Abstract
Acute subdural hemorrhage is typically associated with a history of head trauma, and as such it is a finding with significant potential medicolegal consequences. In this article, 37 adult and post-infantile pediatric sudden death autopsy cases with small volume (“thin film” or “smear”) acute subdural hemorrhage are presented—in which there is either no further evidence of head trauma or only features of minor head injury. The possible underlying pathophysiological mechanisms are explored, and it is concluded that a common thread in many of these cases is likely to have been cranial venous hypertension at around the time of death. These findings may have implications in instances where small volume subdural hemorrhage is identified in the absence of other evidence of significant head injury.
Keywords: Forensic pathology, Neuropathology, Subdural hemorrhage, Etiology, Nontraumatic
Introduction
Intracranial acute subdural hemorrhage is typically associated with trauma (1 –3). Indeed, the association is considered to be so strong that when subdural hemorrhage is encountered at autopsy, many practitioners will readily attribute the bleeding to trauma when they fail to identify another potential cause-even in the absence of other positive markers of head injury.
In cases with a documented history of trauma, the subdural hemorrhage will typically be of large volume and pose a risk of mass effect with elevation of intracranial pressure, particularly when paired with brain swelling. However, in other instances, the subdural hemorrhage encountered is of much smaller volume and is often described as forming only a “thin film” or a ”smear” of blood over the surface of the brain. The smaller volume in such cases might of course be explained by there having been only a short time interval between injury and death, insufficient to permit the accumulation of a significant volume of blood, but on occasion, it may be that different mechanisms come into play-a somewhat contentious area in pediatric (infant) neuropathology in particular (4 –9).
In this article, the potential pathophysiological mechanisms of thin film acute subdural hemorrhage in 37 sudden death autopsy cases performed on adults and children older than five years—in which there is either no further evidence of head trauma or only features of minor head injury-are explored.
Materials and Methods
A retrospective study examining coronial (medicolegal) and noncoronial consented (“hospital”) autopsy cases performed at the Vancouver General Hospital, British Columbia, Canada from January 1, 2011, to July 1, 2018. Autopsies performed under the auspices of the British Columbian coronial legislation include deaths which were sudden and unexpected, and where the cause of death was hitherto unknown; where the cause of death was the result of violence, accident, negligence, misconduct, or malpractice; the result of a self-inflicted illness or injury; when the death occurred during or after pregnancy in circumstances that might reasonably be attributable to pregnancy; or when the death occurred while the deceased was in custody (10). Approximately 500 to 600 autopsies are conducted annually at this institution.
Electronic autopsy report files in the Vancouver General Hospital Pathology Department database were searched using standard Microsoft Word (2003) tools for ”subdural hemorrhage/haemorrhage” and “subdural hematoma/haematoma.” Cases were included in the study if the hemorrhage was described as being acute and of small volume—typically described as either a “film,” “thin film,” or “smear.” Cases were excluded if one or more of the following criteria applied: (a) the deceased was younger than five years (primarily due to the paucity of infant autopsy cases at this institution; but also because the mechanism of hemorrhage in this age-group is notoriously contentious and potentially different from that operative in older age groups), (b) there were changes of more than early decomposition, (c) there were features of more than minor recent head trauma (not including the subdural hemorrhage) (minor head trauma in this context is defined to include localized facial/scalp abrasions, contusions, or superficial lacerations, but to exclude cases with deep facial/scalp lacerations or fracturing of the skull or facial skeleton), (d) there was a history of sizeable acceleration/deceleration forces, such as road traffic incidents and falls from height, or (e) there was evidence of a hemorrhagic diathesis, either congenital or acquired, or the presence of a risk factor for a bleeding disorder—such as hepatic cirrhosis.
The following gross postmortem data were obtained from the autopsy report in each case: age, sex, circumstances surrounding death, other significant medical history, whether or not chest compressions were performed as part of attempted resuscitation, the postmortem interval prior to autopsy, documented features of decomposition, laterality of the subdural hemorrhage, whether or not there were features of established brain swelling, toxicology results, the cause of death as provided by the autopsy pathologist, and other significant postmortem findings, with particular emphasis on craniocervical hemorrhage and features of injury.
Ethical clearance for the study was granted by the Clinical Research Ethics Board of the University of British Columbia (ref: H17-02323). Each case was indexed with reference to the institutional case number only in order to ensure anonymity.
Results
Thirty-seven autopsy cases of thin film/smear subdural hemorrhage were identified during the period January 2011 to July 2018 (Table 1). The age range was seven to 70 years, with a mean of 36.6 years (standard deviation [SD]: 2.2). The sex distribution was 25 male/12 female. All were cases of sudden death, without a protracted prodrome or hospitalization prior to death. As per the study inclusion criteria set out previously, all cases were without evidence—either as suggested by the history or postmortem findings—of more than minor recent head trauma (not including the subdural hemorrhage). None showed features of disseminated intravascular coagulation or other hemorrhagic diathesis. The known or estimated postmortem interval prior to autopsy ranged from one to 13 days, with a mean of 5.7 days (SD: 0.5).
