Abstract
Study Design:
Retrospective observational analysis.
Objectives:
Spinal tuberculosis accounts for about 50% of cases among extra pulmonary osteoarticular tuberculosis. Resistance to drugs in spinal tuberculosis patients is on a rise and there is inadequate literature concentrating on the precise pattern of resistance in Indian subcontinent which harbors 24% of global prevalence. The aim was to study the pattern of drug resistance in spinal tuberculosis among first- and second-line drugs. Drug resistance is common in spinal tuberculosis and we intended to find the prevalence of various drug resistance patterns.
Methods:
Patients with spinal tuberculosis visiting a tertiary center were assessed. Samples were taken from the affected vertebrae and sent for BACTEC mycobacterium growth indicator tube (MGIT) 960 culture. Patients with a positive growth in MGIT were included in the study. All previously treated patients (relapse, treatment after failure, treatment after loss to follow-up and other previously treated patients) were excluded.
Results:
A total of 150 patients with a positive growth in MGIT report were included in the study, of whom 43 patients had some kind of drug resistance. Seven were multidrug resistant (MDR), 9 had preextensive drug resistance (pre-XDR), and 4 had extensive drug resistance (XDR). Seventeen patients had mono-drug resistance, which was most frequently for isoniazid. Resistance among second-line drugs was common in the fluoroquinolone group.
Conclusion:
Drug resistance in spinal tuberculosis was found to be 28.6%. Of these, MDR was in 16.2%, pre-XDR in 20.9%, and XDR in 9.3% patients.
Keywords: spinal tuberculosis, drug-resistant tuberculosis, MDR-TB, XDR-TB
Introduction
The mainstay of treatment in spinal tuberculosis (TB) is medical management in the form of antitubercular drugs and surgery is reserved for patients with complications. Medical treatment for TB of spine is not standardized and is mainly surgeon specific. The main factors contributing to the development of drug resistance are inadequate and incomplete treatment, nonadherence to treatment and genetic predisposition. Nonadherence to treatment is particularly important in patients following alternate-day regimens where they tend to miss the doses. Drug resistance pattern in TB is classified into the following types 1,2 :
Mono-resistance: resistance to any single first-line anti-TB drug.
Poly-resistance: resistance to more than 1 first-line anti-TB drugs, other than both isoniazid (INH) and rifampicin (RIF).
Multi-drug resistance (MDR): resistance to both INH and RIF.
Extensive drug resistance (XDR): resistance to INH and RIF with resistance to any fluoroquinolone (FQ) and at least 1 of the 3 second-line injectable drugs (capreomycin, kanamycin, and amikacin).
Pre-extensive drug resistance (pre-XDR): resistance to INH and RIF (MDR) with resistance to FQ or injectable group (capreomycin, kanamycin, and amikacin).
RIF resistance (RR): resistance to RIF detected using phenotypic or genotypic methods, with or without resistance to other anti-TB drugs.
This study aims to ascertain the resistance pattern among spinal TB patients with a proven growth in TB mycobacterium growth indicator tube (MGIT). This study will further help find the prevalence of MDR TB, XDR TB, Pre-XDR TB, and mono-drug resistance in spinal TB. This pattern of drug resistance will help assess whether there is resistance to any specific drug, which resistance is common among spinal TB, and whether the pattern differs from other types of tuberculosis requiring different protocols for medical management. This becomes important in cases of spinal TB because most clinicians do not follow a uniform set of instructions for the types of drug used and their duration of use. We have also compared the pattern of resistance with previously published studies of drug resistance in pulmonary TB since data for comparison in spinal TB is not yet available.
Materials and Methods
The study was conducted within the premises of a tertiary care center in India. Institutional review board approval was obtained. It was a retrospective observational study carried over a period of 3 years (2016-2018). All the patients with a positive growth on MGIT were included in the study. Patients among all age groups were included in the study. These patients underwent drug sensitivity for first- and second-line drugs. Patients with no growth on MGIT and previously treated patients (relapse, treatment failure patients, treatment after loss to follow-up patients, other previously treated patients) were excluded from the study. We have compared the results of our study with the previous data published by Mohan et al 3 and National Anti-Tuberculosis Drug Resistance Survey of India (2014-2016), 4 for assessing the pattern of resistance to various first- and second-line drugs in terms of the common patterns of drug resistance and individual resistance to drugs in the study. BACTEC (MGIT) 960 is a fully automated system that exploits the fluorescence of an oxygen sensor to detect the growth of mycobacteria in a culture sample. 5 The reports usually include an interim report of primary smear and a culture report after incubation. Following the detection of growth, the sample is tested for drug sensitivity (DST). We routinely perform MGIT, GeneXpert (cartridge-based nucleic acid amplification technique), and histopathological examination in all the patients undergoing a biopsy for suspected tuberculous spondylodiscitis.
