Abstract
In the gut, cathelicidin‐related antimicrobial peptide (CRAMP) has been largely described for its anti‐infective activities. With an increasing recognition of its immune regulatory effects in extra‐intestinal diseases, the role of CRAMP in gluten‐induced small intestinal enteropathy celiac disease remains unknown. This study aimed to investigate the unexplored role of CRAMP in celiac disease. By applying a mouse model of gluten‐induced enteropathy (GIE) recapitulating small intestinal enteropathy of celiac disease, we observed defective CRAMP production in duodenal epithelium during GIE. CRAMP‐deficient mice were susceptible to the development of GIE. Exogenous CRAMP corrected gliadin‐triggered epithelial dysfunction and promoted regulatory immune responses at the intestinal mucosa. Additionally, GIE‐associated gut dysbiosis with enriched Pseudomonas aeruginosa and production of the protease LasB contributed to defective intestinal CRAMP production. These results highlight microbiota‐CRAMP axis in the modulation of barrier function and immune responses in GIE. Hence, modulating CRAMP may represent a therapeutic strategy for celiac disease.
Keywords: antimicrobial peptides, celiac disease, gluten‐induced enteropathy, microbiota, interleukin‐15
Subject Categories: Digestive System; Immunology; Microbiology, Virology & Host Pathogen Interaction
A protective role of cathelicidin‐related antimicrobial peptide (CRAMP) under the influence of gut microbiota in dietary gluten‐induced enteropathy (GIE) is established to provide unique insights into the therapeutic strategy for celiac disease.
The paper explained.
Problem
Celiac disease is a complex immune‐mediated small intestinal enteropathy, for which curative therapeutics are not available. Cathelicidin‐related antimicrobial peptide (CRAMP) plays an indispensable role in intestinal homeostasis. However, the effect of CRAMP in dietary gluten‐induced small intestinal enteropathy (GIE) such as celiac disease remains to be explored.
Results
Defective CRAMP production in duodenal epithelium was correlated with GIE development. CRAMP deficiency aggravated GIE, while exogenous CRAMP treatment protected against it. Furthermore, CRAMP harboured positive modulatory effect on intestinal barrier integrity by counteracting gliadin‐triggered epithelial dysfunction and promoted modulatory immune cell responses at the intestinal mucosa. Lastly, GIE‐associated enrichment of Pseudomonas aeruginosa and increased production of protease LasB contributed to defective intestinal CRAMP production.
Impact
This study provides novel evidence that CRAMP is a key regulatory mediator for GIE. Understanding the impact of gut microbiota‐CRAMP axis on intestinal barrier integrity and immune responses during GIE may shed light on novel therapeutic strategies for clinical celiac disease.
Introduction
Antimicrobial peptides (AMPs) or host defence peptides (HDPs) are evolutionarily conserved peptides as an essential innate defence mechanism found in most all living organisms (Zasloff, 2002; Gallo & Hooper, 2012). Apart from their classical antimicrobial activity, the immune modulatory capacity of AMPs has been increasingly appreciated and they may cause either pro‐inflammatory or anti‐inflammatory effects dependent on the disease model being investigated and their cellular source (Hancock et al, 2016). Among AMPs, we and others have particularly highlighted the role of cathelicidins [the sole member named LL‐37 in humans and cathelicidin‐related antimicrobial peptide (CRAMP) in mice] in autoimmune diseases. Commonly, neutrophil‐derived CRAMP, often citrullinated and when in excess, forms complexes with self‐nucleic acids to activate plasmacytoid dendritic cells (pDCs), inducing deleterious autoimmune responses (Diana et al, 2013; Kahlenberg & Kaplan, 2013). However, we previously demonstrated that pancreatic β‐cells‐derived CRAMP induces regulatory immune cells to shape pancreatic immune microenvironment, thereby protecting against autoimmune diabetes in non‐obese diabetic (NOD) mice (Sun et al, 2015).
Not surprisingly, the mammalian gut epithelium produces a diverse collection of AMPs, responding to the complex environmental cues in the intestine. In the gut, the role of cathelicidins has been largely confirmed to the large intestine for colonic inflammatory diseases or its anti‐infective activities (Mitsutoshi et al, 2005; Raqib et al, 2006; Hing et al, 2013; Mukherjee & Hooper, 2015). To a lesser extent, cathelicidin expression is found in the small intestine (Gallo et al, 1997; Mukherjee & Hooper, 2015). Despite an increasing recognition of its role in autoimmune disease contexts, the role of cathelicidins in the development of dietary gluten‐induced small intestinal enteropathy with autoimmune features such as celiac disease remains to be explored.
Here, we observe defective CRAMP production in duodenal epithelium of mice with gluten‐induced enteropathy (GIE). While CRAMP‐deficient (Cnlp −/−) mice are susceptible to the development of GIE, increasing duodenal CRAMP prevents GIE, by shaping intestinal barrier function and immune response. GIE‐associated dysbiosis with enhanced Pseudomonas aeruginosa (P. aeruginosa) contributes to CRAMP degradation via production of the protease LasB. Thus, the current study reveals a critical role of CRAMP in modulating GIE and supports future therapeutic strategy targeting CRAMP for the prevention of celiac disease.
Results
Epithelial cathelicidin production is defective in dietary gluten‐induced enteropathy (GIE)
To test our hypothesis, we first determined the relevance of cathelicidin production and regulation with GIE in mice. In order to evaluate intestinal CRAMP expression and its effect on gluten‐induced small intestinal enteropathy, a mouse model of GIE by adoptive transfer of gliadin‐presensitized CD4+CD45RBlowCD25− T cells into lymphopenic Rag1 −/− mice, recapitulating intestinal pathology of human celiac disease (Freitag et al, 2009) was applied (Fig 1A). Serum CRAMP levels were found much lower in mice received gliadin‐presensitized T cells and fed with gluten‐containing diet (gluten group) than in those fed with gluten‐free diet (gluten‐free group; Fig 1B). Notably, we observed no difference in serum CRAMP levels in unsensitized mice fed with gluten‐free diet or gluten‐containing diet (Fig EV1A). Western blot experiment confirmed CRAMP expression in duodenum of Rag1 −/−, C57BL/6 and BALB/c mice but not of CRAMP‐deficient mice under steady state (Fig EV1B). After GIE induction, CRAMP became scarcely expressed both for the pro‐form (18 kDa) and the secreted mature form (5 kDa) in duodenal tissue and in ex vivo isolated duodenal epithelial cells (Fig 1C and D). CRAMP may be expressed by epithelial cells and by infiltrating immune cells (Gallo & Hooper, 2012). Immunofluorescent staining confirmed that CRAMP staining (red) was primarily localized in intestinal epithelium and co‐expressed with E‐cadherin‐positive cells, instead of Ly6G+ neutrophils or F4/80+ macrophages which were largely present in the lamina propria (Fig 1E). CRAMP expression by epithelial cells was reduced in mice fed with gluten diet, despite of increased Ly6G and F4/80 staining indicative of increased immune infiltrates. Lastly, we examined whether an association of serum LL‐37 levels, the human ortholog of CRAMP and susceptibility to clinical celiac disease could be established. Serum samples of celiac disease high‐risk subjects positive for anti‐tissue transglutaminase immunoglobulin A antibodies (anti‐tTG IgA) or/and anti‐deamidated gliadin peptide immunoglobulin G antibodies (anti‐DGP IgG)] and double‐negative subjects (anti‐tTG IgA− anti‐DGP IgG−) were obtained. Intriguingly, we found lower levels of serum LL‐37 in celiac disease high‐risk subjects than in double‐negative subjects (Fig EV1C). Together, these results suggest that CRAMP may play a role in GIE and prompt us to investigate how CRAMP modulates GIE development.
Figure 1. Epithelial CRAMP production is defective in dietary gluten‐induced enteropathy (GIE).
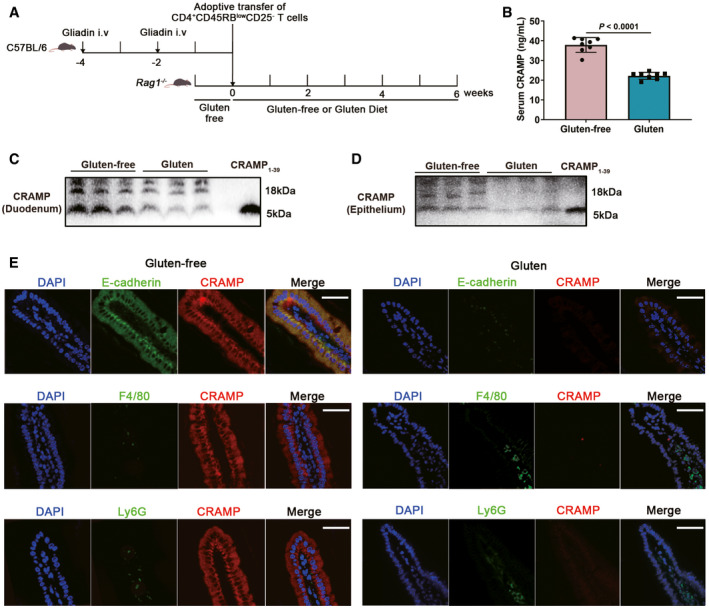
-
AAnimal protocol.
-
BSerum CRAMP determination by ELISA (n = 8).
-
C, DWestern blot of pro‐form CRAMP and mature peptide in duodenum and ex vivo duodenal epithelial cells.
-
ELocalization and expression of CRAMP (red), E‐cadherin (green), F4/80 (green) and Ly6G (green) in duodenum by immunofluorescent staining. Representative photomicrographs of individual and merged staining were shown. Nuclei were stained with DAPI (blue). Scale bar: 50 μm.
Data information: Data in B were representative and were the mean ± SD from three independent experiments. Data (C–E) were representative from three independent experiments. P values were calculated by unpaired two‐tailed t‐test for comparison of two groups.
Source data are available online for this figure.
Figure EV1. The production of cathelicidin (CRAMP in mice and LL‐37 in humans) in mice and in human subjects. Related to Fig 1.