Table 1:
Study Data
| Circumstances surrounding death and scene findings (n = 37) | Quantity | Notes |
|---|---|---|
| Found dead in ‘benign’ circumstances, without obvious cause prior to autopsy | 21 | |
| Witnessed or suspected seizure event | 5 | |
| Suspicious for assault | 2 | |
| Witnessed collapse | 1 | |
| Found in open water, possibly drowned | 2 | |
| Ligature strangulation | 1 | |
| Found submerged in bathtub | 2 | |
| Pinned under heavy load | 1 | |
| Found wedged in open window | 1 | |
| Found dead in house fire | 1 | |
| Resuscitative chest compressions performed | 13 | |
| Changes of early decomposition | 3 | |
| Features of minor recent head injury | 13 | Two of whom also had signs of significant recent trauma elsewhere to the body |
| Primary underlying cause of death, as determined after autopsy, microscopy, and following consideration of the results of ancillary studies as applicable (n = 37) | ||
| Acute drug/alcohol toxicity | 12 | Three opioids and alcohol Two cocaine and opioids Two alcohol and benzodiazepines One cocaine One methamphetamine One opioid, cocaine, and methamphetamine One opioids One clozapine |
| Natural extracranial cardiovascular disease | 5 | One with acute toxic effects of methamphetamine considered contributory to death |
| Seizure disorder | 5 | One suspected stemming from drug/alcohol withdrawal |
| Neck compression | 4 | |
| Drowning | 3 | |
| Smoke inhalation | 1 | |
| Alcohol-withdrawal syndrome | 1 | |
| Drug (likely cocaine)-related acute-on-chronic heart disease, contributed to by acute cocaine toxicity | 1 | This case in addition to the other cases in which cocaine was detected |
| Positional asphyxia | 1 | |
| Traumatic (crush) asphyxia | 1 | |
| Undetermined | 3 |
|
| Other relevant observations | ||
| Petechiae identified within brain parenchyma, thought not due to direct trauma | 3 | Two cases with evidence of neck compression, and 1 case of acute clozapine toxicity |
All of the autopsies included in this study had been undertaken by accredited forensic pathologists, and the large majority had photographs taken at the time of examination. The brain was retained for formal neuropathologic assessment after fixation in 16 of these 37 cases. Histological examination of all the major organs—including the brain—was undertaken in each and every case, but sections of dura were examined in only two instances. Toxicological analyses were performed on all 37 cases. In no cases were radiological studies undertaken either shortly prior to death or postmortem prior to autopsy.
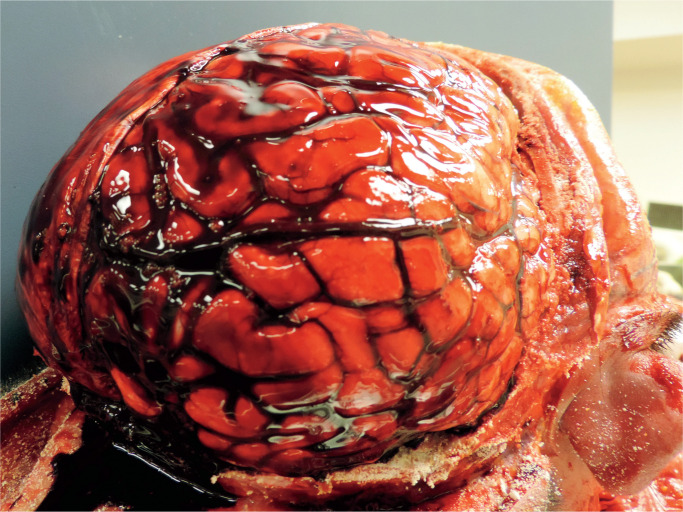
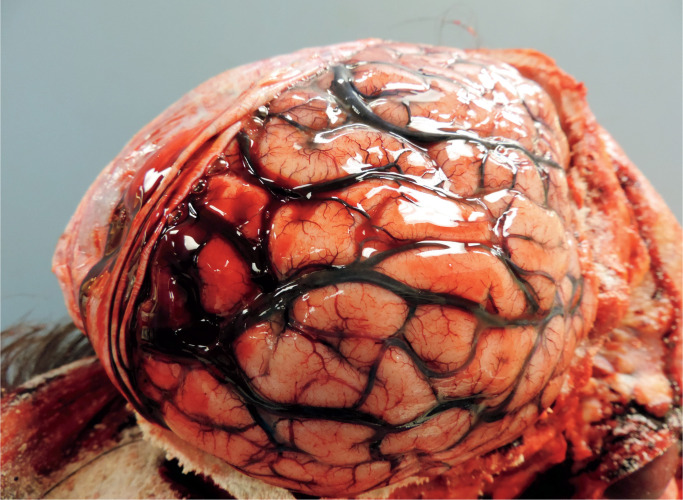
In every case which had been photographed, the hemorrhage was evident while the brain was still in-situ, with the hemispheric dura incised laterally and reflected upwards, and appeared as a thin film of blood over the cerebrum, most likely with a volume of a few milliliters at most. The subdural hemorrhage typically involved the mid to posterior parasagittal regions overlying the cerebral hemispheric convexities. The subdural hemorrhage was bilateral in 19 cases and unilateral in 18. In almost every instance, the brain and/or cerebral vessels were noted to appear congested—either as described in the written report or evident in postmortem images. Features of brain swelling were identified in four cases, though in no cases did this appear pronounced. None of the cases included in this study showed either gross or microscopic features of chronic subdural hemorrhage. Microscopic assessment of the brain revealed scattered petechial hemorrhages within the brain parenchyma in two instances, and focal microscopic cortical hemorrhages in another, but—other than congestive features—no further significant cerebral pathology was identified. In the two cases in which sections of dura were sampled for histological assessment, the dura also appeared congested and adjacent hemorrhage was confirmed, but no further dural pathology was noted. In none of the cases was the source of subdural blood positively identified. See Images 1 and 2 which show typical examples.
