Abstract
Objective
There is an urgent need to assess the impact of immunosuppressive therapies on the immunogenicity and efficacy of SARS-CoV-2 vaccination.
Methods
Serological and T-cell ELISpot assays were used to assess the response to first-dose and second-dose SARS-CoV-2 vaccine (with either BNT162b2 mRNA or ChAdOx1 nCoV-19 vaccines) in 140 participants receiving immunosuppression for autoimmune rheumatic and glomerular diseases.
Results
Following first-dose vaccine, 28.6% (34/119) of infection-naïve participants seroconverted and 26.0% (13/50) had detectable T-cell responses to SARS-CoV-2. Immune responses were augmented by second-dose vaccine, increasing seroconversion and T-cell response rates to 59.3% (54/91) and 82.6% (38/46), respectively. B-cell depletion at the time of vaccination was associated with failure to seroconvert, and tacrolimus therapy was associated with diminished T-cell responses. Reassuringly, only 8.7% of infection-naïve patients had neither antibody nor T-cell responses detected following second-dose vaccine. In patients with evidence of prior SARS-CoV-2 infection (19/140), all mounted high-titre antibody responses after first-dose vaccine, regardless of immunosuppressive therapy.
Conclusion
SARS-CoV-2 vaccines are immunogenic in patients receiving immunosuppression, when assessed by a combination of serology and cell-based assays, although the response is impaired compared with healthy individuals. B-cell depletion following rituximab impairs serological responses, but T-cell responses are preserved in this group. We suggest that repeat vaccine doses for serological non-responders should be investigated as means to induce more robust immunological response.
Keywords: vaccination, rituximab, COVID-19, B-lymphocytes, autoimmune diseases
Key messages.
What is already known about this subject?
There are very few data relating to the effect of immunosuppression on immune responses to SARS-CoV-2 vaccination, as patients receiving immunomodulatory therapies were excluded from all vaccine trials.
What does this study add?
When assessed by both serological and T cell-based assays, most patients (89.3%) develop immune responses following two doses of vaccine, despite immunosuppressive therapies.
B-cell depletion following rituximab treatment was significantly associated with failure to seroconvert, although most of these patients developed T-cell responses to SARS-CoV-2.
Tacrolimus use was associated with impaired T-cell responses.
How might this impact on clinical practice or future developments?
Assessment of both serological and T-cell responses may be necessary to fully define responses to vaccination in immunosuppressed populations.
Administration of additional vaccine (‘booster’) doses may be a potential strategy for serological non-responders.
Introduction
There is an urgent need to understand the impact of immunosuppressive therapies on the efficacy of vaccines to SARS-CoV-2.1 2 Patients with autoimmune diseases have been considered clinically vulnerable to SARS-CoV-2 infection since the onset of the COVID-19 pandemic,3 and population-based and registry-based studies suggest that they experience significant rates of hospitalisation, severe disease and death during its global spread.4–6
Several vaccine candidates have been shown to prevent severe disease in the general population,7–10 although all clinical trials to date excluded patients receiving immunosuppression, who are at risk of diminished vaccine responses. The degree to which the immune response is altered may vary with the specific immunomodulatory regimen and the vaccine used. Published data, for example, indicate impaired humoral responses to influenza and pneumococcal vaccination, especially in those undergoing treatment with rituximab.11–14 However, existing data derived from experience with other vaccine types may not translate to the novel vaccines deployed for COVID-19.
Here, we describe the serological and T-cell responses to first-dose and second-dose vaccines (with either BNT162b2 mRNA or ChAdOx1 nCoV-19 replication-deficient adenoviral vector vaccines) in a cohort of patients with autoimmune glomerular and rheumatic diseases treated with rituximab or other non-biological immunosuppressive therapies, in order to describe the impact of these treatments on vaccine response in this patient population.
Methods
Study participants
Baseline samples were collected from 161 patients with immune-mediated glomerulonephritis and vasculitis who received their first-dose of SARS-CoV-2 vaccination (BNT162b2 mRNA or ChAdOx1 nCoV-19) between 17 January 2o21 and 9 March 2021. For assessment of immunological responses after the first-dose vaccine, 140 patients provided a first follow-up sample at a median of day 28 (IQR 28–30 days) after first-dose administration; 53 of these also provided paired samples for assessment of SARS-CoV-2 T-cell responses. To date, 103 patients in the study have received second-dose vaccine at a median of 30 days (IQR 28–42) after first dose and have provided a subsequent sample for serological analysis at a median of 21 days (IQR 19–28 days) after second-dose administration; 49 also provided paired samples for analysis of T-cell responses.
A group of healthy volunteer (HV) healthcare workers (HCWs) were used as a comparator group for the study (n=70). In this group, assessment of first-dose response was undertaken at a median of 21 days (IQR 19–25 days) after first-dose administration and at a median of 27 days (IQR 21.5–28.0 days) after second-dose administration. This group received second-dose vaccine at a median of 66 days after first-dose (IQR 61–69 days). To control for some of the differences between the cohorts of immunosuppressed (IS) patients (IS group) and the HV group, matching for age and vaccine type was performed.
Separate cohorts of HCWs were used to identify a threshold for positivity on the ELISpot assay in participants who were infection-naïve and unvaccinated (n=30).15
Serological testing
Serum was tested for antibodies to nucleocapsid protein (anti-NP) using the Abbott Architect SARS-CoV-2 IgG two-step chemiluminescent immunoassay (CMIA) according to the manufacturer’s instructions. This is a non-quantitative assay and samples were interpreted as positive or negative with a threshold index value of 1.4. Spike (S) protein antibodies (anti-S IgG) were detected using the Abbott Architect SARS-CoV-2 IgG Quant II CMIA. Anti-S antibody titres are quantitative with a threshold value for positivity of 7.1 binding antibody units (BAU)/mL.
