Abstract
Background and aims:
Currently, there are no definitive therapies for coronavirus disease 2019 (COVID-19). Gut microbial dysbiosis has been proved to be associated with COVID-19 severity and probiotics is an adjunctive therapy for COIVD-19. However, the potential benefit of probiotics in COVID-19 has not been studied. We aimed to assess the relationship of probiotics use with clinical outcomes in patients with COVID-19.
Methods:
We conducted a propensity-score matched retrospective cohort study of adult patients with COVID-19. Eligible patients received either probiotics plus standard care (probiotics group) or standard care alone (non-probiotics group). The primary outcome was the clinical improvement rate, which was compared among propensity-score matched groups and in the unmatched cohort. Secondary outcomes included the duration of viral shedding, fever, and hospital stay.
Results:
Among the propensity-score matched groups, probiotics use was related to clinical improvement rates (log-rank p = 0.028). This relationship was driven primarily by a shorter (days) time to clinical improvement [difference, −3 (−4 to −1), p = 0.022], reduction in duration of fever [−1.0 (−2.0 to 0.0), p = 0.025], viral shedding [−3 (−6 to −1), p < 0.001], and hospital stay [−3 (−5 to −1), p = 0.009]. Using the Cox model with time-varying exposure, use of probiotics remained independently related to better clinical improvement rate in the unmatched cohort.
Conclusion:
Our study suggested that probiotics use was related to improved clinical outcomes in patients with COVID-19. Further studies are required to validate the effect of probiotics in combating the COVID-19 pandemic.
Keywords: clinical improvement, COVID-19, gut microbiota, probiotics
Introduction
The coronavirus disease 2019 (COVID-19) has been considered a global pandemic. 1 Fever, fatigue, and cough are the most common clinical features of COVID-19, but gastrointestinal symptoms (e.g., diarrhea, nausea, vomiting) are increasingly recognized among COVID-19 patients.2,3 Previous studies have shown that positive severe acute respiratory syndrome coronavirus (SARS-CoV-2) in the stool of COVID-19 patients was associated with longer illness duration. 4 Angiotensin-converting enzyme 2 has been found to be abundant in the epithelia of the lungs and intestine, suggesting that there is a subtle link between them. 4 The gut microbiota have been proved to play a critical role in health through the “gut–lung axis”. Thus, SARS-CoV-2 might also affect the gut microbiota in COVID-19. Indeed, microbial dysbiosis was found in some patients with COVID-19. 5 Imbalance of intestinal flora can lead to dysbiosis, which might influence the outcome of the clinical features. A novel and more targeted method may be needed to modulate gut microbiota as one of the treatments for COVID-19 and its comorbidities.
Currently, no definitive drugs are recommended for the treatment of COVID-19. Developing effective new therapies for curing COVID-19 is time-consuming. Therefore, using existing approved therapies or treatment modalities is seen as a more cost-effective strategy for reducing the severity of COVID-19. In early February 2020, China’s National Health Commission and National Administration (version 5) recommended the use of probiotics, gut microecological modulators, in COVID-19 patients to maintain the balance of intestinal microecology. 6 Probiotics have shown good results in regulating innate immunity and improving inflammatory conditions. 7
We hypothesized that probiotics modulate the gut microbiota to protect the respiratory system and would be related to clinical improvement in COVID-19. Thus, we conducted a propensity-score matched retrospective cohort study to assess the association of in-hospital use of probiotics with clinical outcomes.
Methods
Population
We performed a propensity-score matched retrospective cohort study at the Shenzhen Third People’s Hospital (Guangdong, China) from 11 January 2020 to 1 April 2020. Adults (age 18 years or older) were eligible for the study if they tested positive for SARS-CoV-2 according to World Health Organization (WHO) interim guidance. Exclusion criteria were pregnancy or lactation; conditions with gastrointestinal or systemic diseases known to affect gut microbiota composition such as gastrointestinal cancer, inflammatory bowel disease; incomplete information. The definition of disease severity of COVID-19 was based on the Chinese guideline for the management of COVID-19. 6 The study protocols were approved by the Ethics of Research Committee of the Shenzhen Third People’s Hospital (No. 2020-193). A STARD checklist is shown in the Supplemental material online.
