99.4%), “weak or failing kidneys” (P < 0.001; I2 = 99.5%), “kidney problem” (P < 0.001; I2 = 99.2%), and “chronic kidney disease” (P < 0.001; I2 = 99.5%). An individual study may be represented by more than 1 marker if it reported multiple estimates of awareness using different survey questions.
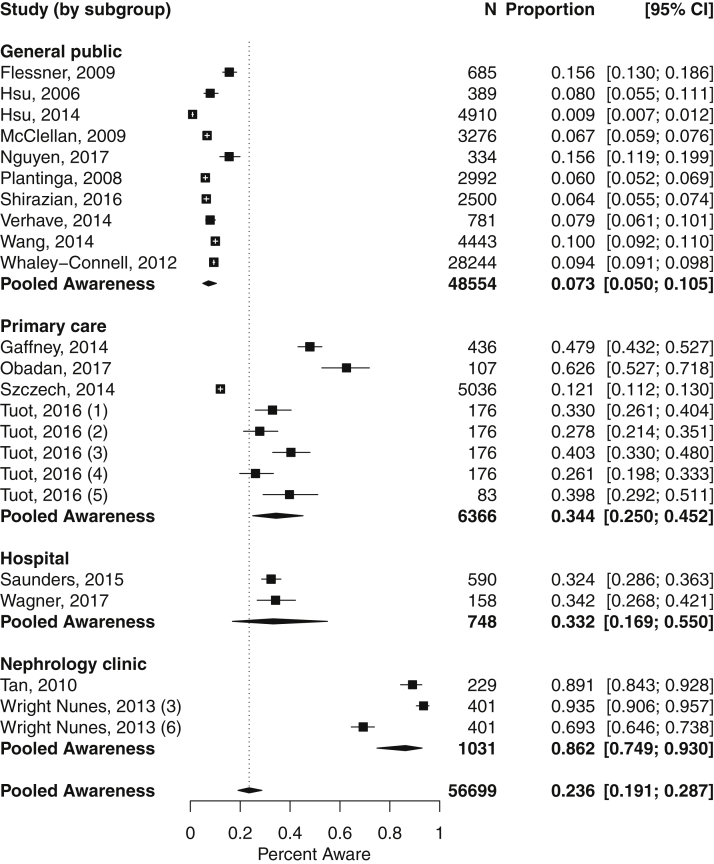
When analyzed by study setting (Fig 4), pooled CKD awareness was highest in the nephrology clinic setting (86.2%; 95% CI, 74.9%-93.0%) and lowest in the general population (7.3%; 95% CI, 5.0%-10.5%). We found significant subgroup heterogeneity by study setting (P < 0.001), though again, significant heterogeneity remained overall (P < 0.001; I2 = 98.2%).
Figure 4.
Meta-analysis of chronic kidney disease awareness prevalence, grouped by study setting. Heterogeneity by setting subgroup: general public (P < 0.001; I2 = 98.9%), primary care (P < 0.001; I2 = 99.3%), hospital (P < 0.001; I2 = 99.3%), and nephrology clinic (P < 0.001; I2 = 98.9%). An individual study may be represented by more than 1 marker if it reported multiple estimates of awareness using different survey questions (numbered [1] weak or failing kidneys; [2] kidney disease; [3] kidney problem; [4] kidney damage; [5] protein in the urine; [6] chronic kidney disease).
In sensitivity analyses restricting to CKD defined by eGFR < 60 mL/min/1.73 m2, there were 18 total estimates of CKD awareness. Pooling estimates from these studies (Fig S1) yielded a prevalence of CKD awareness of 26.5% (95% CI, 11.9%-48.9%). There was also significant heterogeneity among these studies (P < 0.001; I2 = 99.6%). Similar to our primary results, subgrouping by question wording and study setting explained some of the overall heterogeneity, but there remained significant heterogeneity unexplained by subgroup effects (Figs S2 and S3). The comparative pooled prevalence of CKD awareness by wording and by setting were the same as for overall CKD, for which “kidney problem” and nephrology clinic were associated with the highest CKD awareness.
