Abstract
Introduction
Cruciferous vegetables are a rich source of sulforaphane (SFN), which acts as a natural HDAC inhibitor (HDACi). Our previous study found that HDACi could restore histone acetyltransferase/histone deacetylase (HAT/HDAC) balance in the cochlea and attenuate gentamicin-induced hearing loss in guinea pigs. Here, we investigated the protective effect of SFN on cisplatin-induced hearing loss (CIHL).
Methods
Thirty rats were randomly divided into 3 equal groups: the control group, cisplatin group, and SFN+cisplatin group. Rats were injected with SFN (30 mg/kg once a day) and cisplatin (7 mg/kg twice a day) for 7 days to investigate the protective role of SFN on CIHL. We observed auditory brainstem response (ABR) threshold shifts and immunostained cochlear basilar membranes of rats. For in vitro experiments, we treated HEI-OC1 cells and rat cochlear organotypic cultures with SFN (5, 10, and 15 μM) and cisplatin (10 μM). Immunofluorescence, cell viability, and protein analysis were performed to further analyze the protective mechanism of SFN on CIHL.
Results
SFN (30 mg/kg once a day) decreased cisplatin (7 mg/kg twice a day)-induced ABR threshold shifts and outer hair cell loss. CCK-8 assay showed that cisplatin (10 μM) reduced the viability of HEI-OC1 cells to 42%, and SFN had a dose-dependent protective effect. In cochlear organotypic cultures, we found that SFN (10 and 15 μM) increased cisplatin (10 μM)-induced myosin 7a+ cell count and restored ciliary morphology. SFN (5, 10, and 15 μM) reversed the cisplatin (10 μM)-induced increase in HDAC2, -4, and -5 and SFN (15 μM) reversed the cisplatin (10 μM)-induced decrease in H3-Ack9 [acetyl-histone H3 (Lys9)] protein expression in HEI-OC1 cells. Neither cisplatin nor cisplatin combined with SFN affected the expression of HDAC7, or HDAC9.
Conclusion
SFN prevented disruption of the HAT/HDAC balance, protecting against CIHL in rats.
Keywords: histone deacetylase, sulforaphane, hearing loss, histone deacetylase inhibitor, ototoxicity
Introduction
Sulforaphane (SFN; 1-isothiocyanato-4-methylsulfinylbutane) is a nutritious substance found abundantly in cruciferous vegetables. 1 Glucoraphanin, a precursor of SFN, is found in cooked broccoli sprouts and broccoli; myrosinase metabolizes glucoraphanin into active SFN in gastrointestinal microflora, followed by metabolization into sequential metabolites, such as dithiocarbamates.2,3 A previous study showed that SFN exhibited anti-oxidant and anti-inflammatory effects in lipopolysaccharide-treated RAW 264.7 macrophage cells. 4 Additionally, SFN has been reported to trigger G1/S arrest in ductal carcinoma cells (ZR-75-1), 5 as well as exert anti-cancer effects via the Nrf2 pathway preclinically in vivo and in vitro. 6 Recently, a study showed that SFN regulated gene expression via epigenetic mechanisms, especially by inhibiting the activity of histone deacetylase (HDAC) in human lung cancer cells and NOD/SCID mice. 7
Gene expression is known to be regulated by lysine acetylation, a reversible post-transcriptional modification. Basically, histone acetyltransferases (HATs) mediate the conjunction of acetyl groups to histones and, in turn, promote gene expression. Conversely, acetyl group deletion by HDACs represses transcription and inhibits gene expression. HDACs are divided into 5 groups based on sequence homology to the original yeast enzymes and domain organization. 8 Class I includes HDAC1, -2, -3, and -8. Class IIa contains HDAC4, -5, -7, and -9. Class IIb contains HDAC6 and -10. Class III includes HDACs with catalysis dependent on coenzyme I (NAD+) and not Zn2+ and cannot be inhibited by HDAC inhibitors (HDACis). Class IV includes only HDAC11. The histone acetylation levels are regulated by the HAT/HDAC balance. 9
The sensory cells of hearing in the organ of Corti of the cochlea include a row of inner hair cells (IHCs, which convert mechanical acoustic input into receptor potential, which releases neurotransmitters and triggers the action potentials transferred from spiral ganglion neurons to the auditory center of the brain) and 3 rows of outer hair cells (OHCs, which are known to primarily elevate cochlear performance, especially at low sound intensity). Our previous study on guinea pigs demonstrated that the HDACi sodium butyrate increased the histone acetylation level in the organ of Corti. Also, sodium butyrate and gentamicin (a drug with ototoxic side effects)-treated ears showed less auditory brainstem response (ABR) threshold shifts and hair cell loss compared with gentamicin-treated ears. Additionally, sodium butyrate blocked gentamicin-induced increase in HDAC1 expression in OHCs. 10
Studies have shown that SFN is a natural HDACi, which attenuates class IIa HDACs as well as HDAC2 enzyme activities.11,12 The intake of 68 g of broccoli sprouts (rich in SFN) or six supplements (almost 3 g freeze-dried broccoli sprouts) has been shown to decrease HDAC activity in human subjects. 13 Additionally, HDACi has been found to have beneficial effects against in breast cancer cell lines, 14 allergic rhinitis,15,16 noise-induced hearing loss in guinea pigs and mice,17,18 and cisplatin-induced hearing loss (CIHL). 19 Here, we elucidated the protective mechanism exerted by SFN, during the prevention of CIHL.
