Abstract
Background:
Cardiac sarcoidosis (CS) is an increasingly recognized cause of cardiomyopathy; however, data on immunosuppressive strategies are limited. Treatment with tumor necrosis factor (TNF) alpha inhibitors is not well described; moreover, there may be heart failure–related safety concerns.
Methods:
Retrospective multicenter study of patients with CS treated with TNF alpha inhibitors. Baseline characteristics, treatments, and outcomes were adjudicated.
Results:
Thirty-eight patients with CS (mean age 49.9 years, 42% women, 53% African American) were treated with TNF alpha inhibitor (30 infliximab, 8 adalimumab). Prednisone dose decreased from time of TNF alpha inhibitor initiation (21.7 ± 17.5 mg) to 6 months (10.4 ± 6.1 mg, P = .001) and 12 months (7.3 ± 7.3 mg, P = .002) after treatment. On pre-TNF alpha inhibitor treatment positron emission tomography with 18-flourodoxyglucose (FDG-PET), 84% of patients had cardiac FDG uptake. After treatment, there was a significant decrease in number of segments involved (3.5 ± 3.8 to 1.0 ± 2.5, P = .008) and maximum standardized uptake value (3.59 ± 3.70 to 0.57 ± 1.60, P = .0005), with 73% of patients demonstrating complete resolution or improvement of cardiac FDG uptake. The left ventricular ejection fraction remained stable (45.0 ± 16.5% to 47.0 ± 15.0%, P = .10). Four patients required inpatient heart failure treatment, and 8 had infections; 2 required treatment cessation.
Conclusions:
TNF alpha inhibitor treatment guided by FDG-PET imaging may minimize corticosteroid use and effectively reduce cardiac inflammation without significant adverse effect on cardiac function. However, infections were common, some of which were serious, and therefore patients require close monitoring for both infection and cardiac symptoms.
Keywords: Sarcoidosis, heart failure, arrhythmia, inflammation, cytokine
Sarcoidosis is a systemic granulomatous inflammatory disease. Cardiac involvement has been reported in up to 25% of cases of sarcoidosis.1 Cardiac sarcoidosis (CS) is a major cause of death in patients with sarcoidosis, and morbidity is associated with granulomatous inflammation, which results in conduction disease, ventricular arrhythmia (VA), and cardiomyopathy leading to heart failure (HF).2 The mainstay of medical therapy is immunosuppression in the form of corticosteroids with the possible addition of a steroid sparing agent. There is a paucity of data on immunosuppressive regimens in CS; furthermore, long-term treatment with these agents puts patients at risk for significant medication related morbidity. Randomized controlled trials of immunosuppressive strategies in CS are lacking.
Tumor necrosis factor (TNF) alpha is a cytokine released by activated macrophages and dendritic cells and plays a key role in granuloma assembly and maintenance in sarcoidosis and other granulomatous diseases3,4; however, the use of TNF inhibitors for treating extracardiac sarcoidosis has not yet been widely adopted.5–7 Although HF is associated with higher serum levels of TNF,8 early clinical trials to investigate treatment of HF with TNF inhibitors were associated with adverse outcomes.9,10 This outcome has resulted in a black box warning from the US Food and Drug Administration for their use in moderate to severe HF. As a result, there has been hesitancy to expand the use of TNF inhibitors for the treatment of sarcoidosis to those patients with significant cardiac involvement.
With advancements in cardiac imaging, specifically positron emission tomography with 18-flourodoxyglucose (FDG-PET), residual cardiac inflammation after a typical course of immunosuppression can be more precisely detected. Cardiac FDG uptake is associated with poor cardiovascular outcomes, thus prompting need for therapies that more effectively suppress inflammation.11 We, therefore, sought to describe the safety and efficacy of biologic agents, with a focus on TNF alpha inhibitors and FDG-PET responsiveness, in a multicenter cohort of patient with CS. We hypothesized that TNF alpha inhibitors would be generally well-tolerated and result in significantly decreased steroid dosing and cardiac inflammation on FDG-PET imaging in patients with CS.
Methods
Patient Population
We included patients in 2 institutions’ Cardiac Sarcoidosis Registries who were treated for CS with a TNF alpha inhibitor in consultation with a sarcoidosis specialist and cardiologist between 2014 and 2019. Extracardiac organ involvement was determined using World Association of Sarcoidosis and Other Granulomatous Diseases criteria.12 CS was diagnosed based on Heart Rhythm Society (HRS) criteria or Japanese imaging criteria.13 Patients were included if they underwent treatment with TNF alpha inhibitor therapy specifically for cardiac involvement of their sarcoidosis. The study was approved by each institution’s institutional review board.
Clinical Data Collection
Demographic and baseline clinical characteristics at time of diagnosis of CS and time of TNF alpha inhibitor initiation were collected from the electronic medical records. Transthoracic echocardiogram reports were reviewed to determine left ventricular ejection fraction (LVEF) and left ventricular end-diastolic dimension per standard institutional practice and American Society of Echocardiography guidelines.14 Transthoracic echocardiogram reports before and 6–12 months after initiation of TNF alpha inhibitor were included.
CS Treatment Regimens
Each institution follows a standardized approach to immunosuppressive management and monitoring of patients that includes use of oral corticosteroids and steroid-sparing agents (SSA) as well as pre- and post-treatment FDG-PET imaging. Corticosteroid dosing data were collected as follows: (1) maximum daily dose used for the specific indication of cardiac involvement of sarcoidosis, and (2) dose at TNF alpha inhibitor initiation and at 6 and 12 months after treatment initiation. Prednisone dose was typically decreased by 5–10 mg approximately every 4 weeks based on clinical and imaging stability as assessed by treating providers. Details regarding adjunctive SSAs were collected, including dosages and number of agents used. Immunosuppression-related side effects were adjudicated.
Treatment with TNF alpha inhibitors was reserved for (1) persistent cardiac inflammation on FDG-PET despite immunosuppressive treatment, (2) clinically active CS defined by cardiac clinical events (ie, cardiomyopathy, arrhythmia or conduction abnormalities), and/or (3) intolerable side effects from immunosuppression regimens. Dosing, frequency, indication, and side effects were collected and adjudicated. The 2 agents used were infliximab and adalimumab. Infliximab was prescribed as an intravenous infusion in a standard dosing frequency with weight-based dosing. Modifications to frequency of infusions and dose were made by the treating clinician. Adalimumab was prescribed as a standard dose of 40 mg subcutaneously injected every 2 weeks. Laboratories and side effects were monitored per standard guidelines.