Image 1:
A typical case showing a thin film of subdural blood over the cerebral hemispheric convexity and prominent cerebral vascular congestion.
Image 2:
A case of quite localized and minor subdural hemorrhage. Note the characteristic distribution over the mid-posterior parasagittal cerebral hemispheric convexity. Vascular congestion again evident.
In addition to these 37 cases, it is of potential relevance that a further case with unilateral thin film acute subdural hemorrhage was also identified—a 33-year-old male who died during forcible restraint by law enforcement personnel, it seems being held down in a prone position at the time of his death. Autopsy findings in this case included fracturing of the laryngeal skeleton, with adjacent hemorrhage, and there were also features of significant head injuries—including a fairly deep scalp laceration, multifocal facial bruising, and a “blowout” type orbital fracture. Primarily as a result of ongoing uncertainty regarding the precise sequence of events leading up to his death, the cause of death in this individual was considered unascertained but likely multifactorial, with possible contributory factors including chest compression, compression of the neck structures, and excited delirium. As a result of the head injuries, the case was deemed to fall outside the study inclusion criteria.
Discussion
Intracranial acute subdural hemorrhage—that is, bleeding into the potential space between the dura mater and the arachnoid mater surrounding the brain or, to be more anatomically precise, within the plane of weakness within the inner dural border layer (11)—is typically associated with traumatic head injury (1 –3). In the majority of instances of traumatic acute subdural hemorrhage, the forces involved are sizeable, at least in the young and physically robust, with typical scenarios including motor vehicle accidents, falls, assaults, sporting events, and industrial accidents (12). However, other nontraumatic causes have been described, including neoplasia (13), sepsis (14), coagulation disorders (15, 16), high altitude (17), intracranial hypotension (13), and of course extension of hemorrhage from another intracranial source—such as ruptured aneurysms, vascular malformations, and intracerebral bleeds. Some researchers, notably in the field of infantile neuropathology, have also suggested that blood may ooze directly from the dura in the setting of the combination of hypoxic brain damage, brain swelling, and elevated central venous pressure (5 –7, 9, 18)—though this is not without controversy.
The source of the blood in most instances of traumatic, or presumed traumatic, subdural hemorrhage has traditionally been attributed to torn bridging veins traversing from cerebral cortex to dural sinus, or on occasion direct injury to the brain substance—but a definitive site of bleeding is frequently not identified (2). In these traumatic cases, the subdural hemorrhage will typically be of large volume, unilateral, and pose a risk of mass effect with associated elevation of the intracranial pressure, particularly when coupled with brain swelling.
These 37 adult and post-infantile children sudden death cases—none of whom had further evidence of significant recent injury to the head region, either from the history or postmortem findings—were found at autopsy to have small volume (”thin film” or “smear”) acute subdural hemorrhage. These findings are thought to be suggestive of a nontraumatic causal mechanism of accumulation of the blood in the subdural space, distinct from the usual space-occupying type acute subdural hemorrhage encountered in instances of traumatic head injury.
One common theme that emerges in many of these cases is the possibility of venous hypertension in vessels draining the head and neck region, which could have developed by way of a variety of mechanisms— either stemming from direct pressure gradients or consequent upon impaired venous return to the heart. Those cases involving compression of the neck or torso can of course be readily seen to have the potential for direct impairment of cranial venous outflow or venous return to the heart. A Valsalva-type mechanism may be operative in seizure-associated apnea or breath holding in instances of drowning and smoke inhalation (19 –22); a similar mechanism has been invoked by others in instances of subdural hemorrhage associated with paroxysmal coughing (23,24). Independent autonomic effects may additionally be a factor in cases where terminal seizures are suspected (25). It is also conceivable that acute drug toxicity might play a part in the development of acute cranial venous engorgement in the period leading up to death, particularly in instances involving stimulant drug usage with associated excitatory cardiovascular effects. The mechanisms that may be at play in deaths attributed to the acute toxic effects of opioids and other nonstimulatory drugs remain obscure, but in the author’s personal experience profound congestion of the head and upper torso in such instances is a common finding, perhaps reflecting a terminal Valsalva-type mechanism and/or agonal and postmortem positioning, and it is speculated whether such a mechanism may be a factor in these cases. While there is no obvious causal mechanism for the subdural hemorrhage in the deaths attributed to natural cardiovascular causes captured by this study, similar mechanisms may well be operative.
