Abstract
BACKGROUND:
NTRK-rearranged thyroid carcinomas (NRTC), though rare, harbor a potential therapeutic target. The cytomorphologic features by fine needle aspiration (FNA) and the utility of preoperative molecular testing for NRTC remain largely uncharacterized. We provide a detailed cytomorphologic analysis of an institutional NRTC cohort with clinical, radiologic, histopathologic, and molecular correlations.
METHODS:
Our NRTC FNA cohort included 21 specimens from 19 patients. The mean age and female-to-male ratio were 42 years and 2.2:1, respectively. Predominantly alcohol-stained Papanicolaou smears and liquid-based preparations were reviewed for 14 patients with available materials, and histologic review of subsequent resections was conducted for all 19 patients. Imaging and clinical data were accessed through electronic medical records.
RESULTS:
Sonographically, NRTC were hypoechoic (87%), predominantly solid (53%) with limited central vascularity (27%), ill-defined borders (67%), and microcalcifications (67%). Observed cytomorphologic features include mixed architectural patterns (79%), fibrosis (93%), oncocytic and vacuolated cytoplasm (36% and 43%, respectively), and abundant intranuclear pseudoinclusions (14%). Most NRTC FNAs were classified as suspicious for malignancy or malignant (89%). One case classified as atypia of uncertain significance underwent ThyroSeq sequencing where a NTRK1 fusion was identified.
CONCLUSION:
Although NRTC did not show a consistent cytomorphologic signature, mixed architectural patterns, prominent fibrosis and distinct cytoplasmic or nuclear features should raise suspicion for NRTC and, when accompanied by negative BRAFV600E by immunohistochemistry on cell block material, aid in selecting cases for molecular testing. This algorithmic approach may help identify potential NRTC, maximizing treatment options for patients, especially in patients for whom treatment planning is complicated.
Keywords: cytomorphology, FNA, histology, kinase, NTRK, thyroid, thyroid cytology
INTRODUCTION
Signal transduction through extracellular receptor tyrosine kinases (RTK) with temporal and spatial regulation is critical for physiologic growth and development. Therefore, when RTKs are inappropriately expressed or aberrantly activated through gene mutations or genetic translocations, oncologic transformation occurs. However, this tumor-specific protein expression of rearranged RTKs is exactly what may render them susceptible, in isolation, to targeted therapeutic reagents.1 Although rare in thyroid carcinomas, RTK rearrangements are a persistent subset, among them subtypes of the neurotrophic-tropomyosin receptor kinase (NTRK).2,3 The NTRK family includes NTRK1, NTRK2, and NTRK3. NTRK-rearranged thyroid carcinomas (NRTC) account for approximately 2.3% of thyroid carcinomas and occur in all age groups in both radiation-naïve and radiation-exposed patients.4–8 Molecular profiling of NRTC have identified fusions of NTRK1 and NTRK3 but not NTRK2.5 From an oncologic standpoint, NTRK has gained recent attention with notable successes and US Food and Drug Administration approval of targeted therapies, including larotrectnib and entrectinib.4,9,10
While NTRK rearrangements in thyroid carcinomas have been documented in multiple studies, and despite the recent development of NTRK inhibitors, a careful exploration of histologic features that would prompt an initial consideration of NRTC has not been elucidated until recently,4–6,8–14 We recently explored the histologic features, clinical aspects, and treatment outcomes of NRTC in 19 patients.2,3 The cohorts in these studies were predominantly adults; however, similar histologic findings have been reported in pediatric patients with NRTC.6 NRTC demonstrated a multinodular growth pattern with prominent features of lymphatic invasion and intratumoral fibrosis.3 The packeted growth patterns included nodular clusters of papillae and/or microfollicles with a variable but persistent presence of glomeruloid-appearing tumor structures. Three patients in this study who received NTRK inhibitor therapy showed either complete response or a significant decrease in disease burden, highlighting the potential use for NTRK inhibitors in treating NRTC.3
Fine needle aspiration (FNA) remains the current standard of care using the American Thyroid Association guidelines for the initial workup of thyroid nodules, as it is rapid, minimally invasive, and cost effective.15 In addition, preoperative molecular testing on cytology specimens may guide downstream clinical management, including the decision for surgical or medical management depending on patient variables. The disadvantages of FNA include a lack of traditional tissue architecture for evaluation of invasion and cytomorphologic overlap among diagnostic entities with divergent behavior. Although our understanding of the histologic features of NRTC has improved considerably in recent years,3,6,7 the cytomorphologic features of NRTC by FNA remain largely unknown. Further, a discussion about selection of potential cases for preoperative NTRK molecular testing is lacking, especially given the reported potentially aggressive nature of NRTC. In the present study—a follow-up study to 2 recent fusion kinase-related carcinoma studies (an NRTC-specific study by Chu et al3 and a subsequent study by our group2 to include additional cases of NRTC)—we retrospectively review the available FNA material from the same NRTC cohort attempt to address both questions. With close examination of the cytomorphologic features of NTRK1- and NTRK3-rearranged thyroid carcinomas and their histologic correlates, we propose a potential algorithm for preoperative testing in clinically opportune cases.
MATERIALS AND METHODS
In 2 recent studies by Chu et al,2,3 19 patients with thyroid resections with NTRK1 and NTRK3 rearrangements were identified by molecular profiling with Solid Fusion Assay (ArcherDx, Boulder, Colorado)16 on the thyroid resections or lymph node metastases.2,3 A retrospective search of the pathology archives at a single institution (Massachusetts General Hospital, Boston, Massachusetts) identified 21 FNA specimens corresponding to the 19 patients (institutional review board approval 2012P001024 [P.M.S.]). Slides were available for 14 specimens (from 12 patients) and were reviewed by a cytopathology fellow (K.V.) and senior cytopathologist (W.C.F.). FNA specimens were processed as either alcohol-fixed Papanicolaou (Pap) stained slides, air-dried Diff-Quik (2 outside cases), or liquid-based preparations Surepath (Becton Dickinson, East Rutherford, New Jersey) or ThinPrep (Hologic, Marlborough, Massachusetts). A core biopsy was available for 1 case. Given the architectural features recently described by our group for NTRK-related carcinomas,2,3 cytology slides associated with these cases were examined. The alcohol-fixed Pap-stained slides were primarily studied for documenting the cytomorphologic and architectural features of NRTC. Key cytologic features were assessed, specifically: (1) architecture (cell groups and single cells); (2) nuclear features including chromatin quality, nucleoli, intranuclear inclusions, pleomorphism, grooves, and mitoses; (3) cytoplasmic features including quality and quantity/nuclear to cytoplasmic ratio; and (4) background findings such as colloid, inflammation, necrosis, blood, fibrosis, psammoma bodies, and cyst contents. If present, other unique findings were noted. Representative hematoxylin and eosin–stained glass slides of matched thyroid resections were examined to correlate histologic features.3 Clinical parameters and imaging characteristics, when available, were obtained from the medical records and correlated with the cytomorphologic findings. FNA diagnoses were classified using The Bethesda System for Reporting of Thyroid Cytopathology (TBSRTC) criteria.17
RESULTS
Clinicopathologic and Radiologic Features of the NTRK-Fusion Thyroid Carcinoma Cohort
Our NRTC cohort comprised 21 specimens from 19 patients, all definitively diagnosed on histologic resection and with accompanying molecular profiling. Two prior studies by Chu et al, from which our cohort was derived, identified 9 types of NTRK1 and NTRK3 fusions.2,3 On resection, 94.7% (n = 18) cases were classified as papillary thyroid carcinoma, with 5.3% (n = 1) classified as secretory carcinoma of thyroid. Notably, all cases were negative by B-Raf V600E immunohistochemistry on resection.3 The mean age of patients was 42 years (range, 14–74 years) with a 2.2:1 female to male (F:M) ratio and an average tumor size of 4.2 cm (range, 0.9–10.5 cm). Imaging characteristics were available for 79% (n = 15) of NRTC. On ultrasound, 87% (n = 13) were hypoechoic or isoechoic, 53% (n = 8) were solid, 27% (n = 4) showed internal vascular flow, and 67% showed microcalcifications (n = 10). Tumor borders were documented in 9 cases with 67% (n = 6) showing ill-defined borders and 33% showing well-defined borders. Microcalcifications were identified in 67% of patients (n = 10). Among the FNAs performed, 67% (n = 14) had slides available for review. None of the cases had preoperative cell block preparation with B-Raf V600E immunohistochemistry. The clinicopathologic and radiologic findings for the entire cohort are summarized in Table 1. The cytomorphologic findings, histologic correlate, and TBSRTC categorization for each NRTC subset are detailed below.