Results
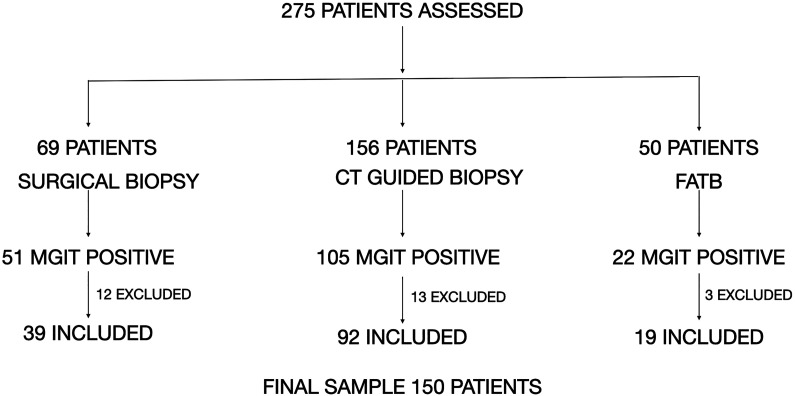
A total of 275 patients with spinal TB were assessed on their routine follow-up. Among the 275 samples, surgical excision biopsy was done for 69 patients, computed tomography (CT)–guided biopsy for 156 patients, and fluoroscopy-assisted transpedicular biopsy (FATB) for 50 patients. Among them, positive MGIT culture for TB was present in 178 patients. Twenty-eight patients were excluded for some form of previous treatment before presenting to us and 150 patients in total were included for final assessment (Figure 1). Among these 150 patients with a positive MGIT, 121 (80.6%) also had a positive histopathological examination for a granulomatous inflammation. GeneXpert was found to be positive in 131 (87.3%) of these 150 patients. Distribution of tuberculosis among male and female sex was 56% and 44%, respectively, in this study (Table 1). In the current study, children younger than 14 years accounted for 1.32% of the total study population, the youngest being 8 years old. Patients in the first 3 decades of life accounted for 44% of the study population. The least number of patients in both the male and the female group belonged to the age group >65 years. This shows that TB can affect all the age groups but is common among those in the second and third decade of life. The involvement of geriatric age does occur but less prevalent as compared with the second to third decade. Table 1 depicts the detailed demographic distribution of the patients included in the study and a comparison with the mentioned studies. Among the 150 samples, 107 patients were susceptible to all the drugs tested by DST and 43 (28.6%) had some kind of drug resistance. Among the drug-resistant patients, 7 (16.27%) had MDR TB, 9 (20.93%) had pre-XDR TB, and 4(9.3%) had XDR TB. Among the 9 patients who had pre-XDR TB, 6 (66.67%) had resistance to FQ and 3 (33.9%) had resistance to an injectable second-line drug. Mono-drug resistance was present in 17 cases (39.5%). Commonest mono-drug resistance was to INH in 7 (41.1%) patients, followed by ethambutol (ETB) in 5 (29.4%) and pyrazinamide (PZN) in 3 (17.64%) patients. The least mono-drug resistance was to linezolid and ofloxacin in 1 (5.8%) patient each. No isolated resistance to RIF was seen. Polydrug resistance was present in the other 6 (13.95%) patients. When analyzing drug resistance among first-line agents, out of 150 patients, 32 (21.34%) patients had resistance to INH, followed by RIF in 20 (13.34%) patients, ETB in 17 (11.34%), and least to PZN in 13 (8.67%) patients (Table 2). INH resistance was the most common among first-line agents. Resistance to RIF was not seen among patients with non-MDR/XDR TB, that is, mono-drug resistance and polydrug resistance. Among the non-MDR/XDR patients, resistance to INH was the highest in 12 (8%) patients (7 cases of mono resistance and 5 of poly resistance), and least was to PZN (mono resistance) in 3 (2%) patients. Among the second-line drugs, resistance to moxifloxacin was found in 7 patients (4.6%) followed by ofloxacin in 6 (4%) patients. Resistance among the injectables group was found most commonly with amikacin in 4 patients (2.6%) followed by capreomycin in 3 (2%) patients and Kanamycin in 3 (2%) patients. Resistance to other second-line drugs was seen with para-amino salicylic acid (PAS) in 4 (2.6%), linezolid in 1 (0.6%) and clofazimine in 2 (1.3%) patients. This data is summarized in Tables 2 and 3 for easier depiction and compared with other mentioned studies.