Materials and methods
Animals
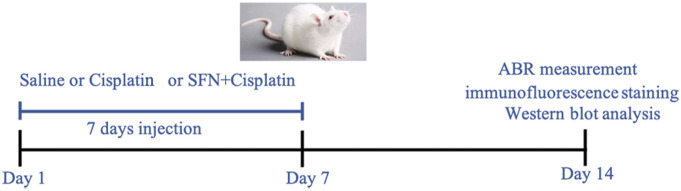
Wistar rats (5-week-old; 150–200 g) were obtained from the Air Force Military Medical University, Xi’an, China. They were raised in a sterile environment at 22°C with food/water ad libitum and in a 12-h light/dark cycle. Thirty rats were randomly divided into three equal groups: the control group (Con group, intraperitoneal injection of 0.5 mL saline once a day); cisplatin group (Cis group, intraperitoneal injection of 7 mg/kg cisplatin twice a day); and SFN+cisplatin group (SFN+Cis group, intraperitoneal injection of 30 mg/kg SFN once a day, and 1 h later, 7 mg/kg cisplatin was injected twice a day). The treatment period was for 7 days. On day 14, the rats were anesthetized (4% chloral hydrate, 400 mg/kg, Sigma-Aldrich, China), subjected to the ABR measurements, and obtained the ABR thresholds at baseline (Figure 1). Next, the rats were sacrificed by cervical dislocation. Ethical approval for this study was obtained from the National Institutes of Health guidelines and approved by the Committee on Animal Research of the Air Force Military Medical University (KJ-2017-XJR4723).
Figure 1.
Road map of the animal experiment.
Drugs and reagents
Jiangsu Haosen (Lianyungang, China) provided cisplatin. SFN (S4441), fluoresceinyl-aminomethyldithiolano-phalloidin (1:60), DAPI (1:1000), 5% bovine serum albumin (BSA), 10 × Basal Medium Eagle, 2% sodium carbonate, 0.1% sodium bicarbonate and horseradish peroxidase (HRP)–conjugated anti-mouse and anti-rabbit IgG antibodies (1:200) were procured from Sigma-Aldrich, China. Anti-HDAC2, -4, -5, -7, and -9 and anti-H3 Ack9 [acetyl-histone H3 (Lys9)] were purchased from Cell Signaling Technology, USA (1:1000). Alexa Fluor 488/594 conjugated donkey anti-rabbit and Alexa Fluor 594/488 conjugated donkey anti-mouse were purchased from Invitrogen/Life technologies, China (1:200). Myosin7a was purchased from Proteus BioSciences (1:1000, USA). Anti-GAPDH was purchased from R&D Systems, USA (1:1000). Collagen gel type I was purchased from Corning (1:1:9 ratio, USA); 1% albumin bovine V was purchased from Amresco (USA); HEI-OC1, an inner ear cell line, was provided by Professor He Zu-hong, Huazhong University of Science and Technology, Wuhan, China; Hanker’s culture medium was purchased from Solarbio, China.