Standard care comprised, as necessary, supplemental oxygen, high-flow nasal cannula (HFNC), antiviral agents, antibiotics, corticosteroids, mechanical ventilation, according to the Chinese guideline for the management of COVID-19. 8 Inclusion criteria for the adjuvant probiotics treatment were: mild to severe patients with COVID-19; there was no history of probiotics or antibiotics use for at least 1 month; did not have allergy to probiotics. Probiotic treatment was administered as 630 mg (three pills) twice daily, using live combined Bifidobacterium, Lactobacillus, and Enterococcus capsules (Bifico) (Shanghai, China). Each capsule is of 210 mg, which contains Bifidobacterium, Lactobacillus and Enterococcus (1.0 × 107 CFU for each ingredient). There was no connection with the probiotic treatment with the gastrointestinal symptoms of COVID-19 patients. The primary exposure was use of probiotics, classified as present if probiotics were received within 48 h of hospital admission and otherwise as absent. The duration of oral probiotics administration was defined as the time from probiotics treatment initiation to viral shedding or death. In the control group, the initial time for the patients without probiotics administration was defined as the time of viral shedding or death.
Clinical data were collected from the Shenzhen Third People’s Hospital (Guangdong, China). Data reliability was verified by two researchers (TWL and LNZ) and difference in interpretation was adjudicated by a third researcher (JYW).
Outcome measures
The primary outcome was the time (days) to clinical improvement. The definition of time to clinical improvement was the time from any treatment initiation to live discharge or an improvement of two points on a seven-point scale, whichever came first according to the previous study. 8 Second outcomes included the duration of hospital stay, fever, viral shedding, and gastrointestinal symptoms.
Laboratory measures
Throat-swab samples were collected for SARS-CoV-2 detection according to WHO interim guidance. Complete blood count, D-dimer, serum biochemical tests, interleukin-6 (IL-6), procalcitonin, CD4+ and CD8+ T cell count were examined. The reference values were as follows: white blood cell (WBC) count: (4–10) × 109; platelet count: (100–300) × 109/l; lymphocyte count: (0.8–4.0) × 109/l; IL-6: (0–7) pg/ml; albumin: 40–55 g/l; D-dimer: 0–0.5 μg/ml; aspartate aminotransferase (AST): 0–45 g/l; alanine transaminase (ALT): 0–45 g/l; procalcitonin: <0.1 ng/ml; cluster of differentiation (CD) 4+ T cell count: >400/mm3; CD8+ T cell count: >150/mm3.
Statistical analysis
Patients in the cohort were classified according to whether probiotics were used or not (probiotics versus non-probiotics). Continuous and categorical variables were shown as medians [interquartile ranges (IQRs) or standard deviations (SDs)] and n (%), respectively. No imputation was performed for missing data, because the patients with insufficient information had been removed during the patient selection. Patients who had a probiotics approach were matched to those who did not receive probiotics therapy 1:1 by greedy matching on the logit of propensity scores with a caliper of 0.2 SD. 9 Variables thought to be potential confounders were enrolled into the propensity score and included socio-demographics [age, sex, body mass index (BMI)], clinical characteristics (disease severity, hypertension, diabetes, coronary disease, radiological findings), laboratory tests (WBC counts, lymphocyte count, IL-6, D-dimer, ALT, AST, CD4+ and CD8+ T cell count), clinical treatments [antibiotics, corticosteroid, intravenous immunoglobin, oxygen therapy, HFNC, non-invasive mechanical ventilation (NIMV), invasive mechanical ventilation (IMV)], and ICU admission. Standardized differences for all covariates were calculated before and after matching, with 10% or more considered indicative of imbalance. 10 The primary outcome was described by Kaplan–Meier plot and compared with a log-rank test after matching. For all outcomes, time to different clinical events was treated as continuous variable, and the difference between the probiotics and non-probiotics groups was analyzed by Hodges–Lehmann estimation in the matched cohort. To further validate the effects of probiotics on clinical improvement, Cox proportional hazard model with hazard ratio (HR) and 95% confidence interval (CI) was used in the unmatched cohort after adjustment for confounding factors. Analysis were performed by R software (version 3.6.1, Vienna, Austria). All tests were two-sided with a significance level of 0.05.
Results
Baseline characteristics of patients before matching
A total of 411 COVID-19 patients were enrolled. Eighteen patients were excluded as they were less than 18 years old, 15 patients had both gastrointestinal disease and tumor, and 10 patients had incomplete information. The final cohort included 375 patients, among which 179 patients received standard care plus probiotics (the probiotics group) and the other 196 cases were treated with standard care alone (the non-probiotics group). The clinical characteristics are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the COVID-19 patients at baseline before and after matching. a .