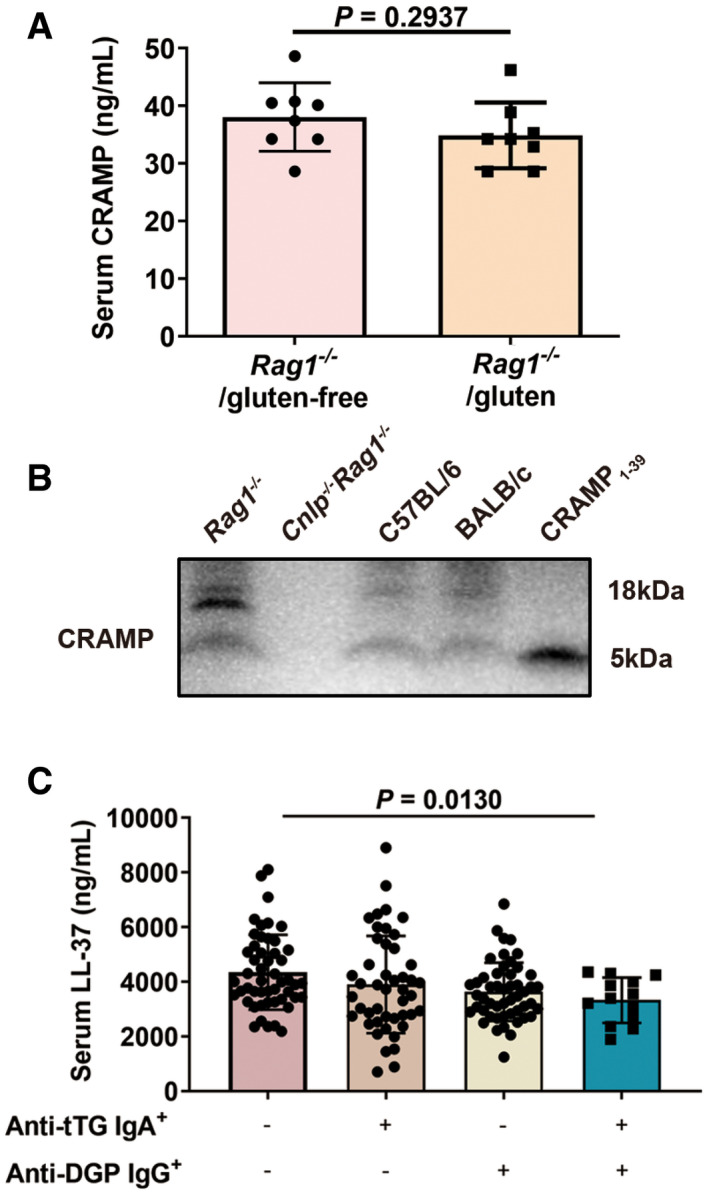
- Serum CRAMP determination by ELISA. Rag1 −/−/gluten‐free: unsensitized Rag1 −/−mice fed with gluten‐free diet. Rag1 −/−/gluten: unsensitized Rag1 −/− mice fed with gluten‐containing diet (n = 8).
- Western blot of pro‐form CRAMP and the mature peptide in duodenum of Rag1 −/−, Cnlp −/− Rag1 −/−, C57BL/6 and BALB/c mice.
- Serum LL‐37 determination by ELISA. Anti‐tTG IgA: anti‐tissue transglutaminase immunoglobulin A antibodies. Anti‐DGP IgG: anti‐deamidated gliadin peptides immunoglobulin G antibodies (Anti‐tTG IgA−Anti‐DGP IgG−, n = 50; Anti‐tTG IgA+Anti‐DGP IgG−, n = 46; Anti‐tTG IgA−Anti‐DGP IgG+, n = 50; Anti‐tTG IgA+Anti‐DGP IgG+, n = 13).
Data information: Data (A, C) were representative and were the mean ± SD from three independent experiments. Data in B were representative from three independent experiments. P values were calculated by unpaired two‐tailed t‐test for comparison of two groups (A) or one‐way ANOVA followed by Tukey’s post hoc test for multiple comparisons (C).
Source data are available online for this figure.
CRAMP deficiency potentiates GIE
As CRAMP production is defective during GIE development, we investigated the impact of CRAMP deficiency on GIE development. Classic symptoms of GIE include weight loss and duodenal histological changes (Freitag et al, 2009; Fig 2A). CRAMP‐deficient mice with GIE (Cnlp −/−/gluten group) exhibited worsened weight loss compared with control mice (gluten group; Fig 2B). Similarly, characteristic histological changes including duodenal crypt hyperplasia, villus atrophy and lymphocytic infiltration (Freitag et al, 2009) were exacerbated in CRAMP‐deficient mice induced with GIE (Cnlp −/−/gluten group; Fig 2C). Intestinal tight junction proteins (TJPs) locate at the apical ends of the lateral membranes of intestinal epithelial cells, including zona occludens‐1 (ZO‐1), ZO‐2, occludin and claudin‐1, and maintain the intestinal barrier integrity (Suzuki, 2013). Dysregulation of small intestinal TJPs leading to increased intestinal permeability is a key pathological event of GIE (Alessio, 2012). As shown in Fig 2D, loss of duodenal TJPs was exacerbated in Cnlp −/−/gluten group, compared with gluten group. Zonulin is a unique protein so far known to reversibly regulate intestinal permeability by modulating intercellular TJP expression (Amit et al, 2009; Alessio, 2012). Duodenal zonulin was found highly produced in Cnlp −/−/gluten group (Fig 2E), leading to suppressed TJP expression and increased intestinal permeability (Fig 2F). Collectively, CRAMP deficiency aggravates GIE.
Figure 2. CRAMP deficiency potentiates GIE.
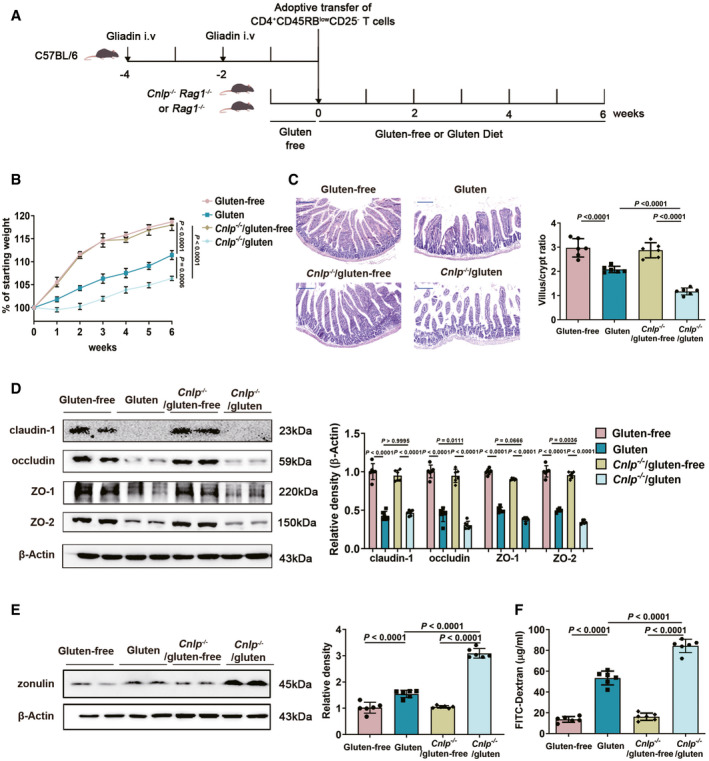
- Animal protocol.
- Changes in body weight relative to starting weight during 6 weeks (n = 6).
- Left, representative images of duodenal damage by H&E staining. Scale bar: 200 μm. Right, graph depicted the ratio of the morphometric assessment of villus height to crypt depth (n = 6).
- Western blot and densitometry analyses of duodenal tight junction proteins (claudin‐1, occludin, ZO‐1 and ZO‐2; n = 6).
- Western blot and densitometry analysis of duodenal zonulin (n = 6).
- Intestinal permeability was assessed by measuring FITC‐Dextran (n = 6).
Data information: Data (B–F) were representative and were the mean ± SD from three independent experiments. P values were calculated by one‐way ANOVA followed by Tukey’s post hoc test for multiple comparisons.
Source data are available online for this figure.
Replenishing duodenal CRAMP is protective against GIE
We further investigated the effect of exogenous CRAMP treatment on GIE development. Mice were intraperitoneally administered with CRAMP (100 µg mouse−1 week−1) either two weeks before (prophylactic treatment) or 6 weeks after (therapeutic treatment) adoptive gliadin‐sensitized T cells transfer (Fig 3A). Small intestinal CRAMP levels were replenished following CRAMP administration (Figs 3B and EV2A and B). GIE‐associated weight loss was notably attenuated by both prophylactic and therapeutic treatment with CRAMP (Fig 3C). We found that increasing duodenal CRAMP protected against gluten‐induced gut barrier dysfunction as evidenced by improved histological markers (duodenum in Fig 3D, jejunum in Fig EV2C and ileum in Fig EV2D), increased expression of TJPs (duodenum in Fig 3E, jejunum in Fig EV2E and ileum in Fig EV2F), decreased duodenal zonulin production (Fig 3F) and reduced intestinal permeability (Fig 3G). Together, these data support a protective role of CRAMP for GIE by shaping intestinal barrier integrity.
Figure 3. Replenishing duodenal CRAMP is protective against GIE.
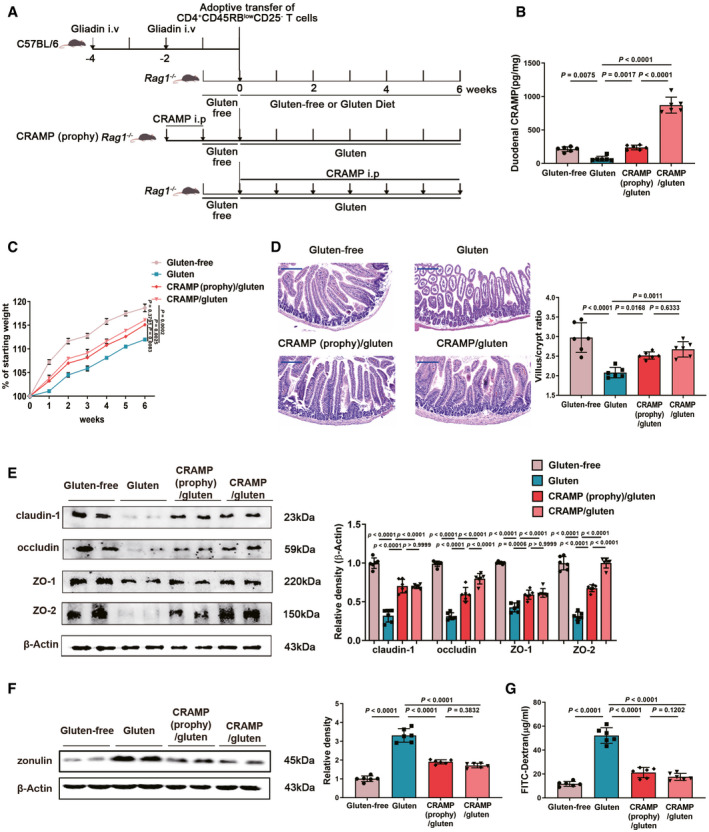
- Animal protocol.
- Duodenal CRAMP determination by ELISA (n = 6).
- Changes in body weight relative to starting weight during 6 weeks (n = 6).
- Left, representative images of duodenal damage by H&E staining. Scale bar: 200 μm. Right, graph depicted the ratio of the morphometric assessment of villus height to crypt depth (n = 6).
- Western blot and densitometry analyses of duodenal tight junction proteins (claudin‐1, occludin, ZO‐1 and ZO‐2; n = 6).
- Western blot and densitometry analysis of duodenal zonulin (n = 6).
- Intestinal permeability was assessed by measuring FITC‐Dextran (n = 6).