TABLE 1.
Clinicopathologic and Radiologic Features of the Patient Cohort by NRTC Subtype
| Patient No. | Age/Sex | Sites Sampled on FNA | Tumor Size, cm | Ultrasound Imaging Findings | Surgical Outcome | Histologic Classification | Additional Treatment |
|---|---|---|---|---|---|---|---|
| ETV6-NTRK3 | |||||||
| 1 | 74/F | Thyroid | 10.5 | Hypoechoic heterogeneous nodule with microcalcifications and nodular borders | Hemithyroidectomy; lateral neck dissection | PTC with follicular, tall cells and focal papillary architecture; lymphovascular invasion extending to the perithyroidal skeletal muscle | RAI, XRT |
| 2 | 32/F | Lymph node | 3.5 | Mixed cystic/solid; isoechoic; internal and peripheral vascularity; associated separate microcalcifications | Total thyroidectomy | PTC, follicular variant; extrathyroidal extension and lymphovascular invasion | RAI |
| 3 | 14/F | Thyroid and lymph node | 4.2 | Poorly marginated; hypoechoic; numerous microcalcifications replacing much of anterior right thyroid | Lobectomy/completion thyroidectomy | PTC; innumerable small nodules involving the entire lobe with extrathyroidal extension | RAI |
| 4 | 23/F | Thyroid | 2 | Heterogeneous; hypoechoic; small echogenic foci | Hemithyroidectomy | PTC, classical type; nodular; surrounded by fibrosis; lymphoplasmacytic infiltrate | None |
| 5 | 71/F | Thyroid | 3.2 | Solid; hypoechoic; no microcalcifications; minimal internal vascularity | Total thyroidectomy | Secretory carcinoma of the thyroid | RAI, XRT, larotrectnib |
| SQSTM1-NTRK3 | |||||||
| 6 | 43/F | Thyroid and lymph node | 6.6 | Heterogeneous mass involving the right thyroid lobe with ETE and LAD | Total thyroidectomy, central neck dissection, modified radical neck dissection | PTC, solid and follicular variant; high-grade features; extensive angioinvasion, lymphatic invasion, intrathyroidal spread, and extrathyroidal extension | RAI |
| 7 | 29/F | Thyroid | 1.4 | Solid; coarse calcifications; hypoechoic; irregular margins; minimal internal vascularity; peripheral vascularity | Total thyroidectomy | PTC, solid variant; microfollicular pattern; nodular architecture and stromal fibrosis, microscopic extrathyroid extension and lymphovascular invasion | RAI |
| 8 | 26/F | Thyroid | 4 | Predominantly solid; isoechoic; heterogeneous nodule with multiple calcifications in the right thyroid midpole | Total thyroidectomy; unilateral central neck dissection | PTC, classical type; well-circumscribed with cystic changes and lymphovascular invasion | Planned RAI |
| RBMPS-NTRK3 | |||||||
| 9 | 22/F | Thyroid | 2.1 | Circumscribed predominantly isoechoic nodule containing psammomatous calcifications | Total thyroidectomy | PTC, classical type; predominant follicular-patterned architecture; prominent lymphovascular invasion and focal extrathyroidal extension | RAI |
| 10 | 60/F | Thyroid | 7 | NA | History of total thyroidectomy; unilateral paratra-cheal neck dissection | PTC; predominant follicular architecture; focal vascular invasion that involves skeletal muscle | RAI |
| TPR1-NTRK1 | |||||||
| 11 | 54/M | Thyroid | 4 | NA | Total thyroidectomy; central compartment dissection; unilateral neck dissection | PTC, classical type; multifocal with lymphovascular invasion and extrathyroidal extension | RAI, larotrectnib |
| 12 | 24/M | Thyroid | 5.4 | Heterogeneous; centrally hypervascular nodule with scattered microcalcifications replacing most of thyroid lobe | Total thyroidectomy; bilateral modified neck dissection | PTC, diffuse sclerosing variant; extensive lymphovascular invasion with intrathyroidal spread and numerous satellite nodules | RAI |
| SQSTM1-NTRK1 | |||||||
| 13 | 37/F | Thyroid | 3.9 | NA | Total thyroidectomy; central compartment dissection | PTC, classical type | RAI |
| 14 | 74/F | Thyroid | 1.5 | Solid; hypoechoic; punctate echogenic foci; microcalcifications | Lobectomy | PTC, classical type; prominent follicular and solid architecture; multinodular with extensive fibrosis and extensive lymphovascular invasion | None |
| TPM3-NTRK1 | |||||||
| 15 | 40/M | Thyroid | 6.2 | Isoechoic; predominantly solid with some cystic areas; irregular contours; minimal vascularity | Total thyroidectomy; central compartment dissection | PTC, classical type; predominantly follicular architecture and focal lymphovascular invasion | RAI |
| 16 | 27/M | Thyroid | 5.7 | Solid; heterogeneous; isoechoic; well-defined margins; tall; marked internal vascularity; numerous microcalcifications | Total thyroidectomy; central compartment dissection; unilateral neck dissection | PTC, classical type with follicular variant component; multinodular, separated by fibrous bands; extensive lymphovascular invasion and multiple satellite tumor nodules | None |
| EML4-NTRK3 | |||||||
| 17 | 42/M | Thyroid | 7 | NA | Brain biopsy; intraventricular lesion | Metastatic papillary carcinoma consistent with primary PTC | RAI, entrectinib |
| IRF2BP2-NTRK1 | |||||||
| 18 | 36/F | Thyroid | 1.2 | Hypoechoic solid nodule; no LAD | Lobectomy | PTC, classical type; focal tall cell features with minimal extrathyroidal extension and lymphovascular invasion | None |
| PPL-NTRK1 | |||||||
| 19 | 62/M | Lymph node | 0.9 | Hypoechoic nodule with microcalcifications with central flow | Total thyroidectomy; bilateral neck dissection | PTC, diffuse sclerosing variant; multifocal lymphovascular invasion | RAI, larotrectnib |
Abbreviations: ETE, extrathyroidal extension; F, female; FNA, fine needle aspiration; LAD, lymphadenopathy; M, male; NA, not available; NRTC, NTRK-related thyroid carcinoma; PTC, papillary thyroid cancer; RAI, radioactive iodine; XRT, external beam radiation therapy.
