Abstract
Glycolipid transfer proteins (GLTPs) were first identified over three decades ago as ~24kDa, soluble, amphitropic proteins that specifically accelerate the intermembrane transfer of glycolipids. Upon discovery that GLTPs use a unique, all-α-helical, two-layer ‘sandwich’ architecture (GLTP-fold) to bind glycosphingolipids (GSLs), a new protein superfamily was born. Structure/function studies have provided exquisite insights defining features responsible for lipid headgroup selectivity and hydrophobic ‘pocket’ adaptability for accommodating hydrocarbon chains of differing length and unsaturation. In humans, evolutionarily-modified GLTP-folds have been identified with altered sphingolipid specificity, e. g. ceramide-1-phosphate transfer protein (CPTP), phosphatidylinositol 4-phosphate adaptor protein-2 (FAPP2) which harbors a GLTP-domain and GLTPD2. Despite the wealth of structural data (> 40 Protein Data Bank deposits), insights into the in vivo functional roles of GLTP superfamily members have emerged slowly. In this review, recent advances are presented and discussed implicating human GLTP superfamily members as important regulators of: i) pro-inflammatory eicosanoid production associated with Group-IV cytoplasmic phospholipase A2; ii) autophagy and inflammasome assembly that drive surveillance cell release of interleukin-1β and interleukin-18 inflammatory cytokines; iii) cell cycle arrest and necroptosis induction in certain colon cancer cell lines. The effects exerted by GLTP superfamily members appear linked to their ability to regulate sphingolipid homeostasis by acting in either transporter and/or sensor capacities. These timely findings are opening new avenues for future cross-disciplinary, translational medical research involving GLTP-fold proteins in human health and disease. Such avenues include targeted regulation of specific GLTP superfamily members to alter sphingolipid levels as a therapeutic means for combating viral infection, neurodegenerative conditions and circumventing chemo-resistance during cancer treatment.
Keywords: Glycolipid transfer protein superfamily, Sphingolipid homeostasis, Autophagy, Inflammasomes, Necroptosis, Eicosanoids, Cytokines, Sphingolipid rheostat, Phosphoglyceride regulatory binding
1. Introduction
Lipid intracellular transport to and from biomembranes occurs by both vesicular and nonvesicular mechanisms. Vesicular mechanisms provide bulk lipid transport that helps establish the compositions and relative amounts of lipids for various organelles including the asymmetric distributions of certain lipids within each half of the membrane bilayer. The asymmetric lipid distributions generally reflect the transmembrane orientations of lipid synthase catalytic sites as well as actions of various energy-dependent, transmembrane lipid transporters now being studied at the molecular level [1–9]. Nonvesicular mechanisms of lipid intermembrane trafficking rely on lipid transfer proteins (LTPs) that function as local curators and sensors of lipid composition and lipid transbilayer asymmetry by acquiring and delivering their specific lipid cargoes during transient interaction with specific membranes [10–17]. LTPs (and LTP domains of complex proteins) are amphitropic proteins that adhere only temporarily to the membrane and function as molecular solubilizers of lipid by enveloping their insoluble lipid cargo during the transfer process. The focus of this review will be on an LTP superfamily composed of family members with specificity for sphingolipids containing two aliphatic chains (i.e. sphingoid and fatty acyl) and either a sugar(s) or phosphate polar headgroup. Members of this sphingolipid transfer protein superfamily share a unique structural fold known as the GLTP-fold which was first discovered for human glycolipid transfer protein (GLTP) over a decade ago [18]. Since then, three new members have been added to the human GLTP superfamily based on their structural architectures which include the presence of an evolutionarily-modified GLTP-fold with altered sphingolipid specificity. As a result, the GLTP superfamily now consists of two distinct families based on their specific targeting of ceramides carrying initial headgroup residues of either carbohydrate (GSLs) or phosphate (ceramide-1-phosphate; C1P). Despite a relative wealth of structural data (> 40 Protein Data Bank deposits), insights into the in vivo functional roles of GLTP superfamily members have been sparse but recently have begun to increase. What is currently known will be the focus of this review.
2. Ceramide-1-phosphate transfer proteins (CPTPs)
2.1. Unique structure and function
Among the most recently characterized LTPs and newest members of the GLTP superfamily are CPTPs which burst onto the scientific landscape a few years ago [19–22]. Ceramide-1-phosphate (C1P) is a sphingolipid (SL) consisting of nonpolar ceramide connected to a polar phosphomonoester (anionic) headgroup (Fig. 1). C1P exerts many bioactive effects including induction of cell proliferation, stem cell mobilization, macrophage migration, activation of IVA phospholipase A2 (cPLA2) for eicosanoid production, and induction of autophagy-mediated, pro-inflammatory cytokine release [23–26]. Prior to the discovery of CPTP, insights into C1P intracellular transport were almost nonexistent [27]. The discovery of human CPTP was stimulated by in silico annotations to the Human Genome Database predicting the existence of the previously unknown gene initially designated at GLTPD1, but later renamed CPTP. PCR testing verified mRNA transcript in various tissues and cells [19]. Molecular cloning of the protein revealed significant homology with a plant orthologue identified a decade earlier as a sphingolipid transfer protein and regulator of the programmed cell death process known as accelerated cell death (ACD)-11 [28,29]. Comprehensive structure/function scrutinization of CPTP and ACD11 established a shared two-layer, all-α-helical protein fold for specific binding of C1P in a ‘sandwich-like’ fashion during transfer [19,20]. The protein fold used by CPTP and ACD11, i.e. GLTP-fold, had originally been discovered by X-ray diffraction studies of human glycolipid transfer protein (GLTP) after molecular cloning [18,30–33]. Subsequent structure determinations revealed the presence of GLTP-fold orthologs in the filamentous fungus, Podospora anserine [34] and in the thermophilic unicellular red alga, Galdieria sulphuraria [35,36]. Structure homology modeling also predicted GLTP-fold involvement in plant glycolipid transport [37] as well as in glucosylceramide (GlcCer) transport during ganglioside synthesis via human 4-phosphate adaptor protein-2 (FAPP2) [38–40], the latter prediction recently verified by X-ray diffraction studies [41]. Yet, in these GLTPs, evolutionary modification enabled binding and transfer of specific glycosphingolipids (GSLs) (Fig. 1) rather than C1P. The findings attest to the evolutionary premium for conservation of the GLTP-fold, which characterizes both human CPTP and GLTP despite being encoded on separate chromosomes by different human genes containing different exon/intron organizational patterns (Fig. 2) that code only 17% identical protein sequences.
Fig. 1. Sphingolipid diagrammatic formula and X-ray structure of CPTP containing bound C1P.
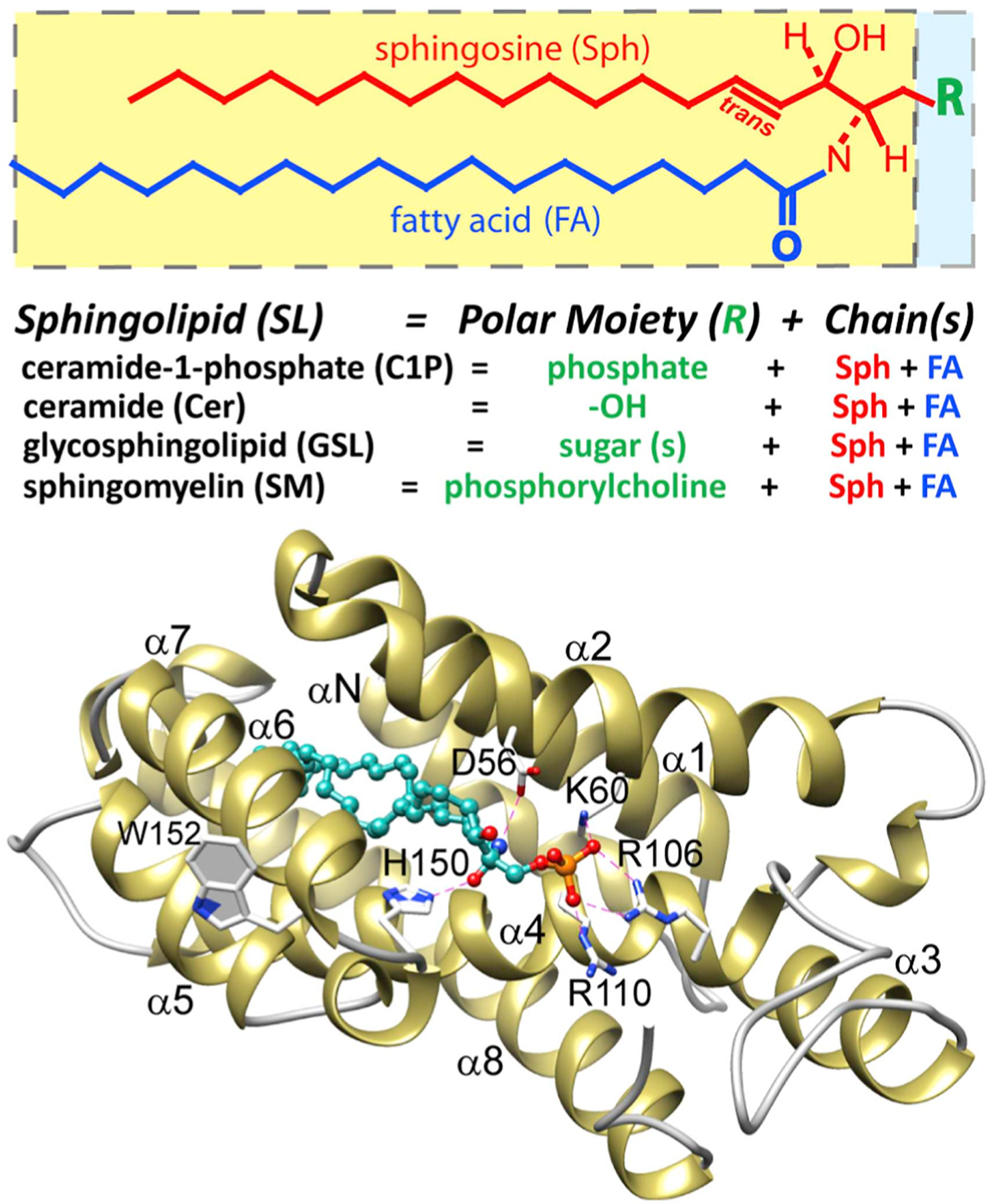
(Upper panel) Sphingolipids are composed of a sphingoid base (red), an amide-linked fatty acyl chain (blue), and polar headgroup (green); (Lower panel) Crystal structure of CPTP in complex with 16:0-C1P (PDB ID: 4K84). A cationic residue triad (K60, R106, R110) provides selectivity for the phosphate headgroup of C1P. D56 and H150 enable selectivity for the ceramide moiety by interacting with the amide linkage. The C1P hydrocarbon chains are ensheathed in a hydrophobic pocket accessed via a cleft-like gate that lies beneath the cationic residue triad that binds the phosphate headgroup.
Fig. 2. Organization of the GLTP domains encoded in human CPTP, PLEKHA8, GLTPD2, and GLTP genes.
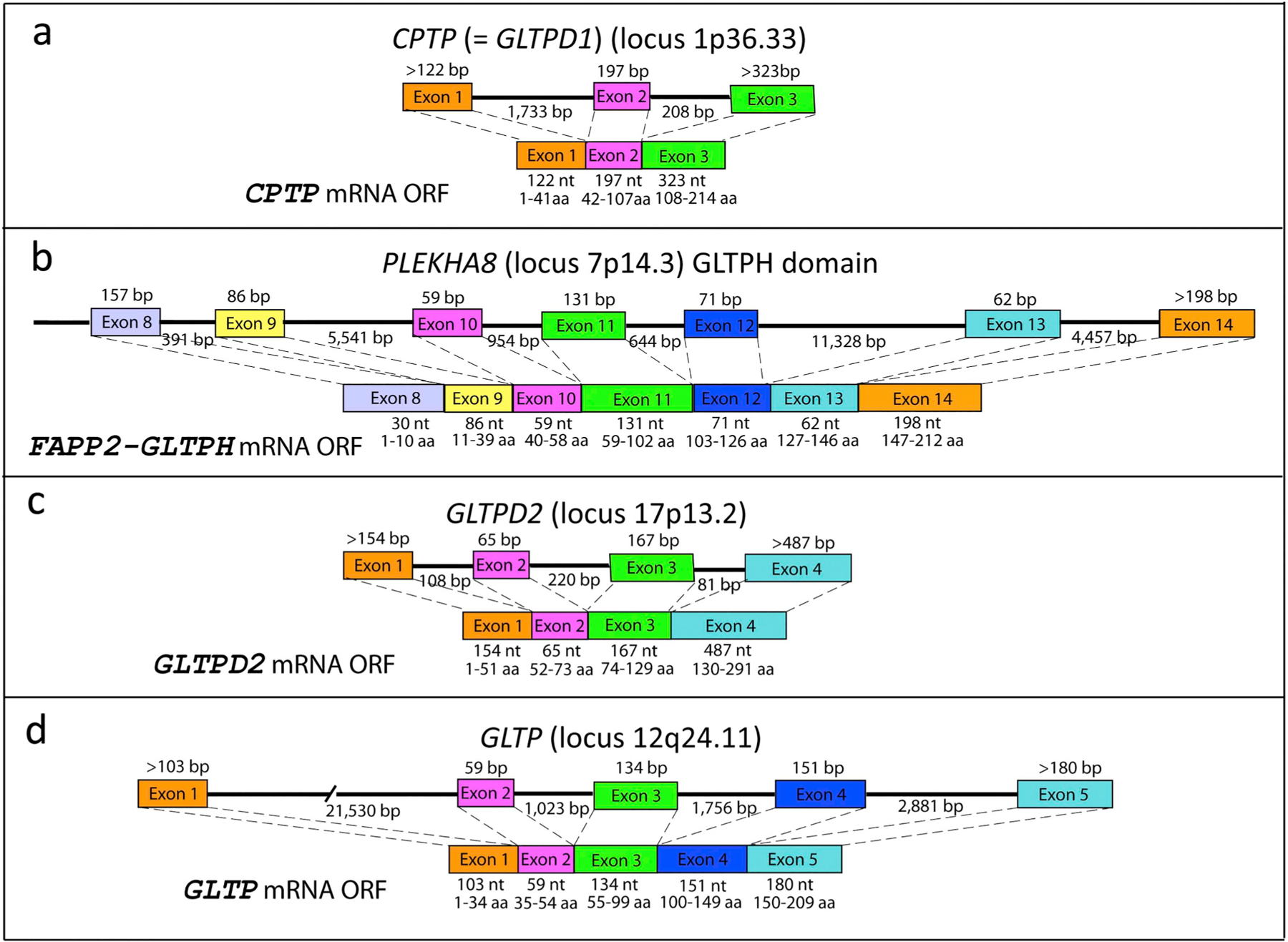
(a) CPTP consists of three exons and two introns and is located on chromosome 1 at locus1p36.33. (b) PLEKHA8 consists of 14 exons and 13 introns and is located at locus 7p14.3 of chromosome 7. The GLTPH domain is encoded by the final 7 exons. A PLEKHA8P1 pseudogene is located at locus 12q12 of chromosome 12 where it encodes a truncated GLTP sequence (141 residues). (c) GLTPD2 consists of four exons and three introns and is located at locus 17p13.2 of chromosome 17. The CPTP-like domain is encoded by the final two exons. (d) GLTP consists of five exons and four introns and is located on chromosome 12 at locus 12q24.11. A transcriptionally-silent GLTP pseudogene (GLTPP1) exists on chromosome 11 at locus 11p15.1. Despite encoding a highly homologous sequence (94%) containing all key amino acid residues involved in glycolipid binding, heterologous expression and purification showed that GLTPP1 is unable to transfer glycolipid. GLTPP1 is found in primates but not in other mammals or other eukaryotes [106]. Gene organizations were determined from on-line sequences provided by the Ensembl genome database.