Cardiac and Whole Body FDG-PET Protocol and Interpretation
Before FDG-PET examination, patients were instructed to follow a high-fat, low-carbohydrate diet for 1 day followed by 12 hours of fasting, to shift the myocardial metabolism to fatty acid use and suppress the uptake of FDG by normal myocardium. Metabolic imaging was performed with whole body and cardiac PET/computed tomography scans (Discovery Rx VCT PET/CT [GE Healthcare, Milwaukee, WI]) using an intravenous dose of 0.135 mCi/kg of FDG, as previously described.15 All FDG-PET examinations were analyzed offline on a dedicated workstation (GE Healthcare) by an experienced radiologist blinded to clinical data. Abnormal myocardial FDG uptake was assessed semiquantitatively as the number of involved segments using a 17-segment model for the left ventricle and maximum standardized uptake value.16 Patients with diffuse cardiac FDG uptake without any areas of focal uptake were presumed to have unsuccessful suppression of myocardial glucose and were not included in the FDG-PET analysis. The LVEF was obtained, when available, from simultaneously performed gated perfusion imaging. Post-treatment scans were compared with pretreatment scans to determine whether patients had complete resolution, partial improvement, no change, or worsening of cardiac FDG uptake. PET 1 was defined as the baseline scan immediately before TNF alpha inhibitor initiation. PET 2 and PET 3 were defined as follow-up scans approximately 6 months after and 12 months after TNF alpha inhibitor initiation, respectively.
Clinical Outcomes
Clinical end points were defined as follows: VA events (defined as ventricular fibrillation, sustained ventricular tachycardia, aborted sudden cardiac death, or any appropriate implantable cardioverter-defibrillator [ICD] therapy), worsening HF (defined as need for hospitalization and change in acute HF therapies), orthotopic heart transplantation, durable left ventricular assist device implantation, and death from any cause.
Statistical Analysis
Continuous data are presented as mean ± standard deviation for normal and as median and interquartile range (IQR) for non-normal data, and categorical variables are presented as frequencies and percentages. Comparisons were performed using the Welch 2-sample t test when comparing 2 separate groups, and the paired t test when comparing 2 time points for the same group. A P value of less than .05 was considered statistically significant. To correct for multiple comparisons, the Bonferroni correction was used, with a more conservative significance level of alpha of 0.05/3.00, or 0.016. All analyses were performed using R Studio (RStudio Team, 2018) and all data visualizations were generated using the package ggplot2 (Wichkam, 2009).
Results
Study Population
A total of 38 patients with CS treated with a TNF alpha inhibitor (30 infliximab, 8 adalimumab) were included in the study cohort. Baseline characteristics at time of CS diagnosis are presented in Table 1. The HRS criteria for CS were met by 37 patients (35 probable and 2 definite) and Japanese imaging criteria by 1 patient for isolated CS.
Table 1.
Characteristics of 38 patients at time of cardiac sarcoidosis diagnosis.
| Characteristics | Mean ± Standard Deviation or n (%) | |
|---|---|---|
| Age, years | 49.9 ± 9.5 | |
| Race | White | 18 (47) |
| African American | 20 (53) | |
| Female | 16 (42) | |
| Cardiac presentation | Ventricular arrhythmia: | 13 (34) |
| Sustained | 7 (18) | |
| Non-sustained | 6 (16) | |
| Atrial fibrillation | 4 (11) | |
| Atrioventricular block: | 10 (26.3) | |
| First degree | 3 (8) | |
| Second degree | 2 (5) | |
| Third degree | 5 (13) | |
| Heart failure | 13 (34) | |
| Bundle Branch Block | Left | 1 (9) |
| Right | 6 (55) | |
| Nonspecific | 1 (9) | |
| Ventricular paced | 3 (27) | |
| Left ventricular ejection fraction, % | 48.5 (15) | |
| Comorbidities | Diabetes mellitus | 8 (21) |
| Coronary artery disease | 4 (11) | |
| Chronic kidney disease | 2 (5) | |
| Prior diagnosis of extracardiac sarcoidosis | 19 (50) | |
| Organs involved | Lung | 29 (76) |
| Liver | 7 (18) | |
| Skin | 11 (29) | |
| Eye | 6 (16) | |
| Spleen | 5 (13) | |
| Neuro | 4 (11) | |
| Lymph nodes | 23 (61) | |
| Sinuses | 3 (8) | |
| Gastrointestinal tract | 1 (3) | |
| Joints | 5 (13) | |
| Bone | 3 (8) | |
| Diagnostic tissue biopsy for sarcoidosis | Lung | 12 (32) |
| Lymph node | 17 (45) | |
| Skin | 3 (8) | |
| Other | 4 (11) | |
| Heart | 2 (5) | |
| Late gadolinium enhancement on cardiac magnetic resonance imaging (n=31) | 24 (77) | |
One-half of the patients had a prior established diagnosis of extracardiac sarcoidosis. At time of CS diagnosis, the majority of patients had extracardiac sarcoidosis, with pulmonary (76%) involvement being common (Table 1).
Almost all patients (n = 37) presented with at least 1 of the following cardiac manifestations: VA, atrioventricular block (AVB), atrial arrhythmia, or HF. Four patients had more than 1 presenting cardiac event as follows: 1 patient had both VA and AVB, 1 patient had both VA and atrial arrhythmia, 1 patient had both AVB and HF, and 1 patient had VA, atrial arrhythmia, and AVB. The last patient presented with palpitations and dyspnea in the context of being treated with infliximab for extracardiac sarcoidosis. In response, FDG-PET was performed and revealed new cardiac FDG uptake, thus prompting increase in dose and frequency of infliximab to specifically treat CS.
Prior Immunosuppression Exposure
By the time of TNF alpha inhibitor treatment, all 38 patients had at some point been treated with prednisone and 37 patients with SSA. Before CS diagnosis, 15 patients were treated with prednisone and 12 were treated with a SSA for extracardiac sarcoidosis. After CS diagnosis, before TNF alpha inhibitor treatment, 36 patients were treated with prednisone (mean max daily dose 45 ± 15.2 mg; range 10–80 mg) and 36 with SSA (15 of which were tried on ≥2 SSA) for CS. Immunosuppressive regimens for CS are described in Table 2. Patient-reported side effects from corticosteroids were common, occurring in 76% of patients, with the most frequent being weight gain (Table 2). SSA side effects were experienced in 30% of patients, with 9 patients requiring permanent discontinuation and/or switch to alternate SSA specifically owing to side effects.
Table 2.