ABR measurement
We used a sound proof chamber to determine auditory thresholds by ABR. A thermostatic heating pad was used to maintain the animals’ body temperature at 38 ± 1°C. We subcutaneously inserted the reference electrode into the mastoid region of the test ear; the active needle electrode into the apex; and the ground electrode into the contralateral mastoid. We used the TDT III auditory evoked potential workstation. The SigGenRZ and BioSigRZ software (Tucker-Davis Technologies, USA) was used for the experimental data generation, presentation, and data acquisition of sound stimulation. We used tone bursts (4, 8, 16, 24, and 32 kHz; 0.5 ms rise/fall time, no plateau, alternating phase) or broad-band clicks (10 ms) presented at 21.97/s to stimulate ABR. The stimulation sound played by RZ6 D/A converter was presented through a high frequency MF1 Multi-Field Magnetic loudspeaker about 2 cm in front of the test ear. We reduced the stimulation in 5 dB steps until the response disappeared. The differential potential was sampled over 10 ms, filtered (low-pass, 4 kHz; high-pass, 100 Hz), and averaged (512 sweeps of alternated stimulus polarity) to obtain the average trajectories at each intensity. The intensity of the last appearance of wave I was used to determine the threshold of ABR.
Indirect immunofluorescence staining
We washed the organ of Corti thrice with 0.01% PBS, followed by treatment with 1% Triton X-100 in 0.01% PBS for 30 min at 37°C. Next, we washed the specimens thrice with 0.01% PBS and treated with 5% BSA at 37°C for 30 min and incubated with myosin7a at 4°C for 48 h. Then, the specimens were treated with the corresponding secondary antibodies at 4°C for 24 h. The specimens were visualized by incubation with fluoresceinyl-aminomethyldithiolano-phalloidin for 8 min and DAPI for 5 min at 37°C. The specimens were rinsed with 0.01% PBS and visualized using a Fluoview 500 confocal microscope and analyzed with the Image J software (NIH, USA).
Cochlear organotypic culture
First, neonatal rats were treated with 4% chloral hydrate (400 mg/kg). The cochlea was dissected, and we used only the basilar membranes for culturing. In Hanker’s culture medium, we used the tip tweezers to open the bone wall of the internal auditory canal to expose the basilar membrane of the cochlear fundus gyrus. We placed the collagen solution (18 μL; 10× BME, 2% sodium carbonate, collagen gel type I) at the center of a culture dish (Corning; 35 mm × 10 mm). Then, serum-free Basal Medium Eagle (1 mL; 1% albumin bovine V, 1% ITS (insulin, transferrin, selenium) liquid media supplement, 5 mg/mL glucose, 0.1% sodium bicarbonate, 2 mM glutamine, and 0.1% penicillin) was added to the culture dish. The Petri dishes were cultured in a 5% CO2 incubator at 37°C. Four hours later, serum-free medium containing varying concentrations of SFN (5, 10, and 15 μM) and/or cisplatin (10 μM) was added and kept for another 48 h.
HEI-OC1 cell culture
The HEI-OC1 cells were grown till semi-confluency under 10% CO2 in DMEM supplemented with 10% heat-inactivated FBS at 33°C. Next, we added varying concentrations of SFN (5, 10, and 15 μM) and/or cisplatin (10 μM). The cells were treated with fresh, pre-warmed control, or treatment-containing medium. Forty-eight hours later, we collected cells for protein extraction and quantification. The procedure was repeated at least thrice.
Western blot analysis
We used a protein extraction kit to extract protein, followed by quantification using the Bio-Rad protein assay kit. The protein samples were electrophoresed (12.5% SDS-PAGE) and then transferred to a nitrocellulose membrane. After blocking with 2.5% nonfat milk at 37°C for 1 h, the membranes were treated overnight with anti-HDAC-2, -4, -5, -7, and -9, anti-H3 Ack9, and anti-GAPDH antibodies at 4°C. After treatment with the HRP-conjugated secondary antibodies at 37°C for 1 h, the bands were visualized using an ECL detection kit.
Cell viability
We added CCK-8 solution (10 μL) and cell culture medium (100 μL) to each well containing 3 × 103 cells/well in a 96-well plate and kept for 1 h in a CO2 incubator. The control well contained cell culture medium and CCK-8 solution except the cells. We measured absorbance at 450 nm (Bio-Rad).
