Abstract
Individuals receiving long-term hemodialysis are at increased risk of developing cardiovascular disease (CVD). Traditional cardiovascular risk factors do not fully explain the high CVD risk in this population. During hemodialysis, blood interacts with the biomaterials of the hemodialysis circuit. This interaction can activate the complement system and the factor XII-driven contact system. FXII activation triggers both the intrinsic pathway of coagulation and the kallikrein-kinin pathway, resulting in thrombin and bradykinin production, respectively. The complement system plays a key role in the innate immune response, but also contributes to the pathogenesis of numerous disease states. Components of the complement pathway, including mannose binding lectin and C3, are associated with CVD risk in people with end-stage kidney disease (ESKD). Both the complement system and the factor XII-driven contact coagulation system mediate proinflammatory and procoagulant responses that could contribute to or accelerate CVD in hemodialysis recipents. This review summarizes what is already known about hemodialysis-mediated activation of the complement system and in particular the coagulation contact system, emphasizing the potential role these systems play in the identification of new biomarkers for CVD risk stratification and the development of potential therapeutic targets or innovative therapies that decrease CVD risk in ESKD patients.
Introduction
More than 2 million people worldwide currently receive dialysis for end-stage kidney disease (ESKD), and this number is expected to increase as rates of diabetes and hypertension continue to grow.1 Furthermore, economic growth and rising per-capita incomes in emerging economies such as the People’s Republic of China, India, and Brazil will likely double the number of individuals on dialysis over the next decade. For people with ESKD, dialysis is a life-sustaining treatment. Nevertheless, long-term hemodialysis, the most commonly employed treatment modality, is associated with a far greater risk of mortality when compared with the general population, primarily due to the elevated cardiovascular disease (CVD) risk. An estimated 39% of individuals undergoing hemodialysis die from CVD,2 and long-term hemodialysis patients face a 30-fold greater risk of CVD-related mortality compared to age, ethnicity and sex-matched controls.3
Factors that contribute to atherosclerosis and CVD in the general population, such as dyslipidemia, diabetes, and hypertension, are very common among ESKD patients. However, these traditional risk factors do not completely account for the exceptionally high CVD risk associated with ESKD.4 Furthermore, when compared with the general population, the pathology, manifestations, and complications of CVD differ in people with kidney disease.5,6 For this reason, there is a growing interest in identifying nontraditional CVD risk factors in this population. Chronic inflammation—as evidenced by elevated levels of interleukin 6 (IL-6), tumor necrosis factor α (TNF-α), and C-reactive protein (CRP), oxidative stress, and endothelial dysfunction—has been shown to predict CVD events in individuals on long-term hemodialysis. Each of these measures have been identified as emerging CVD risk factors for people with chronic kidney disease.4,6,7
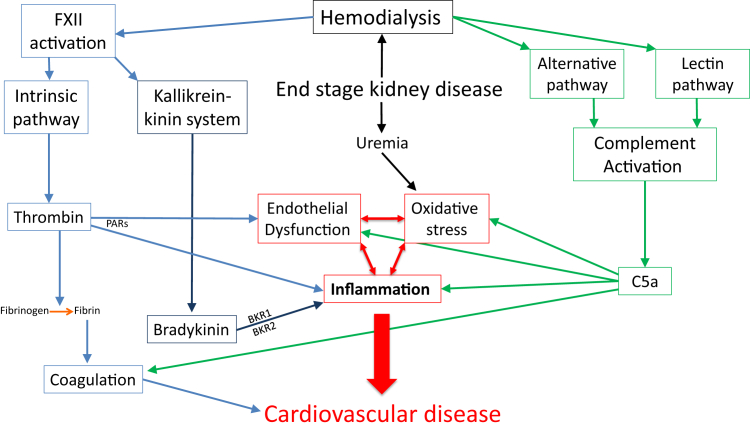
Both disease-related and treatment-related factors contribute to CVD risk in ESKD patients. For example, loss of kidney function results in the retention of uremic toxins and cytokines leading to increased oxidative stress and inflammation.8 On the other hand, interactions between blood and the biomaterials that compose the membrane and tubing of the hemodialysis circuit can trigger systemic cascades of the innate immune response, including the complement and coagulation contact systems (Fig 1).
Figure 1.
Chronic inflammation, oxidative stress, and vascular dysfunction are 3 nontraditional risk factors that contribute to CVD risk in patients on maintenance hemodialysis. Factors related to decreased kidney function in end-stage kidney disease, such as uremia, can lead to increased oxidative stress and inflammation. In addition, hemodialysis brings blood into contact with biomaterials in the dialysis circuit. As a result, both the complement and the contact activation pathways can be activated. Hemodialysis-mediated complement activation includes binding of mannose binding lectin and ficolin 2 to the hemodialysis membrane, leading to activation of the lectin pathway. The lectin pathway, which is fundamentally linked to the alternative pathway via MASP-3, is also simultaneously activated. The binding of properdin and C3b to the membrane may also play a role in activating the alternative pathway. Both the alternative and lectin pathways generate C5a and C5b, which attract and activate polymorphonuclear cells and monocytes, and mediate activation of the membrane attack complex. Complement pathway activation results in inflammation, endothelial injury, oxidative stress, and activation of the coagulation system. Surface activation of FXII initiates both the intrinsic pathway of coagulation, which produces thrombin, and the kallikrein/kinin pathway, which generates bradykinin. In addition to its prothrombotic effects, thrombin can also contribute to inflammation and atherogenesis via interaction with PAR-1 and PAR-4, expressed on platelets, and PAR-1, expressed on endothelial cells. Bradykinin promotes inflammation by binding to BKR1 or BKR2, resulting in leukocyte stimulation, cytokine production, adhesion molecule expression, and NFκB up-regulation. Abbreviations: BKR, bradykinin receptor; CVD, cardiovascular disease; FXII, factor XII; MASP-3, third MBL-associated serine protease of the lectin pathway; NFκB, nuclear factor-κB; PAR, protease-activated receptor.
Dialysis membrane-induced activation of complement has been well documented, and evidence suggests that hemodialysis membranes and tubing can also activate the factor XII (FXII)-driven contact system of coagulation.9, 10, 11 The contact pathway activates both the intrinsic pathway of coagulation and the bradykinin-producing kallikrein/kinin pathway, and can thus drive cardiovascular injury by mediating both procoagulant and proinflammatory responses.12
This review will focus on the potential role of hemodialysis as an activator of the complement and coagulation contact systems. We will also discuss their possible involvement in the elevated CVD risk associated with ESKD and hemodialysis.
The FXII-Driven Contact System
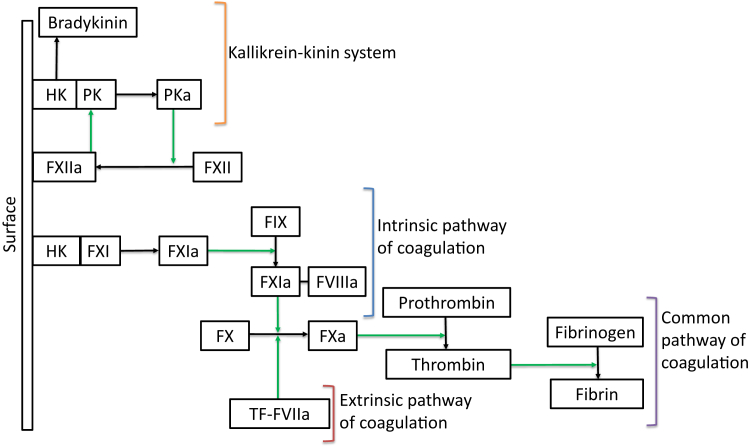
FXII, or Hageman factor, is an 80-kDa plasma protein and the inactive zymogen form of the serine protease factor XIIa (FXIIa). It is produced mainly in the liver and circulates in plasma as a single-chain molecule at a concentration of 40 μg/mL. The contact pathway is a proinflammatory and procoagulant protease cascade, which involves FXII, plasma prekallikrein (PK), and high-molecular-weight kininogen (HK). FXII binding to polyanionic surfaces results in autoactivation of FXII and the generation of FXIIa (Fig 2).
Figure 2.
Summary of FXII-mediated activation of the kallikrein-kinin pathway and the intrinsic pathway of coagulation. FXIIa-mediated activation of the kallikrein-kinin pathway results in bradykinin generation, and the intrinsic pathway of coagulation which leads to thrombin generation. The common pathway of coagulation can also be activated by the tissue factor-factor VIIa complex, via the so-called extrinsic pathway. Abbreviations: FIX, factor IX; FIXa, activated factor IX; FVIIIa, activated factor VIII; FX, factor X; FXa, activated factor X; FXI, factor XI; FXIa, activated factor XI; FXII, factor XII; FXIIa, activated factor XII; HK, high-molecular-weight kininogen; PK, prekallikrein; PKa, kallikrein; TF-FVIIa, tissue factor-factor VIIa complex.