Figure 1.
Flowchart demonstrating the selection of patients in the study.
Table 1.
Demographic Distribution of the Sample and Comparison With the National Study.a
| Age group (years) | Study population | National surveillance data 4 | ||||
|---|---|---|---|---|---|---|
| Total | Males (n = 84) (56%) | Females (n = 66) (44%) | Total | Males (%) (72.01%) | Females (%) (27.99%) | |
| 0-14 | 2 (1.32) | — | 2 (1.32) | 90 (1.7) | 21 (0.60) | 69 (4.70) |
| 15-24 | 28 (18.67) | 10 (6.7) | 18 (12) | 1098 (20.80) | 636 (16.70) | 462 (31.30) |
| 25-34 | 36 (24) | 16 (10.67) | 20 (13.3) | 1134 (21.50) | 757 (19.90) | 377 (25.50) |
| 35-44 | 30 (20) | 19 (12.67) | 11 (7.3) | 963 (18.20) | 752 (19.80) | 211 (14.30) |
| 45-54 | 25 (16.67) | 14 (9.3) | 11 (7.3) | 907 (17.20) | 752 (19.80) | 155 (10.50) |
| 55-64 | 18 (12) | 10 (6.7) | 8 (5.3) | 679 (12.90) | 541 (14.20) | 138 (9.30) |
| ≥65 | 11 (7.3) | 8 (5.3) | 3 (2) | 409 (7.70) | 343 (9.00) | 66 (4.50) |
a Values are presented as number (percentage).
Table 2.
Pattern of Drug Resistance Among First- and Second-Line Drugs.
| Drug | No. of patients resistant (out of 150), n (%) | MDR + XDR + pre-XDR (out of 20), n | Non-MDR/XDR (out of 23), n |
|---|---|---|---|
| INH | 32 (21.34) | 20 | 12 |
| RIF | 20 (13.34) | 20 | 0 |
| PZN | 13 (8.67) | 8 | 5 |
| ETB | 17 (11.34) | 10 | 7 |
| Amikacin | 4 (2.6) | 3 | 1 |
| Kanamycin | 3 (2) | 2 | 1 |
| Capreomycin | 3 (2) | 3 | 0 |
| Ofloxacin | 6 (4) | 4 | 2 |
| Moxifloxacin | 7 (4.6) | 7 | 0 |
| Moxifloxacin (double dose) | 2 (1.3) | 2 | 0 |
| Clofazamine | 2 (1.3) | 2 | 0 |
| Linezolid | 1 (0.6) | 0 | 1 |
| Ethionamide | 3 (2) | 1 | 2 |
| PAS | 4 (2.6) | 4 | 0 |
Abbreviations: MDR, multidrug resistance; XDR, extensive drug resistance; INH, isoniazid; RIF, rifampicin; PZN, pyrazinamide; ETB, ethambutol; PAS, para-amino salicylic acid.
Table 3.
