Abstract
An operationally simple method to employ non-activated carboxylic acids as alkylating agents in N-alkylation of heterocycles is reported through an electrochemically driven anodic decarboxylative process. A wide substrate scope across a range of heterocycles is demonstrated along with a series of applications that significantly reduce the step-count required to access such medicinally relevant structures.
Graphical Abstract
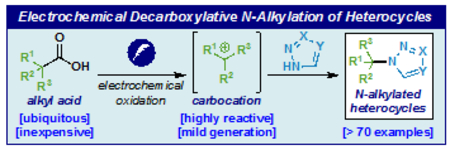
A recent analysis of the reactions conducted by modern medicinal chemists revealed that C–N alkylation, not surprisingly, represents a significant percentage of reactions conducted on a yearly basis.1 Within this class of transformation, the alkylation of unsaturated N-heterocycles is a popular tactic for diversification. Numerous methods exist for to achieve this, the most popular being variants of the SN1 or SN2 reaction (such as Mitsunobu).2 As the starting materials for such common reactions are alkyl halides or alcohols, the development of new entry points for this disconnection have become attractive. Carboxylic acids are perhaps even more widespread than alcohols and over the past decade numerous methods to replace the C–C bond with other valuable substituents have emerged.3,4 Decarboxylative aminations were first reported by Barton in 1992 where his eponymous redox-active ester was converted to the corresponding amine using diazirines as radical traps.5 Recent developments (Figure 1) include the use of intermediary NHPI-based redox active esters or I(III)-based esters in concert with Cu-based photochemical systems (with or without additional photocatalysts) to achieve amine and heterocycle alkylations with broad scope.6 Non-activated approaches that don’t traverse through activated esters are less common.7 For example, decarboxylative methods to convert stabilized acids to N-alkylated products were reported in 20197a,b and earlier this year a photochemical N-alkylation of DTAD (di-tert-butyl azodicarboxylate) was reported and such adducts could be converted to pyrazoles after deprotection/condensation.7c In 2019 our group reported a simple means to prepare hindered ethers through an electrochemical decarboxylative approach wherein an electrogenerated carbocation could be intercepted with an alcohol.8 It was hypothesized that