Alternatively, FXII can be cleaved by kallikrein (PKa), the enzymatically active form of PK, to generate FXIIa. As FXIIa also activates PK, the reciprocal activation of PK by FXIIa and FXII by PKa produces a positive feedback loop.11,13 The net result is PKa-mediated cleavage of HK to generate bradykinin, a potent proinflammatory and proangiogenic mediator.11,13,14
FXIIa simultaneously initiates the intrinsic pathway of coagulation by activating factor XI (FXI), which then mediates limited proteolysis of factor IX (FIX). Active FIX (FIXa) forms a cell-surface complex with activated factor VIII (FVIIIa), known as the “intrinsic tenase” complex.11 Subsequent activation of FX ultimately results in the production of thrombin, which not only leads to fibrin generation and platelet activation, but also has a variety of proinflammatory properties that are mediated by multicellular protease-activated receptors (PARs).15 FXIIa-initiated contact activation is regulated primarily by the serpin, C1-esterase inhibitor (C1INH), the main plasma inhibitor of both FXIIa and PKa.13
Although its essential role in clot formation in vitro has been well described, FXII(a)-driven contact activation was not thought to contribute to coagulation in vivo until relatively recently. This is because both mice and humans lacking FXII do not experience abnormal bleeding.16 However, findings from animal studies published over the last 20 years have revealed that both FXI(a) and FXII(a) may play crucial amplification roles in the genesis of arterial and venous thrombi.13,17,18
Additionally, some studies report that plasma levels of FXI and prekallikrein zymogens are associated with coronary heart disease,19 venous thrombosis,20 and ischemic stroke.21 Conversely, severe FXI deficiency in humans is associated with a decreased risk of stroke and deep vein thrombosis.22 Furthermore, several studies have shown that elevated levels of FXII and FXIIa are associated with adverse cardiovascular outcomes such as myocardial infarction,23,24 coronary heart disease,25 and atherosclerosis.26 However, unlike FXI, current epidemiologic data do not clearly illustrate a link between elevated levels of FXII and thrombotic risk.16 One possible explanation for this is that the methods most commonly used to measure FXII and FXIIa can be unreliable due to ex vivo FXII activation that occurs either during or after blood is drawn.16
The exact (patho)physiological mechanisms that lead to contact activation in vivo remain incompletely understood, but several candidate biological activators have been proposed, including misfolded proteins,27 neutrophil extracellular traps28 and extracellular nucleic acids,29 inorganic polyphosphate,30 glycosaminoglycans,31 phosphatidylserine exposure on apoptotic cells32 and bacterial surface proteins.33 These ongoing studies have served to refocus attention on the role of the contact system in CVD and thrombosis.
Complement Activation in Hemodialysis
Biocompatibility refers to the ability of an artificial surface material to exist in contact with tissues without causing an inappropriate host immune response.34 The complement system plays an important role in mediating the innate immune response to biomaterials because it is capable of identifying “self” from “non-self.”35,36 Three different activation pathways—the classical, lectin, and alternative pathways—can trigger complement activation. Whereas the classical pathway is initiated by the C1q complex, the lectin pathway is initiated by collectin-11, mannose binding lectin (MBL), or ficolins. Both of these pathways lead to the formation of the C3 convertase C4b2b. The alternative pathway is activated by spontaneous hydrolysis of C3 or by properdin binding to certain cell surfaces, resulting in the creation of the C3bBb C3-convertase. The alternative pathway is linked to the lectin pathway because its activity is dependent on the presence of the third MBL-associated serine protease of the lectin pathway (MASP-3).37 Both forms of C3-convertase cleave C3 to generate C3a and C3b.38 Elevated levels of C3b result in the formation of C5 convertase, which cleaves C5, producing C5a and C5b. C5b interacts with C6-9, forming the membrane attack complex.39,40 C5a acts as a potent anaphylotoxin, and recruits more neutrophils and monocytes, thus fueling the inflammatory process.
The potential for hemodialysis membranes to activate the complement system was first documented in the 1970s.41 Following the initial reports, multiple studies were published describing “hypersensitivity reactions” or “first use syndrome” as pseudo-anaphylactic responses that occurred within the first 10 minutes of hemodialysis.42, 43, 44 Today, these allergic-like reactions are generally classified as either type A or type B. Type A reactions, which can involve pruritus, bronchospasm, laryngeal edema, or anaphylactic shock, are typically considered to be mediated by IgE, while type B reactions, which are less severe, are commonly thought to be mediated by complement.45 The mechanisms of complement activation during hemodialysis are not well understood. The lectin pathway is considered to play an important role, but its exact contribution and the potential contributions of other pathways are yet to be fully elucidated.46
Over the last several decades dialysis membranes have been modified to minimize complement activation. Early dialysis membranes were composed of cuprophane, a copper-substituted cellulose with many free hydroxyl (–OH) groups that conferred high surface immunoreactivity. These were eventually replaced by modified cellulose membranes, and later by synthetic membranes, which are commonly composed of polymethylmethacrylate, polyether sulfone, polysulfone, or polyacrylonitrile, and have greatly reduced immune reactivity.47
Modern synthetic membranes can still cause complement activation, and researchers continue to investigate which membranes are truly the most biocompatible, and what characteristics contribute to biocompatibility.48,49 A recent study published by Wagner et al50 compared the biocompatibility of hemodialysis membranes composed of cellulose triacetate (CTA) or polyvinylpyrrolidone: polyarylethersulfone. The results of their morphological, chemical, spectroscopic, and in vitro incubation analyses revealed that the 2 membranes differ in their roughness, fiber diameter, fiber wall thickness, pore size and distribution, hydrophilicity, and biocompatibility. Consequently, the CTA membranes resulted in poorer clearance of uremic toxins, but exhibited lower fibrinogen adsorption and caused a less severe increase in complement and inflammatory factors.51 Studies have also compared the biocompatibility of different types of polysulfone dialyzers.50,52 A study by Koga et al52 showed that 5 different polysulfone hemodialysis membranes caused different levels of cell activation, fibrin absorption, and oxidative stress, with NV-U membranes proving to be the most biocompatible.
Hemodialysis facilities may choose to reuse dialyzers for a variety of reasons, including cost control, waste reduction, and improved dialyzer biocompatibility. Indeed, in the 1980s several studies showed that reusing cuprophane membranes decreased their capacity to activate complement.53, 54, 55 However, additional studies revealed that reused dialyzers should not be assumed to be more biocompatible as biocompatibility depends on the type of dialyzer membrane, the dialysate used, and the methods used to sterilize, store, and process the membranes.56, 57, 58, 59, 60, 61 For example, dialyzers can be sterilized with agents such as ethylene oxide, steam, or radiation (gamma or beta). A study by Lundberg et al61 showed that ethylene oxide sterilization appeared to cause more complement activation than steam sterilization, but only when using bicarbonate dialysate and a cuprophane dialyzer. In addition, ethylene oxide is not considered the preferred method for dialyzer sterilization because it can cause a type A allergic reaction through IgE-mediated hypersensitivity.45 For these reasons, ethylene oxide sterilized dialysis membranes are rarely used today. Hemodialysis tubing is still sterilized using ethylene oxide in some clinics, but this is generally considered to be unproblematic.
