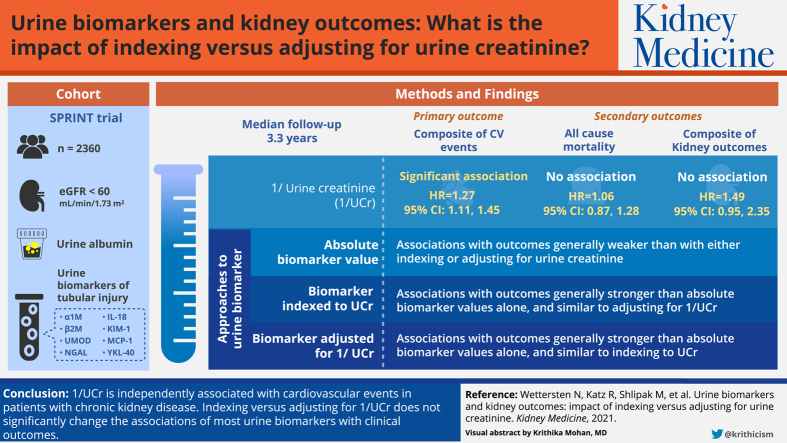
Abstract
Rationale & Objective
Urinary biomarker concentrations are frequently indexed to urinary creatinine (Ucr) concentration in spot samples to account for urine dilution; however, this may introduce biases. We evaluated whether indexing versus adjusting urinary biomarker concentrations for Ucr concentration altered their associations with outcomes.
Study Design
Observational cohort.
Setting & Participants
We analyzed data from 2,360 Systolic Blood Pressure Intervention Trial (SPRINT) participants with estimated glomerular filtration rates < 60 mL/min/1.73 m2 and urinary albumin (UAlb) and 8 urinary kidney tubule biomarkers measured at baseline.
Outcomes
The primary outcome was a composite of cardiovascular disease events; secondary outcomes were all-cause mortality and a composite of kidney outcomes (50% estimated glomerular filtration rate decline, end-stage kidney disease, or transplantation).
Analytical Approach
We used Cox proportional hazards regression to examine the associations of 1/Ucr with outcomes and compared the associations of UAlb and 8 individual urinary tubule biomarkers with outcomes, analyzed by indexing to Ucr, adjusting for 1/Ucr or the biomarker alone (without Ucr concentration).
Results
During a median follow-up of 3.3 years, 307 composite cardiovascular events, 166 deaths, and 34 composite kidney outcomes occurred. After multivariable adjustment, 1/Ucr was significantly associated with cardiovascular events (HR, 1.27 per 2-fold higher; 95% CI, 1.11-1.45), not associated with either mortality (HR, 1.06; 95% CI, 0.87-1.28) or kidney events (HR, 1.49; 95% CI, 0.95-2.35). For UAlb and urinary tubule biomarker concentrations, most risk estimates were not significantly different when indexed to Ucr concentration versus adjusted for 1/Ucr.
Limitations
Cohort excluded patients with diabetes and overall had low levels of albuminuria.
Conclusions
1/Ucr is independently associated with cardiovascular events in trial participants with chronic kidney disease. Indexing versus adjusting for 1/Ucr does not significantly change the associations of most urinary biomarkers with clinical outcomes.
Index Words: Biomarkers, prognosis, cardiovascular disease, mortality, kidney disease
Graphical abstract
Plain-Language Summary.
Urinary biomarkers are often indexed to urinary creatinine concentration to account for urine dilution, but urinary creatinine concentration itself is associated with outcomes. We evaluated associations of urinary creatinine concentration itself and compared the associations of concentrations of urinary albumin and 8 urinary tubular biomarkers with cardiovascular outcomes, kidney outcomes, and mortality when indexed versus adjusted for urinary creatinine concentration among nondiabetic individuals with kidney disease. Urinary creatinine concentration was significantly associated with cardiovascular outcomes. Comparing indexing versus adjusting for urinary creatinine concentration, all biomarkers had directionally consistent associations with the outcomes; risk estimates for most biomarkers did not significantly differ. These findings affirm indexing biomarkers to urinary creatinine concentration clinically, but the impact of indexing versus adjusting for urinary creatinine concentration needs further study.
Multiple urinary biomarkers of kidney tubular health have diagnostic and prognostic utility in various clinical settings.1,2 Although timed urine collection is a more accurate method for measuring urinary biomarker excretion, it is cumbersome and prone to inaccuracies from incomplete urine collection. Thus, spot urine specimens are frequently used in research and clinical practice to assess biomarker concentrations, and their values are usually indexed to urinary creatinine (Ucr) concentration to account for urine dilution, such as with urinary albumin (UAlb) for urinary albumin-creatinine ratio (UACR). Without accounting for urine dilution, the prognostic significance of a urinary biomarker may be masked in an individual by the interindividual variability in urine dilution.
However, indexing a urinary biomarker concentration to Ucr concentration may bias the diagnostic and prognostic ability of that biomarker. Studies have questioned how well Ucr concentration accounts for urine dilution given substantial interindividual variability in creatinine excretion and spot Ucr values and how Ucr indexing may potentially add further errors.3,4 Beyond assessing urine dilution, Ucr concentration directly correlates with muscle mass and physical activity, and lower Ucr values have been associated with higher risk for mortality, coronary artery disease, heart failure (HF), and kidney disease progression in prior studies.5, 6, 7, 8, 9, 10, 11 Furthermore, indexing a biomarker to Ucr concentration compounds the assay imprecisions in the laboratory measurement of the biomarker and Ucr, obscures the relationship of the biomarker in the numerator with Ucr in the denominator for the outcome, and assumes a linear relationship between the biomarker and Ucr concentrations.12,13 Theoretically, the latter may be relevant when indexing Ucr concentration to a distal tubule biomarker. Ucr is subjected to water reabsorption throughout the tubule influencing its concentration, whereas a distal biomarker's concentration will not be influenced by this upstream absorption of water. Consequently, concentrations of a distal tubule biomarker and Ucr are not likely to be similarly influenced by water reabsorption. A recent study using the National Health and Nutrition Examination Survey (NHANES) in 2009 and 2010 showed that the risk estimate of UAlb concentration for death significantly differed when UAlb concentration was indexed to Ucr versus adjusted for Ucr concentration.14 Whether these findings can be recapitulated in other patient samples remains to be determined.