similar conditions might also be amenable to capture with N-heterocycles. In this Letter we report our findings and demonstrate that a simple electrochemically driven approach analogous to ether synthesis can be employed to generate N-alkylated heterocycles. The reaction exhibits a broad substrate scope with regards to the heterocyclic substrate, employs a simple and scalable procedure, and can be used to simplify prior routes to such targets.
FIGURE 1.
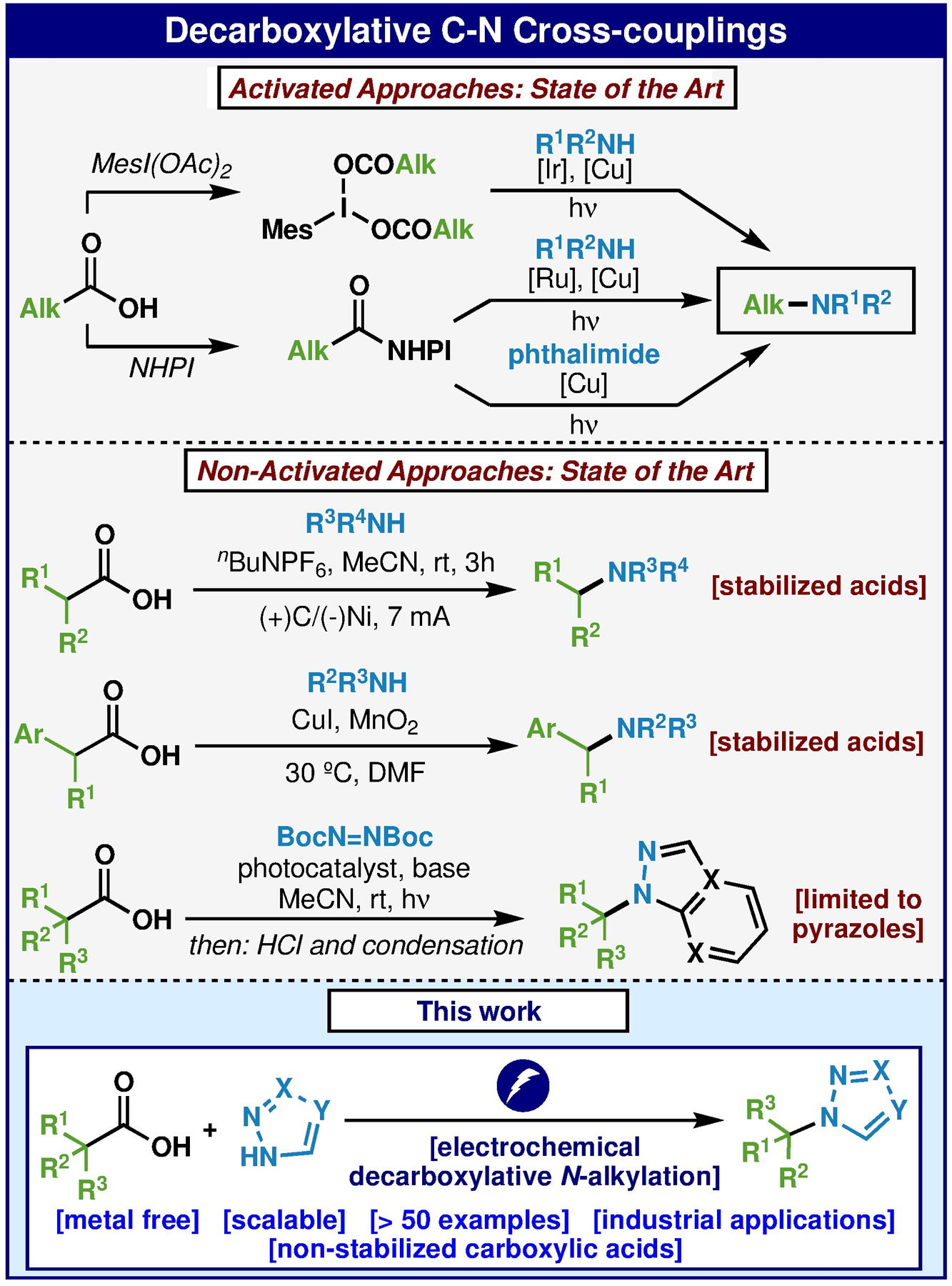
Decarboxylative C-N cross-couplings.
At the outset, optimization was conducted on a medicinally relevant substrate that was employed in the synthesis of a Cereblon binder, pyrazole 1 (Scheme 1A).9 This compound was previously obtained in 6 steps starting from 4-methylpyridine wherein only 2 of those steps contribute to building skeletal bonds (C–C and C–N). Interestingly, the key C–N bond forming step relies on a single electron Mukaiyama-type reaction between an olefin and DTAD. As carboxylic acid 2 is commercial, a far more direct path to 1 would involve direct decarboxylative union with pyrazole 3 (via a 2-electron pathway this time). In its optimized manifestation, this N-alkylation afforded compound 1 in 52% isolated yield. Extensive optimization was conducted on this and other substrates (see SI) to arrive at this final set of conditions, some of which is summarized in Scheme 1B. Notably, both the procedure we reported for etherification and the closest electrochemical precedent only afforded low yield in this transformation (entries 1 and 2). A key departure from etherification occurred when switching the cathode material from graphite (34% yield, entry 4) to nickel. The addition of molecular sieves and collidine were essential relative to the prior e-alkylation approach (entries 5 and 6). The use of DCM, as with etherification, was also essential for the reaction (entry 7). Although lowering the amount of carboxylic acid to one or two equivalents is detrimental for the reaction (entries 8 and 9), the excess acid can be recovered if desired for valuable substrates. Finally, a screen of bases revealed that collidine was optimum (e.g. DBU afforded lower yield, entry 10). Variables such as electrolyte and concentration are discussed further in the SI but had only negligible effect on the reaction.
SCHEME 1.
