Abstract
Rational & Objective
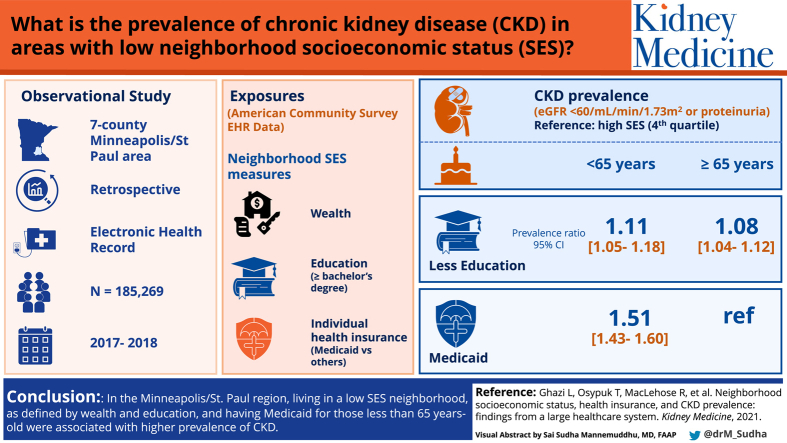
Neighborhood socioeconomic status (SES) and health insurance status may be important upstream social determinants of chronic kidney disease (CKD), but their relationship remains unclear. The aim of this study was to determine whether neighborhood SES and individual-level health insurance status were independently associated with CKD prevalence.
Study Design
Observational study using electronic health records (EHRs).
Setting & Participants
EHRs of patients (n = 185,269) seen at a health care system in the 7-county Minneapolis/St Paul area (2017-2018).
Exposures
Census tract neighborhood SES measures (median value of owner-occupied housing units [wealth], percentage of residents aged >25 years with bachelor’s degree or higher [education]) and individual-level health insurance status (aged <65 years: Medicaid vs other insurance; ≥65 years: Medicare vs Medicare and supplemental insurance plan) were obtained from the American Community Survey and EHR data. Neighborhood SES was operationalized into quartiles, comparing low (first quartile) versus high (fourth quartile) neighborhood SES.
Outcomes
CKD prevalence: estimated glomerular filtration rate < 60 mL/min/1.73 m2 or proteinuria.
Analytic Approach
Multilevel Poisson regression with robust error variance with a random intercept at the census-tract level, adjusted for demographic and clinical covariates, was used to estimate the association between neighborhood SES, insurance, and CKD.
Results
Neighborhood SES and insurance were independently associated with CKD prevalence. In covariate-adjusted models, patients living in low versus high neighborhood SES had a higher CKD prevalence among both younger and older patients. For example, the prevalence ratios of CKD in low versus high neighborhood SES as defined by education among patients younger than 65 and 65 years and older were 1.11 (95% CI, 1.05-1.18) and 1.08 (95% CI, 1.04-1.12), respectively. Patients younger than 65 years receiving Medicaid had higher CKD prevalence versus those with other insurance (1.51 [95% CI, 1.43-1.6]). For patients 65 years and older, insurance was not associated with prevalence of CKD in the fully adjusted model.
Limitations
One health care system and selection bias.
Conclusions
Living in low neighborhood SES as defined by wealth and education and having Medicaid for patients younger than 65 years were associated with higher CKD prevalence.
Index Words: Chronic kidney disease, Medicaid, prevalence, socioeconomic status, electronic health records
Graphical abstract
Plain-Language Summary.
Neighborhood socioeconomic status (SES) and health insurance status may be important upstream social determinants of chronic kidney disease (CKD) but their relationship remains unclear. We used electronic health record data from a large midwestern US metropolitan area to assess whether neighborhood SES and individual-level health insurance status are independently associated with CKD prevalence. We found that patients from low neighborhood SES and Medicaid recipients (among patients aged <65 years) have greater rates of CKD compared with patients from high SES tracts and patients with other insurance. These may be 2 of several socioeconomic and individual factors influencing the complexity of identification, management, and treatment of CKD.
Chronic kidney disease (CKD) is a significant public health problem in the United States affecting 26 million Americans (14.8% of the adult population).1 CKD contributes to poor quality of life, leads to premature death, and is costly for both the public and private sectors.2 Disadvantaged socioeconomic status (SES) populations have a disproportionate burden of CKD.3
Understanding the relationship between SES and CKD is important for informing intervention and policy efforts to reduce CKD burden and deliver appropriate care for vulnerable populations. This is especially relevant as the health care community undertakes initiatives related to the Presidential Executive Order on Advancing American Kidney Health.4 Factors such as disparities in SES, racial discrimination, and being underinsured/uninsured all contribute to the development of CKD.5 A recent systematic review of 3,632,531 participants and 832,497 cases of CKD showed that low SES was associated with CKD (low estimated glomerular filtration rate [eGFR]/high albuminuria; odds ratio, 1.4 [95% CI, 1.2-1.6]).6 Area-level SES was less studied than individual-level SES and most studies focused on end-stage kidney disease (ESKD).7, 8, 9 Most of these previous analyses examined area-level SES using large geographical areas that may mask the variation of CKD within such areas.9, 10, 11 Furthermore, the association of SES and CKD was predominately assessed using population-level data from cohort studies or was limited to veterans only, subsequently limiting the generalizability of results. Moreover, composite measures of neighborhood SES were commonly used, which are less useful for informing policy decisions regarding specific neighborhood SES measures.
Overall, low neighborhood SES is associated with worsening kidney function. Meanwhile, individual-level health insurance also contributes to disparities in clinical care and clinical outcomes, including health outcomes of several diseases (cardiovascular disease and ESKD).12 Receiving Medicaid or Medicare without supplemental insurance may affect health outcomes independent of SES as coverage.13 This is true particularly for Medicaid (aged <65 years) because eligibility is mainly tied to having income below the poverty line.14 Moreover, patients with Medicare (aged >65 years) without supplemental insurance plans have been shown to receive fewer preventive services and have higher deductibles for services and prescription drugs.15, 16, 17, 18
Studies to date have focused mainly on the association of health insurance in ESKD. Overall, both neighborhood SES and individual-level health insurance play an important role in health outcomes. However, research to date has not jointly assessed the independent association of neighborhood SES and individual-level health insurance on CKD prevalence. To our knowledge, no studies have further examined whether the association of neighborhood SES and health insurance with CKD differs between patients with or without hypertension or diabetes and by race.
The aim of this study was to determine whether neighborhood SES and individual-level health insurance status are independently associated with CKD prevalence in a cohort of electronic health records (EHRs) from a large midwestern US metropolitan area.