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Non-probiotics group n = 196 |
Probiotics group n = 179 |
SDM b | Non-probiotics group n = 150 |
Probiotics group n = 150 |
SDM b |
| Male sex, n (%) | 105 (53.6) | 88 (49.2) | 0.09 | 76 (50.7) | 77 (51.3) | 0.01 |
| Age, median (IQR), years | 50 (36–62) | 48 (36–59) | 0.01 | 50 (37–62) | 49 (35–60) | 0.00 |
| BMI, median (IQR) | 23.6 (21.7–26.1) | 22.9 (20.8–25.1) | 0.08 | 23.3 (21.4–25.6) | 23.2 (21.3–25.3) | 0.01 |
| Disease severity, n (%) | ||||||
| Mild | 11 (5.6) | 15 (8.4) | 0.11 | 10 (6.7) | 11 (7.4) | 0.03 |
| Moderate | 174 (88.8) | 157 (87.7) | 0.03 | 135 (90.0) | 134 (89.3) | 0.02 |
| Severe | 11 (5.6) | 7 (3.9) | 0.08 | 5 (3.3) | 5 (3.3) | 0.0 |
| Comorbidity, n (%) | ||||||
| Hypertension | 36 (18.4) | 31 (17.3) | 0.03 | 27 (18.0) | 31 (20.7) | 0.07 |
| Diabetes | 22 (11.2) | 15 (8.4) | 0.09 | 16 (10.7) | 14 (9.3) | 0.05 |
| Coronary disease | 10 (5.1) | 6 (3.4) | 0.08 | 7 (4.7) | 6 (4.0) | 0.03 |
| Laboratory test, median (IQR) | ||||||
| WBC count, ×109/l | 4.6 (3.8–5.7) | 4.8 (3.8–6.0) | 0.09 | 4.7 (3.9–5.8) | 4.7 (3.6–5.0) | 0.00 |
| Lymphocyte count, ×109/l | 1.2 (1.0–1.7) | 1.3 (1.0–1.7) | 0.23 | 1.3 (1.0–1.7) | 1.3 (1.0–1.7) | 0.02 |
| IL-6, pg/ml | 10.2 (3.9–20.7) | 6.3 (3.0–17.1) | 0.01 | 8.8 (3.4–17.3) | 8.5 (3.0–18.9) | 0.00 |
| D-dimer, μg/ml | 0.4 (0.3–0.6) | 0.3 (0.2–0.5) | 0.04 | 0.4 (0.2–0.6) | 0.4 (0.3–0.6) | 0.07 |
| ALT, g/l | 21.0 (15.0–30.8) | 18.0 (12.0–28.0) | 0.00 | 20.0 (13.0–29.0) | 19.0 (13.0–29.5) | 0.00 |
| AST, g/l | 25.0 (19.3–31.0) | 22.0 (19.0–28.0) | 0.01 | 24.0 (19.0–30.0) | 23.0 (19.0–29.3) | 0.01 |
| T cell count, /ml | 967 (592–1261) | 1076 (711–1389) | 0.00 | 988 (628–1373) | 1043 (678–1359) | 0.00 |
| CD4+ T cell count, /ml | 519 (377–735) | 586 (413–780) | 0.00 | 545 (390–759) | 567 (409–780) | 0.00 |
| CD8+ T cell count, /ml | 343 (204–470) | 386 (240–539) | 0.00 | 348 (200–504) | 377 (231–514) | 0.00 |
| Radiological findings, n (%) | ||||||
| Normal | 10 (5.1) | 15 (8.4) | 0.13 | 9 (6.0) | 11 (7.3) | 0.05 |
| Ground-glass opacity | 174 (88.8) | 157 (87.7) | 0.03 | 129 (86.0) | 133 (88.7) | 0.08 |
| Bilateral pulmonary infiltration | 147 (75.0) | 131 (73.3) | 0.04 | 108 (72.0) | 114 (76.0) | 0.09 |
| Duration of illness onset to any treatments initiation, median (IQR), days | 3 (2–6) | 3.5 (2–7) | 0.02 | 3 (2–7)) | 3 (1–5) | 0.05 |
| Treatments, n (%) | ||||||
| Antibiotics | 76 (38.8) | 58 (32.4) | 0.13 | 54 (36.0) | 49 (32.7) | 0.07 |
| Antiviral treatment | 196 (100.0) | 179 (100.0) | 0.00 | 150 (100.0) | 150 (100.0) | 0.00 |
| Corticosteroids | 68 (34.7) | 32 (17.9) | 0.39 | 32 (21.3) | 32 (21.3) | 0.00 |
| Intravenous immunoglobin | 68 (34.7) | 37 (20.7) | 0.32 | 36 (24.0) | 36 (24.0) | 0.00 |
| Oxygen therapy | 147 (75.0) | 102 (57.0) | 0.39 | 101 (67.3) | 102 (68.0) | 0.02 |
| HFNC | 8 (4.1) | 4 (2.2) | 0.11 | 5 (3.3) | 4 (2.7) | 0.04 |
| NIMV | 39 (19.9) | 22 (12.3) | 0.21 | 21 (14.0) | 22 (14.7) | 0.02 |
| IMV | 9 (4.6) | 7 (3.9) | 0.03 | 6 (4.0) | 7 (4.7) | 0.03 |
| ICU admission | 18 (9.2) | 10 (5.6) | 0.14 | 9 (6.0) | 10 (6.7) | 0.03 |
Greedy matching occurred 1:1 on the logit of a propensity score with a caliper of 0.2 SD.