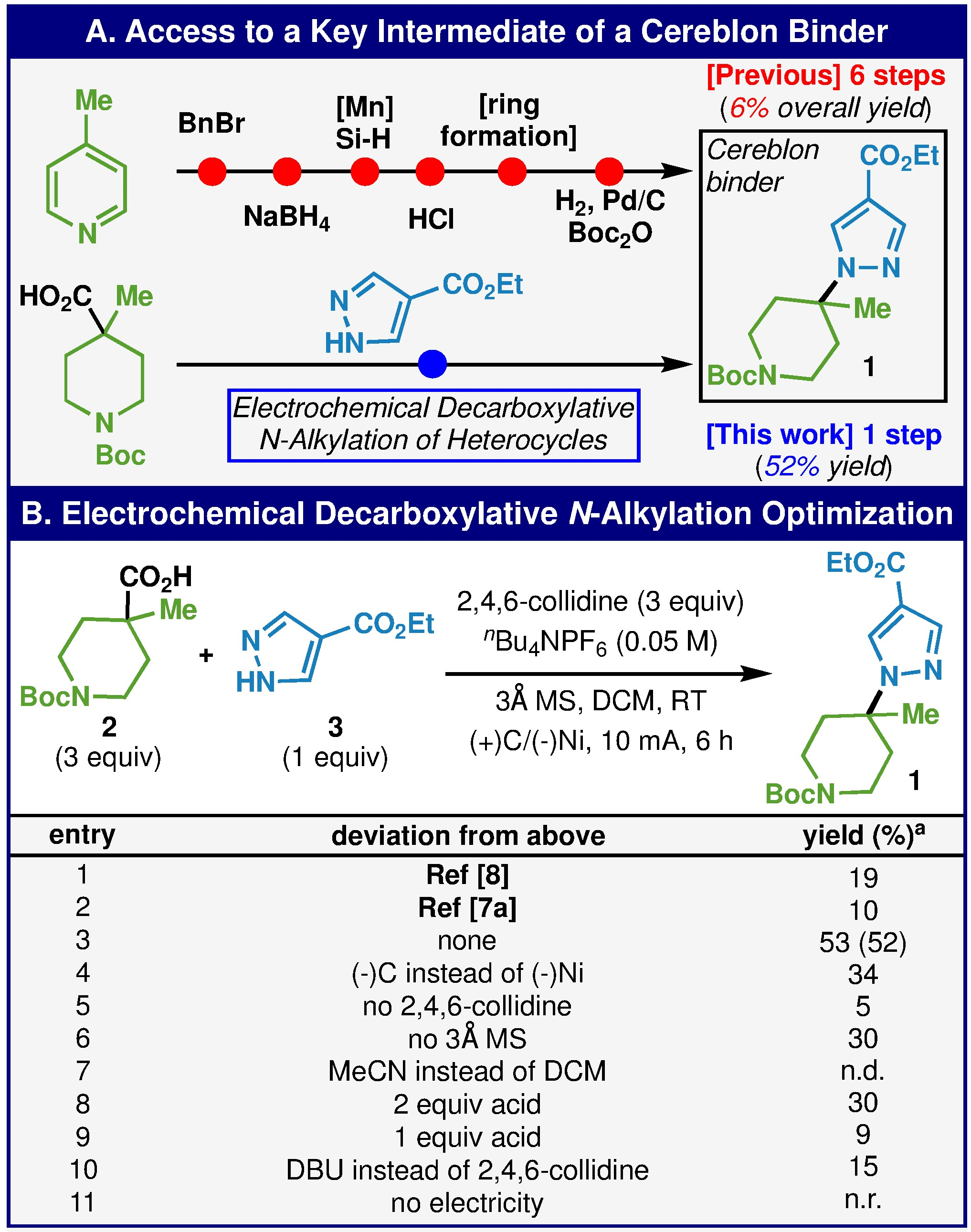
(A) Access to a key intermediate of a Cereblon binder. (B) Optimization of the electrochemical decarboxylative N-alkylation of heterocycles. a Isolated yield.