Statistical analysis
We used two-sided, one-way ANOVA with LSD’s post hoc test for data analysis and presented as means ± standard deviations. The data were normally distributed. The threshold of significance was set as p < 0.05. GraphPad Prism 8 was used for data analysis.
Results
SFN reduced CIHL and hair cell damage in vivo
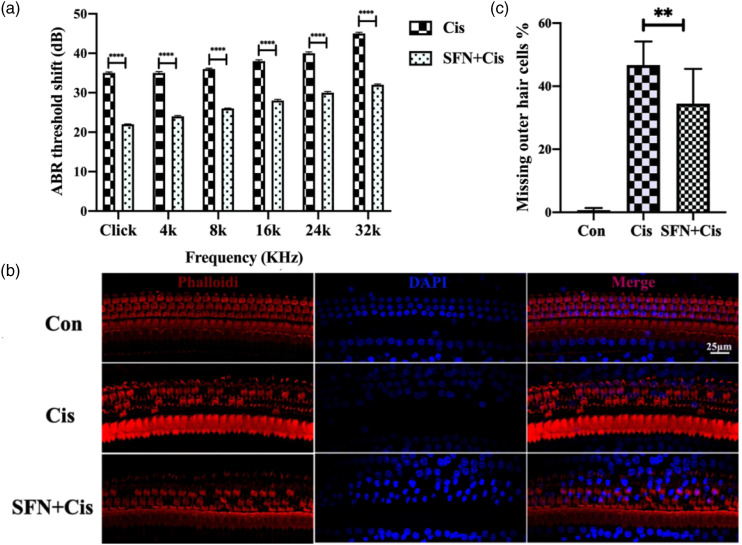
The rats were intraperitoneally injected with saline, cisplatin, or SFN+cisplatin to investigate whether SFN had protective effect on CIHL in vivo. After administration of cisplatin, the ABR threshold shifts increased significantly, including 4, 8, 16, 24, 32 kHz, and broad-band clicks. However, the ABR thresholds increased less, and the shifts were statistically significant when the rats were injected with SFN before cisplatin as compared to rats injected with cisplatin only (Figure 2(a), p < 0.001). Phalloidin staining showed less outer hair cell loss in the middle turn of the basilar membrane when the rats were injected with SFN before cisplatin as illustrated in Figure 2(b). The percentage of missing outer hair cells was significantly less in the SFN+Cis group compared to the Cis group in the middle turn of the basilar membrane (Figure 2(c), p < 0.01).
Figure 2.
Sulforaphane reduced cisplatin-induced hearing loss and hair cell damage in rats in vivo. A: The auditory brainstem response thresholds increased less, the shifts were statistically significant, including 4, 8, 16, 24, 32 kHz, and broad-band clicks, when the rats were injected with sulforaphane before cisplatin as compared to rats injected with cisplatin only (***p < 0.001; n = 60 ears). B: The middle turn of the organ of Corti phalloidin staining indicated that outer hair cell loss was less in the sulforaphane +Cis group (red: phalloidin, blue: DAPI. Scale bar = 25 μm). C: The percentage of missing outer hair cells was significantly less in the sulforaphane +Cis group compared to the Cis group in the middle turn of the basilar membrane (n = 30 ears, **p < 0.01).
SFN protects auditory cells from cisplatin-induced damage in vitro
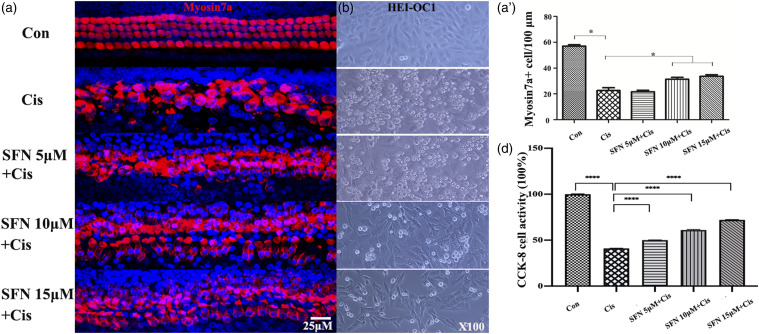
We used increasing concentrations of SFN (5, 10, and 15 μM), to investigate protective effect. In cochlear organotypic culture, cisplatin (10 μM) induced considerable damage and loss of inner and outer hair cells, while the damage was less severe when the cells were incubated with cisplatin combined with SFN as illustrated in Figure 3(a). Myosin 7a+ cell numbers were significantly higher in the SFN+Cis group (10, 15 μM) comparing to the cisplatin (10 μM) group (Figure 3(a)’, p < 0.05). In HEI-OC1 cells, cisplatin (10 μM) promoted HEI-OC1 cell death, observed as round, floating cells in the culture medium. However, incubated with SFN (5, 10, and 15 μM) prevented cisplatin-induced HEI-OC1 cell death (Figure 3(b)). CCK-8 assay showed that cisplatin (10 μM) reduced the viability of HEI-OC1 cells to 42%, and SFN had a dose-dependent protective effect (Figure 3(c), p < 0.0001). Increasing doses of SFN attenuated hair cell damage in vitro.