Comparison of pattern of drug resistance with previously available data.
| Drugs | National surveillance data 4 (691 patients), % | Mohan et al 3 (111 patients), n (%) | This study (43 patients), n (%) |
|---|---|---|---|
| INH | 49.05 | 103 (92.79) | 32 (74.4) |
| RIF | 12.59 | 91 (81.98) | 20 (46.5) |
| PZN | 30.82 | 52 (46.84) | 13 (30.23) |
| ETB | 10.13 | 57 (51.35) | 17 (39.53) |
| Amikacin | 4.34 | 5 (4.5) | 4 (9.30) |
| Kanamycin | 4.48 | 5 (4.5) | 3 (6.97) |
| Capreomycin | 4.63 | 1 (0.9) | 3 (6.97) |
| Ofloxacin | 16.4 | 36 (32.4) | 6 (13.95) |
| Moxifloxacin | 10.28 | 14 (12.6) | 7 (16.27) |
| Moxifloxacin (double dose) | NA | NA | 2 (4.65) |
| Clofazamine | NA | 0 | 2 (4.65) |
| Linezolid | NA | NA | 1 (2.32) |
| Ethionamide | 11.4 | 39 (35.1) | 3 (6.81) |
| PAS | 10.27 | 8 (7.2) | 4 (9.09) |
Abbreviations: INH, isoniazid; RIF, rifampicin; PZN, pyrazinamide; ETB, ethambutol; PAS, para-amino salicylic acid; NA, not applicable.
Discussion
Spinal TB is the most common form of osteoarticular TB. 6 Most authors advice long-term treatment for a period of more than 12 months. 7 MDR TB is most commonly a consequence of inadequate treatment. Johnson et al 8 in a study of 109 culture-positive patients of pulmonary tuberculosis have shown a high incidence of drug resistance in previous treatment defaulters, while very few in new cases. Treatment of spinal TB is similar to other tubercular diseases, that is, with antitubercular drugs. Inappropriate discontinuation or noncompliance to drug therapy causes development of drug resistance. Short-course chemotherapy with drug-resistant strains of the bacilli may create even more resistance to the drugs in use, commonly known as the amplifier effect 9 . Parket al 10 have shown that the susceptibility to MDR TB is strongly related to HLA-DRB1*08 032-DQB1*0601 haplotypes thus making them more vulnerable. Patients having HLA-DRB1*13 and HLA-DRB1*14 types are also reported to have 2 times higher chance of developing MDR TB. 11 Coinfection with HIV is also an important factor related to MDR TB especially in developed countries where primary TB infections are uncommon. 12 TB, in general, is more common among the male population and this accounts for a higher overall prevalence similarly encountered in our study. 13,14
Tubercular lesion in lungs are multibacillary as Mycobacterium tuberculosis and related species are strict aerobes and thus survive at high oxygen tension. In a cancellous vertebral body, the blood supply is abundant but the organism can multiply only moderately, hence much fewer organisms are encountered making it a “paucibacillary” (less than 104 colony-forming units per milliliter) lesion. 14 Mycobacterium bacilli shows a doubling time of 18 to 20 hours thus making it advantageous as the lesions grow slowly. This poses a significant disadvantage as the drugs that act on the rapidly multiplying group of bacteria are consequently less effective. 15 Lipoarabinomannan, a molecule in the cell wall of the bacillus helps the survival of the organism within the macrophages. The macrophages, in an effort to engulf and destroy the mycobacteria, tend to protect them from the usual antibiotics. Several types of bacilli exist in each colony with different growth potential and metabolic properties. Therefore, multiple antibiotics are required to address these demands. 16
Primary and acquired resistance to chemotherapeutic drugs are common in TB. The frequency of resistant mutants in a lesion of tuberculosis is 1 in 106 for INH and 1 in 108 for RIF. The probability of having a bacterium resistant to both is 1 in 1014. Hence, to decrease this drug resistance, a combination of at least 2 drugs is used. The knowledge of common patterns of drug resistance is important to treat the patients empirically especially in the initial phase of treatment where the drug sensitivity report might not be available. It also helps prevent development of further resistance and to prevent the development of amplifier effect. In many countries due to the availability of cartridge-based polymerase chain reaction tests like GeneXpert, these initial tests could demonstrate a possibility of RIF resistance which serves as a surrogate marker for multi drug resistance. In these group of patients, information about the existing drug resistance patterns help us to decide the empirical drug regimen till final drug sensitivity reports are available. The clinical criteria for suspecting drug-resistant cases of spinal TB includes patients of spinal tuberculosis on treatment with first-line drugs for 5 months or more showing one of these findings: poor clinical and radiological response; the development of a fresh lesion of osteoarticular tuberculosis; deterioration of spinal deformity; the appearance of discharging sinus or operated scar wound dehiscence.
