Abstract
Background
Vibrio vulnificus has been reported as the leading causative pathogen of necrotizing fasciitis (NF) and related fatality in the coastal area. Necrotizing fasciitis caused by methicillin-resistant Staphylococcus aureus (MRSA) and V. vulnificus have high mortality rates. The purpose of this prospective study was to clarify the clinical characteristics between death and survival NF patients, to investigate bacteriologic profile and mortality of NF patients, and to compare risk indicators of MRSA and V. vulnificus NF patients.
Methods
This prospective study was conducted in 184 consecutive NF patients over a period of three years in a tertiary coastal hospital. Differences in mortality, laboratory findings, microbiology and clinical outcomes were compared between the death and survival groups, and the V. vulnificus and MRSA subgroups.
Results
Twenty patients died, resulting in a mortality rate of 10.9%, and there were 108 patients with a monomicrobial infection (58.7%). The death group had a significantly higher incidence of shock at emergency room and bacteremia than did the survival group. Vibrio species (40 cases) and S. aureus (31 cases) were the two major pathogens. Significant differences with respect to hepatic dysfunction, shock, the event with seawater or seafood contact, bacteremia, C-reactive protein, mean platelet counts, and the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score were observes between V. vulnificus and MRSA groups.
Conclusions
NF patients with both hepatic dysfunction and diabetes mellitus, bacteremia and shock have significantly higher mortality. We should be aware of the increasing incidence of monomicrobial NF and higher mortality rates of Gram-negative pathogens in the warm coastal area. LRINEC score is not a suitable diagnostic indicator for V. vulnificus NF, which is more rapidly progressive and fulminant than MRSA NF. NF needed team works by early suspicion, immediate surgical intervention and aggressive care, which can successfully decrease mortality.
Keywords: Necrotizing fasciitis, Vibrio vulnificus, MRSA, Monomicrobial, Gram-negative
Background
Necrotizing fasciitis (NF) is a life-threatening soft tissue infection with a high mortality rate of 25.3–73% [1–3]. Early suspicion of NF with emergent surgical debridement and appropriate antibiotic therapy can increase the survival rates and clinical outcomes [4, 5]. To improve the NF diagnosis, several clinical features had been recommended in the clinical diagnosis, including bullae, purple skin discoloration, crepitus, gas on X-Ray, local pain, swelling, erythema, tachycardia, fever, hypotension, and tachypnea [6–9]. NF is generally due to external trauma or skin wound that occurs commonly in patients with pre-existing chronic underlying diseases; however, diabetes mellitus, immunosuppression, chronic renal failure and decompensated liver disease have been reported as the major co-morbidities with poor prognosis in NF patients [6–10].
Depending on microbiological findings, NF is classified into four types—Polymicrobial infection (type I) with signs and symptoms of severe septic shock and multiple organ dysfunction was believed to be the first cause of NF, and followed by group A Streptococcus infection alone or combined with Staphylococcus aureus (type II) [1–10]. However, several studies have found that S. aureus, with or without methicillin resistanc, has emerged as an important monomicrobial infection type for NF [6–15]. Meanwhile, the marine bacterial infection related NF, such as Vibrio species, Aeromonas or Klebsiella species, have been noted as type III, and the fungal infection have been classified as type IV [1–7, 15–18].
In a 10-year study from 2003 to 2013, Streptococcus (48%), Staphylococcus (22%), and Gram-negative bacteria (21%) were the main pathogens to cause death in the patients with NF in the U.S., while monomicrobial NF due to either Staphylococcus or Streptococcus contributed to 69% of deaths with identified microorganisms [11]. In the past decade, methicillin-resistant Staphylococcus aureus (MRSA) was evidenced as an important emerging monomicrobial pathogens with a high mortality rate of up to 15% with a general increasingly range from 4 to 23% [12–15]. Furthermore, MRSA infection has caused a higher amputation rate in patients with deep-seated infection than methicillin-sensitive Staphylococcus aureus (MSSA) did [4, 13, 15].