In this study, we aimed to: (1) examine whether spot Ucr concentration is prognostic for the outcomes of cardiovascular disease (CVD), mortality, and chronic kidney disease (CKD) progression; (2) confirm that accounting for urine dilution changes the risk prediction from urinary biomarkers for these outcomes; and (3) compare these risk estimates when urinary biomarkers are indexed versus adjusted for Ucr concentration in a subset of participants from the Systolic Blood Pressure Intervention Trial (SPRINT) with estimated glomerular filtration rates (eGFRs) < 60 mL/min/1.73 m2.15, 16, 17 These biomarkers have been previously shown in SPRINT to associate with acute kidney injury, CKD progression, cardiovascular events, and mortality.16, 17, 18, 19 A priori, we hypothesized that indexing biomarkers to Ucr concentration would alter their prognostication of outcomes compared with adjustment for Ucr concentration.
Methods
Population
The study design and primary results of SPRINT have been previously published.15,20 Briefly, SPRINT is an open-label clinical trial that randomly assigned persons with systolic blood pressures (SBPs) ≥ 130 mm Hg and at high risk for CVD events to an SBP target of <120 mm Hg (intensive arm) versus <140 mm Hg (standard arm). Participants were recruited from 102 centers in the United States and Puerto Rico. Inclusion criteria were 50 years and older, SBP of 130 to 180 mm Hg, and increased risk for CVD events (prior clinical or subclinical CVD other than stroke, 10-year risk for CVD ≥ 15% based on the Framingham risk score, CKD defined as eGFR of 20-59 mL/min/1.73 m2, or aged ≥75 years). Major exclusion criteria comprised diabetes mellitus, urinary protein excretion > 1 g/d, polycystic kidney disease, prior stroke or transient ischemic attack, symptomatic HF, or left ventricular ejection fraction < 35%. A total of 9,361 participants were enrolled between November 2010 and March 2013. Institutional review boards of all participating institutions approved the study.
Among a subgroup of 2,908 SPRINT participants with eGFRs < 60 mL/min/1.73 m2 calculated using the CKD Epidemiology Collaboration (CKD-EPI) creatinine–cystatin C (eGFRcrCysC) equation, we excluded 449 participants with missing Ucr and 99 with missing urinary biomarker data for the present analysis, resulting in a final analytic sample of 2,360 participants.21
Biomarker Measurements
Spot urine specimens and venous blood specimens were collected at the baseline visit and stored at −80 °C at a central laboratory until measurement of biomarkers. Urinary biomarkers were measured in duplicate and averaged to improve precision at the Laboratory for Clinical Biochemistry Research at the University of Vermont. Ucr was measured enzymatically on a Roche Chemistry Analyzer, with calibration traceable to an isotope-dilution mass spectrometry procedure and an interassay coefficient of variation (CV) of 1.5% to 4.3%. UAlb was measured by nephelometry using the Siemens ProSpec nephelometer, with an interassay CV of 2.2% to 6.9%. The analytic methods for the tubular biomarkers α1-microglobulin, β2-microglobulin (B2M), uromodulin (UMOD), neutrophil gelatinase-associated lipocalin (NGAL), interleukin 18 (IL-18), kidney injury marker 1 (KIM-1), monocyte chemoattractant protein 1 (MCP-1), and chitinase-3-like protein 1 (YKL-40) have been previously described.17, 18, 19 Samples with biomarker values below the limit of detection were assigned a value equivalent to the lower limit of detection divided by the square root of 2. Overall, there was a small percentage of samples with values below the limit of detection, which ranged from 0.1% for NGAL to 3% for B2M.
Outcomes
We used the SPRINT primary outcome, a composite of cardiovascular events consisting of myocardial infarction, acute coronary syndrome, stroke, acute decompensated HF, or cardiovascular death.12 Secondary outcomes included all-cause mortality and a composite kidney outcome used in SPRINT for participants with CKD, defined as a decrease in eGFR ≥ 50%, incident end-stage kidney disease requiring dialysis, or kidney transplantation.
Covariates
Baseline demographic, medical history, and clinical and laboratory characteristics collected at study enrollment were used in this analysis. Demographic characteristics consisted of age, sex, and race. Medical history included smoking status (never, former, and current), history of CVD, and history of HF. Clinical characteristics measured before randomization included body mass index (BMI), SBP, diastolic blood pressure, number of antihypertensive medications, and randomization arm. Laboratory characteristics included baseline values of total serum cholesterol, serum high-density lipoprotein cholesterol, serum low-density lipoprotein cholesterol, and eGFR calculated using the CKD-EPIcrCysC equation.
Statistical Analysis
Participant characteristics were compared by quartiles of baseline Ucr concentrations. Spearman correlation coefficients were used to assess correlations between concentrations of Ucr and unindexed urinary biomarkers. Ucr, UAlb, and urinary biomarker concentrations had a skewed distribution and were log base-2 transformed (calculated as log2[biomarker/Ucr]) so that higher levels could be interpreted as “per 2-fold higher level” of the urinary biomarker value.
For all analyses, we examined the relationship of 1/Ucr (log2[1/Ucr]) with outcomes. We examined 1/Ucr instead of Ucr concentration to be consistent with how Ucr concentration is applied when indexing urinary biomarkers. We used multivariable Cox proportional hazards regression to estimate the hazard ratio and corresponding 95% CI of 1/Ucr with time to the primary and secondary outcomes. We constructed models with sequential multivariable adjustment. Model 1 adjusted for age, sex, race, and randomization arm. Model 2 additionally adjusted for baseline eGFR, smoking status, history of CVD, number of antihypertensive medications, SBP, diastolic blood pressure, BMI, and high-density lipoprotein and total cholesterol levels. Finally, model 3 additionally adjusted for UAlb concentration. To evaluate the functional form of 1/Ucr with outcomes, we used restricted cubic spline functions with knots placed at the quartiles of the 1/Ucr distribution.