In CPTP and ACD11, C1P specificity originates from a few key residue that comprise the SL headgroup recognition center (Fig. 1). These residues are chemically and spatially conserved to selectively anchor the C1P phospho-acyl-amide region rather than the GSL initial sugar-acyl-amide region recognized by GLTP [18–20]. Connected to the recognition center is a hydrophobic pocket that can ensheath the nonpolar SL aliphatic chains of the ceramide moiety of C1P. The hydrophobic pocket is: (i) accessed through a cleft beneath the SL headgroup recognition center, lined almost exclusively with highly nonpolar residue side-chains, and (ii) collapsed when unoccupied, but expands to accommodate the SL hydrocarbon chains in tight-fitting fashion. Within the hydrophobic pocket, Phe side-chains are often positioned to function as ‘gates’ and ‘baffles’ that swing open in adjustable fashion to accommodate the chain(s) in upper regions of the pocket. Certain Phe residues sometimes function to seal bottom regions of the pocket. Detailed descriptions of the structure/function features of CPTP and other GLTP superfamily members are available in recent reviews [21,22] as well as in a recent publication of the X-ray structure of the FAPP2 glycolipid transfer protein homology (GLTPH) domain complexed with monoglycosylceramide [41].
The lipid-phosphate binding site architectures used by CPTP and ACD11 differ substantially from the binding motifs for other known phosphate-modified biomolecules [42]. By virtue of using a cationic residue (arginine/lysine) triad for binding, the lipid phosphate recognition site undergoes minimal conformational change upon C1P binding and is rendered relatively pH-insensitive, thus differing distinctively from the ubiquitous GxGxxG Gly-rich loops and GxxxxGKS/T P-loops that bind phosphate in NADP/NAD and ATP/GTP binding proteins [42]. Also, few similarities exist between CPTP/ACD11 and the ‘venus flytrap’ fold that characterizes the phosphate-binding protein superfamily [43], where two globular domains are hinged together to form a central β-sheet core that needs large conformational changes to function as a phosphate-binding site [44]. The CPTP binding motif also clearly differs from other proteins known to bind lipids containing single-phosphate headgroups. For instance, sphingosine-1-phosphate (S1P) lyase utilizes a ‘phosphate cup’ containing Tyr, His, and Ser along with one Arg, to bind S1P during hydrolysis [45]. In the case of cPLA2α,the protein’s C2-domain, which is the primary driver for membrane interaction, interacts with the C1P phosphate headgroup via a β-groove binding site containing three basic residues (Arg59, Arg61, His62), while the ceramide chains tether the protein to the membrane. The specific interaction with C1P lowers cPLA2α dissociation from the membrane to stimulate phosphoglyceride hydrolysis by the cPLA2α catalytic domain that releases arachidonic acid needed for pro-inflammatory eicosanoid production [46,47]. Altogether, current evidence indicates that the CPTP GLTP-fold represents a new kind of lipid phosphate binding fold that defines a previously unknown branch of the GLTP superfamily.
A surprising aspect of CPTP and GLTP structure is the complete lack of structural homology with ceramide transfer protein (CERT) (Fig. 3), which functions in vivo as a transporter of ceramide (Cer) from the endoplasmic reticulum to the Golgi in conjunction with sphingomyelin production by sphingomyelin synthase [48,49]. CERT or steroidogenic acute regulatory (Star)D11 belongs to the (Star)D2 protein subfamily which also includes members that bind phosphatidylcholine (StarD2, StarD7) or PC and phosphatidylethanolamine (StarD10) [49–51]. Other members of the large StarD protein family bind cholesterol, oxysterols, bile acids, and steroid hormones [50]. CERT consists of an N-terminal pleckstrin homology (PH) domain for binding PI-4P in the Golgi, a middle coiled domain containing a FFAT motif (two phenylalanines (FF) in an acidic tract) for interacting with the ER and a C-terminal, ~240 residue, Star protein-related lipid transfer (START) domain for binding and transferring Cer [51]. Sulfate binding to the PH domain has been proposed to mimic conformational changes induced by phosphatidylinositol 4-phosphate (PI-4P) binding [52]. Moreover, studies of isolated PH and START domains show interaction with each other [53]. In CERT, the START domain binds to the PH domain at the same site as PI-4P, suggesting that the START domain competes with PI-4P for association with the PH domain [53]. Cer binding occurs via a long, large preformed cavity that extends through the middle of the START domain which relies on an α/β-fold built around an incomplete U-shaped β-barrel to form a helix-grip structure in which curved β -sheets are covered by three α-helices and two Ω loops (Fig. 3). The Cer hydroxyl and amide groups form hydrogen bonds with five, deeply buried polar/charged residues in the cavity while the hydrocarbon chains extend back toward the protein surface. No extra space exists at the bottom of the START cavity to accommodate a polar headgroup bulkier than the C1 hydroxyl of Cer. Compared to SLs bound in GLTP-folds, the reversed orientation of bound Cer in CERT, with the hydroxyl/acyl-amide headgroup region buried deeper than the aliphatic chains within the START binding cavity, indicates a fundamentally different mechanism of Cer uptake/release during membrane interaction.
Fig. 3. Structural Differences between CERT and CPTP.

(a) Chemical structure of ceramide containing a palmitoyl (16:0; hexadecanoyl) acyl chain. (b) Structure of CERT START domain containing bound 16:0-Cer (space-filling) (PBD: 2E3O) showing nitrogen (blue), oxygen (red), and hydrocarbon (green) (c) Space-filling structure of CERT containing bound 16:0-Cer (PDB: 4K84). Blue and red equal positive- and negative-charged surface, respectively. (d) Chemical structure of ceramide-1-phosphate (C1P) containing a palmitoyl (16:0; hexadecanoyl) acyl chain. (e) Structure of CPTP containing bound 16:0-C1P (space-filling) showing nitrogen (blue), oxygen (red), and hydrocarbon (yellow). (f) Space-filling structure of CPTP containing bound 16:0-C1P. Blue and red equal positive- and negative-charged surface, respectively.
2.2. Lipid regulators of CPTP
Targeting and activation mechanisms that could potentially help guide CPTP and the plant CPTP ortholog, ACD11, to specific intracellular membrane sites in vivo have recently begun to emerge [54]. Upon initial discovery [19], immunocytochemical microscopy analyses revealed enrichment of cytosolic CPTP at the trans-Golgi and association with early (Rab5) and late (Rab9) endosomes as well as at specific regions of the plasma membrane (Fig. 4). Thus it stands to reason that certain phosphoglycerides located in membranes that face the cytosol might act as regulators of CPTP. Indeed, the presence of physiologically-relevant concentrations of 1-palmitoyl-2-oleoyl phosphatidylserine (POPS) in 1-palmitoyl-2-oleoyl phosphatidylcholine (POPC) vesicles containing C1P increases C1P intermembrane transfer rates by ACD11 and CPTP. Yet, other anionic long-chain phosphoglycerides such as phosphatidic acid or phosphatidylglycerol (PG) have the opposite effect of PS and depress C1P transfer rates [54]. None of these anionic phosphoglycerides affect glycolipid transfer by human GLTP. Notably, ‘soluble’ PS (dihexanoyl-PS) that can only weakly partition to and embed in membrane bilayers fails to stimulate C1P transfer. Surface plasmon resonance analyses reveal that having either POPG or POPS in POPC vesicles increases ACD11 partitioning to the membrane surface [54]. Their opposite effects on C1P transfer rates suggest that POPS may facilitate protein release after C1P acquisition or optimize protein orientation for C1P uptake during membrane contact. In contrast, POPG may enhance protein partitioning in nonspecific ways that mitigate access to the C1P headgroup recognition site, thus negatively regulating ACD11 action [54]. The existence of a PS headgroup-specific site on the surface of ACD11/CPTP (Fig. 5) near the C1P binding site has been hypothesized to explain how PS embedded in the PC membrane can enhance and facilitate a favorable interaction orientation by ACD11 or CPTP. Still, the mechanistic details defining exactly how POPS speeds up CPTP-mediated C1P transfer are in need of clarification. Nonetheless, intracellular membranes containing cytoplasmically oriented long-chain PS, such as the plasma membrane and trans-Golgi network [55,56], could function as targeted ‘hot spots’ for ACD11 and CPTP action in normal cells. A regulatory role for certain phosphoinositides has also emerged in preliminary in vitro studies [57].
Fig. 4. CPTP intracellular localization.

CPTP localizes to the Golgi region, nucleus and plasma membrane in cultured mammalian cells expressing either FLAG-CPTP or GFP-CPTP. (Left panel) At the plasma membrane, CPTP localization appears enhanced at membrane ruffling sites (white arrows). (Right panel) The Golgi localization of CPTP is indicated by anti-TGN46 labeling. The nuclear localization of GFP-CPTP in fixed cells mirrors the localization in live cells (not shown). BSC-1 cells (African Green Monkey kidney cells) are shown on coverslips expressing FLAG-CPTP or GFP-CPTP and fixed in −20 °C methanol. Labelling was performed with anti-FLAG or anti-GFP followed by 2° antibodies coupled to Alexa-488 (green) as well as with anti-γ-tubulin or anti-TGN-46 followed by 2° antibodies coupled to Alexa-594 (red). Cells were counter stained with DAPI (blue), mounted in 10% PBS, 90% glycerol and imaged. Bar = 10 μm. (Adapted from [19]).
Fig. 5. Model depicting PS-induced enhancement of ACD11 membrane interaction.

The model shows how the PS acyl chains remain embedded in the membrane while the PS headgroup (magenta) interacts with a putative surface site in ACD11 (ribbon representation) to help orient the C1P binding site of ACD11 in a way that can improve uptake of the C1P from the membrane. (Adapted from [54]).
2.3. Emerging in vivo functional roles for CPTP
In mammals, C1P anabolic production is known to occur via ceramide kinase [23,58] at the trans-Golgi. As indicated earlier, human CPTP is cytosolic but also associates with select membranes that face the cell cytosol such as the trans-Golgi as well as at select sites along the cytoplasmic side of the plasma membrane (Fig. 4). CPTP down-regulation or expression of point mutants with ablated C1P binding sites affects cells in two major ways [19]: i) C1P levels increase in subcellular fractions enriched in trans-Golgi, but decrease in fractions enriched in plasma membranes; and ii) arachidonic acid and pro-inflammatory eicosanoid levels increase (Fig. 6). The findings are consistent with CPTP acting as a C1P sensor and mediator of C1P transport to the plasma membrane from the trans-Golgi where C1P is produced by ceramide kinase [19]. As illustrated in Fig. 6f, when CPTP is downregulated, C1P accumulates at the trans-Golgi where this SL can enhance membrane partitioning of IVA phospholipase A2 (cPLA2α) via a C1P-specific anchoring site in the protein’s C2-domain [46,47]. The ensuing cPLA2α activation releases arachidonic acid, the essential precursor for downstream pro-inflammatory eicosanoid production by cyclooxygenases and lipoxygenases [59–61]. The ability of changes in CPTP expression to regulate cellular events by altering C1P local levels at distinct sites within the cell has noteworthy ramifications due to the presence of putative C1P membrane interaction motifs (Arg-X-Arg-His) in other proteins involved in important in cell processes [47]. Such proteins include UV-resistance-associated gene (UVRAG; also known as p63), regulator of G-protein signaling 3 (RGP3), and tumor necrosis factor-alpha converting enzyme (TACE).
Fig. 6. CPTP regulation of C1P levels, arachidonic acid levels, and eicosanoid production.

(a) Intracellular C1P levels of siRNA-treated and control A549 cells. X-axis shows acyl composition of C1P species with sphingosine base chains (d18:1). (b, c, d, e) Arachidonic acid (AA) and eicosanoids (COX = cyclooxygenase, LOX = lipoxygenase, CYP = cytochrome P450 pathways) secreted into media by siRNA-treated and control A549 cells. Data for b-e represent averages of 6 experiments by Student’s t-test (* p < 0.05, ** p < 0.01, NS not significant). 6keto-PGF1α, the primary prostacyclin metabolite, is nearly unaffected by siCPTP or siCERK suggesting existence of an AA pool derived independently of C1P-activated cPLA2α. (f) Model for CPTP regulation of eicosanoid production. C1P synthesis occurs via ceramide kinase (CERK) which uses its pleckstrin homology (PH) domain to translocate to the trans-Golgi network (TGN) vicinity upon stimulation. CERK relies on ceramide transported from the ER by ceramide transfer protein (CERT) which also contains a targeting PH domain. After synthesis, C1P is transported to subcellular destinations by CPTP and possibly by vesicular trafficking. RNAi knockdown of CPTP (shown by red Xs) leads to accumulation and elevation of C1P at the Golgi complex, a condition that activates GIVA phospholipase A2 (cPLA2α), generating arachidonic acid for downstream production of pro-inflammatory eicosanoids. cPLA2α activation occurs by translocation from the cytoplasm and/or cis-Golgi (red arrows) through enhanced anchoring to membrane-embedded C1P generated by CERK in the TGN. COX-1 and inducible COX-2 which use arachidonic acid to produce pro-inflammatory prostaglandins, also concentrate in the TGN vicinity during stimulation. For clarity, other eicosanoid generator pathways are not depicted. (Adapted from [19]).
A cellular alteration linked to CPTP depletion is the loss of Golgi cisternal stacking and fragmentation [19], a phenotype also associated with starvation-induced autophagy [62,63]. Recently, autophagy induction and inflammasome assembly/activation, key processes associated with innate immunity and chronic inflammatory disease, have been linked to CPTP expression [26]. Depletion of CPTP (but not GLTP) or expression of CPTP mutants with ablated C1P binding-sites in human epithelial cells stimulates an 8- to 10-fold increase in autophagosome levels as detected by elevated levels of endogenous microtubule-associated protein 1A/1B-light chain 3 (LC3) conjugated to phosphadiylethanolamine (LC3-II) (Fig. 7) and decreased expression of sequestosome 1 (SQSTM1; also known as p62). WT-CPTP overexpression exerts a protective effect against starvation-induced autophagy. Not surprisingly, RNAi-induced CPTP depletion (CPTPi) that results in autophagy requires expression of key assembly and elongation factors [autophagy-related protein 5 (ATG5), ATG7, unc-51-like kinase 1 (ULK1)] that are needed for production of nascent membranes, i.e. phagophores [26]. Notably, autophagy initiation events such as phosphorylation of mammalian-target-of-rapamycin (mTOR) and its downstream target, ribosomal protein S6 kinase (p70S6K), that coordinately regulate the balance between eukaryotic cell growth and autophagy in response to nutritional status, growth factor and stress signals, are suppressed by CPTPi; whereas certain early stage markers of autophagosome formation (Golgi-derived autophagy-related protein 9A (ATG9A)-vesicles, WD repeat domain phosphoinositide-interacting protein 1 (WIPI-1), also known as Atg18, become elevated. The marked increase in ATG9A-vesicles originating from the trans-Golgi is consistent with the disruption of Golgi cisternae stacks and hyper-vesiculation induced by CPTP depletion [19,26]. In macrophage-like THP-1 surveillance cells, CPTP knockdown not only induces autophagy but also elevates caspase-1 levels, and strongly increases cytokine release (interleukin-1β and interleukin-18). The resulting inflammasome activation/assembly is autophagy-dependent and occurs via a stimulatory mechanism that depends on NLR family pyrin domain containing 3 (NLRP3) but not on NLR family CARD domain containing 4 (NLRC4) to ultimately increase cell death via pyroptosis [26].
Fig. 7. Autophagic flux is increased by siCPTP.