Vibrio spp. had been reported as the leading causative pathogen of NF and related fatality in our institution, which is located at warm-water coastal regions in southwest Taiwan [6, 15–18]. Vibrio NF usually occurs through the injuries sustained when handling seafood, wound exposure to seawater, and ingestion of contaminated undercooked seafood [15–18]. We have established a treatment strategy including emergency fasciotomy or amputation, antibiotic therapy with a third-generation cephalosporin plus tetracycline, and admission to the intensive care unit (ICU) for patients with fulminant necrotizing fasciitis, such as Vibrio, MRSA, and Aeromonas infections [6, 15–18]. The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score has been proposed as a tool to identify patients at a higher risk for NF, in which a LRINEC score of 6 or greater would indicate a high risk for the presence of NF and be widely used [1]. However, our previous study revealed that the LRINEC scoring system is not appropriate for determining early diagnosis with Vibrio NF [6]. Our previous study had demonstrated that the clinical features of Vibrio vulnificus infection were more rapidly progressive and fulminant than those of the MRSA or MSSA infection, but it was a retrospective study [15]. Thus, we conducted this prospective study to search out the association between LRINEC scores and early detection of V. vulnificus and MRSA NF.
The purpose of this prospective study was to determine and clarify the clinical characteristics between dead and survival NF patients. We also investigated microbiological features and the association between mortality and different organisms of the consecutive NF patients, and compared the clinical and laboratory risk indicators of the patients with V. vulnificus and MRSA NF on initial examination.
Methods
Ethics
This prospective study for the causes and outcomes of patients with surgical confirmed NF of the extremities was approved by the Ethics Committee and Institutional Review Board of Chang Gung Medical Foundation (103-2081B), and conformed to the Declaration of Helsinki. Informed consent was obtained from all subjects included in this study, and all methods were carried out in accordance with relevant guidelines and regulations.
Patients selection
We included the NF patients who were initially diagnosed by emergency medicine doctors and underwent excisional fasciotomy or immediate limb amputation by orthopedic surgeons admitted to Chia-Yi Chang Gung Memorial Hospital between April 2015 to May 2018. NF was defined by surgically findings: the presence of grayish necrotic soft tissue and hemorrhagic bullaes, loss of resistance of normally adherent fascia to digital blunt dissection, and the appearance of pus with the foul odor of dishwater. The diabetic foot infection and NF patients who did not receive surgery were excluded. A total of the 184 consecutive patients were enrolled into this program. These patients included 120 men and 64 women with a mean age of 66.4 years (range, 19 to 95 years).
Diagnosis and treatment protocol
The most common symptoms of NF patients were pain and swelling of the involved limbs with edematous, patchy, erythematous, and hemorrhagic bullous skin lesions. Contact history with seawater or raw seafood was routinely surveyed. Broad-spectrum antibiotics with usage of ceftriaxone with/without other regimens were initially administered to all the patients, and emergency fasciotomy or immediate limb amputation was performed wherein necrotizing fasciitis was diagnosed at the time of admission to the emergency room (ER) or at the time of consultation in the ward [19]. Surgical debridement was done every other day if progressive necrotic changes were combined with a deteriorating clinical presentation. Initial empiric antibiotics were continued after first surgery and adjusted based on the results of blood cultures and tissue tests a few days later. Hyperbaric oxygen (HBO) therapy and vacuum assisted closure (VAC) therapy was administered in stable NF patients for improving wound healing. Soft tissue reconstructions, such as skin grafts and flap reconstruction, were done until the infected necrotic tissue was controlled and stabilized [6, 16, 19] (Fig. 1).
Fig. 1.