Cytologic Correlate of ETV6-NTRK3 Fusion Thyroid Carcinomas
For ETV6-NTRK3–rearranged thyroid carcinoma FNAs (n = 6 specimens), we had 5 cases available to review in-house and an outside report for 1 case. One patient had 2 FNA specimens, 1 from the thyroid and 1 from a lymph node with metastatic carcinoma (cases 3A and 3B; Table 2). Common mixed architectural patterns included micropapillary (40%, n = 2), sheet-like (40%, n = 2), small crowded groups (40%, n = 2), and a single-cell pattern (60%, n = 3). All cases except case 5 demonstrated typical papillary thyroid carcinoma (PTC)-type nuclei with delicate, even chromatin and typical micronucleoli/chromocenters. Fibrotic fragments were noted in all cases (100%) that had slides available for review. With TBSRTC, cases 1, 3A, 3B, and 4 were classified as malignant (PTC), whereas case 5 was classified as suspicious for malignancy (PTC). Case 2 was considered indeterminate per the outside report and thus could not be classified into a specific TBSRTC category. The corresponding resected NRTC demonstrated multinodular growth, extensive lymphatic invasion, and intratumoral fibrosis. The packeted growth patterns included nodular papillary clusters and/or microfollicles with a variable but persistent presence of glomeruloid-appearing tumor structures.3 One patient had a primary secretory carcinoma of the thyroid (case 5; Table 2), which was confirmed by mammaglobin immunostaining on the resection.3 The cytomorphologic features of the primary thyroid secretory carcinoma resembled that seen in the salivary gland including prominent nucleoli and oncocytic cytoplasm with cytoplasmic vacuoles.
TABLE 2.
Key Histologic Features of Each NRTC Fusion Subset
| Case No. | Site | Slides Available for Review | FNA Diagnosis | TBSRTC Category | Afirma or ThyroSeq Testing | Architecture | Nuclear Features |
Cytoplasmic Features |
Background |
Histologic Featuresa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chromatin | Nucleoli | Grooves | INCI | Mitoses | Pleomorphism | Cytoplasmic Quality | Cytoplasmic Quantity/Nuclear to Cytoplasmic Ratio | Colloid | Inflammation | Necrosis | Blood | Fibrosis | Psammoma Bodies | Histiocytes | Other Notable Findings | ||||||||
| ETV6-NTRK3 | |||||||||||||||||||||||
| 1 | Thyroid | Yes | PTC | M | NA | Micropapillary; single cells and stripped nuclei | Powdery | Small, typical micronucleoli | 1+ | 3+ | No | Mild | Delicate, oncocytic | Moderate | Scant | No | No | Yes | 1+ | Absent | Rare giant cells | NA | Multinodular; predominantly follicular; scattered micropapillary; papillary; psammoma bodies |
| 2 | Lymph node | No (cytologic description from outside report) | Follicular sheets of thyroid; sheets and microfollicles; colloid and numerous macrophages | Indeterminate | NA | Sheets and microfollicles | NA | NA | NA | NA | NA | NA | NA | NA | Present | NA | NA | NA | NA | NA | Numerous | NA | |
| 3A | Lymph node | Yes | PTC | M | NA | Predominantly sheet-like | Powdery | Small, typical micronucleoli | 1+ | 2+ | No | No | Scant, delicate, nonvacuolated | High cellularity/moderate to high | No | No | No | Yes | 1+ | Absent | Absent | NA | |
| 3B | Thyroid | Yes | PTC | M | NA | Predominantly sheet-like; rare single cells | Powdery | Small, typical micronucleoli | 1+ | 2+ | No | No | Scant, delicate, nonvacuolated | High cellularity/moderate to high | Scant | No | No | Yes | 1+ | Absent | Rare giant cells; histiocytes | NA | |
| 4 | Thyroid | Yes | PTC | M | NA | Micropapillary; small, crowded groups; few single cells | Powdery | Small, typical micronucleoli | 1+ | 2+ | No | No | Oncocytic | High cellularity/moderate to high | No | No | No | Yes | 1+ | Absent | Occasional giant cell | NA | |
| 5 | Thyroid | Yes | Atypical elongate to spindle cells with prominent cytoplasmic vacuoles forming signet rings; medullary thyroid carcinoma cannot be entirely excluded | SUS | NA | Small, very crowded groups | Powdery to speckled | Distinct nucleoli | 1+ | 1+ | No | No | Moderate, delicate with prominent cytoplasmic vacuoles | Moderate | Yes, on core biops | Yes, on core biopsy | No | Yes | 2+ | Absent | Absent | NA | Multinodular growth; prominent intratumoral fibrosis; mixed microcystic, tubular and papillary patterns; finely stippled chromatin; prominent nucleoli; grooves |
| SQSTM1-NTRK3 | |||||||||||||||||||||||
| 6A | Thyroid | Yes | PTC | M | NA | Small, crowded groups; papillary; low cellularity | Variable hyperchromasia | Small, typical micronucleoli | 1+ | 1+ | No | No | Scant, delicate, oncocytic | Low cellularity/moderate to high | No | No | No | Yes | 1+ | Absent | Absent | NA | Oncocytic tumor islands in solid, trabecular and insular patterns; PTC-type nuclei; increased mitotic activity |
| 6B | Lymph node | Yes | PTC | M | NA | Crowded groups; single cells | Variable hyperchromasia | Small, typical micronucleoli | 1+ | 1+ | No | No | Delicate, oncocytic | Moderate cellularity/moderate to high | No | No | No | Yes | 2+ | Absent | Absent | NA | |
| 7 | Thyroid | No | NA | SUS | Afirma: Suspicious | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| 8 | Thyroid | Yes | PTC with cystic degeneration | M | NA | Papillary fragments | Powdery to clustered | Small, typical micronucleoli | 1+ | 1+ | No | Mild | Histiocytoid cells, microvacuolate | Moderate cellularity/low to moderate | Scant | No | No | Yes | 2+ | Present | Cystic degeneration | NA | |
| RBMPS-NTRK3 | |||||||||||||||||||||||
| 9 | Thyroid | No | PTC | M | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | Multinodular growth; predominant follicular pattern; scattered papillary structures |
| 10 | Thyroid | Yes | PTC | M | NA | Crowded groups; single cells; stripped nuclei | Variable hyperchromasia | Small, typical micronucleoli | 2+ | 1+ | No | No | Scant to moderate, delicate | High cellularity/moderate to high | No | No | No | Yes | 2+ | Absent | Absent | NA | |
| TPR-NTRK1 | |||||||||||||||||||||||
| 11 | Thyroid | Yes | PTC | M | NA | Single cells; small, crowded groups; micropapillary | Powdery | Small, typical micronucleoli | 3+ | 1+ | No | No | Microvacuolated, abundant, well-defined, polygonal | Low to moderate | No | No | No | Yes | 2+ | Present | Absent | Squamoid morular structures | Multinodular; packeted papillae; glomeruloid bodies/pseudocribriform structures; intratumoral fibrosis; psammoma bodies |