Methods
Data
Patients were identified from the EHR database of Fairview Health Services, the primary affiliate of the University of Minnesota. The Institutional Review Board at the University of Minnesota approved this study (ID: 1502M63126). Written informed consent at the patient level was waived by the Institutional Review Board. We identified patients from June 1, 2017, through December 31, 2018. The EHR data incorporate all outpatient visits, laboratory tests, billing data, geocoded addresses, and census tracts of patients’ residences.19
Sample Definition
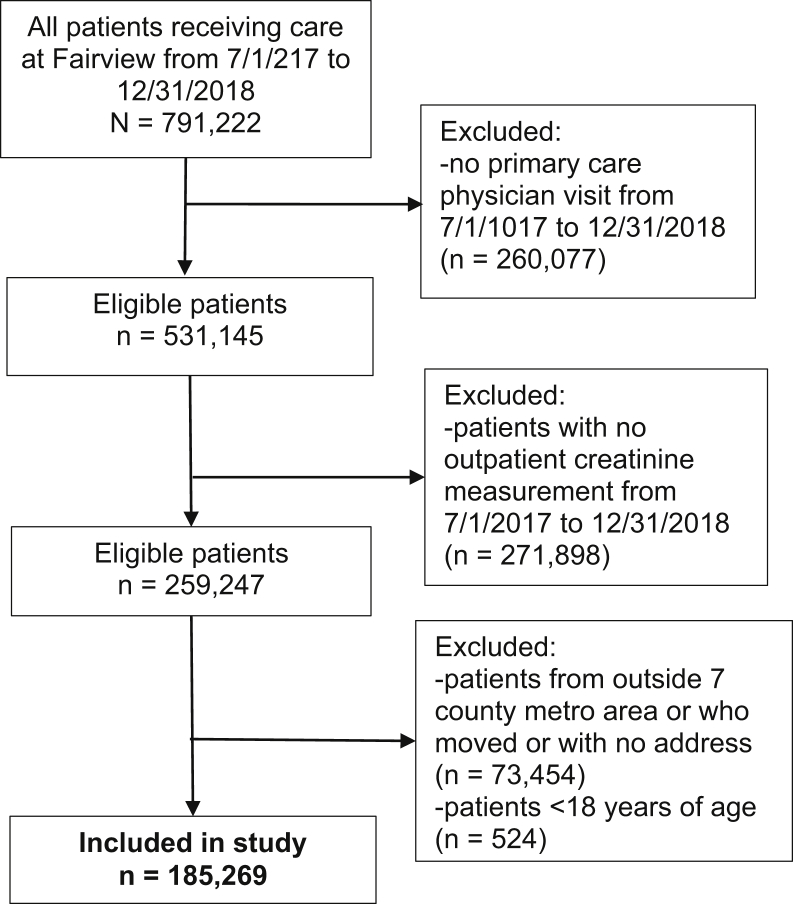
The sample included all adult patients (aged ≥18 years) who had their address geocoded and are from the 7-county Minneapolis/St Paul Minnesota metropolitan area and who had at least 1 measurement of outpatient creatinine level during the included period (ie, excluded patients with no available outpatient creatinine level). Patients without outpatient creatinine levels were excluded. Patients were included if they had insurance data available (available for all patients) and had at least 1 primary care physician visit in the Fairview health system during this period (Figs 1 and S1). We defined index creatinine level as the last available creatinine level during this period. Patients who opted out of research or moved from June 1, 2017, to December 31, 2018, were excluded.
Figure 1.
Cohort flow chart of Fairview patients from the 7-county Minneapolis/St Paul metropolitan (metro) area.
Geographic Unit and Neighborhood SES
We selected census tracts as the geographic unit of analysis, which are small relatively stable areas designed to include homogeneous populations.20 We linked each patient’s residence to the appropriate census tract and tract characteristics. We included 3 measures to operationalize neighborhood SES at the tract level: median value of owner-occupied housing units, percentage of residents older than 25 years with a bachelor’s degree or higher, and median household income, all as identified from the 2012 American Community Survey 5-year data (2008-2012).21, 22, 23, 24 These measures reflect wealth, education, and income of the tract.23 We divided neighborhood SES into quartiles and primarily compared the 2 extremes of the neighborhood SES distribution, with low and high neighborhood SES defined as belonging to the first (Q1) and fourth (Q4) quartiles of the distribution of each of the measures using American Community Survey data of the metropolitan area (specifically defined with tract wealth as <$165,200 [Q1] or ≥$231,300 [Q4]; tract education as <20.4% [Q1] or ≥48.1% [Q4], and tract income as <$35,935 [Q1] or ≥$62,343 [Q4]). We present Q2 and Q3 neighborhood SES results as well for completeness.
Health Insurance
We defined a patient’s most recent insurance status from the EHR, stratified by age. For individuals younger than 65 years, insurance was defined as either having Medicaid versus other insurance; for those 65 years and older, insurance was defined as having Medicare (Part A, B, or C) versus having Medicare and supplemental insurance plan for individuals (Item S1).
CKD Outcome
eGFR was calculated using the CKD Epidemiology Collaboration (CKD-EPI) equation.25 CKD was defined as having an outpatient index eGFR < 60 mL/min/1.73 m2 or having proteinuria (last measure available of urinary albumin-creatinine ratio > 30 mg/g or urinary protein-creatinine ratio > 150 mg/g or urinalysis > 30 mg/g between June 1, 2017, and December 31, 2018). We manually abstracted clinical and laboratory data for 100 random patient charts and compared them with data pulled directly from EHRs to ensure data quality.
Covariates
We identified sex and race from the EHR. Age and smoking status were defined using the last value before or at the time of the index creatinine level. All creatinine measures were traceable to an isotope-dilution mass spectrometry reference measurement. Comorbid conditions were considered present if at least 2 International Classification of Diseases, Ninth/Tenth Revision (ICD-9/10) codes for that condition were present at or before the date of the index creatinine level.26 We used the last value at or before the date of the index creatinine level for each of the following covariates: body mass index, urinary albumin-creatinine ratio, urinary protein-creatinine ratio, and urinalysis. All laboratory values were derived from outpatient clinical visits.
Statistical Analysis
We evaluated the possible sparsity of data by using contingency to assess the number of participants for each insurance status by tract SES.27,28 We assessed baseline demographic and clinical characteristics of the cohort. Categorical variables were presented as frequencies, and continuous variables, as mean (standard deviation) values. Pearson correlations were used to test associations among neighborhood SES variables (as continuous measures).
