Abstract
Background
Bone marrow infiltration (BMI) is a devastating stage of paediatric lymphoma. Prompt diagnosis of BMI in newly diagnosed paediatric lymphoma patients is critical but can be very challenging at present.
Methods
We systematically retrieved studies from PubMed, EMBASE, and the Cochrane Library. Data extraction and quality assessment were performed by two reviewers independently. A total of nine eligible studies were included in the quantitative analysis.
Results
The pooled sensitivity and specificity of FDG-PET/CT for diagnosing BMI in newly diagnosed paediatric lymphoma patients were 0.97 (95% confidence interval [CI], 0.93 to 0.99) and 0.99 (95% CI, 0.98 to 0.99), respectively. The pooled PLR, NLR, and DOR were 79.9 (95% CI, 42.7 to 149.6), 0.03 (95% CI, 0.01 to 0.17), and 2414.6 (95% CI, 989.6 to 5891.4), respectively. The AUC of FDG-PET/CT for BMI was 1.00 (95% CI, 0.99 to 1.00). Compared with FDG-PET/CT, BMB had a lower pooled sensitivity (0.44, 95% CI, 0.34 to 0.55) and comparable pooled specificity (1.00, 95% CI, 0.92 to 1.00).
Conclusion
Compared with BMB, FDG-PET/CT was a more valuable diagnostic method for evaluating BMI in paediatric Hodgkin and non-Hodgkin lymphoma patients with extremely high diagnostic accuracy.
Keywords: Paediatric lymphoma, Bone marrow involvement, FDG-PET/CT, Bone marrow biopsy, Meta-analysis
Introduction
Lymphoma is one of the most common paediatric malignancies, with a prevalence rate of 12–15%, following acute leukaemia and malignant brain tumours [1]. The detection of bone marrow involvement (BMI) in lymphoma is important for accurate staging and management of the disease because the presence of BMI indicates the highest stage of lymphoma (Ann Arbor stage IV), which influences both treatment and prognosis [2]. The gold standard procedure for evaluating BMI is bone marrow biopsy (BMB) [3]. However, BMB explores only a limited part of the bone marrow, generally the unilateral or bilateral iliac crest, so focal bone marrow involvement may be missed. In addition, as an invasive procedure, BMB can cause pain to patients [4]. Despite these defects, BMB has been routinely used to assess BMI for many years.
As a glucose analogue, F-18 FDG provides information about glucose metabolism in normal and abnormal tissues, particularly in FDG-avid malignancies. F-18 FDG positron emission tomography/computed tomography (FDG-PET/CT) can simultaneously assess the structural anatomy and metabolic activity level of a tumour, which may be very useful for detecting BMI in paediatric lymphoma patients and may eliminate the need for BMB [5]. Because lymphoma is almost always FDG-avid, FDG-PET/CT has been widely used to stage newly diagnosed lymphoma, including the detection of BMI, and it has been reported to have high sensitivity and accuracy [6].
The clinical value of FDG-PET/CT in assessing paediatric lymphoma BMI is still under debate and investigation. In recent years, several studies have been published on the application of FDG-PET/CT for detecting BMI in paediatric lymphoma patients. However, because of the heterogeneity of study quality and low incidence of BMI in these patients [1], the results of these studies are inconclusive. The aim of this systematic review and meta-analysis was to synthesize published data on the accuracy of FDG-PET/CT in detecting BMI in newly diagnosed paediatric lymphoma patients and to determine whether BMB is still necessary for these patients.
Material and methods
The methodological approach to evidence searching and synthesis described in this article was based on the Cochrane Collaboration’s diagnostic test accuracy method [7]. We performed the current systematic review in accordance with the standards of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) in reporting the findings of this review [8]. No ethical approval or informed consent was required for this article because all data were retrieved from published literature. Searching for studies, identifying eligibility, extracting data, and assessing quality were performed by two investigators independently. Any disagreement was resolved through discussion, and the two researchers had to come to a consensus.
Search strategy
Three electronic databases, PubMed, EMBASE, and Cochrane Library, were searched for entries recorded from the time of database inception to March 10, 2021. Vocabulary and syntax were specifically adapted according to the database. We used “bone marrow infiltration” or “bone marrow involvement” as our diagnosis of interest and “positron emission tomography” or “PET” or “positron emission tomography/computed tomography” or “PET/CT” as our target index. The following group terms were used for searching: ((((child or teen or adolescent or paediatric or infant or newborn)) AND (“positron emission tomography” or “PET” or “positron emission tomography/computed tomography” or “PET/CT”)) AND (“bone marrow infiltration” or “bone marrow involvement”)) AND (Lymphoma or “Hodgkin Lymphoma” or “Non-Hodgkin Lymphoma”). Reference lists of relevant articles were also screened manually for any additional possible records.
Inclusion criteria
Studies included in this systematic review met the following criteria: (1) enrolled patients were diagnosed with paediatric lymphoma, including Hodgkin lymphoma and non-Hodgkin lymphoma; (2) studies investigated the diagnostic performance of FDG-PET/CT in the detection of BMI; (3) BMB was used as a (part of the) reference standard; and (4) sufficient data could be extracted to construct a 2 × 2 contingency table. Case reports, commentaries, expert opinion, narrative reviews, and studies carried out in animals and patients who had undergone systemic therapy were excluded. If more than one study provided overlapping data, only the most comprehensive or latest study was included.