T-cell ELISpot
SARS-CoV-2-specific T-cell responses were detected using the T-SPOT Discovery SARS-CoV-2 (Oxford Immunotec) according to the manufacturer’s instructions. In brief, peripheral blood mononuclear cells (PBMCs) were isolated from whole blood samples with the addition of T-Cell Select (Oxford Immunotec) where indicated. A total of 250 000 PBMCs were plated into individual wells of a T-SPOT Discovery SARS-CoV-2 plate. The assay measures immune responses to five different SARS-CoV-2 structural peptide pools: S1 protein, S2 protein, NP protein, M protein (membrane), a mixed panel and positive (phytohaemagglutinin) and negative controls. Cells were incubated and interferon-γ secreting T cells were detected. Spot-forming units (SFUs) were detected using an automated plate reader (Autoimmun Diagnostika). Infection-naïve, unvaccinated participants were used to identify a threshold for a positive response using mean+3 SD SFU/106 PBMC for S peptide pools. This resulted in a cut-off for positivity of 40 SFU/106 PBMC for S protein responses.15
Statistical analysis
Statistical analysis was conducted using Prism V.9.0 (GraphPad Software, San Diego, California, USA). Unless otherwise stated, all data are reported as median with IQR. Where appropriate, Mann-Whitney U and Kruskal-Wallis tests were used to assess the difference between 2 or >2 groups, with Dunn’s post hoc test to compare individual groups. For paired analysis, Wilcoxon test was used. Multivariate analysis was carried out using multiple logistic regression using variables which were found to be significant on univariate analysis.
Patient involvement
The initial study proposal was supported and funded by the West London Kidney Patient Association. Patients were not directly involved in the experimental design or in performing the study.
Results
Sample collection and baseline data
A total of 140 IS patients provided samples at baseline and at 28-40 days after first vaccine dose; 103 patients provided a further sample 18–29 days after second-dose vaccine (administered at a median of 32 and 30 days after first dose for ChAdOx1 and BNT162b2, respectively). Clinical characteristics and immunosuppressive treatments are summarised in online supplemental table S1. One hundred and fourteen patients (81.4%) previously received rituximab, of whom 56.1% (64/114) were treated within the last 6 months, and 60.5% (69/114) were B-cell deplete (circulating CD19 <10 cells/µL) at the time of vaccination. All 69 patients who were B-cell deplete had received treatment with rituximab, 69.6% (48/69) within the last 6 months. Nineteen patients (13.6%) had evidence of previous SARS-CoV-2 infection on baseline testing—in keeping with the low prevalence of disease previously described in our cohort16—and these were analysed separately from those who were infection-naive. Two further patients developed anti-NP IgG after vaccination, indicating SARS-CoV-2 infection at or since vaccination and were excluded from analysis.
annrheumdis-2021-220626supp001.pdf (71KB, pdf)
Immunological response to first-dose vaccine in infection-naïve patients
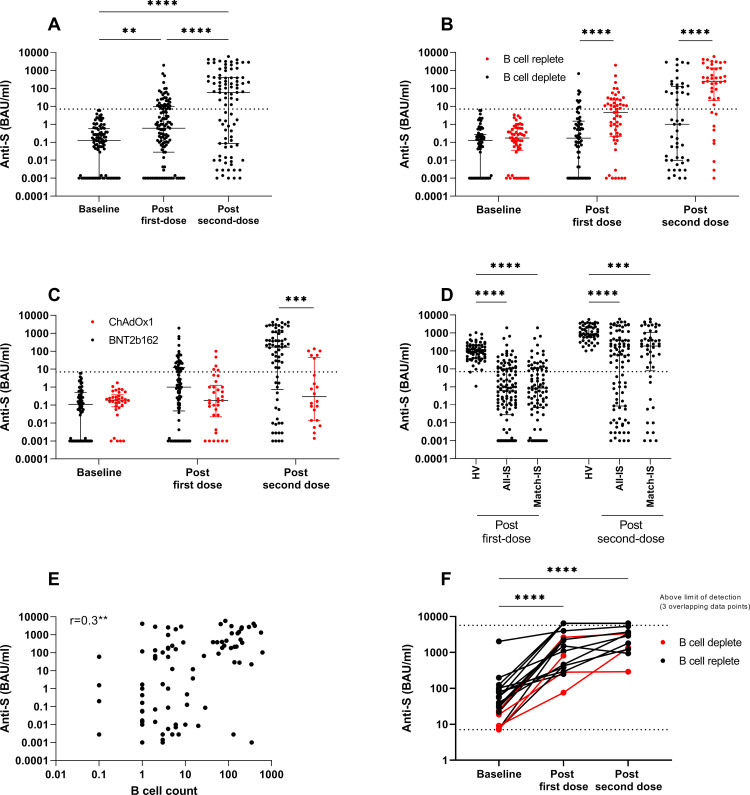
One hundred and nineteen infection-naïve patients were included in the analysis of response to first-dose vaccine. At 28–40 days, 28.6% (34/119) had detectable anti-S IgG (figure 1A; median 0.61 BAU/mL (IQR 0.03–9.8)). By univariate analysis, ChAdOx1 vaccine, prior cyclophosphamide treatment, prior rituximab treatment, and current B-cell depletion were all associated with a decreased likelihood of seroconversion (figure 1B, C). In the group of patients who had received rituximab, treatment within the last 6 months was associated with decreased rates of seroconversion (table 1), and the median anti-S titre was significantly lower in this group (0.12 and 1.1 BAU/mL in those treated <6 and >6 months, respectively, p=0.01). By multivariate analysis, B-cell depletion at the time of vaccination was associated with non-seroconversion (figure 1B; OR 0.3, p=0.03).