We used a multilevel model with a random intercept for census tract to estimate the association between neighborhood SES and insurance status association and CKD prevalence. Models were stratified by age (<65 and ≥65 years). Because CKD prevalence was a common outcome, we estimated the prevalence ratio and 95% CI using Poisson regression with robust error variance.29, 30, 31 We fit the following models: model 1, neighborhood SES (each measure separately) and health insurance status; model 2, model 1 plus age, sex, race, obesity, and smoking; and model 3, model 2 plus history of cardiovascular disease, stroke, cancer, hyperlipidemia, hypertension, and diabetes. Effect modification (on the multiplicative and additive scale) of the neighborhood SES or insurance-CKD relationship was examined by race, hypertension, and diabetes (Item S1).32
Because we included only patients who had their kidney function measured, our analysis may be subject to selection bias if, for example, people in higher SES neighborhoods and people with CKD are more likely to get measured. We assessed the possible impact that selection bias could have had on our results using sensitivity analyses with different selection probabilities.33 We estimated an adjusted crude prevalence ratio by multiplying each cell of the 2×2 table (exposure: patients in low vs high SES; outcome: patients with and without CKD) with a selection factor (probability of a patient being included in cohort given CKD presence and tract characteristics). A range of subjectively specified selection factors was used to ascertain the possible sensitivity of our results to a range of possible selection probabilities.
We undertook preplanned analysis to assess the sensitivity of how and/or when CKD, insurance, and the cohort were defined: limiting our analyses to using a more restrictive definition of CKD (eGFR < 45 mL/min/1.73 m2 per year or urinary albumin-creatinine ratio ≥ 300 mg/g or urinary protein-creatinine ratio ≥ 500 mg/g or urinalysis ≥ 100 mg/g); only including creatinine measured during routine clinic visits (ie, laboratory tests measured during annual clinic well-being visits); defining CKD as having 2 consecutive eGFRs more than 3 months apart <60 mL/min/1.73 m2; defining insurance status as having continuous coverage by a single provider from June 1, 2017, to December 31, 2019 (more restrictive definition); and changing the cohort inclusion period from 18 to 12 months (December 31, 2017, to December 31, 2018). We accounted for missing data by using “missingness” as a variable to adjust for rather than by imputation, which is limited to the assumption of randomness rather than dropping missing data out of the cohort.34,35 We used an indicator variable for missingness for obesity (9% missing) and systolic and diastolic blood pressure (3% missing). Statistical analyses were conducted using R (R Core Team) and Stata (StataCorp).36,37
Results
Cohort Characteristics
There were 791,222 patients identified from the EHRs. After excluding most records due to no primary care physician visit and no creatinine measurement from June 1, 2017, to December 31, 2018, we included 185,269 in our cohort (Fig 1). Patients were on average 55 years old (69% < 65 years), 9% were Black, 45% were men, and 20% had CKD (Table 1). Fairview patients resided in 676 of the 704 tracts in the metropolitan area, with a median of 187 and interquartile range of 106 to 365 for the number of patients per tract. Compared with the metropolitan area population, our cohort had a similar distribution of patients by county and by percent Blacks, but our cohort consisted of an older population with lower rates of Medicaid enrollment (Table S1). Tract income was moderately correlated with tract wealth and education (r=0.33 and r=0.30, respectively). However, tract wealth was more strongly correlated with tract education (r=0.68).
Table 1.
Characteristics of the Fairview Cohort Overall and by Age (<65 and ≥65 years)
| Overall (N = 185,269) | Age < 65 y (n = 127,977) | Age ≥ 65 y (n = 57,292) | |
|---|---|---|---|
| eGFR, mL/min/1.73 m2 | 82.4 [68.6-97.9] | 87.6 [75.2-103.1] | 69.8 [56.9-82.9] |
| CKD | 37,098 (20%) | 15,374 (12%) | 21,724 (38%) |
| Individual-level characteristics | |||
| Age, y | 55.0 ± 17.8 | 45.9 ± 12.7 | 75.38 ± 7.7 |
| Male sex | 84,116 (45%) | 58,667 (46%) | 25,449 (44%) |
| Race | |||
| Black | 16,130 (9%) | 13,660 (11%) | 2,470 (4%) |
| White | 146,563 (79%) | 95,995 (75%) | 50,568 (88%) |
| Ever smokers | 77,867 (42%) | 49,076 (38%) | 28,791 (50%) |
| Insurance status | |||
| Medicaid (among patients <65 y) | 5,259 (4%) | 5,259 (4%) | — |
| Medicare (among patients ≥65 y) | 11,719 (20%) | — | 11,719 (20%) |
| BP | |||
| Systolic BP, mm Hg | 127.2 ± 17.8 | 125.27 ± 17.0 | 131.5 ± 18.8 |
| Diastolic BP, mm Hg | 77.0 ± 11.3 | 78.3 ± 11.3 | 74.2 ± 10.9 |
| Medical history | |||
| Hypertension | 83,270 (45%) | 41,746 (33%) | 41,524 (73%) |
| Diabetes | 29,913 (16%) | 15,847 (12%) | 14,066 (25%) |
| Obese (BMI ≥ 30 kg/m2) | 67,467 (40%) | 48,651 (42%) | 18,816 (36%) |
| Cardiovascular disease | 26,789 (15%) | 8,639 (7%) | 18,150 (32%) |
| Stroke | 7,447 (4%) | 2,285 (2%) | 5,162 (9%) |
| Hyperlipidemia | 80,636 (44%) | 40,281 (32%) | 40,355 (70%) |
| Cancer | 14,609 (8%) | 5,127 (4%) | 9,482 (17%) |
| Median value of owner-occupied housing units | |||
| Q1: <$165,200 | 16,625 (9%) | 12,149 (10%) | 4,476 (8%) |
| Q2: $165,200-$188,100 | 22,475 (12%) | 15,758 (12%) | 6,717 (12%) |
| Q3: $188,100-$231,300 | 63,198 (34%) | 43,073 (34%) | 20,125 (35%) |
| Q4: ≥$231,300 | 82,940 (45%) | 56,973 (45%) | 25,967 (45%) |
| Residents > 25 y with ≥bachelor’s degree | |||
| Q1: <20.4% | 19,825 (11%) | 14,637 (11%) | 5,188 (9%) |
| Q2: 20.4%-34.1% | 46,939 (25%) | 32,831 (26%) | 14,108 (25%) |
| Q3: 34.1%-48.1% | 61,027 (33%) | 41,261 (32%) | 19,766 (35%) |
| Q4: ≥48.1% | 57,452 (31%) | 39,229 (31%) | 18,223 (32%) |
| Median household income | |||
| Q1: <$35,935 | 21,363 (12%) | 15,025 (12%) | 6,338 (11%) |
| Q2: $35,935-$47,379 | 23,344 (13%) | 16,057 (13%) | 7,287 (13%) |
| Q3: $47,379-$62,343 | 40,829 (22%) | 27,648 (22%) | 13,181 (23%) |
| Q4: ≥$62,343 | 99,590 (52%) | 69,141 (54%) | 30,449 (53%) |
Note: Values expressed as median [interquartile range], number (percent), or mean ± standard deviation.