Data information: Data (B–G) were representative and were the mean ± SD from three independent experiments. P values were calculated by one‐way ANOVA followed by Tukey’s post hoc test for multiple comparisons.
Source data are available online for this figure.
Figure EV2. Replenishing CRAMP is protective against jejunal and ileal barrier damage during GIE. Related to Fig 3.
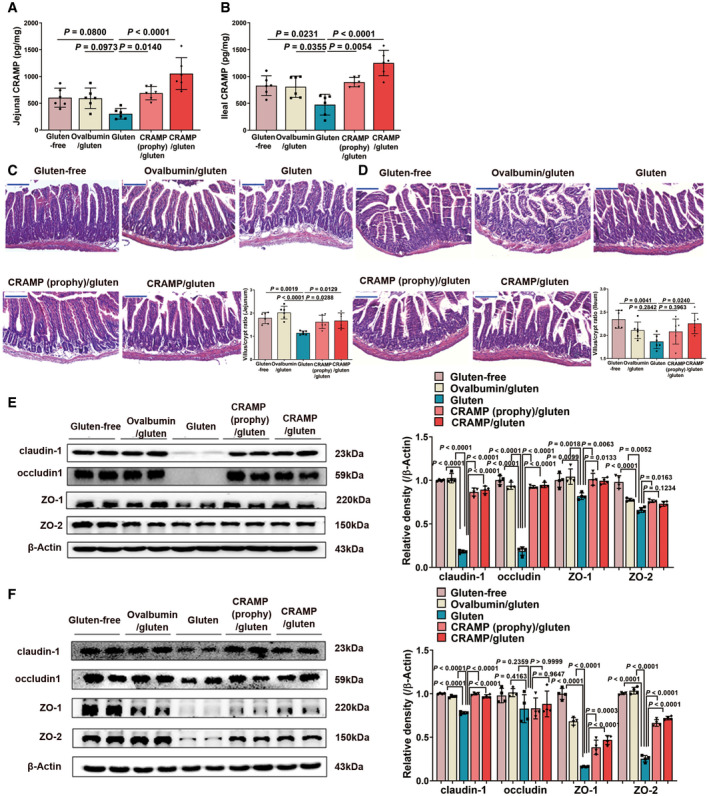
-
A, BCRAMP determinations in (A) jejunum and (B) ileum by ELISA (n = 6).
-
C, DRepresentative images of (C) jejunal and (D) ileal damage by H&E staining. Scale bar: 200 μm. The graphs depicted the ratio of the morphometric assessment of villus height to crypt depth (n = 6).
-
E, FWestern blot and densitometry analyses of tight junction proteins (claudin‐1, occludin, ZO‐1 and ZO‐2) in (E) jejunum and (F) ileum (n = 4).
Data information: Data were representative and were the mean ± SD from three (A–D) or two (E, F) independent experiments. P values were calculated by one‐way ANOVA followed by Tukey’s post hoc test for multiple comparisons.
Source data are available online for this figure.
CRAMP counteracts gliadin‐triggered inflammatory signalling to protect epithelial function
Next, we attempted to decipher signalling mechanism underlying the regulatory effect of CRAMP on epithelial barrier function. Gliadin, the undigested immunogenic peptide from gluten, is known to induce prolonged epidermal growth factor receptor (EGFR) activation at Tyr1068, leading to downstream phosphoinositide‐3‐kinase/protein kinase B (Pi3K/Akt)‐nuclear factor kappa light chain enhancer of activated B cells (NF‐κB) signalling or a myeloid differentiation factor 88 (MyD88)‐dependent zonulin production, triggering epithelial barrier disruption (Thomas et al, 2006; Barone et al, 2007; Lammers et al, 2008; Yan et al, 2011). Then, using ex vivo isolated duodenal epithelial cells to investigate how CRAMP may affect gliadin‐triggered cellular signalling. We observed that CRAMP inhibited gliadin‐triggered phosphorylation of EGFR at Tyr1068, Pi3K/Akt activation, MyD88/TNF receptor‐associated factor 6 (TRAF6) expression and NF‐κB activation in duodenal epithelial cells ex vivo, while deficiency CRAMP intensified gliadin‐triggered inflammation (Fig 4A). Similarly, an inhibitory effect of LL‐37, the human ortholog of CRAMP, on pepsin/trypsin digested (PT)‐gliadin‐induced signalling was demonstrated on human epithelial cells (Fig 4B). To further determine the mechanism by which LL‐37 inhibits gliadin‐induced signalling, human epithelial cells were pre‐treated with Ilomastat before stimulation with PT‐gliadin. Ilomastat, an inhibitor of EGFR transactivation, was used to test whether LL‐37 acts via a competitive binding (Tjabringa et al, 2003; Sho et al, 2005). The results showed that the inhibitory effect of LL‐37 on PT‐gliadin‐induced signalling was abolished by Ilomastat (Fig 4B), supporting that CRAMP counteracts gliadin‐induced signalling by inducing a competitive binding to interrupt prolonged pathological activation of EGFR by gliadin.
Figure 4. CRAMP counteracts gliadin‐triggered inflammatory signalling to protect epithelial function.
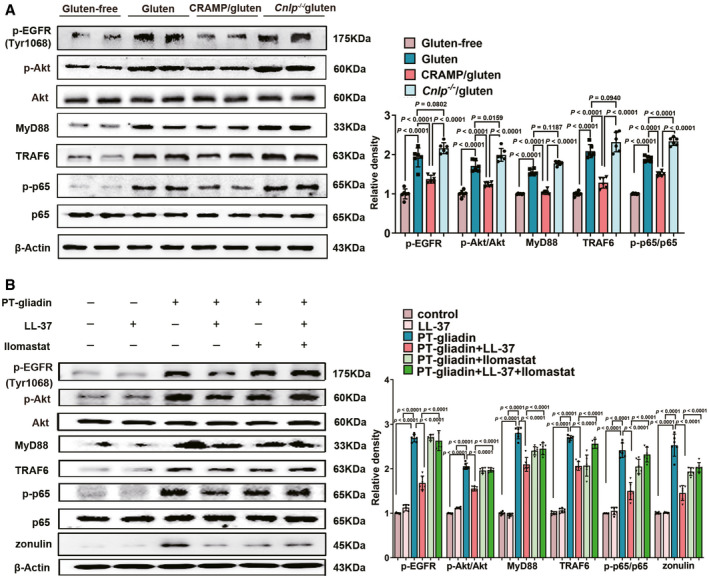
- Western blot and densitometry analyses of gliadin‐triggered inflammatory signalling [p‐EGFR (Tyr1068), p‐Akt, Akt, MyD88, TRAF6, p‐p65 and p65] in epithelial cells ex vivo (n = 6).
- Western blot and densitometry analyses of gliadin‐triggered inflammatory signalling [p‐EGFR (Tyr1068), p‐Akt, Akt, MyD88, TRAF6, p‐p65, p65 and zonulin] in vitro (n = 6).
Data information: Data (A and B) were representative and were the mean ± SD from three independent experiments. P values were calculated by one‐way ANOVA followed by Tukey’s post hoc test for multiple comparisons.
Source data are available online for this figure.
CRAMP modulates intestinal immune disorder in GIE
In addition to barrier dysfunction, gliadin also triggers epithelial cell to release interleukin‐15 (IL‐15) which importantly activates intraepithelial lymphocytes (IELs) (Villella et al, 2019). IL‐15 activated IELs acquire cytotoxic properties by upregulating natural‐killer group 2 member D (NKG2D) receptor, responsible for epithelial cell damage, leading to dysregulated immune responses at intestinal mucosa in celiac disease (Qingsheng et al, 2006; Valérie & Bana, 2014). We examined whether CRAMP impacts on IL‐15 production and mucosal immune imbalance during GIE. Therapeutic treatment of cathelicidin significantly reduced Il15/IL15 expression in mouse duodenum and in human epithelial cells (Fig EV3A and B). Ex vivo examination also confirmed that CRAMP downregulated IL‐15 expression in duodenal epithelial cells (Fig 5A). Consequently, this led to attenuated percentage of NKG2D+ IELs (Fig 5B). The regulatory effect of CRAMP on IL‐15 production and cytotoxic IELs expansion was further confirmed by the observation that CRAMP‐deficient mice with GIE (Cnlp −/−/gluten group) exhibited a significantly increased percentage of NKG2D+ IELs (Fig 5B).
Figure EV3. Cathelicidin regulates cytokine expression in GIE and IL‐15 expression in PT‐gliadin‐stimulated human epithelial cells. Related to Fig 5.
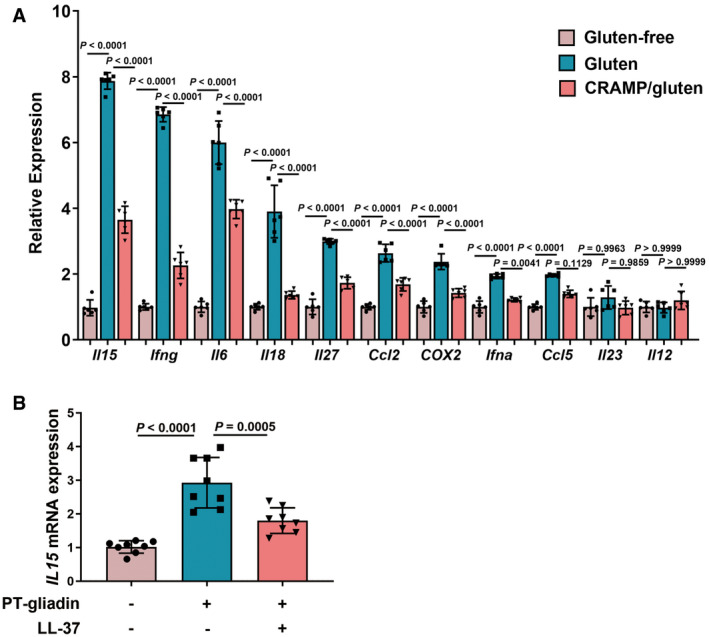
- The mRNA levels of cytokines in duodenum were measured by RT–qPCR (n = 6).
- The mRNA levels of IL15 in vitro were measured by RT–qPCR (n = 8).
Data information: Data were representative and were the mean ± SD from three (A) or four (B) independent experiments. P values were calculated by one‐way ANOVA followed by Tukey’s post hoc test for multiple comparisons.
Source data are available online for this figure.
Figure 5. CRAMP modulates intestinal immune disorder in GIE.