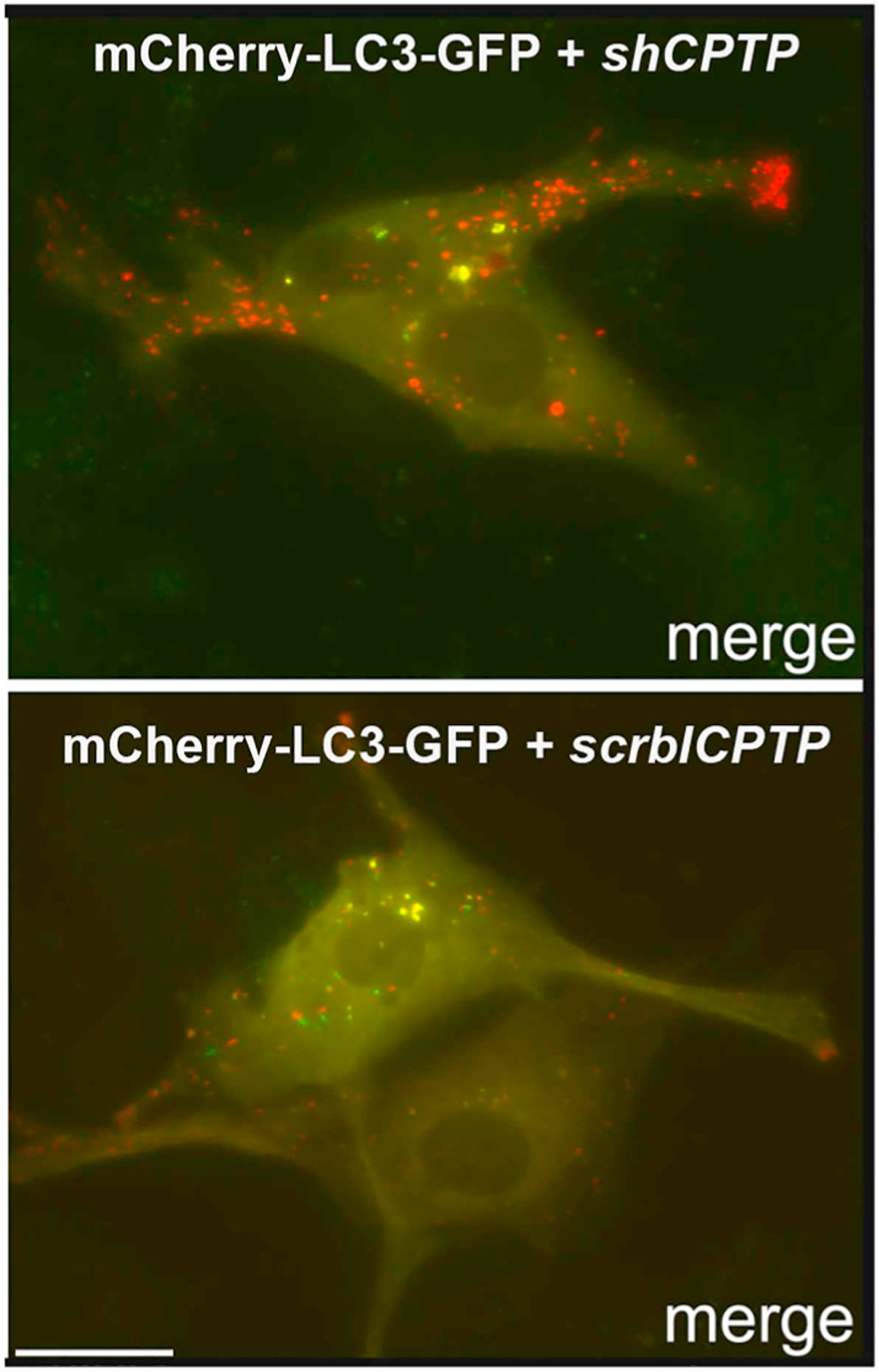
Knockdown of CPTP in cultured mammalian cells transfected with dual-label pBABE-puro-mCherry-EGFP-LC3 plus shCPTP plasmid provides evidence of increased autophagic flux. (shCPTP = short-hairpin CPTP; scrbl-CPTP = scrambled-CPTP) The green and red signals for LC3 combine to produce yellow puncta until conversion to LC3-II and acidification occurs. The low pH environment of the mature autophagosomes quenches the green GFP signal resulting in orange and eventually red puncta, verifying increased autophagic flux. Bar = 10 μm. (For complete description, see [26]).
Pyroptosis is a primary cellular response to the sensing of potentially damaging insults, such as pathogen ligands, damage-associated molecular patterns (DAMPs), altered levels of host metabolites and environmental irritants [64–66]. Execution of this highly inflammatory form of programmed cell death is mediated by caspase-1 and/or caspase-11 which cleave and activate members of the gasdermin gene family. The activation induces their binding to acidic phospholipids located on the inner leaflet of the plasma membrane to form oligomeric death-inducing pores that release cell contents. Interestingly, in CPTP-depleted surveillance cells, the induction of autophagy-dependent, pro-inflammatory cytokine release that ultimately leads to pyroptosis appears to be related to elevation of C1P. Indeed, elevation of intracellular C1P by exogenous C1P treatment using a dodecane/ethanol delivery system [67–69] rather than by CPTP depletion also was found to induce autophagy and IL1β release. The findings identify CPTP as an endogenous regulator of early-stage autophagosome assembly that helps drive inflammasome-mediated release of pro-inflammatory cytokines such as IL-1β and IL-18 [26].
The new data linking CPTP expression to autophagy induction and inflammasome regulation provide insights that could help advance understanding of disease-related microarray data showing changes in the expression of CPTP (known as GLTPD1 before renaming by the Human Genome Organization (HUGO) Gene Naming Committee (https://www.genenames.org). For instance, in age-related macular degeneration (AMD), CPTP is among various genes that are significantly downregulated [70]. AMD development correlates with drusen levels in nearby cells. Drusen are yellow and white deposits of extracellular materials (protein and lipid) that build up under the retina. Drusen-derived amyloid β1–40 peptide, a known trigger of inflammation, negatively affects retinal pigment epithelial cells [70]. Whether CPTP expression regulates production of drusen-derived amyloid β1–40 peptide has not yet been explored.
Other recent studies indicate that patients with severe acute pancreatitis triggered by bacterial infiltration into the blood exhibit inflammatory damage linked to downregulated CPTP expression and abnormally high expression levels of IVA cPLA2 (cPLA2α). Also found was downregulated expression of occluding and zonula occludens-1 proteins needed for viable tight junctions in intestinal mucosal epithelial cells [71]. The authors concluded that CPTP, by upregulating IVA cPLA2 expression, may trigger inflammation that alters the expression of tight junction proteins needed to protect the intestinal epithelial barrier [71]. To what extent the downregulated CPTP expression affects sphingolipid levels (e.g. C1P, Cer, sphingosine-1-phosphate (S1P)) remains to be determined. Notably, the upregulation of IVA cPLA2 transcript (PLA2G4A mRNA) in response to CPTP down-regulation has also been reported in T47D and SKBR3 breast cancer cells [72]. Both of these breast cancer cell lines express the estrogen and progesterone receptors but differ dramatically in their expression of human epidermal growth factor receptor 2 (HER2) gene product. Unlike T47D cells, SKBR3 cells overexpress the HER2 gene product, which has been implicated in the regulation of breast cancer proliferation pathways such as the Ras/Raf/mitogen-activated protein kinase and phosphatidylinositol-3 kinase/protein kinase-B/mammalian target of rapamycin pathways.
Another medical condition involving loss of CPTP expression is Chromosome 1p36 deletion syndrome (MIM 607872), one of the most common terminal deletion genetic birth defect in humans, occurring in one in 5,000 to 10,000 individuals [73]. The disorder generally results in severe intellectual disability, limited speech ability, seizures, auditory and visual impairment, and delayed growth with varying symptoms depending on the exact location of the chromosomal deletion. The DNA deletion occurs on the outermost band on the short arm (p) of chromosome 1. The breakpoints for 1p36 deletion syndrome vary but are most commonly found from 1p36.13 to 1p36.33. The size of the deletion ranges from approximately 1.5 million to greater than 10 million base pairs. The deletion can include the 1p36.32–1p36.33 region, the location of CPTP [73]. Additional research is needed to pinpoint the specific physiologic changes that are attributable to the loss of CPTP.
3. Phosphatidylinositol 4-phosphate adaptor protein-2 (FAPP2)
Among GLTP superfamily members, the global structural organization of FAPP2 differs dramatically from other family members and is similar to CERT. Both FAPP2 and CERT utilize N-terminal pleckstrin homology (PH) domains that are connected via linker regions to their SL binding domains [49] and thus are classified as LTPs anchored at membrane contact sites (LAMs) [10–17]. Yet, in FAPP2, the linker region attaches to a GLTP-homology (GLTPH) domain rather than a START domain as occurs in CERT to enable binding and transfer of GSL rather than Cer, respectively. FAPP2 was discovered because of its key role in vesicular anterograde trafficking from the Golgi and specific recognition of phosphatidylinositol 4-phosphate (PI-4P) via its N-terminal PH domain [74–76]. The protein was subsequently found to be important in GSL biosynthesis by functioning as a shuttle for delivering glucosylceramide (GlcCer) to appropriate Golgi sites for the production of complex GSLs [38,39,77]. Cell permeabilization that enables entry of recombinant FAPP2 loaded with fluorescent GlcCer revealed delivery of the fluorescent glycolipid cargo to the Golgi area in HeLa cells [38]. Moreover, the intra-Golgi distribution of GlcCer became altered by FAPP2 knockdown resulting in loss of GlcGer at the TGN and accumulation at the cis Golgi region [39]. Whether FAPP2-mediates direct transfer of GlcCer between the cis- and trans-Golgi [38,39] or via an additional intermediary transfer to the ER [77] has been debated. Regardless, the data provide strong support for FAPP2 being a genuine GlcCer transfer protein in vitro and in vivo.
In PLEKHA8−/− (= FAPP2−/−) mouse knockout studies [39], no overt phenotype change was evident. In tissues (e.g. kidney) where FAPP2 is normally highly expressed, a decrease in globotriaosylceramide (Gb3) occurred as well as lowered upstream levels of GlcCer and lactosylceramide (LacCer) while no effect was observed on ganglioside GM3 levels. This finding is consistent with cell studies showing that loss of FAPP2 or GLTP becomes lethal only when both proteins are knocked down [77]. To further evaluate the GSL synthetic branch point involving GlcCer and LacCer for Gb3 or GM3 production, GlcCer synthesis was bypassed by labelling HeLa cells with fluorescent-GlcCer. Upon FAPP2 knockdown, selective inhibition was observed for the synthesis of fluorescent Gb3, but not GM3. When combined with brefeldin A and dicoumarol inhibitor studies [39], the findings revealed that GlcCer transported by FAPP2 feeds a LacCer pool specifically destined to Gb3 synthesis; whereas a vesicular GlcCer pool feeds GM3 synthesis. Further examination revealed that GlcCer binding by FAPP2 positively regulates its localization to the trans-Golgi involving PI-4P targeting mediated via the N-terminal PH domain binding of FAPP2. In contrast, apo-FAPP2 preferred to associate with the cis-Golgi where it acquires GlcCer which then increased FAPP2 affinity for the PI-4P-enriched trans-Golgi, resulting in an FAPP2 cycle for continuing the transfer of GlcCer [39].
From the human molecular genetics standpoint, it is clear that the C-terminal GLTPH domain of FAPP2 is not a splice variant arising from the GLTP gene [40]. Rather, FAPP2 originates from the single-copy PLEKHA8 gene on human chromosome 7 (locus 7p21-p11.2) (Fig. 2). The final 7 exons in mature PLEKHA8 transcript (14 exons) are involved in coding the GLTPH domain [40] which is highly conserved among various organisms [76]. Glycolipid transfer activity by the isolated FAPP2-GLTPH domain became evident only after cloning of the bona fide full-length protein (519 residues). This protein included 12 residues missing from the C-terminus of an earlier FAPP2 clone, explained by a cloning-induced mutation artefact involving a premature TTA stop codon rather than the authentic downstream TGA stop codon in final exon 14 [40]. In agreement with GLTP data [21,36], FAPP2-GLTPH clones containing the missing 12 C-terminal residues were found to actively transfer glycolipid, enabling detailed functional analysis of the GLTPH domain compared to GLTP [40]. GLTPH exhibits much greater selectivity for uncharged monoglycosylated ceramides (e.g. GlcCer and GalCer) than for more complex GSLs including sulfatide and gangliosides compared to GLTP (Table 1). Interestingly, little selectivity difference between GlcCer and GalCer is detectable in the in vitro intermembrane transfer rates of GLTP or FAPP2-GLTPH implying that the in vivo preference of FAPP2 for GlcCer reflects the cytosolic orientation/accessibility of GlcCer during synthesis rather than fundamental structural differences in the GLTPH domain [40].
Table 1.
Sphingolipid Transfer Specificity for Human GLTP Superfamily Members
| Human GLTP superfamily member | Sphingolipid(s) binding or transfer specificity between PC membranes | References |
|---|---|---|
| CPTP | Fast for C1P Very Slow for SM & S1P None for GalCer LacCer, Cer, PA, PC |
[19] |
| FAPP2 | Fast for GlcCer & GalCer Moderate for LacCer Very slow for SM or sulfatide None for Cer |
[38], [40], [41] |
| GLTPD2 | Unknown | |
| GLTP | Fast for GlcCer, GalCer, LacCer, GM1, asialo-GM1 Moderate for sulfatide Very slow for SM None for Cer |
see [169] and refs therein for detailed comprehensive coverage of GLTP studies of GSL specificity |
It is noteworthy that a putative FAPP2-like protein known as PLEKHA9 has been identified as containing an uncharacterized GLTP-like domain (141 amino acid sequence) by BLAST analyses of the human genome [49]. Yet, the gene encoding this putative protein has hallmarks of a transcriptionally-silent processed pseudogene, and thus has been designated PLEKHA8P1. Despite encoding many key residues of the glycolipid headgroup binding site, PLEKHA8P1, which is located on human chromosome 12 at locus 12q12, lacks coding sequence for the last 49 amino acids (~25%) present in FAPP2-GLTPH and thus is predicted to lack glycolipid transfer activity. However, experimental verification is needed.
A recent X-ray study of FAPP2-GLTPH domain [41] derived from PLEKHA8 has confirmed the global structure to be a GLTP-fold as predicted by earlier structural homology modelling [38,40]. The highly resolved (1.45 Å) crystal structure of FAPP2-GLTPH complexed with N-oleoyl-GalCer shows the conserved structural arrangement and identical nature of the key residues required for binding the initial ceramide-linked sugar to the FAPP2 GLTPH domain (Fig. 8). Notably, the structural basis for the differing GSL selectivity between FAPP2-GLTPH and GLTP with respect to their vastly differing capacity for transferring gangliosides and sulfatide has been linked to the conformationally different α3/α4 connecting loops, i.e. ID-loops, in their respective GLTP-folds [41]. This study, along with earlier structural data of GLTP complexed with various simple GSL species [19–21,35,36] also provides insights into the accommodation of the GSL hydrocarbon chains within the hydrophobic pockets of GLTP and FAPP2. Phe side-chains positioned at strategic points within the hydrophobic pocket function as ‘gates’ and ‘baffles’ that swing open to accommodate the SL chain(s) and that also often seal bottom regions of the hydrophobic pockets.
Fig. 8. Structure comparison for GLTP domains of human GLTP superfamily members.

The acyl-amide linkage in the SLs is specifically targeted by the D/E and H residues that function in ‘pincher-like’ fashion in all family members (Upper panel) Superposition of FAPP2-GLTPH domain (sky blue) containing bound GalCer acylated with an oleoyl (18:1) chain (ball-and-stick) colored as nitrogen (blue), oxygen (red), and hydrocarbon (green) (PDB: 5KDI). GLTP (beige) containing bound GlcCer acylated with an oleoyl (18:1) chain (ball-and-stick) colored as nitrogen (blue), oxygen (red), and hydrocarbon (yellow) (PDB: 3SOK). (Lower panel) Superposition of predicted GLTPD2 (grey) architecture based on structural homology modeling using I-Tasser [170]compared with CPTP (yellow) containing bound C1P with a palmitoyl (16:0) chain (ball-and-stick) (PDB ID: 4K84) colored as nitrogen (blue), oxygen (red), and hydrocarbon (green) (PDB: 4K84). Superposition of the structures was performed using Chimera 1.11.2.