Abbreviations and Definitions: Cardiovascular disease, includes congestive heart failure, acute myocardial infarction, ischemic heart disease, and peripheral vascular disease; BMI, body mass index; BP, blood pressure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; Q, quartile.
Among younger patients (aged <65 years), 5,259 had Medicaid (21% with CKD) and 118,430 had other insurance (12% with CKD). Among older patients (aged ≥65 years), 11,718 were receiving Medicare (Parts A, B, or C; 40% with CKD) and 45,573 had Medicare and supplemental insurance plans (37% with CKD). Patients who lived in low neighborhood SES tracts, Whites, and patients with Medicaid or Medicare without a supplemental insurance plan all exhibited a higher prevalence of CKD and comorbid conditions (Tables S2-S5).
Neighborhood SES
Among patients both younger than 65 and 65 years and older, low neighborhood SES defined by wealth and separately by education was associated with greater rates of prevalent CKD compared with high neighborhood SES, after adjusting for covariates (Tables 2 and 3). For example, the prevalence ratio of CKD in low versus high neighborhood SES defined by education among patients younger than 65 years was 1.11 (95% CI, 1.05-1.18), and among patient 65 years and older was 1.08 (95% CI, 1.04-1.12). However, we found no association between CKD and tract income when comparing low versus high neighborhood SES. The prevalence ratio magnitude of the association of CKD with the Q2 and Q3 of tract wealth SES and tract education SES versus Q4 was smaller than that observed comparing the Q1 versus Q4 of tract wealth and tract education SES.
Table 2.
Multilevel Regression Model for the Association of Tract-Level SES and Insurance Status With CKD Prevalence in Individuals Younger Than 65 Years
| Median Value of Owner-Occupied Housing Units |
Insurance |
|||||
|---|---|---|---|---|---|---|
| High SES: Q4 (n = 55,332) | Q3 (n = 41,562) | Q2 (n = 15,145) | Low SES: Q1 (n = 11,626) | Other Insurance (n = 118,430) | Medicaid (n = 5,259) | |
| Model 1 | 1.00 | 1.14 (1.09-1.19) | 1.24 (1.17-1.31) | 1.38 (1.29-1.48) | 1.00 | 1.71 (1.62-1.81) |
| Model 2 | 1.00 | 1.16 (1.11-1.21) | 1.26 (1.20-1.33) | 1.42 (1.32-1.52) | 1.00 | 1.82 (1.74-1.92) |
| Model 3 | 1.00 | 1.04 (1.00-1.08) | 1.07 (1.02-1.13) | 1.16 (1.09-1.24) | 1.00 | 1.51 (1.42-1.59) |
| % > 25 y With ≥ Bachelor’s Degree |
Insurance |
|||||
|---|---|---|---|---|---|---|
| High SES (n = 38,118) | Q3 (n = 39,882) | Q2 (n = 31,605) | Low SES (n = 14,084) | Other Insurance (n = 118,430) | Medicaid (n = 5,259) | |
| Model 1 | 1.00 | 1.18 (1.13-1.23) | 1.28 (1.21-1.34) | 1.39 (1.30-1.50) | 1.00 | 1.72 (1.62-1.82) |
| Model 2 | 1.00 | 1.17 (1.13-1.23) | 1.28 (1.22-1.34) | 1.38 (1.29-1.49) | 1.00 | 1.83 (1.72-1.94) |
| Model 3 | 1.00 | 1.07 (1.03-1.12) | 1.08 (1.04-1.13) | 1.11 (1.05-1.18) | 1.00 | 1.51 (1.43-1.60) |
| Median Household Income |
Insurance |
|||||
|---|---|---|---|---|---|---|
| High SES (n = 67,029) | Q3 (n = 26,600) | Q2 (n = 15,434) | Low SES (n = 14,626) | Other Insurance (n = 118,430) | Medicaid (n = 5,259) | |
| Model 1 | 1.00 | 1.13 (1.07-1.18) | 1.14 (1.07-1.21) | 1.02 (0.95-1.09) | 1.00 | 1.75 (1.65-1.85) |
| Model 2 | 1.00 | 1.15 (1.09-1.20) | 1.16 (1.09-1.23) | 1.04 (0.97-1.12) | 1.00 | 1.85 (1.75-1.96) |
| Model 3 | 1.00 | 1.09 (1.04-1.13) | 1.08 (1.03-1.14) | 0.99 (0.94-1.06) | 1.00 | 1.51 (1.43-1.60) |
Note: Values expressed as prevalence ratio of CKD for individual in low SES tract versus high SES tract (95% CI). Median value of owner-occupied housing units: high SES (Q4), $231,300; Q3, $188,100 to $231,300; Q2, $165,200 to $188,100; low SES (Q1), <$165,200; percent older than 25 years with a bachelor’s degree or more: high SES (Q4), ≥48.1%; Q3, 34.1% to 48.1%; Q2, 20.4% to 34.1%; low SES (Q1), <20.4%; median household income: high SES (Q4), ≥$62.343; Q3, $47,379 to $62,343; Q2, $35,935 to $47,379; low SES (Q1), <$35,935. Model 1, tract SES and insurance status. Model 2, model 1 plus race, sex, and age. Model 3, model 2 plus obesity, smoking, history of cardiovascular disease, stroke, cancer, hyperlipidemia, hypertension, and diabetes. No interaction between tract SES (any of the 3 measures) and race or diabetes (multiplicative and additive scale). There is an interaction between education and income with hypertension history (multiplicative scale and on additive scale). No interaction between insurance and race; there is an interaction between insurance and hypertension and diabetes on multiplicative and additive scale.
Abbreviations: CKD, chronic kidney disease; Q, quartile; SES: socioeconomic status.
Table 3.