Data extraction
Requisite data extracted and recorded in standardized Excel files included surname of the first author, publication year, study inclusion interval, country, study design, demographic information of participants, number of BMI/total patients, interval between FDG-PET/CT and BMB, time between FDG administration and scanning, FDG dose, image analysis, criteria for positivity, standard reference, and number of false/true-positive and false/true-negative cases.
Quality assessment
The methodological quality of the included studies was appraised according to the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 tool, which consists of four key domains (i.e. patient selection, index test, reference standard, and flow and timing). Risk of bias was assessed in each domain, and concerns about applicability were assessed in the first three domains with signalling questions. These questions were answered with “yes” for a low risk of bias/concern, “no” for a high risk of bias/concern, or “unclear” when relevant information was not clearly provided [9].
Statistical analyses
Pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were calculated using the bivariate meta-analysis framework. In addition, summarized receiver operating characteristic (sROC) curves were constructed, with the area under the curve (AUC) depicting the accuracy of the tests. Heterogeneity among the included studies was assessed using the I2 statistic. An I2 value of 0% implied no observed heterogeneity, and values of > 50% indicated substantial heterogeneity. For studies with substantial heterogeneity, we performed meta-regression analyses using a bivariate model to find the source of variability.
A two-sided p value < 0.05 was considered statistically significant in all statistical tests. Stata version 14 (StataCorp, College Station, TX) was used to analyse data from the included studies, and Review Manager Software version 5.3 (Cochrane Collaboration, Oxford, UK) was used to assess the methodological quality of the included studies.
Results
Search results and study selection
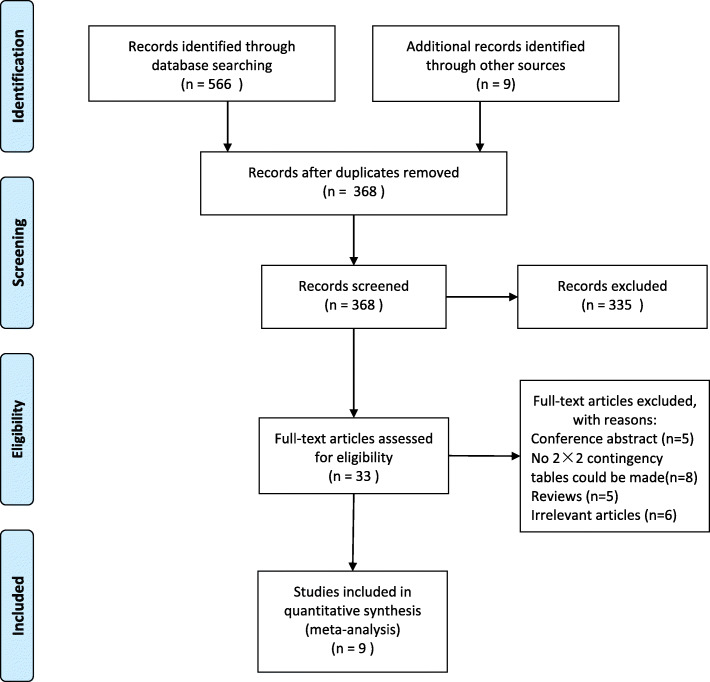
A total of 1985 records were identified by searching databases and removing duplicates. After initial screening of titles and abstracts, 36 articles were further assessed by scrutinizing the full texts against the predesigned criteria, and 9 articles [11–19] were eventually included in the quantitative analysis. The selection processes for the eligible studies are depicted in Fig. 1.
Fig. 1.
Selection process of included studies
Study characteristics
One study [18] was prospective, eight studies [11–17, 19] were retrospective, and all the studies were cohort studies. Nine studies involving a total of 1640 patients (326 patients with BMI) explored the diagnostic accuracy of FDG-PET/CT for BMI in paediatric lymphoma patients; four of these studies [12, 16–18] were about Hodgkin’s lymphoma (HL), one study [14] was about non-Hodgkin’s lymphoma (NHL), and four studies [11, 13, 15, 19] were about both HL and NHL. The mean ages of the included patients ranged from 4 to 14.8 years, and the proportion of males ranged from 48.1 to 83.3%. The time between FDG administration and scanning ranged from 45 to 90 min, and the interval between FDG-PET/CT and BMB was within 14 days. The main characteristics of the included studies are summarized in Table 1.
Table 1.