Although dialyzer reuse was once a highly prevalent practice in the United States, its popularity has steadily declined over the last 20 years due to the improved biocompatibility and decreased price of currently used membranes. Compared to the United States, dialyzer reuse is even less prevalent in Europe and Japan, but is still common in some areas of the world where availability and price remain an issue.62
Hemodialysis is the most common form of kidney replacement therapy. However, many individuals with ESKD are treated with peritoneal dialysis, a common alternative to hemodialysis. A recent study aimed to determine whether peritoneal dialysis was associated with less severe complement activation.63 The possible cause of low biocompatibility in peritoneal dialysis is the dialysis fluid. In this study, both forms of kidney replacement therapy altered the concentrations of C3a, C5a, and C5b-9. However, complement activation was greatest in hemodialysis patients. The results of this study indicate that dialysis modality is an important factor to take into account when considering dialysis biocompatibility.63
The complement system is not only a crucial part of the body’s innate immune system, but also plays a key role in the pathology of multiple diseases, including CVD. A number of studies have explored the potential prognostic utility of complement biomarkers for predicting CVD risk in ESKD patients.46,49 For example, MBL, which mediates the host inflammatory response against infections, is generally elevated in hemodialyzed patients compared with healthy controls.64 However, low levels of MBL have been shown to be associated with an elevated risk of CVD events and all-cause mortality.64,65 This could be because low levels of MBL could result in higher rates of infection in hemodialyzed individuals, thereby promoting the pathogenesis of atherosclerosis.64 Furthermore, the addition of plasma MBL levels to risk prediction models significantly improved prediction of CVD risk beyond traditionally used clinical markers in patients on maintenance dialysis.49
Another recent study linked C3 with proinflammatory and prothrombotic activity in hemodialysis. The study reported that hemodialysis patients who developed CVD events had significantly elevated C3 activation in the first 30 minutes of hemodialysis, followed by an increased IL-6/IL-10 ratio and elevated concentrations of TNF-α and von Willebrand factor. Furthermore, the authors used an ex vivo hemodialysis model to demonstrate that complement inhibition significantly decreased markers of inflammation and coagulation activation.46 These findings are in accordance with a study by Lines and Carter,66 which showed that higher baseline levels of C3 were associated with subsequent CVD events. On the other hand, people on hemodialysis with intermediate levels of SC5b-9 had significantly lower CVD mortality than those with low or high levels.66
Atherosclerotic CVD risk has also been shown to be positively correlated with the binding complex formed by adiponectin and C1q in human blood (C1q-APN).67 Furthermore, polymorphisms of genes that code for components of the complement pathway, including the Y402H polymorphism in the complement inhibitor factor H (CFH) gene and C5507G polymorphism of the complement receptor 1 gene, were associated with CVD risk in people with ESKD.68,69 Overall, these results indicate that altered complement pathway activation could increase CVD risk in people treated with hemodialysis, and certain complement biomarkers could be prognostic indicators of CVD risk in these individuals.
Contact Activation in Hemodialysis
Despite the many technological advances that have been made, hemodialysis still results in robust intraprocedural thrombin production in the absence of anticoagulation. For this reason, anticoagulation is often required during hemodialysis to prevent the formation of thromboemboli, and the subsequent obstruction of the extracorporeal hemodialysis filter and circuit. As expected, the contact and intrinsic pathways have been implicated as the primary cause of coagulation activation during hemodialysis.11 Even so, our understanding of hemodialysis-mediated contact system activation remains limited, as relatively few published studies have addressed this topic (Table 1).
Table 1.
Studies on Hemodialysis-Mediated Contact Activation
| Study | Year | In Vivo or Ex Vivo | Study Question | Does the Evidence Support Contact Activation? |
|---|---|---|---|---|
| Matsuo et al70 | 1997 | In vivo | Is FXIIa elevated in patients receiving long-term hemodialysis? | Yes |
| Svensson et al71 | 1996 | In vivo | Is FXIIa activity increased after 4 hours of hemodialysis? | Yes |
| Bouman et al72 | 2006 | In vivo | Does continuous venovenous hemofiltration using cellulose triacetate filters cause contact activation? | No |
| Salmon et al73 | 1997 | In vivo | Does continuous venovenous hemofiltration using polyacrylonitrile filters cause contact activation? | No |
| Francois et al74 | 2020 | In vivo | Does routine hemodialysis increase FXII, FXI, or PK activation compared to baseline levels or compared to control participants? | No |
| Papageorgiou et al75 | 2014 | In vivo | Is FXIIa higher in patients with ESKD receiving long-term hemodialysis compared with healthy controls? | Yes |
| Hong et al76 | 2001 | Ex vivo | Does FXIIa play a role in thrombin generation when whole blood is incubated with PVC? | Yes |
| Lamba et al77 | 2000 | Ex vivo | Does incubation of whole blood or plasma with PVC result in contact activation? | Yes |
| Frank et al78 | 2013 | Ex vivo | Does PVC tubing cause FXII activation and trigger the coagulation cascade? | Yesa |
| Renaux et al43 | 1999 | Ex vivo | Mechanisms of dialysis membrane-mediated activation of the kallikrein-kinin system | Yes |
| Frank et al79 | 2001 | Ex vivo | Role of contact system activation in dialysis membrane-mediated thrombin production | Yes |
| Schulman et al80 | 1993 | Ex vivo | Mechanisms of bradykinin generation caused by dialysis membranes | Yes |
Abbreviations: ESKD, end-stage kidney disease; FXI, factor XI; FXII, factor XII; FXIIa, activated factor XII; PK, prekallikrein; PVC, polyvinylchloride.
Potentially contradictory findings were reported in the study.
Studies addressing in vivo FXII activation in patients undergoing hemodialysis have reported inconsistent results. Matsuo et al70 showed that FXIIa was elevated in patients with and without diabetes receiving long-term hemodialysis. Similarly, in another study conducted in 9 patients undergoing long-term hemodialysis, circulating FXIIa activity increased after 4 hours of hemodialysis.71 However, 2 studies conducted in critically ill patients with acute kidney failure reported that venovenous hemofiltration did not have any effect on contact activation, measured by FXIIa-C1 inhibitor and PKa-C1 inhibitor complexes.72,73 Furthermore, a recent study conducted in 10 long-term hemodialysis patients reported that routine hemodialysis did not increase FXII, FXI, or PK activation compared with baseline levels or compared with control participants.74 On the other hand, an additional study showed that FXIIa was 3 times higher in patients with ESKD receiving long-term hemodialysis compared with healthy controls.75 The contradictory findings of these studies could be attributed to the differing populations, the small sample sizes, or the difficulties associated with measuring contact activation in vivo, including the sensitivity of different laboratory markers as well as the potential for artifactual (ex vivo) activation of FXII after blood has been drawn.
A number of ex vivo studies have evaluated contact activation during hemodialysis. Several of these studies focused on the role of polyvinylchloride (PVC), the standard polymer used for hemodialysis circuit tubing, as a trigger of contact activation. Although PVC lacks an electronegative charge, incubation of whole blood or plasma with PVC results in thrombin generation, which is abolished by the addition of corn trypsin inhibitor, a specific inhibitor of FXIIa.76,77 A more recent in vitro study showed that direct inhibition of FXIIa, but not FVIIa, decreased thrombin generation in a chandler loop model. However, this study was unable to detect any changes in FXIIa activity, free FXIIa levels, or FXIIa-inhibitor complexes.78 These findings confirm that FXII plays an integral role in PVC-mediated thrombin generation, while drawing attention to the challenges related to measuring systemic FXII activation in vivo.78 The results indicate that previous clinical studies relying only on levels of FXIIa or FXIIa-inhibitor complexes should be interpreted with caution.
Additional ex vivo studies have focused on the activation of the contact pathway by hemodialysis membranes. These studies have indicated that the circulation of plasma in contact with hemodialysis membranes results in increased levels of FXIIa and PKa paired with bradykinin release, especially on membranes with a greater negative charge.43,79,80 A study by Reneaux et al43 tested 6 different hemodialysis membranes and observed that levels of bradykinin and PKa were highest in plasma that was perfused through membranes with the greatest electronegative charge. The same study indicated that both the pH and the concentration of the plasma flowing through an extracorporeal hemodialysis circuit can also affect the degree of contact activation.43
Possible Contributions of Contact System Activation to CVD Risk
Contact activation triggers both the intrinsic pathway of coagulation and the kallikrein-kinin pathway, with net prothrombotic and proinflammatory effects. The enzymes that activate these 2 branches of the contact system, FXIIa, FXIa, and PKa, have been implicated in atherosclerosis and myocardial infarction,23,24,26 and FXI deficiency is related to lower rates of cardiovascular and venous thromboembolic events.81 Therefore, it is possible that repetitive hemodialysis-mediated contact activation could contribute to the genesis of CVD and/or exacerbate pre-existing CVD. In this section, we will briefly address how the principal products of the 2 branches of the contact system—namely, thrombin and bradykinin—could contribute to CVD risk by promoting thrombosis, inflammation, vascular dysfunction, and atherosclerosis.
Thrombin
Thrombin is a multifunctional serine protease that contributes to hemostasis and inflammation in several ways. First, it proteolytically cleaves fibrinogen to form fibrin monomers, which, via the activity of factor XIIIa, become the cross-linked fibrin matrix in mature blood clots. Thrombin also helps to modulate downstream anticoagulant and fibrinolytic pathways whenever coagulation is activated.82
In addition to its roles in coagulation and fibrinolysis, thrombin further contributes to inflammation and atherogenesis. Thrombin signaling is mediated via protease-activated receptor 1 (PAR-1), PAR-3, and PAR-4, which are expressed by platelets, endothelial cells, leukocytes, and smooth muscle cells.83 For example, thrombin cleavage of PAR-1 or PAR-4 on platelets results in platelet activation, aggregation, and adhesion to the endothelium.84 Thrombin activation of PAR-1 on endothelial cells leads to the release of von Willebrand factor and the expression of adhesion molecules, including E-selectin, P-selectin, intracellular cell adhesion molecule-1, and vascular cell adhesion molecule-1, which promote the adhesion, rolling, attachment, and activation of platelets and leukocytes.85,86 Endothelial cell activation by thrombin also results in the increased expression of cytokines, chemokines, and genes related to hemostasis, such as tissue factor and plasminogen activator inhibitor-1 (PAI-1).