FAPP2 intracellular targeting has been comprehensively investigated. In silico analyses predict two ‘weak’ ER-targeting sequences, i.e. diphenylalanines-in-an-acidic tract (FFAT motifs), and a third very weak FFAT-like motif in human FAPP2 [78,79]. However, testing of the putative TFFST-N FFAT-like sequence motif revealed no targeting to the ER, but pseudo-phosphorylation of the Ser residue by mutation to Asp, i.e. TFFDA-N, did result in weak interaction with vesicle-associated membrane protein-associated proteins (VAMP-associated proteins or VAPs) localized in the ER cytosolic surface [80]. In vivo, the intracellular localization of FAPP2 is regulated by its PH domain which targets PI-4P embedded in Golgi membranes [38,39]. Interestingly, the presence of PI-4P in membranes induces FAPP2 dimerization that stimulates membrane tubulation. The resulting wedge-like structure of the dimerized PH-domains is proposed to drive the membrane tubulation activity [81,82]. It remains unclear whether the tubulation activity, which may be needed for formation of Golgi-derived trafficking vesicles that export protein and lipid to the cell surface [81–84], is also required for FAPP2-mediated GlcCer transfer that drives GSL synthesis.
In any case, the PH domain appears to play a key role in directing FAPP2 with bound GlcCer from the cis-Golgi to the trans-Golgi [38,39]. Interestingly, binding of GlcCer synthesized on the cis-Golgi cytosolic face by the GLTPH domain ‘activates’ the PH domain by enhancing its ability to interact with PI-4P located in the trans-Golgi. In this way, FAPP2 functions as a GlcCer transfer protein that stimulates the synthesis of globo- and asialo-GSLs, but not ganglio-GSL synthesis which relies on GlcCer delivered by a vesicular mechanism that may route through the Golgi cisternae [39] or involve the endoplasmic reticulum [27].
It is noteworthy that PI-4P is not the only molecular determinant that regulates the ability of FAPP2 to transfer GlcCer [85]. Acyl-coenzyme A binding domain containing 3 (ACBD3) has recently been identified as a FAPP2-interacting partner. ACBD3 helps maintain Golgi structure via interaction of its C-terminal domain with the Golgi integral protein giantin as well as by organizing the Golgi stacking proteins and a Ras-related Rab33b-guanosine triphosphatase–activating protein (GAP) at the medial-Golgi. ACBD3 knockdown is known to negatively affect PI-4 kinase localization to the trans-Golgi and has recently been shown to lead to dramatic Golgi fragmentation and subsequent triggering of FAPP2 dispersal throughout the cytoplasm and decreased localization at the trans-Golgi network [85]. Not surprisingly, the resulting changes in FAPP2 distribution profoundly affect SL metabolism including GlcCer elevation but lowering of LacCer as well as downstream globoside Gb3 and ganglioside GM3.
Given the important role played by FAPP2 in GSL metabolism, it is not surprising that this protein has been linked to the regulation of programmed cell death processes. For instance, upon FAPP2 knockdown via siRNA, apoptotic cell death significantly increases in certain glioma and breast tumor cells incubated with soluble Fas ligand (FasL), a type-II transmembrane protein of the tumor necrosis factor (TNF) family that binds its receptor to induce apoptosis [86]. Yet, the apoptosis does not necessarily correlate with increased Fas expression. Nonetheless, the data reveal a potential role for FAPP2 in conferring resistance to apoptosis [86]. Whether resistance to necroptosis can also be conferred by FAPP2 knockdown was not specifically addressed and needs further study which could be the case for the especially high death efficiency imparted to U-87MG glioma cells expressing high levels of receptor-interacting protein kinase 3 (RIPK-3) [87].
Necroptosis is an inflammatory form of cell death that is frequently observed when apoptotic death receptor signaling components are rendered nonfunctional [88–91]. This form of cell death serves as a backup cell death defense mechanism and is highlighted by caspase-8 inhibition. Necroptosis requires assembly of a signaling complex comprised of RIPK-1 and RIPK-3 along with mixed lineage kinase domain-like (MLKL) protein. High expression of RIPK-3 and a phosphorylation cascade that includes RIPK3-mediated phosphorylation of MLKL results in MLKL translocation to the plasma membrane. MLKL oligomerization creates pores that induce membrane damage and shedding, resulting in cell death [88–91].
FAPP2 also has been found to play important roles in certain pathophysiological conditions such as that involving hepatitis C virus (HCV) [92]. FAPP2 depletion via shRNA attenuates HCV infectivity and impedes HCV RNA synthesis. As discussed earlier, FAPP2 has separate lipid-binding domains: a PH domain specific for PI-4P and a GLTPH domain specific for GSLs. So while expression of FAPP2 containing mutant PH domain with ablated PI4P-binding was expected to inhibit HCV replication, what was not expected was the marked drop in replication efficiency also observed upon mutational ablation of GSL-binding by the GLTPH domain. These data support the crucial role played by both domains of FAPP2 in HCV genome replication. HCV infections also were found to significantly increase some GSL levels. Adding these lipids to FAPP2-depleted cells partially rescued replication, further arguing for the importance of GSLs in HCV RNA synthesis. Thus, HCV hijacks FAPP2 for virus genome replication via PI4P binding and GSL transport to the HCV replication complex.
4. Glycolipid transfer protein domain-containing protein-2 (GLTPD2)
The least studied and most mysterious member of the human GLTP superfamily is encoded by GLTPD2, a gene originally predicted by computer-generated annotation of the Human Genome over a decade ago. A short time later, PCR analyses verified the presence of GLTPD2 mRNA in certain tissues with especially high expression in liver tissue (X. Zou & R.E. Brown, unpublished observation). In humans, BLAST analysis indicates that GLTPD2 is located on chromosome 17 and contains 4 exons encoding a 291 residue protein (Fig. 2). Sequence alignment shows that the GLTP-domain of GLTPD2 consists of the final 211 residues coded by exons 3 and 4. Structural homology modeling reveals that the GLTP-fold of GLTPD2 more closely resembles C1P-specific CPTP and ACD11 than glycolipid-selective GLTPs at key interaction positions in the putative sphingolipid headgroup recognition center (Fig. 8). Notably, however, the N-terminal 80 residue region preceding the GLTP-like domain contains a 20 residue stretch that is not present in other human GLTP superfamily members and is predicted to be a transmembrane helix. A helix such as this could stabilize membrane interaction by GLTPD2 and enable this GLTP superfamily member to function by swinging back and forth in gap areas near membrane contact sites where membranes are closely apposed in similar manner as so-called LTPs anchored at membrane contact sites (LAMs) [10–17]. Experimental evidence in support of this proposed mechanism for GLTPD2 function is currently lacking and in need of pursuit. Structure/function studies have been hampered by heterologous expression problems after cloning (Y-G. Gao & R.E. Brown, unpublished observation).
The Human Protein Atlas web site indicates high expression of GLTPD2 mRNA in liver cancer. GLTPD2 gene is also among the top 15 genes upregulated during necrotizing enterocolitis in neonatal mice [93] and is one of the top 12 genes upregulated in endothelial progenitor cells treated with monomeric but not pentameric C-reactive protein (CRP) [94]. Dissociation of pentameric CRP to monomeric CRP is thought to be involved in local pro-inflammatory reactions at the site of developing atherosclerotic plaques. In a recent gene-wide association study [95], GLTPD2 has been identified as one of 10 gene variants linked to cardiovascular disease risk from among 35 lipid-species-associated gene loci. Interestingly, the lipid species most strongly affected by an intronic substitution of thymidine for guanosine in the variant GLTPD2 gene are specific sphingomyelins (SMs) i.e. N-palmitoyl (16:0) dihydroSM, N-behenoyl (22:0) SM, and N-nervonoyl (24:1) SM. The decreased SM levels found associated with this GLTPD2 variant are proposed to be indicative of reduced risk of atherosclerosis. Interestingly, ACD11, the plant CPTP orthologue that resembles GLTPD2 more closely than GLTPs, is able to transfer SM slowly [28] and a crystal complex of ACD11 and lysoSM (PDB 4NT2) has been resolved to 2.4 Å [20]. Future studies will be needed to test whether the subtle differences in the putative sphingolipid binding sites of ACD11 (or CPTP) compared to GLTPD2 enable this protein to function as a bona fide SM transfer protein or as a transporter of SM building block metabolites.
5. Glycolipid transfer protein (GLTP)
Whereas research on CPTP, FAPP2, and GLTPD2 is relatively recent and has occurred almost entirely during the current millennium, studies of mammalian GLTPs began in the 1980s [96–105]. The 1990s were largely a dormant period for GLTP research until successful cloning of mammalian GLTPs by hot-start, semi-nested PCR and rapid-amplification-of-cDNA-ends (RACE)-PCR overcame problems associated with the high guanine-cytosine content of the GLTP exon 1 and promoter regions [32,33,106]. This achievement along with the development of a robust fluorescence resonance energy transfer assay capable of tracking GLTP activity in real time while using low and physiologically-relevant GSL membrane concentrations [107] reignited research in the field. Successful heterologous expression in E. coli enabled rapid purification of milligram protein amounts providing the avenue for achieving high resolution crystal structures of human GLTP complexed with various GSLs [30,31,35,36]. The novel all-α-helical, two-layer ‘sandwich’ architecture used by GLTP to encapsulate a single GSL molecule became designated as the GLTP-fold and human GLTP was denoted as the prototype and founding member of the GLTP superfamily. Structure/function analyses enabled mapping of the GSL binding site [30,31,35,36,108,109] as well as identification of the protein’s membrane interaction region [18,21,22,33,110–114]. Membrane structural properties (e.g. phase state, curvature stress) [99,101,115,116] and lipid composition [110,117–120] are also known regulators of GLTP transfer activity. For further details regarding GLTP biophysics, readers are referred to various reviews [21,22,121–123] as well as a recent paper reporting the X-ray structure of the FAPP2 GLTPH domain complexed with monoglycosylceramide [41].
5.1. GLTP – GSL transport between membranes
The cell biological function(s) of GLTP have remained a subject of investigation and debate for many years. In vivo lipid trafficking measurements showed that the arrival of some GlcCer at the plasma membrane persists in the presence of inhibitors that block vesicular trafficking [124] or when FAPP2 is depleted by RNAi [77], consistent with GLTP involvement in GlcCer transfer to the Golgi, ER, and plasma membrane. In GLTP, diphenylalanine-in-an-acidic tract (FFAT)-like motifs have been identified and evaluated for ER targeting via interaction with ER-associated vesicle-associated membrane protein (VAMP)-associated proteins (VAPs) [78–80,125]. Interestingly, binding of GlcCer to GLTP has recently been shown to weaken its interaction with VAP-A, a resident ER transmembrane protein [109]. In another recent study [126], bicylol, an antiviral drug, has been found to upregulate GLTP expression. The increased GLTP competes for VAP-A binding, thus interrupting formation of the VAP-A/NS5A complex that is essential for hepatitis C virus replication. NA5A is a nonstructural, zinc-binding and proline-rich hydrophilic phosphoprotein that plays a key role in Hepatitis C virus RNA replication.
The ability of GLTP to transfer various GSLs including gangliosides between liposomal membranes has been known for many years from in vitro studies [121]. In early studies, red cell ghosts and neuronal membranes sometimes served as acceptor membranes for GSLs transferred by GLTP [97,99,102–104]. Yet, the ability of GLTP to transfer ganglioside from natural donor membranes has been demonstrated more recently [127]. When donor- and acceptor-membranes are naturally-derived, GLTP is able to equilibrate the ganglioside GM1 concentration between the membranes as well as transfer GM1 between membranes from different cell types. GLTP also has the capacity to increase GM1 levels above endogenous levels in either side of the natural membrane leaflet, i.e., external or cytosolic, when used in concert with GM1-containing donor liposomes. Glycolipid transport by GLTP is highly efficient, requires no cofactors, is driven solely by the chemical potential of GM1 and can involve either the extra- or intracellular membrane leaflet in permeabilized cells [127]. Aside from the plasma membrane, GM1 is also known to reside at mitochondria-associated ER membranes as well as in nuclear membranes [128–132]. Thus, exploration of GLTP involvement appears warranted since the FAPP2 GLTPH domain transfers simple uncharged monoglycosylceramides (e.g. GlcCer and GalCer) but not sulfatide or gangliosides [38–41]. Interestingly, relocation of ganglioside GD3 to mitochondria is known to induce apoptosis in several cell types, including human hematopoietic cells and neural cells [132,133]. GD3 has also been implicated in autophagy via physical interaction with MAP1LC3/LC3 as well as beclin-1-regulated autophagy (AMBRA1) and WIPI-1, two important effectors of autophagosome formation [134]. GD3 transport reportedly can occur to destination membranes independent of vesicular trafficking disrupted by brefeldin A after synthesis in the trans-Golgi [135]. GLTP is expected to be able to bind and transfer GD3. However, whether interaction between GLTP and GD3 is prevented by virtue of residing on opposite sides of intracellular membranes remains a possibility that needs further study.
5.2. GLTP and other superfamily members – human gene locations & organization
Among GLTP superfamily members, the human GLTP gene and a related pseudogene, GLTPP1, are the only genes that have been carefully characterized [106]. Insights into the organization and locations of human genes encoding CPTP, FAPP2-GLTPH, and GLTPD2 have been deduced using the NCBI Basic Local Alignment Search Tool (BLAST) to analyze the human genome (Fig. 2), but detailed investigations are currently lacking. A single-copy GLTP gene located at locus 12q24.11 is transcribed to produce human mRNA encoding active GLTP. Five interspersed nucleotide sequences on chromosome 12 encode the full-length GLTP cDNA ORF (630 bases), consistent with the GLTP gene consisting of five exons separated by four introns (Fig. 2). When joined in 5’-to-3’ fashion, the five exons encode the same 209 amino acid GLTP sequence determined earlier by RT-PCR using purified mRNA [32,33]. All exon/intron boundaries are characterized by classic consensus nucleotide sequences expected for splice sites, i.e. introns containing 5’ GT and 3’ AG dinucleotides as well as upstream pyrimidine tracts.
Phylogenetic/evolutionary analyses have indicated that the 5-exon/4-intron organizational pattern and encoded sequence of 12q24.11 GLTP are highly conserved among therian mammals and other vertebrates [106]. A genetic element at locus 11p15.1 was found to exhibit several features of a potentially active single-copy GLTP retrogene, including a highly homologous (~94% identity), full-length single-exon coding sequence containing all key amino acid residues involved in glycolipid liganding. Yet analyses of transcriptional activity for each human GLTP gene by in silico EST evaluations, RT-PCR amplifications of GLTP transcript(s), and methylation status of regulator CpG islands using various human cells indicated active transcription only for 12q24.11 GLTP and not for 11p15.1 GLTP. Heterologous expression and purification of the GLTP paralogs showed glycolipid intermembrane transfer activity only for 12q24.11 GLTP. The transcriptionally-silent11p15.1 GLTP gene, which was found only in primates and not in other mammals, was identified as a pseudogene (GLTPP1) [106].
Two GLTP splice variants, GLTP_v1, and GLTP_v2, linked to the human GLTP gene arise via alternative cis-splicing (X. Zou and R.E. Brown, unpublished). As shown in Fig. 9, exon1 is the only exon common to the GLTP, GLTP_v1, and GLTP_v2 transcripts. GLTP_v1 transcript (GenBank JN039379) consists of exons 1 and 2 as well as a new exon (exon N) derived from nucleotide sequence previously identified as intron 2 (1,021nt) of the GLTP gene [106]. Only a small portion (~10%) of exon N (339nt) represents open reading frame (39nt) due to an in-frame stop codon (TAA) that renders subsequent sequence as well as that of exons 3, 4, and 5 as noncoding. The encoded GLTP_v1 protein is predicted to consist of 67 amino acids. PCR template analyses of Human Multiple Tissue cDNA panels revealed high expression of GLTP_v1 in spleen and testis and lower expression in placenta, liver, pancreas, prostate, ovary, colon, and polymorphonucleocytes (Fig. 9). The other novel splice variant, GLTP_v2 (GenBank JN052207) retains exons 1, 4, and 5 of GLTP, but lacks exons 2, N, and 3 (Fig. 9). A splice-induced, reading frameshift confers novel coding of exons 4 and 5. The open reading frame of GLTP_v2 encodes a 97 amino-acid protein. Expression of GLTP_v2 is strong in pancreas and detectable in skeletal muscle and peripheral blood leukocytes (Fig. 9). Neither of the splice variants is capable of binding/transferring glycolipids and their cellular functions remain unexplored. To date, human genetic abnormalities directly involving the human GLTP gene have yet to be reported in the scientific literature.
