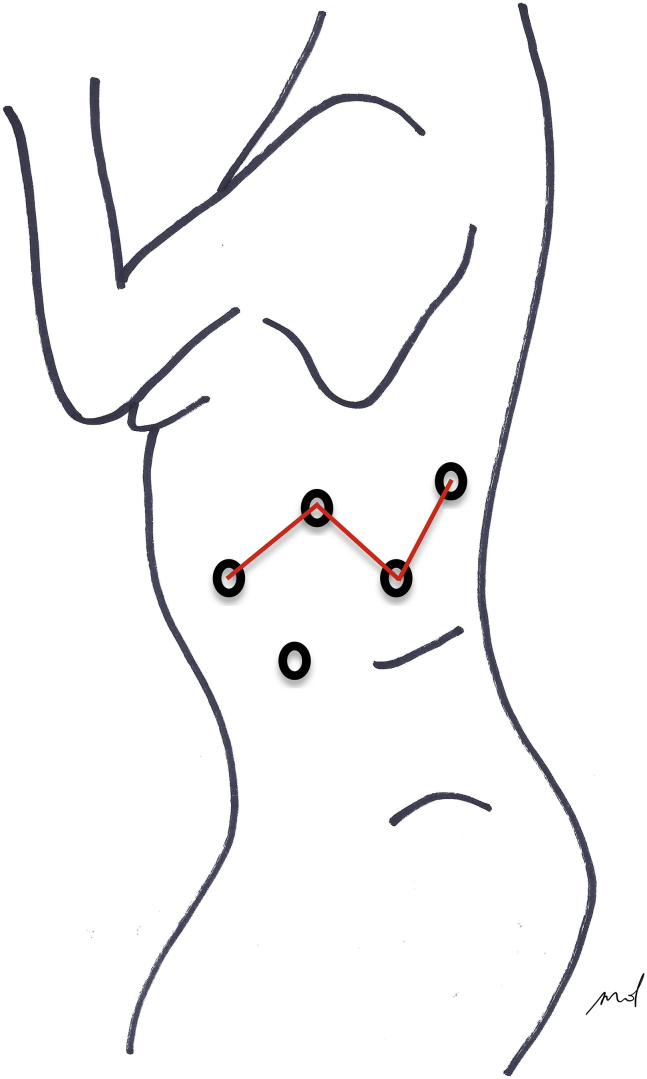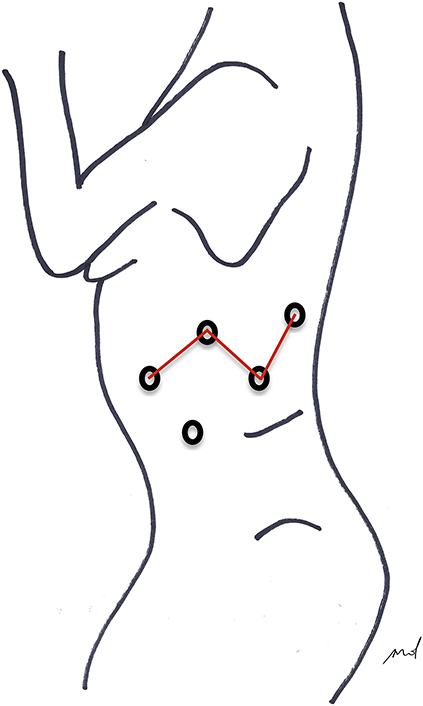
Port placement scheme.
Central Message.
Segmentectomy is an increasing used surgical approach in thoracic surgery. We describe and discuss technical steps using a 4-arm completely portal approach with a Da Vinci Xi system.
Clinical Vignette
We present a video of an 84-year-old woman, a former smoker (30 packs per year) diagnosed with suspected stage I (cT2 N0 M0) primary lung cancer in the left segment 3 (S3). The patient's medical history included moderate chronic obstructive pulmonary disease, obstructive sleep apnea syndrome, and cerebral aneurysm. Considering the high surgical risk (eg, age, diffusing capacity for carbon monoxide, and pathological underlying lungs) she was scheduled for a robotic S3 segmentectomy with radical node harvest.1 We describe and discuss technical steps using a 4-arm completely portal approach with a Da Vinci Xi system (Intuitive Surgical, Sunnyvale, Calif) using near-infrared fluorescence with indocyanine green (ICG) for intersegmental plan definition.
Surgical Technique
As for any lung resection, patient's position and port setting were done as previously described2,3 (Figure 1).
Figure 1.
Port placement scheme.
Firstly, inferior exposure was achieved to perform the triangular ligament dissection, visualization of the inferior pulmonary vein and zone 8 and 9 node harvest. Secondly, zone 7, 10, and 4L node harvest was completed through posterior exposure and caution was exercised to preserve the vagus nerve. Thirdly, through fissure exposure, spotting the artery and zone 11, 12 node harvest were performed.