| 12 | Thyroid | No | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| SQSTM1-NTRK1 | |||||||||||||||||||||||
| 13 | Thyroid | No | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | Multinodular; micropapillary; papillary; glomeruloid structures; absent psammoma bodies |
| 14 | Thyroid | Yes | PTC | M | NA | Single cells; crowded groups; micropapillary | Powdery | Small, typical micronucleoli | 3+ | 2+ | No | No | Oncocytic | Moderate | Scant | No | No | Yes | 2+ | Absent | Absent | NA | |
| TPM3-NTRK1 | |||||||||||||||||||||||
| 15 | Thyroid | Yes | Macrofollicular nodule with a subset of follicles with enlarged, pale, crowded nuclei, rare nuclear grooves, and no inclusions; concerning for PTC | AUS | ThyroSeq: NTRK1 fusion identified | Glomeruloid/micropapillary structures; small, crowded groups | Variable hyperchromasia | Small, typical micronucleoli | 3+ | 0 | No | Mild | Bubbly, vacuolated | Low to moderate | Scant | No | No | Scant | 1+ | Absent | Scattered giant cells | NA | Multinodular; intratumoral fibrosis; follicular; papillae; classical type PTC nuclei; scattered psammoma bodies |
| 16 | Thyroid | Yes | PTC | M | NA | Larger papillary structures; few single cells | Powdery | Small, typical micronucleoli | 2+ | 3+ | No | No | Delicate, scant | Moderate to high | No | No | No | Yes | 3+ | Absent | Absent | NA | |
| EML4-NTRK3 | |||||||||||||||||||||||
| 17 | Thyroid | No | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | Oncocytic; microfollicles; papillae |
| IRF2BP2-NTRK1 | |||||||||||||||||||||||
| 18 | Thyroid | No (cytologic description from outside report) | Atypical follicular groups; vaguely papillary clusters; slightly enlarged nuclei; occasional nuclear grooves; small prominent nucleoli; nuclear crowding and INCI | SUS | NA | Follicular groups; vaguely papillary | NA | Small, prominent nucleoli | 1+ | 1+ | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | Classical type PTC with focal tall cell features |
| PPL-NTRK1 | |||||||||||||||||||||||
| 19 | Lymph node | Yes | Metastatic PTC | M | NA | Micropapillary; small, crowded groups | Variable hyperchromasia | Small, typical micronucleoli | 1+ | 1+ | No | Mild | Oncocytic-type, delicate, microvacuolated | Moderate | Scant | No | No | Yes | 0 | Present | Absent | NA | Multifocal; predominantly follicular with oncocytic features and focal papillary with squamous metaplasi |
Abbreviations: AUS, atypia of uncertain significance; FNA, fine needle aspiration; INCI, intranuclear pseudoinclusion; M, malignant; NA, not applicable; PTC, papillary thyroid carcinoma; SUS, suspicious for papillary thyroid cancer; TBSRTC, The Bethesda System for Reporting Thyroid Cytology.
Cytologic Correlate of SQSTM1-NTRK3 Fusion Thyroid Carcinomas
For SQSTM1-NTRK3 fusion thyroid carcinomas (n = 4), we had slides from 3 FNAs from 2 patients available to review in-house (cases 6A, 6B, and 8; Table 2). One patient had 2 FNA specimens, 1 from the thyroid and 1 from a lymph node (cases 6A and 6B; Table 2). All 3 available FNAs showed fibrotic fragments (100%) with mixed architectural patterns that included crowded small groups (cases 6A and 6B), papillary architecture (cases 6A and 8), and single cells (case 6B). The cytoplasm was oncocytic in 2 sites from the same patient (cases 6A and 6B; Table 2) and microvacuolated in the third patient. Cases 6A, 6B, and 8 were all classified as malignant (PTC) with TBSRTC. While no cytomorphologic information was available on case 7, it was classified as suspicious for malignancy (PTC) based on the Afirma molecular testing. Resections from the 4 patients showed islands of oncocytic tumor cells in varying solid, trabecular, and insular patterns; PTC-type nuclei; and increased mitotic activity.3
Cytologic Correlate of RBPMS-NTRK3 Fusion Thyroid Carcinomas
Our cohort had 2 patients with RBPMS-NTRK3 fusion primary thyroid carcinomas that on histology showed multinodular growth, follicular architecture, and scattered papillary structures (cases 9 and 10).3 FNA slides for 1 RBPMS-NTRK3 thyroid carcinoma were available for review and showed high cellularity with small crowded groups, single cells, and stripped nuclei. The nuclei showed variable hyperchromasia, grooves, occasional intranuclear pseudoinclusions, and typical micronucleoli. The background showed prominent fibrotic fragments, blood, and scant colloid. Both FNAs were classified as malignant (PTC) using TBSRTC.
Cytologic Correlate of TPR-NTRK1 Fusion Thyroid Carcinomas
For TPR-NTRK1 fusion thyroid carcinomas (n = 2 specimens), we had slides from 1 FNA (case 11) available for review. The smear preparation revealed a mixed architectural pattern that included single cells, small crowded groups, and micropapillary fragments in a background showing fibrinous debris, psammoma bodies, and no colloid (Fig. 1A,C). The case was classified as malignant (PTC) using TBSRTC. Thyroid resections available for both cases showed a multinodular growth pattern with prominent intratumoral fibrosis, packeted papillae, pseudocribriform/glomeruloid structures, and psammoma bodies. A unique feature seen in this FNA case was the presence of squamoid-type morules with abundant, microvacuolated cytoplasm and well-defined polygonal cell borders (Fig. 1B). The precise histologic correlate of these squamoid-type morules are unclear. They may either represent fragments of papillae with abundant cytoplasm or they may correspond to the pseudocribriform/glomeruloid-type structures (Fig. 1D).
Figure 1.

Distinct mixed architectural patterns in NTRK-rearranged thyroid carcinomas. (A, D) Mixed papillary (A, white arrow) and micropapillary (A, black arrow) patterns in NTRK-related thyroid carcinoma (NRTC) with TPR-NTRK1 on fine needle aspiration (FNA) (Papanicolaou stain, original magnification ×200) and its corresponding histology (D, black and white arrows; hematoxylin and eosin [H&E] stain, original magnification ×100). (B, E) Unusual squamoid morule-type structures in NRTC with TPR-NTRK1 on FNA (B, black arrow; Papanicolaou stain, original magnification ×200) and possible corresponding histology (E, black arrow; H&E stain, original magnification ×200). (C, F) Micropapillary/small crowded group pattern in several NRTCs and shown here in an ETV6-NTRK3 NRTC FNA (C, Papanicolaou stain, original magnification×100), and the corresponding histologic correlate (F, black arrow; H&E stain, original magnification ×100).