Next, we evaluated the associations of concentrations of UAlb and each urinary tubular biomarker with the primary and secondary outcomes using a fully adjusted model with a parallel approach. First, the biomarker’s absolute value; second, the biomarker indexed to Ucr concentration; and third, the biomarker adjusted for 1/Ucr as a separate covariate. The multivariable Cox proportional hazards regression model adjusted for age, sex, race, randomization arm, baseline eGFR, smoking status, history of CVD, baseline number of antihypertensive medications, SBP, diastolic blood pressure, BMI, and high-density lipoprotein and total cholesterol levels. To compare hazard ratios of the markers indexed to Ucr concentration versus adjusted for 1/Ucr, we used the method of Lin et al22 that assesses the difference in surrogate markers. The method constructs an artificial bivariate survival function formulated from 2 proportional hazards functions; one for the indexed biomarker and the other for the biomarker adjusted for Ucr concentration. The joint distribution of the β coefficients for the biomarkers allows us to make inferences and compare the strengths of the 2 markers.
All analyses were performed with SPSS, version 26.0 (IBM Corp), and R, version 3.6.0 (https://www.R-project.org/). A 2-sided P0.05 was considered significant for all analyses.
Results
Patient Characteristics
Among the 2,360 participants included in this study, average age ± standard deviation at baseline was 73 ± 9 years (Table 1). Twenty-five percent of participants had prevalent CVD, 6% had prevalent HF, and 55% were current or former smokers. Mean eGFR was 46 ± 11 mL/min/1.73 m2, and median Ucr concentration was 112 (interquartile range [IQR], 75-161) mg/dL. Values were higher in men (112; IQR, 81-171 mg/dL) than women (98; IQR, 65-171 mg/dL) and among Black individuals (126; IQR, 87-190 mg/dL) compared with White individuals (108; IQR, 72-154 mg/dL), Hispanic individuals (105; IQR, 79-153 mg/dL), and other races (110; IQR, 57-136 mg/dL). Table 1 displays demographic and clinical characteristics stratified by Ucr quartiles. Participants in the lower Ucr quartiles were on average older, more often women, and of White race and had lower BMI values compared with higher quartiles. Although median serum creatinine values were highest among persons in the higher Ucr quartiles, median serum cystatin C values were lower in persons within the higher Ucr quartiles.
Table 1.
Baseline Characteristics by Quartiles of Urinary Creatinine
| N | Q1 |
Q2 |
Q3 |
Q4 |
Total |
|---|---|---|---|---|---|
| 590 | 590 | 590 | 590 | 2,360 | |
| Range, mg/dL | <75 | 75-112 | 113-161 | ≥162 | |
| Randomization arm | |||||
| Standard | 289 (49%) | 286 (49%) | 285 (48%) | 300 (51%) | 1,160 (49%) |
| Intensive | 301 (51%) | 304 (51%) | 305 (52%) | 290 (49%) | 1,200 (51%) |
| Age, y | 75 ± 9 | 74 ± 9 | 73 ± 9 | 71 ± 9 | 73 ± 9 |
| Female sex | 307 (52%) | 247 (42%) | 195 (33%) | 192 (33%) | 941 (40%) |
| Race/ethnicity | |||||
| Non-Hispanic White | 428 (73%) | 398 (68%) | 392 (66%) | 340 (58%) | 1,558 (66%) |
| Non-Hispanic Black | 114 (19%) | 134 (23%) | 147 (25%) | 210 (36%) | 605 (26%) |
| Hispanic | 35 (6%) | 52 (9%) | 36 (6%) | 36 (6%) | 159 (7%) |
| Other | 13 (2%) | 6 (1%) | 15 (3%) | 4 (1%) | 38 (2%) |
| Smoking | |||||
| Never | 273 (46%) | 271 (46%) | 250 (42%) | 270 (46%) | 1,064 (45%) |
| Former | 279 (47%) | 258 (44%) | 289 (49%) | 261 (44%) | 1,087 (46%) |
| Current | 38 (6%) | 61 (10%) | 51 (9%) | 59 (10%) | 209 (9%) |
| BMI, kg/m2 | 28.8 ± 5.8 | 29.4 ± 6.1 | 29.8 ± 5.7 | 30.2 ± 5.9 | 29.5 ± 5.9 |
| History of cardiovascular disease | 130 (22%) | 167 (28%) | 159 (27%) | 141 (24%) | 597 (25%) |