The study conducted by Mohan et al 3 among 111 spinal TB patients who had some kind of drug resistance on culture showed that 103 (92.7%) had resistance to INH and 91 (81.9%) had resistance to RIF. Least resistance among the first-line drugs was to PZN in 52 (46.8%) patients. Mono-drug resistance to INH was in 4 (3.6%) patients, RIF in 1 (0.9%) and PZN in 2 (1.8%) patients. Among the second-line drugs, 36 (32.4%) had resistance to ofloxacin and 14 (12.6%) were resistant to moxifloxacin. In the injectable group, amikacin and kanamycin resistance was present in 5 (4.5%) patients and capreomycin resistance in 1 (0.9%) patient. Other drugs in the second line that showed resistance were: PAS in 8 (7.2%) and ethionamide in 39 (35.1%) patients. This study when compared with our study showed results on similar lines (Table 3). In the Indian National Drug Resistance Survey conducted by the Ministry of Health and Family Welfare, the study enrolled a total of 5280 patients with positive sputum microbiological examination. 4 Among these, 4957 patients underwent DST. Among the 3065 newly diagnosed patients, 2374 (77.46%) were susceptible to all drugs and 691 (22.54%) had some kind of resistance. A total of 87 (2.84%) patients had MDR TB, 27 (0.88%) had pre-XDR, and 2 (2.30%) had XDR TB. Among the first-line drugs, resistance to INH was found in 49.05% patients and to RIF in 12.59% patients whereas least drug resistance was found to ETB in 10.13% patients. Mono-drug resistance to INH was in 3.85% patients and least mono-drug resistance among the first-line drugs was to ETB in 0.23% patients. There was no mono-drug resistance to RIF. A detailed comparison of drug resistance between this study, drug resistance in spinal TB by Mohan et al 3 and that of the Indian Drug Resistance Survey 4 is shown in Table 3.
In both combined and mono-drug resistance patterns, resistance to INH was highest in all 3 studies. There was no mono-drug resistance to RIF in this study. It is also noted that all patients who had resistance to RIF have either MDR/XDR TB and hence it becomes an important surrogate marker for detecting drug-resistant tuberculosis as justified in cartridge-based polymerase chain reaction techniques like GeneXpert. There was a single case of mono-drug resistance to RIF reported in the drug surveillance conducted in India but it was from a group of previously treated patients. Moreover, there was one case of mono-drug resistance to RIF in the study conducted by Mohan et al. 3 Even though RIF resistance is considered as a proxy for MDR TB, there are reports of mono-drug resistance to RIF. Coovadia et al 17 conducted a study among the South African population and a total of 16 748 patients were assessed for susceptibility to RIF and INH. They concluded that mono-drug resistance to RIF existed in 8.8% of their study population. This provides a mandate for performing drug susceptibility testing for other drugs before considering the patient as a case of MDR TB. Another study conducted by Sharma et al 18 studied the pattern of drug resistance in second-line drugs in MDR TB patients. They concluded that 40% of the patients started empirically with multidrug regimens were already resistant to one or the other drugs. Both these studies showed the need for detailed DST in every patient of tuberculosis.
There are other studies conducted among patients with pulmonary TB demonstrating maximum resistance to INH among first-line drugs as shown by Fodor et al. 19 In this study, the bacillus was isolated from 264 newly diagnosed and 147 previously treated patients. All strains were tested for resistance against INH, RIF, streptomycin, and ETB. Primary resistance to INH was found in 4% patients, to streptomycin in 2%, to RIF in 0.4% and to ETB in 0.4%. Among the second-line drugs, resistance to FQ was highest in spinal as well as pulmonary tuberculosis. While FQ is used as a second-line drug, resistance to FQ has a considerable impact on the outcome of drug-resistant TB as shown by Falzon et al. 20 In their meta-analysis of drug resistance and their treatment outcome of 6724 cases between 1980 and 2009, they showed that patients of pre-XDR TB and XDR TB with resistance to injectable group had a better outcome as compared to patients with resistance to FQ group. There has been an increase in the prescription of FQ as a broad-spectrum antibiotic for many infections 19 and could be a reason for the increase in resistance to this group.