Cytologic Correlate of SQSTM1-NTRK1 Fusion Thyroid Carcinomas
For SQSTM1-NTRK1 fusion carcinomas (n = 2 specimens), we had slides from 1 FNA (case 14) available for review. The smear preparation revealed a mixed architectural pattern that included single cells, small crowded groups, and micropapillary fragments with oncocytic cytoplasm and typical PTC-type nuclei in a background of fibrinous debris, scant colloid, and no psammoma bodies. The case was classified as malignant (PTC) using TBSRTC. On resections from both patients, the tumor was multinodular with micropapillary, papillary, and glomeruloid patterns and without psammoma bodies.
Cytologic Correlate of TPM3-NTRK1 Fusion Thyroid Carcinoma
For TPM3-NTRK1 fusion carcinomas (n = 2 specimens), we had slides from both FNAs (cases 15 and 16) available for review. The smear preparation revealed a mixed architectural pattern that included small crowded groups, papillary, glomeruloid/micropapillary fragments, and a few single cells. The cytoplasmic quality was delicate to vacuolated, and nuclei showed PTC-type features. The background showed fibrosis without psammoma bodies and scant to absent colloid. One case (case 15) was classified as atypia of undetermined significance with TBSRTC and underwent molecular testing with ThyroSeq that identified the NTRK1 fusion. Case 16, on the other hand, was classified as malignant (PTC). On resections from both patients, the tumor was multinodular with micropapillary, papillary, and glomeruloid patterns without psammoma bodies.
Cytologic Correlate of IRF2BP2-NTRK1 Fusion Thyroid Carcinoma
For IRF2BP2-NTRK1 fusion thyroid carcinomas (n = 1, case 18), FNA slides were not available; therefore, we used descriptors from the outside cytology report. The outside institution noted mixed architectural patterns including follicular and vaguely papillary clusters, enlarged nuclei, nuclear crowding, small nucleoli, and occasional intranuclear inclusions and nuclear grooves. No information was available on the background contents. The case was categorized as suspicious for malignancy (PTC) using TBSRTC per the outside report. On the corresponding thyroid resection, the tumor showed features of classic papillary thyroid carcinoma with focal tall cell features in a background of chronic lymphocytic thyroiditis.
Cytologic Correlate of PPL-NTRK1 Fusion Thyroid Carcinoma
For the PPL-NTRK1 fusion thyroid carcinomas (n = 1, case 19), we had 1 FNA specimen from a lymph node metastasis at the time of presentation. On Pap-stained smears, tumor cells were arranged in micropapillary and small crowded groups with oncocytic, delicate, microvacuolated cytoplasm and moderate nuclear to cytoplasmic ratio. Nuclei showed mild pleomorphism and were slightly hyperchromatic with micronucleoli, occasional nuclear grooves, and intranuclear pseudoinclusions. The background showed minimal colloid and scattered psammoma bodies. No fibrosis was identifiable in this case. The case was classified as malignant (PTC) using TBSRTC. On thyroid resection, the tumor was multifocal with extensive lymphovascular invasion and extrathyroidal extension. The tumoral architecture was follicular with focal oncocytic features and papillary with areas of squamous metaplasia.
Overall, among NRTC FNAs with slides available for review (n = 14), fibrosis (93%, n = 13) and mixed architectural patterns (79%, n = 11) (Figs. 1 and 2), followed by oncocytic cytoplasm (43%, n = 6; Fig. 3A,B), vacuolated (microvacuoles and larger vacuoles) cytoplasm (36%, n = 5; Fig. 3A–D), and abundant intranuclear pseudoinclusions (14%, n = 2; Fig. 4) were observed most frequently. The findings for each of the NRTC types are summarized in Table 3.
Figure 2.
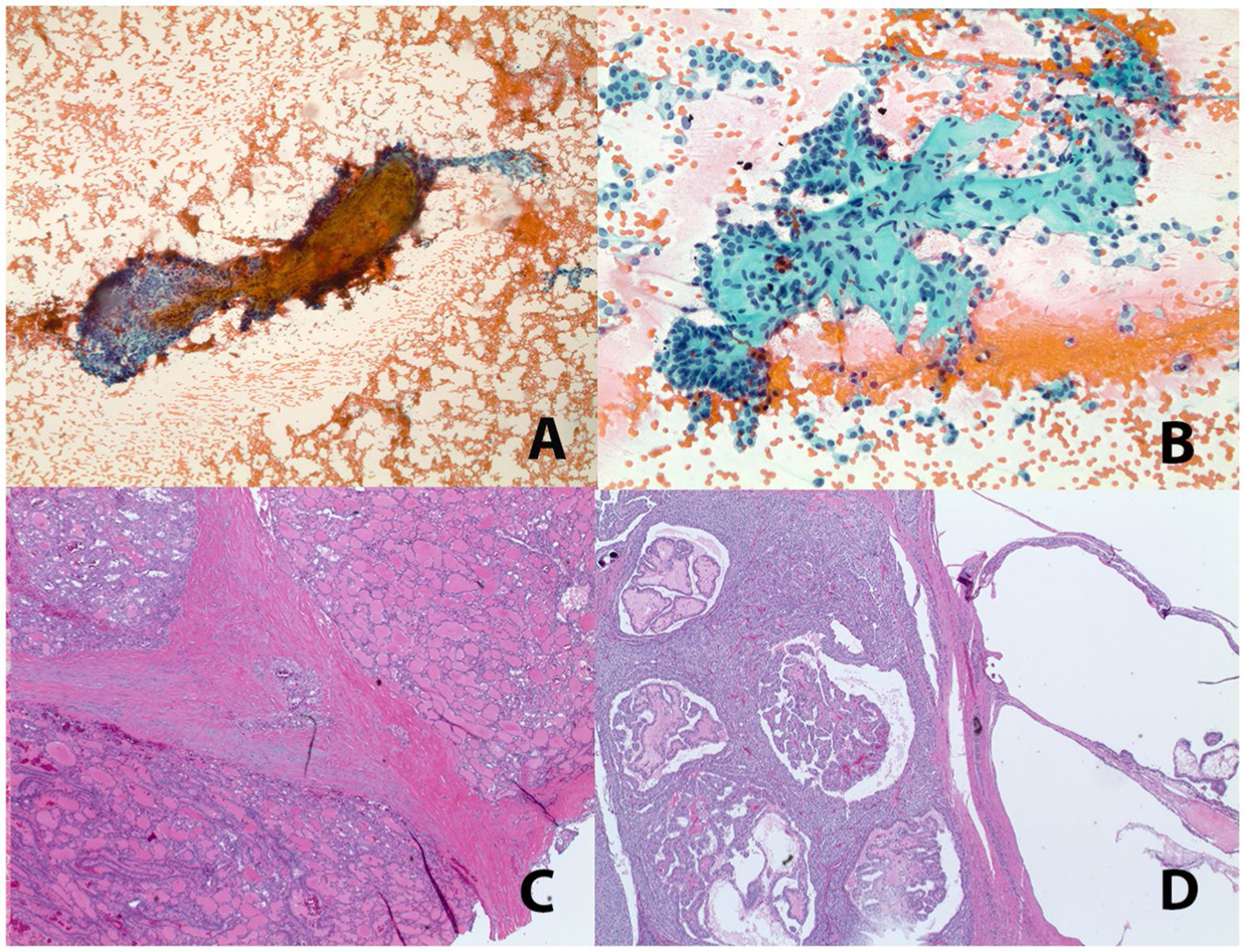
Fibrotic fragments are prominent in NTRK-rearranged thyroid carcinomas. Representative examples of fibrotic fragments from case 16 (A, TPM3-NTRK1) and case 14 (B, SQSTM1-NTRK1) (Papanicolaou stain, original magnification ×40 and ×200, respectively) are shown with their corresponding histologic features (C, D; hematoxylin and eosin stain, original magnification ×20).