| History of heart failure | 51 (9%) | 34 (6%) | 31 (5%) | 35 (6%) | 151 (6%) |
| Systolic BP, mm Hg | 142 ± 17 | 140 ± 16 | 138 ± 15 | 138 ± 16 | 139 ± 16 |
| Diastolic BP, mm Hg | 73 ± 12 | 73 ± 12 | 74 ± 12 | 76 ± 13 | 74 ± 12 |
| No. of antihypertensive medications | |||||
| 0 | 26 (4%) | 27 (5%) | 25 (4%) | 19 (3%) | 97 (4%) |
| 1 | 115 (20%) | 115 (20%) | 145 (25%) | 149 (25%) | 524 (22%) |
| 2 | 202 (34%) | 232 (39%) | 212 (36%) | 215 (36%) | 861 (37%) |
| 3 | 187 (32%) | 162 (28%) | 166 (28%) | 163 (28%) | 678 (29%) |
| ≥4 | 60 (10%) | 54 (9%) | 42 (7%) | 44 (8%) | 200 (9%) |
| RAAS-I | 362 (61%) | 374 (63%) | 377 (64%) | 354 (60%) | 1,467 (62%) |
| Diuretics | 373 (63%) | 322 (55%) | 302 (51%) | 294 (50%) | 1,291 (55%) |
| Total cholesterol, mg/dL | 186 ± 39 | 184 ± 42 | 182 ± 40 | 183 ± 41 | 184 ± 41 |
| LDL cholesterol, mg/dL | 105 ± 33 | 106 ± 34 | 106 ± 34 | 107 ± 36 | 106 ± 35 |
| HDL cholesterol, mg/dL | 56 ± 15 | 53 ± 15 | 51 ± 14 | 50 ± 13 | 52 ± 14 |
| Median serum creatinine, mg/dL | 1.30 [1.11-1.57] | 1.33 [1.16-1.58] | 1.35 [1.19-1.60] | 1.36 [1.20-1.58] | 1.34 [1.17-1.59] |
| Median serum cystatin C, mg/L | 1.45 [1.27-1.79] | 1.45 [1.28-1.74] | 1.39 [1.25-1.66] | 1.36 [1.23-1.56] | 1.41 [1.26-1.69] |
| eGFR, mL/min/1.73 m2 | 44 ± 11 | 44 ± 11 | 46 ± 10 | 48 ± 9 | 46 ± 11 |
| Median urinary creatinine, mg/dL | 51 [37-65] | 94 [85-103] | 133 [121-148] | 206 [180-250] | 112 [75-161] |
| β2-microglobulin, ng/mL | 87 [30-355] | 132 [48-461] | 90 [41-299] | 102 [28-244] | 101 [36-325] |
| α1-microglobulin, mg/L | 8 [4-14] | 15 [8-25] | 15 [9-28] | 21 [10-35] | 14 [7-25] |
| YKL-40, ng/mL | 269 [113-689] | 448 [188-1,073] | 603 [304-1,144] | 1,080 [561-2,135] | 555 [223-1,267] |
| IL-18, ng/mL | 13 [8-23] | 26 [17-42] | 37 [24-59] | 60 [39-108] | 31 [17-57] |
| UMOD, ng/mL | 4 [3-6] | 6 [5-9] | 8 [6-11] | 9 [6-12] | 7 [4-10] |
| MCP-1, pg/mL | 64 [32-102] | 149 [102-242] | 221 [152-331] | 389 [269-602] | 185 [94-331] |
| KIM-1, pg/mL | 276 [110-520] | 717 [424-1,094] | 1,080 [702-1,643] | 1,902 [1,266-3,043] | 872 [411-1,618] |
| NGAL, ng/mL | 17 [9-39] | 26 [15-56] | 31 [18-60] | 39 [23-77] | 28 [15-60] |
| Urinary albumin, mg/L | 11 [4-35] | 14 [7-57] | 17 [8-56] | 24 [13-67] | 16 [8-52] |
Note: Data presented as mean ± standard deviation, number (percent), or median [interquartile range].
Abbreviations BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Q, quartile; RAAS-I, renin-angiotensin-aldosterone system inhibitor; IL-18, interleukin 18; KIM-1, kidney injury marker-1; MCP-1, monocyte chemoattractant protein 1; NGAL, neutrophil gelatinase-associated lipocalin; UMOD, uromodulin; YKL-40, chitinase-3-like protein-1.
Urinary Biomarker Correlations
Ucr concentration was significantly and positively correlated with concentrations of all biomarkers except B2M (ρ=0.03). Spearman correlation coefficients from weakest to strongest were 0.26 for UAlb, 0.30 for NGAL, 0.36 for α1-microglobulin, 0.38 for YKL-40, 0.46 for UMOD, 0.61 for IL-18, 0.71 for KIM-1, and 0.74 for MCP-1. Thus, with the exception of MCP-1, KIM-1, and potentially IL-18, most urinary biomarkers were only moderately to weakly correlated with Ucr concentration.
Associations of Ucr With Cardiovascular Events, Mortality, and Kidney Outcomes
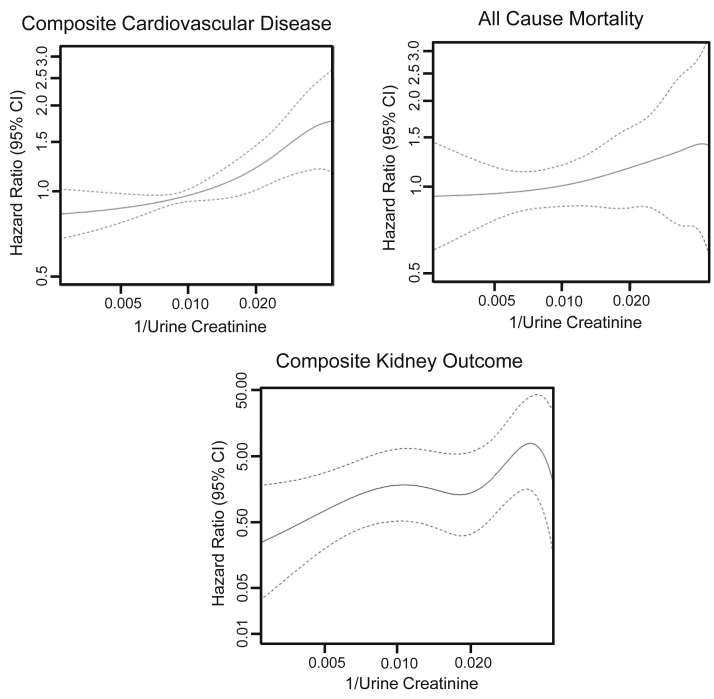
During a median 3.3 (IQR, 2.8-3.9) years of follow-up, 307 participants experienced the composite CVD outcome, 166 died, and 34 experienced the composite kidney outcome. Higher 1/Ucr (ie, lower Ucr concentration) was associated with incrementally higher risk for the composite CVD outcome and mortality end points but with variable risk for the composite kidney outcome (Fig 1).
Figure 1.