Fig. 9. Genetic origins and organization of human GLTP transcripts.

(Upper panel) GLTP organization on chromosome 12 at locus 12q24.11. Exons are shown as color-filled rectangles with connecting lines representing introns. Mature GLTP transcript consisting of 5 exons encodes active protein that is able to transfer GSLs. The GLTP gene is also the source of two additional transcripts (GLTP_v1 and GLTP_v2) that encode significantly truncated proteins and are not expected to function as glycolipid transfer proteins. Their cellular function(s) remain to be explored. (Lower panel) GLTP, GLTP_v1, and GLTP_v2 transcript levels in human tissues were determined by PCR template analyses of Human Multiple Tissue cDNA panels described in [19] by amplification using primer-pairs 5′tcaaagctgtgtacgacaccaacc3′/5′tctcgtagatgacat-cgatggtcg3′ for GLTP; 5′cagtgtttactcccatcaaggcagac3’/5’cttatatgatcctggtggca-aacctg3’ for GLTP_v1; and 5′aatggcgctgctggccgaacac-3′/5′tgtttctccatgtggccacg-agtc3′ for GLTP_v2.
Interestingly, the molecular evolution of GLTP has been linked to TRPV4 which encodes a membrane cation channel sensory protein that localizes specifically to cholesterol-enriched lipid rafts to form a signaling complex that includes membrane components (e.g. cholesterol) and sub-membranous cytoskeleton [136]. TRPV4 promotes cell-cell junction formation in skin keratinocytes and regulates expression of pro-inflammatory chemokines and cytokines in adipocytes. The TRPV4 gene is a close neighbor of the GLTP gene at locus 12q24.11 of chromosome 12. Synteny analysis indicates that TRPV4 has coevolved with GLTP as well as the sterol biosynthetic gene for mevalonate kinase [136]. The findings suggest a possible involvement of GLTP in the formation and/or maintenance cholesterol-enriched lipid rafts, an idea in need of further exploration.
5.3. GLTP – regulatory sensor of cellular sphingolipid homeostasis
The essence of much debate regarding GLTP in vivo function is whether this protein actually functions as an intermembrane transporter of GSLs or as a regulatory sensor of sphingolipid metabolic homeostasis [108,121,123,137,138]. Feedback regulation of GLTP mRNA at the transcriptional level by certain sphingolipids has been revealed by characterization of the GLTP gene promoter [139]. The study identified the constitutive and basal (225 bp; ~78% guanine (G) + cystosine (C)) human GLTP promoters along with adjacent regulatory elements using luciferase and GFP reporters in concert with deletion mutants. Despite high G+C content, translational regulation by the mTOR pathway is not observed. Testing of sphingolipids (GlcCer, sulfatide, ganglioside GM1, S1P, sphingosine, C1P, dihydroceramide, ceramide) showed that only ceramide, a nonglycosylated precursor metabolite, can induce GLTP promoter activity despite being unable to bind to GLTP protein. Elevated ceramide was found to up-regulate GLTP promoter activity as well as mitigate decreases in promoter activity induced by knockdown of transcription factors such as specificity protein 1/specificity protein 3 (Sp1/Sp3). Four GC-boxes serve as functional Sp1/Sp3 transcription factor binding sites. Sp1/Sp3 RNA silencing and mithramycin-A treatment significantly inhibit GLTP promoter activity. Interestingly, ceramide treatment alters the GLTP promoter binding affinity for Sp1 and Sp3 rather than altering their endogenous levels. In the case of Sp3, the altered binding affinity is linked to ceramide-induced changes in acetylated Sp3 levels, a modification known to regulate Sp3 transcriptional activity. It is noteworthy that the GeneCards on-line human database currently indicates that the GLTP promoter region may also be regulated by transcription factors such as Arnt, c-Myc, GATA-1, Max, Pax-4a, RFX1, Sox5, Sox9, and YY1 based on Qiagen bioinformatics data predictions.
Direct evidence for a potential role in the regulation of SL homeostasis in vivo by GLTP began to emerge when SL metabolic labeling concurrent with GLTP overexpression revealed changes in cellular de novo sphingolipid production. The changes included increased GlcCer synthesis and decreased sphingomyelin synthesis, but no changes in GalCer or LacCer synthesis [140]. Subsequent studies by the Mattjus lab have shown that treatment of human skin fibroblasts with Golgi/ER vesicle transport inhibitors (brefeldin A and monesin) elevate the cellular levels of simple SLs (GlcCer > GalCer > LacCer > Cer > SM) as well as GLTP expression at the transcript and protein levels [137]. Yet, inhibition of GSL degradation with conduritol-B-epoxide that results in GlcCer accumulation in the lysosomes exerts no effect on GLTP levels. Blocking GlcCer synthase activity with N-butyl-deoxynojirimycin or 1-phenyl–2-decanoylamino-3-morpholino-1-propanol or by RNAi-knockdown of GlcCer synthase also decreases both GlcCer and GLTP levels [137]. In a lipidomics analysis of HeLa cells [138], up- and down-regulation of GLTP expression were found to affect GlcCer and Gb3 levels but exerted no change to GalCer and minimal changes to LacCer and Cer levels. To explain their findings, Mattjus and co-workers suggest that GLTP may function as an in vivo sensor to regulate GlcCer production levels in the ER/cis-Golgi. In their view [138] the increased Gb3 levels in HeLa cells overexpressing GLTP could reflect GLTP involvement in enabling increased GlcCer at the ER/cis-Golgi for FAPP2 to subsequently transfer to the trans-Golgi for production of a LacCer pool that is available for Gb3 synthesis. As discussed earlier, the role of FAPP2 in transferring GlcCer to the trans-Golgi and existence of a LacCer pool for the production of Gb3 has been investigated in detail by De Matteis and colleagues [38,39,76]. In any case, while the mechanism of GLTP involvement remains to be further explored, the impact of GLTP expression on the homeostatic levels of certain GSLs is becoming clear.
Recent proteomic studies in mice provide evidence for Gltp involvement in the neuronal myelination process [141–143]. One study is especially noteworthy [143]. In Niemann-Pick Type-C disease (NPC), hypomyelination occurs via mutations in the intracellular cholesterol transporter 1 or 2 (Npc1 or Npc2) that deliver the high cholesterol levels needed for proper myelin formation. In conditional npc-1-knockout mice, the impaired cholesterol transport leads to delayed and reduced myelination [e.g. 143 and references therein]. A recent proteomics study involving identification of > 3000 proteins in the corpus callosum of NPC mice brains provides new possibilities for GLTP in vivo function [143]. The corpus callosum is a wide, thick nerve tract, beneath the cerebral cortex in the brain found only in placental mammals. It consists of an axon fiber bundle, supported by neurons, astrocytes, oligodendrocytes, and microglia, that connects the left and right cerebral hemispheres, enabling communication between them. As expected NPC mice exhibited diminished levels of various myelin structural proteins. In addition, downregulation of ceramide synthase 2 (CerS2), UDP glycosyltransferase 8 (Ugt8), and glycolipid transfer protein (Gltp) was observed [140]. Notably, Gltp levels in NPC mice were only 32% of normal WT mice. The major contributions of GalCer and sulfatide, i.e. 3-SO3-GalCer, to myelin structure and function are well known [144,145]. The new finding suggests the need for Gltp transport of sulfatide and GalCer produced by Ugt8 and CerS2 during myelin formation/maintenance. It is noteworthy that in vitro studies clearly show that GLTP and FAPP2 both transfer GalCer efficiently but only GLTP transfers sulfatide well [35,36,40,41,102].
5.4. GLTP induction of necroptosis
Certain sphingolipids are established regulators of various PCD processes as well as senescence [146–152]. Gaining insights into the mechanisms by which sphingolipid effectors can trigger apoptosis have been pursued due to the therapeutic potential for combatting various pathological conditions [e.g. [153,154]]. In particular, ceramide as well as S1P and C1P have been well studied as respective cell pro-death and pro-life effectors. The ratio between S1P (and C1P) and ceramide, i.e. the ‘sphingolipid rheostat’, correlates with cell survival versus PCD [155–159]. Recent interest has broadened to PCD mechanisms other than apoptosis, i.e. autophagy, pyroptosis, necroptosis, that can help induce the therapeutic killing of apoptotic-resistant cells as potential alternate means for stimulating cell death when apoptosis induction becomes compromised such often as occurs in cancer. Necroptosis, a regulated form of necrosis, has recently generated much interest in cancer therapeutics as a potential means for induction of non-apoptotic death for cells that become apoptosis-resistant during chemotherapy [160–163].
Hints of GLTP’s possible involvement in programmed cell death processes began to be explored when changes in GLTP expression were found to dramatically affect the phenotype of certain cells. Overexpression of human GLTP in HeLa cells and HEK-293 cells resulted in cell rounding (~70% transformation) at 24 h post-transfection [164]. In contrast, overexpression of W96A-GLTP, a ligand-site point mutant with abrogated glycolipid transfer ability, failed to alter cell shape. The round adherent cells remained viable but exhibited diminished motility in wound healing assays and could not endocytose cholera toxin suggesting transition to a quiescent state. Regulation of the GLTP-induced cell rounding response occurred via interaction with δ-catenin. Co-expression of GLTP with δ-catenin accelerated the transition to a rounded phenotype but δ-catenin overexpression alone induced dendritic outgrowths from the cell surface [164]. The phenotypic changes triggered by GLTP overexpression did not reflect increased poly(ADP-ribose) polymerase cleavage (PARP) by caspase-3 activation, a hallmark indicator for cell apoptosis and critical executioner of both the extrinsic (death ligand) and intrinsic (mitochondrial) apoptotic pathways.
Recent studies of colon cancer cells have yielded exciting new findings that link GLTP expression to cell cycle progression and PCD processes [165]. Human GLTP overexpression has been found to inhibit the growth of human colon carcinoma cells (HT-29; HCT-116) while sparing normal colonic cells (CCD-18Co). The growth inhibition by GLTP overexpression reflects arrest of the cell cycle at the G1/S checkpoint via upregulation of cyclin-dependent kinase inhibitor-1B (Kip1/p27) and cyclin-dependent kinase inhibitor 1A (Cip1/p21) at the protein and mRNA levels, and downregulation of cyclin-dependent kinase-2 (CDK2), cyclin-dependent kinase-4 (CDK4), cyclin E and cyclin D1 protein levels. Interestingly, the biological fate of the GLTP-induced, growth-arrested HCT-116 and HT-29 cells differs. HCT-116 cells overexpressing GLTP showed no increase in cell death suggesting transition to a quiescent state. Yet, HT-29 cells overexpressing GLTP underwent cell death by necroptosis as revealed by phosphorylation of human mixed lineage kinase domain-like protein (pMLKL) via receptor-interacting protein kinase-3 (RIPK-3), elevated cytosolic calcium, and plasma membrane permeabilization by pMLKL oligomerization. The GLTP-induced necroptosis was abrogated by RNAi depletion of RIPK-3 or MLKL but not by pretreatment of the cells with pan caspase inhibitor zVAD. Overexpression of W96A-GLTP, an ablated GSL binding site mutant, did not arrest the cell cycle or induce necroptosis. Sphingolipid analyses (ceramide, monohexosylceramide, sphingomyelin, C1P, sphingosine, and S1P) of colon carcinoma cells overexpressing GLTP revealed interesting changes in sphingolipid metabolic homeostasis especially with respect to altered S1P and ceramide levels [165]. Whereas HCT-116 cells overexpressing GLTP displayed no significant change in S1P and only slightly increased 16:0-Cer, large decreases in S1P along with minimal change in 16:0-ceramide (> 5-fold or 85%) were found in HT-29 cells overexpressing GLTP, tipping the ‘sphingolipid rheostat’ (S1P/16:0-Cer ratio) towards cell death (Fig. 10). The findings establish GLTP upregulation as a previously unknown suppressor of human colon carcinoma HT-29 cell growth via interference with cell cycle progression and induction of necroptosis [165]. The discovery of necroptosis induction by changes in GLTP expression provides a potential new avenue for combating cancer progression in cells that become chemo-resistant to apoptosis induction.
Fig. 10. Changes in ceramides and S1P induced by GLTP overexpression.

(Upper panels) HCT-116 colon cancer cells and (Lower panels) HT-29 colorectal adenocarncinoma cells. Both cell lines have epithelial morphology. Figure adapted from [165]. Sphingolipidomics analyses were carried out by UPLC-mass spectrometry. Analyses of sphingomyelin, monohexosylceramide, and ceramide-1-phosphate species as well as sphingosine and sphinganine levels can be found in Supplementary Figure S6 of [165].
6. Closing comments
It is becoming increasingly clear that GLTP superfamily proteins have the potential to impact intracellular SL homeostasis by functioning in a sensor capacity to regulate SL metabolism. Whether this ‘sensor function’ directly involves the transfer of key metabolites (e.g. GlcCer or C1P) to specific sites within cells is not yet confirmed. The SL metabolic ‘sensor’ function of GLTP superfamily members is expected to be of utmost importance in cells because of the life-or-death processes in which SLs participate, i.e., cell proliferation, differentiation, development, apoptosis, autophagy, inflammation, pyroptosis, and necroptosis. The phylogenetic conservation of the GLTP-fold, which extends from fungi to humans, attests to the importance of the GLTP superfamily. Evolutionary modification has led to human GLTP-folds with specificity for C1P (e.g. CPTP) or with variable selectivity for GSLs (e.g. FAPP2 and GLTP) that are encoded by single-copy genes with differing intron/exon organizations on different human chromosomes.
A remaining question is whether additional sphingolipid transfer proteins exist that have eluded detection to date. This question is especially relevant to mono-chain SLs such as lyso-GSLs which have very low binding affinity for GLTP [166] as well as sphingosine and related sphingoid metabolites. Especially notable is the case for S1P and for sphingosine. Unresolved questions persist regarding the mechanism used by cells transport sphingosine generated via the salvage pathway back to the endoplasmic reticulum from the lysosomes [167]. Thus, the search for new sphingolipid transfer proteins needs to continue.
In any case, the recent in vivo discoveries involving GLTP superfamily members establish a translational bridge between the world of GLTP-fold basic science and disease-related pathologies. Notable examples include CPTP downregulation stimulating pro-inflammatory eicosanoid production and autophagy that drives inflammasome assembly for pro-inflammatory interleukin release. Moreover, GLTP up-regulation leads to lower levels of S1P without affecting ceramide levels in HT-29 colon cancer cells. The end result for RIPK-3 high expressers such as HT-29 cells is induction of necroptosis. These timely findings are opening new avenues for future cross-disciplinary, translational medical research involving GLTP-fold proteins in human health and disease [72,165,168]. Such avenues include targeted regulation of specific GLTP superfamily members to alter sphingolipid levels as a therapeutic means for combating viral infection, neurodegenerative conditions and circumventing chemo-resistance during cancer treatment.