Figure 3.
Sulforaphane protected against cisplatin-induced hair cell damage in vitro. A: Cochlear organotypic culture, with myosin7a staining. A’: Myosin 7a+ cell counting. After cisplatin (10 μM) treatment, the number of myosin 7a+ cells decreased significantly. Myosin 7a+ cell numbers were significantly higher in the sulforaphane +Cis group (10 and 15 μM) comparing to the cisplatin (10 μM) group. B: HEI-OC1 cells culture. Cisplatin (10 μM) induced HEI-OC1 cell death. However, incubated with sulforaphane (5, 10, and 15 μM) prevented cisplatin-induced HEI-OC1 cell death. C: HEI-OC1 cell viability. After treatment with cisplatin (10 μM), HEI-OC1 cell viability decreased to 42%, while sulforaphane (5, 10, and 15 μM) significantly prevented the decrease induced by cisplatin (Scale bar = 25 μm. Red indicates myosin7a, blue indicates DAPI. *p < 0.05, ****p < 0.0001, versus cisplatin group; 3 in each group, n = 15).
SFN can prevent the cisplatin-induced imbalance of HAT/HDAC expression in HEI-OC1 cells
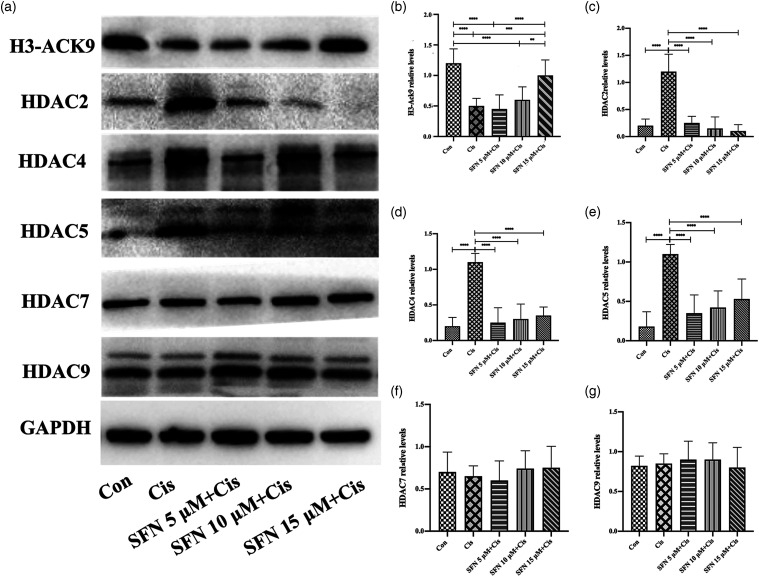
After treatment with cisplatin (10 μM), the acetylated H3-Ack9 expression decreased significantly (Figure 4(a) and (b), p < 0.0001). The addition of SFN 15 μM significantly inhibited the decrease of H3-Ack9 expression (Figure 4(a) and (b), p < 0.001). The addition of SFN (5, 10, and 15 μM) significantly inhibited the increase of HDAC2, HDAC4, and HDAC5 expression induced by cisplatin (10 μM) (Figure 4(a), (c)–(e), p < 0.0001). There was no observed difference between the different SFN concentrations. Neither cisplatin nor cisplatin combined with SFN affected the expression of HDAC7 (Figure 4(a) and (f)) or HDAC9 (Figure 4(a) and (g)). These results indicated that SFN exerted a protective effect against CIHL by preventing the disruption of the HAT/HDAC balance (Figure 4).