Figure 1.
Humoral responses to SARS-CoV-2 vaccination in IS patients. (A) Anti-S titre at baseline, following first-dose and second-dose vaccine in patients who were infection-naïve. (B) Anti-S titre by B-cell status at the time of vaccination in infection-naïve patients at baseline, 28–40 days following first-dose vaccine and 18–29 days after second-dose vaccine. (C) Anti-S titre by vaccine type at the time of vaccination in infection-naïve patients at baseline, 28–40 days following first-dose vaccine and 18–29 days after second-dose vaccine. (D) Anti-S titre following first-dose and second-dose vaccinations in healthy volunteers (HVs), IS patients and a matched cohort of IS patients. (E) Correlation of anti-S titre after second-dose vaccination and B-cell count at the time of vaccination in IS patients. (F) Anti-S titre in patients with previous natural infection at baseline, following first-dose and second-dose vaccines. Dotted line indicates 7.1 BAU/mL, the threshold for detectable anti-S antibodies. For visualisation of data on a log scale, values=0 are represented by 0.001, which is below the lower limit of the assay (0.00142). HV, healthy volunteer; IS, immunosuppressed; S, spike. **p<0.01, ***p<0.001, ****p<0.0001.
Table 1.
Patient characteristics by serological status in those with no evidence of previous natural infection
| Characteristics | n | First dose (n=119) | n | Second dose (n=91) | |||||
| Non-seroconversion | Seroconversion | P value | Non-seroconversion | Seroconversion | P value | ||||
| n=85 (71.4%) | n=34 (28.6%) | n=37 (40.7%) | n=54 (59.3%) | ||||||
| Gender | Male | 62 | 44 (71.0) | 18 (29.0) | 49 | 20 (40.8) | 29 (59.2) | ||
| Female | 57 | 41 (71.9) | 16 (28.1) | 42 | 17 (40.5) | 25 (59.5) | |||
| Age | Years (IQR) | 52.0 (39.9–63.9) | 56.2 (36.1–60.7) | 60.5 (43.8–69.5) | 51.8 (37.3–60.2) | 0.05 | |||
| Ethnicity | White | 62 | 44 (71.0) | 18 (29.0) | 50 | 22 (44.0) | 28 (56.0) | ||
| Black | 10 | 7 (70.0) | 3 (30.0) | 7 | 1 (14.8) | 6 (85.2) | |||
| South Asian | 34 | 24 (70.6)) | 10 (19.6) | 27 | 12 (44.4) | 15 (55.6) | |||
| Mixed-race | 7 | 6 (85.7) | 1 (14.3) | 3 | 1 (33.3) | 2 (66.7) | |||
| Other | 6 | 4 (66.7) | 2 (33.3) | 4 | 1 (25.0) | 3 (75.0) | |||
| Diagnosis | AAV and anti-GBM disease | 45 | 35 (77.8) | 10 (22.2) | 34 | 17 (50.0) | 17 (50.0) | ||
| Podocytopathy* | 28 | 18 (64.3) | 10 (35.7) | 25 | 12 (48.0) | 13 (52.0) | |||
| Membranous GN | 23 | 15 (65.2) | 8 (34.8) | 21 | 6 (28.6) | 15 (71.4) | |||
| SLE | 19 | 14 (73.7) | 5 (26.3) | 8 | 1 (12.5) | 7 (87.5) | |||
| Other† | 4 | 3 (75.0) | 1 (25.0) | 3 | 1 (33.3) | 2 (66.7) | |||
| Comorbidities | Diabetes | 19 | 17 (89.5) | 2 (10.5) | 16 | 9 (56.3) | 7 (43.7) | ||
| Asthma/COPD | 25 | 17 (68.0) | 8 (32.0) | 14 | 4 (28.6) | 10 (71.4) | |||
| Previous malignancy | 6 | 4 (66.7) | 2 (33.3) | 4 | 3 (75.0) | 1 (25.0) | |||
| Immunotherapy | Previous rituximab | 99 | 77 (77.8) | 22 (22.2) | 0.002 | 75 | 35 (46.7) | 40 (53.3) | 0.01 |
| Last 6 months | 56 | 49 (87.5) | 7 (12.5) | 0.016 | 44 | 26 (59.1) | 18 (40.9) | 0.0007 | |
| Tacrolimus | 23 | 17 (73.9) | 6 (26.0) | 21 | 7 (33.3) | 14 (67.7) | |||
| Azathioprine | 13 | 6 (46.1) | 7 (53.9) | 8 | 3 (37.5) | 5 (62.5) | |||
| MMF | 7 | 5 (71.4) | 2 (29.6) | 13 | 6 (46.2) | 7 (53.8) | |||
| Methotrexate | 3 | 2 (66.7) | 1 (33.3) | 2 | 0 | 2 (100) | |||
| Prednisolone | 52 | 40 (76.9) | 12 (23.1) | 36 | 14 (38.9) | 22 (61.1) | |||
| ≥10 mg | 19 | 13 (68.4) | 6 (31.6) | 11 | 5 (45.5) | 6 54.5) | |||
| Belimumab | 4 | 3 (75.0) | 1 (25.0) | 1 | 0 | 1 (100) | |||
| No current IS | 4 | 1 (25.0) | 3 (75.0) | 4 | 1 (25.0) | 3 (75.0) | |||
| Previous CYP | 58 | 47 (81.0) | 11 (19.0) | 0.03 | 41 | 18 (43.9) | 23 66.1) | ||
| Vaccine | AZ/ChAdOx1 | 34 | 29 (85.3) | 5 (14.7) | 0.04 | 22 | 16 (72.3) | 6 (37.7) | 0.0009 |
| Pfizer/ BNT162b2 | 85 | 56 (65.9) | 29 (34.1) | 69 | 21 (30.4) | 48 (69.6) | |||
| Clinical parameter | B-cell depletion | 64 | 54 (63.5) | 10 (29.4) | 0.001 | 49 | 28 (57.1) | 21 (42.9) | 0.0006 |
| Hypogammaglobulinaemia | 25 | 19 (76.0) | 6 (34.0) | 22 | 12 (54.5) | 10 (45.5) | |||
Comparison between groups by χ2 test.