Acknowledgements
We are grateful to Xin Lin, Taeowan Chung, and Helen Pike for their pioneering molecular biological and cell biological studies of GLTP and CPTP as members of the REB lab. Our studies also benefited from collaborative research on the plant CPTP orthologue, ACD11, with the John Mundy lab via Nikolaj Petersen, Daniel Hofius, and David Munch. We are thankful for biophysical studies carried out by Xiuhong Zhai, Dhirendra Simanshu, Ivan Boldyrev, Ravi-Kanth Kamlekar, Roop Kenoth, Margarita Malakhova, Chetan Rao, Xin-Min Li, and Peter Mattjus as well as to other long-standing collaborators (Julian G. Molotkovsky and Dinshaw J. Patel) who played key roles in elucidating GLTP-fold structure/function relationships. We especially appreciate the long-term interest and support of GLTP superfamily research by Dr. Jean Chin while administering NIH RO1-GM45928 and also are grateful for support received from RO1-CA121493, RO1-HL125353, Southern Minnesota Paint-the-Town-Pink Grant Awards, and the Hormel Foundation.
Abbreviations:
- ACD11
accelerated cell death-11
- ACBD3
acyl-coenzyme A binding domain containing 3
- AMD
age-related macular degeneration
- ATG5
autophagy-related protein 5
- ATG9A
autophagy-related protein 9A
- Cer
ceramide
- CPTP
ceramide-1-phosphate transfer protein
- CERT
ceramide transfer protein
- CRP
C-reactive protein
- ER
endoplasmic reticulum
- GalCer
galactosylceramide
- Gb3
globotriaosylceramide
- GlcCer
glucosylceramide
- GLTP
glycolipid transfer protein
- GLTPD2
glycolipid transfer protein domain-containing protein-2
- GLTPH
glycolipid transfer protein homology
- GSLs
glycosphingolipids
- HCV
hepatitis C virus
- HER2
human epidermal growth factor receptor 2
- IL-1β
interleukin-1β
- IL-18
interleukin-18
- LacCer
lactosylceramide
- LTPs
lipid transfer proteins
- LAMs
LTPs anchored at membrane contact sites
- mTOR
mammalian-target-of-rapamycin
- MLKL
mixed lineage kinase domain-like
- GD3
disialosyllactosylceramide
- GM3
monosialosyldihexosylganglioside
- GM1
monosialosyltetrahexosylganglioside
- LC3
microtubule-associated protein 1A/1B-light chain 3
- LC3-II
LC3-conjugated to phosphadiylethanolamine
- NLRP3
NLR family pyrin domain containing 3
- CARD
NLR family caspase recruitment domain
- NLRC4
containing 4
- POPC
1-palmitoyl-2-oleoyl phosphatidylcholine
- POPG
1-palmitoyl-2-oleoyl phosphatidylglycerol
- POPS
1-palmitoyl-2-oleoyl phosphatidylserine
- cPLA2α
IVA phospholipase A2
- PI-4P
phosphatidylinositol 4-phosphate
- FAPP2
phosphatidylinositol 4-phosphate adaptor protein-2
- PH
pleckstrin homology
- PCD
programmed cell death
- (RACE)-PCR
rapid-amplification-of-cDNA-ends
- GAP
Ras-related Rab33b-guanosine triphosphatase–activating protein
- RIPK-3
receptor-interacting protein kinase 3
- RGP3
regulator of G-protein signaling 3
- p70S6K
ribosomal protein S6 kinase
- FasL
soluble Fas ligand
- SQSTM1 or p62
sequestosome 1
- CPTPi
RNAi-induced CPTP depletion
- Sp1/Sp3
specificity protein 1/specificity protein 3
- SMs
sphingomyelins
- SL
sphingolipid
- S1P
sphingosine-1-phosphate
- START
steroidogenic acute regulatory (Star) protein-related lipid transfer
- TNF
tumor necrosis factor
- TACE
tumor necrosis factor-alpha converting enzyme
- ULK1
unc-51-like kinase 1
- UVRAG
UV-resistance-associated gene
- VAMP-associated proteins or VAPs
vesicle-associated membrane protein-associated proteins
- WIPI-1 or Atg18
WD repeat domain phosphoinositide-interacting protein 1
Footnotes
Declaration of Competing Interest
The authors declare no conflicts of interest and have no relevant financial or nonfinancial relationships to disclose.
References
- [1].van Meer G, Voelker DR, Feigenson GW, Membrane lipids: where they are and how they behave, Nat Rev Mol Cell Biol 9 (2008) 112–124, 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Casares D, Escribá PV, Rosselló CA, Membrane lipid composition: effect on membrane and organelle structure, function and compartmentalization and therapeutic avenues, Int J Mol Sci 20 (2019) 2167, 10.3390/ijms20092167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Andersen JP, Vestergaard AL, Mikkelsen SA, Mogensen LS, Chalat M, Molday RS, P4-ATPases as phospholipid flippases—structure, function,and enigmas, Front Physiol 7 (2016) 275, 10.3389/fphys.2016.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Takada N, Naito T, Inoue T, Nakayama K, Takatsu H, Shin H-W, Phospholipid-flipping activity of P4-ATPase drives membrane curvature, EMBO J 37 (2018) e97705, 10.15252/embj.201797705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hiraizumi M, Yamashita K, Nishizawa T, Nureki O, Cryo-EM structures capture the transport cycle of the P4-ATPase flippase, Science 365 (2019) 1149–1155, 10.1126/science.aay3353. [DOI] [PubMed] [Google Scholar]
- [6].Theorin L, Faxén K, Sørensen DM, Migotti R, Dittmar G, Schiller J, et al. , The lipid head group is the key element for substrate recognition by the P4 ATPase ALA2: a phosphatidylserine flippase, Biochem J 476 (2019) 783–794, 10.1042/BCJ20180891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Locher KP, Mechanistic diversity in ATP-binding cassette (ABC) transporters, Nat Struct Mol Biol 23 (2016) 487–493, 10.1038/nsmb.3216. [DOI] [PubMed] [Google Scholar]
- [8].Neumann J, Rose-Sperling D, Hel UA, Diverse relations between ABC transporters and lipids: An overview, Biochim Biophys Acta 2017 (1859) 605–618 10.1016/j.bbamem.2016.09.023. [DOI] [PubMed] [Google Scholar]
- [9].Qian H, Zhao X, Cao P, Lei J, Yan N, Gong X, Structure of the human lipid exporter ABCA1, Cell 169 (2017) 1228–1239, 10.1016/j.cell.2017.05.020. [DOI] [PubMed] [Google Scholar]
- [10].Holthuis JCM, Menon AK, Lipid landscapes and pipelines in membrane homeostasis, Nature 510 (2014) 48–57, 10.1038/nature13474. [DOI] [PubMed] [Google Scholar]
- [11].Prinz WA, Bridging the gap: membrane contact sites in signaling, metabolism, and organelle dynamics, J Cell Biol 205 (2014) 759–769, 10.1083/jcb.201401126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wong LH, Čopič A, Levine TP, Advances on the transfer of lipids by lipid transfer proteins, Trends Biochem Sci 42 (2017) 516–530, 10.1016/j.tibs.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Muallem S, Chung WY, Jha A, Ahuja M, Lipids at membrane contact sites: cell signaling and ion transport, EMBO Rep 18 (2017) 1893–1904, 10.15252/embr.201744331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jain A, Holthuis JCM, Membrane contact sites, ancient and central hubs of cellular lipid logistics, Biochim Biophys Acta Mol Cell Res 2017 (1864) 1450–1458 10.1016/j.bbamcr.2017.05.017. [DOI] [PubMed] [Google Scholar]
- [15].Handa K, Lipid transfer proteins rectify interorganelle flux and accurately deliver lipids at membrane contact sites, J Lipid Res 59 (2018) 1341–1366, 10.1194/jlr.R085324 And errata: J Lipid Res 2018;59:2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Luo J, Jiang L-Y, Yang H, Song B-L, Intracellular cholesterol transport by sterol transfer proteins at membrane contact sites, Trends Biochem Sci 44 (2019) 272–292, 10.1016/j.tibs.2018.10.001. [DOI] [PubMed] [Google Scholar]
- [17].Wong LH, Gatta AT, Levine TP, Lipid transfer proteins: the lipid commute via shuttles, bridges, and tubes, Nat Rev Mol Cell Biol 20 (2019) 85–101, 10.1038/s41580-018-0071-5. [DOI] [PubMed] [Google Scholar]
- [18].Malinina L, Malakhova ML, Teplov A, Brown RE, Patel DJ, Structural basis for glycosphingolipid transfer specificity, Nature 430 (2004) 1048–1053, 10.1038/nature02856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Simanshu DK, Kamlekar R-K, Wijesinghe DS, Zou X, Zhai X, Mishra SK, et al. , Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids, Nature 500 (2013) 463–467, 10.1038/nature12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Simanshu DK, Zhai X, Munch D, Hofius D, Markham JE, Bielawski J, et al. , Arabidopsis accelerated cell death 11, ACD11, is a ceramide-1-phosphate transfer protein and intermediary regulator of phytoceramide levels, Cell Rep 6 (2014) 388–399, 10.1016/j.celrep.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Malinina L, Simanshu DK, Zhai X, Samygina VR, Kamlekar R-K, Kenoth R, et al. , Sphingolipid transfer proteins defined by the GLTP-fold, Q Rev Biophys 48 (2015) 281–322, 10.1017/S003358351400016X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Malinina L, Patel DJ, Brown RE, How α-helical motifs form functionally diverse lipid-binding compartments, Annu Rev Biochem 86 (2017) 609–636, 10.1146/annurev-biochem-061516-044445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hoeferlin LA, Wijesinghe DS, Chalfant CE, The role of ceramide-1-phosphate in biological functions, in: Gulbins E, Petrache I (Eds.), Handbook of Experimental Pharmacology, 215 Springer-Verlag Wien, 2013, pp. 153–165, , 10.1007/978-3-7091-1368-4_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gomez-Muñoz A, Presa N, Gomez-Larrauri A, Rivera I-G, Trueba M, Ordoñez M, Control of inflammatory responses by ceramide, sphingosine 1-phosphate and ceramide 1-phosphate, Prog Lipid Res 61 (2016) 51–62, 10.1016/j.plipres.2015.09.002. [DOI] [PubMed] [Google Scholar]
- [25].Gomez-Muñoz A, The role of ceramide 1-phosphate in tumor cell survival and dissemination, Adv Cancer Res 140 (2018) 217–234, 10.1016/bs.acr.2018.04.012. [DOI] [PubMed] [Google Scholar]
- [26].Mishra SK, Gao Y-G, Deng Y, Chalfant CE, Hinchcliffe EH, Brown RE, CPTP: a sphingolipid transfer protein that regulates autophagy and inflammasome activation, Autophagy 14 (2018) 862–879, 10.1080/15548627.2017.1393129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Boath A, Graf C, Lidome E, Ullrich T, Nussbaumer P, Bornancin F, Regulation and traffic of ceramide 1-phosphate produced by ceramide kinase, J Biol Chem 283 (2008) 8517–8526, 10.1074/jbc.M707107200. [DOI] [PubMed] [Google Scholar]
- [28].Brodersen P, Petersen M, Pike HM, Olszak B, Skov S, Ødum N, et al. , Knockout of Arabidopsis ACCELERATED-CELL-DEATH11 encoding a sphingosine transfer protein causes activation of programmed cell death and defense, Genes Dev 16 (2002) 490–502, 10.1101/gad.218202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Petersen NHT, McKinney LV, Pike H, Hofius D, Zakaria A, Brodersen P, et al. , Human GLTP and mutant forms of ACD11 suppress cell death in the Arabidopsis acd11 mutant, FEBS J 275 (2008) 4378–4388, 10.1111/j.1742-4658.2008.06584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Malinina L, Malakhova ML, Kanack AT, Lu M, Abagyan R, Brown RE, et al. , The liganding of glycolipid transfer protein is controlled by glycolipid acyl structure, PLoS Biol 4 (2006) e362, , 10.1371/journal.pbio.0040362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Airenne TA, Kidron H, Nymalm Y, Nylund M, West G, Mattjus P, et al. , Structural evidence for adaptive ligand binding of glycolipid transfer protein, J Mol Biol 355 (2006) 224–236, 10.1016/j.jmb.2005.10.031. [DOI] [PubMed] [Google Scholar]
- [32].Lin X, Mattjus P, Pike HM, Windebank AJ, Brown RE, Cloning and expression of glycolipid transfer protein from bovine and porcine brain, J Biol Chem 275 (2000) 5104–5110, 10.1074/jbc.275.7.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li X-M, Malakhova ML, Lin X, Pike HM, Chung T, Molotkovsky JG, et al. , Human glycolipid transfer protein: Probing conformation using fluorescence spectroscopy, Biochemistry 43 (2004) 10285–10294, 10.1021/bi0495432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kenoth R, Simanshu DK, Kamlekar R-K, Pike HM, Molotkovsky JG, Benson LM, et al. , Structural determination and tryptophan fluorescence of heterokaryon incompatibility C2 protein (HET-C2), a fungal glycolipid transfer protein (GLTP), provide novel insights into glycolipid specificity and membrane interaction by the GLTP-fold, J Biol Chem 285 (2010) 13066–13078, 10.1074/jbc.M109.093203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Samygina VR, Popov AN, Cabo-Bilbao A, Ochoa-Lizarralde B, Goni-de-Cerio F,Zhai X, et al. , Enhanced selectivity for sulfatide by engineered human glycolipid transfer protein, Structure 19 (2011) 1644–1654, 10.1016/j.str.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Samygina VR, Ochoa-Lizarralde B, Popov AN, Cabo-Bilbao A, Goni-de-Cerio F, Molotkovsky JG, et al. , Structural insights into lipid-dependent reversible dimerization of human GLTP, Acta Crystallogr D69 (2013) 603–616, 10.1107/S0907444913000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].West G, Viitanen L, Alm C, Mattjus P, Salminen TA, Edqvist J, Identification of a glycosphingolipid transfer protein GLTP1 in Arabidopsis thaliana, FEBS J 275 (2008) 3421–3437, 10.1111/j.1742-4658.2008.06498.x. [DOI] [PubMed] [Google Scholar]
- [38].D’Angelo G, Polishchuk E, Di Tullio G, Santoro M, Di Campli A, Godi A, et al. , Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide, Nature 449 (2007) 62–67, 10.1038/nature06097. [DOI] [PubMed] [Google Scholar]
- [39].D’Angelo G, Uemura T, Chuang CC, Polishchuk E, Santoro M, Ohvo-Rekilä H, et al. , Vesicular and non-vesicular transport feed distinct glycosylation pathways in the Golgi, Nature 501 (2013) 116–120, 10.1038/nature12423. [DOI] [PubMed] [Google Scholar]
- [40].Kamlekar R-K, Simanshu DK, Gao Y-G, Kenoth R, Pike HM, Prendergast FG, et al. , The glycolipid transfer protein (GLTP) domain of phosphoinositol 4-phosphate adaptor protein-2 (FAPP2): Structure drives preference for simple neutral glycosphingolipids, Biochim Biophys Acta 2013 (1831) 417–427, 10.1016/j.bbalip.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ochoa-Lizarralde B, Popov AN Gao Y-G, Samygina VR, Zhai X, Mishra SK, Boldyrev IA, et al. , Structural analyses of 4-phosphate adaptor protein 2 yield mechanistic insights into sphingolipid recognition by the glycolipid transfer protein family, J Biol Chem 293 (2018), 10.1074/jbc.RA117.000733 16709–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hirsch AK, Fischer FR, Diederich F, Phosphate recognition in structural biology, Angew Chem Int Ed 46 (2007) 338–352, 10.1002/anie.200603420. [DOI] [PubMed] [Google Scholar]
- [43].Berna A, Bernier F, Chabriere E, Perera T, Scott K, DING proteins; novel members of a prokaryotic phosphate-binding protein superfamily which extends into the eukaryotic kingdom, Int J Biochem Cell Biol 40 (2008) 170–175, 10.1016/j.biocel.2007.02.004. [DOI] [PubMed] [Google Scholar]
- [44].Luecke H, Quiocho FA, High specificity of a phosphate transport protein determined by hydrogen bonds, Nature 347 (1990) 402–406. [DOI] [PubMed] [Google Scholar]
- [45].Bourquin F, Riezman H, Capitani G, Grutter MG, Structure and function of sphingosine-1-phosphate lyase, a key enzyme of sphingolipid metabolism, Structure 18 (2010) 1054–1065, 10.1016/j.str.2010.05.011. [DOI] [PubMed] [Google Scholar]