Immunosuppression Regimen and Side Effects Before Tumor Necrosis Factor Alpha Inhibitor Initiation in 38 Patients With Cardiac Sarcoidosis*
| Variables | Mean ± Standard Deviation or n (%) | |
|---|---|---|
| Maximum prednisone dose for CS, mg | 45 ± 15.2 | |
| No. of SSA used for CS | 0 | 2 (5) |
| 1 | 21 (55) | |
| 2 | 12 (32) | |
| 3 | 2 (5) | |
| 4 | 1 (3) | |
| First SSA agent used for CS [average maximum dose] | Mycophenolate | 16 (44) [2059 mg/d] |
| Methotrexate | 11 (31) [16.7 mg/wk] | |
| Azathioprine | 8 (22) [171.4 mg/d] | |
| Hydroxychloroquine | 1 (3) | |
| Prednisone side effect experienced | 29 (76) | |
| Multiple prednisone side effects experienced | 21 (55) | |
| Prednisone side effects | Weight gain | 22 (58) |
| Gastroesophageal reflux | 9 (24) | |
| Mood lability | 7 (19) | |
| Osteopenia/osteoporosis | 6 (17) | |
| Fluid retention | 4 (11) | |
| Appetite change | 3 (8) | |
| Diabetes | 3 (8) | |
| Skin fragility/bruising | 3 (8) | |
| Myopathy | 2 (5) | |
| Diaphoresis | 2 (5) | |
| Insomnia | 2 (6) | |
| Diabetic ketoacidosis/hyperglycemia | 1 (3) | |
| Infection | 0 (0) | |
| SSA side effect experienced | 11 (30) | |
| Multiple SSA side effects experienced | 2 (5) | |
| SSA side effects | Infection | 4 (11) |
| Gastrointestinal intolerance | 4 (11) | |
| Transaminitis | 2 (5) | |
| Mouth sores | 1 (3) | |
| Pruritis | 1 (3) | |
| Hair loss | 1 (3) | |
| Thrombocytopenia | 1 (3) | |
| Anemia | 0 | |
| Leukopenia | 0 | |
Steroid data presented with denominator of 38 patients, all of whom had steroid treatment at some point in clinical course, and 36 of whom had steroid treatment for cardiac sarcoidosis (CS) specifically. Steroid-sparing agent (SSA) data are presented with denominator of 37 patients who received SSA treatment.
TNF Alpha Inhibitor Treatment Course
Patients were started on a TNF alpha inhibitor a median of 490 days (IQR 727 days) after CS diagnosis. More than one-third of patients had more than 1 of the 3 outlined study indications for starting TNF alpha inhibitor treatment for their CS, as detailed in Table 3. At the time of treatment initiation, pharmacologic and device therapy for cardiac complications was common (Table 3). ICD implantation (n = 27) and resultant ICD therapy for VA (n = 10) were common. Echocardiogram features before initiation of TNF alpha inhibitor were notable for an average LVEF of 48 ± 16%, with 16 patients (43%) having an LVEF of less than 45%.
Table 3.
Clinical Characteristics and Treatment Regimen of 38 Patients With Cardiac Sarcoidosis at the Time of Tumor Necrosis Factor Alpha Inhibitor Treatment
| Clinical Cardiac Characteristics | Mean ± Standard Deviation or n (%) | |
|---|---|---|
| Antiarrhythmic drug | 11 (29) | |
| ICD | 27 (71) | |
| ICD therapy delivered | 10 (37) | |
| VT ablation | 2 (5) | |
| PPM | 4 (10.5) | |
| CRT | 5 (13) | |
| Left ventricular ejection fraction, % (n=37) | 48 (16) | |
| Left ventricular diastolic dimension, cm (n=37) | 4.90 ± 0.94 | |
| Moderate to severe right ventricular dysfunction (n=30) | 2 (7) | |
| Tricuspid annular plane of systolic excursion, mm (n=16) | 20 ± 4.1 | |
| Heart Failure Medications | Beta-blocker | 24 (63) |
| ACE-I/ARB | 16 (42) | |
| Mineralocorticoid antagonist | 4 (11) | |
| Diuretic | 14 (37) | |
| New York Heart Association Class | I | 14 (37) |
| II | 18 (47) | |
| III | 6 (16) | |
| IV | 0 (0) | |
| TNF alpha inhibitor treatment characteristics | ||
| TNF alpha inhibitor agent Infliximab | 30 (79) | |
| Adalimumab | 8 (21) | |
| Time from CS diagnosis to TNF alpha inhibitor treatment, days* | 490 (727) | |
| Indication for TNF alpha inhibitor treatment | Persistent cardiac FDG uptake | 22 (58) |
| Cardiac events | 13 (34) | |
| Intolerance to other regimen | 17 (45) | |
| Multiple indications | 14 (37) | |
| TNF alpha inhibitor maximum dose | Infliximab, mg/kg | 6.1 ± 2.2 |
| 3 mg/kg | 1 (3) | |
| 5 mg/kg | 21 (70) | |
| 6 mg/kg | 1 (3) | |
| 10 mg/kg | 7 (23) | |
| Adalimumab, mg | 40 ± 0 | |
| Dose increased during treatment | 7 (18) | |
| Frequency increased during treatment | 4 (11) | |
Continuous variables are expressed as mean ± standard deviation if normal distribution or * median [IQR] if non-normal. Categorical variables are expressed as number (%).
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CRT, cardiac resynchronization therapy; CS, cardiac sarcoidosis; FDG, 18F-fluorodeoxyglucose; ICD, implantable cardiac defibrillator; PPM, permanent pacemaker; TNF, tumor necrosis factor; VT, ventricular tachycardia
All patients had an FDG-PET scan (PET 1) before initiation of TNF alpha inhibitor (at a median of 74.0 days before [IQR 76.5 days before]). One was uninterpretable owing to poor dietary preparation. Of the remaining patients, 31 (84%) had cardiac FDG uptake as summarized in Table 4 and 21 (55%) had extracardiac FDG uptake.
Table 4.
FDG-PET Findings in 38 Patients Undergoing Treatment With Tumor Necrosis Factor Alpha Inhibitor for Cardiac Sarcoidosis
| PET 1 (Pretreatment) (n = 38) | PET 2 (n = 28) | PET 3 (n = 15) | |
|---|---|---|---|
| Time from TNF alpha inhibitor initiation, days* | −74 [76.5] | 176 [54] | 370 [212] |
| Perfusion defect present | 23 (61%) (n = 38) | 14 (54%) (n = 26) | 12 (80%) (n = 15) |
| Cardiac FDG uptake present | 31 (84%) (n = 37) | 8 (31%) (n = 26) | 4 (29%) (n = 14) |
| Number of involved segments | 3.95 ± 3.8) (n = 23) | 0.72 ± 2.2 (n = 22) | 0.7 ± 2.2 (n = 10) |
| SUVmax | 4.1 ± 4.5 (n = 34) | 0.54 ± 1.6 (n = 23) | 0.65 ± 1.5 (n = 11) |
| RV FDG uptake present | 4 (11%) (n = 36) | 0 (n = 27) | 0 (n = 15) |
| LVEF, % | 52.6 ± 15.9 (n = 37) | 53.8 ± 17.1 (n = 26) | 49.3 ± 16.1 (n = 15) |
| Extracardiac FDG uptake present (pretreatment) or increased from prior PET | 21 (55%) (n = 38) | 8 (29%) (n = 28) | 6 (40%) (n = 15) |
Continuous variables are expressed as mean ± standard deviation if normal distribution or * median [IQR] if non-normal. Categorical variables are expressed as number (%). FDG, 18F-fluorodeoxyglucose; LVEF, left ventricular ejection fraction; PET: positron emission tomography; RV, right ventricular; SUVmax, maximum standardized uptake value; TNF, tumor necrosis factor
TNF alpha inhibitor dosing is described in Table 3. There were no significant infusion site reactions. There were 8 patients who had infections during the course of treatment (infliximab: 3 shingles, 1 metapneumovirus pneumonia, 1 urinary tract infection and 1 intra-abdominal collection presumed to be infectious; adalimumab: 1 aseptic meningitis, 1 Mycobacterium avium complex). Of these 8 patients, the patient with an intra-abdominal collection required temporary infliximab discontinuation for 4 months and the patient with aseptic meningitis opted to discontinue adalimumab.