Association of 1/urinary creatinine (Ucr) with cardiovascular events, mortality, and compositive kidney outcome. Because Ucr concentration is in the denominator, smaller Ucr values are reflected as larger values on the right side of the x-axis. Risk generally increases as Ucr values are lower.
When adjusted for age, sex, race, and randomization arm (model 1), per 2-fold higher, 1/Ucr was associated with 23% higher risk for CVD events (Table 2). The risk estimate was lower but remained statistically significant after multivariable adjustment for CVD risk factors (model 2). When 1/Ucr was additionally adjusted for UAlb concentration (model 3), the risk estimate of 1/Ucr was numerically the highest of all models. Thus, higher 1/Ucr (ie, lower Ucr concentration) was independently associated with higher CVD risk in the fully adjusted model. In contrast, 1/Ucr was not significantly associated with all-cause mortality in any model, but the risk estimate changed in a similar manner as with CVD events with sequential adjustments. Although 1/Ucr was significantly associated with kidney outcomes in model 1, it was not significant in models 2 or 3.
Table 2.
Associations of 1/Urinary Creatinine With Composite CVD Events, Mortality, and the Composite Kidney Outcome
| HR (95% CI) |
HR (95% CI) |
HR (95% CI) |
|
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Primary CVD events (307 events) | 1.23 (1.08-1.39) | 1.15 (1.01-1.32) | 1.27 (1.11-1.45) |
| All-cause mortality (166 events) | 1.07 (0.89-1.27) | 0.96 (0.80-1.15) | 1.06 (0.87-1.28) |
| Composite kidney outcome (34 events) | 1.75 (1.25-2.44) | 1.11 (0.75-1.65) | 1.49 (0.95-2.35) |
Note: All biomarker HRs are per 2-fold increase. Model 1: age, sex, race, and randomization arm. Model 2: model 1 plus baseline estimated glomerular filtration rate, smoking status, history of CVD, baseline number of antihypertensive medications, systolic blood pressure, diastolic blood pressure, body mass index, and high-density lipoprotein and total cholesterol levels. Model 3: adjusted for all factors plus urinary albumin level.
Abbreviation: CVD, cardiovascular disease; HR, hazard ratio.
Effect of Adjusting Versus Indexing for Ucr on the Association of UAlb and Tubule Biomarkers With Cardiovascular Events, Mortality, and Kidney Outcomes
To examine how the risk estimates for UAlb and each urinary tubule biomarker’s association with outcomes differed by how Ucr concentration was modeled, we analyzed each biomarker alone, indexed to Ucr concentration, or adjusted for 1/Ucr within a fully adjusted model (Table 3). For the composite CVD outcome, 5 of the 9 biomarkers were significantly associated with this outcome. When indexed to Ucr concentration, risk estimates for all biomarkers except B2M, UMOD, and KIM-1 were significantly higher for the composite CVD outcome (Table S1). Similarly, when adjusting for 1/Ucr, risk estimates for all biomarkers except B2M and UMOD were significantly higher for the composite CVD outcome (Table S1). However, risk estimates for biomarkers indexed to Ucr concentration versus adjusted for 1/Ucr were not significantly different except for UMOD, MCP-1, and KIM-1, though the direction of change of risk estimates for both MCP-1 and KIM-1 were similar (Table 3). Patterns were similar for the all-cause mortality and kidney outcomes (Tables S1 and 3). For these 2 outcomes, differences in risk estimates between the indexed or adjusted biomarker concentrations were not significant.
Table 3.
Urinary Biomarker Associations With Longitudinal Outcomes Based on the Absolute Value of Biomarker, Biomarker Indexed to Ucr, and Biomarker Adjusted for 1/Ucr
| Biomarker | HR (95% CI) |
HR (95% CI) |
HR (95% CI) |
P |
|---|---|---|---|---|
| Absolute Biomarker Level | Biomarker/Ucr | Biomarker Adjusted for 1/Ucr | Indexed vs Adjusted HR | |
| Composite Cardiovascular Disease Outcome (n=307) | ||||
| β2-microglobulin | 1.05 (1.01-1.10) | 1.06 (1.02-1.11) | 1.05 (1.01-1.10) | 0.29 |
| α1-microglobulin | 1.28 (1.16-1.42) | 1.43 (1.29-1.60) | 1.44 (1.29-1.62) | 0.69 |
| YKL-40 | 1.06 (1.00-1.11) | 1.09 (1.03-1.15) | 1.09 (1.03-1.15) | 0.23 |
| IL-18 | 1.07 (0.98-1.16) | 1.21 (1.09-1.34) | 1.21 (1.09-1.34) | 0.88 |
| UMOD | 0.80 (0.72-0.90) | 0.86 (0.75-0.98) | 0.79 (0.68-0.91) | <0.01 |
| MCP-1 | 1.03 (0.96-1.11) | 1.17 (1.05-1.31) | 1.23 (1.10-1.37) | 0.01 |
| KIM-1 | 0.99 (0.93-1.06) | 1.05 (0.95-1.15) | 1.10 (0.99-1.22) | 0.03 |
| NGAL | 1.07 (0.99-1.14) | 1.11 (1.04-1.19) | 1.10 (1.02-1.18) | 0.21 |
| Albumin | 1.14 (1.08-1.21) | 1.18 (1.11-1.24) | 1.17 (1.10-1.24) | 0.23 |
| All-Cause Mortality (n=166) | ||||
| β2-microglobulin | 0.98 (0.93-1.04) | 0.99 (0.94-1.04) | 0.99 (0.93-1.04) | 0.80 |
| α1-microglobulin | 1.25 (1.09-1.44) | 1.29 (1.11-1.50) | 1.33 (1.13-1.56) | 0.29 |
| YKL-40 | 1.11 (1.04-1.19) | 1.13 (1.05-1.21) | 1.13 (1.05-1.21) | 0.94 |
| IL-18 | 1.15 (1.03-1.29) | 1.27 (1.10-1.46) | 1.27 (1.10-1.46) | 0.95 |
| UMOD | 0.88 (0.76-1.04) | 0.87 (0.72-1.04) | 0.84 (0.69-1.02) | 0.50 |
| MCP-1 | 1.09 (0.98-1.21) | 1.21 (1.04-1.41) | 1.23 (1.06-1.43) | 0.48 |
| KIM-1 | 1.07 (0.98-1.18) | 1.17 (1.01-1.34) | 1.20 (1.03-1.39) | 0.41 |
| NGAL | 1.12 (1.02-1.23) | 1.13 (1.03-1.24) | 1.13 (1.03-1.25) | 0.70 |
| Albumin | 1.18 (1.09-1.27) | 1.18 (1.09-1.27) | 1.18 (1.10,1.28) | 0.37 |
| Composite Kidney Outcome (n=34) | ||||
| β2-microglobulin | 1.11 (0.98-1.26) | 1.11 (0.98-1.25) | 1.10 (0.96-1.25) | 0.58 |
| α1-microglobulin | 1.18 (0.87-1.58) | 1.44 (1.03-1.20) | 1.40 (0.99-1.97) | 0.45 |
| YKL-40 | 1.11 (0.98-1.26) | 1.17 (1.02-1.34) | 1.17 (1.03-1.34) | 0.84 |
| IL-18 | 1.21 (0.95-1.55) | 1.43 (1.09-1.87) | 1.40 (1.07-1.84) | 0.42 |