With an optimized set of conditions in hand, the scope of this transformation was explored as illustrated in Scheme 2. In addition to the ester moiety, a variety of functional groups were tolerated such as those sensitive to hydrolytic (cyano, 43 and 46), oxidative (BPin, 9 and 50), reductive (nitro, 48), and acidic conditions (acetal, Boc-protected amines, 30, 31, 33, 35, and 36). Aryl halides (F, Cl, Br, and I, 10, 13, 17, 27, 49 and 50) and fluoroalkyl substituents were also unharmed in this reaction (11, 25, 47, and 67). To our knowledge this represents the first use of non-stabilized/non-bridged tertiary acids in a direct decarboxylative N-alkylation process (4, 6 and 7). With regards to the scope of heterocycles, aside from pyrazoles, (benzo)triazoles (40 and 41), tetrazoles (42), imidazoles (43), 1,2,4-triazoles (53 and 54), indazoles (56 and 57), xanthones (58), oxazolidinones (59 and 69), γ-lactams (60), succinimides (61), pyridones (62), 2-aminopyrimidines (63), and oxindoles (65) could all be employed. The operationally simple protocol (setup in ca. 5–10 minutes) could be conducted on a commercial potentiostat without exclusion of air and was amenable to scale up (8, 69% yield on 1 mmol scale) without significant diminishment in yield. In cases where the reaction only proceeds to a modest extent (for example cyclobutane 22 and 23), the remaining pyrazole substrate (limiting reagent) can be recovered.
SCHEME 2.
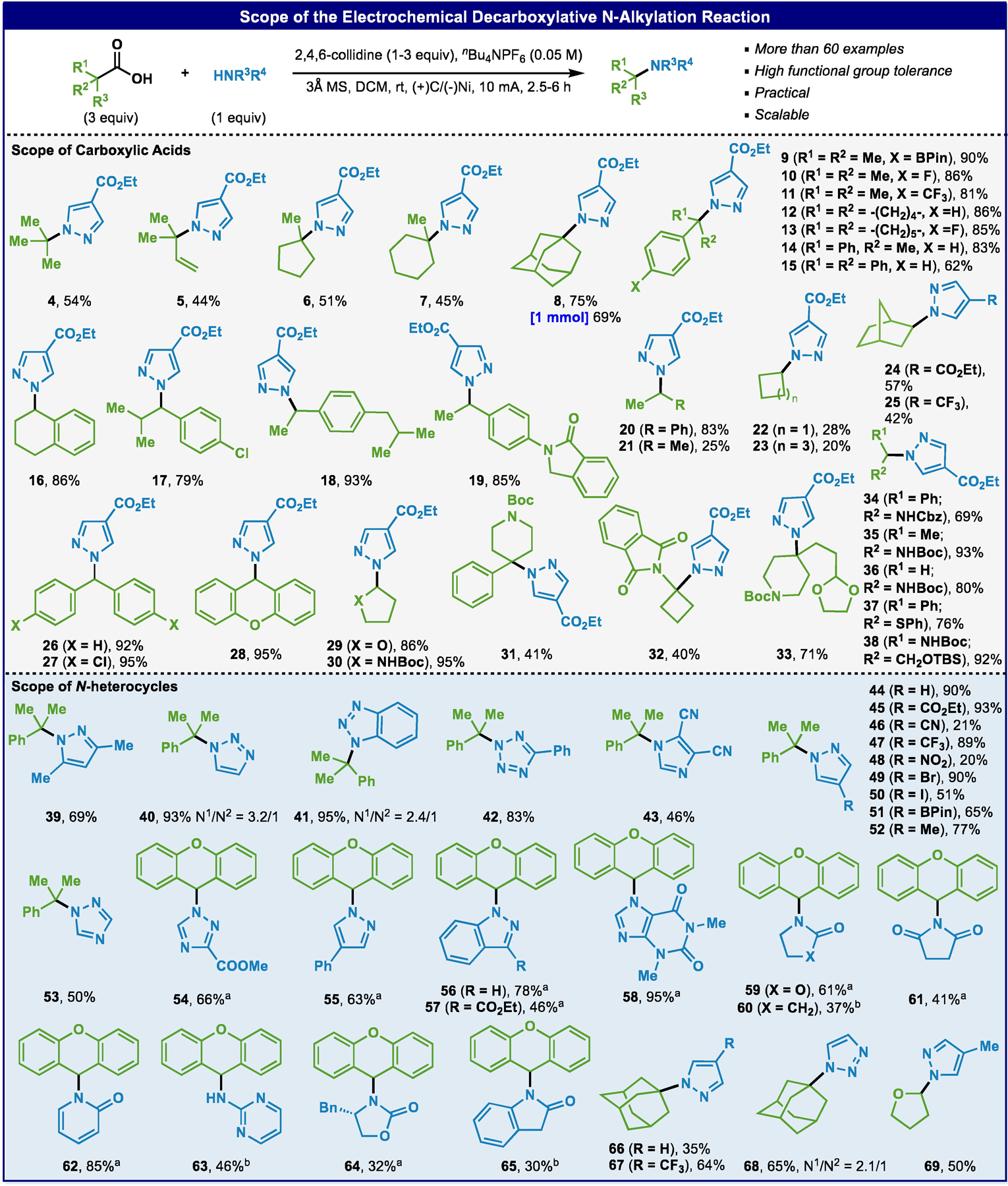
Scope of the electrochemical decarboxylative N-alkylation of heterocycles. a2 equivalent of acid instead of 3. b3 equivalent of N-heterocycle and 1 equivalent of acid.