Thirty-three patients were treated with prednisone while starting TNF alpha inhibitor. Mean prednisone dose at time of TNF alpha inhibitor initiation was 21.7 ± 17.5 mg, with a significant decrease to 10.4 ± 6.1 mg (P = .001) and 7.3 ± 7.3 mg (P = .002) at 6 and 12 months after treatment initiation, respectively, when compared with baseline (Fig. 1). These Pvalues remained significant after correction for multiple comparisons. A total of 10 patients were completely off prednisone at end of study: 5 patients who were off prednisone by the time of TNF alpha inhibitor initiation and 5 who were weaned off during treatment. SSA was continued in 32 patients.
Fig. 1.
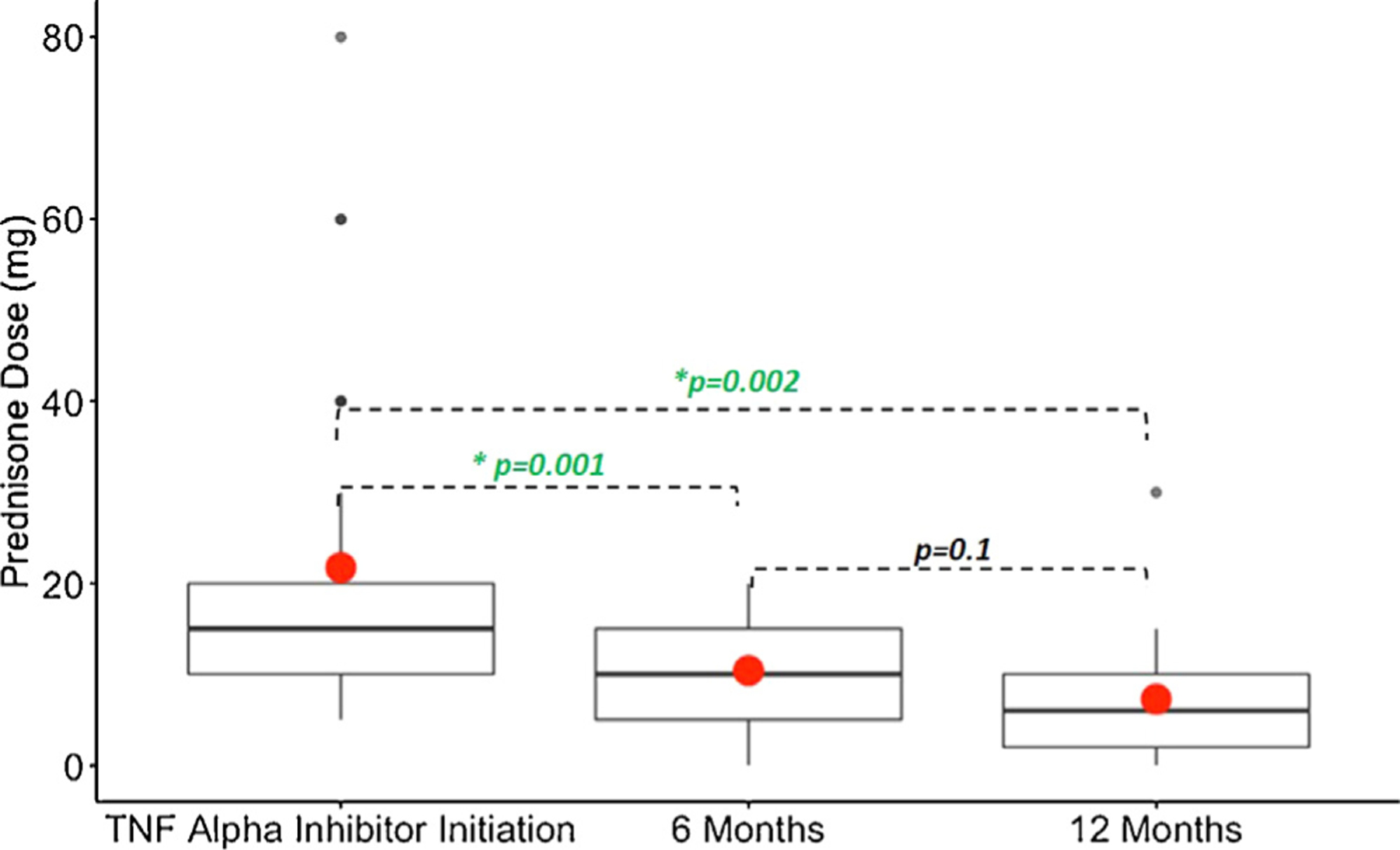
Change in prednisone dose before and after treatment of cardiac sarcoidosis with tumor necrosis factor (TNF) alpha inhibitor. Boxplot of prednisone dose with median and interquartile range (black line and rectangles) and mean (red dots) dose. Prednisone dose at time of TNF alpha inhibitor initiation (n = 33) decreased significantly at both 6 months (n = 31, P = .001) and 12 months (n = 17, P = .002) when compared with the baseline dose.
Imaging Outcomes After Treatment With TNF Alpha Inhibitor
There was no significant change in LVEF on echocardiogram before and after treatment (from 45 ± 16.5% to 47 ± 15.0%, P = .10, n = 29) (Fig. 2). Seven patients had an absolute LVEF improvement of 10% or greater. Only 1 patient had a decrement in absolute LVEF of more than 10% (from 35% to 20%, with left ventricular end-diastolic dimension increasing from 5.4 to 6.3 cm). Of the patients that did not have a post-treatment echocardiogram, 5 had an LVEF of 65% or greater on follow-up FDG-PET scan, 2 had clinical follow-up with documented stability and lack of HF signs or symptoms, and 1 had undergone heart transplantation. In the subset of patients that had right ventricular function assessments on echocardiogram, the frequency of moderate to severe right ventricular dysfunction was 3 of 31 vs 3 of 29 (P = .97) and tricuspid annular plane of systolic excursion was 20 ± 4.1 mm (n = 16) vs 22 ± 3.7 mm (n = 15) (P = .17).
Fig. 2.