It is proposed that cranial venous hypertension, when pronounced, can result in seepage of blood from engorged intracranial, possibly peridural or dural-based, vascular channels—perhaps too small to be appreciated with the naked eye or upon routine histological assessment (though interestingly, in a recent study involving a small number of infantile “triad” cases, three of which had no further features of head trauma, it is reported that “torn/ruptured small and medium-sized veins” were identified histologically in sections from deep to the subdural blood in a paramedian distribution (26)). After all, it is very well recognized that petechiae can develop within the skin and mucous membranes of the head and neck in association with venous hypertension in instances of compression of the neck and/or torso—indeed, petechial hemorrhages are frequently used as a supportive diagnostic sign in cases such as these. It would seem logical that the same processes may well apply to the cranial vasculature in instances of venous hypertension. Such an explanation for the origin of the subdural hemorrhage in the cases identified in this study would appear to be supported by the finding of perivascular intracerebral hemorrhages in two of the cases involving compression of the neck structures—an observation that has previously been reported by others in instances of traumatic (crush) asphyxia (27).
The lack of a protracted survival period in any of these cases would likely weigh against the possibility that hypoxic damage to the tissues might have been a significant factor in the origin of hemorrhage, in these cases at least—a mechanism that has previously been put forward by others (5, 9, 28).
This venous hypertension hypothesis in the formation of acute subdural hemorrhage would of course also provide a ready explanation for the hemorrhage being of only small volume—namely, developing as an ooze from engorged venous channels, in contrast to flow of blood from a frank vascular tear as is thought to be the case in most instances of typical space-occupying, trauma-related subdural hemorrhage.
There is scarce prior mention of this proposed mechanism of subdural hemorrhage in the published literature. However, it is notable that, shortly after the turn of the twentieth century, Cushing observed that subdural hemorrhage ”may occur when too great strain has been put upon the vessels by the profound venous stasis of postpartum asphyxiation; just as in later months they may rupture under the passive congestion brought about by a paroxysm of whooping-cough or a severe convulsion” (29). In addition, Spitz and Fisher make reference to small amounts of subarachnoid bleeding in some instances of asphyxial death (30). As noted previously, some workers in the field of infantile neuropathology have also suggested that subdural hemorrhage may develop consequent upon a combination of hypoxic tissue damage and elevated intravascular pressure (5 –7, 9, 18).
Notwithstanding the preceding commentary, consideration must also be given to the possibility that the subdural bleeding identified in at least some of these cases might be explained by postmortem phenomena. The so-called hypostatic hemorrhage has been described in the soft tissues in gravitationally dependent parts of the body after death (31), and animal models have pointed to the same occurrence in brain tissue (32). Despite fairly lengthy postmortem intervals prior to autopsy in many of these cases, the exclusion of cases from this study showing significant changes in decomposition would likely minimize the risk of such artefact. Nonetheless, it is perhaps of relevance that in several of these cases, the body of the deceased was described as being prone or slumped over at the scene, and of course the two bodies recovered from open water are likely to have adopted a backup/head down position prior to recovery of the remains. It is also possible that the subdural blood may have collected as an artefact during the autopsy process-most likely stemming from incisions through, or by way of traction upon, vascular structures at the time of brain removal, perhaps exacerbated by a reduction in pressure inside the head during removal of the calvarium. However, the fact that the observations were made by experienced forensic pathologists and neuropathologists who are well-versed in postmortem artefact would likely weigh against such an explanation.
Although the observations described in this study are of interest, it is acknowledged that it would be desirable to collect a larger number of cases for assessment in future studies, ideally with histological examination of the dura in every case. Shorter postmortem intervals and radiological imaging prior to instrumentation at autopsy may assist further with the assessment of the possibility of artefactual origin of the subdural blood. Cases with evidence of head trauma, however minimal, should ideally also be excluded from subsequent projects.
It is of interest that cardiopulmonary resuscitation had been attempted in 14 of the 37 cases described in this study. This observation raises the possibility that, in at least some of the cases described, the subdural hemorrhage may have been in part or wholly related to an elevation of intracranial intravascular pressure stemming from chest compressions—a mechanism that data from other published studies would perhaps support (33), and which has previously been proposed by others (34). However, the data set out herein are not thought to provide meaningful further assistance in assessment of this theory.
The possibility of subdural bleeding developing in association with cranial venous hypertension adds another dimension to the ongoing vigorous debate regarding infantile subdural hemorrhage, particularly in instances where there is no, or only limited additional evidence of trauma (4 –6, 8, 9, 18, 35)—an issue of critical relevance given the widely accepted strong association between subdural hemorrhage and nonaccidental head injury (36 –38). Is it possible that it is cranial venous hypertension may be a factor, at least in some cases—rather than head trauma by way of impact injury or forcible and repetitive shaking? This cranial venous hypertension theory may go some way in explaining the apparent discrepancy between forces required experimentally to rupture dural bridging veins and the quantification of forces actually generated in models of abusive infantile shaking (39 –42), as well as the limited radiological and pathological data suggestive of neck injury in cases of infant deaths traditionally attributed to shaking (27, 43). However, it is acknowledged that different mechanistic processes may be operative in infant cases from the post-infantile pediatric and adult cases described in this study.