Finally, thrombin activation results in increased permeability of the endothelial cell monolayer, alterations in vascular tone, and vascular remodeling.84 Although a healthy endothelium can adapt to these stimuli by regulating the vascular milieu, ESKD and repeated exposure to hemodialysis may dysregulate the vascular response.87 Indeed, the intense vascular inflammation and oxidative stress caused by ESKD can lead to endothelial dysfunction, a precursor event in the pathogenesis of CVD. Additional thrombin production occurring with each hemodialysis session could compound these issues by repeatedly up-regulating the expression of proinflammatory cytokines, accelerating changes in the vasculature, and promoting a procoagulant environment.
Bradykinin
The kallikrein/kinin system is an endogenous metabolic cascade in which HK is cleaved by PKa, resulting in the release of bradykinin. As discussed previously, in vitro studies have indicated that hemodialysis results in FXII activation, PKa generation, and bradykinin production.43,79,80 Bradykinin is a potent proinflammatory nanopeptide that activates complex signaling cascades through two G protein-coupled receptors, bradykinin receptor 1 (BKR1) and BKR2. BKR2 is constitutively expressed by many mammalian cells, whereas BKR1 expression is inducible in endothelial and smooth muscle cells in the context of inflammation.88,89 Bradykinin plays an important role in the body’s vascular responses to inflammation by mediating vasodilation and increasing microvascular permeability and vascular remodeling, thereby promoting angiogenesis.89
The effects of bradykinin are highly complex, and can be physiological and cardioprotective, or pathological, depending on the context. Both BKR2 (through endothelial nitric oxide synthase) and BKR1 (through cytokine-inducible nitric oxide synthase) generate nitric oxide (NO), endothelium hyperpolarizing factor (EDHF), and prostacyclin (PGI2). These 3 molecules stimulate smooth muscle relaxation, resulting in vasodilation, and can also exert antithrombotic effects.90 However, in the presence of oxidative stress, NO is degraded by superoxide to peroxynitrite (ONOO–), a highly potent oxidant that contributes to endothelial dysfunction.91,92 Both BKR2 and BKR1 can also mediate protective and defensive roles of bradykinin by promoting angiogenesis following ischemia.90,93 These beneficial actions may contribute to the pharmacologic effects of angiotensin-converting enzyme (ACE) inhibitors.90
The pathological actions of bradykinin that are mediated by BKR1 and BKR2 include stimulation of the migration of leukocytes from blood to tissues, promotion of synthesis of proinflammatory cytokines and prostaglandins, pain, and edema. BKR1, in particular, plays an important role in atherogenesis by up-regulating nuclear factor-κB (NFκB) activity, leading to proinflammatory cytokine production and enhanced interactions between polymorphonuclear cells and endothelial cells.94,95
In vivo studies have underlined the complexity of the actions of the kallikrein/kinin system in the vasculature. For example, a clinical study of BKR2 inhibition in patients receiving hemodialysis showed that the use of a bradykinin antagonist decreased concentrations of monocyte chemotactic protein 1 ((MCP-1) and PAI-1 after hemodialysis.96 PAI-1 expression is stimulated by cytokines including IL-6, TNF-α, and IL-1β,97 and independently predicts thrombotic events in ESKD patients on hemodialysis.98 On the other hand, BKR2 inhibition caused circulating F2-isoprostane and P-selectin concentrations to increase, suggesting that endogenous bradykinin inhibits platelet activation. Overall, these results indicate that, despite a potentially beneficial effect on platelet aggregation, the elevated levels of bradykinin repeatedly produced during each hemodialysis session could paradoxically increase CVD risk.96
Human and animal studies also indicate that bradykinin’s effects on vasomotion can be complex. Animal studies have suggested that transient increases in bradykinin cause acute hypotension, whereas continuous bradykinin infusion results in increased mean arterial pressure.99 Recent work by Papageorgiou et al75 showed that during hemodialysis the activation of FXII positively correlated with mean arterial pressure and peripheral resistance in humans. Furthermore, in a mouse model of ESKD, FXIIa-kininogen-mediated vasoconstriction contributed significantly to hypertension. Overall, these findings suggest that sustained FXIIa-mediated bradykinin production could contribute to hypertension in ESKD.
Bradykinin also plays an important role in certain kinds of hypersensitivity reactions in patients on hemodialysis.44,100 Dialysis-associated reactions are generally acute events, and patients present with a variety of symptoms, ranging from mild (urticaria, erythema, flushing, and fainting) to severe (hypotension, bronchospasm, laryngeal edema, bradycardia, and even cardiac and/or respiratory arrest and death).101
Although there are many different types and potential causes of dialysis-related hypersensitivity reactions, contact activation leading to bradykinin production has been identified as one such mechanism.100, 101, 102 Early studies identified negatively charged dialysis membranes, especially the AN-69 membrane, as a source of FXII activation that may promote bradykinin generation, which could lead to acute vasodilation and hypotension.43,44,80 Another type of dialysis-related contact activation was observed in 2007 when hundreds of patients in the United States and Europe experienced hypotension, angioedema, and swelling of the larynx within minutes of initiating hemodialysis.102 A contaminant in heparin—namely, oversulfated chondroitin sulfate (OSCS)—was eventually identified as the cause of this reaction.103 A subsequent study showed that OSCS and other polyanions, such as dextran sulfate, trigger FXII-mediated activation of the kallikrein/kinin system resulting in bradykinin production.104 Interestingly, heparin is a known potential activator of FXII, but only polyanions with a high enough degree of sulfation (4.0 sulfates per disaccharide)—as is the case with OSCS and dextran sulfate—activate FXII in plasma. Furthermore, any alteration that reduces kinin degradation, such as ACE inhibitor therapy, could increase bradykinin to pathological levels, and thus increase the risk of developing hypersensitivity reactions that occur as a result of contact system activation.102,105
In addition, some evidence suggests that FXIIa-mediated bradykinin production could also play a role in hypersensitivity reactions driven by IgE.106,107 Anaphylaxis is commonly mediated through immune IgE-dependent mechanisms that lead to the release of glycosaminoglycan heparin from mast cells. Findings from a study conducted by Sala-Cunill et al107 suggest that mast cell-derived heparin provides the negatively charged surface for the binding and consequent activation of FXII, thereby triggering the kallikrein-kinin system and resulting in the generation of bradykinin. These data suggest that the contact system could potentially play a role in type A IgE-mediated allergic reactions during hemodialysis. Furthermore, IgE mediates allergic reactions that are caused by hemodialysis membranes sterilized using ethylene oxide. Therefore, it is also possible that the FXII contact system could also play a role in these reactions. However, due to their propensity to activate complement, ethylene oxide sterilized membranes are no longer recommended for use.45
Complement, Coagulation, and CVD in ESKD
Although numerous studies have illustrated an association between complement activation and CVD events in hemodialysis patients,46,64,65,67, 68, 69 the exact mechanisms linking complement to CVD risk in this population remain poorly described.66 One possible link could be the relationship between complement activation, inflammation, and prothrombotic processes.10 A study by Poppelaars et al46 showed that hemodialysis patients who later developed CVD events experienced a significant peak in complement activation during dialysis, which was not seen in those who did not end up developing CVD events. Furthermore, complement activation was accompanied by significantly higher circulating levels of proinflammatory cytokines and von Willebrand factor. These proinflammatory and prothrombotic responses were shown to be subsequent to the complement activation.46
The findings of Poppelaars et al46 are in accordance with other studies that have demonstrated a link between complement activation and prothrombotic activity.108, 109, 110 For example, Schuett et al110 showed that elevated levels of C3 were associated with the formation of a denser clot structure, which increases CVD risk in people undergoing hemodialysis. Another study by Kourtzelis et al109 showed that complement pathway activation caused by hemodialysis biomaterials resulted in the expression of active tissue factor in peripheral blood neutrophils.