Data from National Anti-TB Drug Resistance Survey 2014-20 164 shows the prevalence of MDR TB in previously untreated patients to be 2.84% as compared to our study with around 16.27% cases. XDR TB in the national survey was 1.3% and in the current study was 9.3%. Pre-XDR FQ resistance was found to be more than the pre-XDR injectables group in both the studies. This pattern suggests that resistance to FQ is higher than the injectable aminoglycoside group. The higher number of MDR cases in this study as compared with the national tuberculosis survey might be due to the fact that our center is a tertiary care referral center and receives patients from all over the country. Many of the patients presenting to us have already been started on antitubercular therapy for variable duration as empirical management without a biopsy, which could be a source of potential bias in the study. Once the patients reach our center, we subject all of them for a biopsy and drug sensitivity by MGIT. It could also be accounted to the fact that this study was conducted for spinal TB and it has been compared with pulmonary TB due to the scarcity of literature pertaining to similar data in spinal TB. Of the 43 patients with drug resistance in the current study, resistance to INH was the highest (74.4%) and the least resistance was to PZN (30.23%), among the primary drugs. These correlate with the previous studies, conducted by Mohan et al 3 in their pattern of drug resistance in tuberculosis spine (INH 92.7%, and PZN 46.84%). INH and PZN resistance according to Indian national survey 4 were present in 49.05% and 30.82% patients, respectively. The least resistance was to ETB in 10.13% patients. However, ETB resistance in the study conducted by Mohan et al 3 and the current study was on a higher side, that is, 51.35% and 39.53%, respectively. Drug resistance among spinal cases when compared with that of pulmonary cases showed an increase in resistance to all primary drugs. There is an increase in the prevalence of drug resistance among the FQ group as compared with the injectable second line group. Among the FQ, moxifloxacin (4.6%) followed by ofloxacin (4%) showed the highest number of resistance. There is considerable cross-resistance among the FQ group as shown by Sanders. 21 This increased resistance might be due to the common usage of these drugs in the treatment of upper and lower respiratory tract infection.
Limitations
This study highlights the present trends of increase in resistance to the first-line antitubercular drugs, but the sample size is small and further multi centric studies on large scales are required to provide similar corroborative evidence regarding the trends in drug resistance pattern. We have not included patients with conventional culture in our study. This gives rise to a group of patients who did not show a positive MGIT but were diagnosed to have TB by other methods (conventional culture, histopathology, GeneXpert) and these patients could not be included in the sample population. Although our tertiary care center (K.E.M Hospital, Mumbai) receives patients from throughout the country, the sample may not be representative of the entire population. The pattern of resistance is a dynamic concept and the trends may have changed over the course of time after the data was collected.
Conclusion
Prevalence of MDR TB in the spine seems to be higher than that of pulmonary TB. Among the 43 drug-resistant patients, 7(16.27%) had MDR TB, 9 (20.93%) had pre-XDR TB, and 4(9.3%) had XDR TB. Among the 9 patients who had pre-XDR TB, 6 (13.95%) had resistance to FQ and 3 (6.97%) had resistance to an injectable second-line drug. Mono-drug resistance was present in 17 cases (39.5%) and polydrug resistance was present in 6 (13.95%) patients. The most common drug resistance among the first line agents was encountered was with isoniazid (74.4%) and least with pyrazinamide (30.23%). Among the second-line agents, the drug resistance was more common with the FQ group as compared with second-line injectables. Resistance to RIF in the study was less as compared with that of INH, showing that INH resistance is more prevalent and examining patients with GeneXpert for RIF resistance alone will not prevent amplifier effect.