SDMs of 0.1 or more represent meaningful difference in covariates between groups before and after matching.
ALT, alanine transaminase; AST, aspartate aminotransferase; BMI, body mass index; CD, cluster of differentiation; HFNC, high-flow nasal cannula; ICU, intensive care unit; IL-6, interleukin-6; IMV, invasive mechanical ventilation; IQR, interquartile range; NIMV, non-invasive mechanical ventilation; SDM, standard difference of the mean; WBC, white blood cell.
Before probiotics treatment, the number of patients with diarrhea was 20 (10.2%) in the non-probiotics group and 13 (7.3%) in the probiotics group (p = 0.10); the number of patients with abdominal pain was three (1.5%) in the non-probiotics group and three (1.7%) in the probiotics group (p = 0.12); the number of patients with nausea and vomiting was eight (4.1%) in the non-probiotics group and four (2.2%) in the probiotics group (p = 0.12). After probiotics treatment, gastrointestinal symptoms of these patients improved and symptoms disappeared. We did not observe any side effects of probiotic usage. Compared with patients who received probiotics therapy, those who had not received probiotics had lower rates of mild type (5.6% versus 8.4%; standardized difference, 0.11) and normal radiological findings (5.1% versus 8.4%; 0.13), as well as a lower lymphocyte count [median, 1.2 × 109/l (1.0–1.7) versus 1.3 × 109/l (1.0–1.7); 0.23]. With respect to clinical therapy, patients in the non-probiotics group had higher rates of antibiotics (38.8% versus 32.4%; 0.13), corticosteroids (34.7% versus 17.9%; 0.39), intravenous immunoglobin (34.7% versus 20.7%; 0.32), oxygen therapy (75.0% versus 57.0%; 0.39), HFNC (4.1% versus 2.2%; 0.11), NIMV (19.9% versus 12.3%; 0.21), and ICU admission (9.2% versus 5.6%; 0.14) as compared with those in the probiotics group. There was no significant difference in antibiotics use between the two groups. The type, number, and combinations of administered drugs are shown in Supplemental Table 1.
Primary outcome after matching
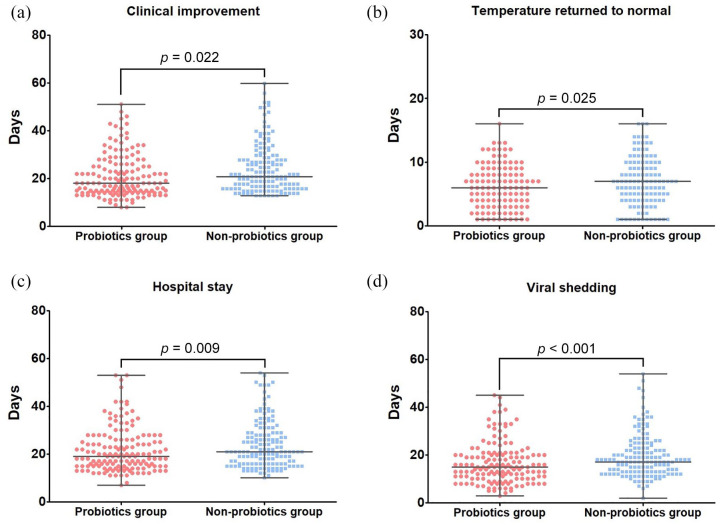
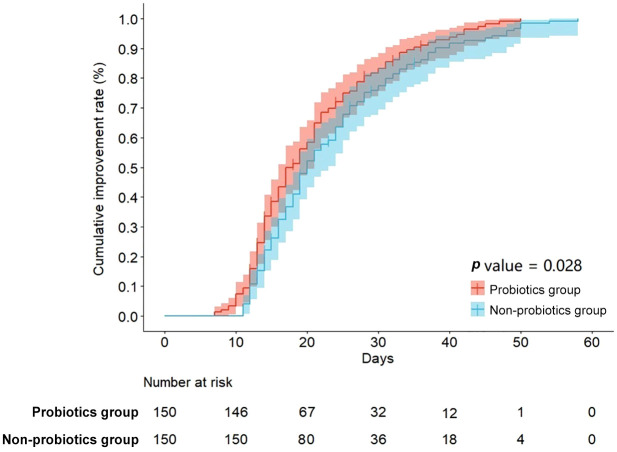
A total of 150 patients (83.8%) who had undergone a probiotics approach were each matched to a patient who had not received probiotics therapy with standardized difference of less than 10% for all covariates, suggesting an adequate match (Table 1). Compared with the non-probiotics group, the cumulative improvement rate was higher in the probiotics group with a log-rank p value of 0.028 (Figure 1). Patients in the probiotics group also have an accelerated time to clinical improvement after matching [median, 18 days (IQR: 14–28) versus 21 days (IQR: 17–29)] with a median difference of −3 days (95% CI: −4 to −1; p = 0.022) [Figure 2(a) and Table 2].