Aside from the highlighted application in Scheme 1A (pyrazole 1), a small selection of additional known compounds were targeted such as pyrazoles 70-72 (Scheme 3A).10–12 Hemi-aminals 70 and 71 could be accessed in high yield in one simple step versus multistep procedures used in the past. Finally, the tert-butylated pyrazole 72 could be accessed in a single step (although in low yield due to an extended reaction time needed with pivalic acid).
SCHEME 3.
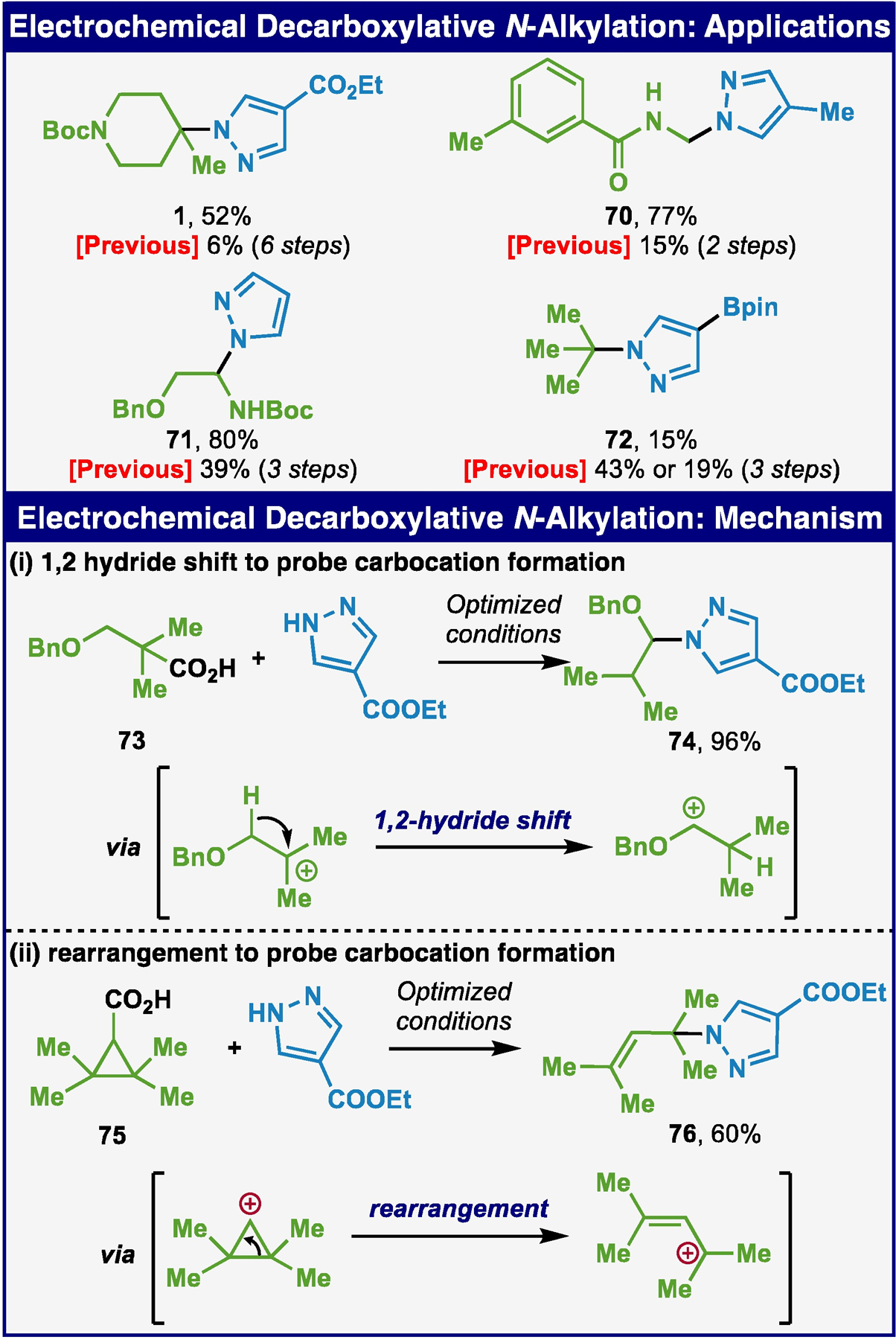
(A) Applications. (B) Mechanistic investigation.