The fact that subdural hemorrhage has been documented to develop in association with otherwise “benign” birthing processes—including instances of not only vaginal delivery, with and without instrumentation, but also in association with cesarean delivery (44, 45)—would tend to suggest that subdural hemorrhage occurs more readily in infants than adults. Spontaneous infantile subdural hemorrhage has also been described in association with a variety of natural conditions (2), most notably in the presence of benign enlargement of the subarachnoid space (46, 47) — though it has been stressed that spontaneous infantile subdural hemorrhage ought not be definitively diagnosed simply because of the absence of markers of injury, but only with positive evidence of predisposing conditions such as macrocrania, arachnoidomegaly, or severe dehydration (48).
Those used to handling young infants will no doubt be familiar with the quite profound vascular engorgement of the face that can occur in association with bouts of heavy and protracted crying, possibly at least in part reflecting a Valsalva-like physiological mechanism. It may be the case that this congestion primes the infantile head to hemorrhage with additional mechanically activated venous hypertension, perhaps by way of compression of the infant torso. Similarly, compression of the infantile frame may also be a factor in the development of subdural hemorrhage during passage through the birth canal.
Infantile rib fractures, especially posteriorly sited fractures, are traditionally frequently thought to be caused by way of forcible gripping of the chest and are typically described as having high specificity for nonaccidental injury (49 –51). Forcible compression of the infant chest can of course be readily seen to have much in common with the cases of compression of the torso identified in this adult study. Similarly, and as touched upon previously, parallels could arguably also be drawn with instances of resuscitative compression of the infant chest. This observation, combined with the frequent combination of rib fractures and acute subdural hemorrhage in many cases of apparent abusive infantile head trauma, raises the possibility that in at least some of these cases the subdural bleeding may be consequent upon compression of the infant chest, perhaps by way of forcible gripping (maybe ”squeezed infant syndrome” would be an appropriate moniker)—rather than head injury caused by impact and/or forcible and repetitive shaking. Such an “asphyxia” mechanism would also provide a ready explanation for the typical abrupt-onset nature of the symptomatology in these cases—presumably by way of acute cardiorespiratory compromise—and the subsequent development of ischemic/hypoxic encephalopathy rather than diffuse traumatic axonal injury (52).
It is further hypothesized that cranial venous hypertension may also be a factor in instances of extravasation of blood in other locations in the infantile head and neck—such as the retina, optic nerve sheath, and possibly also the cervical spinal nerve roots. Of note, while typically described in instances of alleged shaking, extensive multilayered retinal hemorrhage has been described in a young child whose head and chest were crushed by a television (53). Valsalva hemor rhagic retinopathy may have some parallels here too (54). Cerebral sinovenous thrombosis has also been proposed as a possible cause of subdural hemorrhage in infancy (55), and such a possibility would appear to be supported by the findings in this study and the proposed role of cranial venous hypertension in the origin of subdural hemorrhage.
Conclusion
The autopsy cases described in this study highlight the fact that acute subdural hemorrhage is not always traumatic in nature and reinforce the need to consider other etiologies in the differential diagnosis. One possible explanation for these observations, at least in some instances, is that cranial venous hypertension results in oozing of blood from engorged, possibly dural-based, venous channels. Although this study focused on adults and post-infantile children, the hypothesis proposed may be of relevance to the ongoing debate regarding subdural hemorrhage in infants.
Acknowledgement
The author is grateful to Dr. Michael Rodriguez for his kind review of the manuscript prior to submission, and his useful and constructive comments.
Authors
Matthew M. Orde MBChB FRCPath FRCPA DMJ (Path) Dip For Med (SA) MFFLM LLDip PgDLS, Forensic Pathology, Vancouver General Hospital
Roles: Project conception and/or design, data acquisition, analysis and/or interpretation, manuscript creation and/or revision, approved final version for publication, accountable for all aspects of the work, principal investigator of the current study.