*Podocytopathy included minimal change disease and focal segmental glomerulosclerosis.
†Other diagnoses included C3 glomerulopathy and IgG4-related disease.
AAV, ANCA-associated vasculitis; COPD, chronic obstructive pulmonary disease; CYP, cyclophosphamide; GBM, glomerular basement membrane; IS, immunosuppressed; MMF, mycophenolate mofetil; SLE, systemic lupus erythematosus.
The rate and magnitude of serological responses in the IS group were significantly lower than those in an HV group (online supplemental table S2) at a similar time point after first-dose vaccine (figure 1D; 97.1% (68/70) seroconversion in the HV group, median anti-S titre 90 BAU/mL (IQR 40.7–199.8), p<0.0001 compared with IS cohort). In the IS cohort, we did not identify any correlation between serological response to first-dose vaccine and age, although we and others have reported this in healthy individuals.15 17 The group of HVs included in this study is significantly younger than the IS group (online supplemental table S2; median age 41.4 and 53.7 years for HV and IS groups, respectively; p<0.0001). However, when an age-matched cohort of IS patients (median age 46.2 years) is used for comparison, serological responses were not significantly different from the whole IS cohort and remained lower than those in HV (figure 1D; median 0.85 BAU/mL (IQR 0.07–10.9), p<0.0001 compared with HV). This suggests that the overall younger age of our HV cohort does not fully account for the significant difference in serological response.
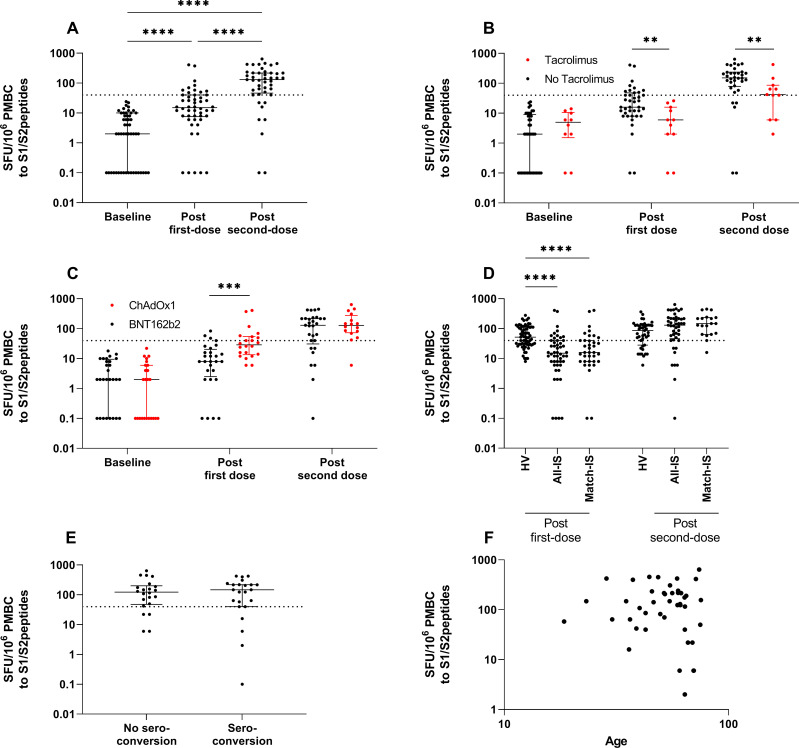
T-cell responses were assessed in 50/119 infection-naïve patients following first-dose vaccine. Only 26.0% (13/50) had detectable T-cell responses (>40 SFU/106 PBMC) (figure 2A and table 2). Patients receiving tacrolimus were less likely to have T-cell responses above the threshold for positivity: 0% (0/13) and 29.7% (11/37) of patients in T-cell responder and non-responder groups, respectively, were receiving tacrolimus (p=0.05) (figure 2B; median 6 and 16 SFU/106 PBMC in those receiving tacrolimus vs those who were not, p=0.003). Patients receiving ChAdOx1 were more likely to mount T-cell responses following first-dose vaccine: 69.2% (9/13) and 35.1% (13/37) of T-cell responders and non-responders, respectively, received ChAdOx1 vaccine (p=0.05) (figure 2C; median SFU/106 PBMC 8 and 29 for BNT162b2 and ChAdOx1, p=0.0007). Similar to serological responses after first-dose vaccine, T-cell responses were poorer in the IS group compared with HV (figure 2D; 61.1% (41/67) of HV had detectable responses, median 15 and 52 SFU/106 PBMC for IS and HV, respectively; p<0.0001).
Figure 2.