Figure 1.
Secondary outcomes after matching. (a) Time to clinical improvement, (b) time to temperature return to normal, (c) duration of hospital stay, (d) duration of viral shedding.
Figure 2.
Kaplan–Meier estimates of cumulative clinical improvement rate.
Table 2.
Clinical outcomes after the propensity-score matching.
| Characteristic | Total N = 300 |
Non-probiotics group n = 150 |
Probiotics group n = 150 |
Difference | p value |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Time to clinical improvement, days | 20.0 (15.0–28.0) | 21.0 (17.0–29.0) | 18.0 (14.0–28.0) | –3 (–4 to –1) | 0.022 |
| Secondary outcomes | |||||
| Hospital stay, days | 21.0 (15.0–28.0) | 22.0 (16.0–31.0) | 19.0 (15.0–25.0) | –3 (–5 to –1) | 00.009 |
| Duration of viral shedding, days | 16.0 (12.0–22.0) | 18.0 (13.0–24.0) | 15.0 (10.0–20.0) | –3 (–6 to –1) | <0.001 |
| Duration of fever, days | 7.0 (4.0–9.0) | 7.0 (4.0–10.0) | 6.0 (3.0–9.0) | –1.0 (–2.0 to 0.0) | 00.025 |
| ICU stay, days | 15.0 (10.0–20.0) | 16.0 (13.0–30.0) | 13.0 (7.0–17.0) | –4 (–9 to 2) | 0.397 |
| Duration of NIMV, days | 6.0 (4.75–8.0) | 6.0 (4.0–8.0) | 7.0 (5.0–9.5) | 1 (–1 to 3) | 0.545 |
| Duration of IMV, days | 19.0 (10.5–28.5) | 24.0 (19.0–33.0) | 12.0 (7.0–25.5) | –11 (–20 to 1) | 0.615 |
| Oxygen support, days | 14.0 (8.0–21.0) | 14.5 (7.25–20.0) | 14.0 (8.0–21.0) | 0 (–3 to 2) | 0.081 |
Data are median (interquartile range).
ICU, intensive care unit; IMV, invasive mechanical ventilation; NIMV, non-invasive mechanical ventilation.
Secondary outcomes after matching
The secondary outcomes of the probiotics and non-probiotics groups after matching are shown in Table 2. Among patients who experienced fever symptoms, the duration of fevers in the probiotics group was also shorter than that in the non-probiotics group (median, 6 days versus 7 days) with a significant difference in median −1 day (IQR: −2 to 0 days; p = 0.025) [Figure 1(b)]. The probiotics group was superior to the non-probiotics group in shortening the duration of hospital stay [median, 17 days versus 20 days; difference, −3 days (95% CI: −5 to −1), p = 0.009] [Figure 1(c)] and the duration of viral shedding [median, 15 days versus 18 days; difference, −3 days (95% CI: −6 to −1), p < 0.001] [Figure 1(d)]. However, there was no significance in the ICU stay length, duration of NIMV, duration of IMV, and duration of oxygen support between the propensity-score matched groups (p > 0.05) (Table 2).
Effects of probiotics on clinical improvement in the unmatched cohort
The effects of probiotics on clinical improvement analyzed by Cox regression analyses in the unmatched cohort are shown in Table 3. A crude model of univariate analysis showed that the application of probiotics in COVID-19 patients was dramatically related to a better clinical improvement (HR, 1.35; p = 0.006). Using further multivariate analyses we found that patients who underwent probiotics therapy were likely to experience a favorable clinical improvement compared with those not treated with probiotics, even after adjusting for age, sex, BMI [Model 1; adjusted HR (aHR), 1.33; p = 0.007]; further adjusted for disease severity, comorbidity, radiological findings based on Model 1 (Model 2; aHR, 1.32; p = 0.012); based on Model 2, further adjusted for laboratory test results (Model 3; aHR, 1.28; p = 0.026). We further additionally adjusted for clinical treatments and ICU admission based on Model 3 (Model 4; aHR, 1.30; p = 0.017).