Footnotes
Ethical Approval: As per Journal Policies, ethical approval was not required for this manuscript
Statement of Human and Animal Rights: This article does not contain any studies conducted with animals or on living human subjects
Statement of Informed Consent: No identifiable personal data were presented in this manuscript
Disclosures & Declaration of Conflicts of Interest: The author, reviewers, editors, and publication staff do not report any relevant conflicts of interest
Financial Disclosure: The author has indicated that he does not have financial relationships to disclose that are relevant to this manuscript
References
- 1). Dolinak D, Matshes EW, Lew EO, editors. Forensic pathology: principles and practice. San Diego: Elsevier Academic Press; 2005. 616 p. [Google Scholar]
- 2). Leestma JE. Forensic neuropathology. 3rd ed. Boca Raton: CRC Press; 2014. 811 p. [Google Scholar]
- 3). Oehmichen M, Auer RN, König HG. Forensic neuropathology and associated neurology. Berlin: Springer; 2009. 660 p. [Google Scholar]
- 4). Case ME, Graham MA, Handy TC, et al. Position paper on fatal abusive head injuries in infants and young children. Am J Forensic Med Pathol. 2001. Jun; 22(2):112–22. PMID: 11394743. 10.1097/00000433-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 5). Geddes JF, Tasker RC, Hackshaw AK, et al. Dural haemorrhage in non-traumatic infant deaths: does it explain the bleeding in “shaken baby syndrome”? Neuropathol Appl Neurobiol. 2003. Feb; 29(1): 14–22. PMID: 12581336. 10.1046/j.1365-2990.2003.00434.x. [DOI] [PubMed] [Google Scholar]
- 6). Squier W, Mack J. The neuropathology of infant subdural haemorrhage. Forensic Sci Int. 2009. May 30; 187(1-3):6–13. PMID: 19303229. 10.1016/j.forsciint.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 7). Cohen MC, Scheimberg I. Evidence of occurrence of intradural and subdural hemorrhage in the perinatal and neonatal period in the context of hypoxic ischemic encephalopathy: an observational study from two referral institutions in the United Kingdom. Pediatr Dev Pathol. 2009. May-Jun; 12(3):169–76. PMID: 19007301. https://doi.Org/10.2350/08-08-0509.1. [DOI] [PubMed] [Google Scholar]
- 8). Pollanen MS. Subdural hemorrhage in infancy: keep an open mind. Forensic Sci Med Pathol. 2011. Sep; 7(3):298–300. PMID: 21479950. 10.1007/s12024-011-9238-5. [DOI] [PubMed] [Google Scholar]
- 9). Scheimberg I, Cohen MC, Zapata Vazquez RE, et al. Nontraumatic intradural and subdural hemorrhage and hypoxic ischemic encephalopathy in fetuses, infants, and children up to three years of age: analysis of two audits of 636 cases from two referral centers in the United Kingdom. Pediatr Dev Pathol. 2013. May-Jun; 16(3):149–59. PMID: 23113698. 10.2350/12-08-1232-OA.!. [DOI] [PubMed] [Google Scholar]
- 10). British Columbia Coroners Act, 2007 [Internet]. Victoria (BC): Queen’s Printer; 2007. [cited 21 Nov 2018]. Available from: http://www.bclaws.ca/EPLibraries/bclaws_new/document/ID/freeside/00_07015_01. [Google Scholar]
- 11). Haines DE, Harkey HL, al-Mefty O. The (Subdural’ space: a new look at an outdated concept. Neurosurgery. 1993. Jan; 32(1):111–20. PMID: 8421539. 10.1227/00006123-199301000-00017. [DOI] [PubMed] [Google Scholar]
- 12). Spitz WU, Spitz DJ, Fisher RS. Spitz and Fisher’s Medicolegal investigation of death: guidelines for the application of pathology to crime investigation. 4th ed. Springfield (IL): Charles C. Thomas; 2006. 1325 p. [Google Scholar]
- 13). Fischbein NJ, Wijman CA. Nontraumatic intracranial hemorrhage. Neuroimaging Clin N Am. 2010. Nov; 20(4):469–92. PMID: 20974372. 10.1016/j.nic.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 14). Geisenberger D, Huppertz LM, Buchsel M, et al. Non-traumatic subdural hematoma secondary to septic brain embolism: A rare cause of unexpected death in a drug addict suffering from undiagnosed bacterial endocarditis. Forensic Sci Int. 2015. Dec; 257: e1–e5. PMID: 26296471. 10.1016/j.forsciint.2015.07.055. [DOI] [PubMed] [Google Scholar]
- 15). Miyao M, Abiru H, Ozeki M, et al. Subdural hemorrhage: a unique case involving secondary vitamin k deficiency bleeding due to biliary atresia. Forensic Sci Int. 2012. Sep 10; 221(1-3): e25–9. PMID: 22607980. 10.1016/j.forsciint.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 16). Luongo M, Pizzuti M, Godano U. Bilateral chronic subdural non-traumatic hematoma associated with von Willebrand’s type I disease: a case report. Acta Neurochir (Wien). 2012. Jun; 154(6):1087–8. PMID: 22392015. 10.1007/s00701-012-1310-8. [DOI] [PubMed] [Google Scholar]
- 17). Ganau L, Prisco L, Ganau M. High altitude induced bilateral nontraumatic subdural hematoma. Aviat Space Environ Med. 2012. Sep; 83(9):899–901. PMID: 22946355. 10.3357/asem.3331.2012. [DOI] [PubMed] [Google Scholar]