Cellular responses to SARS-CoV-2 vaccination in IS patients. (A) T-cell responses to spike protein peptides of SARS-CoV-2 in infection-naïve patients at baseline, 28–40 days following first-dose vaccine and 18–29 days after second-dose vaccine. (B) T-cell responses in those receiving tacrolimus therapy versus those who were not in infection-naïve participants at baseline, after first-dose vaccine and after second-dose vaccine. (C) T-cell responses by vaccine type in infection-naïve participants at baseline, after first-dose vaccine and after second-dose vaccine. (D) T-cell responses following first-dose and second-dose vaccinations in healthy volunteers (HVs), IS patients and a matched cohort of IS patients. (E) T-cell responses following second-dose vaccine in those who did and did not also seroconvert. (F) Correlation of T-cell responses after second-dose vaccination and age at time of vaccination. Dotted line indicates mean plus 3 SDs for spike peptide pool reactivity calculated from infection-naïve, non-vaccinated individuals (40 SFU/106 PBMC). For visualisation of data on a log scale, values=0 are represented by 0.1. HV, healthy volunteer; IS, immunosuppressed; PMBC, peripheral blood mononuclear cell; SFU, spot-forming unit.
Table 2.
Patient characteristics by T-cell responses in those with no evidence of previous natural infection
| Characteristics | First dose (n=50) | Second dose (n=46) | |||||||
| n | No T-cell response | T-cell response | P value | n | No T-cell response | T-cell response | P value | ||
| n=37 (74.0%) | n=13 (26.0%) | n=8 (17.4%) | n=38 (82.6%) | ||||||
| Gender | Male | 31 | 24 (77.4) | 7 (22.5) | 27 | 3 (11.1) | 24 (88.9) | ||
| Female | 19 | 13 (68.4) | 6 (31.6) | 19 | 5 (26.3) | 14 (73.7) | |||
| Age | Years (IQR) | 54.9 (42.7–63.9) | 49.4 (39.8–62.8) | 65.1 (61.4–70.0) | 51.9 (42.1–75.3) | 0.02 | |||
| Ethnicity | White | 28 | 20 (71.4) | 8 (28.6) | 24 | 4 (16.7) | 20 (83.3) | ||
| Black | 1 | 1 (100) | 0 | 2 | 0 | 2 (100) | |||
| South Asian | 19 | 15 (78.9) | 4 (21.1) | 18 | 4 (22.2) | 14 (77.8) | |||
| Mixed-race | 2 | 1 (50.0) | 1 (50.0) | 1 | 0 | 1 (100) | |||
| Other | 0 | 0 | 0 | 1 | 0 | 1 (100) | |||
| Diagnosis | AAV and anti-GBM disease | 24 | 14 (58.3) | 10 (41.7) | 19 | 4 (21.1) | 15 (78.9) | ||
| Podocytopathy* | 15 | 13 (86.7) | 2 (13.3) | 15 | 2 (13.3) | 13 (86.7) | |||
| Membranous GN | 10 | 9 (90.0) | 1 (10.0) | 9 | 2 (22.2) | 7 (77.8) | |||
| SLE | 0 | 0 | 0 | 2 | 0 | 2 (100) | |||
| Other† | 1 | 1 (100) | 0 | 1 | 0 | 1 (100) | |||
| Comorbidities | Diabetes | 10 | 7 (70.0) | 3 (30.0) | 8 | 3 (37.5) | 5 (62.5) | ||
| Asthma/COPD | 10 | 7 (70.0) | 3 (30.0) | 11 | 2 (18.2) | 9 (81.8) | |||
| Previous malignancy | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Immunotherapy | Rituximab | 44 | 31 (70.5) | 13 (29.5) | 41 | 7 (17.1) | 34 (82.9) | ||
| Last 6 months | 32 | 21 (65.6) | 11 (34.4) | 28 | 4 (14.3) | 24 (85.7) | |||
| Tacrolimus | 11 | 11 (100) | 0 | 0.04 | 12 | 3 (25.0) | 9 (75.0) | ||
| Azathioprine | 4 | 2 (50.0) | 2 (50.0) | 3 | 0 | 3 (100) | |||
| MMF | 5 | 3 (60.0) | 2 (40.0) | 4 | 1 (25.0) | 3 (75.0) | |||
| Methotrexate | 0 | 0 | 0 | 1 | 1 (100) | 0 | |||
| Prednisolone | 17 | 14 (82.3) | 3 (17.6) | 14 | 2 (14.3) | 12 (85.7) | |||
| ≥10 mg | 5 | 4 (80.0) | 1 (20.0) | 3 | 1 (33.3) | 2 (66.7) | |||
| Belimumab | 0 | 0 | 0 | 0 | 0 | 0 | |||
| No IS | 1 | 1 (100) | 0 | 1 | 0 | 1 (100) | |||
| Previous CYP | 25 | 15 (60.0) | 10 (40.0) | 20 | 3 (15.0) | 17 (85.0) | |||
| Vaccine | AZ/ChAdOx1 | 22 | 13 (59.1) | 9 (40.9) | 0.05 | 17 | 1 (5.9) | 16 (94.1) | |
| Pfizer/ BNT162b2 | 28 | 24 (85.7) | 4 (14.3) | 29 | 7 (24.1) | 22 (75.9) | |||
| Clinical parameter | B-cell depletion | 33 | 22 (66.7) | 11 (33.3) | 30 | 5 (16.7) | 25 (83.3) | ||
| Hypogammaglobulinaemia | 13 | 10 (76.9) | 3 (13.1) | 9 | 3 (33.3) | 6 (66.7) | |||
*Podocytopathy included minimal change disease and focal segmental glomerulosclerosis.