Characteristics Associated With CKD Awareness
Studies that examined characteristics associated with CKD awareness consistently found that increasing severity of CKD (whether by lower eGFR or higher albuminuria) was associated with greater awareness. In 1 NHANES study, awareness was 41.8% in CKD G4 compared with 7.8% in CKD G3,21 and in a KEEP study, awareness was 32.1% compared with 5.4%, respectively, in CKD G4 versus CKD G3.38 Comorbid conditions, including hypertension, diabetes, and cardiovascular disease, were also associated with greater CKD awareness across multiple studies. The association between sex and CKD awareness was less consistent because some reported greater CKD awareness with female sex,16,42 whereas others reported that males were more likely to be aware of CKD.27,28,40,41 Three studies reported factors related to health care access that were associated with greater CKD awareness, including having had a health examination in the previous 2 years,26 having health insurance,13 and self-reported difficult access to care.43
Risk-of-Bias Assessment
We assessed the risk of bias of individual studies using the JBI critical appraisal checklist (Table S2). Most included studies (28 of 32) satisfied at least 7 of the 9 checklist domains, suggesting a low overall risk of bias. All studies had their target population appropriately framed, identified CKD using appropriate measures, and used appropriate statistical methods for reporting prevalence of CKD awareness. Across all studies, the most common potential sources of bias were unclear or bias-prone sampling methods (ie, convenience sampling), unclear representativeness of sample subgroups (termed “coverage” in the JBI checklist), and failure to report response rates or manage low response rates.
Discussion
In this systematic review of studies estimating CKD awareness, we identified 32 reports providing a total of 39 estimates of CKD awareness in a variety of study populations. Estimates of CKD awareness from these studies demonstrate low awareness (pooled CKD awareness, 19.2%), with most studies reporting <50% of individuals with CKD being aware of their condition.
Studies of CKD awareness often used the KDIGO (Kidney Disease: Improving Global Outcomes) definitions for CKD, which describe a heterogeneous population ranging from advanced CKD with high cardiovascular and kidney failure risk to very low-risk disease with preserved eGFR (>60 mL/min/1.73 m2) but elevated albuminuria.44 Individuals in the latter category comprise >40% of prevalent CKD, but low albuminuria testing rates may preclude both provider and patient awareness of CKD in this population.1,45 As a result, reports of CKD awareness are typically very low when such individuals are included in the denominator. However, limiting analyses only to CKD defined by eGFR < 60 mL/min/1.73 m2 yielded modestly higher awareness, from 19.2% to 26.5%. Even among studies using the most sensitive wording to describe kidney disease (“kidney problem”), only about two-thirds (66.2%) of individuals with eGFRs < 60 mL/min/1.73 m2 were found to be aware of CKD. Awareness has been shown to be low even in individuals with CKD at high risk for progression to end-stage kidney disease,6 and it is disappointing to see this finding reproduced across many studies in this systematic review.
We found a wide range of CKD awareness based on wording, from pooled estimates of 12.3% (“weak or failing kidneys”) to 58.7% (“kidney problem”). In studies that examined multiple questions simultaneously, greater awareness was found with “kidney problem” compared with “chronic kidney disease” in a nephrology clinic setting (93.5% vs 69.3%).19 “Kidney problem” similarly outperformed “weak or failing kidneys,” “kidney disease,” and “kidney damage” in primary care settings (40.1% vs 33.2%, 27.7%, and 26.4%, respectively).8 These data are consistent with results from a more recently published study of community-dwelling adults with CKD that demonstrated that “kidney problem” was the most sensitive individual descriptor of CKD.9 However, in that same study, a compound question asking about “weak kidneys, failing kidneys, or kidney disease” was far more sensitive than the individual descriptor, though still only 19.5% of adults with CKD were aware when asked using this compound question.9 In this review, 1 study evaluated the potential performance of compound questions, finding higher potential sensitivity when compared with individual descriptors: the best combination descriptor was estimated to be “kidney problem, protein in the urine, or kidney disease.”8 Although we did not have sufficient data to conduct a pooled analysis of compound descriptors to estimate CKD awareness, future studies should investigate the potential added utility of compound questions for assessing CKD awareness in surveillance efforts.