Characteristics of the included studies
| Study (Published year) | Inclusion interval | Country | No. of patients | Male/Female | Mean Age (y) (range) | Study design/type | Time between FDG administration and scanning(min) |
| Yaǧci-Küpeli et al. [10] | 2014.07-2014.12 | Turkey | 63 | 43/20 | 8.7 (2-17) | RCohort | 60 |
| Zapata et al. [11] | 2009.01-2014.10 | USA | 69 | 32/37 | 9.6 (0.5-21) | RCohort | 60 |
| Cistaro et al. [12] | NR | Italy | 224 | NR | 14 (4-18) | RCohort | 60-90 |
| Chen et al. [13] | 2010.05-2017.02 | China | 93 | 66/27 | 8 (1-21) | RCohort | 60 |
| Badr et al. [14] | 2010.02-2015.12 | Egypt | 140 | 105/35 | 8.6 (2-17) | RCohort | 45-60 |
| Hassan et al. [15] | 2010.07-2015.06 | Pakistan | 784 | 653/131 | 10.3 (2-18) | RCohort | 60 |
| Agrawal et al. [16] | NR | India | 38 | 30/8 | 9.8 (3-18) | RCohort | 45-60 |
| Purz et al. [17] | 2002-2006 | Germany | 175 | 89/86 | 14.6 | PCohort | 40-90 |
| Cheng et al. [18] | 2007.07-2008.12 | USA | 54 | 26/28 | 14.8 (6-24) | RCohort | 60-90 |
| Study (Published year) | Interval between FDG-PET/CT and BMB | FDG dose | Criteria for positivity | Reference standard | Lymphoma type | ||
| Yaǧci-Küpeli et al, [10] | NR | 185 MBq | Bone marrow uptake was higher than the liver | BMB; PET/CT; follow-up | HL and NH | ||
| Zapata et al, [11] | NR | NR | FDG avidity was equal to primary tumor or greater than adjacent tissues | BMB; PET/CT; follow-up | HL and NHL | ||
| Cistaro et al, [12] | <15 days | weight-adapteda | isolated/multiple focal uptake was higher than the liver or spleenb | BMB; PET/CT; follow-up | HL | ||
| Chen et al, [13] | 1-14 days | 5.18 MBq/Kg | Focal or multifocal abnormally increased FDG uptake | BMB; PET/CT; follow-up; MRI | NHL | ||
| Badr et al, [14] | <14 days | 5 to 10 MBq/kg, | Bone marrow uptake was higher than the liver | BMB; PET/CT; followup; MRI | HL and NHL | ||
| Hassan et al, [15] | <14 days | weight-based (90–270 MBq) | one or more bifocal uptakeb | BMB; PET/CT; follow-up | HL | ||
| Agrawal et al, [16] | NR | 3.7 MBq/kg | focal uptake was higher than the liverb | BMB; PET/CT; follow-up | HL | ||
| Purz et al, [17] | NR | weight–adapted | isolated/multiple focal uptake was higher than the liver | BMB; PET/CT; follow-up | HL | ||
| Cheng et al, [18] | NR | 5.18 MBq/kg or 0.14 mCi/kg | focal or multifocal abnormally increased FDG uptake | BMB; PET/CT; follow-up | HL and NHL | ||
P prospective, R retrospective, NR not reported, BMB bone marrow biopsy, CT computed tomography, FDG 18F-fluoro-2-deoxy-D-glucose, PET positron emission tomography, BMI bone marrow involvement, MRI magnetic resonance imaging
a Weight-adapted FDG dosage recommended according to the manufacturer guidelines for each scan model
b Diffusely/homogeneously increased bone marrow FDG uptake was also regarded as positive for BMI
Results of quality assessment
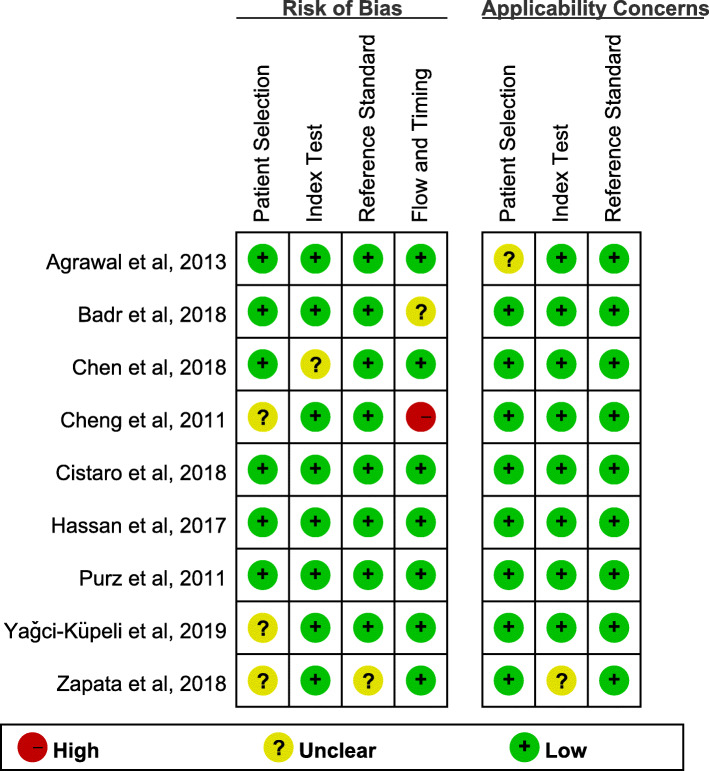
The results of the QUADAS-2 assessments for each included study are displayed in Fig. 2. In almost every key domain, the proportion of high-risk and unclear studies was less than 20%, which indicated that the quality of the included studies was good.