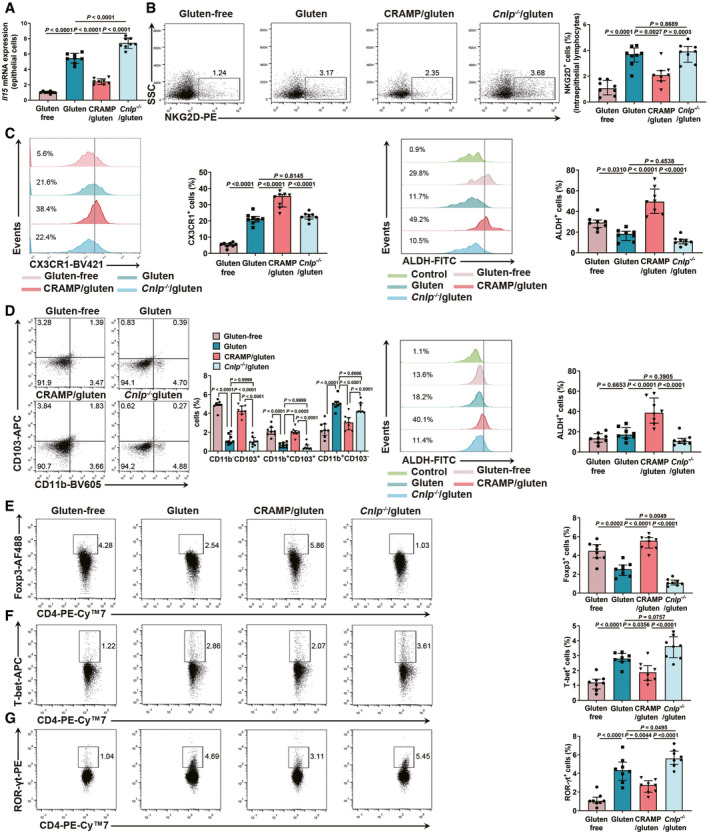
-
AIl15 expression in duodenal epithelial cells ex vivo (n = 8).
-
BThe frequency of NKG2D+ IELs among CD3+CD4+ population (n = 8).
-
CThe frequency of CX3CR1+ macrophages in CD45+CD11b+ population and ALDH activity in CD45+CD11b+CX3CR1+ macrophages in lamina propria (n = 8).
-
DThe percentages of three subsets of CD45+CD11c+ DCs and ALDH activity in CD45+CD11c+ DCs in lamina propria (n = 8).
-
E–GThe percentages of (E) Foxp3+ Treg, (F) T‐bet+ Th1 and (G) RORγt+ Th17 among CD3+CD4+ population in lamina propria were shown by flow cytometry (n = 8).
Data information: Data in A were representative and were the mean ± SD from two independent experiments. Data (B–G) were representative and were the median ± interquartile range from three independent experiments. P values were calculated by one‐way ANOVA followed by Tukey’s post hoc test for multiple comparisons.
Source data are available online for this figure.
Innate and adaptive immune cells in lamina propria (LP), including macrophages and dendritic cell (DCs) of different phenotypes as well as differentially polarized T cells, play important roles in development of celiac disease (Meresse et al, 2012). We observed that CRAMP induced CD45+CD11b+CX3CR1+ macrophages and conventional CD45+CD11c+DCs (cDCs) exhibiting a high aldehyde dehydrogenase (ALDH) activity (Fig 5C and D). CRAMP treatment stimulated the numbers of regulatory CD11b−CD103+ and CD11b+CD103+DCs and decreased inflammatory CD11b+CD103− DCs (Fig 5D). Accordingly, CRAMP treatment induced regulatory T cells (Treg) (Fig 5E) and reduced T helper 1 (Th1) (Fig 5F) and Th17 cells (Fig 5G). While restoring duodenal CRAMP harbours positive immunoregulatory effects, CRAMP deficiency further exacerbated gluten‐induced immune dysregulation (Fig 5A–G). Collectively, CRAMP shapes intestinal mucosal immune environment by decreasing cytotoxic NKG2D+ IELs and modulating DCs and macrophages phenotypes to induce regulatory T cells.
Gut dysbiosis contributes to CRAMP degradation
Finally, we aimed to determine why CRAMP production in epithelium is defective in GIE. Our as well as other groups have demonstrated that CRAMP expression may be regulated by gut microbiota and metabolites such as short‐chain fatty acids or proteases (Jan & Pike, 2009; Sun et al, 2015). Patients with celiac disease have altered faecal and duodenal microbiota compositions compared to healthy individuals, which is partially restored after treatment with a gluten‐free diet, suggesting the gut microbiota might play a vital role in celiac disease (Verdu et al, 2015). To test how GIE‐associated dysbiosis contributes to defective CRAMP production, we analysed gliadin‐induced perturbation of intestinal microbiota composition. Principal coordinates analysis (PCoA) showed that the gut microbiota communities in mice fed with gluten‐containing diet were remarkably different from gluten‐free diet group (Fig 6A). Analyses of microbiota composition at phylum level (Fig 6B), genus level (Fig 6C) and species level (Fig 6D) revealed that P. aeruginosa, an opportunistic pathogen unique to celiac disease patients and increased by gliadin (Caminero et al, 2019a), was increased, while Akkermansia muciniphila (A. muciniphila) was significantly decreased in gluten group. Butyrate‐producing bacteria did not show any significant change during GIE (Fig 6C), neither did faecal levels of short‐chain fatty acids (SCFAs) (Fig EV4). Pathogenic bacteria, including P. aeruginosa, are able to counteract host antimicrobial mechanism by producing proteases to cleave and inactivate cathelicidins (Jan & Pike, 2009; Cole & Nizet, 2016). Indeed, we found that the production of LasB, a protease of P. aeruginosa, was markedly increased in gluten group (Fig 6E). Moreover, at genus level, CRAMP was negatively associated with P. aeruginosa while positively correlated with A. muciniphila (Fig 6F). To confirm that P. aeruginosa could contribute to reduced CRAMP production, mice were orally supplemented with a highly virulent or a moderately virulent strain of P. aeruginosa, PA14 or PAO1 (Fig 6G and H). As shown in Fig 6I, reduced CRAMP production was observed in duodenum with colonization of the two P. aeruginosa strains, with a more pronounced effect observed with PA14. These data support that GIE‐associated gut dysbiosis with increased P. aeruginosa contribute to defective CRAMP production.
Figure 6. Gut dysbiosis contributes to CRAMP degradation.
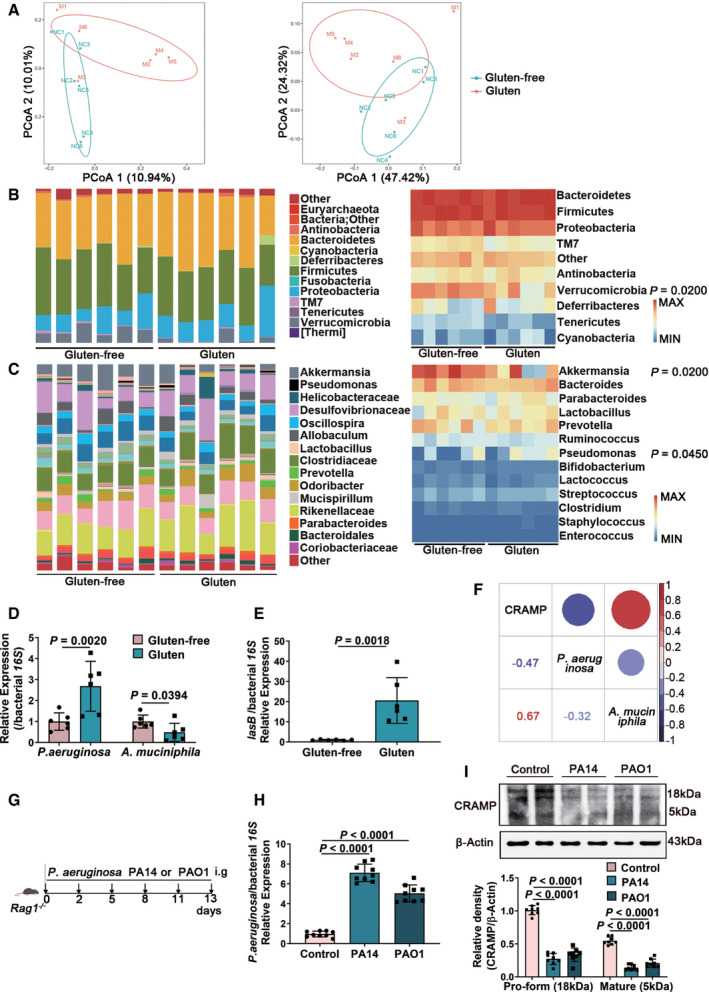
- The unweighted (left) and weighted (right) UniFrac distances based on OTU abundance (NC: gluten‐free, M: gluten; n = 6).
- The taxonomic composition distribution at phylum level (n = 6).
- The taxonomic composition distribution at genus level (n = 6).
- Duodenal colonizing P. aeruginosa and A. muciniphila as determined by RT–qPCR using bacterial‐specific species gene primers (n = 6).
- The production of LasB (a protease of P. aeruginosa) was examined in duodenum (n = 6).
- Dot plot of Pearson correlation coefficients among CRAMP, P. aeruginosa and A. muciniphila at species level. The size of each point represented the correlation coefficient and the colour represented positive (red) or negative (purple) relationship (n = 6).
- Animal protocol.
- Duodenal colonizing PA14 and PAO1 as determined by RT–qPCR using P. aeruginosa‐specific species gene primer (n = 9).
- Western blot and densitometry analyses of pro‐form CRAMP and mature peptide in duodenum (n = 8).
Data information: Data (D, E, H, I) were representative and were the mean ± SD from three independent experiments. P values were calculated by unpaired two‐tailed t‐test for comparison of two groups (B–E) or one‐way ANOVA followed by Tukey’s post hoc test for multiple comparisons (H, I).
Source data are available online for this figure.
Figure EV4. Faecal content of short‐chain fatty acids in GIE. Related to Fig 6.
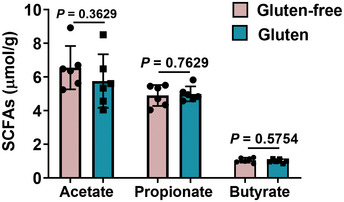
Faecal levels of short‐chain fatty acids (acetic acid, propionic acid and butyric acid) were measured by gas chromatography–mass spectrometer (n = 6). Data were representative and were the mean ± SD from three independent experiments. P values were calculated by unpaired two‐tailed t‐test for comparison of two groups.
Source data are available online for this figure.
Pseudomonas aeruginosa depletion restores CRAMP production and dampens GIE
To finally confirm that gut dysbiosis favours defective CRAMP production and GIE development, we applied an antibiotic cocktail (ABX) to deplete mice of primary P. aeruginosa and increase A. muciniphila (Fig 7A) (Hill et al, 2010). We found that the ABX cocktail effectively reduced highly resistant pathogenic bacteria P. aeruginosa with an ability to cleave CRAMP, while keeping the beneficial bacteria, such as A. muciniphila intact (Fig EV5A–C), which consequently led to increased CRAMP production in serum (Fig 7B) and in duodenum (Fig 7C), supporting a direct effect of dysbiosis on duodenal CRAMP expression. Accordingly, ABX treatment alleviated the barrier damage (Fig 7D–F) and immune cell dysregulation in epithelium (Fig 7G) and lamina propria (Fig 7H–L) caused by gluten, reducing NKG2D+ IELs and promoting modulatory phenotypes of macrophages, DCs and T cells. Together, our results support that gut microbiota via intestinal CRAMP production, impact mucosal barrier and immune environment and thus the development of GIE.