Of note, none of the studies based in English-speaking countries used the “renal disease” wording. The broader issue of nomenclature in kidney disease has received greater attention recently as the subject of an international consensus conference, which emphasized the importance of a consistent patient-centered nomenclature for facilitating awareness, education, and self-management for kidney disease.46 Accordingly, nomenclature guidelines from this conference, informed extensively by results from patient and caregiver focus groups,47 recommend avoiding using “renal” when “kidney” could be appropriately substituted.46 Beyond the recommendations about “renal,” a consistent nomenclature that is aligned across domains of clinical care, patient education, and public awareness campaigns could also improve national surveillance efforts, much of which has been based on studies using NHANES given its continuous and nationally representative nature.48 However, although the NHANES questionnaire asks participants to report whether they have been told of having “diabetes” or “hypertension, also called high blood pressure” (a compound descriptor), the questionnaire uses “weak or failing kidneys” to assess kidney disease—an imprecise descriptor that may not consistently correspond to how clinicians describe CKD to patients, how clinicians describe CKD to one another, or how kidney disease is described in patient education materials. This may contribute in part to the finding in NHANES that CKD awareness has consistently been substantially lower than awareness of hypertension or diabetes.6 With the Healthy People 2030 initiative, CKD awareness continues to be prioritized as a national public health objective by the US Department of Health and Human Services.49 CKD awareness has been further underscored as a goal in the Public Awareness Initiative (as part of the Advancing American Kidney Health executive order). Taken together, there is a pressing need for a consistent nomenclature for patients and clinicians that facilitates accurate measurement for surveillance efforts. Perhaps a transition to new phraseology might include both old and new questions allowing extrapolation of past to new phraseology.
The highest estimates of awareness were from studies in the nephrology clinic setting; the lowest awareness estimates were from surveys of the general population. This result is unsurprising because studies in health care settings—and particularly the nephrology clinic setting—are likely to select for a population with greater engagement in CKD care and more likely to be aware of CKD. Patient awareness of CKD in the health care setting is critical for engagement with clinicians about CKD education, which can facilitate healthy self-management behaviors to reduce progression or associated complications.50, 51, 52 In studies based in health care settings in which CKD is diagnosed, patient unawareness of CKD may represent a failure of effective education or communication between clinicians and patients. By contrast, survey-based studies (such as NHANES), which examine the overall population outside the health care setting, perform laboratory testing broadly for participants and thus do not rely on clinicians having diagnosed kidney disease in individual patients. Low estimates of CKD awareness in NHANES may thus reflect inadequacies in kidney disease detection, communication of the diagnosis to patients, or both. In this manner, studies of CKD awareness in the general population are important for surveillance and public health efforts. In both the health care or general public settings, using consistent and precise terminology for CKD diagnosis would likely be beneficial for effective communication, education, and surveillance.
This study had several limitations. There was significant heterogeneity overall, and although we explored sources of heterogeneity through subgroup analyses, there was significant heterogeneity remaining across all examined subgroups. As a result, overall and subgroup summary estimates should be interpreted with caution. Some wording variants were used by very few studies, precluding their inclusion in meta-analysis. There were insufficient studies to reliably examine differences in terminology within each study setting. Given the limited number of wordings used in certain study settings, the effects seen in pooled estimates for wording may reflect some effect of setting (and vice versa). The generalizability of this review may also be limited by our inclusion criteria requiring studies to have at least 100 participants to limit imprecise estimates from small studies. Requiring studies to be published in 2000 or later is intended to increase relevance to current trends and policies but could potentially exclude informative earlier studies. Because of our focus on how questions were asked, we required the exact wording of the question used to ascertain awareness to be provided by each study, recognizing that this may limit the number of studies eligible. Although we required all studies to define CKD using laboratory-based criteria (as opposed to less reliable methods such as chart review or diagnostic codes), the definition of CKD was variable across studies (ie, inclusion of proteinuria or albuminuria criteria), as was the severity of CKD in each study population.
Although many of the studies reviewed have likely informed public health and preventive care efforts for patients with CKD, the 2019 Advancing American Kidney Health initiative and the accompanying Public Awareness campaign have refocused and reinforced attention on CKD awareness as a priority in the cascade of delivering effective care to populations at risk for kidney disease. Going forward, reliable surveillance is contingent on identifying the most meaningful ways to ascertain CKD awareness, as well as developing ways to leverage awareness to advance patient engagement and health—a scenario unlikely to be realized if studies and surveillance of CKD awareness continue to rely on questions using insensitive wording and an incomplete understanding of the multiple determinants affecting awareness.
Article Information
Authors’ Full Names and Academic Degrees
Chi D. Chu, MD, MAS, Michael H. Chen, Charles E. McCulloch, PhD, Neil R. Powe, MD, MPH, MBA, Michelle M. Estrella, MD, MHS, Michael G. Shlipak, MD, MPH, and Delphine S. Tuot, MDCM, MAS.