Fig. 2.
Quality assessment of included studies using QUADAS-2 tool criteria. Red in figure indicates high risk, yellow represents unclear risk and green means low risk
Diagnostic performance
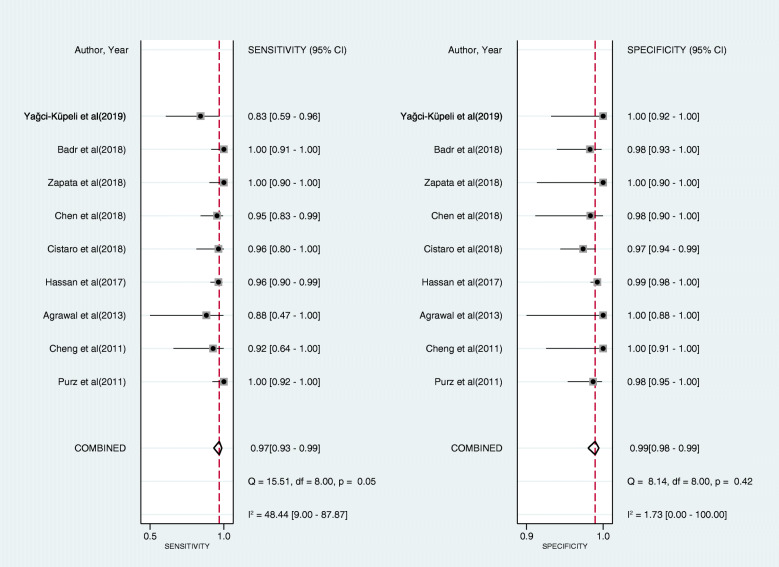
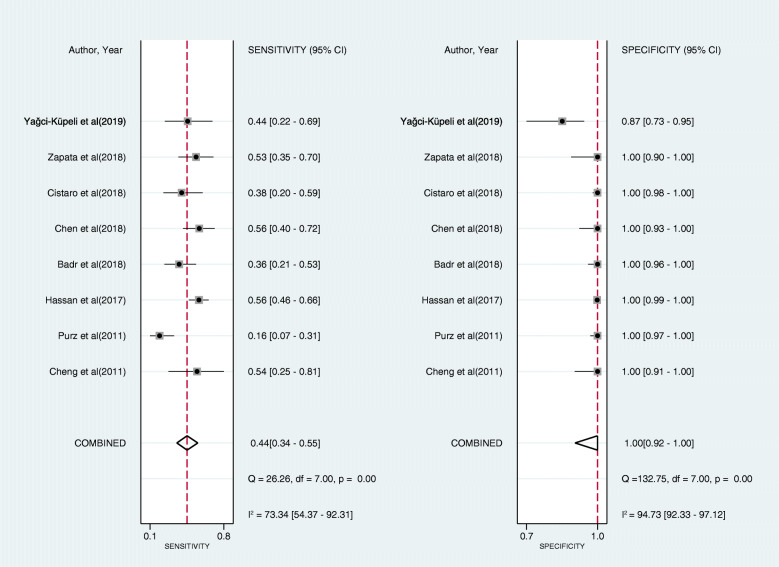
As shown in Fig. 3, the pooled sensitivity and specificity of FDG-PET/CT for diagnosing BMI in newly diagnosed paediatric lymphoma patients were 0.97 (95% CI, 0.93 to 0.99) and 0.99 (95% CI, 0.98 to 0.99), respectively. The pooled PLR, NLR, and DOR were 79.9 (95% CI, 42.7 to 149.6), 0.03 (95% CI, 0.01 to 0.17), and 2414.6 (95% CI, 989.6 to 5891.4), respectively. The AUC of FDG-PET/CT for BMI was 1.00 (95% CI, 0.99 to 1.00) (Fig. 4). The I2 statistics for sensitivity and specificity values were 48.44% (95% CI, 9.00% to 87.87%, p value = 0.05) and 1.73% (95% CI, 0.00% to 100.00%, p value = 0.42), respectively, which indicated no substantial heterogeneity among the included studies. Compared with PTE/CT, BMB had a lower pooled sensitivity (0.44, 95% CI, 0.34 to 0.55) and comparable pooled specificity (1.00, 95% CI, 0.92 to 1.00) (Fig. 5). The summary results of bivariate model analysis and subgroup analysis are presented in Table 2.
Fig. 3.
Forest plots of the sensitivity and specificity of FDG-PET/CT for bone marrow infiltration in the newly diagnosed paediatric lymphoma across all included studies. Diamonds in the central vertical lines represent pooled sensitivities or specificities with corresponding 95% confidence interval
Fig. 4.