Mechanistically we hypothesize that a cationic intermediate is formed after decarboxylation analogous to etherification (Scheme 3B). To lend evidence for this hypothesis, acids 73 and 75 were exposed to the standard conditions and the resulting products 74 and 76 formed as a result of cationic rearrangement. The limitations of this reaction are thus linked to this mechanistic requirement in that the carboxylic acid donor must be able to generate a reasonably long-lived carbocation following decarboxylation. A full summary of failed substrates is listed in the SI to aid the practitioner.
To conclude, a useful method for the direct decarboxylative N-alkylation of heterocycles has been developed. This direct anodic electrochemical process exhibits a wide substrate scope with regards to the carboxylic acid (stabilized and non-stabilized) and heterocycle. As such, application to the synthesis of valuable medicinal and agrochemicals can be foreseen.
Supplementary Material
ACKNOWLEDGMENT
Financial support for this work was provided by NIH (GM-118176), China Scholarship Council (predoctoral fellowship to T.S.), Shanghai Institute of Organic Chemistry (postdoctoral fellowship to H-J.Z.), Zhejiang Yuanhong Medicine Technology Co. Ltd. (postdoctoral fellowship to M.S), and Jiangsu Industrial Technology Research Institute (postdoctoral fellowship to C.H.). We thank D.-H. Huang and L. Pasternack (Scripps Research Institute) for assistance with NMR spectroscopy and J. S. Chen, B. B. Sanchez, and E. Sturgell (Scripps Research Institute) for assistance with LCMS analysis and HRMS analysis.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Additional optimization data, complete experimental procedures, characterization data, Frequently Asked Questions section and NMR spectra (PDF)
REFERENCES
- (1).(a) Roughley SD; Jordan AM The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem 2011, 54, 3451–3479. [DOI] [PubMed] [Google Scholar]; (b) Brown DG; Boström J Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone? J. Med. Chem 2016, 59, 4443–4458 [DOI] [PubMed] [Google Scholar]
- (2).(a) Salvatore RN; Yoon CH; Jung KW. Synthesis of secondary amines. Tetrahedron 2001, 57, 7785–7811. [Google Scholar]; (b) Swamy KCK; Kumar NNB; Balaraman E; Kumar KVPP Mitsunobu and related reactions: advances and applications. Chem. Rev 2009, 109, 2551–2651. [DOI] [PubMed] [Google Scholar]
- (3).For selected reviews of decarboxylative cross-coupling reactions:; (a) Rodriíguez N; Goossen LJ. Decarboxylative coupling reactions: a modern strategy for C-C bond formation. Chem. Soc. Rev 2011, 40, 5030–5048. [DOI] [PubMed] [Google Scholar]; (b) Weaver JD; Recio A; Grenning AJ; Tunge JA Transition metal-catalyzed decarboxylative allylation and benzylation reactions. Chem. Rev 2011, 111, 1846–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Xuan J; Zhang Z-G; Xiao W-J Visible-light-induced decarboxylative functionalization of carboxylic acids and their derivatives. Angew. Chem., Int. Ed 2015, 54, 15632–15641. [DOI] [PubMed] [Google Scholar]; (d) Kumar NYP; Bechtoldt A; Raghuvanshi K; Ackermann L Ruthenium(II)-catalyzed decarboxylative C−H activation: versatile routes to meta-alkenylated arenes. Angew. Chem., Int. Ed 2016, 55, 6929–6932. [DOI] [PubMed] [Google Scholar]; (e) Wei Y; Hu P; Zhang M; Su W Metal-catalyzed decarboxylative C-H functionalization. Chem. Rev 2017, 117, 8864–8907. [DOI] [PubMed] [Google Scholar]; (f) Arshadi S; Ebrahimiasl S; Hosseinian A; Monfared