Table 3.
Time-varying analysis for the effect of probiotics on clinical improvement in the unmatched cohort.
| Models | Hazard ratio (95% CI) | p value |
|---|---|---|
| Crude model | 1.35 (1.09–1.67) | 0.006 |
| Model 1 a | 1.33 (1.09–1.67) | 0.007 |
| Model 2 b | 1.32 (1.06–1.64) | 0.012 |
| Model 3 c | 1.28 (1.04–1.59) | 0.026 |
| Model 4 d | 1.30 (1.04–1.61) | 0.017 |
Model 1 was adjusted for age, sex, and body mass index.
Model 2 was additionally adjusted for disease severity, comorbidity (including hypertension, diabetes, and coronary disease), and radiological findings (including normal, ground-glass opacity, and bilateral pulmonary infiltration).
Model 3 was additionally adjusted for laboratory test results (including white blood cell count, lymphocyte count, interleukin-6, D-dimer, alanine transaminase, aspartate aminotransferase, T cell count, CD4+ T cell count, and CD8+ T cell count).
Model 4 was additionally adjusted for clinical treatments (including antibiotics, corticosteroid, intravenous immunoglobin, oxygen therapy, high-flow nasal cannula, non-invasive mechanical ventilation, and invasive mechanical ventilation), and intensive care unit admission.
CI, confidence interval.
Discussion
In the present study, we found that probiotics added to standard care was superior to standard care alone in time to clinical improvement. The significant reduction in duration of viral shedding was related to clinical improvement as shown by the significant reductions in duration of fever and hospital stay.
Gastrointestinal symptoms with a 70% relative increased risk for positive detection of COVID-19. The presence of diarrhea was associated with the severity of respiratory symptoms. 2 The gut microbiota are considered to have an important role on the innate and adaptive immune system. 16 Gut–lung crosstalk may affect the gut microbiota in patients with COVID-19. 4 It has been shown that modulating intestinal flora can avoid early replication of influenza virus in lung epithelia and reduce ventilator-associated pneumonia.17,18 Currently, no specific treatment has been recommended for COVID-19. Probiotics have been recommended for maintain the balance of gut microbiota in COVID-19 patients and prevent secondary bacterial infection. 6 Probiotics, including bacteria and yeast, are living microorganisms that have been shown to be beneficial to human health. 17 In vivo experiments and clinical trials expand our current understanding of the critical role of probiotics in human intestinal flora-related diseases. Many clinical trials have shown that probiotics can shape the intestinal flora, making it possible to control a variety of intestinal diseases and promote overall health. 19 The presence of gastrointestinal symptoms was related to a 70%increased risk for positive detection of COVID-19.
COVID-19 infection affects lung tissue and intestines, thereby activating inflammation. Probiotics supplementation may improve the ability of the gastrointestinal microbiota to regulate immune activity, thereby preventing viral infections, including COVID-19. Although many experimental and clinical studies support the possible role of probiotic microorganisms in protecting the host against viral infections (e.g., colds and flu),20–23 no research has reported the use of probiotics to treat or prevent COVID-19. In our study, higher percentages of antibiotics, antiviral treatment corticosteroids, intravenous immunoglobin, oxygen therapy, HFNC, NIMV, IMV, and ICU admission in the non-probiotics group before matching imply that disease was much more severe in the non-probiotics group compared with the probiotic group. Similarly, higher percentages of antibiotics were found in the non-probiotics group after matching. We found that patients in the probiotics plus standard care group had shorter time to clinical improvement. The significant reduction in duration of viral shedding was related to clinical improvement as shown by the significant reductions in duration of fever and hospital stay. These results may lead to a better understanding of probiotics use in COVID-19 patients. According to our study, the indication of probiotics is mild to severe patients with COVID-19 and the contraindication is patients who are allergic to probiotics. The strains selection may contain Bifidobacterium, Lactobacillus, and Enterococcus, based on our current study. The dosage of probiotics is given according to the specifications. However, these findings are observational, and we cannot exclude the possibility of unmeasured confounders or hidden bias that account for the association between probiotics use and improved outcomes. Further randomized controlled trials (RCTs) are needed to prospectively explore the benefit of probiotics in combating the COVID-19 pandemic. Our study may also provide a theoretical basis for the next prospective randomized controlled studies. Currently, three registered trials to evaluate probiotics administration to COVID-19 patients are ongoing and these studies could help to address the scientific questions.24–26
Our study has several limitations. First, the cohort study design limits interpretation of results and confirmation of efficacy will require controlled trials; second, this study was limited to one single medical institution in Shenzhen and therefore may have limited external generalizability to other medical settings; third, critically ill patients are underrepresented, which did not allow the generalization of our findings to critical cases.