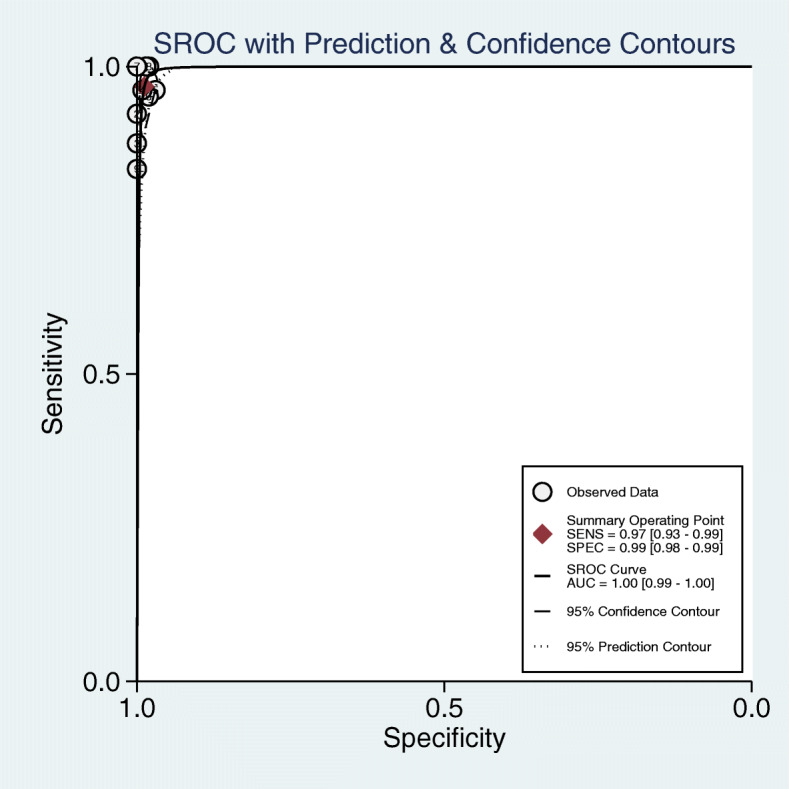
Summarized receiver operating characteristic curve (sROC) of FDG-PET/CT for bone marrow infiltration in the newly diagnosed paediatric lymphoma with corresponding 95% confidence region and the 95% prediction region
Fig. 5.
Forest plots of the sensitivity and specificity of bone marrow biopsy for bone marrow infiltration in the newly diagnosed paediatric lymphoma across all included studies. Diamonds in the central vertical lines represent pooled sensitivities or specificities with corresponding 95% confidence interval
Table 2.
Summary results of bivariate model analysis and sub-group analysis
| Summary results of bivariate model analysis | ||||||
|---|---|---|---|---|---|---|
| Bivariate Model Analysis | ||||||
| Sen (95% CI) | Spe (95% CI) | PLR (95% CI) | NLR (95% CI) | DOR (95% CI) | AUC (95% CI) | |
| PET/CT | ||||||
| 0.97(0.93-0.99) | 0.99(0.98-0.99) | 79.9(42.7-149.6) | 0.03(0.01-0.17) | 2414.6(989.6-5891.4) | 1.00(0.99-1.00) | |
| BMB | ||||||
| 0.44(0.34-0.55) | 1.00(0.92-1.00) | 1277.1(4.8-337317.5) | 0.56(0.46-0.68) | 2274(9-596153) | 0.71(0.67-0.75) | |
| Subgroup Analyses | ||||||
| Subgroup | Sen (95% CI) | Spe (95% CI) | PLR (95% CI) | NLR (95% CI) | DOR (95% CI) | AUC (95% CI) |
| PET/CT | ||||||
| HL | 0.97(0.93-0.99) | 0.99(0.97-0.99) | 66.4(36.7-120.3) | 0.03(0.01-0.07) | 2532(865-7412) | 1.00(0.99-1.00) |
| NHL | 0.94(0.85-0.97) | 0.99(0.94-1.00) | 68.9(15.7-302.9) | 0.06(0.03-0.16) | 1062(193-5853) | 0.99(0.98-1.00) |
| BMB | ||||||
| HL | 0.32(0.18-0.50) | 1.00(0.91-1.00) | 231.8(2.9-18534.3) | 0.68(0.53-0.87) | 341(4-30241) | 0.65(0.61-0.69) |
| NHL | 0.55(0.45-0.64) | 0.99(0.95-1.00) | 77.1(10.8-547.6) | 0.46(0.37-0.56) | 169(23-1250) | 0.98(0.96-0.99) |
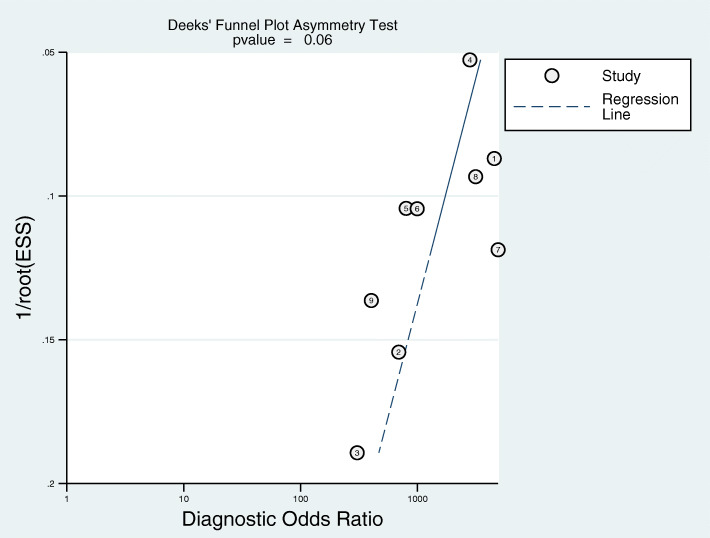
Publication bias
Deek’s funnel plot asymmetry test indicated no evidence of significant publication bias (p = 0.06) (Fig. 6).