| UMOD | 0.78 (0.57-1.08) | 0.90 (0.61-1.33) | 0.82 (0.54-1.24) | 0.10 |
| MCP-1 | 1.21 (0.94-1.55) | 1.68 (1.22-2.32) | 1.72 (1.25-2.36) | 0.52 |
| KIM-1 | 1.14 (0.90-1.43) | 1.69 (1.17-2.44) | 1.84 (1.27-2.66) | 0.16 |
| NGAL | 1.19 (0.97-1.46) | 1.27 (1.04-1.56) | 1.25 (1.02-1.54) | 0.45 |
| Albumin | 1.37 (1.15-1.63) | 1.44 (1.20-1.73) | 1.44 (1.20-1.73) | 0.53 |
Note: All biomarker HRs are per 2-fold higher. All models adjusted for age, sex, race, randomized treatment arm, baseline estimated glomerular filtration rate, smoking status, history of cardiovascular disease, baseline number of antihypertensive medications, systolic blood pressure, diastolic blood pressure, body mass index, and high-density lipoprotein and total cholesterol levels.
Abbreviations: HR, hazard ratio; IL-18, interleukin 18; KIM-1, kidney injury marker 1; MCP-1, monocyte chemoattractant protein-1; NGAL, neutrophil gelatinase-associated lipocalin; Ucr, urinary creatinine; UMOD, uromodulin; YKL-40, chitinase-3-like protein-1.
Discussion
In this study of SPRINT participants with CKD, we found that lower spot Ucr concentration was independently associated with CVD events. Ucr concentration was not significantly associated with all-cause mortality or kidney events; however, our findings suggest an association with Ucr concentration for kidney events that likely did not reach statistical significance because of a limited number of events. Contrary to our initial hypothesis, indexing to versus adjusting for 1/Ucr resulted in directionally similar changes in risk estimates for biomarkers with similar magnitudes of risk for UAlb and most tubular biomarkers for all 3 outcomes.
Multiple studies have shown that Ucr concentration is associated with risk for CVD, HF, CKD progression, and mortality.7, 8, 9, 10, 11 We also observed that lower Ucr concentration was independently associated with CVD; however, in contrast to these prior studies, we found that Ucr concentration was not significantly associated with mortality. Ucr concentration was nearly associated with kidney events, with a relative hazard of 1.49, but the CIs were too wide to rule out the possibility of no effect. With only 34 kidney events, these findings should therefore be interpreted cautiously. Our findings may differ from prior studies due to differences in the timing of urine specimens. Most prior studies used 24-hour Ucr measurements and examined Ucr excretion, whereas we evaluated spot Ucr concentrations.7, 8, 9, 10 Although both 24-hour Ucr excretion and spot Ucr concentration are influenced by muscle mass and activity, the spot Ucr concentration is more susceptible to fluctuations throughout the day due to differences in hydration status and urine dilution.3 This may have biased the association of Ucr concentration with outcomes toward the null in our study. Additionally, our cohort was restricted to patients with CKD, which may also have influenced Ucr excretion and risks for adverse outcomes.23
UACR is a well-established biomarker ratio associated with increased mortality and kidney disease progression.24 When we evaluated UAlb concentration, risk estimates did not substantially differ whether we indexed to Ucr concentration or adjusted for 1/Ucr. Prior studies have also found that UACR is prognostic for outcomes across a spectrum of different Ucr concentrations despite low Ucr concentration itself being prognostic for outcomes.25,26 Recently, an analysis of 5,642 participants in NHANES in 2009 and 2010 showed that the risk estimate of UAlb concentration for death significantly differed when UAlb concentration was indexed to Ucr versus adjusted for Ucr concentration.14 This contrasts with our findings, likely due to differences in study populations. The NHANES analysis had a study population inclusive of individuals with diabetes and only 8% of participants had eGFRs < 60 mL/min/1.73 m2. Conversely, our population comprised older, hypertensive, and nondiabetic individuals with CKD. These discordant findings imply that the approaches to account for urine dilution using Ucr concentration may yield varying estimates based on the study population and setting.
Although UACR has been extensively studied with well-described prognostic implications, how to appropriately control for urine dilution with novel urinary biomarkers remains unclear. Indexing to Ucr concentration has been recognized as an imperfect option.3,4,27,28 Our findings affirm that accounting for urine dilution is important because nearly all urinary biomarker concentrations had the lowest risk estimates with all 3 outcomes when they were examined individually, and risk estimates significantly increased after controlling for Ucr concentration either by indexing or adjusting for most biomarkers. These observations indicate that we unmasked associations that would have been biased to the null without accounting for urine dilution. However, except for UMOD, KIM-1, and MCP-1 for the composite CVD outcome, the magnitude of risk estimates was not significantly different when biomarkers were indexed to Ucr concentration versus adjusted for 1/Ucr in models. Thus, in this study, indexing to Ucr concentration did not substantially affect urinary biomarkers’ prognostic utility despite well-founded statistical concerns for indexing and finding that Ucr concentration itself had associations with certain outcomes. Whether indexing and adjusting for Ucr concentration will yield similar estimates in other patient populations or with other urinary biomarkers, as seen with UAlb concentration in the NHANES analysis, requires further investigation.