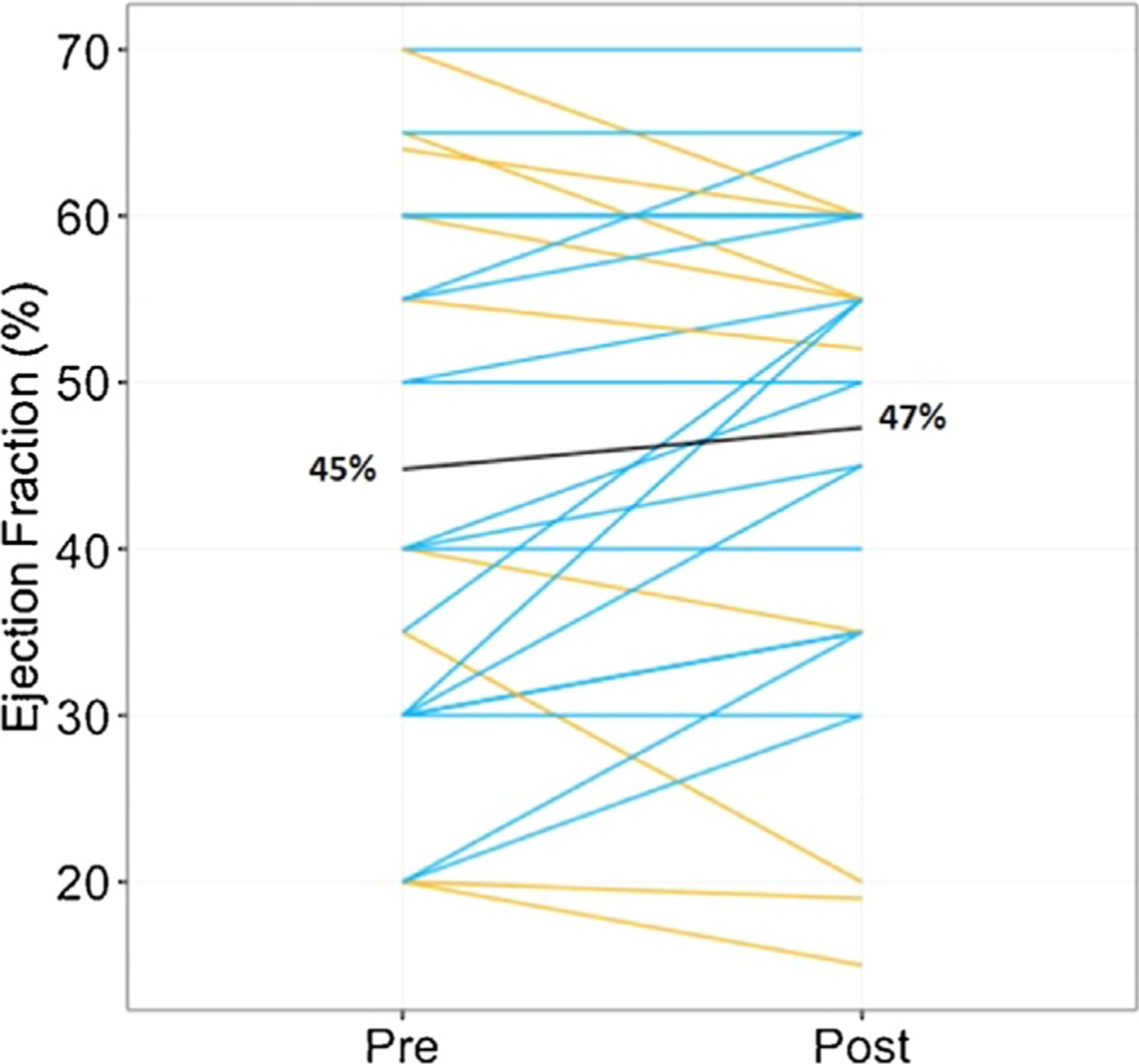
Change in left ventricular ejection fraction (LVEF) by echocardiographic assessment from before to after treatment of cardiac sarcoidosis with tumor necrosis factor alpha inhibitor treatment. The black line indicates overall cohort mean LVEF before and after treatment (n = 29, P = .1). The blue lines indicate patients with improved or stable LVEF, and the yellow lines indicate patients with decline in LVEF.
Thirty-two patients had at least 1 follow-up FDG-PET scan, with the first follow-up scan timing being approximately 6 months for the majority (n = 28, PET 2) (Table 4). Eleven patients also had a second follow-up scan (PET 3). There was a decrease in all cardiac FDG uptake parameters on post-treatment PET scans (Table 4). We then assessed paired comparisons for patients who had semiquantitative measures available at both baseline and first follow-up FDG-PET scan. There was a statistically significant decrease in number of involved segments (from 3.5 ± 3.8 to 1 ± 2.5, P = .008) and maximum standardized uptake value (from 3.59 ± 3.7 to 0.57 ± 1.6, P = .0005), with a trend toward a LVEF increase (50.6 ± 16.5 vs 53.9 ± 17.4, P = .07) (Figure 3). There was a numerical but not statistically significant difference in the proportion of patients who had cardiac perfusion defects at baseline vs follow-up scan (23/38 [61%] vs 8/18 [44%], P = .13)
Fig. 3.
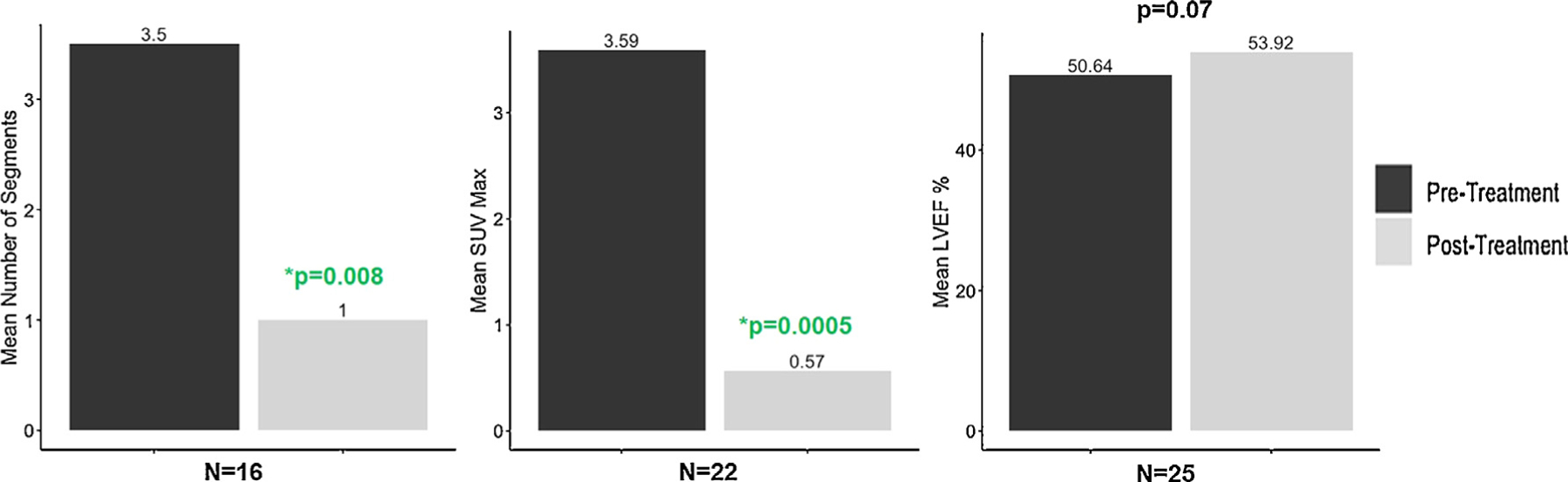
Changes in quantitative 18-flourodoxyglucose positron emission tomography (FDG-PET) assessments before and after treatment of cardiac sarcoidosis with tumor necrosis factor (TNF) alpha inhibitor treatment.