†Other diagnoses included C3 glomerulopathy and IgG4-related disease. Comparison between groups by χ2 test.
AAV, ANCA-associated vasculitis; COPD, chronic obstructive pulmonary disease; CYP, cyclophosphamide; GBM, glomerular basement membrane; GN, glomerulonephritis; IS, immunosuppression; MMF, mycophenolate mofetil; SLE, systemic lupus erythematosus.
In patients for whom both serological and T-cell assessments were available, 64.0% (32/50) did not have a demonstrable response to first-dose vaccine by either measure (online supplemental table S3).
Immunological response to second-dose vaccine in infection-naïve patients
Ninety-one patients were included in the analysis of response to second-dose vaccine. At 18–29 days after second-dose vaccine, the proportion of patients with detectable anti-S IgG increased to 59.4% (54/91, figure 1A). In contrast, all HV individuals had detectable anti-S IgG after second-dose vaccine. The median anti-S titre after second-dose vaccine was significantly lower in IS patients than in HV, whether analysed as the whole cohort, or as an age-matched and vaccine-matched subgroup (figure 1D; median 58.7 (IQR 0.8–437.2), median 189.3 (IQR 7.9–1090) and median 877 (IQR 575–2203) BAU/mL for IS total cohort, IS matched grou and HV, respectively; p<0.0001).
Within the IS group, in those who had already seroconverted following first-dose vaccine, anti-S titres increased significantly in all patients. In those who were seronegative after first dose, a further 42.4% (28/66) now had detectable anti-S IgG. In keeping with our findings after first-dose vaccine, ChAdOx1 vaccine, prior rituximab treatment and current B-cell depletion were associated with a decreased likelihood of seroconversion, as was increasing age (figure 1B, C, and table 1). There was moderate correlation between serological response to second-dose vaccine and peripheral B-cell count at the time of vaccination (figure 1E). In the group of patients treated with rituximab, administration within the last 6 months was significantly associated with failure to seroconvert; 40.9% (18/44) vs 71.0% (22/31) seroconversion in those treated <6 months and >6 months previously, respectively (p=0.02). By multivariate analysis, B-cell depletion at the time of vaccination (OR 0.32, p=0.04) was significantly associated with non-seroconversion.
T-cell responses were assessed in 46/91 patients following second-dose vaccine and were detected in 82.6% (38/46, figure 2A). There were no differences in the rate or magnitude of T-cell response between those who seroconverted (81.2% (18/22), median SFU/106 PBMC 123) and those who did not (83.3% (20/24), median SFU/106 PBMC 148) (figure 2E and table 2). The number of patients without detectable T-cell responses following second-dose was small (n=8), and age was the only parameter significantly associated with absence of T-cell response to vaccination, although there was no correlation between age and magnitude of response (figure 2F and table 2; median age 51.9 and 61.5 years for those with T-cell responses above and below threshold, respectively; p=0.05). Although there was no significant difference in the proportion of patients with T-cell responses above threshold, the magnitude of response was significantly lower in patients treated with tacrolimus (figure 2B; median 53 and 152 SFU/106 PBMC for those treated with tacrolimus and not, p=0.01).
In infection-naïve patients for whom both serological and T-cell assessments were available, 47.8% (22/46) had negative serological responses after second-dose vaccine. Of these patients, 81.8% (18/22) had detectable T-cell responses. In patients who were B-cell deplete, an assessment of both serological and T-cell assessments were available in 30 patients, 60.0% (18/30) of whom had negative serological responses. In this B-cell deplete group with no serological response to vaccine, 83.3% (15/18) had detectable T-cell responses.
Comparing the HV and IS group, there was no significant difference in the proportion with T-cell responses to second-dose vaccine (figure 1D, 74.4% (32/43) of HV had T-cell responses above threshold) or in the magnitude of response (median 130 and 86 SFU/106 PBMC for IS and HV, respectively; p=not significant (ns)). Since second-dose vaccine samples in HV were limited to individuals who received BNT162b2; an analysis of age-matched and vaccine-matched IS patients was performed, and there were no significant differences in response (median 140 and 86 SFU/106 PBMC for matched IS and HV, respectively, p=ns; the numerical differences in T-cell number between these groups were not statistically significant and may reflect a degree of T cell enrichment in PMBC preparations from B-cell deplete IS patients).
In infection-naïve patients for whom both serological and T-cell assessments were available, the response rate (by one or both immunological parameters) increased significantly following each dose (36.0% (18/50) and 91.3% (42/46), respectively; p<0.0001). The four patients with no immunological response after second-dose were significantly older than those with a response by either measure; all four had received rituximab previously, although one was no longer B-cell deplete (online supplemental table S3).
Immunological response to vaccination in patients with prior natural infection
In keeping with our previous report in healthy individuals,15 the 19 participants with evidence of prior SARS-CoV-2 infection mounted robust serological responses to first-dose vaccination, including those who had previously received rituximab (n=13/19) or who were B-cell deplete (n=4/19, figure 1F). In 12 patients, serology was available following second-dose vaccine. Anti-S titre increased further following second-dose vaccine (‘third’ S protein challenge) in 8/12, remained above the limit of detection in 2/12, and declined or plateaued in only 2/12 (figure 1F). Due to the number of patients with responses above the threshold of detection of the assay, it was not possible to compare median anti-S titres following first-dose and second-dose vaccine in this group. T-cell responses were available for three patients in this cohort; all mounted robust cellular immunity to both first-dose and second-dose vaccines (60–616 and 300–580 SFU/106 PBMC after first and second doses, respectively).