In summary, our study has demonstrated that the use of probiotics was associated with a shorter time to clinical improvement including fever, hospital stay, and viral shedding in hospitalized COVID-19 subjects. This information may help clinicians to consider the use of probiotics in patients with mild to severe COVID-19. Further RCTs are needed to prospectively explore the benefit of probiotics in combating the COVID-19 pandemic.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848211035670 for Probiotics use is associated with improved clinical outcomes among hospitalized patients with COVID-19 by Lina Zhang, Huanqin Han, Xuan Li, Caozhen Chen, Xiaobing Xie, Guomei Su, Shicai Ye, Cuili Wang, Qing He, Fang Wang, Fang Huang, Zhaoqin Wang, Jiayuan Wu and Tianwen Lai in Therapeutic Advances in Gastroenterology
Footnotes
Author contributions: Contributors: LNZ, HQH, XL, and CZC served as co-first authors. TWL, JYW, and ZQW served as co-corresponding authors. TWL and ZQW designed the study, interpreted the results, the accuracy of the data analysis, and drafted the manuscript. LNZ, JYW, XL, and HQH contributed to data collection, data analysis, and data interpretation. XBX, CLW, SCY, QH, FW, FH, and GMS contributed to literature search and data collection. All authors approved the final version of the manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Data availability statement: The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the Guangdong Basic and Applied Basic Research Foundation (No. 2020B1515020004, No. 2021A1515011056, No. 2018A0303130269), the National Natural Science Foundation of China (81873404); Guangdong Medical Science and Technology Research Fund (No. B2021206, No. A2018162); Project of Young Innovative Talents in Colleges and Universities in Guangdong Province (No. 2018KQNCX095); Shenzhen Key Medical Discipline Construction Fund (No. SZXK076) and Sanming Project of Medicine in Shenzhen (No. SZSM201612014).
ORCID iD: Tianwen Lai  https://orcid.org/0000-0001-9921-3425
https://orcid.org/0000-0001-9921-3425
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Lina Zhang, Department of Liver Diseases, National Clinical Research Center for Infectious Disease, Shenzhen Third People’s Hospital, The Second Affiliated Hospital, School of Medicine, Southern University of Science and Technology, Shenzhen, Guangdong, China.
Huanqin Han, Infectious Diseases Center, Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, China.
Xuan Li, Department of Liver Diseases, National Clinical Research Center for Infectious Disease, Shenzhen Third People’s Hospital, The Second Affiliated Hospital, School of Medicine, Southern University of Science and Technology, Shenzhen, Guangdong, China.
Caozhen Chen, Department of Breast Surgery, the Second Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, China.
Xiaobing Xie, Department of Respiratory and Critical Care Medicine, Affiliated Hospital, Institute of Respiratory Diseases, Guangdong Medical University, Zhanjiang, Guangdong, China.
Guomei Su, Department of Respiratory and Critical Care Medicine, Affiliated Hospital, Institute of Respiratory Diseases, Guangdong Medical University, Zhanjiang, Guangdong, China.
Shicai Ye, Department of Gastroenterology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, China.
Cuili Wang, Department of Respiratory and Critical Care Medicine, Affiliated Hospital, Institute of Respiratory Diseases, Guangdong Medical University, Zhanjiang, Guangdong, China.
Qing He, Department of Liver Diseases, National Clinical Research Center for Infectious Disease, Shenzhen Third People’s Hospital, The Second Affiliated Hospital, School of Medicine, Southern University of Science and Technology, Shenzhen, Guangdong, China.
Fang Wang, Department of Liver Diseases, National Clinical Research Center for Infectious Disease, Shenzhen Third People’s Hospital, The Second Affiliated Hospital, School of Medicine, Southern University of Science and Technology, Shenzhen, Guangdong, China.
Fang Huang, Department of Liver Diseases, National Clinical Research Center for Infectious Disease, Shenzhen Third People’s Hospital, The Second Affiliated Hospital, School of Medicine, Southern University of Science and Technology, Shenzhen, Guangdong, China.
Zhaoqin Wang, Department of Liver Diseases, National Clinical Research Center for Infectious Disease, Shenzhen Third People’s Hospital, The Second Affiliated Hospital, School of Medicine, Southern University of Science and Technology, Shenzhen 518000, Guangdong, China.
Jiayuan Wu, Department of Respiratory and Critical Care Medicine, Affiliated Hospital, Institute of Respiratory Diseases, Guangdong Medical University, Zhanjiang, Guangdong, China.