In recent years, new drug regimens are being evaluated for drug-resistant pulmonary TB based on drug sensitivity pattern, while no such study has been performed for spinal TB. This study provides pattern of drug resistance in patients with drug-resistant spinal TB.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Aditya Raj, MS (Ortho)  https://orcid.org/0000-0003-0407-3496
https://orcid.org/0000-0003-0407-3496
Nandan Marathe, MS (Ortho)  https://orcid.org/0000-0002-8939-2690
https://orcid.org/0000-0002-8939-2690
References
- 1. World Health Organization. Guidelines for Surveillance of drug Resistance in Tuberculosis. World Health Organization; 2009. [Google Scholar]
- 2. Banerjee R, Allen J, Westenhouse J, et al. Extensively drug-resistant tuberculosis in California, 1993-2006. Clin Infect Dis. 2008;47:450–457. [DOI] [PubMed] [Google Scholar]
- 3. Mohan K, Rawall S, Pawar UM, et al. Drug resistance patterns in 111 cases of drug-resistant tuberculosis spine. Eur Spine J. 2013;22(suppl 4):647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ministry of Health and Family Welfare, Government of India. Report of the first national anti-tuberculosis drug resistance survey: India 2014-16, 2018. Accessed July 7, 2020. https://tbcindia.gov.in/showfile.php?lid=3315
- 5. Tortoli E, Cichero P, Piersimoni C, Simonetti MT, Gesu G, Nista D. Use of BACTEC MGIT 960 for recovery of mycobacteria from clinical specimens: multicenter study. J Clin Microbiol. 1999;37:3578–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gautam MP, Karki P, Rijal S, Singh R. Pott’s spine and Pott’s paraplegia. J Nep Med Assoc. 2005;44:106–115. [PubMed] [Google Scholar]
- 7. Donald PR. The chemotherapy of osteo-articular tuberculosis with recommendations for treatment of children. J Infect. 2011;62:411–439. [DOI] [PubMed] [Google Scholar]
- 8. Johnson J, Kagal A, Bharadwaj R. Factors associated with drug resistance in pulmonary tuberculosis. Indian J Chest Dis Allied Sci. 2003;45:105-109. [PubMed] [Google Scholar]
- 9. Falzon D, Jaramillo E, Schünemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011;38:516–528. [DOI] [PubMed] [Google Scholar]
- 10. Park MH, Song EY, Park HJ, Kwon SY, Han SK, Shim YS. HLA-DRB1 and DQB1 gene polymorphism is associated with multidrug-resistant tuberculosis in Korean patients. Hum Immunol. 2002;63:S33. [Google Scholar]
- 11. Sharma SK, Turaga KK, Balamurugan A, et al. Clinical and genetic risk factors for the development of multi-drug resistant tuberculosis in non-HIV infected patients at a tertiary care center in India: a case-control study. Infect Genet Evol. 2003;3:183–188. [DOI] [PubMed] [Google Scholar]
- 12. Kant S, Maurya AK, Kushwaha RAS, Nag VL, Prasad R. Multi-drug resistant tuberculosis: an iatrogenic problem. Biosci Trends. 2010;4:48–55. [PubMed] [Google Scholar]
- 13. Javaid A, Hasan R, Zafar A, et al. Prevalence of primary multidrug resistance to anti-tuberculosis drugs in Pakistan. Int J Tuberc Lung Dis. 2008;12:326–331. [PubMed] [Google Scholar]
- 14. Nhamoyebonde S, Leslie A. Biological differences between the sexes and susceptibility to tuberculosis. J Infect Dis. 2014;209(suppl 3):S100–S106. [DOI] [PubMed] [Google Scholar]
- 15. Toman K, Frieden TR. Toman’s Tuberculosis: Case Detection, Treatment and Monitoring: Questions and Answers. 2nd ed. World Health Organization; 2004. [Google Scholar]
- 16. Rajasekaran S, Khandelwal G. Drug therapy in spinal tuberculosis. Eur Spine J. 2013;22(suppl 4):587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coovadia YM, Mahomed S, Pillay M, Werner L, Mlisana K. RIF mono-resistance in Mycobacterium tuberculosis in KwaZulu-Natal, South Africa: a significant phenomenon in a high prevalence TB-HIV region. PLoS One. 2013;8:e77712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sharma AK, Gupta N, Kala DK, et al. A study on pattern of resistance to second line anti tubercular drugs among multi drug resistant tuberculosis patients. Indian J Tuberc. 2018;65:233–236. [DOI] [PubMed] [Google Scholar]
- 19. Fodor T, Vadász I, Lõrinczi I. Drug-resistant tuberculosis in Budapest. Int J Tuberc Lung Dis. 1998;2:732–735. [PubMed] [Google Scholar]
- 20. Falzon D, Gandhi N, Migliori GB, et al. Resistance to FQ and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J. 2013;42:156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanders CC. Mechanisms responsible for cross-resistance and dichotomous resistance among the quinolones. Clin Infect Dis. 2001;32(suppl 1):S1–S8. [DOI] [PubMed] [Google Scholar]