Figure 7. Pseudomonas aeruginosa depletion restores CRAMP production and dampens GIE.
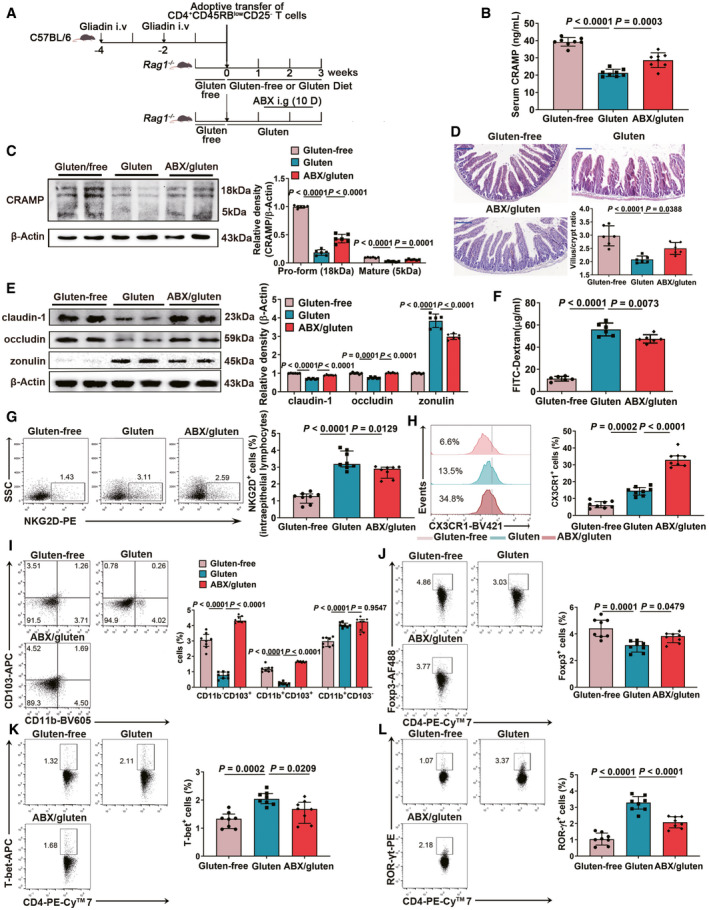
-
AAnimal protocol.
-
BThe levels of CRAMP in serum (n = 8).
-
CThe levels of CRAMP in duodenum (n = 6).
-
DRepresentative images of intestinal damage by H&E staining. Scale bar: 200 μm. The graph depicted the ratio of the morphometric assessment of villus height to crypt depth (n = 6).
-
EWestern blot and densitometry analyses of tight junction proteins (claudin‐1 and occludin) and zonulin in duodenum (n = 6).
-
FIntestinal permeability was assessed by measuring FITC‐Dextran (n = 6).
-
GThe frequency of NKG2D+ IELs among CD3+CD4+ population (n = 8).
-
HThe frequency of CX3CR1+ macrophages among CD45+CD11b+ population (n = 8).
-
IThe percentages of three subsets of CD45+CD11c+ DCs (n = 8).
-
J–LThe percentages of (J) Foxp3+ Treg, (K) T‐bet+ Th1 and (L) RORγt+ Th17 among CD3+CD4+ population in lamina propria were shown by flow cytometry (n = 8).
Data information: Data (B–F) were representative and were the mean ± SD from three independent experiments. Data (G–L) were representative and were the median ± interquartile range from three independent experiments. P values were calculated by one‐way ANOVA followed by Tukey’s post hoc test for multiple comparisons.
Source data are available online for this figure.
Figure EV5. The antibiotic cocktail treatment modulates gut microbiota composition, causing reduced P. aeruginosa and increased A. muciniphila during GIE. Related to Fig 7.
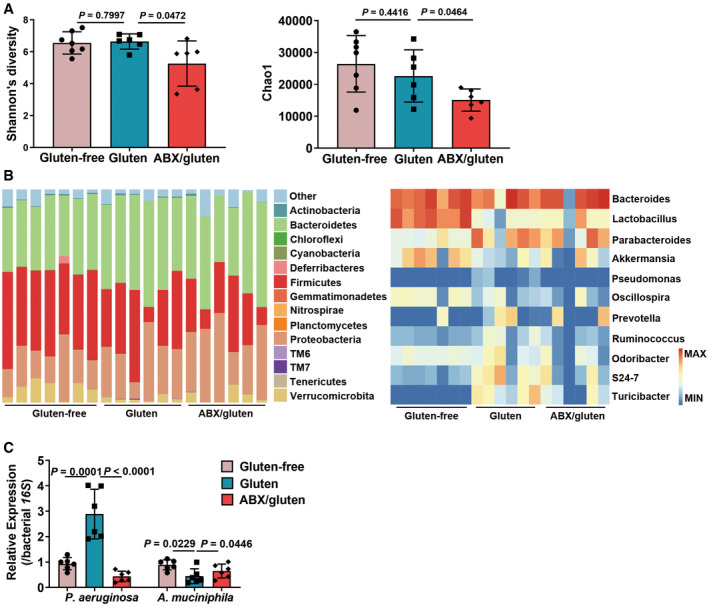
- Strain diversity (Left, Shannon’s diversity; Right, Chao1 index; Gluten‐free, n = 7; Gluten, n = 6; ABX/gluten, n = 6).
- The taxonomic composition distributions at phylum (left) and genus (right) levels (Gluten‐free, n = 7; Gluten, n = 6; ABX/gluten, n = 6).
- Duodenal colonizing P. aeruginosa and A. muciniphila as determined by RT–qPCR using bacterial‐specific species gene primers (n = 6).
Data information: Data in A were representative and were the mean ± SD. Data in C were representative and were the mean ± SD from three independent experiments. P values were calculated by one‐way ANOVA followed by Tukey’s post hoc test for multiple comparisons.
Source data are available online for this figure.
Discussion
Our study supports that perturbation of gut microbiota composition during GIE contributes to defective production of epithelial CRAMP. CRAMP promotes epithelial barrier integrity and function by counteracting gliadin‐triggered signalling. Additionally, CRAMP harbours positive immunoregulatory effects at intestinal mucosa by downregulating epithelial IL‐15 and promoting modulatory phenotypes of macrophages and DCs to induce Treg differentiation. Thus, a dysregulated immune‐gut microbiota axis triggered by gliadin results in low duodenal CRAMP levels, an unopposed mucosal barrier and immune dysregulation, and the development of GIE.
The mammalian gut epithelium produces a diverse collection of AMPs, to confront the complex microbial challenges. Although the small intestine is not considered as the primary site for cathelicidin production (Gallo & Hooper, 2012) compared to the colon, CRAMP is found to be expressed by small intestinal epithelial cells of adult mice (Gallo et al, 1997; Mukherjee & Hooper, 2015). Our data confirm its expression and secretion from epithelial cells in the duodenum of Rag1 −/−, BALB/c and C57BL/6 mice but not of CRAMP‐deficient mice (Fig EV1A). In addition, host immune state has a profound impact on gut microbiota (Wu & Wu, 2012). Upon induction of GIE immunopathology, distorted gut microbiota composition was observed three weeks after adoptive transfer of gliadin‐restricted T cells, accompanied by subsequently reduced CRAMP production, suggesting an immune‐gut microbiota axis to regulate endogenous antimicrobial peptide.
Effects of gliadin on epithelial dysfunction during GIE may be mediated via prolonged EGFR activation or a MyD88‐dependent mechanism to release zonulin (Thomas et al, 2006; Lammers et al, 2008). Gliadin triggered EGFR activation involves its phosphorylation at Tyr1068 (Barone et al, 2007), which leads to downstream Pi3K/Akt‐NF‐κB activation and inflammatory responses (Yan et al, 2011). Zonulin is the only protein known to date to reversibly regulate intestinal permeability by modulating intercellular TJPs (Amit et al, 2009). Gliadin‐induced zonulin release is thought to be mediated via C‐X‐C motif chemokine receptor 3 (CXCR3) and MyD88 signalling (Lammers et al, 2008), which enhances intestinal permeability, a key pathologic event of GIE. Indeed, it has been suggested that gliadin acts by prolonging binding and activation of EGFR by its native ligand EGF and delays receptor inactivation by interfering its endocytic pathway (Barone et al, 2007). Interestingly, cathelicidins (CRAMP or LL‐37) may also activate EGFR (Tjabringa et al, 2003; Sho et al, 2005; Niyonsaba et al, 2007; Sun et al, 2015) through a “trans‐activation” mechanism involving EGFR phosphorylation at a different tyrosine site (Sho et al, 2005). EGFR phosphorylation at different loci is associated with different cellular responses (Avraham & Yarden, 2011). Gliadin‐induced EGFR phosphorylation at Tyr1068 mediates intestinal dysfunction (Barone et al, 2007). In contrast, EGFR phosphorylation at Tyr100 induced by probiotic‐derived protein protects the intestinal barrier (Shen et al, 2018). While the exact mechanism remains to be determined, cathelicidin‐induced EGFR trans‐activation has been shown to depend on metalloproteinase (MMP)‐mediated HB‐EGF shedding and binding to EGFR (Tjabringa et al, 2003), which is in agreement with our observation that the inhibitory effect of LL‐37 on gliadin‐induced EGFR phosphorylation (Tyr1068) was abolished by a MMP inhibitor Ilomastat. Thus, LL‐37 counteracts gliadin‐induced signalling by inducing a competitive binding to interrupt prolonged pathological activation of EGFR by gliadin. In addition, earlier evidence has shown that LL‐37 inhibits MyD88 expression (Pinheiro et al, 2009). This agrees with our data that LL‐37 inhibits gliadin‐induced MyD88 expression, thus contributing to reduced zonulin production. Together, it may be postulated that LL‐37, by preventing gliadin‐induced EGFR Tyr1068‐phosphorylation associated with pathologic consequences and by inhibiting MyD88‐dependent zonulin, protects against gliadin‐induced epithelial dysfunction in GIE.