Figure 4.
Sulforaphane prevented disruption of the histone deacetylase / histone deacetylase balance. The addition of sulforaphane 15 μM significantly inhibited the decrease of H3-Ack9 expression induced by cisplatin (10 μM). The addition of sulforaphane (5, 10, and 15 μM) significantly inhibited the increase of HDAC2, -4, and -5 expression induced by cisplatin (10 μM). There was no observed difference between the different sulforaphane concentrations. The addition of sulforaphane or treatment with cisplatin (10 μM) and the expression of HDAC7 and HDAC9 did not change significantly. A: Western blot. The relative protein expression levels of H3-Ack9 (B), HDAC2 (C), HDAC4 (D), HDAC5 (E), HDAC7 (F), and HDAC9 (G) were quantified using Image-Pro Plus 6.0 and normalized to GAPDH (**p < 0.01, ***p < 0.001, ****p < 0.0001; n = 15).
Discussion
Cisplatin is used alone or combined with other chemotherapeutics for the treatment of cancer. Cisplatin-induced nephrotoxicity and ototoxicity are not uncommon. From ABR measurements and phalloidin staining of hair cells, we found that SFN reduced CIHL in vivo. In the most accepted model for hair cell mechanotransduction, a cluster of myosin motors located at the stereocilia upper tip link density keeps the tip link under tension at rest. Myosin7a localizes to the upper tip link density. 20 An early functional study in Myosin7a mutant mice showed that Myosin7a was essential to keep tip links under tension at rest. 21 Myosin7a is implicated in Usher type I syndrome, 22 characterized by deafness, vestibular dysfunction, and retinopathy leading to blindness. Myosin7a is critical for the development and arrangement of hair bundles. 23 Cisplatin is known to induce a decrease in Myosin 7a+ cell count in rats cochlear organotypic culture. 24 Based on the cochlear organotypic culture, HEI-OC1 cell culture, and CCK-8 detection of cell viability, we found that SFN protected hair cell count, increased Myosin 7a+ cell count, prevented HEI-OC1 cell death, and increased cell viability in vitro.
SFN, as a natural HDACi, has the potential to decrease HDAC activity to protect ethanol-exposed neural crest cells 25 ; inhibit HDAC activity to prevent vestibular schwannoma growth in vitro and in vivo 26 ; trigger DNA damage; and hinder DNA repair in colon cancer cells by alter HAT and HDAC activities. 27 Our previous study showed that the HDACi suberoylanilide hydroxamic acid could decrease high HDAC1 and HDAC4 levels and increase low H3-Ack9 levels to alleviate the noise-induced hearing loss. 18 We also showed that the HDACi sodium butyrate decreased high HDAC1 levels and increased low H3-Ack9 levels to alleviate gentamicin-induced hearing loss. 10 The present study showed that SFN minimized the cisplatin-induced expression of HDAC2, -4, and -5. Additionally, SFN prevented the decrease of cisplatin-induced expression of H3-Ack9. The literature showed that SFN could downregulate HDAC2 expression to induce preventive and therapeutic effects on breast cancer.28,29 Both SFN and its metabolites produced an increase in acetylated histone H3. 30 The inhibition of HDAC5-lysine-specific demethylase 1 axis with SFN blocked breast cancer growth. 12 SFN decreased the enzyme activities of HDAC2, -4, -5, -7, and -9 of primary vascular smooth muscle cells. 31 However, in this study, we did not find the expression changes of HDAC7 and HDAC9 in HEI-OC1 cells.
Although SFN can inhibit the growth of breast cancer cells, it has no inhibitory effect on normal breast epithelial cells. 12 The negative effects of SFN on auditory cells need further study. Also, we need to further study the signal pathway of SFN affecting the balance of HAT/HDAC in auditory cells. In vivo study, the results should be further divided into left ear and right ear.
Conclusions
SFN decreased cisplatin-induced ABR threshold shifts and outer hair cell loss. SFN had a dose-dependent protective effect on HEI-OC1 cells. In cochlear organotypic cultures, SFN increased cisplatin-induced myosin 7a+ cell count and restored ciliary morphology. SFN reversed the cisplatin-induced increase in HDAC2, -4, and -5 and decrease in H3-Ack9 protein expression in HEI-OC1 cells. SFN prevented the HAT/HDAC imbalance, protecting against CIHL in rats.