The overall clinical impression of first follow-up FDG-PET scan for 30 patients with interpretable pre- and post-FDG-PET scans was as follows: 16 had complete resolution of cardiac FDG uptake, 6 had improvement, 6 were stable, and 2 were worse. One of the 2 patients who had worsening cardiac inflammation did not have cardiac FDG uptake on a pretreatment scan with the follow-up PET scan performed 3 months after completion of a 9-month course of infliximab. The other patient was treated with adalimumab for 9 months, during which time PET 2 and PET 3 had increased cardiac FDG uptake compared with PET 1. Adalimumab was then discontinued owing to infectious issues, and a fourth PET scan less than 3 months later showed complete resolution of cardiac FDG uptake.
Clinical Outcomes After Treatment
During a median follow-up time of 486 days (IQR 405 days) after TNF alpha inhibitor initiation, 4 patients had worsening HF occurring at 90, 180, 185, and 472 days. The first patient, described elsewhere in this article with decrease in LVEF from 35% to 20% had experienced progressive HF before TNF alpha inhibitor treatment, and ultimately required inotropic support and evaluation for heart transplantation. The second patient (stable LVEF 35%) was admitted with HF approximately 1 month after a 6-month course of TNF alpha inhibitor treatment. The third patient who developed worsening HF (stable pre- and post-treatment LVEF 20%) went on to receive a left ventricular assist device and is waitlisted for heart transplantation. Treatment with adalimumab in this patient resulted in reduction in cardiac FDG uptake, improved neurosarcoidosis symptoms which contributed to improved functional status and thus operative candidacy. The fourth patient (pretreatment LVEF of 30%, post-treatment LVEF of 35%) was treated for decompensated HF with intravenous diuretics during an admission for pneumonia. One additional patient underwent heart transplantation 3 months after starting TNF alpha inhibitor treatment. There were no deaths.
Despite the prevalence of VA before TNF alpha inhibitor treatment, only 3 patients had new events after treatment at 46, 273, and 356 days after treatment initiation. Two of the patients had a baseline LVEF of 30% and a history of VA with ICD therapy and the third patient had a LVEF of 60% with a history of ICD implantation but no prior sustained VA events. All 3 had evidence of late gadolinium enhancement on cardiac magnetic resonance imaging. None of the 5 patients with complete heart block on initial presentation had recovery of AV nodal conduction at the time of follow-up.
Discussion
The present study represents the first multicenter analysis to assess the usefulness of TNF alpha inhibitor therapy in CS. Additionally, this is the largest study to report on cardiac FDG-PET response after treatment with TNF alpha inhibitor in a cohort meeting the HRS diagnostic criteria for CS. We demonstrate that TNF alpha inhibitor treatment (1) allowed significant decrease in corticosteroid dose, (2) was tolerated from a HF standpoint in the majority of patients, and (3) significantly decreased cardiac FDG uptake and numerically decreased clinical cardiac events.
Treatment of CS: Current Strategies and Limitations
Immunosuppressive treatment strategies for CS rely on noncardiac sarcoidosis literature or limited retrospective cohorts, resulting in a wide variation in clinical practice.17,18 Generally, immunosuppression in the form of corticosteroids remains the mainstay therapy, with the possible addition of a SSA, which may have limited efficacy but mitigate untoward side effects of chronic corticosteroid use. For example, more than three-quarters of our cohort had a reported side effect, with more than one-half having multiple side effects. Additionally, upfront corticosteroid treatment dose is usually higher for cardiac involvement compared with other organs (mean initial dose of 45 mg in our cohort), making consideration of alternate therapies even more important when the heart is involved. In the present study, we demonstrated a significant decrease in prednisone dose after treatment with TNF alpha inhibitor therapy that was not achieved with concomitant SSA therapy alone.
Role of TNF Alpha Inhibition in the Treatment of Sarcoidosis
TNF alpha plays a central role in sarcoidosis related granuloma formation and maintenance. Excess or increased levels of TNF have been found in the blood and bronchial alveolar lavage fluid of sarcoidosis patients.19–21 The intravenous anti-TNF alpha monoclonal antibody infliximab has been shown to be effective in treatment of refractory sarcoidosis, including cutaneous and neurologic involvement.7,22,23 In the single randomized trial demonstrating a benefit of TNF alpha inhibitor therapy in chronic pulmonary sarcoidosis, Baughman and colleagues5 demonstrated a modest but statistically significant improvement in the primary end point (change in percent of predicted forced vital capacity from baseline) after treatment with infliximab when compared with placebo. Adalimumab, a subcutaneously delivered TNF alpha inhibitor (IgG monoclonal antibody), has similarly proven to be effective in refractory pulmonary sarcoidosis and as an alternative therapy for patients intolerant to infliximab.24,25 A recent systematic review suggested that TNF alpha inhibitors are effective and safe in the treatment of sarcoidosis.26
Despite the observed benefits, challenges exist with TNF alpha inhibitor administration. These include infections (including mycobacterial disease),27 increased risk of malignancy,28 and infusion reactions, as well as cost. In our cohort, it was possible the infections observed were attributable to TNF alpha inhibition; however, before initiation 4 patients on SSAs had infectious complications and most patients remained on corticosteroids and/or SSAs at time of TNF alpha inhibition. Our findings are in line with prior studies and emphasize the need to ensure up-to-date vaccinations and close monitoring for infections and malignancies.