Fig. 6.
Deek’s funnel plot asymmetry test indicated no evidence of significant publication bias
Discussion
Accurate and fast diagnosis of BMI in newly diagnosed lymphoma patients remains a challenging problem. The present staging and response criteria for HL and NHL published by the National Cancer Institute Working Group and revised by the International Working Group (IWG) in 2007 have been adopted by most physicians [20]. BMB remains the most commonly used method and gold standard for the clinical assessment of BMI. Despite the guidelines issued by the European Society for Medical Oncology (ESMO) in 2011 and 2018 indicating that FDG-PET/CT is sufficient for BMI assessment in adults, the effectiveness of FDG-PET/CT in evaluating paediatric lymphoma is still ambiguous since relevant data are scarce [20]. Our results revealed that FDG-PET/CT was highly sensitive and specific in the BMI evaluation of newly diagnosed paediatric lymphoma, with a pooled sensitivity of 0.97 (95% CI, 0.93~0.99) and pooled specificity of 0.99 (95% CI, 0.98~0.99).
As a systemic metabolic imaging technique that has been widely used in metastatic and invasive malignancies, FDG-PET/CT has been considered to have the potential for evaluating BMI in lymphoma patients. In 2010, Riad et al. [20] compared the role of FDG-PET/CT versus CT in the evaluation of paediatric lymphoma at the initial, intermediate chemotherapy, end of treatment, and recurrence stages based on 152 patients. In the initial staging, FDG-PET/CT staging was more accurate than CT in 11 of 41 patients. They demonstrated that FDG-PET/CT is of great significance in the early evaluation of paediatric lymphoma. The results of the present meta-analysis showed that the pooled specificity of FDG-PET/CT and BMB in the diagnosis of BMI in newly diagnosed paediatric lymphoma patients was approximate, but the pooled sensitivity of FDG-PET/CT was significantly higher than that of BMB. This suggests that FDG-PET/CT may be an option to avoid BMB in children with newly diagnosed lymphoma. In 2013, Adams et al. [21] found that in evaluating BMI in lymphoma, FDG-PET/CT could detect BMI patients who were not detected by BMB. This indicates that FDG-PET/CT can accurately detect BMI in lymphoma patients.
Previous studies have confirmed that FDG-PET/CT has a high sensitivity and specificity for evaluating BMI in HL patients, while its effectiveness for evaluating BMI in NHL patients has not been confirmed [21]. Our subgroup analysis found that the pooled sensitivity (0.97 and 0.94, respectively) and specificity (0.99 and 0.99, respectively) of FDG-PET/CT for evaluating BMI in HL and NHL patients were both high. In addition, the sensitivity of FDG-PET/CT-assessed HL and NHL was significantly higher than that of BMB (0.32 and 0.55, respectively). In addition, although the sensitivity of FDG-PET/CT for evaluating BMI in NHL patients was slightly lower than that of HL, there was no difference in specificity. The heterogeneity and publication bias of this study were very small; in fact, after removing the study by Yaǧci-Küpeli et al. [10], both the heterogeneity and publication bias vanished. The I2 of sensitivity and specificity were 48.44% and 1.73%, and the P values were 0.05 and 0.42, respectively. After removing the study by Yaǧci-Küpeli et al. [10], the I2 of sensitivity and specificity became 13.21% and 3.79%, and the P value changed to 0.33 and 0.40, respectively. There was no threshold effect for evaluating BMI in lymphoma patients using FDG-PET/CT or BMB, so no further threshold effect analysis was performed.
BMB is an invasive test that causes pain to the patient [22] and may cause some complications that cannot be ignored [23]. Studies have shown that BMB assesses bone marrow involvement by detecting only a small fraction of the bone marrow and is therefore prone to sampling errors [24]. For newly diagnosed paediatric lymphoma patients, false-negative BMI is more likely to be assessed using BMB. In contrast, FDG-PET/CT is a non-invasive systemic metabolic imaging technique that visualizes the entire bone marrow and has the advantages of non-invasive and error-free sampling over BMB [21]. Given all the paediatric lymphomas that are metabolically FDG-avid, FDG-PET/CT is valuable for the evaluation of lymphoma infiltration, especially BMI. The diagnosis of BMI is critical in assessing the disease status of lymphoma, guiding Ann Arbor staging, and influencing prognosis and treatment. Adequate examination before treatment can reduce the risk of litigation [10, 25]. In addition, the accuracy of FDG-PET/CT in BMI diagnosis is better than that of BMB, so FDG-PET/CT should be used instead of BMB.