Footnotes
Author contributions: JW produced the animal model and was a major contributor in writing the manuscript. KYT analyzed the gene data. YF performed the histological examination and western blotting. HMC performed the CCK-8 detection of cell viability. YNH performed the western blotting. FQC helped with the design, implementation, and revision of important contents of the experiments. JW, KYT, and YF contributed equally to this work. All authors read and approved the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was supported by the National Natural Science Foundation of China (No. 81670925, 81271069, 82000959), Natural Science Foundation of Shaanxi Province (No.2019JQ-434, 2020JM-606), Xi’an Health and Family Planning Commission (No. J201902034), and Shaanxi Provincial Department of Science and Technology key industry innovation chain (Group)—social development field (2021ZDLSF02-12).
Ethics approval: Ethical approval for this study was obtained from the National Institutes of Health guidelines and approved by the Committee on Animal Research of the Air Force Military Medical University (KJ-2017-XJR4723).
Animal welfare: The present study followed the National Institutes of Health guidelines for humane animal treatment and complied with relevant legislation.
Availability of data and materials: The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
ORCID iD
Fu-quan Chen https://orcid.org/0000-0002-4625-073X
References
- 1.Costa V, Casamassimi A, Ciccodicola A. (2010) Nutritional genomics era: opportunities toward a genome-tailored nutritional regimen. Journal of Nutritional Biochemistry 21: 457–467. [DOI] [PubMed] [Google Scholar]
- 2.Song L, Thornalley PJ. (2007) Effect of storage, processing and cooking on glucosinolate content of brassica vegetables. Food Chem Toxicol 45: 216–224. [DOI] [PubMed] [Google Scholar]
- 3.Amjad AI, Parikh RA, Appleman LJ, et al. (2015) Broccoli-derived sulforaphane and chemoprevention of prostate cancer: from bench to bedside. Current Pharmacology Reports 1: 382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vuong LD, Nguyen QN, Truong VL. (2019) Anti-inflammatory and anti-oxidant effects of combination between sulforaphane and acetaminophen in LPS-stimulated RAW 264.7 macrophage cells. Immunopharmacol Immunotoxicol 41: 413–419. [DOI] [PubMed] [Google Scholar]
- 5.Cheng AC, Shen CJ, Hung CM, et al. (2019) Sulforaphane decrease of SERTAD1 expression triggers G1/S arrest in breast cancer cells. Journal of Medicinal Food 22: 444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo M, Spagnuolo C, Russo GL, et al. (2018) Nrf2 targeting by sulforaphane: a potential therapy for cancer treatment. Critical Reviews in Food Science and Nutrition 58: 1391–1405. [DOI] [PubMed] [Google Scholar]
- 7.Jiang LL, Zhou SJ, Zhang XM, et al. (2016) Sulforaphane suppresses in vitro and in vivo lung tumorigenesis through downregulation of HDAC activity. Biomed Pharmacother 78: 74–80. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Liu Y, Han R, et al. (2015) FOXP3+ regulatory T cell development and function require histone/protein deacetylase 3. Journal of Clinical Investigation 125: 1111–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H, Xia A, Ye M, et al. (2020) Opposing functions of Fng1 and the Rpd3 HDAC complex in H4 acetylation in Fusarium graminearum. PLoS Genetics 16(11): e1009185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Wang Y, Chen X, et al. (2015) Histone deacetylase inhibitor sodium butyrate attenuates gentamicin-induced hearing loss in vivo. American Journal Ontolaryngol 36: 242–248. [DOI] [PubMed] [Google Scholar]
- 11.Choi SY, Kee HJ, Jin L, et al. (2018) Inhibition of class IIa histone deacetylase activity by gallic acid, sulforaphane, TMP269, and panobinostat. Biomed Pharmacother 101: 145–154. [DOI] [PubMed] [Google Scholar]