In addition, the complement and coagulation pathways can activate each other via crosstalk on both stationary and circulating cells as well as on artificial surfaces.111 Notably, both the complement and contact pathways are regulated by C1 esterase inhibitor and are activated by the gC1q receptor on the endothelium.111 Moreover, evidence from Irmsher et al112 illustrated that, in addition to cleaving kininogen, kallikrein can also cleave and activate C3 in vitro. Finally, recent work by Lopatko Fagerström et al113 demonstrated that reducing contact pathway signaling using B1R and B2R kinin receptor antagonists or C1INH results in decreased complement levels. Their findings indicate that blocking the kallikrein-kinin pathway could reduce complement-mediated endothelial cell injury.
Therapeutic Implications
Research on the complement and FXII signaling cascades could open the door to new potential therapeutic approaches that target these pathways to decrease inflammation and CVD risk in people with ESKD. For example, treatments that target the complement system are considered a promising therapeutic option for people undergoing hemodialysis. Potential complement therapeutics can be grouped into 3 different treatment strategies that have been comprehensively reviewed elsewhere.46
First, anticoagulants with complement inhibitory activities, such as citrate and heparin, could be used. Citrate reduces complement activity through its calcium chelating properties. Although some studies have shown attenuated complement activation with the use of citrate anticoagulation, the results of other studies have been inconsistent.46,114,115 The potential effects of heparin as a complement inhibitor during hemodialysis have not yet been tested.46,65
Second, systemic complement inhibitors could be used. Inhibitors of C3, C5, and C5a receptor have been suggested as particularly attractive options to attenuate complement activation and the resulting inflammation and pro-thrombotic activity.46,107,116 Third, complement inhibitors could be used locally to improve the biocompatibility of hemodialysis membranes. In a previous study, 5C6 coated to a polystyrene surface was shown to bind factor H and inhibit complement activation when exposed to human plasma, thereby enhancing biocompatibility.117
Therapies directed at reducing contact pathway activation could provide additional approaches worthy of consideration. For instance, because the anticoagulants routinely used during hemodialysis are associated with an increased risk of bleeding, therapeutic anticoagulation strategies targeting FXIa are currently under study in patients on hemodialysis (eg, NCT02902679, NCT03787368, NCT02553889).118 FXIIa could prove to be an additional therapeutic target for decreasing both inflammation and coagulation activation without increasing bleeding risk.13,119 Additionally, kinin B2 antagonists and C1INH are commercially available agents that have been shown to attenuate complement activation.113 Future studies could explore the potential of these inhibitors to reduce vascular inflammation in people with ESKD.
Conclusions
Both ESKD and long-term hemodialysis are associated with an elevated risk of CVD. Moreover, the pathogenesis, progression, and manifestations of CVD differ in individuals with chronic kidney disease. Thus, understanding the drivers of CVD development in this population remains an issue of utmost importance.
Although the potential for hemodialysis to activate the complement and coagulation contact systems has been recognized for decades, a growing number of recent studies have underscored the possible links between hemodialysis membrane-induced activation of these systems and CVD pathogenesis. Further research focused on deepening our understanding of hemodialysis-mediated activation of the contact pathways could potentially play an important role in preventing and/or treating CVD disease in ESKD. For example, studies examining the complement and FXII-mediated contact systems in hemodialysis patients could lead to the identification of alternative biomarkers for CVD risk stratification that perform better than the traditional clinical markers used to predict CVD risk in this population. Additionally, both the contact and complement pathways could present future avenues for therapeutic approaches to reduce the proinflammatory and procoagulant responses associated with hemodialysis.
Finally, there is currently a renewed demand for innovation in kidney replacement therapy, with a special focus on developing novel treatment solutions, including miniaturized, portable, wearable, and implantable kidney replacement therapy options. Developing a better understanding of the mechanisms that trigger complement and FXII activation in ESKD patients could prove to be essential to creating improved hemodialysis technologies or prescriptions that would limit this activation, and thereby potentially decrease CVD risk in this population.120
Article Information
Authors’ Full Names and Academic Degrees
Sarah C. Skinner, PhD, Vimal K. Derebail, MD, Caroline J. Poulton, MSW, Donna C. Bunch, PhD, Prabir Roy-Chaudhury, MD, and Nigel S. Key, MD
Support
SCS was supported by NIH grant T32 HL007149. The funders had no role in the study design, analysis, reporting, or decision to submit the manuscript for publication.
Financial Disclosure
Dr Derebail has received consultant fees from Novartis, Bayer, and Retrophin. Dr Roy-Chaudhury has participated as a consultant or on the advisory board for WL Gore, Medtronic, BD, Cormedix, Bayer, Akebia, Humacyte, Inregen, and Reata, and is the CSO and founder of Inovasc LLC. The remaining authors declare that they have no relevant financial interests.
Peer Review
Received December 23, 2020, as a submission to the expedited consideration track with 2 external peer reviews. Direct editorial input from an Associate Editor and the Editor-in-Chief. Accepted in revised form April 9, 2021.
Footnotes
Complete author and article information provided before references.
References
- 1.Liyanage T., Ninomiya T., Jha V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System (USRDS) National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018. 2018 USRDS annual data report: Epidemiology of kidney disease in the United States. [Google Scholar]
- 3.Jha V., Garcia-Garcia G., Iseki K. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 4.Stenvinkel P., Ketteler M., Johnson R.J. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int. 2005;67:1216–1233. doi: 10.1111/j.1523-1755.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 5.Herzog C.A., Asinger R.W., Berger A.K. Cardiovascular disease in chronic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2011;80:572–586. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- 6.Valdivielso J.M., Rodríguez-Puyol D., Pascual J. Atherosclerosis in chronic kidney disease: more, less, or just different? Arterioscler Thromb Vasc Biol. 2019;39:1938–1966. doi: 10.1161/ATVBAHA.119.312705. [DOI] [PubMed] [Google Scholar]
- 7.Himmelfarb J., Hakim R.M. Oxidative stress in uremia. Curr Opin Nephrol Hypertens. 2003;12(6):593–598. doi: 10.1097/00041552-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Betjes M.G. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9:255–265. doi: 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- 9.Craddock P.R., Fehr J., Brigham K.L., Kronenberg R.S., Jacob H.S. Complement and leukocyte-mediated pulmonary dysfunction in hemodialysis. N Engl J Med. 1977;296:769–774. doi: 10.1056/NEJM197704072961401. [DOI] [PubMed] [Google Scholar]
- 10.Ekdahl K.N., Soveri I., Hilborn J., Fellström B., Nilsson B. Cardiovascular disease in haemodialysis: role of the intravascular innate immune system. Nat Rev Nephrol. 2017;13:285–296. doi: 10.1038/nrneph.2017.17. [DOI] [PubMed] [Google Scholar]
- 11.Smith S.A., Travers R.J., Morrissey J.H. How it all starts: initiation of the clotting cascade. Crit Rev Biochem Mol Biol. 2015;50:326–336. doi: 10.3109/10409238.2015.1050550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmaier A.H. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost. 2016;14:28–39. doi: 10.1111/jth.13194. [DOI] [PubMed] [Google Scholar]
- 13.Maas C., Renné T. Coagulation factor XII in thrombosis and inflammation. Blood. 2018;131:1903–1909. doi: 10.1182/blood-2017-04-569111. [DOI] [PubMed] [Google Scholar]