Another hallmark of celiac disease is NF‐κB‐regulated IL‐15 expression. IL‐15 may be released from epithelium upon its stimulation by gliadin (Valérie & Bana, 2014) and plays a critical role in IEL‐mediated epithelial cell damage and in dysregulated immune responses during celiac disease. IL‐15 is transcriptionally regulated by NF‐κB. The p65 subunit of NF‐κB binds to the IL‐15 NF‐κB motif (Villella et al, 2019). Additional, MyD88‐dependent signalling is important for regulation of IL‐15 production and in maintenance of IELs (Qingsheng et al, 2006). Cathelicidins have been shown to suppress the translocation of NF‐κB subunits (Mookherjee et al, 2006) as well as MyD88 expression. Consistently, we observed that cathelicidin antagonized gliadin‐induced NF‐κB activation and MyD88 signalling and suppressed epithelial IL‐15 production. Following its release from epithelial cells, IL‐15 activates intraepithelial lymphocytes responsible for epithelial cell damage (Valérie & Bana, 2014). Furthermore, IL‐15 treatment reversed the effect of retinoic acid (RA) by altering the tolerogenic phenotype of intestinal DCs, hence preventing the generation of inducible Treg cells to dietary gluten and promoting the development of Th1 and Th17 inflammatory immune responses (Valérie & Bana, 2014). Here, our data support that CRAMP reduces epithelial toxic NKG2D+ IELs by IL‐15 inhibition. Additionally, our data demonstrate that CRAMP induces ALDH+ macrophages and ALDH+ cDCs. ALDH is an important enzyme present in innate immune cells important for the production of RA, which is a key mechanism of these cells to induce Treg cells (Guilliams et al, 2010) and to inhibit the development of Th17 (Daniel et al, 2007). In addition, at intestinal mucosa, cDCs may be subdivided into three subsets based on the expression of CD11b and CD103: CD11b−CD103+, CD11b+CD103− and CD11b+CD103+ DCs, exhibiting inflammatory or modulatory properties. The CD11b+ CD103+DCs are so far found to be unique to the intestines and to preferentially drive the development of Foxp3+ Treg, which is dependent on RA (Denning et al, 2007; Sun et al, 2007). We observe that CRAMP induces the regulatory subsets of cDCs (CD11b−CD103+ and CD11b+CD103+) and reduces the inflammatory subsets (CD11b+CD103−), suggesting its additional immune modulatory mechanism. All these mechanisms contribute to regulatory T cells polarization induced by CRAMP.
Here, we demonstrate that a dysregulated immune‐gut microbiota axis contributes to epithelial CRAMP loss. To be noted, cathelicidin antimicrobial peptide is part of host defence mechanisms to exhibit broad‐spectrum activities against microbial invasions. Nevertheless, during co‐evolution with the host, opportunistic pathogens have also developed a variety of means to resist AMPs (Nizet, 2006). For examples, proteases of P. aeruginosa, Staphylococcus aureus, Streptococcus pyogenes, Porphyromonas gingivalis and Enterococcus faecalis lead to degradation of LL‐37 and consequently abolish antimicrobial activity (Jan & Pike, 2009). Celiac disease is associated with typical gut dysbiosis (Sanchez et al, 2013; Verdu et al, 2015), and the role of gut microbiota appears to be complex. Mice colonized with a microbiota devoid of opportunistic pathogens develop attenuated dietary gluten‐induced immunopathology compared with germ‐free mice and mice that harbour a complex microbiota that includes opportunistic pathogens (Galipeau et al, 2015). Specific gut bacteria may promote or protect celiac disease‐associated immunopathology (Caminero et al, 2019a; Caminero et al, 2019b). We observed that gut dysbiosis developed with induction of GIE, with increased P. aeruginosa and reduced A. muciniphila, which were similarly observed in clinical celiac disease (Bodkhe et al, 2019; Caminero et al, 2019a). Duodenal tissue was detected with increased abundance of P. aeruginosa, confirming its ability of translocating from colon to duodenum. Recently, the pathogenic mechanism of P. aeruginosa in celiac disease has been revealed (Caminero et al, 2016; Caminero et al, 2019a). In mice expressing celiac disease risk genes, the metabolite elastase, also called LasB of P. aeruginosa synergizes with gluten to induce severe inflammation (Caminero et al, 2019a). It is suggested that P. aeruginosa‐derived LasB promotes the propagation of celiac disease mediated by the protease‐activated receptor 2 (PAR2) pathway, which interacts with zonulin (Amit et al, 2009; Caminero et al, 2019a). Also, P. aeruginosa produces LasB with demonstrated ability to degrade CRAMP (Jan & Pike, 2009). Indeed, we observed increased LasB in duodenal tissue, corresponding to reduced CRAMP. We further confirmed this by applying antibiotic treatment able to deplete P. aeruginosa. Such treatment dampened the development of GIE, by preventing P. aeruginosa‐mediated degradation of CRAMP, confirming the role of gut dysbiosis in defective CRAMP production. The effect of antibiotic therapy in the development of GIE is complex, dependent on its targeted gut bacteria. Accordingly, selective antibiotic treatment targeting different bacterial subgroups may yield varying outcome on celiac disease (Hansen et al, 2013; Dydensborg Sander et al, 2019). Perinatal antibiotic treatment, which led to increased numbers of Proteobacteria, enhanced the severity of GIE. Vancomycin treatment that propagates A. muciniphila, reduces interferon‐gamma (IFN‐γ) and IL‐15 levels in the intestine and NKG2D ligand expression on intestinal epithelial cells (IECs), thus protecting GIE (Hansen et al, 2013). While P. aeruginosa is resistant to most antibiotics (Breidenstein et al, 2011), our combinatory antibiotics effectively reduce P. aeruginosa, which is attributable for its protective effect on GIE.
Altogether, in current GIE model we demonstrate that the undigested immunogenic gliadin from gluten triggers a dysregulated immune‐gut microbiota axis that negatively impacts intestinal CRAMP production, consequently inducing pathological dysregulation of barrier function and immune environment at intestinal mucosa and unopposed development of GIE. It should be interesting to determine in other experimental models of celiac disease and in susceptible individuals whether similar effect and mechanism of CRAMP exist. Finally, our data support that manipulation of gut microbiota and the production of intestinal AMPs may represent an attractive therapeutic strategy to maintain intestinal homeostasis and prevent the development of celiac disease.
Materials and Methods
Reagents and Tools table
| Reagent/Resource | Reference or Source | Identifier or Catalog Number |
|---|---|---|
| Experimental Models | ||
| Mouse: C57BL/6J: | GemPharmatech | C57BL/6JGpt; Cat# N000295; RRID: SCR_017239 |
| Mouse: Cnlp −/−: | Jackson Laboratory | B6.129X1‐Camptm1Rlg/J Mus musculus; Cat# JAX:017799; RRID: IMSR_JAX:017799 |
| Mouse: Rag1 −/−: | GemPharmatech | B6/JGpt‐Rag1em1Cd /Gpt; Cat# T004753; RRID: SCR_017239 |
| Mouse: Cnlp −/− Rag1 −/− | This study | N/A |
| Cell lines: Caco‐2 | ATCC | Cat# HTB‐37; RRID: CVCL_0025 |
| Patient samples: Human serum | Second Affiliated Hospital of Nanchang University | Ethics number: no. [2010] 041 |
| Bacterial strains: P. aeruginosa PAO1 | ATCC | Cat# 47085 |
| Bacterial strains: P. aeruginosa PA14 | Isolated from patient | N/A |
| Antibodies | ||
| Anti‐CRAMP (1‐39) antibody | Innovagen | Cat# PA‐CRPL‐100 |
| Anti‐claudin‐1 antibody | Abcam | Cat# ab180158 |
| Anti‐occludin antibody | Abcam | Cat# ab216327; RRID: AB_2737295 |
| Anti‐zonulin antibody | Abcam | Cat# ab131236; RRID: AB_11157376 |
| Anti‐TRAF6 antibody | Abcam | Cat# ab40675; RRID: AB_778573 |
| Anti‐p65 antibody | Abcam | Cat# ab32536; RRID: AB_776751 |
| Anti‐p‐AKT antibody | Cell Signaling Technology | Cat# 4060; RRID: AB_2315049 |
| Anti‐AKT antibody | Cell Signaling Technology | Cat# 4685; RRID: AB_2225340 |
| Anti‐MyD88 antibody | Cell Signaling Technology | Cat# 4283; RRID: AB_10547882 |
| Anti‐p‐p65 antibody | Cell Signaling Technology | Cat# 3033; RRID: AB_331284 |
| Anti‐ZO‐1 antibody | Thermo Fisher Scientific | Cat# 40‐2200; RRID: AB_2533456 |
| Anti‐ZO‐2 antibody | Thermo Fisher Scientific | Cat# 71‐1400; RRID: AB_88012 |
| Anti‐ACTB antibody | ABclonal | Cat# AC026; RRID: AB_2768234 |
| Alexa Fluor® 700 anti‐mouse CD45 | Biolegend | Cat# 103128; RRID: AB_493715 |
| Brilliant Violet® 711 anti‐mouse CD11c | Biolegend | Cat# 117349; RRID: AB_2563905 |
| Brilliant Violet® 605 anti‐mouse CD11b | Biolegend | Cat# 117349; RRID: AB_2563905 |
| APC anti‐mouse CD103 | Biolegend | Cat# 12141; RRID: AB_1227503 |
| Brilliant Violet® 421 anti‐mouse CX3CR1 | Biolegend | Cat# 149023; RRID: AB_2565706 |
| Brilliant Violet® 421 anti‐mouse CD3e | Biolegend | Cat# 100341; RRID: AB_2562556 |
| PE anti‐mouse CD314 (NKG2D) | Biolegend | Cat# 130207; RRID: AB_1227713 |
| APC anti‐T‐bet | Biolegend | Cat# 644813; RRID: AB_10896913 |
| Alexa Fluor® 488 anti‐mouse FOXP3 | Biolegend | Cat# 126405; RRID: AB_1089114 |
| PE anti‐mouse ROR GAMMA (T) (B2D) | Thermo Fisher Scientific | Cat# 12‐6981‐80; RRID: AB_10805392 |
| PE‐Cy™7 anti‐mouse CD4 | BD biosciences | Cat# 552775; RRID: AB_394461 |
| anti‐CRAMP pAb | Proteintech | Cat# 12009‐1‐AP; RRID: AB_908736 |
| anti‐E‐cadherin pAb | BD biosciences | Cat# 610181; RRID: AB_397580 |
| anti‐F4/80 mAb | Abcam | Cat# ab6640; RRID: AB_1140040 |
| anti‐Ly6G mAb | Abcam | Cat# ab25377; RRID: AB_470492 |
| CD8a Antibody, Biotin | Miltenyi Biotec | Cat# 130‐118‐147; RRID: AB_2733536 |
| CD11b Antibody, Biotin | Miltenyi Biotec | Cat# 130‐113‐233; RRID: AB_2726044 |
| CD11c Antibody, Biotin | Miltenyi Biotec | Cat# 130‐113‐578; RRID: AB_2726178 |
| CD19 Antibody, Biotin | Miltenyi Biotec | Cat# 130‐113‐729; RRID: AB_2726270 |
| CD45R(B220) Antibody, Biotin | Miltenyi Biotec | Cat# 130‐123‐853; RRID: AB_2819526 |
| CD49b(DX5) Antibody, Biotin | Miltenyi Biotec | Cat# 130‐101‐934; RRID: AB_2660466 |
| CD105 Antibody, Biotin | Miltenyi Biotec | Cat# 130‐101‐992; RRID: AB_2660100 |
| MHC Class II Antibody, Biotin | Miltenyi Biotec | Cat# 130‐101‐849; RRID: AB_2660066 |
| Ter‐119 Antibody, Biotin | Miltenyi Biotec | Cat# 130‐120‐828; RRID: AB_2784480 |
| TCRγ/δ Antibody, Biotin | Miltenyi Biotec | Cat# 130‐114‐028; RRID: AB_2733574 |
| CD25 Antibody, Biotin | Miltenyi Biotec | Cat# 130‐092‐569; RRID: AB_871645 |
| Streptavidin MicroBeads | Miltenyi Biotec | Cat# 130‐048‐101 |
| CD45RB Antibody, FITC | Miltenyi Biotec | Cat# 130‐102‐461; RRID: AB_2658357 |
| Anti‐FITC MicroBeads antibody | Miltenyi Biotec | Cat# 130‐048‐701; RRID: AB_244371 |
| Oligonucleotides and other sequence‐based reagents | ||
| PCR primers | This study | Table EV2 |
| Chemicals, Enzymes and other reagents | ||
| CRAMP | GL biochem | Cat#088328 |
| Gliadin from wheat | Sigma | Cat#G3375‐25G |
| Gluten from wheat | Sigma | Cat#G5004‐500G |
| Software | ||
| FlowJo | BD Bioscience | RRID: SCR_008520; https://www.flowjo.com/solutions/flowjo/downloads |
| GraphPad Prism | GraphPad | RRID: SCR_002798; https://www.graphpad.com/ |
| Image J | Image J | RRID:SCR_003070; https://imagej.en.softonic.com/ |
| R project | R Project | RRID:SCR_001905; https://www.r‐project.org/ |
| FluorChem FC3 | ProteinSimple | RRID:SCR_013724; http://www.proteinsimple.com/fluorchem_e.html |
| ZEN | Zeiss | RRID:SCR_018163; http://stmichaelshospitalresearch.ca/wp‐content/uploads/2015/09/ZEN‐Black‐Quick‐Guide.pdf |
| Other | ||
| Illumina MiSeq | Illumina |
RRID:SCR_010233 |
| CRAMP ELISA kit | My biosource | Cat# MBS7700441 |
| LL‐37 ELISA kit | My biosource | Cat# MBS3800861 |
Methods and Protocols
Animals and treatments
All animal experimental protocols were approved by the Animal Ethics Committee of Jiangnan University [JN. No20181230c0500815(303) and JN. No20170614‐20190225 (77)] and were performed in accordance with the guidelines. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, 2010). Male C57BL/6 mice, Rag1 −/− mice (N000295; T004753; GemPharmatech Co., Ltd, Jiangsu, China), CRAMP‐deficient Cnlp −/− mice (C57BL/6 background; JAX:017799; The Jackson Laboratory, CA, USA) and Cnlp −/− Rag1 −/− mice were maintained at the Animal Housing Unit of Jiangnan University (Jiangsu, China) under a controlled temperature (23−25°C) and a 12 h light–12 h dark cycle. The Cnlp −/− Rag1 −/− mice were generated by backcrossing of Cnlp −/− mice with Rag1 −/− mice for more than 10 generations. All experiments were performed with mice at age 6–8 weeks.