A; Vessally E Recent developments in decarboxylative cross-coupling reactions between carboxylic acids and N-H compounds. RSC Adv. 2019, 9, 8964–8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).For selected decarboxylative cross-coupling works from our lab, see:; (a) Qin T; Cornella J; Li C; Malins LR; Edwards JT; Kawamura S; Maxwell BD; Eastgate MD; Baran PS A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science 2016, 352, 801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang J; Qin T; Chen T-G; Wimmer L; Edwards JT; Cornella J; Vokits B; Shaw SA; Baran PS Nickel-catalyzed cross-coupling of redox-active esters with boronic acids. Angew. Chem. Int. Ed 2016, 55, 9676–9679. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Qin T; Malins LR; Edwards JT; Merchant RR; Novad AJE; Zhong JZ; Mills RB; Yan M; Yuan C; Eastgate MD; Baran PS Nickel-catalyzed Barton decarboxylation and Giese reactions: a practical take on classic transforms. Angew. Chem. Int. Ed 2017, 56, 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Li C; Wang J; Barton LM; Yu S; Tian M; Peters DS; Kumar M; Yu AW; Johnson KA; Chatterjee AK; Yan M; Baran PS Decarboxylative borylation. Science, 2017, 356, eaam 7355. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Edwards JT; Merchant RR; McClymont KS; Knouse KW; Qin T; Malins LR; Vokits B; Shaw SA; Bao D-H; Wei F-L; Zhou T; Eastgate MD; Baran PS Decarboxylative alkenylation. Nature 2017, 545, 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Smith JM; Qin T; Merchant RR; Edwards JT; Malins LR; Liu Z; Che G; Shen Z; Shaw SA; Eastgate MD; Baran PS Decarboxylative alkynylation. Angew. Chem. Int. Ed 2017, 56, 11906–11910. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Wang J; Shang M; Lundberg H; Feu KS; Hecker SJ; Qin T; Blackmond DG; Baran PS Cu-catalyzed decarboxylative borylation. ACS Catal. 2018, 8, 9537–9542. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Ni S; Garriso-Castro AF; Merchant RR; deGtuyter JN; Schmitt DC; Mousseau JJ; Gallego GM; Yang S; Collins MR; Qiao JX; Yeung K-S; Langley DR; Poss MA; Scola PM; Qin T; Baran PS A general amino acid synthesis enabled by innate radical cross-coupling. Angew. Chem. Int. Ed 2018, 57, 14560–14565. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Kingston C; Wallace MA; Allentoff AJ; deGruyter JN; Chen JS; Gong SX; Bonacorsi S Jr.; Baran PS Direct carbon isotope exchange through decarboxylative carboxylation. J. Am. Chem. Soc 2019, 141, 774–779. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Chen T-G; Zhang H; Mykhailiuk PK; Merchant RR; Smith CA; Qin T; Baran PS Quaternary centers by nickel-catalyzed cross-coupling of tertiary carboxylic acids and (hetero)aryl zinc reagents. Angew. Chem. Int. Ed 2019, 58, 2454–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Ni S; Padial NM; Kingston C; Vantourout JC; Schmitt DC; Edwards JT; Kruszyk MM; Merchant RR; Mykhailiuk PK; Sanchez BB; Yang S; Perry MA; Gallego GM; Mousseau JJ; Collins MR; Cherney RJ; Lebed PS; Chen JS; Qin T; Baran PS. A radical approach to anionic chemistry: synthesis of ketones, alcohols, and amines. J. Am. Chem. Soc 2019, 141, 6726–6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Barton DHR; Jaszberenyi J Cs.; Theodorakis, E. A. Nitrogen transfer to carbon radicals. J. Am. Chem. Soc 1992, 114, 5904–5905. [Google Scholar]