- 14.Hofman Z., de Maat S., Hack C.E., Bradykinin Maas C. inflammatory product of the coagulation system. Clin Rev Allergy Immunol. 2016;51:152–161. doi: 10.1007/s12016-016-8540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coughlin S.R. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 16.Key N.S. Epidemiologic and clinical data linking factors XI and XII to thrombosis. Hematology Am Soc Hematol Educ Program. 2014;2014:66–70. doi: 10.1182/asheducation-2014.1.66. [DOI] [PubMed] [Google Scholar]
- 17.Matafonov A., Leung P.Y., Gailani A.E. Factor XII inhibition reduces thrombus formation in a primate thrombosis model. Blood. 2014;123:1739–1746. doi: 10.1182/blood-2013-04-499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen E.D., Gailani D., Castellino F.J. FXI is essential for thrombus formation following FeCl3-induced injury of the carotid artery in the mouse. Thromb Haemost. 2002;87:774–776. [PubMed] [Google Scholar]
- 19.Merlo C., Wuillemin W.A., Redondo M. Elevated levels of plasma prekallikrein, high molecular weight kininogen and factor XI in coronary heart disease. Atherosclerosis. 2002;161:261–267. doi: 10.1016/s0021-9150(01)00666-9. [DOI] [PubMed] [Google Scholar]
- 20.Meijers J.C., Tekelenburg W.L., Bouma B.N., Bertina R.M., Rosendaal F.R. High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med. 2000;342:696–701. doi: 10.1056/NEJM200003093421004. [DOI] [PubMed] [Google Scholar]
- 21.Suri M.F., Yamagishi K., Aleksic N., Hannan P.J., Folsom A.R. Novel hemostatic factor levels and risk of ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Cerebrovasc Dis. 2010;29:497–502. doi: 10.1159/000297966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salomon O., Steinberg D.M., Koren-Morag N., Tanne D., Seligsohn U. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood. 2008;111:4113–4117. doi: 10.1182/blood-2007-10-120139. [DOI] [PubMed] [Google Scholar]
- 23.Doggen C.J., Rosendaal F.R., Meijers J.C. Levels of intrinsic coagulation factors and the risk of myocardial infarction among men: opposite and synergistic effects of factors XI and XII. Blood. 2006;108:4045–4051. doi: 10.1182/blood-2005-12-023697. [DOI] [PubMed] [Google Scholar]
- 24.Grundt H., Nilsen D.W., Hetland Ø, Valente E., Fagertun H.E. Activated factor 12 (FXIIa) predicts recurrent coronary events after an acute myocardial infarction. Am Heart J. 2004;147:260–266. doi: 10.1016/j.ahj.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Lowe G.D., Rumley A., McMahon A.D., Ford I., St J O’Reilly D., Packard C.J. West of Scotland Coronary Prevention Study Group. Interleukin-6, fibrin D-dimer, and coagulation factors VII and XIIa in prediction of coronary heart disease. Arterioscler Thromb Vasc Biol. 2004;24:1529–1534. doi: 10.1161/01.ATV.0000135995.39488.6c. [DOI] [PubMed] [Google Scholar]
- 26.Colhoun H.M., Zito F., Chan N.N., Rubens M.B., Fuller J.H., Humphries S.E. Activated factor XII levels and factor XII 46C>T genotype in relation to coronary artery calcification in patients with type 1 diabetes and healthy subjects. Atherosclerosis. 2002;163:363–369. doi: 10.1016/s0021-9150(02)00022-9. [DOI] [PubMed] [Google Scholar]
- 27.Maas C., Govers-Riemslag J.W., Bouma B. Misfolded proteins activate factor XII in humans, leading to kallikrein formation without initiating coagulation. J Clin Invest. 2008;118:3208–3218. doi: 10.1172/JCI35424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Von Brühl M.L., Stark K., Steinhart A. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kannemeier C., Shibamiya A., Nakazawa F. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A. 2007;104:6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller F., Mutch N.J., Schenk W.A. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hojima Y., Cochrane C.G., Wiggins R.C., Austen K.F., Stevens R.L. In vitro activation of the contact (Hageman factor) system of plasma by heparin and chondroitin sulfate. E. Blood. 1984;63:1453–1459. [PubMed] [Google Scholar]
- 32.Yang A., Chen F., He C. The procoagulant activity of apoptotic cells is mediated by interaction with factor XII. Front Immunol. 2017;8:1188. doi: 10.3389/fimmu.2017.01188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herwald H., Mörgelin M., Olsén A. Activation of the contact-phase system on bacterial surfaces—a clue to serious complications in infectious diseases. Nat Med. 1998;4:298–302. doi: 10.1038/nm0398-298. [DOI] [PubMed] [Google Scholar]
- 34.Williams D.F. On the mechanisms of biocompatibility. Biomaterials. 2008;29:2941–2953. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 35.Atkinson J.P., Farries T. Separation of self from non-self in the complement system. Immunol Today. 1987;8:212–215. doi: 10.1016/0167-5699(87)90167-8. [DOI] [PubMed] [Google Scholar]
- 36.Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dobó J., Szakács D., Oroszlánet G. MASP-3 is the exclusive pro-factor D activator in resting blood: the lectin and the alternative complement pathways are fundamentally linked. Sci Rep. 2016;6:31877. doi: 10.1038/srep31877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nilsson B., Ekdahl K.N., Mollnes T.E., Lambris J.D. The role of complement in biomaterial-induced inflammation. Mol Immunol. 2007;44:82–94. doi: 10.1016/j.molimm.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Hein E., Garred P. The lectin pathway of complement and biocompatibility. Adv Exp Med Biol. 2015;865:77–92. doi: 10.1007/978-3-319-18603-0_5. [DOI] [PubMed] [Google Scholar]
- 40.Poppelaars F., Faria B., Gaya da Costa M. The complement system in dialysis: a forgotten story? Front Immunol. 2018;9:71. doi: 10.3389/fimmu.2018.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poothullil J., Shimizu A., Day R.P., Dolovich J. Anaphylaxis from the product(s) of ethylene oxide gas. Ann Intern Med. 1975;82:58–60. doi: 10.7326/0003-4819-82-1-58. [DOI] [PubMed] [Google Scholar]
- 42.Pegues D.A., Oettinger C.W., Bland L.A. A prospective study of pyrogenic reactions in hemodialysis patients using bicarbonate dialysis fluids filtered to remove bacteria and endotoxin. J Am Soc Nephrol. 1992;3:1002–1007. doi: 10.1681/ASN.V341002. [DOI] [PubMed] [Google Scholar]
- 43.Renaux J.L., Thomas M., Crost T., Loughraieb N., Vantard G. Activation of the kallikrein-kinin system in hemodialysis: role of membrane electronegativity, blood dilution, and pH. Kidney Int. 1999;55:1097–1103. doi: 10.1046/j.1523-1755.1999.0550031097.x. [DOI] [PubMed] [Google Scholar]
- 44.Verresen L., Waer M., Vanrenterghem Y., Michielsen P. Angiotensin-converting-enzyme inhibitors and anaphylactoid reactions to high-flux membrane dialysis. Lancet. 1990;336:1360–1362. doi: 10.1016/0140-6736(90)92904-v. [DOI] [PubMed] [Google Scholar]
- 45.Saha M., Allon M. Diagnosis, treatment, and prevention of hemodialysis emergencies. Clin J Am Soc Nephrol. 2017;12:357–369. doi: 10.2215/CJN.05260516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poppelaars F, Gaya da Costa M, Faria B, et al. Intradialytic complement activation precedes the development of cardiovascular events in hemodialysis patients. Front Immunol. 9:2070. [DOI] [PMC free article] [PubMed]
- 47.Kerr P.G., Huang L. Review: membranes for haemodialysis. Nephrology (Carlton) 2010;15:381–385. doi: 10.1111/j.1440-1797.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- 48.Hempel J.C., Poppelaars F., Gaya da Costa M. Distinct in vitro complement activation by various intravenous iron preparations. Am J Nephrol. 2017;45:49–59. doi: 10.1159/000451060. [DOI] [PubMed] [Google Scholar]
- 49.Poppelaars F., Gaya da Costa M., Berger S.P. Strong predictive value of mannose-binding lectin levels for cardiovascular risk of hemodialysis patients. J Transl Med. 2016;14:236. doi: 10.1186/s12967-016-0995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner S., Zschätzsch S., Ansgar Erlenkoette A. Hemocompatibility of polysulfone hemodialyzers—exploratory studies on impact of treatment modality and dialyzer characteristics. Kidney360. 2020;1(1):25–35. doi: 10.34067/KID.0000342019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westphalen H., Saadati S., Eduok U. Case studies of clinical hemodialysis membranes: influences of membrane morphology and biocompatibility on uremic blood-membrane interactions and inflammatory biomarkers. Sci Rep. 2020;10(1):14808. doi: 10.1038/s41598-020-71755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koga Y., Fujieda H., Meguro H. Biocompatibility of polysulfone hemodialysis membranes and its mechanisms: involvement of fibrinogen and its integrin receptors in activation of platelets and neutrophils. Artif Organs. 2018;42(9):E246–E258. doi: 10.1111/aor.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chenoweth D.E., Cheung A.K., Ward D.M., Henderson L.W. Anaphylatoxin formation during hemodialysis: comparison of new and re-used dialyzers. Kidney Int. 1983;24:770–774. doi: 10.1038/ki.1983.226. [DOI] [PubMed] [Google Scholar]