These findings raise the question of which is preferable; to index or adjust for Ucr concentration? With indexing, Ucr concentration reflects not only urine dilution but also muscle mass and is itself independently associated with adverse clinical outcomes. These independent prognostic associations could influence the risk estimate of a biomarker ratio with Ucr concentration, which is one of the potential statistical errors introduced by use of ratios.12,13 Ratios obscure whether relationships are driven by the numerator, denominator, or both but are widely interpreted by focusing on the numerator only. An approach that statistically adjusts for Ucr concentration allows differentiation between the associations of the biomarker (numerator) from Ucr (denominator) with each outcome. Ratios compound errors that occur with laboratory assay imprecision for measuring the biomarker in the numerator and denominator, risking imprecision of the risk estimate. That is, as every assay has a CV, the imprecision of measuring the numerator is compounded with the imprecision of measuring the denominator. As stated in our methods, UAlb concentration has a CV up to 6.9%, and Ucr concentration, up to 4.3%; imprecisions in both biomarkers are compounded when UAlb is indexed to Ucr concentration. Furthermore, ratios assume a linear association between the numerator and denominator, which likely does not exist for most biomarkers, and analyses for biomarkers frequently require log transformation for normality, which is not done in clinical practice. Conversely, adjusting considers the average effect of a biomarker for the whole population, while indexing evaluates on an individual patient level. In considering these differences in methodologies for epidemiologic studies, accounting for Ucr concentration as a separate covariate seems the preferable approach when the goal is to determine the independent relationship of the biomarker in the numerator. However, adjustment is not readily feasible in clinical practice or necessarily specific to an individual patient. Thus, our results are reassuring for clinical contexts because the interpretation of the indexed urinary biomarker was consistently similar to that of the biomarker separately adjusted for 1/Ucr.
Strengths of this study include its evaluation of a relatively large sample of persons with CKD, availability of measurements of multiple important confounding variables, and evaluation of multiple urinary biomarkers such that the consistency of the findings could be compared across different urinary markers.
This study also has important limitations. All participants had CKD, individuals with diabetes and proteinuria with protein excretion > 1 g were excluded, and all were clinical trial participants. Our findings will therefore require confirmation in other settings. Although we had a large number of events in the primary composite CVD outcome, there were only a moderate number of deaths and very few composite kidney outcome events. Thus, null findings for these end points should be interpreted within the confines of their respective 95% CIs. Significant associations remain plausible and require future study in settings with larger numbers of these end points.
Spot Ucr concentration is independently associated with CVD events and has a signal for an association with kidney events. Although Ucr concentration has prognostic associations, risk estimates across multiple different urinary biomarkers did not substantially change whether they were adjusted versus indexed for Ucr concentration. Despite similarities in risk estimates, adjusting for Ucr concentration is likely a more appropriate approach in epidemiologic studies because Ucr concentration itself carries prognostic value, there are several well-described statistical issues with using ratios, and other studies have shown that indexing versus adjusting can alter risk estimates.12, 13, 14
Article Information
Authors’ Full Names and Academic Degrees
Nicholas Wettersten, MD, Ronit Katz, DPhil, Michael G. Shlipak, MD, Rebecca Scherzer, PhD, Sushrut S. Waikar, MD, MPH, Joachim H. Ix, MD, and Michelle M. Estrella, MD.
Authors’ Contributions
Research idea and study design: NW, RK, MGS, JHI, MME; data acquisition: MGS, JHI, MME; data analysis/interpretation: NW, RK, MGS, RS, SSW, JHI, MME; supervision: MGS, JHI, MME. All authors contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
The primary funding sources for this ancillary study were supported by an R01 award from the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) to Drs Ix and Shlipak (R01DK098234) and an American Heart Association award to Dr Ix (I4EIA18560026). Dr Ix was supported by a midcareer mentoring award from the NIDDK (K24DK110427). Dr Estrella is supported by an R01 from the NIDDK (R01DK103574). Dr Wettersten and this work were supported (or supported in part) by Career Development Award Number IK2 CX002105 from the US Department of Veterans Affairs Clinical Sciences R&D Service. The contents do not represent the view of the US Department of Veterans Affairs or the US government. The study funders did not play an role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit this manuscript for publication. SPRINT is funded with federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute, the NIDDK, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke, under contract numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and interagency agreement number A-HL-13-002-001. It also was supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals Int, Inc, Osaka, Japan. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the US Department of Veterans Affairs, or the US Government. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list at https://www.sprinttrial.org/public/dspScience.cfm.
Financial Disclosure
Dr Ix has received funding for an Investigator Initiated Research Study from Baxter Int and is a Data and Safety Monitoring Board member for Sanifit Pharmaceuticals and an Advisory Board member for AstraZeneca. The remaining authors declare that they have no relevant financial interests.
Peer Review
Received November 12, 2020. Evaluated by 3 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form February 15, 2021.
Footnotes
Complete author and article information provided before references.