- 12.Cao C, Wu H, Vasilatos SN, et al. (2018) HDAC5-LSD1 axis regulates antineoplastic effect of natural HDAC inhibitor sulforaphane in human breast cancer cells. International Journal of Cancer 143: 1388–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke JD, Riedl K, Bella D, et al. (2011) Comparison of isothiocyanate metabolite levels and histone deacetylase activity in human subjects consuming broccoli sprouts or broccoli supplement. Journal of Agricultural and Food Chemistry 59: 10955–10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bian X, Liang Z, Feng A, et al. (2018) HDAC inhibitor suppresses proliferation and invasion of breast cancer cells through regulation of miR-200c targeting CRKL. Biochem Pharmacol 147: 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Wen LT, Wang Y, et al. (2016) Therapeutic effect of histone deacetylase inhibitor, sodium butyrate, on allergic rhinitis in vivo. DNA Cell Biology 35: 4–6. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Cui M, Sun F, et al. (2020) HDAC inhibitor sodium butyrate prevents allergic rhinitis and alters lncRNA and mRNA expression profiles in the nasal mucosa of mice. Journal of Molecular Medicine 45: 1150–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang DH, Xie J, Liu K, et al. (2017) The histone deacetylase inhibitor sodium butyrate protects against noise-induced hearing loss in guinea pigs. Neuroscience Letters 660: 140–146. [DOI] [PubMed] [Google Scholar]
- 18.Wen LT, Wang J, Wang Y, et al. (2015) Association between histone deacetylases and the loss of cochlear hair cells: role of the former in noise-induced hearing loss. International Journal of Molecular Medicine 36: 534–540. [DOI] [PubMed] [Google Scholar]
- 19.Drottar M, Liberman MC, Ratan RR, et al. (2006) The histone deacetylase inhibitor sodium butyrate protects against cisplatin-induced hearing loss in guinea pigs. Laryngoscope 116: 292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grati M, Kachar B. (2011) Myosin VIIa and sans localization at stereocilia upper tip-link density implicates these Usher syndrome proteins in mechanotransduction. Proceedings of the National Academy of Sciences of the United States of America 108: 11476–11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kros CJ, Marcotti W, van Netten SM, et al. (2002) Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nature Neuroscience 5: 41–47. [DOI] [PubMed] [Google Scholar]
- 22.Yan D, Liu XZ. (2010) Genetics and pathological mechanisms of Usher syndrome. Journal of Human Genetics 55: 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YY, Nam H, Jung H, et al. (2017) Over-expression of myosin7A in cochlear hair cells of circling mice. Laboratory Animal Research 33: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian Ky, Chang HM, Wang J, et al. (2019) Inhibition of DHCR24 increases the cisplatin-induced damage to cochlear hair cells in vitro. Neuroscience Letters 706: 99–104. [DOI] [PubMed] [Google Scholar]
- 25.Yuan F, Chen X, Liu J, et al. (2017) Sulforaphane restores acetyl-histone H3 binding to Bcl-2 promoter and prevents apoptosis in ethanol-exposed neural crest cells and mouse embryos. Experimental Neurology 300: 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim BG, Fujita T, Stankovic KM, et al. (2016) Sulforaphane, a natural component of broccoli, inhibits vestibular schwannoma growth in vitro and in vivo. Scientific Reports 6: 36215–36218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okonkwo A, Mitra J, Johnson GS, et al. (2018) Heterocyclic analogs of sulforaphane trigger DNA damage and impede DNA repair in colon cancer cells: interplay of HATs and HDACs. Molecular Nutrition & Food Research 62: e1800228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paul B, Li Y, Tollefsbol TO. (2018) The effects of combinatorial genistein and sulforaphane in breast tumor inhibition: role in epigenetic regulation. International Journal of Molecular Sciences 19: 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Royston KJ, Paul B, Nozell S, et al. (2018) TO tollefsbol: Withaferin A and sulforaphane regulate breast cancer cell cycle progression through epigenetic mechanisms. Experimental Cell Research 368: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myzak MC, Tong P, Dashwood WM, et al. (2007) Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Experimental Biology and Medicine 232: 227–234. [PMC free article] [PubMed] [Google Scholar]
- 31.Yong CS, Jin KH, Li J, et al. (2018) Inhibition of class IIa histone deacetylase activity by gallic acid, sulforaphane, TMP269, and panobinostat. Biomedicine & Pharmacotherapy 101: 145–154. [DOI] [PubMed] [Google Scholar]