Discussion
The immune response to first-dose BNT162b2 mRNA or ChAdOx1 nCoV-19 vaccine was poor in patients receiving immunosuppression, with only 28.6% of patients having detectable humoral or T-cell responses. These rates compare poorly to a cohort of non-IS HVs. Reassuringly, immune responses were augmented by second-dose vaccine, increasing the seroconversion and T-cell response rates to 59.4% and 82.6%, respectively. Only 8.7% of patients had neither antibody nor T-cell responses following second-dose vaccine. These findings indicate that both vaccines are immunogenic in patients receiving immunosuppression, but that protocolised two-dose vaccination schedules are required. The augmented response to second-dose vaccine (and ‘third’ challenge in patients with prior natural infection) suggests that repeat boost strategies could be considered in this patient group, to induce more robust immune responses in the future.
B-cell depletion (following prior rituximab treatment) at the time of vaccination was the strongest predictor of failure to seroconvert, in keeping with data on impaired humoral responses to other vaccines in patients treated with rituximab. These studies found that time since rituximab treatment was a determinant of serological response,13 14 consistent with our finding of lower response rates in those who were currently B-cell deplete versus those who had repopulated peripheral B cells. Current guidelines differ regarding the timing of SARS-CoV-2 vaccination after rituximab.18–20 While our data suggest that better serological responses may be achieved by delaying vaccination until B-cell reconstitution has occurred, it may not be ethical to do so when community transmission rates are high (or to defer rituximab treatment when needed for disease control). We therefore suggest that additional courses of vaccination should be made available to these patients between or after completed rituximab cycles.
While current vaccine efforts have focused on the induction of neutralising antibodies to SARS-CoV-2, T-cell immunity may also provide protection from infection. Experimental data suggest that CD8+ T-cell responses in particular may have a protective role in the presence of waning or subprotective antibody titres.21 In addition, patients with agammaglobulinaemia have been described to recover from COVID-19 in the absence of a serological response, suggesting T-cell responses may be sufficient to mount protection or aid recovery from disease.22–24 It is reassuring that vaccine-induced T-cell responses were detected in most of our study cohort, including those who were B-cell deplete at the time of vaccination, and those who failed to seroconvert. Tacrolimus use was associated with impaired T-cell response, and further studies are needed to investigate the impact of calcineurin inhibitors and other T cell-directed therapies on vaccine response in more detail.
The immune correlates of protection from disease, however, are not clearly defined. Published trials have not reported antibody measurements of participants who contracted COVID-19 following vaccination, and in vitro assessments of antibody neutralising activity have not been correlated with clinical outcomes. Robust CD8 and CD4 T-cell responses to BNT162b2/ChAdOx1 were reported in early-phase clinical studies,25 26 although all participants also mounted neutralising antibody responses. Thus, further work is needed to determine whether the serological or T-cell response observed in our cohort will confer protection from clinical disease and whether the longevity of the immune response in this group is comparable to that in healthy individuals.
A limitation of our study is that only a small proportion of patients were treated with conventional synthetic disease-modifying antirheumatic drugs such as methotrexate or MMF, and some conditions such as systemic lupus erythematosus are under-represented. While we observed possible differences between vaccine types (with stronger serological responses in patients receiving BNT2b162 and better T-cell responses in those receiving ChAdOx1), our study is underpowered to determine if vaccine choice should be influenced by underlying disease or immunosuppressive treatment. Further studies in larger cohorts will be required to understand the impact of these factors and whether there are preferred vaccine types in these high-risk patient groups. In addition, the HV group in our study is not ideally matched to the IS cohort; individuals are younger, and an assessment of second-dose response was only available in participants receiving BNT162b2. The HV group also received second-dose vaccination after a longer time period than the IS cohort (67 and 30 days, respectively). We have undertaken limited matching based on age and vaccine type, but sufficiently detailed data for the HV cohort is not available to provide a more accurate comparator group.
Despite these limitations, our data confirm the immunogenicity of SARS-CoV-2 vaccination in an IS cohort, finding that B-cell depletion following rituximab impairs serological responses, but T-cell responses are preserved in this group. Reassuringly, our data confirm an immunological response in most patients, when assessed by a combination of serological and cell-based assays. Our findings support SARS-CoV-2 vaccination in this patient group; however, since the overall quality of response was impaired compared with healthy individuals, we suggest that repeat vaccine doses may be necessary to optimise the immunological response and to induce more robust serological responses in particular, for these vulnerable patients.
Acknowledgments
We thank the patients who participated in this study; Dr Genevieve Small (NWLCCG lead); Pippa Nightingale (NWL secondary care lead); Dr Naomi Katz; and Anne-Marie McCooey, Ian Bateman, Shona Maxwell and Sarah Kelsall, who facilitated access to the vaccine; and Graham Pickard and Donald Mokreri for assistance with the spike protein antibody assay and research nurses at Royal Brompton and Harefield NHS Trust for assisting with patient recruitment. MW acknowledges support from Sidharth and Indira Burman and the Nan Diamond Fund. CC is supported by an Auchi Fellowship. MP is supported by a National Institute for Health Research (NIHR) Clinical Lectureship. PK acknowledges support from the Wellcome Trust and North West London Pathology Trust. We acknowledge support from the Imperial Healthcare Charity Auchi Fund and the NIHR Imperial Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Footnotes
Handling editor: Josef S Smolen
Twitter: @kidneydoc101
MP and CC contributed equally.