Authors’ Contributions
Research idea and study design: CDC, DST; data acquisition: CDC, MHC; data analysis/interpretation: CDC, MHC, CEM, NRP, MME, MGS, DST; statistical analysis: CDC, CEM, DST; supervision or mentorship: NRP, DST. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s own contributions, and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
Dr Chu was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under the Ruth L. Kirschstein National Research Service Award (F32DK122629-01). The funders of this study had no role in the design of this study; collection, analysis, or interpretation of data; writing the report; or the decision to submit this report for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
We thank academic research librarian Evans Whitaker for assistance with developing literature database searches.
Peer Review
Received December 20, 2020, as a submission to the expedited consideration track with 2 external peer reviews. Direct editorial input from an Associate Editor and the Editor-in-Chief. Accepted in revised form March 21, 2021.
Footnotes
Complete author and article information provided before references.
Figure S1: Meta-analysis of awareness prevalence of chronic kidney disease defined by eGFR < 60 mL/min/1.73 m2
Figure S2: Meta-analysis of awareness prevalence of chronic kidney disease defined by eGFR < 60 mL/min/1.73 m2, by question wording
Figure S3: Meta-analysis of awareness prevalence of chronic kidney disease defined by eGFR < 60 mL/min/1.73 m2, by study setting
Item S1: Detailed search strategies
Table S1: Full data abstraction table
Table S2: Risk of bias assessment
Supplementary Material
Figure S1-S3, Item S1, Table S1, Table S2
References
- 1.US Renal Data Service . 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018. [Google Scholar]
- 2.Narva A.S., Briggs M. The National Kidney Disease Education Program: improving understanding, detection, and management of CKD. Am J Kidney Dis. 2009;53(3 suppl 3):S115–S120. doi: 10.1053/j.ajkd.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 3.McCullough P.A., Brown W.W., Gannon M.R. Sustainable community-based CKD screening methods employed by the National Kidney Foundation’s Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2011;57(3 suppl 2):S4–S8. doi: 10.1053/j.ajkd.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Chin H.J., Ahn J.M., Na K.Y. The effect of the World Kidney Day campaign on the awareness of chronic kidney disease and the status of risk factors for cardiovascular disease and renal progression. Nephrol Dial Transplant. 2010;25(2):413–419. doi: 10.1093/ndt/gfp512. [DOI] [PubMed] [Google Scholar]
- 5.Levey A.S., Andreoli S.P., DuBose T., Provenzano R., Collins A.J. Chronic kidney disease: common, harmful and treatable--World Kidney Day 2007. Am J Nephrol. 2007;27(1):108–112. doi: 10.1159/000099801. [DOI] [PubMed] [Google Scholar]
- 6.Chu C.D., McCulloch C.E., Banerjee T. CKD awareness among US adults by future risk of kidney failure. Am J Kidney Dis. 2020;76(2):174–183. doi: 10.1053/j.ajkd.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bieber S.D., Gadegbeku C.A. A call to action for the kidney community: nephrologists’ perspective on Advancing American Kidney Health. Clin J Am Soc Nephrol. 2019;14(12):1799–1801. doi: 10.2215/CJN.10470919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuot D.S., Zhu Y., Velasquez A. Variation in patients’ awareness of CKD according to how they are asked. Clin J Am Soc Nephrol. 2016;11(9):1566–1573. doi: 10.2215/CJN.00490116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuot D.S., Wong K.K., Velasquez A. CKD awareness in the general population: performance of CKD-specific questions. Kidney Med. 2019;1(2):43–50. doi: 10.1016/j.xkme.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munn Z., Moola S., Lisy K., Riitano D., Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. JBI Evidence Implementation. 2015;13(3):147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 11.Higgins J.P.T., Thompson S.G., Spiegelhalter D.J. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172(1):137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obadan N.O., Walker R.J., Egede L.E. Independent correlates of chronic kidney disease awareness among adults with type 2 diabetes. J Diabetes Complications. 2017;31(6):988–991. doi: 10.1016/j.jdiacomp.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen H., Anderson C., Miracle C., Rifkin D. The association between depression, perceived health status, and quality of life among individuals with chronic kidney disease: an analysis of the National Health and Nutrition Examination Survey 2011-2012. Nephron. 2017;136(2):127–135. doi: 10.1159/000455750. [DOI] [PubMed] [Google Scholar]