The strengths of the current study lie in the following two aspects. First, we innovatively demonstrated the efficacy of FDG-PET/CT in evaluating the BMI of newly diagnosed paediatric malignant lymphoma. Second, a subgroup analysis was conducted; thus, we demonstrated that FDG-PET/CT is effective in assessing BMI in NHL patients, which is unprecedented. Potential limitations of this meta-analysis should also be considered. The included studies were mainly retrospective studies, with only one prospective study lacking prospective confirmatory studies. Research on FDG-PET/CT assessment of inert lymphoma is still insufficient, and further studies are needed to obtain more data in the future.
Conclusions
Based on the results of the current meta-analysis, it could be concluded that compared with BMB, FDG-PET/CT was a more valuable diagnostic method for evaluating BMI in paediatric Hodgkin and non-Hodgkin lymphoma patients with extremely high diagnostic accuracy.
Acknowledgements
This study was supported by the Beijing Natural Science Foundation (7174346, 7182146), National Natural Science Foundation of China (81672236, 81802224, 81871830), and Graduate Innovation Foundation of Peking Union Medical College (2019-1002-91).
Authors’ contributions
SW contributed to the conception of the study. LZZ, LCX, and CBR contributed significantly to literature search, data extraction, quality assessment, data analyses, and manuscript preparation. SLJ and WPX helped perform the analysis with constructive discussions. SW and GFQ revised the manuscript and approved the final version. The authors read and approved the final manuscript.
Funding
Beijing Natural Science Foundation (7174346, 7182146), National Natural Science Foundation of China (81672236, 81802224, 81871830), Graduate Innovation Foundation of Peking Union Medical College (2019-1002-91).
Availability of data and materials
The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.
Declarations
.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhizhuo Li and Chengxin Li are joint first authors.
References
- 1.Buhtoiarov IN. Pediatric lymphoma. Pediatrics in review. 2017;38(9):410-423. Epub 2017/09/03. doi: 10.1542/pir.2016-0152. PubMed PMID: 28864732. [DOI] [PubMed]
- 2.El-Galaly TC, Gormsen LC, Hutchings M. PET/CT for staging; past, present, and future. Seminars in nuclear medicine. 2018;48(1):4-16. Epub 2017/12/03. doi: 10.1053/j.semnuclmed.2017.09.001. PubMed PMID: 29195617. [DOI] [PubMed]
- 3.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J clin oncol : official journal of the American Society of Clinical Oncology. 2014;32(27):3059-3068. Epub 2014/08/13. doi: 10.1200/jco.2013.54.8800. PubMed PMID: 25113753; PubMed Central PMCID: PMCPMC4979083. [DOI] [PMC free article] [PubMed]
- 4.Kwee TC, de Klerk JM, Nievelstein RA. Imaging of bone marrow involvement in lymphoma: state of the art and future directions. TheScientificWorldJournal. 2011;11:391-402. Epub 2011/02/22. doi: 10.1100/tsw.2011.40. PubMed PMID: 21336455; PubMed Central PMCID: PMCPMC5719992. [DOI] [PMC free article] [PubMed]
- 5.Ziai P, Hayeri MR, Salei A, Salavati A, Houshmand S, Alavi A, Teytelboym OM Role of optimal quantification of FDG PET imaging in the clinical practice of radiology. Radiographics : a review publication of the Radiological Society of North America, Inc. 2016;36(2):481-496. Epub 2016/03/11. doi: 10.1148/rg.2016150102. PubMed PMID: 26963458. [DOI] [PubMed]
- 6.Adams HJ, Kwee TC, de Keizer B, Fijnheer R, de Klerk JM, Littooij AS, et al. Systematic review and meta-analysis on the diagnostic performance of FDG-PET/CT in detecting bone marrow involvement in newly diagnosed Hodgkin lymphoma: is bone marrow biopsy still necessary? Ann oncol : official journal of the European Society for Medical Oncology. 2014;25(5):921-927. Epub 2013/12/20. doi: 10.1093/annonc/mdt533. PubMed PMID: 24351400. [DOI] [PubMed]
- 7.Leeflang MMG, Deeks JJ, Gatsonis C, Bossuyt PMM, Cochrane Diagnostic Test A. Systematic reviews of diagnostic test accuracy. Ann Int Med. 2008;149(12):889-+. doi: 10.7326/0003-4819-149-12-200812160-00008. PubMed PMID: WOS:000261771900006. [DOI] [PMC free article] [PubMed]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012. doi: 10.1016/j.jclinepi.2009.06.005. PubMed PMID: WOS:000270250500003. [DOI] [PubMed]
- 9.Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529-U104. doi: 10.7326/0003-4819-155-8-201110180-00009. PubMed PMID: WOS:000296066300018. [DOI] [PubMed]