To recapitulate gliadin‐induced small intestinal enteropathy and the gliadin‐specific T‐cell responses in active celiac disease patients, we chose an experimental model of dietary gluten‐induced enteropathy by adoptive gliadin‐specific T cells transfer as previously described (Freitag et al, 2009). Briefly, C57BL/6 donor mice were maintained on a gluten‐free standardized diet (AIN‐76A; Research Diets, NJ, USA) from birth. Mice were given 100 μg gliadin (G3375‐25G) or ovalbumin (negative control antigen) in Complete Freund’s Adjuvant at tail base on day 0 and 50 μg gliadin or ovalbumin in Incomplete Freund’s Adjuvant on day 14 (all Sigma, Shanghai, China). Rag1 −/− and Cnlp −/− Rag1 −/− recipient mice were changed to AIN‐76A on day 21. For sensitization, on day 28, Rag1 −/− and Cnlp −/− Rag1 −/− recipient mice were injected intraperitoneally with 4.5 × 105 splenic CD4+CD45RBlowCD25− T cells from C57BL/6 donor mice. Mice were divided randomly into experimental groups by different diet: gluten‐free diet (AIN‐76A) and gluten‐containing diet [based on AIN‐76A, containing 2.5 g wheat gluten (G5004‐500G; Sigma, Shanghai, China) per kg] (n = 6‐8). Unsensitized mice did not receive splenic CD4+CD45RBlowCD25− T cells and were fed with either gluten‐free diet or gluten‐containing diet. CRAMP (the mature form; 088328; GL biochem, Shanghai, China) was intraperitoneal administered (100 µg mouse−1 week−1) either two weeks before (prophylactic treatment) or 6 weeks after (therapeutic treatment) received adoptive T cells transfer. For antibiotic treatment (Hill et al, 2010), three weeks after adaptive transfer, mice were given 1 g l−1 metronidazole, 1 g l−1 gentamicin, 0.5 g l−1 vancomycin, 1 g l−1 ampicillin and 1 g l−1 neomycin (all in Sigma, Shanghai, China) by daily oral gavage of 200 μl in antibiotic solution (100% H2O) for 10 days (Hill et al, 2010). Mice were sacrificed by a lethal dose of pentobarbital sodium (90 mg kg−1; Sigma, Shanghai, China).
Human samples
This study was approved by the ethics committee of the Second Affiliated Hospital of Nanchang University [no. (2010) 041]. All subjects provided informed consent before participation and the experiments conformed to the principles set out in the WMA Declaration of Helsinki and the Department of Health and Human Services Belmont Report. The characteristics of participants were given in Appendix Table S1.
Cell culture and treatment
Human intestinal epithelial cells Caco‐2 (HTB‐37) were obtained from the American Type Culture Collection (ATCC, VA, USA) and maintained in DMEM containing 1,800 mg l−1 NaHCO3, supplemented with 10% FBS, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin at 37°C in a humidified atmosphere with 5% CO2. LL‐37 (GL biochem, Shanghai, China), a broad‐spectrum matrix metalloproteinase inhibitor Ilomastat (Selleck, Shanghai, China) and PT‐gliadin were dissolved in distilled water. For treatment, cells were pre‐incubated with Ilomastat (5 μM) for 1 h, subsequently stimulated with PT‐gliadin (1 mg ml−1) and LL‐37 (10 µg ml−1) for 3 h. Cells were then collected for measurements. All cell lines were regularly tested negative for mycoplasma contamination.
Bacterial supplementations
Pseudomonas aeruginosa PA14 (patient isolate) and PAO1 (47085; ATCC, WA, USA) were synthesized and kindly provided by Dr Lvyan Ma’s laboratory in Chinese Academy of Sciences (Beijing, China). Rag1 −/− mice were supplemented by oral gavage with P. aeruginosa PA14 or PAO1 three times a week for 2 weeks (1010 cfu mouse−1).
RNA isolation and reverse transcription real‐time quantitative polymerase chain reaction (RT–qPCR)
Total RNA was extracted from tissues and cells using TRIzol following the manufacturer’s protocol, and cDNAs were synthesized by a reverse transcription reagent kit (RR036A; TaKaRa, Kyoto, Japan). Gene expression levels were analysed by RT–qPCR using the Bio‐Rad CFX Connect Real‐Time System (CA, USA). After the preparation of the duodenal content (bacterial DNA extraction), the quantity of total bacteria, P. aeruginosa and A. muciniphila at species level were determined using bacterial‐specific species gene primers and bacterial 16S gene sequence universal primer. Primer sequences were given in Appendix Table S2.
Preparation of T cells fractions for adoptive transfer experiments
CD4+CD45RBlowCD25− subset T cells were isolated from spleens as described (Mottet et al, 2003). In brief, single cell suspensions were stained with biotinylated anti‐CD8a (130‐118‐147), CD11b (130‐113‐233), CD11c (130‐113‐578), CD19 (130‐113‐729), CD45R(B220) (130‐123‐853), CD49b(DX5) (130‐101‐934), CD105 (130‐101‐992), MHC Class II (130‐101‐849), Ter‐119 (130‐120‐828), TCRγ/δ (130‐114‐028) and CD25 (130‐092‐569) followed by streptavidin MACS beads (130‐048‐101) and sorted on an AutoMACS (all Miltenyi Biotec, Bergisch Gladbach, Germany). The CD4+CD25− fraction was then stained with anti‐CD45RB‐FITC (130‐102‐461), followed by incubation with anti‐FITC MACS beads (130‐048‐701) and sorted on an AutoMACS.
Statistical analyses
Data were expressed as mean ± SD. P < 0.05 was considered statistically significant. Difference between two groups was determined using unpaired two‐tailed t‐test. Differences among three or more groups were determined analysis of variance (ANOVA) followed by Tukey’s post hoc test. For flow cytometry statistical analyses, data were expressed as median ± interquartile range. The Pearson correlation coefficients between CRAMP and microbiota were analysed by the R Project (NJ, USA). All data were analysed using GraphPad Prism 8 software (CA, USA).
Author contributions
ZR, L‐LP and YH performed experiments and analysed data. HC and YL helped with obtaining the human samples. HL, XT, YL, BL, XD and XP assisted the experiments. YF and HFL helped with bacteria culture. BA and JD contributed to the data acquisition and critically reviewed the manuscript. ZR, JS and JD designed and interpreted experiments. JS, ZR and L‐LP wrote the paper.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Source Data for Expanded View
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6
Source Data for Figure 7
Acknowledgements
We would like to thank Prof Lvyan Ma from Chinese Academy of Sciences (Beijing, China) for kindly providing two strains of P. aeruginosa PA14 and PAO1. The work was supported by funds from the National Natural Science Foundation of China (Grant Nos: 80270666, 81870439, 81973322, 91642114, 31570915, and National Youth 1000 Talents Plan), the Natural Science Foundation for Distinguished Young Scholars of Jiangsu Province (Grants No.: BK20200026), Jiangsu Province Recruitment Plan for High‐level, Innovative and Entrepreneurial Talents (Innovative Research Team), Wuxi Social Development Funds for International Science & Technology Cooperation (Grant No: WX0303B010518180007PB), Jiangsu Province “Six Summit Talents” programme (YY‐038), Jiangsu Province Qing Lan Project, National First‐class Discipline Program of Food Science and Technology (Grant No: JUFSTR20180103), the Fundamental Research Funds for the Central Universities (Grant Nos.: JUSRP221037, JUSRP22007), Postgraduate Research & Practice Innovation Program of Jiangsu Province (Grant No: KYCX20_1876), Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province and Wuxi Taihu Talent Project.
EMBO Mol Med (2021) 13: e14059.
Contributor Information
Julien Diana, Email: julien.diana@inserm.fr.
Jia Sun, Email: jiasun@jiangnan.edu.cn.