- 15.Saunders M., Kim S., Patel N., Meltzer D., Chin M. Hospitalized patients frequently unaware of their chronic kidney disease. J Hosp Med. 2015;10(9):619–622. doi: 10.1002/jhm.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaffney H., Blakeman T., Blickem C. Predictors of patient self-report of chronic kidney disease: baseline analysis of a randomised controlled trial. BMC Fam Pract. 2014;15:196. doi: 10.1186/s12875-014-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright Nunes J., Greene J.H., Wallston K. Pilot study of a physician-delivered education tool to increase patient knowledge about CKD. Am J Kidney Dis. 2013;62(1):23–32. doi: 10.1053/j.ajkd.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright Nunes J., Wallston K., Eden S., Shintani A., Ikizler T., Cavanaugh K. Associations among perceived and objective disease knowledge and satisfaction with physician communication in patients with chronic kidney disease. Kidney Int. 2011;80(12):1344–1351. doi: 10.1038/ki.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright J.A., Wallston K., Elasy T., Ikizler T., Cavanaugh K.A. Development and results of a kidney disease knowledge survey given to patients with CKD. Am J Kidney Dis. 2011;57(3):387–395. doi: 10.1053/j.ajkd.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan A., Hoffman B., Rosas S. Patient perception of risk factors associated with chronic kidney disease morbidity and mortality. Ethn Dis. 2010;20(2):106–110. [PubMed] [Google Scholar]
- 21.Plantinga L., Boulware L., Coresh J. Patient awareness of chronic kidney disease: Trends and predictors. Arch Intern Med. 2008;168(20):2268–2275. doi: 10.1001/archinte.168.20.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner M., Wanner C., Schich M. Patient’s and physician’s awareness of kidney disease in coronary heart disease patients - a cross-sectional analysis of the German subset of the EUROASPIRE IV survey. BMC Nephrol. 2017;18(1):321. doi: 10.1186/s12882-017-0730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dharmarajan S.H., Bragg-Gresham J.L., Morgenstern H. State-level awareness of chronic kidney disease in the U.S. Am J Prev Med. 2017;53(3):300–307. doi: 10.1016/j.amepre.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirazian S., Diep R., Jacobson A.M., Grant C.D., Mattana J., Calixte R. Awareness of chronic kidney disease and depressive symptoms: National Health and Nutrition Examination Surveys 2005-2010. Am J Nephrol. 2016;44(1):1–10. doi: 10.1159/000446929. [DOI] [PubMed] [Google Scholar]
- 25.Szczech L.A., Stewart R.C., Su H.-L. Primary care detection of chronic kidney disease in adults with type-2 diabetes: the ADD-CKD Study (awareness, detection and drug therapy in type 2 diabetes and chronic kidney disease) PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0110535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang F., Zhang L., Wang H., China National Survey of CKD Working Group Awareness of CKD in China: a national cross-sectional survey. Am J Kidney Dis. 2014;63(6):1068–1070. doi: 10.1053/j.ajkd.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Verhave J.C., Troyanov S., Mongeau F. Prevalence, awareness, and management of CKD and cardiovascular risk factors in publicly funded health care. Clin J Am Soc Nephrol. 2014;9(4):713–719. doi: 10.2215/CJN.06550613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu Y.-C., Lee P.-H., Lei C.-C., Shih Y.-H., Lin C.-L. Analgesic use, parents’ clan, and coffee intake are three independent risk factors of chronic kidney disease in middle and elderly-aged population: a community-based study. Ren Fail. 2014;36(3):361–366. doi: 10.3109/0886022X.2013.866017. [DOI] [PubMed] [Google Scholar]
- 29.Wright-Nunes J.A., Luther J.M., Ikizler T.A., Cavanaugh K.L. Patient knowledge of blood pressure target is associated with improved blood pressure control in chronic kidney disease. Patient Educ Couns. 2012;88(2):184–188. doi: 10.1016/j.pec.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whaley-Connell A., Shlipak M.G., Inker L.A. Awareness of kidney disease and relationship to end-stage renal disease and mortality. Am J Med. 2012;125(7):661–669. doi: 10.1016/j.amjmed.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agrawal V., Jaar B.G., Frisby X.Y. Access to health care among adults evaluated for CKD: findings from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2012;59(3 suppl 2):S5–S15. doi: 10.1053/j.ajkd.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuot D.S., Plantinga L.C., Hsu C., Powe N.R. Is awareness of chronic kidney disease associated with evidence-based guideline-concordant outcomes? Am J Nephrol. 