- [46].Stahelin RV, Subramanian P, Vora M, Cho W, Chalfant CE, Ceramide-1-phosphate binds Group IVA cytosolic phospholipase A2 via a novel site in the C2 domain, J Biol Chem 282 (2007) 20467–20474, 10.1074/jbc.M701396200. [DOI] [PubMed] [Google Scholar]
- [47].Ward KE, Bhardwaj N, Vora M, Chalfant CE, Lu H, Stahelin RV, The molecular basis of ceramide-1-phosphate recognition by C2 domains, J Lipid Res 54 (2013) 636–648, 10.1194/jlr.M031088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, et al. , Molecular machinery for non-vesicular trafficking of ceramide, Nature 426 (2003) 803–809, 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- [49].Yamaji T, Hanada K, Sphingolipid metabolism and interorganellar transport: localization of sphingolipid enzymes and lipid transfer proteins, Traffic 16 (2015) 101–122, 10.1111/tra.12239. [DOI] [PubMed] [Google Scholar]
- [50].Thorsell A-G, Lee W-H, Persson C, Siponen MI, Nilsson M, Busam RD, et al. , Comparative structural analysis of lipid binding START domains, PLoS One 6 (2010) e19521, , 10.1371/journal.pone.0019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kudo N, Kumagai K, Tomishige N, Yamaji T, Wakatsuki S, Nishijima M, et al. , Structural basis for specific lipid recognition by CERT responsible for nonvesicular trafficking of ceramide, Proc Natl Acad Sci U S A 105 (2008) 488–493, 10.1073/pnas.0709191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Prashek J, Truong T, Yao X, Crystal structure of the pleckstrin homology domain from the ceramide transfer protein: implications for conformational change upon ligand binding, PLoS One 8 (2013) e79590, , 10.1371/journal.pone.0079590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Prashek J, Bouyain S, Fu M, Li Y, Berkes D, Yao X, Interaction between the PH and START domains of ceramide transfer protein competes with phosphatidylinositol 4-phosphate binding by the PH domain, J Biol Chem 292 (2017) 14217–14228, 10.1074/jbc.M117.780007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhai X, Gao Y-G, Mishra SK, Simanshu DK, Boldyrev IA, Benson LM, et al. , Phosphatidylserine stimulates ceramide 1-phosphate (C1P) intermembrane transfer by C1P transfer proteins, J Biol Chem 292 (2017) 2531–2541, 10.1074/jbc.M116.760256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Leventis PA, Grinstein S, The distribution and function of phosphatidylserine in cellular membranes, Annu Rev Biophys 39 (2010) 407–427, 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- [56].Fairn GD, Schieber NL, Ariotti N, Murphy S, Kuerschner L, Webb RI, et al. , High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine, J Cell Biol 194 (2011) 257–275, 10.1083/jcb.201012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhai X, Simanshu DK, Boldyrev IA, Pike HM, Mundy J, Malinina L, et al. , Sphingolipid transfer by GLTP-fold proteins is differentially regulated by phosphatidylinositol derivatives, Biophys J 112 (2017) 229a. [Google Scholar]
- [58].Bornancin F, Ceramide kinase: the first decade, Cell Signal 23 (2011) 999–1008, 10.1016/j.cellsig.2010.11.012. [DOI] [PubMed] [Google Scholar]
- [59].Channon JY, Leslie CC, A calcium-dependent mechanism for associating a soluble arachidonoyl-hydrolyzing phospholipase A2 with membrane in the macrophage cell line RAW 264.7, J Biol Chem 265 (1990) 5409–5413. [PubMed] [Google Scholar]
- [60].Clark JD, Lin L-L, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, et al. , A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP, Cell 65 (1991) 1043–1051, 10.1016/0092-8674(91)90556-E. [DOI] [PubMed] [Google Scholar]
- [61].Harizi H, Corcuff J-B, Gualde N, Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology, Trends Mol Med 14 (2008) 461–469, 10.1016/j.molmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- [62].Takahashi Y, Meyerkord CL, Hori T, Runkle K, Fox TE, Kester M, et al. , Bif-1 regulates Atg9 trafficking by mediating the fission of Golgi membranes during autophagy, Autophagy 7 (2011) 61–73, 10.4161/auto.7.1.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lamb CA, Yoshimori T, Tooze SA, The autophagosome: origins unknown, biogenesis complex, Nat Rev Mol Cell Biol 14 (2013) 759–774, 10.1038/nrm3696. [DOI] [PubMed] [Google Scholar]
- [64].Bergsbaken T, Fink SL, Cookson BT, Pyroptosis: host cell death and inflammation, Nat Rev Microbiol 7 (2009) 99–109, 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Frank D, Vince JE, Pyroptosis versus necroptosis: similarities, differences, and crosstalk, Cell Death Differ 26 (2019) 99–114, 10.1038/s41418-018-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].de Vasconcelos NM, Lamkanfi M, Recent Insights on Inflammasomes, gasdermin pores, and pyroptosis, Cold Spring Harb Perspect Biol (2019), 10.1101/cshperspect.a036392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wijesinghe DS, Subramanian P, Lamour NF, Gentile LB, Granado MH, Bielawska A, et al. , Chain length specificity for activation of cPLA2α by C1P: use of the dodecane delivery system to determine lipid-specific effects, J Lipid Res 50 (2009) 1986–1995, 10.1194/jlr.M800367-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Pettus BJ, Bielawska A, Subramanian P, Wijesinghe DS, Maceyka M, Leslie CC, et al. , Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2, J Biol Chem 279 (2004) 11320–11326, 10.1074/jbc.M309262200. [DOI] [PubMed] [Google Scholar]
- [69].Pettus BJ, Bielawska A, Spiegel S, Roddy P, Hannun YA, Chalfant CE, Ceramide kinase mediates cytokine and calcium ionophore-induced arachidonic acid release, J Biol Chem 2783 (2003) 38206–38213, 10.1074/jbc.M304816200. [DOI] [PubMed] [Google Scholar]
- [70].Kurji KH, Cui JZ, Lin T, Harriman D, Prasad SS, Kojic L, et al. , Microarray analysis identifies changes in inflammatory gene expression in response to amyloid-β stimulation of cultured human retinal pigment epithelial cells, Invest Ophthalmol Vis Sci 51 (2010) 1151–1163, 10.1167/iovs.09-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wang J, Li C, Jiang Y, Zheng H, Li D, Liang Y, et al. , Effect of ceramide-1-phosphate transfer protein on intestinal bacterial translocation in severe acute pancreatitis, Clin Res Hepatol Gastroenterol 41 (2017) 86–92, 10.1016/j.clinre.2016.08.003. [DOI] [PubMed] [Google Scholar]
- [72].Mishra SK, Brown RE, Sphingolipids transfer proteins (GLTP and CPTP) regulate the neoplastic progression of colon and breast cancer cells, Cancer Res 77 (Suppl) (2017), 10.1158/1538-7445.AM2017-1123 Abstr.1123. [DOI] [Google Scholar]
- [73].D’Angelo CS, Kohl I, Varela MC, de Castro CIE, Kim CA, Bertola DR, et al. , Extending the phenotype of monosomy 1p36 syndrome and mapping of a critical region for obesity and hyperphagia, Am J Med Genet Part A 152A (2010) 102–110, 10.1002/ajmg.a.33160. [DOI] [PubMed] [Google Scholar]
- [74].Dowler S, Currie RA, Campbell DG, Deak M, Kular G, Downes CP, et al. , Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities, Biochem J 351 (2000) 19–31, 10.1042/0264-6021:3510019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Godi A, Di Campli A, Konstantakopoulos A, Di Tullio G, Alessi DR, Kular GS, et al. , FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P, Nat Cell Biol 6 (2004) 393–404, 10.1038/ncb1119. [DOI] [PubMed] [Google Scholar]
- [76].D’Angelo G, Rega LR, De Matteis MA, Connecting vesicular transport with lipid synthesis: FAPP2, Biochim Biophys Acta 2012 (1821) 1089–1095, 10.1016/j.bbalip.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Halter D, Neumann S, van Dijk SM, Wolthoorn J, de Mazière AM, Vieira OV, et al. , Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis, J Cell Biol 179 (2007) 101–115, 10.1083/jcb.200704091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Loewen CJR, Roy A, Levine TP, A conserved ER targeting motif in three families of lipid binding proteins and in Opi1p binds VAP, EMBO J 22 (2003) 2025–2035, 10.1093/emboj/cdg201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kaiser SE, Brickner JH, Reilein AR, Fenn TD, Walter P, Brunger AT, Structural basis of FFAT motif-mediated ER targeting, Structure 13 (2005) 035–1045, 10.1016/j.str.2005.04.010. [DOI] [PubMed] [Google Scholar]
- [80].Mikitova V, Levine TP, Analysis of the key elements of FFAT-Like motifs identifies new proteins that potentially bind VAP on the ER, including two AKAPs and FAPP2, PLoS One 7 (2012) e30455, , 10.1371/journal.pone.0030455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Cao X, Coskun Ü, Rössle M, Buschhorn SB, Grzybek M, Dafforn TR, et al. , Golgi protein FAPP2 tubulates membranes, Proc Natl Acad Sci U S A 106 (2009) 21121–21125, 10.1073/pnas.0911789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lenoir M, Coskun Ü, Grzybek M, Cao X, Buschhorn SB, James J, et al. , Structural basis of wedging the Golgi membrane by FAPP pleckstrin homology domains, EMBO Rep 11 (2010) 279–284, 10.1038/embor.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Yui N, Okutsu R, Sohara E, Rai T, Ohta A, Noda Y, et al. , FAPP2 is required for aquaporin-2 apical sorting at trans-Golgi network in polarized MDCK cells, Am J Physiol Cell Physiol 297 (2009) C1389–C1396, 10.1152/ajpcell.00098.2009. [DOI] [PubMed] [Google Scholar]
- [84].Mayinger P, Signaling at the Golgi, Cold Spring Harb Perspect Biol 3 (2011) a005314, 10.1101/cshperspect.a005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Liao J, Guan Y, Chen W, Shi C, Yao D, Wang F, et al. , ACBD3 is required for FAPP2 transferring glucosylceramide through maintaining the Golgi integrity, J Mol Cell Biol 11 (2019) 107–117, 10.1093/jmcb/mjy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Tritz R, Hickey MJ, Lin AH, Hadwiger P, Sah DWY, Neuwelt EA, et al. , FAPP2 gene downregulation increases tumor cell sensitivity to Fas-induced apoptosis, Biochem Biophys Res Commun 383 (2009) 167–171, 10.1016/j.bbrc.2009.03.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Morgan MJ, Kim Y-S, The serine threonine kinase RIP3: lost and found, BMB Rep 48 (2015) 303–312, 10.5483/BMBRep.2015.48.6.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].He S, Wang L, Miao L, Wang T, Du F, Zhao L, et al. , Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α, Cell 137 (2009) 1100–1111, 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- [89].Ros U, Peña-Blanco A, Hänggi K, Kunzendorf U, Krautwald S, Wong W-WL, et al. , Necroptosis execution is mediated by plasma membrane nanopores independent of calcium, Cell Rep 19 (2017) 175–187, 10.1016/j.celrep.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Tummers B, Green DR, Caspase-8: regulating life and death, Immunol Rev 277 (2017) 76–89, 10.1111/imr.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Choi ME, Price DR, Ryter SW, Choi AMK, Necroptosis: a crucial pathogenic mediator of human disease, JCI Insight 4 (2019) e128834, , 10.1172/jci.insight.128834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Khan I, Katikaneni DS, Han Q, Sanchez-Felipe L, Hanada K, Ambrose RL, et al. , Modulation of hepatitis C Virus genome replication by glycosphingolipids and four-phosphate adaptor protein 2, J Virol 88 (2014) 12276–12295, 10.1128/JVI.00970-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Jung K, Kim J-H, Cheong HS, Shin E, Kim S-H, Hwang J-Y, et al. , Gene expression profile of necrotizing enterocolitis model in neonatal mice, Int J Surg 23 (2015) 28–34, 10.1016/j.ijsu.2015.09.049. [DOI] [PubMed] [Google Scholar]
- [94].Ahrens I, Domeij H, Eisenhardt SU, Topcic D, Albrecht M, Leitner E, et al. , Opposing effects of monomeric and pentameric C-reactive protein on endothelial progenitor cells, Basic Res Cardiol 106 (2011) 879–895, 10.1007/s00395-011-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Tabassum R, Rämö JT, Ripatti P, Koskela JT, Kurki M, Karjalainen J, et al. , Genetic architecture of human plasma lipidome and its link to cardiovascular disease, Nature Com 10 (2019) 4329, 10.1038/s41467-019-11954-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Metz RJ, Radin NS, Glucosylceramide uptake from spleen cytosol, J Biol Chem 255 (1980) 4463–4467. [PubMed] [Google Scholar]
- [97].Metz RJ, Radin NS, Purification and properties of a cerebroside transfer protein, J Biol Chem 257 (1982) 12901–12907. [PubMed] [Google Scholar]
- [98].Abe A, Yamada K, Sasaki T, A protein purified from pig brain accelerates the intermembranous translocation of mono- and dihexosylceramides, but not the translocation of phospholipids, Biochem Biophys Res Commun 104 (1982) 1386–1393, 10.1016/0006-291x(82)91403-6. [DOI] [PubMed] [Google Scholar]
- [99].Wong M, Brown RE, Barenholz Y, Thompson TE, Glycolipid transfer protein from bovine brain, Biochemistry 23 (1984) 6498–6505, 10.1021/bi00321a035. [DOI] [PubMed] [Google Scholar]
- [100].Abe A, Sasaki T, Purification and some properties of the glycolipid transfer protein from pig brain, J Biol Chem 260 (1985) 11231–11239. [PubMed] [Google Scholar]
- [101].Brown RE, Stephenson FA, Markello T, Barenholz Y, Thompson TE, Properties of a specific glycolipid transfer protein from bovine brain, Chem Phys Lipids 38 (1985) 79–93, 10.1016/0009-3084(85)90059-3. [DOI] [PubMed] [Google Scholar]
- [102].Yamada K, Abe A, Sasaki T, Specificity of glycolipid transfer protein from pig brain, J Biol Chem 260 (1986) 4615–4621. [PubMed] [Google Scholar]
- [103].Gammon CM, Ledeen RW, Evidence for the presence of a ganglioside transfer protein in brain, J Neurochem 44 (1985) 979–981, 10.1111/j.1471-4159.1985.tb12912.x. [DOI] [PubMed] [Google Scholar]
- [104].Gammon CM, Vaswani KK, Ledeen RW, Isolation of two glycolipid transfer proteins from bovine brain: reactivity towards gangliosides and neutral glycosphingolipids, Biochemistry 26 (1987) 6239–6243, 10.1021/bi00393a043. [DOI] [PubMed] [Google Scholar]
- [105].Brown RE, Jarvis KL, Hyland KJ, Purification and characterization of glycolipid transfer protein from bovine brain, Biochim Biophys Acta 1044 (1990) 77–83, 10.1016/0005-2760(90)90221-I. [DOI] [PubMed] [Google Scholar]
- [106].Zou X, Chung T, Lin X, Malakhova ML, Pike HM, Brown RE, Human glycolipid transfer protein (GLTP) genes: organization, transcriptional status and evolution, BMC Genomics 9 (2008) 72, 10.1186/1471-2164-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Mattjus P, Molotkovsky JG, Smaby JM, Brown RE, A fluorescence resonance energy transfer approach for monitoring protein-mediated glycolipid transfer between vesicle membranes, Anal Biochem 268 (1999) 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Malakhova ML, Malinina L, Pike HM, Kanack AT, Patel DJ, Brown RE, Point mutational analysis of the liganding site in human glycolipid transfer protein: Functionality of the complex, J Biol Chem 280 (2005) 26312–26320, 10.1074/jbc.M500481200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Backman APE, Halin J, Nurmi H, Möuts A, Kjellberg MA, Mattjus P, Glucosylceramide acyl chain length is sensed by the glycolipid transfer protein, PLoS One 13 (2018) e0209230, , 10.1371/journal.pone.0209230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Rao CS, Chung T, Pike HM, Brown RE, Glycolipid transfer protein interaction with bilayer vesicles: modulation by changing lipid composition, Biophys J 89 (2005) 4017–4028, 10.1529/biophysj.105.070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].West G, Nylund M, Slotte JP, Mattjus P, Membrane interaction and activity of the glycolipid transfer protein, Biochim Biophys Acta 2006 (1758) 1732–1742, 10.1016/j.bbamem.2006.06.020. [DOI] [PubMed] [Google Scholar]