- (6).(a) Zhao W; Wurz RP; Peters JC; Fu GC Photoinduced, copper-catalyzed decarboxylative C–N coupling to generate protected amines: an alternative to the Curtius rearrangement. J. Am. Chem. Soc 2017, 139, 12153–12156. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mao R; Frey A; Balon J; Hu X Decarboxylative C(sp3)-N cross-coupling via synergetic photoredox and copper catalysis. Nat. Catal 2018, 1, 120–126. [Google Scholar]; (c) Liang Y; Zhang X; MacMillan DWC Decarboxylative sp3 C-N coupling via dual copper and photoredox catalysis. Nature 2018, 559, 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Mao R; Balon J; Hu X Cross-coupling of alkyl redox-active esters with benzophenone imines: tandem photoredox and copper catalysis. Angew. Chem. Int. Ed 2018, 57, 9501–9504. [DOI] [PubMed] [Google Scholar]
- (7).(a) Shao X; Zheng Y; Tian L; Martín-Torres I; Echavarren AM; Wang Y Decarboxylative Csp3-N bond formation by electrochemical oxidation of amino acids. Org. Lett 2019, 21, 9262–9267. [DOI] [PubMed] [Google Scholar]; (b) Kong D; Moon PJ; Bsharat O; Lundgren RJ Direct catalytic decarboxylative amination of aryl acetic acids. Angew. Chem. Int. Ed 2020, 59, 1313–1319. [DOI] [PubMed] [Google Scholar]; (c) Merchant RR; Lang SB; Yu T; Zhao S; Qi Z; Suzuki T; Bao J A general one-pot protocol for hindered N-alkyl azaheterocycles from tertiary carboxylic acids. Org. Lett 2020, 22, 4180–4184. [DOI] [PubMed] [Google Scholar]
- (8).Xiang J; Shang M; Kawamata Y; Lundberg H; Reisberg S; Chen M; Mykhailiuk P; Beutner G; Collins M; Davies A; Del Bel M; Gallego G; Spangler J; Starr JT; Yang S; Blackmond D; Baran PS Hindered dialkyl ether synthesis via electrogenerated carbocations. Nature 2019, 573, 398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Veits GK; He M; Henderson JA; Nasveschuk CG; Phillips AJ; Good AC Cereblon binders for the degradation of Ikaros cross-reference to related applications. WO 2019/191112, October 3, 2019. [Google Scholar]
- (10).(a) Patel MV; Kolasa T; Mortell K; Matulenko MA; Hakeem AA; Rohde JJ; Nelson SL; Cowart MD; Nakane M; Miller LN; Uchic ME; Terranova MA; El-Kouhen OF; Donnelly-Roberts DL; Namovic MT; Hollingsworth PR; Chang R; Martino BR; Wetter JM; Marsh KC; Martin R; Darbyshire JF; Gintant G; Hsieh GC; Moreland RB; Sullivan JP; Brioni JD; Stewart AO Discovery of 3-methyl-N-(1-oxy-3’,4’,5’,6’-tetrahydro-2’H-[2,4’-bipyridine]-1’-ylmethyl)benzamide (ABT-670), an orally bioavailable Dopamine D4 agonist for the treatment of erectile dysfunction. J. Med. Chem 2006, 49, 7450–7465. [DOI] [PubMed] [Google Scholar]; (b) Labanauskas L; Mazeikaite R; Striela R; Gedrimaite O; Urbelis G; Zilinskas A Nuclephilic substitution of the acetoxy group in 3-methylbenzoylaminomethyl acetate. Russ. Chem. Bull., Int. Ed 2011, 60, 1672–1676. [Google Scholar]
- (11).(a) George N; Bekkaye M; Masson G; Zhu J A practical, one-pot multicomponent synthesis of α-amidosulfides and their application as latent N-acylimines in the Friedel–Crafts reaction. Eur. J. Org. Chem 2011, 3695–3699. [Google Scholar]; (b) Jarrige L; Levitre G; Masson G Visible-light photoredox-catalyzed coupling reaction of azoles with alfa-carbamoyl sulfides. J. Org. Chem 2016, 81, 7230–7236. [DOI] [PubMed] [Google Scholar]
- (12).(a) Atkinson FL; Barker MD; Douault C; Garton NS; Liddle J; Patel VK; Preston AGS; Wilson DM 7-(1H-Pyrazole-4-yl)-1,6-naphthyridine compounds as syk inhibitors. WO 2011/134971, November 3, 2011. [Google Scholar]; (b) Do S; Hu H; Kolesnikov A; Tsui VH; Wang X 5-Azaindazole compounds and methods of use. WO 2014/001377, January 3, 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