Figure 3.

Distinct cytoplasmic features noted in few NTRK-rearranged thyroid carcinomas. (A, D) Microvacuolated and oncocytic cytoplasm noted in a SQSTM1-NTRK1 fusion NTRK-related thyroid carcinoma (NRTC) (A, black arrow; Papanicolaou stain, original magnification ×600) and its histologic correlate (D, black arrow; hematoxylin and eosin [H&E] stain, original magnification ×400). (B, E) Microvacuolated and oncocytic cytoplasm noted in a SQSTM1-NTRK3 fusion NRTC (B, Papanicolaou stain, ×200 original magnification [inset: SurePathpreparation, original magnification ×600]) and its histologic correlate (E, black arrow; H&E stain, original magnification×400). (C, F) Prominent cytoplasmic vacuoles were most notable in case 5, an ETV6-NTRK3 rearranged primary secretory carcinoma of the thyroid (C, black arrow; Papanicolaou stain, original magnification×400) and its histologic correlate (F, black arrow; H&E stain, original magnification ×40).
Figure 4.

Representative example of extensive intranuclear pseudoinclusions noted in 2 NTRK-rearranged thyroid carcinomas. Extensive intranuclear pseudoinclusions in case 1 (A, Papanicolaou stain, original magnification ×400) and its histologic correlate (B, hematoxylin and eosin stain, original magnification × ×200) are shown.
TABLE 3.
Summary of Features for Each NRTC Subtype
| NRTC Fusion | Architecture | Nuclear Features | Cytoplasmic Features | Background |
|---|---|---|---|---|
| ETV6-NTRK3 | Mixed: follicular; micropapillary; sheet-like; single cells; crowded groups | PTC-like nuclei | Delicate oncocytic cytoplasm (exception: case 5 [secretory carcinoma of thyroid with cytoplasmic vacuoles]) | Fibrosis; scant to absent colloid; blood; no inflammation; no necrosis |
| SQSTM1-NTRK3 | Mixed: papillary; single cells; crowded groups | Variable hyperchromasia, but otherwise PTC-like nuclei | Delicate oncocytic/microvacuolated cytoplasm | Fibrosis; scant to absent colloid; blood; no inflammation; no necrosis; psammoma bodies present in 1 case |
| RBMPS-NTRK3 | Mixed: crowded groups; single cells; stripped nuclei | Variable hyperchromasia, but otherwise PTC-like nuclei | Delicate cytoplasm | Fibrosis; scant to absent colloid; blood |
| TPR1-NTRK1 | Mixed: single cells; crowded groups; micropapillary; squamoid morules | PTC-like nuclei | Microvacuolated abundant cytoplasm | Fibrosis; scant to absent colloid; blood; no inflammation; no necrosis; psammoma bodies |
| SQSTM1-NTRK1 | Mixed: single cells; crowded groups; micropapillary | PTC-like nuclei | Oncocytic cytoplasm | Fibrosis; scant to absent colloid; blood; no inflammation; no necrosis |
| TPM3-NTRK1 | Mixed: glomeruloid/micropapillary; papillary; small, crowded groups | PTC-like nuclei | Delicate scant/vacuolated cytoplasm | Fibrosis; scant to absent colloid; blood; no inflammation; no necrosis |
| IRF2BP2-NTRK1 | Mixed (per outside report): follicular; vaguely papillary | PTC-like nuclei (per outside report) | NA | NA |
Abbreviations: NA, not available; NRTC, NTRK-related thyroid carcinoma; PTC, papillary thyroid carcinoma.
For the EML4-NTRK3 fusion-related thyroid carcinoma metastatic to the brain (patient 19, case 17; Tables 1 and 2),3 a corresponding FNA aspirate was not available with morphology based on intraoperative smear (Table 2).
DISCUSSION
Recently, it has become possible to consider the use of rational therapeutics that target specific cancer-derived products rather than systemic traditional chemotherapies that target rapidly dividing cells and have broad side effect profiles. The discovery of primary cancers driven by kinase fusion-derived oncogenes provides an opportunity to employ targeted therapeutics with limited side effect profiles in a subset of fusion-related carcinomas—including recurrent/advanced disease, which was previously considered unresectable or untreatable.1 NTRK subtypes are well known, and kinase fusion partners are emerging in multiple malignancies, including secretory carcinomas of the breast, salivary gland, and thyroid, along with infantile fibrosarcoma.4,5,8 The targeting of NTRK fusions has gained traction over the past several years with the successful NTRK inhibitors etrectinib and larotrectinib.4,9,10 Notably, although NTRK fusions appear to be enriched in thyroid carcinomas, a key challenge is the selection of cases to increase the pretest probability of identifying cases that may harbor an NTRK fusion.5,8 Surprisingly, while the histologic features for resected NRTC have been documented in several recent studies,2,3,6,7 whether a cytologic correlate for NTRK-rearranged thyroid carcinomas could be preoperatively determined by FNA and the implications for preoperative molecular testing remain unknown. Thus, we chose to examine these aspects in this study.