Tianwen Lai, Department of Respiratory and Critical Care Medicine, Affiliated Hospital, Institute of Respiratory Diseases, Guangdong Medical University, Zhanjiang, Guangdong, China.
References
- 1. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ 2020; 369: m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nobel YR, Phipps M, Zucker J, et al. Gastrointestinal symptoms and COVID-19: case-control study from the United States. Gastroenterology 2020; 159: 373–375.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dhar D, Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Res 2020; 285: 198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu K, Cai H, Shen Y, et al. Management of COVID-19: the Zhejiang experience (in Chinese). Zhejiang Da Xue Xue Bao Yi Xue Ban 2020; 49: 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Health Commission of the People’s Republic of China. Chinese guideline for the management Covid-19 (version 5.0), http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a/files/ab6bec7f93e64e7f998d802991203cd6.pdf. (2020, accessed 19 April 2020) (In Chinese).
- 7. Su S, Shen J, Zhu L, et al. Involvement of digestive system in COVID-19: manifestations, pathology, management and challenges. Therap Adv Gastroenterol 2020; 13: 1756284820934626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020; 382: 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28: 3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eder P, Łodyga M, Dobrowolska A, et al. Addressing multiple gastroenterological aspects of coronavirus disease 2019. Pol Arch Intern Med 2020; 130: 420–430. [DOI] [PubMed] [Google Scholar]
- 12. Wan Y, Li J, Shen L, et al. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol Hepatol 2020; 5: 534–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jin X, Lian JS, Hu JH, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2018 (COVID-19) with gastrointestinal symptoms. Gut 2020; 69: 1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheung KS, Hung IF, Chan PP, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from Hong Kong Cohort and systematic review and meta-analysis. Gastroenterology 2020; 159: 81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pazgan-Simon M, Rorat M, Buczyńska I, et al. Gastrointestinal symptoms as the first, atypical indication of SARS-CoV-2 infection. Pol Arch Intern Med 2020; 130: 338–339. [DOI] [PubMed] [Google Scholar]
- 16. Negi S, Das DK, Pahari S, et al. Potential role of gut microbiota in induction and regulation of innate immune memory. Front Immunol 2019; 10: 2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dumas A, Bernard L, Poquet Y, et al. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol 2018; 20: e12966. [DOI] [PubMed] [Google Scholar]
- 18. Dickson RP. The microbiome and critical illness. Lancet Respir Med 2016; 4: 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alberca RW, Oliveira LM, Branco ACCC, et al. Obesity as a risk factor for COVID-19: an overview. Crit Rev Food Sci Nutr 2021; 61: 2262–2276. [DOI] [PubMed] [Google Scholar]
- 20. Leyer GJ, Li S, Mubasher ME, et al. Probiotic effects on cold and influenza-like symptom incidence and duration in children. Pediatrics 2009; 124: e172–e179. [DOI] [PubMed] [Google Scholar]
- 21. Rautava S, Salminen S, Isolauri E. Specific probiotics in reducing the risk of acute infections in infancy-a randomised, double-blind, placebo-controlled study. Br J Nutr 2009; 101: 1722–1726. [DOI] [PubMed] [Google Scholar]
- 22. Hatakka K, Savilahti E, Pönkä A, et al. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. Br Med J 2001; 322: 1327–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanders ME, Guarner F, Guerrant R, et al. An update on the use and investigation of probiotics in health and disease. Gut 2013; 62: 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Biosearch SA. Evaluation of the probiotic lactobacillus coryniformis K8 on COVID-19 prevention in healthcare, https://clinicaltrials.gov/ct2/show/NCT04366180?cond=NCT04366180&draw=2&rank=1 (accessed 5 December 2020).
- 25. University of Roma La Sapienza. Bacteriotherapy in the treatment of COVID-19, https://clinicaltrials.gov/ct2/show/NCT04368351?term=lactobacillus&cond=COVID&draw=2 (accessed 5 December 2020).
- 26. Poscia R. Oxygen-ozone as adjuvant treatment in early control of COVID-19 progression and modulation of the gut microbial flora (PROBIOZOVID), https://clinicaltrials.gov/ct2/show/NCT04366089?term=lactobacillus&cond=COVID&draw=2 (accessed 5 December 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848211035670 for Probiotics use is associated with improved clinical outcomes among hospitalized patients with COVID-19 by Lina Zhang, Huanqin Han, Xuan Li, Caozhen Chen, Xiaobing Xie, Guomei Su, Shicai Ye, Cuili Wang, Qing He, Fang Wang, Fang Huang, Zhaoqin Wang, Jiayuan Wu and Tianwen Lai in Therapeutic Advances in Gastroenterology