Multilevel Regression Model for Association of Tract-Level SES and Insurance Status With CKD Prevalence in Individuals 65 Years and Older
| Median Value of Owner-Occupied Housing Units |
Insurance |
|||||
|---|---|---|---|---|---|---|
| High SES: Q4 (n = 25,967) | Q3 (n = 20,125) | Q2 (n = 6,717) | Low SES: Q1 (n = 4,483) | Medicare and Supplemental Insurance Plan (n = 45,573) | Medicare (n = 11,719) | |
| Model 1 | 1.00 | 1.13 (1.09-1.16) | 1.12 (1.07-1.18) | 1.18 (1.12-1.23) | 1.00 | 1.04 (1.02-1.05) |
| Model 2 | 1.00 | 1.09 (1.07-1.12) | 1.10 (1.05-1.15) | 1.13 (1.05-1.21) | 1.00 | 1.00 (0.99-1.02) |
| Model 3 | 1.00 | 1.03 (1.03-1.07) | 1.04 (1.00-1.09) | 1.07 (1.02-1.12) | 1.00 | 1.01 (0.99-1.03) |
| % >25 y With ≥Bachelor’s Degree |
Insurance |
|||||
|---|---|---|---|---|---|---|
| High SES (n = 18,223) | Q3 (n = 19,766) | Q2 (n = 14,108) | Low SES (n = 5,195) | Medicare and Supplemental Insurance Plan (n = 45,573) | Medicare (n = 11,719) | |
| Model 1 | 1.00 | 1.10 (1.06-1.15) | 1.16 (1.11-1.20) | 1.19 (1.14-1.25) | 1.00 | 1.04 (1.02-1.05) |
| Model 2 | 1.00 | 1.07 (1.04-1.11) | 1.13 (1.09-1.17) | 1.18 (1.14-1.23) | 1.00 | 1.00 (0.99-1.03) |
| Model 3 | 1.00 | 1.03 (1.00-1.06) | 1.05 (1.02-1.08) | 1.08 (1.04-1.12) | 1.00 | 1.01 (1.00-1.02) |
| Median Household Income |
Insurance |
|||||
|---|---|---|---|---|---|---|
| High SES (n = 30,449) | Q3 (n = 13,181) | Q2 (n = 7,287) | Low SES (n = 6,375) | Medicare and Supplemental Insurance Plan (n = 45,573) | Medicare (n = 11,719) | |
| Model 1 | 1.00 | 1.05 (1.01-1.09) | 1.07 (1.03-1.12) | 0.99 (0.95-1.05) | 1.00 | 1.03 (1.02-1.05) |
| Model 2 | 1.00 | 1.01 (0.98-1.04) | 1.04 (1.00-1.07) | 0.98 (0.94-1.03) | 1.00 | 1.01 (0.99-1.02) |
| Model 3 | 1.00 | 1.00 (0.98-1.03) | 1.01 (0.98-1.04) | 1.00 (0.98-1.03) | 1.00 | 1.01 (0.99-1.02) |
Note: Values expressed as prevalence ratio of CKD for individual in low SES tract versus high SES tract (95% CI). Median value of owner-occupied housing units: high SES (Q4), ≥$231,300; Q3, $188,100 to $231,300; Q2, $165,200 to $188,100; low SES (Q1), <$165,200; percent aged 25 years with a bachelor’s degree or more: high SES (Q4), ≥48.1%; Q3, 34.1% to 48.1%; Q2, 20.4% to 34.1%; low SES (Q1), <20.4%; median household income: high SES (Q4), ≥$62.343; Q3, $47,379 to $62,343; Q2, $35,935 to $47,379; low SES (Q1), <$35,935. Model 1, tract SES and insurance status. Model 2, model 1 plus race, sex, and age. Model 3, model 2 plus obesity, smoking, history of cardiovascular disease, stroke, cancer, hyperlipidemia, hypertension, and diabetes. No interaction between tract SES (any of the 3 measures) and race, hypertension, or diabetes (on both multiplicative and additive scale). No interaction between insurance (any of the 3 measures) and race, hypertension, or diabetes (on both multiplicative and additive scale).
Abbreviations: CKD, chronic kidney disease; Q, quartile; SES, socioeconomic status.
Hypertension modified the association between tract SES (education and income) with CKD prevalence (on additive and multiplicative scale; Table S6; Item S1). Among patients with a history of hypertension, those living in low education SES tracts had a 5% (95% CI, 0.98-1.13) greater prevalence of CKD compared with those living in high education SES tracts. Conversely, among patients without a history of hypertension, those living in low education SES tracts had a 19% (95% CI, 1.08-1.32) greater prevalence of CKD compared with those living in high education SES tracts. There was no evidence of effect modification (on a multiplicative and additive scale) of the neighborhood SES-CKD association by race or diabetes.
Health Insurance
In fully adjusted models, receiving Medicaid was associated with a great prevalence ratio of CKD compared with patients with other insurance (1.51 [95% CI, 1.42-1.59]; Table 2). There was effect modification of the Medicaid-CKD association by hypertension and diabetes for patients younger than 65 years on the additive and multiplicative scales (Tables S6 and S7). Among patients with and without a history of hypertension, those with Medicaid had 35% and 81% greater prevalence rates of CKD, respectively, compared with patients who have other insurance. Similarly, among patients with and without a diabetes history, those with Medicaid had 23% and 81% greater prevalence of CKD, respectively, compared with patients who have other insurance. We found no association between insurance status (Medicare vs Medicare and supplemental insurance plan) and CKD in patients ≥65 years (Table 3).
Selection Bias
In our selection bias analyses, over a range of selection probabilities, we found that the selection bias–adjusted prevalence ratios for the association of tract SES/insurance status with CKD were attenuated relative to observed prevalence ratios (Tables S8 and S9), suggesting that we may be overestimating associations. For example, among those younger than 65 years, the prevalence ratio for the association of neighborhood SES for wealth (low vs high) and insurance (Medicaid vs other) with CKD was 1.2 and 1.5 in the fully adjusted model, not accounting for selection bias. However, after adjusting for selection bias, prevalence ratios ranged between 0.8 to 1.1 and 1.1 to 1.5 for tract low wealth and insurance, respectively.
Our results were consistent when using a more restrictive definition of CKD, including only routine creatinine measurements, defining CKD using 2 eGFR measurements < 60 mL/min/1.73 m2 more than 3 months apart, using a more restrictive definition for insurance coverage and changing inclusion time.
Discussion
Our study found that both low (vs high) neighborhood SES, specifically measures of tract wealth and separately, tract education, were associated with a higher prevalence of CKD. Moreover, having Medicaid for health insurance coverage versus other insurance among younger patients (<65 years) was also associated with a higher prevalence of CKD.
Because we included only patients who had their eGFR measured, our results were potentially influenced by selection bias. Included patients were older and more likely to be smokers, have Medicare without a supplemental insurance plan, and have more comorbid conditions than excluded patients. However, the percentage of patients in each quartile of tract SES was similar for included and excluded patients (Table S10). To examine the effect of selection bias from different inclusion patterns, we estimated a selection bias–adjusted prevalence ratio as sensitivity analysis. The magnitude of the association of tract SES status/insurance status with CKD was attenuated after adjustment for the potential selection bias; however, the extent and even direction of the change in the parameter estimate depended on hypothetical selection probabilities, for which there is little information to guide our analysis. Our results should therefore be interpreted with caution. We note also that because of this potential bias, in addition to standard limitations deriving from observational studies, we are only able to infer associations and not causal effects of neighborhood SES and insurance with CKD.