In our NRTC cohort, FNA samples showed adequate cellularity with prominent fragments of fibrosis and mixed architectural patterns (Figs. 1 and 2). Oncocytic cytoplasm and the presence of cytoplasmic vacuoles were the second most frequent finding followed by prominent intranuclear pseudoinclusions (Figs. 3 and 4). Our cytomorphologic findings parallel the histologic features described in recent studies.2,3,6 Our studies,2,3 along with Prasad et al,6 highlighted multinodular growth, mixed architectural patterns, intratumoral fibrosis, and extensive lymphatic invasion as defining factors for NTRK and other kinase fusion-related thyroid cancers. Similar to their studies,6 we did not identify a unifying signature for all NRTC. Rather, consistent themes of follicular, papillary, micropapillary and small crowded group patterns and intratumoral fibrosis in the form of fibrous debris were appreciated in most of our NRTC aspirates. It should not be surprising that the multinodular growth pattern is not overtly identifiable by aspirate, as tissue architecture is unavailable, a limitation akin to distinguishing encapsulated and invasive thyroid carcinomas by FNA. Oncocytic cytoplasm was noted in NRTC with SQSTM1-NTRK3, ETV6-NTRK3, SQSTM1-NTRK1, and PPL-NTRK1 rearrangements. Vacuolated cytoplasm was noted in the ETV6-NTRK3 rearranged secretory carcinoma and in occasional NRTC with SQSTM1-NTRK3, TPR-NTRK1, and TPM3-NTRK1 rearrangements. Both of these cytoplasmic features noted by FNA were correlated with their histologic counterpart (Fig. 3). We also noted 2 cases with extensive intranuclear pseudoinclusions, similar to hyalinizing trabecular tumors, except that these were present as both single and multiple intranuclear pseudoinclusions, imparting a “soap bubble” appearance in most cells (Fig. 4). However, the mechanisms underlying the varied morphologies in each NTRK translocation subset requires additional study.
Despite the unique architectural and morphological features of NRTC, distinction from other variants of PTC can be quite challenging due to cytomorphologic overlap. In fact, one NRTC (case 5, secretory carcinoma of the thyroid) was first diagnosed as PTC with hobnail and signet ring features prior to clarification with molecular testing. Both the tall cell variant and the hobnail variants of PTC can have abundant oncocytic cytoplasm. In addition, the tall cell variant of PTC is known to have abundant soap bubble inclusions. Squamoid-type morules/glomerular structures (Fig. 1B) seen in cases 11 (TPR-NTRK1) and 15 (TPM3-NTRK1) could be misinterpreted as squamous morules that are a defining characteristic of the cribriform–morular variant of PTC, although this variant is strikingly rare, much more so than NRTC. Fibrotic fragments and/or fibrous debris alone may be seen in benign, inflammatory conditions, including Riedel thyroiditis and the fibrous variant of Hashimoto thyroiditis, as well as in malignant entities, such as diffuse sclerosing variant of PTC, although this variant is also, in its true form, among the fusion-related thyroid carcinomas.2 Thus, it is important to realize that cytomorphologic features in our NRTC cohort are not entirely specific. Regardless, if these unusual cytomorphologic features are encountered, especially when present in combination, a diagnosis of NRTC should be in the differential, and appropriate ancillary testing should be considered.
An important consideration is whether there is utility in the preoperative determination of NTRK fusion with molecular testing of a positive FNA specimen. Two scenarios in which such testing might be justified are if the NTRK rearrangement may (1) impact surgical or medical management and (2) if neoadjuvant therapy comes into consideration. Per the current TBSRTC and American Thyroid Association guidelines, molecular testing with Afirma or ThyroSeq is recommended only for the indeterminate categories of atypia of undetermined significance (AUS)/follicular lesion of undetermined significance and suspicious for follicular neoplasm.15,17 In our cohort, only 1 case was classified as AUS, with the remaining cases classified as suspicious for malignancy or malignant. The AUS case was subjected to ThyroSeq testing that identified the NTRK1 fusion. A second case in an outside institution that was classified as suspicious for malignancy and subjected to Afirma testing did not identify a B-Raf V600E or RET alteration, but also did not identify the NTRK rearrangement. It is possible that the earlier version of Afirma may not have included NTRK, data that would now be included with the Xpression Atlas.18 However, patients with lesions classified as suspicious for malignancy or malignant based on cytomorphologic features are likely to forego molecular testing and go directly for surgical resection given the risk of malignancy associated with these categories. However, the growing body of knowledge about NRTC may prospectively influence the algorithmic approach to molecular testing.
Whether preoperative knowledge of NTRK will impact the extent of surgery is unclear. Within our cohort, 79% had a total thyroidectomy with or without radioactive iodine treatment, whereas 21% had a hemithyroidectomy or lobectomy. For most cases, NTRK fusions were identified following resection and thus, it is unclear how the management would have been impacted had the NTRK status been determined preoperatively. In addition, for the 2 cases with preoperative molecular testing, the results did not appear to affect the extent of surgery which was governed more by the advanced disease stage. That said, as our understanding of NTRK inhibitors continues to develop and as more clinical trial data are generated, preoperative NTRK testing—especially on FNA material—may become more significant, particularly for cases that present with advanced disease (eg, undifferentiated [anaplastic] thyroid carcinoma) or cases that are considered clinically inoperable.19
Another rationale for preoperative testing for NTRK rearrangements is the potential for neoadjuvant treatment with NTRK inhibitors. In a recent clinical trial (ClinicalTrials.gov identifier NCT02637687), 5 pediatric patients with infantile fibrosarcoma or soft tissue sarcoma harboring the NTRK fusion that were refractory to standard therapy showed partial response upon neoadjuvant larotrectinib treatment. Subsequently, all 5 patients could obtain limb-sparing surgical resections instead of amputation.12 Significant responses to larotrectinib in patients with disease recurrences or metastatic disease has also been documented in several other studies.9–11,13 In our NRTC cohort, most patients presented with advanced stage disease and underwent a total thyroidectomy with or without a neck lymph node dissection followed by radioactive iodine treatment. Three patients who had refractory disease who were treated with NTRK inhibitors showed either complete resolution or significant reduction in disease burden, which raises the possibility of using NTRK inhibitors preoperatively to reduce the disease burden and may impact the extent of surgical resection or enable surgery in cases that would otherwise be unresectable.3 The data to support these considerations are quite limited; however, there are multiple ongoing basket clinical trials to explore the utility of neoadjuvant NTRK inhibition in malignancies with the rearrangement, including NRTC.
If preoperative molecular testing for NRTC can be justified, another important question is how to select FNA cases for molecular testing20. We recently proposed a schema in which thyroid carcinoma resections cases with notable multinodular growth pattern, intratumoral fibrosis, lymphovascular invasion, and mixed architectural patterns on histology should first be tested for B-Raf V600E by immunohistochemistry and, if negative, should then proceed to molecular testing for potential kinase-rearrangement fusions, including NTRK.2 One can envision an analogous approach to cytologic and small biopsy specimens (Fig. 5) that would be comparatively straight-forward using current commercially available molecular testing platforms such as ThyroSeq version 3 or Afirma GSC and Xpression Atlas. Alternatively, if sufficient material is present on smears or a liquid based preparation, and if any unusual cytomorphologic features are noted, BRAF immunocytochemistry testing may be considered, either on a cell block or on smear preparations.21,22 Further, B-Raf V600E-negative thyroid carcinomas might undergo additional sequencing studies that include a panel to detect kinase fusions, including NTRK. However, a direct molecular approach may be most practical given caveats in BRAF testing on cytology specimens.18,23 Similarly, while NTRK IHC/immunocytochemistry is a consideration, there is limited to no data on its utility in cytologic specimens. In the event that an NTRK fusion is identified, it then provides additional avenues for treatment either preoperatively or postoperatively, especially in the subset of patients for whom immediate surgery is either contra-indicated or suboptimal due to comorbidity or other prohibitive or adverse circumstances.