Table S1: Urinary Biomarker Associations With Longitudinal Outcomes Based on the Absolute Value of Biomarker, Biomarker Indexed to Urinary Creatinine, and Biomarker Adjusted for 1/Urinary Creatinine
Supplementary Material
Table S1
References
- 1.Fassett R.G., Venuthurupalli S.K., Gobe G.C., Coombes J.S., Cooper M.A., Hoy W.E. Biomarkers in chronic kidney disease: a review. Kidney Int. 2011;80(8):806–821. doi: 10.1038/ki.2011.198. [DOI] [PubMed] [Google Scholar]
- 2.Tesch G.H. Review: serum and urine biomarkers of kidney disease: a pathophysiological perspective. Nephrology (Carlton) 2010;15(6):609–616. doi: 10.1111/j.1440-1797.2010.01361.x. [DOI] [PubMed] [Google Scholar]
- 3.Boeniger M.F., Lowry L.K., Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993;54(10):615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- 4.Barr D.B., Wilder L.C., Caudill S.P., Gonzalez A.J., Needham L.L., Pirkle J.L. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113(2):192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z.M., Gallagher D., Nelson M.E., Matthews D.E., Heymsfield S.B. Total-body skeletal muscle mass: evaluation of 24-h urinary creatinine excretion by computerized axial tomography. Am J Clin Nutr. 1996;63(6):863–869. doi: 10.1093/ajcn/63.6.863. [DOI] [PubMed] [Google Scholar]
- 6.Heymsfield S.B., Arteaga C., Mcmanus C., Smith J., Moffitt S. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr. 1983;37(3):478–494. doi: 10.1093/ajcn/37.3.478. [DOI] [PubMed] [Google Scholar]
- 7.Ix J.H., De Boer I.H., Wassel C.L., Criqui M.H., Shlipak M.G., Whooley M.A. Urinary creatinine excretion rate and mortality in persons with coronary artery disease: the Heart and Soul Study. Circulation. 2010;121(11):1295–1303. doi: 10.1161/CIRCULATIONAHA.109.924266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oterdoom L.H., Gansevoort R.T., Schouten J.P., De Jong P.E., Gans R.O., Bakker S.J. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207(2):534–540. doi: 10.1016/j.atherosclerosis.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Di Micco L., Quinn R.R., Ronksley P.E. Urine creatinine excretion and clinical outcomes in CKD. Clin J Am Soc Nephrol. 2013;8(11):1877–1883. doi: 10.2215/CJN.01350213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson F.P., Xie D., Anderson A.H. Urinary creatinine excretion, bioelectrical impedance analysis, and clinical outcomes in patients with CKD: the CRIC Study. Clin J Am Soc Nephrol. 2014;9(12):2095–2103. doi: 10.2215/CJN.03790414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ter Maaten J.M., Maggioni A.P., Latini R. Clinical and prognostic value of spot urinary creatinine in chronic heart failure-an analysis from GISSI-HF. Am Heart J. 2017;188:189–195. doi: 10.1016/j.ahj.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Jasienski M., Bazzaz F.A. The fallacy of ratios and the testability of models in biology. Oikos. 1999;84(2):321–326. [Google Scholar]
- 13.Curran-Everett D. Explorations in statistics: the analysis of ratios and normalized data. Adv Physiol Educ. 2013;37(3):213–219. doi: 10.1152/advan.00053.2013. [DOI] [PubMed] [Google Scholar]
- 14.Koopman J.J.E., Scherzer R., Ix J.H., Shlipak M.G., Waikar S.S. A comparison of different estimates of albuminuria in association with mortality in epidemiologic research. Clin J Am Soc Nephrol. 2020;15(12):1814–1816. doi: 10.2215/CJN.07290520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SPRINT Research Group. Wright J.T., Jr., Williamson J.D., Whelton P.K. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garimella P.S., Lee A.K., Ambrosius W.T. Markers of kidney tubule function and risk of cardiovascular disease events and mortality in the SPRINT trial. Eur Heart J. 2019;40(42):3486–3493. doi: 10.1093/eurheartj/ehz392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malhotra R., Craven T., Ambrosius W.T. Effects of intensive blood pressure lowering on kidney tubule injury in CKD: a longitudinal subgroup analysis in SPRINT. Am J Kidney Dis. 2019;73(1):21–30. doi: 10.1053/j.ajkd.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W.R., Craven T.E., Malhotra R. Kidney damage biomarkers and incident chronic kidney disease during blood pressure reduction: a case-control study. Ann Intern Med. 2018;169(9):610–618. doi: 10.7326/M18-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee A.K., Katz R., Jotwani V. Distinct dimensions of kidney health and risk of cardiovascular disease, heart failure, and mortality. Hypertension. 2019;74(4):872–879. doi: 10.1161/HYPERTENSIONAHA.119.13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambrosius W.T., Sink K.M., Foy C.G. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clin Trials. 2014;11(5):532–546. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inker L.A., Schmid C.H., Tighiouart H. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin D.Y., Fleming T.R., De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med. 1997;16(13):1515–1527. doi: 10.1002/(sici)1097-0258(19970715)16:13<1515::aid-sim572>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Jain R.B. Associated complex of urine creatinine, serum creatinine, and chronic kidney disease. Epidemiol Open Access. 2016;06(02) [Google Scholar]
- 24.Levey A.S., Becker C., Inker L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults. JAMA. 2015;313(8):837–846. doi: 10.1001/jama.2015.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter C.E., Gansevoort R.T., Scheven L. Influence of urine creatinine on the relationship between the albumin-to-creatinine ratio and cardiovascular events. Clin J Am Soc Nephrol. 2012;7(4):595–603. doi: 10.2215/CJN.09300911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter C.E., Katz R., Kramer H. Influence of urine creatinine concentrations on the relation of albumin-creatinine ratio with cardiovascular disease events: the Multi-Ethnic Study Of Atherosclerosis (MESA) Am J Kidney Dis. 2013;62(4):722–729. doi: 10.1053/j.ajkd.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ginsberg J.M., Chang B.S., Matarese R.A., Garella S. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med. 1983;309(25):1543–1546. doi: 10.1056/NEJM198312223092503. [DOI] [PubMed] [Google Scholar]
- 28.Schwab S.J., Christensen R.L., Dougherty K., Klahr S. Quantitation of proteinuria by the use of protein-to-creatinine ratios in single urine samples. Arch Intern Med. 1987;147(5):943–944. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1