Our study found that low neighborhood SES, as defined by wealth and education, was associated with a higher prevalence of CKD. However, we did not find associations for tract income. We hypothesize that neighborhood wealth and education are more embedded as major components of residential segregation, whereas income is part of social determinants of health that might be overcome to a greater level by individual actions. The variation of the association of neighborhood SES and individual SES with CKD has been shown in several narrative literature reviews.38, 39, 40 A recent meta-analysis showed that low individual-level SES (income and education) and low combined-level SES (>1 individual SES or summary score of area-level SES indicators) were associated with CKD prevalence.41 Importantly, this meta-analysis did not differentiate between area- and individual-level SES effect on CKD due to the scarcity and heterogeneity of the data. However, making such a distinction will be important as evidence builds. Because we did not have measures of individual-level SES, our neighborhood SES measure could be capturing individual SES associations.
Only 4 cohort studies in the United States have studied the association of area-level SES with CKD prevalence. Unlike our analysis, these studies were population based and were able to adjust for individual-level SES.10,42, 43, 44 For example, in the Atherosclerosis Risk in Communities Study, the association of neighborhood SES (measures of income, wealth, education, and occupation for census block) and CKD progression was studied. Living in the lowest versus highest quartile of tract neighborhood SES was not associated with CKD in Blacks or White women; it was associated with progression only in White men (hazard ratio [HR], 1.6 [95% CI, 1.0, 2.5]).42 However, in a study of 4,735 participants from the elderly Cardiovascular Health Study (aged ≥65 years), people living in the lowest neighborhood SES quartile (based on a composite SES measure at the census block group level) had 40% greater risk for progressive CKD (creatinine elevation ≥ 0.4 mg/dL or CKD hospitalization) after adjusting for demographics, individual-level SES, hypertension, and diabetes (HR, 1.4; 95% CI, 1.0-1.7). In all, the role of neighborhood SES and kidney function remains unclear and findings from the literature and our study are mixed.
Our study tested how health insurance was associated with CKD. Previous studies have reported worse health outcomes (hospitalizations and myocardial infarction) for patients receiving Medicaid or Medicare.45, 46, 47, 48, 49 As far as kidney health, efforts have focused on ESKD and Medicaid expansion programs. For instance, patients with ESKD and Medicaid or who are uninsured and live in more generous Medicaid eligibility states have better outcomes (early transplant and permanent vascular access).50 Uninsured patients receive worse predialysis care. We found that insurance (Medicaid vs other insurance) was independently associated with CKD after adjustment, but only for patients younger than 65 years. Insurance coverage does not fully capture an individual’s SES. It may be viewed as a measure of SES, but more importantly, it has been associated with clinical care and health outcomes.12, 13, 14, 15, 16, 17, 18 Medicaid coverage among patients younger than 65 years might reflect lower individual-level SES, limited access to care and preventative services, worse health literacy, and few community and family resources that promote good health, limited use of medical services, and worse overall health not related to aging (unlike Medicare coverage for individuals ≥65 years) especially because you can enroll into Medicaid retroactively, that is, after you become ill. Therefore, our results indicating that Medicaid is a strong predictor of CKD might be an overall reflection of SES and the availability, accessibility, and acceptability of care.51
Interestingly, we also found that the prevalence of CKD in patients with Medicaid versus other insurance (aged <65 years) without a history of hypertension or diabetes was greater than patients with these comorbid conditions. Similarly, the prevalence of CKD in patients living in low versus high tract education without a history of hypertension was greater than patients with hypertension. This could be because patients with hypertension or diabetes have been advised to avoid nephrotoxic medications and are receiving medications that protect the kidney, such as renin-angiotensin system inhibitors.52,53 This might also be due to misclassifying people in low SES tracts as not having hypertension or diabetes due to lack of screening. Although health insurance coverage is a measure of access to care, there is an array of socioeconomic and individual factors that contribute to CKD. A more comprehensive look at barriers to CKD care is needed.
Our study has several limitations. First, we studied a single health care system in Minnesota, which may not be representative of the general population and other states. However, we have shown that patients included in this analysis are similar to those in the 7-county metropolitan area.
Second, several limitations derive from using EHR data, including limited ability to assess individual measures of SES, a coarse race measure that does not capture mixed race, and inability to measure total time that patients lived in a certain neighborhood. Our results therefore reflect the general state of EHR studies given the data collected for health care delivery; certainly this and other EHR studies would be greatly strengthened by collecting more complete demographic and socioeconomic data, including with supplementation by surveys or data linkage. Of note, some researchers have used individual insurance status as a proxy for individual-level SES, but the validity of this proxy is debated.47, 48, 49
Third, we were unable to assess the neighborhood SES-insurance association for the uninsured due to a limited number of uninsured patients in our sample. Fourth, we are assuming that the most recent insurance status of patients reflects their past coverage as well. Given the similar findings when using a restrictive definition of insurance to the main analyses, we believe our findings are robust. Fifth, for our main analyses we used 1-time eGFR measurement to determine CKD prevalence. However, our findings were consistent when using a more restrictive definition of CKD and among a subset of patients who had 2 consecutive eGFRs measured more than 3 months apart that were <60 mL/min/1.73 m2 (Tables S11 and S12). Sixth, we used 2008 to 2012 American Community Survey results to determine neighborhood SES. However, we might be subject to misclassification bias if neighborhood SES changed over time. Seventh, we dichotomized insurance coverage to Medicaid/other and Medicare/Medicare and supplemental insurance and were unable to delineate the specific medical reasons patients were receiving Medicaid/Medicare. Finally, we used ICD-9/10 codes to determine comorbid conditions. To minimize misclassification bias by ICD-9/10 codes, we used at least 2 ICD-9/10 codes to confirm diagnoses. We also adjudicated 100 patient charts and found that ICD-9/10 code diagnoses were consistent with clinic notes.
A major strength of this study is the use of a large EHR data set from routine clinical practice that, unlike structured cohort studies, reflects the population of this health care system and the population it is serving. We used small geographic areas of tracts to determine area-level SES. Most studies regarding kidney disease have focused on ESKD and dialysis facilities and used the US Renal Data System. This study has the benefit of using observational data found in a large EHR health system with no recruitment limitations and encompasses all CKD stages.
In conclusion, we found that patients from low SES tracts and Medicaid recipients (among patients aged <65 years) have greater rates of CKD compared with patients from high SES tracts and patients with other insurance. These may be 2 of several socioeconomic and individual factors influencing the complexity of identification, management, and treatment of CKD. Further research is needed to identify neighborhood structural factors and individual and clinical factors that could be the target of public health interventions to reduce the burden of CKD.