Collaborators: Imperial Renal Vaccine Group: staff within the Imperial College Renal and Transplant Centre who helped with the vaccination of the participants in this study: Rute Cardoso De Aguiar, Amrita Dhutia, Tabitha Turner-Stokes, Katie Tansey, Caroline Clerkin, Fabiana Costa, Rafael Santiago, Dejin Yang, David Thomas, Tom Cairns, Megan Griffith, Jeremy Levy, James Tomlinson, Marie Condon, Frederick Tam, Nicholas Medjeral-Thomas, Marina Loucaidou, Frank Dor, Anand Muthasamy and Rishana Shuaib.
Contributors: All authors contributed to the intellectual content of the submission and preparation of the manuscript, and all have approved the final version for submission.
Funding: PK and MW have received support to use the T-SPOT Discovery SARS-CoV-2 by Oxford Immunotec.
Competing interests: PK and MW received support to use the T-SPOT Discovery SARS-CoV-2 by Oxford Immunotec.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study for these participants was approved by the NHS Health Research Authority, research ethics committee (reference 20/WA/0123 and reference 20/SC/0208).
References
- 1.Arnold J, Winthrop K, Emery P. COVID-19 vaccination and antirheumatic therapy. Rheumatology 2021. 10.1093/rheumatology/keab223. [Epub ahead of print: 12 Mar 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kronbichler A, Anders H-J, Fernandez-Juárez GM, et al. Recommendations for the use of COVID-19 vaccines in patients with immune-mediated kidney diseases. Nephrol Dial Transplant 2021;36:1160–8. 10.1093/ndt/gfab064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UK Department of Health and Social Care; Public Health England . Guidance on shielding and protecting people who are clinically extremely vulnerable from COVID-19, 2021. Available: https://wwwgovuk/government/publications/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19
- 4.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waldman M, Soler MJ, García-Carro C, et al. Results from the IRoc-GN international registry of patients with COVID-19 and glomerular disease suggest close monitoring. Kidney Int 2021;99:227–37. 10.1016/j.kint.2020.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutherford MA, Scott J, Karabayas M, et al. Risk factors for severe outcomes in patients with systemic vasculitis & COVID-19: a bi-national registry-based cohort study. Arthritis Rheumatol 2021. 10.1002/art.41728. [Epub ahead of print: 22 Mar 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voysey M, Costa Clemens SA, Madhi SA, et al. Single-Dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 2021;397:881–91. 10.1016/S0140-6736(21)00432-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–16. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an RAD26 and RAD5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021;397:671–81. 10.1016/S0140-6736(21)00234-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oren S, Mandelboim M, Braun-Moscovici Y, et al. Vaccination against influenza in patients with rheumatoid arthritis: the effect of rituximab on the humoral response. Ann Rheum Dis 2008;67:937–41. 10.1136/ard.2007.077461 [DOI] [PubMed] [Google Scholar]
- 12.Hua C, Barnetche T, Combe B, et al. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res 2014;66:1016–26. 10.1002/acr.22246 [DOI] [PubMed] [Google Scholar]
- 13.Arad U, Tzadok S, Amir S, et al. The cellular immune response to influenza vaccination is preserved in rheumatoid arthritis patients treated with rituximab. Vaccine 2011;29:1643–8. 10.1016/j.vaccine.2010.12.072 [DOI] [PubMed] [Google Scholar]
- 14.van Assen S, Holvast A, Benne CA, et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum 2010;62:75–81. 10.1002/art.25033 [DOI] [PubMed] [Google Scholar]
- 15.Prendecki M, Clarke C, Brown J, et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet 2021;397:1178–81. 10.1016/S0140-6736(21)00502-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner-Stokes T, Jiang E, Johnson N, et al. Serologic screening for coronavirus disease 2019 in patients with glomerular disease. Kidney Int Rep 2021;6:1402–6. 10.1016/j.ekir.2021.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu Jabal K, Ben-Amram H, Beiruti K, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill 2021;26:2100096. 10.2807/1560-7917.ES.2021.26.6.2100096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtis JR, Johnson SR, Anthony DD, et al. American College of rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 1. Arthritis Rheumatol 2021;73:1093–107. 10.1002/art.41734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arthritis and Musculoskeletal Alliance . Principles for COVID-19 vaccination in musculoskeletal and rheumatology for clinicians (version 3), 2021. Available: http://armauknet/covid-19-vaccination-and-msk
- 20.Furer V, Rondaan C, Agmon-Levin N, et al. Point of view on the vaccination against COVID-19 in patients with autoimmune inflammatory rheumatic diseases. RMD Open 2021;7:e001594. 10.1136/rmdopen-2021-001594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021;590:630–4. 10.1038/s41586-020-03041-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinti I, Lougaris V, Milito C, et al. A possible role for B cells in COVID-19? lesson from patients with agammaglobulinemia. J Allergy Clin Immunol 2020;146:211–3. 10.1016/j.jaci.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soresina A, Moratto D, Chiarini M, et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr Allergy Immunol 2020;31:565–9. 10.1111/pai.13263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breathnach AS, Duncan CJA, Bouzidi KE, et al. Prior COVID-19 protects against reinfection, even in the absence of detectable antibodies. J Infect 2021. 10.1016/j.jinf.2021.05.024. [Epub ahead of print: 28 May 2021]. [DOI] [PubMed] [Google Scholar]
- 25.Sahin U, Muik A, Vogler I. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. medRxiv 2020:2020.12.09.20245175. [Google Scholar]
- 26.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020;396:467–78. 10.1016/S0140-6736(20)31604-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2021-220626supp001.pdf (71KB, pdf)
Data Availability Statement
Data are available upon reasonable request.