- 18). Cohen MC, Sprigg A, Whitby EH. Subdural hemorrhage, intradural hemorrhage and hypoxia in the pediatric and perinatal post mortem: are they related? An observational study combining the use of post mortem pathology and magnetic resonance imaging. Forensic Sci Int. 2010. Jul 15; 200(1-3):100–7. PMID: 20510556. 10.1016/j.forsciint.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 19). Pstras L, Thomaseth K, Waniewski J, et al. Review: the Valsalva manoeuvre: physiology and clinical examples. Acta Physiol (Oxf). 2016. Jun; 217(2):103–19. PMID: 26662857. 10.1111/apha.12639. [DOI] [PubMed] [Google Scholar]
- 20). Gindea AJ, Slater J, Kronzon I. Doppler echocardiographic flow velocity measurements in the superior vena cava during the Valsalva maneuver in normal subjects. Am J Cardiol. 1990. Jun 1; 65(20):1387–91. PMID: 2343828. 10.1016/0002-9149(90)91333-2. [DOI] [PubMed] [Google Scholar]
- 21). Devinsky O. Sudden, unexpected death in epilepsy. N Engl J Med. 2011. Nov 10; 365(19):1801–11. PMID: 22070477. 10.1056/NEJMra1010481. [DOI] [PubMed] [Google Scholar]
- 22). Bierens JJ, Lunetta P, Tipton M, Warner DS. Physiology of drowning: a review. Physiology (Bethesda). 2016. Mar; 31(2):147–66. PMID: 26889019. 10.1152/physiol.00002.2015. [DOI] [PubMed] [Google Scholar]
- 23). Talbert DG. Paroxysmal cough injury, vascular rupture and ‘shaken baby syndrome’. Med Hypotheses. 2005; 64(1):8–13. PMID: 15533602. 10.1016/j.mehy.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 24). Geddes JF, Talbert DG. Paroxysmal coughing, subdural and retinal bleeding: a computer modelling approach. Neuropathol Appl Neurobiol. 2006. Dec; 32(6):625–34. PMID: 17083477. 10.1111/j.1365-2990.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- 25). Freeman R. Cardiovascular manifestations of autonomic epilepsy. Clin Auton Res. 2006. Feb; 16(1):12–7. PMID: 16477490. 10.1007/s10286-006-0278-y. [DOI] [PubMed] [Google Scholar]
- 26). Ramsay DA. IAFS 2017 abstracts: Histology of tears of small veins under subdural hematomas in the infantile ‘triad syndrome’. Forensic Sci Int. 2017; 277(suppl 1):161. 10.1016/j.forsciint.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 27). Al-Sarraj S, Laxton R, Swift B, et al. Neuropathology and brain weight in traumatic-crush asphyxia. J Forensic Leg Med. 2017. Nov; 52:110–5. PMID: 28892750. 10.1016/j.jflm.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 28). Matshes EW, Evans RM, Pinckard JK, et al. Shaken infants die of neck trauma, not of brain trauma. Acad Forensic Pathol. 2011. Jul; 1(1):82–91. 10.23907/2011.009. [DOI] [Google Scholar]
- 29). Goodrich JT. Reprint of (Concerning surgical intervention for the intracranial hemorrhages of the new-born’ by Harvey Cushing, MD. 1905. Childs Nerv Syst. 2000;16(8):484–92. 10.1007/s003810000255. [DOI] [PubMed] [Google Scholar]
- 30). Mechanical Injuries of Brain and Meninges. In: Spitz WU, Spitz DJ, Fisher RS. Spitz and Fisher’s Medicolegal investigation of death: guidelines for the application of pathology to crime investigation. 4th ed. Springfield (IL): Charles C. Thomas; 2006. p. 598. [Google Scholar]
- 31). Pollanen MS, Perera SD, Clutterbuck DJ. Hemorrhagic lividity of the neck: controlled induction of postmortem hypostatic hemorrhages. Am J Forensic Med Pathol. 2009. Dec; 30(4):322–6. PMID: 19901802. 10.1097/PAF.0b013e3181c17ec2. [DOI] [PubMed] [Google Scholar]
- 32). Xiang L, Zhou G, Xia S, et al. Could postmortem hemorrhage occur in the brain? A preliminary study on the establishment and investigation of postmortem hypostatic hemorrhage using rabbit models. Am J Forensic Med Pathol. 2013. Jun; 34(2):147–9. PMID: 23629388. 10.1097/PAF.0b013e31828877f0. [DOI] [PubMed] [Google Scholar]
- 33). Matshes EW, Shuman M, Lew EO. Retinal and optic nerve sheath hemorrhages are not pathognomonic of abusive pediatric head injury. Paper presented at: American Academic of Forensic Sciences Annual Scientific Meeting; 2010. Feb 22-27; Seattle. [Google Scholar]
- 34). Squier W, Mack J, Jensen AC. Infants dying suddenly and unexpectedly share demographic features with infants who die with retinal and dural bleeding: a review of neural mechanisms. Dev Med Child Neurol. 2016. Dec; 58(12):1223–34. PMID: 27435495. 10.1111/dmcn.13202. [DOI] [PubMed] [Google Scholar]
- 35). Case ME. Inflicted traumatic brain injury in infants and young children. Brain Pathol. 2008. Oct; 18(4):571–82. PMID: 18782169. 10.1111/j.1750-3639.2008.00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Matschke J, Voss J, Obi N, et al. Nonaccidental head injury is the most common cause of subdural bleeding in infants < 1 year of age. Pediatrics. 2009. Dec; 124(6):1587–94. PMID: 19948629. 10.1542/peds.2008-3734. [DOI] [PubMed] [Google Scholar]