- 54.Hakim R.M., Fearon D.T., Lazarus J.M. Biocompatibility of dialysis membranes: effects of chronic complement activation. Kidney Int. 1984;26:194–200. doi: 10.1038/ki.1984.155. [DOI] [PubMed] [Google Scholar]
- 55.Stroncek D.F., Keshaviah P., Craddock P.R., Hammerschmidt D.E. Effect of dialyzer reuse on complement activation and neutropenia in hemodialysis. J Lab Clin Med. 1984;104:304–311. [PubMed] [Google Scholar]
- 56.Dumler F., Zasuwa G., Levin N.W. Effect of dialyzer reprocessing methods on complement activation and hemodialyzer-related symptoms. Artif Organs. 1987;11:128–131. doi: 10.1111/j.1525-1594.1987.tb02643.x. [DOI] [PubMed] [Google Scholar]
- 57.Gagnon J. Structure and activation of complement components C2 and factor B. Philos Trans R Soc Lond B Biol Sci. 1984;306:301–309. doi: 10.1098/rstb.1984.0091. [DOI] [PubMed] [Google Scholar]
- 58.Gagnon J., Arlaud G.J. Primary structure of the A chain of human complement-classical-pathway enzyme C1r. N-terminal sequences and alignment of autolytic fragments and CNBr-cleavage peptides. Biochem J. 1985;225:135–142. doi: 10.1042/bj2250135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klinkmann H., Grassmann A., Vienken J. Dilemma of membrane biocompatibility and reuse. Artif Organs. 1996;20:426–432. doi: 10.1111/j.1525-1594.1996.tb04527.x. [DOI] [PubMed] [Google Scholar]
- 60.Kuwahara T., Markert M., Wauters J.P. Biocompatibility aspects of dialyzer reprocessing: a comparison of 3 re-use methods and 3 membranes. Clin Nephrol. 1989;32:139–143. [PubMed] [Google Scholar]
- 61.Lundberg L., Johansson G., Karlsson L., Stegmayr B.G. Complement activation is influenced by the membrane material, design of the dialyser, sterilizing method, and type of dialysate. Nephrol Dial Transplant. 1994;9:1310–1314. [PubMed] [Google Scholar]
- 62.Upadhyay A., Jaber B.L. Reuse and biocompatibility of hemodialysis membranes: clinically relevant? Semin Dial. 2017;30:121–124. doi: 10.1111/sdi.12574. [DOI] [PubMed] [Google Scholar]
- 63.Stepniewska J., Dolegowska B., Golembiewska E. The activation of complement system in different types of renal replacement therapy. J Physiol Pharmacol. 2020;71(2):275–281. doi: 10.26402/jpp.2020.2.12. [DOI] [PubMed] [Google Scholar]
- 64.Satomura A., Endo M., Fujita T. Serum mannose-binding lectin levels in maintenance hemodialysis patients: impact on all-cause mortality. Nephron Clin Pract. 2006;102(3-4):c93–c99. doi: 10.1159/000089666. [DOI] [PubMed] [Google Scholar]
- 65.Poppelaars F., Damman J., de Vrij E.L. New insight into the effects of heparinoids on complement inhibition by C1-inhibitor. Clin Exp Immunol. 2016;184:378–388. doi: 10.1111/cei.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lines S.W., Carter A.M. Complement and cardiovascular disease—the missing link in haemodialysis patients? Nephron. 2016;134:104. doi: 10.1159/000448741. [DOI] [PubMed] [Google Scholar]
- 67.Kishida K., Kishida N., Arima M. Serum C1q- binding adiponectin in maintenance hemodialysis patients. BMC Nephrol. 2013;14:50. doi: 10.1186/1471-2369-14-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buraczynska M., Ksiazek P., Wacinski P., Zukowski P., Dragan M., Bednarek-Skublewska A. Complement receptor 1 gene polymorphism and cardiovascular disease in dialyzed end-stage renal disease patients. Hum Immunol. 2010;71:878–882. doi: 10.1016/j.humimm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 69.Buraczynska M., Ksiazek P., Zukowski P., Benedyk-Lorens E., Orlowska-Kowalik G. Complement factor H gene polymorphism and risk of cardiovascular disease in end-stage renal disease patients. Clin Immunol. 2009;132:285–290. doi: 10.1016/j.clim.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 70.Matsuo T., Koide M., Kario K., Suzuki S., Matsuo M. Extrinsic coagulation factors and tissue factor pathway inhibitor in end-stage chronic renal failure. Haemostasis. 1997;27:163–167. doi: 10.1159/000217449. [DOI] [PubMed] [Google Scholar]
- 71.Svensson M., Friberger P., Lundström O., Stegmayr B. Activation of FXII during haemodialysis. Scand J Clin Lab Invest. 1996;56:649–652. doi: 10.3109/00365519609090600. [DOI] [PubMed] [Google Scholar]
- 72.Bouman C.S., de Pont A.C., Meijers J.C. The effects of continuous venovenous hemofiltration on coagulation activation. Crit Care. 2006;10:R150. doi: 10.1186/cc5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salmon J., Cardigan R., Mackie I., Cohen S.L., Machin S., Singer M. Continuous venovenous haemofiltration using polyacrylonitrile filters does not activate contact system and intrinsic coagulation pathways. Intensive Care Med. 1997;23:38–43. doi: 10.1007/s001340050288. [DOI] [PubMed] [Google Scholar]
- 74.Francois K., Orlando C., Jochmans K. Hemodialysis does not induce detectable activation of the contact system of coagulation. Kidney Int Rep. 2020;5:831–838. doi: 10.1016/j.ekir.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Papageorgiou P.C., Chan C.T., Yeo E.L., Backx P.H., Floras J.S. Coagulation factor XIIa-kinin-mediated contribution to hypertension of chronic kidney disease. J Hypertens. 2014;32:1523–1533. doi: 10.1097/HJH.0000000000000192. [discussion: 1533] [DOI] [PubMed] [Google Scholar]
- 76.Hong J., Larsson A., Ekdahl K.N., Elgue G., Larsson R., Nilsson B. Contact between a polymer and whole blood: sequence of events leading to thrombin generation. J Lab Clin Med. 2001;138:139–145. doi: 10.1067/mlc.2001.116486. [DOI] [PubMed] [Google Scholar]
- 77.Lamba N.M., Courtney J.M., Gaylor J.D., Lowe G.D. In vitro investigation of the blood response to medical grade PVC and the effect of heparin on the blood response. Biomaterials. 2000;21:89–96. doi: 10.1016/s0142-9612(99)00145-3. [DOI] [PubMed] [Google Scholar]
- 78.Frank R.D., Mueller U., Lanzmich R., Floege J. Factor XII activation markers do not reflect FXII dependence of thrombin generation induced by polyvinylchloride. J Mater Sci Mater Med. 2013;24:2561–2566. doi: 10.1007/s10856-013-5002-6. [DOI] [PubMed] [Google Scholar]
- 79.Frank R.D., Weber J., Dresbach H., Thelen H., Weiss C., Floege J. Role of contact system activation in hemodialyzer-induced thrombogenicity. Kidney Int. 2001;60:1972–1981. doi: 10.1046/j.1523-1755.2001.00009.x. [DOI] [PubMed] [Google Scholar]
- 80.Schulman G., Hakim R., Arias R., Silverberg M., Kaplan A.P., Arbeit L. Bradykinin generation by dialysis membranes: possible role in anaphylactic reaction. J Am Soc Nephrol. 1993;3:1563–1569. doi: 10.1681/ASN.V391563. [DOI] [PubMed] [Google Scholar]
- 81.Preis M., Hirsch J., Kotler A. Factor XI deficiency is associated with lower risk for cardiovascular and venous thromboembolism events. Blood. 2017;129:1210–1215. doi: 10.1182/blood-2016-09-742262. [DOI] [PubMed] [Google Scholar]
- 82.Mann K.G. Thrombin: can't live without it; probably die from it. Chest. 2003;124:1s–3s. doi: 10.1378/chest.124.3_suppl.1s. [DOI] [PubMed] [Google Scholar]
- 83.Coughlin S.R. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 84.Martorell L., Martínez-González J., Rodríguez C., Gentile M., Calvayrac O., Badimon L. Thrombin and protease-activated receptors (PARs) in atherothrombosis. Thromb Haemost. 2008;99:305–315. doi: 10.1160/TH07-08-0481. [DOI] [PubMed] [Google Scholar]
- 85.Kaplanski G., Marin V., Fabrigoule M. Thrombin-activated human endothelial cells support monocyte adhesion in vitro following expression of intercellular adhesion molecule-1 (ICAM-1; CD54) and vascular cell adhesion molecule-1 (VCAM-1; CD106) Blood. 1998;92:1259–1267. [PubMed] [Google Scholar]
- 86.Minami T., Miura M., Aird W.C., Kodama T. Thrombin-induced autoinhibitory factor, Down syndrome critical region-1, attenuates NFAT-dependent vascular cell adhesion molecule-1 expression and inflammation in the endothelium. J Biol Chem. 2006;281:20503–20520. doi: 10.1074/jbc.M513112200. [DOI] [PubMed] [Google Scholar]