Data availability
Mouse gut microbiota sequencing and assembly: Sequence Read Archive PRJNA686187 (https://www.ncbi.nlm.nih.gov/bioproject/?term=prjna686187).
The main data supporting the findings of this study are available within the article and its Expanded View Figures. Extra data are available from the corresponding author upon request.
References
- Abadie V, Jabri B (2014) IL‐15: a central regulator of celiac disease immunopathology. Immunol Rev 260: 221–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham R, Yarden Y (2011) Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nat Rev Mol Cell Biol 12: 104–117 [DOI] [PubMed] [Google Scholar]
- Barone MV, Gimigliano A, Castoria G, Paolella G, Maurano F, Paparo F, Maglio M, Mineo A, Miele E, Nanayakkara M et al (2007) Growth factor‐like activity of gliadin, an alimentary protein: implications for coeliac disease. Gut 56: 480–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkhe R, Shetty SA, Dhotre DP, Verma AK, Bhatia K, Mishra A, Kaur G, Pande P, Bangarusamy DK, Santosh BP et al (2019) Comparison of small gut and whole gut microbiota of first‐degree relatives with adult celiac disease patients and controls. Front Microbiol 10: 164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenstein EB, de la Fuente‐Nunez C , Hancock RE (2011) Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol 19: 419–426 [DOI] [PubMed] [Google Scholar]
- Caminero A, Galipeau HJ, McCarville JL, Johnston CW, Bernier SP, Russell AK, Jury J, Herran AR, Casqueiro J, Tye‐Din JA et al (2016) Duodenal bacteria from patients with celiac disease and healthy subjects distinctly affect gluten breakdown and immunogenicity. Gastroenterology 151: 670–683 [DOI] [PubMed] [Google Scholar]
- Caminero A, McCarville JL, Galipeau HJ, Deraison C, Bernier SP, Constante M, Rolland C, Meisel M, Murray JA, Yu XB et al (2019a) Duodenal bacterial proteolytic activity determines sensitivity to dietary antigen through protease‐activated receptor‐2. Nat Commun 10: 1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminero A, McCarville JL, Zevallos VF, Pigrau M, Yu XB, Jury J, Galipeau HJ, Clarizio AV, Casqueiro J, Murray JA et al (2019b) Lactobacilli degrade wheat amylase trypsin inhibitors to reduce intestinal dysfunction induced by immunogenic wheat proteins. Gastroenterology 156: 2266–2280 [DOI] [PubMed] [Google Scholar]
- Cole JN, Nizet V (2016) Bacterial evasion of host antimicrobial peptide defenses. Microbiol Spectr 4: 1–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B (2007) Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17‐producing T cell responses. Nat Immunol 8: 1086–1094 [DOI] [PubMed] [Google Scholar]
- Diana J, Simoni Y, Furio L, Beaudoin L, Agerberth B, Barrat F, Lehuen A (2013) Crosstalk between neutrophils, B‐1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med 19: 65–73 [DOI] [PubMed] [Google Scholar]
- Dydensborg Sander S, Nybo Andersen A‐M, Murray JA, Karlstad Ø, Husby S, Størdal K (2019) Association between antibiotics in the first year of life and celiac disease. Gastroenterology 156: 2217–2229 [DOI] [PubMed] [Google Scholar]
- Fasano A (2012) Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin Gastroenterol Hepatol 10: 1096–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag TL, Rietdijk S, Junker Y, Popov Y, Bhan AK, Kelly CP, Terhorst C, Schuppan D (2009) Gliadin‐primed CD4+CD45RBlowCD25‐ T cells drive gluten‐dependent small intestinal damage after adoptive transfer into lymphopenic mice. Gut 58: 1597–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galipeau HJ, McCarville JL, Huebener S, Litwin O, Meisel M, Jabri B, Sanz Y, Murray JA, Jordana M, Alaedini A et al (2015) Intestinal microbiota modulates gluten‐induced immunopathology in humanized mice. Am J Pathol 185: 2969–2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo RL, Hooper LV (2012) Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol 12: 503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo RL, Kim KJ, Bernfield M, Kozak CA, Zanetti M, Merluzzi L, Gennaro R (1997) Identification of CRAMP, a cathelin‐related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem 272: 13088 [DOI] [PubMed] [Google Scholar]
- Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, de Bovis B, Alexopoulou L, Dalod M, Malissen B (2010) Skin‐draining lymph nodes contain dermis‐derived CD103(‐) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood 115: 1958–1968 [DOI] [PubMed] [Google Scholar]
- Hancock RE, Haney EF, Gill EE (2016) The immunology of host defence peptides: beyond antimicrobial activity. Nat Rev Immunol 16: 321–334 [DOI] [PubMed] [Google Scholar]
- Hansen CHF, Holm TL, Krych Ł, Andresen L, Nielsen DS, Rune I, Hansen AK, Skov S (2013) Gut microbiota regulates NKG2D ligand expression on intestinal epithelial cells. Eur J Immunol 43: 447–457 [DOI] [PubMed] [Google Scholar]
- Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, Kirn TJ, Bushman FD, Artis D (2010) Metagenomic analyses reveal antibiotic‐induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol 3: 148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hing TC, Samantha H, Shih DQ, Ryan I, Michelle C, Jeremy C, Xinhua C, Ivy L, Robert N, Kelly CP (2013) The antimicrobial peptide cathelicidin modulates Clostridium difficile‐associated colitis and toxin A‐mediated enteritis in mice. Gut 62: 1295–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iimura M, Gallo RL, Hase K, Miyamoto Y, Eckmann L, Kagnoff MF (2005) Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. Journal of Immunology 174: 4901–4907 [DOI] [PubMed] [Google Scholar]
- Kahlenberg JM, Kaplan MJ (2013) Little peptide, big effects: the role of LL‐37 in inflammation and autoimmune disease. J Immunol 191: 4895–4901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers KM, Lu R, Brownley J, Lu B, Gerard C, Thomas K, Rallabhandi P, Shea‐Donohue T, Tamiz A, Alkan S et al (2008) Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 135: 194–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meresse B, Malamut G, Cerf‐Bensussan N (2012) Celiac disease: an immunological jigsaw. Immunity 36: 907–919 [DOI] [PubMed] [Google Scholar]
- Mookherjee N, Brown KL, Bowdish DME, Doria S, Falsafi R, Hokamp K, Roche FM, Mu R, Doho GH, Pistolic J et al (2006) Modulation of the TLR‐mediated inflammatory response by the endogenous human host defense peptide LL‐37. Journal of Immunology 176: 2455–2464 [DOI] [PubMed] [Google Scholar]
- Mottet C, Uhlig HH, Powrie F (2003) Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol 170: 3939–3943 [DOI] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H (2007) Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317: 256–260 [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Hooper LV (2015) Antimicrobial defense of the intestine. Immunity 42: 28–39 [DOI] [PubMed] [Google Scholar]
- Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, Nagaoka I, Okumura K, Ogawa H (2007) Antimicrobial peptides human beta‐defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol 127: 594–604 [DOI] [PubMed] [Google Scholar]
- Nizet V (2006) Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr Issues Mol Biol 8: 11–26 [PubMed] [Google Scholar]
- Pinheiro da Silva F, Gallo RL, Nizet V (2009) Differing effects of exogenous or endogenous cathelicidin on macrophage toll‐like receptor signaling. Immunol Cell Biol 87: 496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa J, Pike RN (2009) Corruption of innate immunity by bacterial proteases. J Innate Immun 1: 70–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qingsheng Y, Ce T, Sun X, Toshiki Y, Kiyoshi T, Yasunobu Y (2006) MyD88‐dependent signaling for IL‐15 production plays an important role in maintenance of CD8 alpha alpha TCR alpha beta and TCR gamma delta intestinal intraepithelial lymphocytes. J Immunol 176: 6180–6185 [DOI] [PubMed] [Google Scholar]
- Raqib R, Sarker P, Bergman P, Ara G, Lindh M, Sack DA, Nasirul Islam KM, Gudmundsson GH, Andersson J, Agerberth B (2006) Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc Natl Acad Sci USA 103: 9178–9183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez E, Donat E, Ribes‐Koninckx C, Fernández‐Murga ML, Sanz Y (2013) Duodenal‐mucosal bacteria associated with celiac disease in children. Appl Environ Microbiol 79: 5472–5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Liu L, Peek RM, Acra SA, Moore DJ, Wilson KT, He F, Polk DB, Yan F (2018) Supplementation of p40, a Lactobacillus rhamnosus GG‐derived protein, in early life promotes epidermal growth factor receptor‐dependent intestinal development and long‐term health outcomes. Mucosal Immunol 11: 1316–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C‐M, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y (2007) Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 204: 1775–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Furio L, Mecheri R, van der Does AM , Lundeberg E, Saveanu L, Chen Y, van Endert P , Agerberth B, Diana J (2015) Pancreatic beta‐cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity 43: 304–317 [DOI] [PubMed] [Google Scholar]
- Suzuki T (2013) Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci 70: 631–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KE, Sapone A, Fasano A, Vogel SN (2006) Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88‐dependent: role of the innate immune response in Celiac disease. J Immunol 176: 2512–2521 [DOI] [PubMed] [Google Scholar]
- Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sørensen OE, Borregaard N, Rabe KF, Hiemstra PS (2003) The antimicrobial peptide LL‐37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol 171: 6690–6696 [DOI] [PubMed] [Google Scholar]
- Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, Yahata Y, Dai X, Tohyama M, Nagai H et al (2005) Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL‐37. Journal of Immunology 175: 4662–4668 [DOI] [PubMed] [Google Scholar]
- Tripathi A, Lammers KM, Goldblum S, Shea‐Donohue T, Netzel‐Arnett S, Buzza MS, Antalis TM, Vogel SN, Zhao A, Yang S et al (2009) Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin‐2. Proc Natl Acad Sci USA 106: 16799–16804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdu EF, Galipeau HJ, Jabri B (2015) Novel players in coeliac disease pathogenesis: role of the gut microbiota. Nat Rev Gastroenterol Hepatol 12: 497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villella VR, Venerando A, Cozza G, Esposito S, Ferrari E, Monzani R, Spinella MC, Oikonomou V, Renga G, Tosco A et al (2019) A pathogenic role for cystic fibrosis transmembrane conductance regulator in celiac disease. Embo J 38: e100101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H‐J, Wu E (2012) The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 3: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F, Cao H, Cover TL, Washington MK, Shi Y, Liu L, Chaturvedi R, Peek RM Jr, Wilson KT, Polk DB (2011) Colon‐specific delivery of a probiotic‐derived soluble protein ameliorates intestinal inflammation in mice through an EGFR‐dependent mechanism. J Clin Investig 121: 2242–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415: 389–395 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Source Data for Expanded View
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6
Source Data for Figure 7
Data Availability Statement
Mouse gut microbiota sequencing and assembly: Sequence Read Archive PRJNA686187 (https://www.ncbi.nlm.nih.gov/bioproject/?term=prjna686187).
The main data supporting the findings of this study are available within the article and its Expanded View Figures. Extra data are available from the corresponding author upon request.