Efficacy and Safety of TNF Alpha Inhibition in HF and CS
In addition to its role in sarcoidosis, TNF has been found to be upregulated in chronic HF.8 Thus, the RECOVER and RENAISSANCE studies were designed to assess the usefulness of etanercept, a subcutaneously delivered recombinant human TNF receptor, in patients with chronic systolic HF (LVEF of <30%, New York Heart Association functional class II–IV).10 In the prespecified combined analysis, there was no effect on death or HF hospitalization. Infliximab also failed to demonstrate a benefit in a randomized study of patients with moderate to severe chronic systolic HF (LVEF of <35%, New York Heart Association functional class III–IV), with an increased risk of death and HF hospitalization for those randomized to the higher dose of 10 mg/kg.9 Thus, concerns regarding TNF inhibition and the development of HF have resulted in published warnings highlighting this risk.29
Despite these prior studies in HF patients, we did not see a significant decrease in cardiac function after treatment in our patients, almost one-half of whom had a decreased baseline left ventricular systolic function. In fact, a proportion of patients had a clinically significant improvement in LVEF after treatment. Thus, it may be that because TNF inhibition is targeting the specific process leading to active CS, these patients benefit from such therapy, and at lower doses studied in HF cohorts. However, even with treatment, patients with CS, including some in our cohort, may develop progressive cardiomyopathy or refractory VA, and advanced HF therapies such as left ventricular assist device or heart transplantation may be considered.30,31 Candidacy for these therapies relies on controlled extracardiac disease and comorbidities, including those that are steroid induced. Thus, judicious use of TNF alpha inhibition may be helpful in these cases, as we found in 3 of our patients who subsequently became candidates for advanced HF therapies. It should be noted that TNF alpha inhibitor treatment in our cohort did not reverse those with advanced cardiomyopathy or heart block, raising the question of its efficacy at these later stages of disease. Earlier introduction of TNF alpha inhibitors, currently considered third-line agents in sarcoidosis, to alter disease course warrants further investigation.
FDG-PET Imaging in the Treatment of CS
Cardiac FDG-PET imaging is increasingly used in the diagnosis and monitoring of CS.16 FDG is a glucose analogue taken up by macrophages, the drivers of granulomatous inflammation in sarcoidosis.32 Serial FDG-PET examinations help to monitor response to immunosuppressive therapy and may guide treatment changes.33–35 In our cohort, almost all patients had cardiac FDG uptake despite previous immunosuppressive treatment, in line with prior studies demonstrating corticosteroid resistance on FDG-PET scan.36 The presence of cardiac FDG uptake has been associated with increased rates of cardiac events and mortality, therefore raising the question of whether one metric of adequate treatment should be suppression of cardiac inflammation.11 We demonstrated significant decrease in cardiac FDG uptake after TNF alpha inhibitor treatment using both established semiquantitative and qualitative methods, with the majority of patients experiencing improvement or complete resolution of cardiac FDG uptake. Notably, FDG-PET results provided us with an objective marker of cardiac response to treatment to allow significant decreases in corticosteroid dosing.
Our study adds incremental value to only recently published limited case series of TNF alpha inhibition in CS.37–39 In the previous largest series, Harper and colleagues37 retrospectively demonstrated that 75% of patients with refractory CS (defined by World Association of Sarcoidosis and Other Granulomatous Diseases criteria) responded to infliximab therapy based on one of the following, without worsening in the other 2 categories: prednisone dose reduction, LVEF improvement by 5% or greater, or a decrease in dysrhythmia burden.38 FDG-PET data were unavailable. In a subsequent series of 20 patients with CS receiving a variety of TNF alpha inhibitors (infliximab, adalimumab, or golimumab), patients experienced qualitative improvement on cardiac FDG-PET scan, a decrease in mean prednisone dose, and stable LVEF, notably including 7 of 20 patients who did not meet HRS criteria (defined as “possible” CS).38 A third study included 19 methotrexate-treated patients who received adalimumab, with 63% showing complete resolution based on FDG imaging.39 No significant toxicity was noted (including infection or worsening HF). Our current study is the largest study of TNF alpha inhibitor use in CS, that also includes a multi-institutional cohort, both infliximab- and adalimumab-treated patients, and rigorous imaging data including both echocardiography and FDG-PET assessments over time.
Implications for Clinical Practice
These data support the need for a standardized approach to immunosuppressive treatment of CS, including determination of optimal corticosteroid dosing and weaning as well as timing and indications for consideration of TNF alpha inhibitor therapy. Additionally, it is possible that the quality of life for patients receiving TNF antagonist therapy (particularly adalimumab given the mode of administration) may be improved relative to those CS patients on chronic prednisone therapy. Ultimately, hypotheses-driven prospective studies in sarcoidosis are needed to identify optimal treatment strategies and will require multicenter, organ-specific investigations.
Limitations
This study has several limitations. First, although it comprises 2 large sarcoidosis centers with expertise in treatment of CS and similar practices, generalizability may be limited by referral bias and sample size. Second, major barriers exist in access to TNF alpha inhibitor treatment including insurance coverage, and it was not feasible to capture all patients for whom this treatment may have been considered. Third, this was a retrospective cohort study and we did not have a control group. Therefore, it is possible that a treatment effect may not be entirely attributable to TNF alpha inhibition. However, the majority of patients had active cardiac disease, as evidenced by pretreatment cardiac FDG uptake, despite optimized immunosuppressive strategies directed by a specialty sarcoidosis clinic and collaborating HF cardiologists. Last, we cannot make conclusions as to the long-term effects of TNF alpha inhibition given the relatively short follow-up duration.
Conclusions
We demonstrate that TNF alpha inhibitor treatment (1) allowed significant decrease in corticosteroid dose, (2) was tolerated from a HF standpoint in the majority of patients but infections were common, and (3) significantly reduced cardiac FDG uptake and numerically decreased clinical cardiac events.
Footnotes
Disclosures
None.