- 37). Sun DT, Zhu XL, Poon WS. Non-accidental subdural haemorrhage in Hong Kong: incidence, clinical features, management and outcome. Childs Nerv Syst. 2006. Jun; 22(6):593–8. PMID: 16544146. 10.1007/s00381-006-0094-7. [DOI] [PubMed] [Google Scholar]
- 38). Feldman KW, Bethel R, Shugerman RP, et al. The cause of infant and toddler subdural hemorrhage: a prospective study. Pediatrics. 2001. Sep; 108(3):636–46. PMID: 11533330. 10.1542/peds.1083.636. [DOI] [PubMed] [Google Scholar]
- 39). Duhaime AC, Gennarelli TA, Thibault LE, et al. The shaken baby syndrome. A clinical, pathological, and biomechanical study. J Neurosurg. 1987. Mar; 66(3):409–15. PMID: 3819836. 10.3171/jns.1987.663.0409. [DOI] [PubMed] [Google Scholar]
- 40). Duhaime AC, Christian CW, Rorke LB, Zimmerman RA. Nonaccidental head injury in infants-the (shaken-baby syndrome’. N Engl J Med. 1998. Jun 18; 338(25):1822–9. PMID: 9632450. 10.1056/NEJM199806183382507. [DOI] [PubMed] [Google Scholar]
- 41). Ommaya AK, Goldsmith W, Thibault L. Biomechanics and neuropathology of adult and paediatric head injury. Br J Neurosurg. 2002. Jun; 16(3):220–42. PMID: 12201393. 10.1080/02688690220148824. [DOI] [PubMed] [Google Scholar]
- 42). Prange MT, Coats B, Duhaime AC, Margulies SS. Anthropomorphic simulations of falls, shakes, and inflicted impacts in infants. J Neurosurg. 2003. Jul; 99(1):143–50. PMID: 12854757. 10.3171/jns.2003.99.1.0143. [DOI] [PubMed] [Google Scholar]
- 43). Hadley MN, Sonntag VK, Rekate HL, Murphy A. The infant whiplash-shake injury syndrome: a clinical and pathological study. Neurosurgery. 1989. Apr; 24(4):536–40. PMID: 2710298. 10.1227/00006123-198904000-00008. [DOI] [PubMed] [Google Scholar]
- 44). Rooks VJ, Eaton JP, Ruess L, et al. Prevalence and evolution of intracranial hemorrhage in asymptomatic term infants. AJNR Am J Neuroradiol. 2008. Jun; 29(6):1082–9. PMID: 18388219. 10.3174/ajnr.A1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Towner D, Castro MA, Eby-Wilkens E, Gilbert WM. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999. Dec 2; 341(23):1709–14. PMID: 10580069. 10.1056/NEJM199912023412301. [DOI] [PubMed] [Google Scholar]
- 46). McNeely P, Atkinson J, Saigal G, et al. Subdural hematomas in infants with benign enlargement of the subarachnoid spaces are not pathognomonic for child abuse. AJNR Am J Neuroradiol. 2006. Sep; 27(8):1725–8. PMID: 16971622. [PMC free article] [PubMed] [Google Scholar]
- 47). Ghosh PS, Ghosh D. Subdural hematoma in infants without accidental or nonaccidental injury: benign external hydrocephalus, a risk factor. Clin Pediatr (Phila). 2011. Oct; 50(10):897–903. PMID: 21576186. 10.1177/0009922811406435. [DOI] [PubMed] [Google Scholar]
- 48). Vinchon M, Delestret I, DeFoort-Dhellemmes S, et al. Subdural hematoma in infants: can it occur spontaneously? Data from a prospective series and critical review of the literature. Childs Nerv Syst. 2010. Sep;26(9):1195–205. PMID: 20195617. 10.1007/s00381-010-1105-2. [DOI] [PubMed] [Google Scholar]
- 49). Kleinman PK, Marks SC, Jr, Spevak MR, Richmond JM. Fractures of the rib head in abused infants. Radiology. 1992. Oct; 185(1):119–23. PMID: 1523293. 10.1148/radiology.185.1.1523293. [DOI] [PubMed] [Google Scholar]
- 50). Kleinman PK, Marks SC, Jr, Nimkin K, et al. Rib fractures in 31 abused infants: postmortem radiologic-histopathologic study. Radiology. 1996. Sep; 200(3):807–10. PMID: 8756936. 10.1148/radiology.200.3.8756936. [DOI] [PubMed] [Google Scholar]
- 51). Kleinman PK, Schlesinger AE. Mechanical factors associated with posterior rib fractures: laboratory and case studies. Pediatr Radiol. 1997. Jan; 27(1):87–91. PMID: 8995179. 10.1007/s002470050073. [DOI] [PubMed] [Google Scholar]
- 52). Geddes JF, Vowles GH, Hackshaw AK, et al. Neuropathology of inflicted head injury in children II. Microscopic brain injury in infants. Brain. 2001. Jul; 124(Pt 7):1299–306. PMID: 11408325. 10.1093/brain/124.7.1299. [DOI] [PubMed] [Google Scholar]
- 53). Lantz PE1, Sinal SH, Stanton CA, Weaver RG, Jr. Perimacular retinal folds from childhood head trauma. BMJ. 2004. Mar 27; 328(7442): 754–6. PMID: 15044292. PMCID: PMC381329. 10.1136/bmj.328.7442.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54). Duane TD. Valsalva hemorrhagic retinopathy. Trans Am Ophthalmol Soc. 1972; 70:298–313. PMID: 4663671. PMCID: PMC1310456. [PMC free article] [PubMed] [Google Scholar]
- 55). Hedlund GL. Cerebral sinovenous thrombosis in pediatric practice. Pediatr Radiol. 2013. Jan; 43(2):173–88. PMID: 23212594. 10.1007/s00247-012-2486-z. [DOI] [PubMed] [Google Scholar]