- [112].Zhai X, Malakhova ML, Pike HM, Benson LM, Bergen III HR, Sugár IP, et al. , Glycolipid acquisition by human glycolipid transfer protein dramatically alters intrinsic tryptophan fluorescence: Insights into glycolipid binding affinity, J Biol Chem 284 (2009) 13620–13628, 10.1074/jbc.M809089200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Kamlekar R-K, Gao Y, Kenoth R, Molotkovsky JG, Prendergast FG, Malinina L, et al. , Human GLTP: three distinct functions for the three tryptophans in a novel peripheral amphitropic fold, Biophys J 99 (2010) 2626–2635, 10.1016/j.bpj.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ohvo-Rekilä H, Mattjus P, Monitoring glycolipid transfer protein activity and membrane interaction with the surface plasmon resonance technique, Biochim Biophys Acta 2011 (1808) 47–54, 10.1016/j.bbamem.2010.08.018. [DOI] [PubMed] [Google Scholar]
- [115].Rao CS, Lin X, Pike HM, Molotkovsky JG, Brown RE, Glycolipid transfer protein mediated transfer of glycosphingolipids between membranes: a model for action based on kinetic and thermodynamic analyses, Biochemistry 43 (2004) 13805–13815, 10.1021/bi0492197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Nylund M, Fortelius C, Palonen EK, Molotkovsky JG, Mattjus P, Membrane curvature effects on glycolipid transfer protein activity, Langmuir 23 (2007) 11726–11733, 10.1021/la701927. [DOI] [PubMed] [Google Scholar]
- [117].Mattjus P, Pike HM, Molotkovsky JG, Brown RE, Charged membrane surfaces impede the protein-mediated transfer of glycosphingolipids between phospholipid bilayers, Biochemistry 39 (2000) 1067–1075, 10.1021/bi991810u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Mattjus P, Kline A, Pike HM, Molotkovsky JG, Brown RE, Probing for preferential interactions among sphingolipids in bilayer vesicles using the glycolipid transfer protein, Biochemistry 41 (2002) 266–273, 10.1021/bi015718l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Nylund M, Kjellberg MA, Molotkovsky JG, Byun HS, Bittman R, Mattjus P, Molecular features of phospholipids that affect glycolipid transfer protein-mediated galactosylceramide transfer between vesicles, Biochim Biophys Acta 2006 (1758) 807–812, 10.1016/j.bbamem.2006.04.023. [DOI] [PubMed] [Google Scholar]
- [120].Nylund M, Mattjus P, Protein mediated glycolipid transfer is inhibited FROM sphingomyelin membranes but enhanced TO sphingomyelin containing raft like membranes, Biochim Biophys Acta 2005 (1669) 87–94, 10.1016/j.bbamem.2004.12.014. [DOI] [PubMed] [Google Scholar]
- [121].Brown RE, Mattjus P, Glycolipid transfer proteins, Biochim Biophys Acta 2007 (1771) 746–760, 10.1016/j.bbalip.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Mattjus P, Glycolipid transfer proteins and membrane interaction, Biochim Biophys Acta 2009 (1788) 267–272, 10.1016/j.bbamem.2008.10.003. [DOI] [PubMed] [Google Scholar]
- [123].Tuuf J, Mattjus P, Membranes and mammalian glycolipid transferring proteins, Chem Phys Lipids 178 (2014) 27–37, 10.1016/j.chemphyslip.2013.10.013. [DOI] [PubMed] [Google Scholar]
- [124].Warnock DE, Lutz MS, Blackburn WA, Young WW Jr., J.U. Baenziger, Transport of newly synthesized glucosylceramide to the plasma membrane by a non-Golgi pathway, Proc Natl Acad Sci U S A 91 (1994) 2708–2712, 10.1073/pnas.91.7.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Tuuf J, Wistbacka L, Mattjus P, The glycolipid transfer protein interacts with the vesicle-associated membrane protein-associated protein VAP-A, Biochem Biophys Res Commun 388 (2009) 395–399, 10.1016/j.bbrc.2009.08.023. [DOI] [PubMed] [Google Scholar]
- [126].Huang M-H, Li H, Xue R, Li J, Wang L, Cheng J, et al. , Up-regulation of glycolipid transfer protein by bicyclol causes spontaneous restriction of hepatitis C virus replication, Acta Pharm Sin B 9 (2019) 769–781, 10.1016/j.apsb.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Lauria I, van Üüm J, Mjumjunov-Crncevic E, Walrafen D, Spitta L, Thiele C, et al. , GLTP mediated non-vesicular GM1 transport between native membranes, PLoS One 8 (2013) e59871, , 10.1371/journal.pone.0059871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Sano R, Annunziata I, Patterson A, Moshiach S, Gomero E, Opferman J, et al. , GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis, Mol Cell 36 (2009) 500–511, 10.1016/j.molcel.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Ledeen RW, Wu G, Nuclear sphingolipids: metabolism and signaling, J Lipid Res 49 (2008) 1176–1186, 10.1194/jlr.R800009-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Ledeen RW, Wu G, New findings on nuclear gangliosides: overview on metabolism and function, J Neurochem 116 (2011) 714–720, 10.1111/j.1471-4159.2010.07115.x. [DOI] [PubMed] [Google Scholar]
- [131].Annunziata I, Sano R, d’Azzo A, Mitochondria-associated ER membranes (MAMs) and lysosomal storage diseases, Cell Death Dis 9 (2018) 328, 10.1038/s41419-017-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].De Maria R, Lenti L, Malisan F, d’Agostino F, Tomassini B, Zeuner A, et al. , Requirement for GD3 ganglioside in CD95- and ceramide-induced apoptosis, Science 277 (1997) 1652–1655, 10.1126/science.277.5332.1652. [DOI] [PubMed] [Google Scholar]
- [133].Rippo MR, Malisan F, Ravagnan L, Tomassini B, Condo I, Costantini P, et al. , GD3 ganglioside directly targets mitochondria in a bcl-2-controlled fashion, FASEB J 14 (2000) 2047–2054, 10.1096/fj.99-1028com. [DOI] [PubMed] [Google Scholar]
- [134].Matarrese P, Garofalo T, Manganelli V, Gambardella L, Marconi M, Grasso M, et al. , Evidence for the involvement of GD3 ganglioside in autophagosome formation and maturation, Autophagy 10 (2014) 750–765, 10.4161/auto.27959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Crespo PM, Iglesias-Bartolomè R, Daniotti JL, Ganglioside GD3 traffics from the trans-Golgi network to plasma membrane by a Rab11-independent and Brefeldin A-insensitive exocytic pathway, J Biol Chem 279 (2004) 7610–47618, 10.1074/jbc.M407181200. [DOI] [PubMed] [Google Scholar]
- [136].Kumari S, Kumar A, Sardar P, Yadav M, Majhi RK, Kumar A, et al. , Influence of membrane cholesterol in the molecular evolution and functional regulation of TRPV4, Biochem Biophys Res Commun 456 (2015) 312–319, 10.1016/j.bbrc.2014.11.077. [DOI] [PubMed] [Google Scholar]
- [137].Kjellberg MA, Mattjus P, Glycolipid transfer protein expression is affected by glycosphingolipid synthesis, PLoS One 8 (2013) e70283, , 10.1371/journal.pone.0070283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Kjellberg MA, Backman APR, Ohvo-Rekilä H, Mattjus P, Alternation in the glycolipid transfer protein expression causes changes in the cellular lipidome, PLoS One 9 (2014) e97263, , 10.1371/journal.pone.0097263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Zou X, Gao Y-G, Ruvolo VR, Gardner TL, Ruvolo PR, Brown RE, Human glycolipid transfer protein gene (GLTP) expression is regulated by Sp1 and Sp3: involvement of the bioactive sphingolipid ceramide, J Biol Chem 286 (2011) 1301–1311, 10.1074/jbc.M110.127837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Tuuf J, Mattjus P, Human glycolipid transfer protein – intracellular localization and effects on the sphingolipid synthesis, Biochim Biophys Acta 2007 (1771) 1353–1363, 10.1016/j.bbalip.2007.09.001. [DOI] [PubMed] [Google Scholar]
- [141].Sharma K, Schmitt S, Bergner CG, Tyanova S, Kannaiyan N, Manrique-Hoyos N, et al. , Cell type– and brain region–resolved mouse brain proteome, Nat Neurosci 18 (2015) 1819–1831, 10.1038/nn.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Yellajoshyula D, Liang C-C, Pappas SS, Penati S, Yang A, Mecano R, et al. , The DYT6 dystonia protein THAP1 regulates myelination within the oligodendrocyte lineage, Dev Cell 42 (2017) 52–67, 10.1016/j.devcel.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Yang F, Guan Y, Feng X, Rolfs A, Schlüter H, Luo J, Proteomics of the corpus callosum to identify novel factors involved in hypomyelinated Niemann-Pick Type C disease mice, Mol Brain 12 (2019) 17, 10.1186/s13041-019-0440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Marcus J, Popko B, Galactolipids are molecular determinants of myelin development and axo–glial organization, Biochim Biophys Acta 1573 (2002) 406–413. [DOI] [PubMed] [Google Scholar]
- [145].Boggs JM, Gao W, Zhao J, Park H-J, Liu Y, Basu A, Participation of galactosylceramide and sulfatide in glycosynapses between oligodendrocyte or myelin membranes, FEBS Lett 584 (2010) 1771–1778, 10.1016/j.febslet.2009.11.074. [DOI] [PubMed] [Google Scholar]
- [146].Hannun YA, Obeid LM, Sphingolipids and their metabolism in physiology and disease, Nat Rev Mol Cell Biol 19 (2018) 175–191, 10.1038/nrm.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Trayssac M, Hannun YA, Obeid LM, Role of sphingolipids in senescence: implication in aging and age-related diseases, J Clin Invest 128 (2018) 2702–2712, 10.1172/JCI97949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Kurz J, Parnham MJ, Geisslinger G, Schiffmann S, Ceramides as novel disease biomarkers, Trends Mol Med 25 (2019) 20–32, 10.1016/j.molmed.2018.10.009. [DOI] [PubMed] [Google Scholar]
- [149].Lewis AC, Wallington-Beddoe CT, Powell JA, Pitson SM, Targeting sphingolipid metabolism as an approach for combination therapies in haematological malignancies, Cell Death Dis 4 (2018) 72, 10.1038/s41420-018-0075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Young MM, Kester M, Wang H-G, Sphingolipids: regulators of crosstalk between apoptosis and autophagy, J Lipid Res 54 (2013) 5–19, 10.1194/jlr.R031278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Young MM, Wang HG, Sphingolipids as regulators of autophagy and endocytic trafficking, Adv Cancer Res 140 (2018) 27–60, 10.1016/bs.acr.2018.04.008. [DOI] [PubMed] [Google Scholar]
- [152].Sentelle RD, Senkal CE, Jiang W, Ponnusamy S, Gencer S, Selvam SP, et al. , Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy, Nat Chem Biol 8 (2012) 831–838, 10.1038/nchembio.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Mullen TD, Obeid LM, Ceramide and apoptosis: exploring the enigmatic connections between sphingolipid metabolism and programmed cell death, Anticancer Agents Med Chem 12 (2012) 340–363. [DOI] [PubMed] [Google Scholar]
- [154].Ogretmen B, Sphingolipid metabolism in cancer signalling and therapy, Nat Rev Cancer 18 (2018) 33–50, 10.1038/nrc.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind JS, et al. , Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate, Nature 381 (1996) 800–803. [DOI] [PubMed] [Google Scholar]
- [156].Gómez-Muñoz A, Ceramide 1-phosphate/ceramide, a switch between life and death, Biochim Biophys Acta 2006 (1758) 2049–2056, 10.1016/j.bbamem.2006.05.011. [DOI] [PubMed] [Google Scholar]
- [157].Taniguchi M, Kitatani K, Kondo T, Hashimoto-Nishimura M, Asano S, Hayashi A, et al. , Regulation of autophagy and its associated cell death by “Sphingolipid Rheostat”: Reciprocal role of ceramide and sphingosine-1-phosphate in the mammalian target of rapamycin pathway, J Biol Chem 287 (2012) 39898–39910, 10.1074/jbc.M112.416552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Espaillat MP, Shamseddine AA, Adada MM, Hannun YA, Obeid LM, Ceramide and sphingosine-1-phosphate in cancer, two faces of the sphinx, Transl Cancer Res 4 (2015) 484–499. [Google Scholar]
- [159].Newton J, Lima S, Maceyka M, Spiegel S, Revisiting the sphingolipid rheostat: evolving concepts in cancer therapy, Exp Cell Res 333 (2015) 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Su Z, Yang Z, Xie L, DeWitt JP, Chen Y, Cancer therapy in the necroptosis era, Cell Death Differ 23 (2016) 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Shan B, Pan H, Najafov A, Yuan J, Necroptosis in development and diseases, Genes Dev 32 (2018) 327–340, 10.1101/gad.312561.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Gong Y, Fan Z, Luo G, Yang C, Huang Q, Fan K, et al. , The role of necroptosis in cancer biology and therapy, Mol Cancer 18 (2019) 100, 10.1186/s12943-019-1029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Nganga R, Oleinik N, Kim J, Selvam SP, De Palma R, Johnson KA, et al. , Receptor-interacting Ser/Thr kinase 1 (RIPK1) and myosin IIA–dependent ceramidosomes form membrane pores that mediate blebbing and necroptosis, J Biol Chem 294 (2019) 502–519, 10.1074/jbc.RA118.005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Gao Y-G, Chung T, Zou X, Pike HM, Brown RE, Human glycolipid transfer protein (GLTP) expression modulates cell shape, PLoS One 6 (2011) e19990, , 10.1371/journal.pone.0019990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Mishra SK, Stephenson DJ, Chalfant CE, Brown RE, Upregulation of human glycolipid transfer protein (GLTP) induces necroptosis in colon carcinoma cells, Biochim Biophys Acta Mol Cell Biol Lipids 2019 (1864) 158–167, 10.1016/j.bbalip.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Zhai X, Malakhova ML, Pike HM, Benson LM, Bergen III HR, Sugár IP, et al. , Glycolipid acquisition by human glycolipid transfer protein dramatically alters intrinsic tryptophan fluorescence: Insights into glycolipid binding affinity, J Biol Chem 284 (2009) 13620–13628, 10.1074/jbc.M809089200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Kitatani K, Idkowiak-Baldys J, Hannun YA, The sphingolipid salvage pathway in ceramide metabolism and signaling, Cell Signal 20 (2008) 1010–1018, 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Samaha D, Hamdo HH, Wilde M, Prause K, Arenz C, Sphingolipid-transporting proteins as cancer therapeutic targets, Int J Mol Sci 20 (2019) 3554, 10.3390/ijms20143554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Mattjus P, Specificity of the mammalian glycolipid transfer proteins, Chem Phys Lipids 194 (2016) 72–78, 10.1016/j.chemphyslip.2015.07.018. [DOI] [PubMed] [Google Scholar]
- [170].Roy A, Kucukural A, Zhang Y, I-TASSER: a unified platform for automated protein structure and function prediction, Nat Protoc 5 (2010) 725–738, 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]