Article Information
Authors’ Full Names and Academic Degrees
Lama Ghazi, MD, PhD, Theresa L. Osypuk, SD, Richard F. MacLehose, PhD, Russell V. Luepker, MD, MS, and Paul E. Drawz, MD, MS.
Authors’ Contributions
Research area and study design: LG, TLO, RFM, RVL, PED; data acquisition: LG, PED; data analysis and interpretation: LG, TLO, RFM, RVL, PED; statistical analysis: LG, PED; supervision or mentorship: TLO, RFM, RVL, PED. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This research was primarily supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant UL1TR002494, and the University of Minnesota Doctoral Dissertation Fellowship (for Dr Ghazi). The content is views of the National Institutes of Health’s National Center for Advancing Translational Sciences. Dr Osypuk was supported by the National Institute of Child Health and Human Development (R01HD090014). Dr MacLehose was supported by the U.S. National Library of Medicine (R01LM013049). None of the funders had any role in the study design; data collection, analysis, or reporting; or decision to submit the application.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received November 11, 2020. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form March 18, 2021.
Footnotes
Complete author and article information provided before references.
Figure S1: Cohort Construction
Item S1: Details of insurance coverage and methods
Table S1: Comparison of Fairview patients to the 7-county Minneapolis/St Paul metropolitan area
Table S2: Characteristics of population by tract socioeconomic status in the Twin Cities metropolitan area for individuals <65 years
Table S3: Characteristics of population by tract socioeconomic status in the Twin Cities metropolitan area for individuals ≥65 years
Table S4: Characteristics of population by race in the Twin Cities metropolitan area
Table S5: Characteristics of population by insurance status in the Twin Cities metropolitan area
Table S6: Multilevel regression model for the association of tract-level socioeconomic status and insurance status with CKD prevalence in individuals <65 years and by hypertension status
Table S7: Multilevel regression model for the association of tract-level socioeconomic status and insurance status with CKD prevalence in individuals <65 years and by diabetes status
Table S8: Quantitative selection bias analysis for association of tract-level socioeconomic status and insurance status with CKD prevalence for individuals <65 years
Table S9: Quantitative selection bias analysis for association of tract-level socioeconomic status with CKD prevalence for individuals ≥65 years
Table S10: Comparison of patients included in analyses versus excluded from analyses
Table S11: Multilevel regression model for the association of tract-level socioeconomic status and insurance status with CKD prevalence (defined as having 2 consecutive eGFRs >3 months apart <60 mL/min/1.73 m2) in individuals <65 years
Table S12: Multilevel regression model for the association of tract-level socioeconomic status and insurance status with CKD prevalence (defined as having 2 consecutive eGFRs > 3 months apart <60 mL/min/1.73 m2) in individuals ≥ 65 years
Supplementary Material
Figure S1, Item S1, Tables S1-S12
References
- 1.Murphy D., McCulloch C.E., Lin F. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med. 2016;165(7):473–481. doi: 10.7326/M16-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Executive summary. Accessed June 6, 2021. https://usrds.org/media/2371/2019-executive-summary.pdf.
- 3.Crews D.C., Charles R.F., Evans M.K., Zonderman A.B., Powe N.R. Poverty, race, and CKD in a racially and socioeconomically diverse urban population. Am J Kidney Dis. 2010;55(6):992–1000. doi: 10.1053/j.ajkd.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Advancing American Kidney Health Initiative. https://www.kidney.org/advocacy/advancing-american-kidney-health-initiative
- 5.Nicholas S.B., Kalantar-Zadeh K., Norris K.C. Socioeconomic disparities in chronic kidney disease. Adv Chronic Kidney Dis. 2015;22(1):6–15. doi: 10.1053/j.ackd.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vart P., Gansevoort R.T., Joosten M.M., Bültmann U., Reijneveld S.A. Socioeconomic disparities in chronic kidney disease: a systematic review and meta-analysis. Am J Prev Med. 2015;48(5):580–592. doi: 10.1016/j.amepre.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Volkova N., McClellan W., Klein M. Neighborhood poverty and racial differences in ESRD incidence. J Am Soc Nephrol. 2008;19(2):356–364. doi: 10.1681/ASN.2006080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garrity B.H., Kramer H., Vellanki K., Leehey D., Brown J., Shoham D.A. Time trends in the association of ESRD incidence with area-level poverty in the US population. Hemodial Int. 2016;20(1):78–83. doi: 10.1111/hdi.12325. [DOI] [PubMed] [Google Scholar]
- 9.Bowe B., Xie Y., Xian H., Lian M., Al-Aly Z. Geographic variation and US county characteristics associated with rapid kidney function decline. Kidney Int Rep. 2017;2(1):5–17. doi: 10.1016/j.ekir.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoham D.A., Vupputuri S., Diez Roux A.V. Kidney disease in life-course socioeconomic context: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2007;49(2):217–226. doi: 10.1053/j.ajkd.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Crews D.C., Gutiérrez O.M., Fedewa S.A. Low income, community poverty and risk of end stage renal disease. BMC Nephrol. 2014;15:192. doi: 10.1186/1471-2369-15-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Care Without Coverage: Too Little, Too Late. National Academies Press (US); 2002. Institute of Medicine (US) Committee on the Consequences of Uninsurance. 3, Effects of health insurance on health.https://www.ncbi.nlm.nih.gov/books/NBK220636/ [PubMed] [Google Scholar]
- 13.Ross C.E., Mirowsky J. Does medical insurance contribute to socioeconomic differentials in health? Milbank Q. 2000;78(2):291–321. doi: 10.1111/1468-0009.00171. 151-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenbaum S. Medicaid. N Engl J Med. 2002;346(8):635–640. doi: 10.1056/NEJM200202213460825. [DOI] [PubMed] [Google Scholar]
- 15.Fang J., Alderman M.H. Does supplemental private insurance affect care of Medicare recipients hospitalized for myocardial infarction? Am J Public Health. 2004;94(5):778–782. doi: 10.2105/ajph.94.5.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrasquillo O., Lantigua R.A., Shea S. Preventive services among Medicare beneficiaries with supplemental coverage versus HMO enrollees, Medicaid recipients, and elders with no additional coverage. Med Care. 2001;39(6):616–626. doi: 10.1097/00005650-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Pourat N., Rice T., Kominski G., Snyder R.E. Socioeconomic differences in Medicare supplemental coverage. Health Aff (Millwood) 2000;19(5):186–196. doi: 10.1377/hlthaff.19.5.186. [DOI] [PubMed] [Google Scholar]