- 87.Jackson S.P., Darbousset R., Schoenwaelder S.M. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133:906–918. doi: 10.1182/blood-2018-11-882993. [DOI] [PubMed] [Google Scholar]
- 88.Koumbadinga G.A., Désormeaux A., Adam A., Marceau F. Effect of interferon-γ on inflammatory cytokine-induced bradykinin B1 receptor expression in human vascular cells. Eur J Pharmacol. 2010;647:117–125. doi: 10.1016/j.ejphar.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 89.Marceau F., Bachelard H., Bouthillier J. Bradykinin receptors: agonists, antagonists, expression, signaling, and adaptation to sustained stimulation. Int Immunopharmacol. 2020;82:106305. doi: 10.1016/j.intimp.2020.106305. [DOI] [PubMed] [Google Scholar]
- 90.Regoli D., Plante G.E., Gobeil F., Jr. Impact of kinins in the treatment of cardiovascular diseases. Pharmacol Ther. 2012;135:94–111. doi: 10.1016/j.pharmthera.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 91.Szabo C., Ischiropoulos H., Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 92.Xu J., Carretero O.A., Shesely E.G. The kinin B1 receptor contributes to the cardioprotective effect of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in mice. Exp Physiol. 2009;94:322–329. doi: 10.1113/expphysiol.2008.045583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hillmeister P., Gatzke N., Dülsner A. Arteriogenesis is modulated by bradykinin receptor signaling. Circ Res. 2011;109:524–533. doi: 10.1161/CIRCRESAHA.111.240986. [DOI] [PubMed] [Google Scholar]
- 94.Figueroa C.D., Matus C.E., Pavicic F. Kinin B1 receptor regulates interactions between neutrophils and endothelial cells by modulating the levels of Mac-1, LFA-1 and intercellular adhesion molecule-1. Innate Immun. 2015;21:289–304. doi: 10.1177/1753425914529169. [DOI] [PubMed] [Google Scholar]
- 95.Leeb-Lundberg L.M., Kang D.S., Lamb M.E., Fathy D.B. The human B1 bradykinin receptor exhibits high ligand-independent, constitutive activity: roles of residues in the fourth intracellular and third transmembrane domains. J Biol Chem. 2001;276:8785–8792. doi: 10.1074/jbc.M007396200. [DOI] [PubMed] [Google Scholar]
- 96.Marney A.M., Ma J., Luther J.M., Ikizler T.A., Brown N.J. Endogenous bradykinin contributes to increased plasminogen activator inhibitor 1 antigen following hemodialysis. J Am Soc Nephrol. 2009;20:2246–2252. doi: 10.1681/ASN.2009050505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lijnen H.R. Pleiotropic functions of plasminogen activator inhibitor-1. J Thromb Haemost. 2005;3:35–45. doi: 10.1111/j.1538-7836.2004.00827.x. [DOI] [PubMed] [Google Scholar]
- 98.Tomura S., Nakamura Y., Doi M. Fibrinogen, coagulation factor VII, tissue plasminogen activator, plasminogen activator inhibitor-1, and lipid as cardiovascular risk factors in chronic hemodialysis and continuous ambulatory peritoneal dialysis patients. Am J Kidney Dis. 1996;27:848–854. doi: 10.1016/s0272-6386(96)90523-5. [DOI] [PubMed] [Google Scholar]
- 99.Hoagland K.M., Maddox D.A., Martin D.S. Bradykinin B2-receptors mediate the pressor and renal hemodynamic effects of intravenous bradykinin in conscious rats. J Auton Nerv Syst. 1999;75:7–15. doi: 10.1016/s0165-1838(98)00166-0. [DOI] [PubMed] [Google Scholar]
- 100.Butani L., Calogiuri G. Hypersensitivity reactions in patients receiving hemodialysis. Ann Allergy Asthma Immunol. 2017;118:680–684. doi: 10.1016/j.anai.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 101.Ebo D.G., Bosmans J.L., Couttenye M.M., Stevens W.J. Haemodialysis-associated anaphylactic and anaphylactoid reactions. Allergy. 2006;61:211–220. doi: 10.1111/j.1398-9995.2006.00982.x. [DOI] [PubMed] [Google Scholar]
- 102.Warkentin T.E., Greinacher A. Heparin-induced anaphylactic and anaphylactoid reactions: two distinct but overlapping syndromes. Exp Opin Drug Saf. 2009;8:129–144. doi: 10.1517/14740330902778180. [DOI] [PubMed] [Google Scholar]
- 103.Guerrini M., Beccati D., Shriver Z. Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nat Biotechnol. 2008;26:669–675. doi: 10.1038/nbt1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kishimoto T.K., Viswanathan K., Ganguly T. Contaminated heparin associated with adverse clinical events and activation of the contact system. N Engl J Med. 2008;358:2457–2467. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tielemans C., Madhoun P., Lenaers M., Schandene L., Goldman M., Vanherweghem J.L. Anaphylactoid reactions during hemodialysis on AN69 membranes in patients receiving ACE inhibitors. Kidney Int. 1990;38:982–984. doi: 10.1038/ki.1990.301. [DOI] [PubMed] [Google Scholar]
- 106.Pinckard RN, Tanigawa C, Halonen M. IgE-induced blood coagulation alterations in the rabbit: consumption of coagulation factors XII, XI, and IX in vivo. J Immunol. ep115:525-532. [PubMed]
- 107.Sala-Cunill A., Björkqvist J., Senter R. Plasma contact system activation drives anaphylaxis in severe mast cell-mediated allergic reactions. J Allergy Clin Immunol. 2015;135:1031–1043.e1036. doi: 10.1016/j.jaci.2014.07.057. [DOI] [PubMed] [Google Scholar]
- 108.Hamad O.A., Mitroulis I., Fromell K. Contact activation of C3 enables tethering between activated platelets and polymorphonuclear leukocytes via CD11b/CD18. Thromb Haemost. 2015;114:1207–1217. doi: 10.1160/TH15-02-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kourtzelis I., Markiewski M.M., Doumas M. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood. 2010;116:631–639. doi: 10.1182/blood-2010-01-264051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schuett K., Savvaidis A., Maxeiner S. Clot structure: a potent mortality risk factor in patients on hemodialysis. J Am Soc Nephrol. 2017;28:1622–1630. doi: 10.1681/ASN.2016030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wiegner R., Chakraborty S., Huber-Lang M. Complement-coagulation crosstalk on cellular and artificial surfaces. Immunobiology. 2016;221:1073–1079. doi: 10.1016/j.imbio.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 112.Irmscher S., Döring N., Halder L.D. Kallikrein cleaves C3 and activates complement. J Innate Immunol. 2018;10:94–105. doi: 10.1159/000484257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lopatko Fagerström I., Ståhl A.L., Mossberg M. Blockade of the kallikrein-kinin system reduces endothelial complement activation in vascular inflammation. EBioMedicine. 2019;47:319–328. doi: 10.1016/j.ebiom.2019.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dhondt A., Vanholder R., Tielemans C. Effect of regional citrate anticoagulation on leukopenia, complement activation, and expression of leukocyte surface molecules during hemodialysis with unmodified cellulose membranes. Nephron. 2000;85:334–342. doi: 10.1159/000045683. [DOI] [PubMed] [Google Scholar]
- 115.Huang S., Sandholm K., Jonsson N. Low concentrations of citrate reduce complement and granulocyte activation in vitro in human blood. Clin Kidney J. 2015;8:31–37. doi: 10.1093/ckj/sfu127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reis E.S., DeAngelis R.A., Chen H., Resuello R.R., Ricklin D., Lambris J.D. Therapeutic C3 inhibitor Cp40 abrogates complement activation induced by modern hemodialysis filters. Immunobiology. 2015;220:476–482. doi: 10.1016/j.imbio.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu Y.Q., Qu H., Sfyroera G. Protection of nonself surfaces from complement attack by factor H-binding peptides: implications for therapeutic medicine. J Immunol. 2011;186:4269–4277. doi: 10.4049/jimmunol.1003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dager W.E., Tsu L.V., Pon T.K. Considerations for systemic anticoagulation in ESRD. Semin Dial. 2015;28:354–362. doi: 10.1111/sdi.12376. [DOI] [PubMed] [Google Scholar]
- 119.Kenne E., Nickel K.F., Long A.T. Factor XII: a novel target for safe prevention of thrombosis and inflammation. J Intern Med. 2015;278:571–585. doi: 10.1111/joim.12430. [DOI] [PubMed] [Google Scholar]
- 120.Bonventre J.V., Hurst F.P., West M., Wu I., Roy-Chaudhury P., Sheldon M. A technology roadmap for innovative approaches to kidney replacement therapies: a catalyst for change. Clin J Am Soc Nephrol. 2019;14:1539–1547. doi: 10.2215/CJN.02570319. [DOI] [PMC free article] [PubMed] [Google Scholar]