A recurrent lingula artery had to be ligated to avoid its tear during later fissure stapling. Subsequently, anterior exposure was conducted. This allowed the completion of node harvest (zone 5, 12, and 13) and S3 vein, artery, and bronchus control. In a sequence, vein 3 and artery 3 were controlled and manually ligated (silk 0 size, 10 cm length). Bronchus 3 was mechanically stapled through the port access (8.5 mm Echelon Powered35-mm white load, Ethicon, Somerville, NJ). S3 lung mapping was completed 20 seconds after ICG intravenous injection (20 mg; ie, 8 mL followed by 10 mL saline flush). Both intersegmental plans stapling required 5 60-mm green loads from the right-hand port (12 mm Echelon Powered Flex). Airtight patency was checked under water, specimen removed in a bag through the port access hole, and chest closed with a 24F chest tube through the anterior port.
Postoperative course was uneventful. Chest tube was removed on postoperative day 1 and the patient was discharged on day 3. Final pathology confirmed pT2 N0 R0 squamous cell carcinoma. Institutional review board approval was not required for the publication of the study data, and the patient's informed consent was received.
Comments
Segmentectomy is a increasingly used surgical approach in thoracic surgery because it combines en bloc resection with radical node harvest and lung sparing strategy.4 It is admitted for a <2 cm lesion but will also be considered for larger tumors in comorbid patients as a best-care strategy versus radiotherapy.4 Culmenectomy was considered due to the large size of the tumor (40 mm), but a 8 Charlson index score and a strictly centred S3 location in the emphysematous zone led us to prefer a segmentectomy.
Complex anatomical segmentectomy can be very challenging using traditional video-assisted thoracoscopic surgery or even open surgery (ie, sizing of structure, versatility of exposure, and lung mapping). As of today, we failed to find a technical description for such a procedure. S3 segmentectomy can be tricky, especially on the left side as upper lobe vascularization might be complex and delicate to control. The robotic system offers significant technical skills and vision that ensure its achievement as demonstrated in Video 1.
Several technique-specific characteristics can be summarized:
-
•
Trocar placement The partial W port setting allows 30°camera visual control of the whole chest cavity sparing tools conflict.5 A distant/lower port access offers the assistant a larger movement range (Figure 1).
-
•
Reproducibility In our experience this positioning is the same for any procedure.
-
•
Utility incision A 15-mm port ensures airtight patency and affords a low-pressure capnothorax (5 mm Hg).
-
•
Robotic stapling sparing Sparing is not only a cost issue. Robotic stapling requires 2 12-mm ports, considering that segmentectomies need both anterior and posterior axes of stapling. Posterior port might be painful especially for a patient with smaller intercostal spaces. The more distal the dissection, the more manual targeting and ligation of vessels are required. Manual vessel ligation is also a safety key point and skills concern; the versatility of hand-wrist instruments allows to maintain the vessel's axis as well as ensure routine surgeon hard skills practice.
-
•
ICG We use a 8 mL (20 mg) dose for each patient, whatever weight and sex, waiting no more than 20 seconds before marking to avoid product spreading. We also routinely check the partial wash out of ICG due to cardiac output to be sure of the venous patency on the remaining segments.
Conclusions
Robotic hand–wrist surgery offers optimal technical conditions for a safe and rigorous vessel control and node harvest during a complex segmentectomy.
Footnotes
Disclosures: The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Supplementary Data
Four-arm robotic left S3 segmentectomy. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00376-X/fulltext.
References
- 1.Durand M. Four arm robotic double right segmentectomies S3 & S7–10 with fluorescence lung mapping. J Vis Surg. 2020;6:40. [Google Scholar]
- 2.Durand M. Four-arm robotic sleeve right upper lobectomy. Ann Cardiothorac Surg. 2019;8:286–287. doi: 10.21037/acs.2018.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novellis P., Bottoni E., Alloisio M., Velez-Cubian F.O., Toloza E.M., Veronesi G. Robotic-assisted pulmonary segmentectomies. J Vis Surg. 2018;4:166. [Google Scholar]
- 4.Cao C., Chandrakumar D., Gupta S., Yan T.D., Tian D.H. Could less be more? A systematic review and meta-analysis of sublobar resections versus lobectomy for non–small cell lung cancer according to patient selection. Lung Cancer. 2015;89:121–132. doi: 10.1016/j.lungcan.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Durand M., Dabboura E., Lamonerie L., Herkert A., Zarka V., Carrier A.S. Four-arm robotic lung resection versus muscle-sparing mini-thoracotomy: retrospective experience. J Thorac Dis. 2019;11:1433–1442. doi: 10.21037/jtd.2019.03.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Four-arm robotic left S3 segmentectomy. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00376-X/fulltext.