- 18.Christensen S., Shinogle J. Effects of supplemental coverage on use of services by Medicare enrollees. Health Care Financ Rev. 1997;19(1):5–17. [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical Data Repository. https://www.ctsi.umn.edu/researcher-resources/clinical-data repository
- 20.US Census Bureau http://www.census.gov/
- 21.Manson S., Schroeder J., Van Riper D., Ruggles S. University of Minnesota; Minneapolis, MN: 2018. IPUMS National Historical Geographic Information System: Version 13.0 [Database] [Google Scholar]
- 22.Diez Roux A.V. Investigating neighborhood and area effects on health. Am J Public Health. 2001;91(11):1783–1789. doi: 10.2105/ajph.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oates J.M., Andrade K.E. Chapter Two: The measurement of socioeconomic status. In: Oakes J.M., Kaufman J.M., editors. Methods in Social Epidemiology. 2nd ed. John Wiley & Sons; Hoboken, NJ: 2017. pp. 23–42. [Google Scholar]
- 24.Messer L., Kaufman J.S. Using census data to approximate neighborhood effects. In: Oakes J.M., Kaufman J.S., editors. Methods in Social Epidemiology. 1st ed. John Wiley & Sons; 2006. pp. 209–236. [Google Scholar]
- 25.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Medicare & Medicaid Services Original Chronic Conditions Data Warehouse Chronic Condition Algorithms. Chronic conditions data warehouse. https://www.ccwdata.org/web/guest/condition-categories
- 27.Messer L.C., Oakes J.M., Mason S. Effects of socioeconomic and racial residential segregation on preterm birth: a cautionary tale of structural confounding. Am J Epidemiol. 2010;171(6):664–673. doi: 10.1093/aje/kwp435. [DOI] [PubMed] [Google Scholar]
- 28.Osypuk T.L., Acevedo-Garcia D. Beyond individual neighborhoods: a geography of opportunity perspective for understanding racial/ethnic health disparities. Health Place. 2010;16(6):1113–1123. doi: 10.1016/j.healthplace.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNutt L.A., Wu C., Xue X., Hafner J.P. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 30.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 31.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004;160(4):301–305. doi: 10.1093/aje/kwh221. [DOI] [PubMed] [Google Scholar]
- 32.VanderWeele T.J., Knol M.J. A tutorial on interaction. Epidemiol Methods. 2014;3:33–72. [Google Scholar]
- 33.Lash T.L., Fox M.P., Fink A.K. Springer; 2009. Applying Quantitative Bias Analysis to Epidemiologic Data. [Google Scholar]
- 34.Rossi P.H., Wright J.D., Anderson A.B. Academic Press; 1983. Handbook of Survey Research; pp. 415–492. [Google Scholar]
- 35.Chow W. The RAND Corp; Santa Monica, CA: 1979. A Look at Various Estimators in Logistic Models in the Presence of Missing Values; pp. 417–420. [Google Scholar]
- 36.R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing V, Austria. https://www.R-project.org/
- 37.StataCorp. 2017. Stata Statistical Software: Release 15. College Station TSL.
- 38.Shoham D.A., Vupputuri S., Kshirsagar A.V. Chronic kidney disease and life course socioeconomic status: a review. Adv Chronic Kidney Dis. 2005;12(1):56–63. doi: 10.1053/j.ackd.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Patzer R.E., McClellan W.M. Influence of race, ethnicity and socioeconomic status on kidney disease. Nat Rev Nephrol. 2012;8(9):533–541. doi: 10.1038/nrneph.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plantinga L.C. Socio-economic impact in CKD. Nephrol Ther. 2013;9(1):1–7. doi: 10.1016/j.nephro.2012.07.361. [DOI] [PubMed] [Google Scholar]
- 41.Zeng X., Liu J., Tao S., Hong H.G., Li Y., Fu P. Associations between socioeconomic status and chronic kidney disease: a meta-analysis. J Epidemiol Community Health. 2018;72(4):270–279. doi: 10.1136/jech-2017-209815. [DOI] [PubMed] [Google Scholar]
- 42.Merkin S.S., Coresh J., Diez Roux A.V., Taylor H.A., Powe N.R. Area socioeconomic status and progressive CKD: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2005;46(2):203–213. doi: 10.1053/j.ajkd.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 43.Merkin S.S., Diez Roux A.V., Coresh J., Fried L.F., Jackson S.A., Powe N.R. Individual and neighborhood socioeconomic status and progressive chronic kidney disease in an elderly population: the Cardiovascular Health Study. Soc Sci Med. 2007;65(4):809–821. doi: 10.1016/j.socscimed.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 44.McClellan W.M., Newsome B.B., McClure L.A. Poverty and racial disparities in kidney disease: the REGARDS study. Am J Nephrol. 2010;32(1):38–46. doi: 10.1159/000313883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schechter M.S., Shelton B.J., Margolis P.A., Fitzsimmons S.C. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med. 2001;163(6):1331–1337. doi: 10.1164/ajrccm.163.6.9912100. [DOI] [PubMed] [Google Scholar]
- 46.Morgan M.A., Behbakht K., Benjamin I., Berlin M., King S.A., Rubin S.C. Racial differences in survival from gynecologic cancer. Obstet Gynecol. 1996;88(6):914–918. doi: 10.1016/s0029-7844(96)00342-0. [DOI] [PubMed] [Google Scholar]
- 47.Ayanian J.Z., Kohler B.A., Abe T., Epstein A.M. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med. 1993;329(5):326–331. doi: 10.1056/NEJM199307293290507. [DOI] [PubMed] [Google Scholar]
- 48.Harnick D.J., Cohen J.L., Schechter C.B., Fuster V., Smith D.A. Effects of practice setting on quality of lipid-lowering management in patients with coronary artery disease. Am J Cardiol. 1998;81(12):1416–1420. doi: 10.1016/s0002-9149(98)00209-4. [DOI] [PubMed] [Google Scholar]
- 49.Shen J.J., Wan T.T., Perlin J.B. An exploration of the complex relationship of socioecologic factors in the treatment and outcomes of acute myocardial infarction in disadvantaged populations. Health Serv Res. 2001;36(4):711–732. [PMC free article] [PubMed] [Google Scholar]
- 50.Kurella-Tamura M., Goldstein B.A., Hall Y.N., Mitani A.A., Winkelmayer W.C. State Medicaid coverage, ESRD incidence, and access to care. J Am Soc Nephrol. 2014;25(6):1321–1329. doi: 10.1681/ASN.2013060658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Penchansky R., Thomas J.W. The concept of access: definition and relationship to consumer satisfaction. Med Care. 1981;19(2):127–140. doi: 10.1097/00005650-198102000-00001. [DOI] [PubMed] [Google Scholar]
- 52.American Diabetes Association Cardiovascular disease and risk management: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S86–S104. doi: 10.2337/dc18-S009. [DOI] [PubMed] [Google Scholar]
- 53.Whelton P.K., Carey R.M., Aronow W.S. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269–1324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, Item S1, Tables S1-S12