Figure 5.

Proposed algorithm for triaging potential NTRK-rearranged thyroid carcinoma fine needle aspiration (FNA). The proposed FNA NTRK algorithm parallels the recently published proposed RTK testing algorithm.2 If the cytomorphology on the FNA preparations demonstrates a mixed architectural pattern, fibrotic fragments, and any unusual cytoplasmic or nuclear features (eg, abundant intranuclear pseudoinclusions), a dedicated FNA pass may prove useful for BRAF testing or direct molecular testing with a larger panel to include NTRK on residual liquid-based material with platforms such as Afirma or ThyroSeq or on cell block material for patients who either have advanced disease or may benefit from neoadjuvant chemotherapy or are inoperable/suboptimal candidates for surgery.
In conclusion, our study provides a detailed examination of the cytomorphologic features of NRTC. Although no specific cytomorphologic signatures unique to NTRK subtype translocations were identified, we have highlighted several notable features, including mixed architectural patterns, fibrotic fragments, oncocytic and/or vacuolated cytoplasm, and least common yet unusual extensive intranuclear pseudoinclusions. Potential limitations of the study are the relatively small cohort size at a single institution and the retrospective nature of the review. Whether these interesting cytomorphologic features could be used to help identify NRTC in a blinded, prospective manner has yet to be studied. However, having an awareness of these cytomorphologic features of NRTC is important, and encountering these unusual features should prompt cytopathologists to consider the possibility of NRTC. Further, preoperative molecular testing, if indicated, should also be considered in the face of poor surgical candidates or in a neoadjuvant setting, even with a suspicious for malignancy or malignant cytologic diagnosis. This will likely become especially important as more clinical and outcome data on the NTRK inhibitors in the context of thyroid carcinoma come to the forefront and impact medical and surgical management.
Acknowledgments
FUNDING SUPPORT
Drs. Sadow and Faquin receive funding from the National Cancer Institute of the National Institutes of Health, 1P01CA240239–01.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Yamaoka T, Kusumoto S, Ando K, Ohba M, Ohmori T. Receptor tyrosine kinase-targeted cancer therapy. Int J Mol Sci. 2018;19:3491. doi: 10.3390/ijms19113491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu Y-H, Wirth LJ, Farahani AA, et al. Clinicopathologic features of kinase fusion-related thyroid carcinomas: an integrative analysis with molecular characterization. Mod Pathol. Published online July 31, 2020. doi: 10.1038/s41379-020-0638-5 [DOI] [PMC free article] [PubMed]
- 3.Chu YH, Dias-Santagata D, Farahani AA, et al. Clinicopathologic and molecular characterization of NTRK-rearranged thyroid carcinoma (NRTC). Mod Pathol. Published online May 26, 2020. doi: 10.1038/s41379-020-0574-4 [DOI] [PMC free article] [PubMed]
- 4.Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15:731–747. doi: 10.1038/s41571-018-0113-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okamura R, Boichard A, Kato S, Sicklick JK, Bazhenova L, Kurzrock R. Analysis of NTRK alterations in pan-cancer adult and pediatric malignancies: implications for NTRK-targeted therapeutics. JCO Precis Oncol. 2018;2018. doi: 10.1200/PO.18.00183 [DOI] [PMC free article] [PubMed]
- 6.Prasad ML, Vyas M, Horne MJ, et al. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer. 2016;122:1097–1107. doi: 10.1002/cncr.29887 [DOI] [PubMed] [Google Scholar]
- 7.Seethala RR, Chiosea SI, Liu CZ, Nikiforova M, Nikiforov YE. Clinical and morphologic features of ETV6-NTRK3 translocated papillary thyroid carcinoma in an adult population without radiation exposure. Am J Surg Pathol. 2017;41:446–457. doi: 10.1097/PAS.0000000000000814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon JP, Linkov I, Rosado A, et al. NTRK fusion detection across multiple assays and 33,997 cases: diagnostic implications and pitfalls. Mod Pathol. 2020;33:38–46. doi: 10.1038/s41379-019-0324-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21:271–282. doi: 10.1016/S1470-2045(19)30691-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Chi P. Basket trial of TRK inhibitors demonstrates efficacy in TRK fusion-positive cancers. J Hematol Oncol. 2018;11:78. doi: 10.1186/s13045-018-0622-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DuBois SG, Laetsch TW, Federman N, et al. The use of neoadjuvant larotrectinib in the management of children with locally advanced TRK fusion sarcomas. Cancer. 2018;124:4241–4247. doi: 10.1002/cncr.31701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong DS, DuBois SG, Kummar S, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21:531–540. doi: 10.1016/S1470-2045(19)30856-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penault-Llorca F, Rudzinski ER, Sepulveda AR. Testing algorithm for identification of patients with TRK fusion cancer. J Clin Pathol. 2019;72:460–467. doi: 10.1136/jclinpath-2018-205679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dias-Santagata D, Akhavanfard S, David SS, et al. Rapid targeted mutational analysis of human tumours: a clinical platform to guide personalized cancer medicine. EMBO Mol Med. 2010;2:146–158. doi: 10.1002/emmm.201000070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2017;27:1341–1346. doi: 10.1089/thy.2017.0500 [DOI] [PubMed] [Google Scholar]
- 18.Krane JF, Cibas ES, Endo M, et al. The Afirma Xpression Atlas for thyroid nodules and thyroid cancer metastases: Insights to inform clinical decision-making from a fine-needle aspiration sample. Cancer Cytopathol. 2020;128:452–459. doi: 10.1002/cncy.22300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suh HJ, Moon HJ, Kwak JY, Choi JS, Kim EK. Anaplastic thyroid cancer: ultrasonographic findings and the role of ultrasonography-guided fine needle aspiration biopsy. Yonsei Med J. 2013;54:1400–1406. doi: 10.3349/ymj.2013.54.6.1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander EK, Kennedy GC, Baloch ZW, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367:705–715. doi: 10.1056/NEJMoa1203208 [DOI] [PubMed] [Google Scholar]
- 21.Smith AL, Williams MD, Stewart J, et al. Utility of the BRAF p.V600E immunoperoxidase stain in FNA direct smears and cell block preparations from patients with thyroid carcinoma. Cancer Cytopathol. 2018;126:406–413. doi: 10.1002/cncy.21992 [DOI] [PubMed] [Google Scholar]
- 22.Wobker SE, Kim LT, Hackman TG, Dodd LG. Use of BRAF v600e immunocytochemistry on FNA direct smears of papillary thyroid carcinoma. Cancer Cytopathol. 2015;123:531–539. doi: 10.1002/cncy.21575 [DOI] [PubMed] [Google Scholar]
- 23.Nikiforova MN, Lepe M, Tolino LA, et al. Thyroid cytology smear slides: An untapped resource for ThyroSeq testing. Cancer Cytopathol. Published online July 22, 2020. doi: 10.1002/cncy.22331 [DOI] [PubMed]