2012;35(2):191–197. doi: 10.1159/000335935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plantinga L., Grubbs V., Sarkar U. Nonsteroidal anti-inflammatory drug use among persons with chronic kidney disease in the United States. Ann Fam Med. 2011;9(5):423–430. doi: 10.1370/afm.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuot D.S., Plantinga L.C., Hsu C.-Y. Chronic kidney disease awareness among individuals with clinical markers of kidney dysfunction. Clin J Am Soc Nephrol. 2011;6(8):1838–1844. doi: 10.2215/CJN.00730111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whaley-Connell A., Sowers J.R., McCullough P.A. Diabetes mellitus and CKD awareness: the Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) Am J Kidney Dis. 2009;53(4 suppl 4):S11–S21. doi: 10.1053/j.ajkd.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Flessner M.F., Wyatt S.B., Akylbekova E.L. Prevalence and awareness of CKD among African Americans: the Jackson Heart Study. Am J Kidney Dis. 2009;53(2):238–247. doi: 10.1053/j.ajkd.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McClellan W.M., Newsome B.B., McClure L.A. Chronic kidney disease is often unrecognized among patients with coronary heart disease: the REGARDS Cohort Study. Am J Nephrol. 2008;29(1):10–17. doi: 10.1159/000148645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saab G., Whaley-Connell A.T., McCullough P.A., Bakris G.L. CKD awareness in the United States: the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2008;52(2):382–383. doi: 10.1053/j.ajkd.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 39.Sarafidis P.A., Li S., Chen S.-C. Hypertension awareness, treatment, and control in chronic kidney disease. Am J Med. 2008;121(4):332–340. doi: 10.1016/j.amjmed.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 40.Hsu C.-C., Hwang S.-J., Wen C.-P. High prevalence and low awareness of CKD in Taiwan: a study on the relationship between serum creatinine and awareness from a nationally representative survey. Am J Kidney Dis. 2006;48(5):727–738. doi: 10.1053/j.ajkd.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 41.Coresh J., Byrd-Holt D., Astor B.C. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16(1):180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 42.Nickolas T.L., Frisch G.D., Opotowsky A.R., Arons R., Radhakrishnan J. Awareness of kidney disease in the US population: findings from the National Health and Nutrition Examination Survey (NHANES) 1999 to 2000. Am J Kidney Dis. 2004;44(2):185–197. doi: 10.1053/j.ajkd.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 43.Shah A., Fried L.F., Chen S.-C. Associations between access to care and awareness of CKD. Am J Kidney Dis. 2012;59(3 suppl 2):S16–S23. doi: 10.1053/j.ajkd.2011.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.KDIGO CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 45.Lee J., Chu C., Guzman D. Albuminuria testing by race and ethnicity among patients with hypertension with and without diabetes. Am J Nephrol. 2019;50(1):48–54. doi: 10.1159/000500706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levey A.S., Eckardt K.-U., Dorman N.M. Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020;97(6):1117–1129. doi: 10.1016/j.kint.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 47.Tong A., Levey A.S., Eckardt K.-U. Patient and caregiver perspectives on terms used to describe kidney health. Clin J Am Soc Nephrol. 2020;15(7):937–948. doi: 10.2215/CJN.00900120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention Chronic Kidney Disease (CKD) Surveillance System-United States. http://www.cdc.gov/ckd Published 2020. Accessed October 1, 2010.
- 49.Centers for Disease Control and Prevention Increase the proportion of adults with chronic kidney disease who know they have it — CKD-02 - Healthy People 2030 | health.gov. https://health.gov/healthypeople/objectives-and-data/browse-objectives/chronic-kidney-disease/increase-proportion-adults-chronic-kidney-disease-who-know-they-have-it-ckd-02
- 50.Narva A.S., Norton J.M., Boulware L.E. Educating patients about CKD: the path to self-management and patient-centered care. Clin J Am Soc Nephrol. 2016;11(4):694–703. doi: 10.2215/CJN.07680715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peng S., He J., Huang J. Self-management interventions for chronic kidney disease: a systematic review and meta-analysis. BMC Nephrol. 2019;20:142. doi: 10.1186/s12882-019-1309-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schrauben S.J., Cavanaugh K.L., Fagerlin A. The relationship of disease-specific knowledge and health literacy with the uptake of self-care behaviors in CKD. Kidney Int Rep. 2019;5(1):48–57. doi: 10.1016/j.ekir.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1-S3, Item S1, Table S1, Table S2