References
- 1.Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation 2009;120:1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation 2015;131:624–32. [DOI] [PubMed] [Google Scholar]
- 3.Bachwich PR, Lynch JP, Larrick J, Spengler M, Kunkel SL. Tumor necrosis factor production by human sarcoid alveolar macrophages. Am J Pathol 1986;125:421–5. [PMC free article] [PubMed] [Google Scholar]
- 4.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 1995;2:561–72. [DOI] [PubMed] [Google Scholar]
- 5.Baughman RP, Drent M, Kavuru M, Judson MA, Costabel U, du Bois R, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med 2006;174:795–802. [DOI] [PubMed] [Google Scholar]
- 6.Judson MA, Baughman RP, Costabel U, Flavin S, Lo KH, Kavuru MS, et al. Efficacy of infliximab in extrapulmonary sarcoidosis: results from a randomised trial. Eur Respir J 2008;31:1189–96. [DOI] [PubMed] [Google Scholar]
- 7.Gelfand JM, Bradshaw MJ, Stern BJ, Clifford DB, Wang Y, Cho TA, et al. Infliximab for the treatment of CNS sarcoidosis: a multi-institutional series. Neurology 2017;89:2092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990;323:236–41. [DOI] [PubMed] [Google Scholar]
- 9.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 2003;107:3133–40. [DOI] [PubMed] [Google Scholar]
- 10.Mann DL, McMurray JJV, Packer M, Swedberg K, Borer JS, Colucci WS, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 2004;109:1594–602. [DOI] [PubMed] [Google Scholar]
- 11.Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014;63:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Judson MA, Costabel U, Drent M, Wells A, Maier L, Koth L, et al. The WASOG Sarcoidosis Organ Assessment Instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis 2014;31:19–27. [PubMed] [Google Scholar]
- 13.Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014;11:1304–23. [DOI] [PubMed] [Google Scholar]
- 14.Douglas PS, Carabello BA, Lang RM, Lopez L, Pellikka PA, Picard MH, et al. 2019 ACC/AHA/ASE key data elements and definitions for transthoracic echocardiography: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards) and the American Society of Echocardiography. Circ Cardiovasc Imaging 2019;12:e000027. [DOI] [PubMed] [Google Scholar]
- 15.Kruse MJ, Kovell L, Kasper EK, Pomper MG, Moller DR, Solnes L, et al. Myocardial blood flow and inflammatory cardiac sarcoidosis. JACC Cardiovasc Imaging 2017;10:157–67. [DOI] [PubMed] [Google Scholar]
- 16.Chareonthaitawee P, Beanlands RS, Chen W, Dorbala S, Miller EJ, Murthy VL, et al. Joint SNMMI-ASNC expert consensus document on the role of 18F-FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J Nucl Med 2017;58:1341–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadek MM, Yung D, Birnie DH, Beanlands RS, Nery PB. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can J Cardiol 2013;29:1034–41. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Lower EE, Li H, Costea A, Attari M, Baughman RP. Cardiac sarcoidosis: the impact of age and implanted devices on survival. Chest 2017;151:139–48. [DOI] [PubMed] [Google Scholar]
- 19.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 1989;56:731–40. [DOI] [PubMed] [Google Scholar]
- 20.Baughman RP, Strohofer SA, Buchsbaum J, Lower EE. Release of tumor necrosis factor by alveolar macrophages of patients with sarcoidosis. J Lab Clin Med 1990;115:36–42. [PubMed] [Google Scholar]
- 21.Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet 2003;361:1111–8. [DOI] [PubMed] [Google Scholar]
- 22.Baughman RP, Lower EE. Infliximab for refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2001;18:70–4. [PubMed] [Google Scholar]
- 23.Baughman RP, Judson MA, Lower EE, Drent M, Costabel U, Flavin S, et al. Infliximab for chronic cutaneous sarcoidosis: a subset analysis from a double-blind randomized clinical trial. Sarcoidosis Vasc Diffuse Lung Dis 2016;32:289–95. [PubMed] [Google Scholar]
- 24.Minnis PA, Poland M, Keane MP, Donnelly SC. Adalimumab for refractory pulmonary sarcoidosis. Ir J Med Sci 2016;185:969–71. [DOI] [PubMed] [Google Scholar]
- 25.Crommelin HA, van der Burg Leone M, Vorselaars ADM, Drent M, van Moorsel HM, et al. Efficacy of adalimumab in sarcoidosis patients who developed intolerance to infliximab. Respir Med 2016;115:72–7. [DOI] [PubMed] [Google Scholar]
- 26.Adler BL, Wang CJ, Bui T, Schilperoort HM, Armstrong AW. Anti-tumor necrosis factor agents in sarcoidosis: a systematic review of efficacy and safety. Semin Arthritis Rheum 2019;48:1093–104. [DOI] [PubMed] [Google Scholar]
- 27.Minozzi S, Bonovas S, Lytras T, Pecoraro V, González-Lorenzo M, Bastiampillai AJ, et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf 2016;15(uppl 1):11–34. [DOI] [PubMed] [Google Scholar]
- 28.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295:2275–85. [DOI] [PubMed] [Google Scholar]
- 29.Page RL, O’Bryant CL, Cheng D, Dow TJ, Ky B, Stein CM, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation 2016;134:32. [DOI] [PubMed] [Google Scholar]
- 30.Fussner LA, Karlstedt E, Hodge DO, Fine NM, Kalra S, Carmona EM, et al. Management and outcomes of cardiac sarcoidosis: a 20–year experience in two tertiary care centres. Eur J Heart Fail 2018;20:1713–20. [DOI] [PubMed] [Google Scholar]
- 31.Crawford TC, Okada DR, Magruder JT, Fraser C, Patel N, Houston BA, et al. A contemporary analysis of heart transplantation and bridge-to-transplant mechanical circulatory support outcomes in cardiac sarcoidosis. J Card Fail 2018;24:384–91. [DOI] [PubMed] [Google Scholar]
- 32.Pellegrino D, Bonab AA, Dragotakes SC, Pitman JT, Mariani G, Carter EA. Inflammation and infection: imaging properties of 18F-FDG-labeled white blood cells versus 18F-FDG. J Nucl Med 2005;46:1522–30. [PubMed] [Google Scholar]
- 33.Ning N, Guo HH, Iagaru A, Mittra E, Fowler M, Witteles R. Serial cardiac FDG-PET for the diagnosis and therapeutic guidance of patients with cardiac sarcoidosis. J Card Fail 2019;25:307–11. [DOI] [PubMed] [Google Scholar]
- 34.Okada DR, Saad E, Wand AL, Griffin JM, Kasper EK, Chen EH, et al. Effect of corticosteroid dose and duration on 18-fluorodeoxyglucose positron emission tomography in cardiac sarcoidosis. JACC Cardiovasc Imaging 2020;13:1280–2. [DOI] [PubMed] [Google Scholar]
- 35.Lee P, Cheng G, Alavi A. The role of serial FDG PET for assessing therapeutic response in patients with cardiac sarcoidosis. J Nucl Cardiol 2017;24:19–28. [DOI] [PubMed] [Google Scholar]
- 36.Shelke AB, Aurangabadkar HU, Bradfield JS, Ali Z, Kumar KS, Narasimhan C. Serial FDG-PET scans help to identify steroid resistance in cardiac sarcoidosis. Int J Cardiol 2017;228:717–22. [DOI] [PubMed] [Google Scholar]
- 37.Harper LJ, McCarthy M, Ribeiro Neto ML, Hachamovitch R, Pearson K, Bonanno B, et al. Infliximab for refractory cardiac sarcoidosis. Am J Cardiol 2019;124:1630–5. [DOI] [PubMed] [Google Scholar]
- 38.Baker MC, Sheth K, Witteles R, Genovese MC, Shoor S, Simard JF. TNF-alpha inhibition for the treatment of cardiac sarcoidosis. Semin Arthritis Rheum 2019;50:546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenthal DG, Parwani P, Murray TO, Petek BJ, Benn BS, De Marco T, et al. Long-term corticosteroid-sparing immunosuppression for cardiac sarcoidosis. J Am Heart Assoc 2019;8: e010952. [DOI] [PMC free article] [PubMed] [Google Scholar]