- 10.Yaǧci-Küpeli B, Koçyiǧit-Deveci E, Adamhasan F, Küpeli S. The value of 18F-FDG PET/CT in detecting bone marrow involvement in childhood cancers. J pediatric hematol/oncol. 2019;41(6):438–441. doi: 10.1097/MPH.0000000000001499. [DOI] [PubMed] [Google Scholar]
- 11.Zapata CP, Cuglievan B. PET/CT versus bone marrow biopsy in the initial evaluation of bone marrow infiltration in various pediatric malignancies. 2018;65(2). doi: 10.1002/pbc.26814. PubMed PMID: 28901637. [DOI] [PubMed]
- 12.Cistaro A, Cassalia L, Ferrara C, Quartuccio N, Evangelista L, Bianchi M, et al. Italian multicenter study on accuracy of (18)F-FDG PET/CT in assessing bone marrow involvement in pediatric Hodgkin lymphoma. Br J Haematol. 2018;18(6):e267-e73. Epub 2018/05/01. 10.1016/j.clml.2018.04.002. [DOI] [PubMed]
- 13.Chen S, Wang S, He K, Ma C, Fu H. Wang H. PET/CT predicts bone marrow involvement in paediatric non-Hodgkin lymphoma and may preclude the need for bone marrow biopsy in selected patients. 2018;28(7):2942–50. 29383519. 10.1007/s00330-018-5306-5. [DOI] [PubMed]
- 14.Badr S, Kotb M, Elahmadawy MA, Moustafa H. Predictive value of FDG PET/CT versus bone marrow biopsy in pediatric lymphoma. Clin Nucl Med. 2018;43(12):e428-ee38. Epub 2018/10/26. doi: 10.1097/rlu.0000000000002315. PubMed PMID: 30358625. [DOI] [PubMed]
- 15.Hassan A, Siddique M, Bashir H, Riaz S, Wali R, Mahreen A, Nawaz M.K. (18)F-FDG PET-CT imaging versus bone marrow biopsy in pediatric Hodgkin's lymphoma: a quantitative assessment of marrow uptake and novel insights into clinical implications of marrow involvement. Eur J Nucl Med Mol Imaging. 2017;44(7):1198-1206. Epub 2017/02/24. doi: 10.1007/s00259-017-3647-y. PubMed PMID: 28229191. [DOI] [PubMed]
- 16.Agrawal K, Mittal BR, Bansal D, Varma N, Srinivasan R, Trehan A, Manohar K, Kashyap R, Bhattacharya A, Marwaha RK Role of F-18 FDG PET/CT in assessing bone marrow involvement in pediatric Hodgkin's lymphoma. Ann Nucl Med. 2013;27(2):146-151. Epub 2012/11/13. doi: 10.1007/s12149-012-0665-5. PubMed PMID: 23143537. [DOI] [PubMed]
- 17.Purz S, Mauz-Körholz C, Körholz D, Hasenclever D, Krausse A, Sorge I, et al. [18F]fluorodeoxyglucose positron emission tomography for detection of bone marrow involvement in children and adolescents with Hodgkin's lymphoma. J Clin Oncol. 2011;29(26):3523–8. 10.1200/JCO.2010.32.4996. [DOI] [PubMed]
- 18.Cheng G, Chen W, Chamroonrat W, Torigian DA, Zhuang H, Alavi A. Biopsy versus FDG PET/CT in the initial evaluation of bone marrow involvement in pediatric lymphoma patients. Eur J Nucl Med Mol Imaging. 2011;38(8):1469-1476. Epub 2011/04/21. doi: 10.1007/s00259-011-1815-z. PubMed PMID: 21505896. [DOI] [PubMed]
- 19.Riad R, Omar W, Kotb M, Hafez M, Sidhom I, Zamzam M, et al. Role of PET/CT in malignant pediatric lymphoma. Eur J Nucl Med Mol Imaging. 2010;37(2):319–29. 10.1007/s00259-009-1276-9. [DOI] [PubMed]
- 20.Adams HJA, Kwee TC, de Keizer B, Fijnheer R, de Klerk JMH, Littooij AS, et al. Systematic review and meta-analysis on the diagnostic performance of FDG-PET/CT in detecting bone marrow involvement in newly diagnosed Hodgkin lymphoma: is bone marrow biopsy still necessary? Annals of Oncol. 2014;25((5)):921–7. 10.1093/annonc/mdt533. [DOI] [PubMed]
- 21.Vanhelleputte P, Nijs K, Delforge M, Evers G, Vanderschueren S. Pain during bone marrow aspiration: prevalence and prevention. J Pain Symptom Manage. 2003;26(3):860–6. 10.1016/S0885-3924(03)00312-9. [DOI] [PubMed]
- 22.Bain BJ. Morbidity associated with bone marrow aspiration and trephine biopsy - a review of UK data for 2004. Haematologica. 2006;91(9):1293–1294. [PubMed] [Google Scholar]
- 23.Wang J, Weiss LM, Chang KL, Slovak ML, Gaal K, Forman SJ, et al. Diagnostic utility of bilateral bone marrow examination: significance of morphologic and ancillary technique study in malignancy. Cancer. 2010;94(5):1522–1531. doi: 10.1002/cncr.10364. [DOI] [PubMed] [Google Scholar]
- 24.Tevanov I, Liciu E, Chirila MO, Dusca A, Ulici A. The use of 3D printing in improving patient-doctor relationship and malpractice prevention. Rom J Leg Med. 2017;25(3):279–82. 10.4323/rjlm.2017.279.
- 25.Liciu E, Frumueanu B, Popescu BM, Florea DC, Ulici A. Personalized surgical planning – the use of 3D printing in oncological pathology. Eur J Orthop Surg Traumatol. 2018;1((Supplement)):40.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.