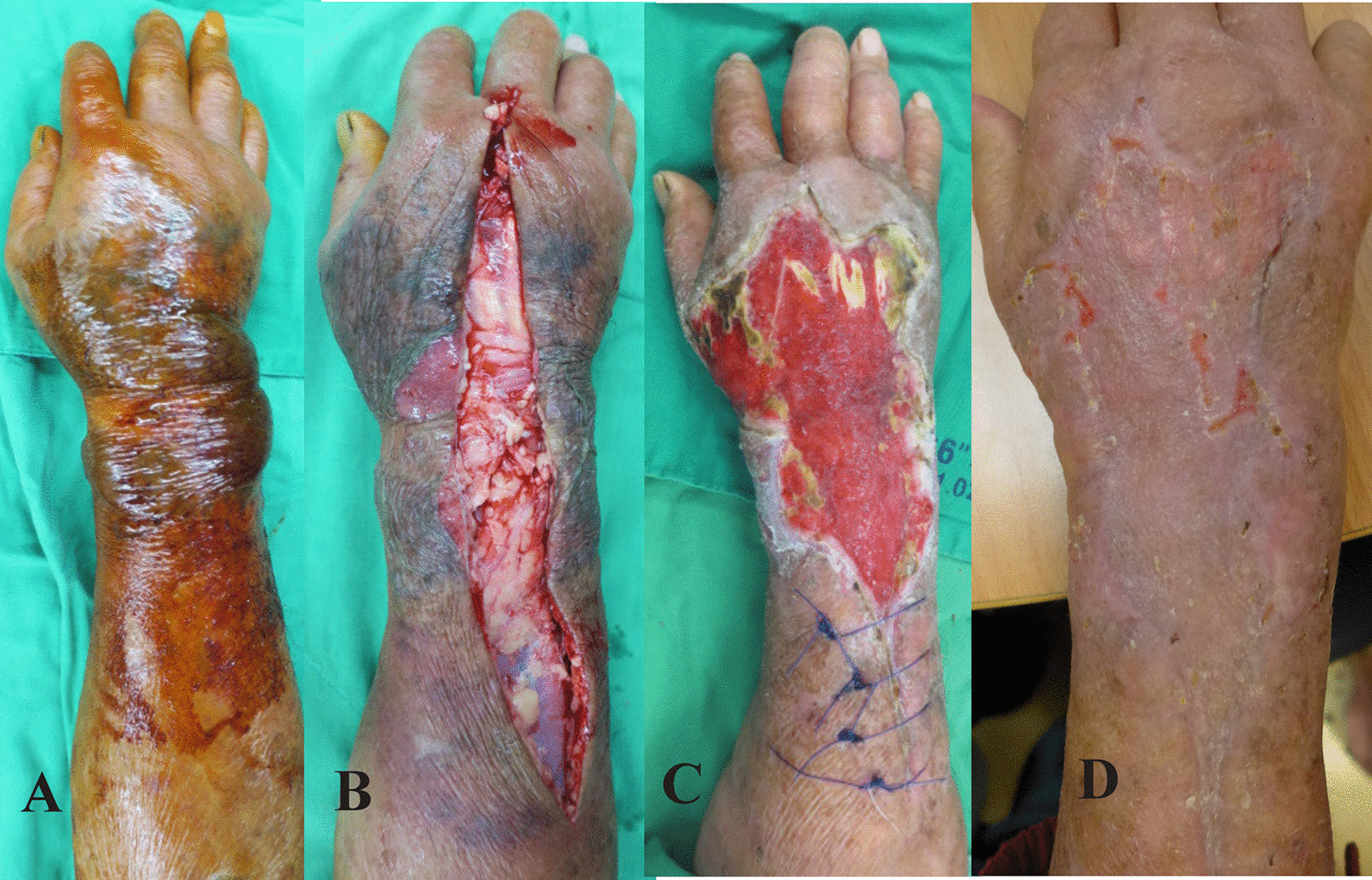
A 73 year-old male fishman with a history of diabete mellitus and oral cancer had right hand pain and swelling for 2 days after handling fish. A Preoperative photographs of the right forearm revealed severe patchy purpura, hemorrhagic bullae and edema in the emergency room. B After emergency fasciotomy, the forearm revealed extensive necrosis of underlying skin and turbid fascial layer. Three days later, the wound cultures confirmed the presence of Vibrio vulnificus. C He had received repeated debridement and vacuum assisted closure (VAC) therapy. He had received skin graft on the 42th day after fasciotomy and discharged on the 50th day. D He was followed up with a good skin growth of right forearm in the clinic
Demographic and clinical variables of the 184 patients were evaluated and recorded after confirming the diagnosis of NF by histopathological examination. The cultured specimens, obtained from the wounds or the blood, were confirmed by microbiologic evaluation. Identification of these microorganisms was based on standard phenotypic tests used in clinical microbiology laboratories. The specimens of the patients were classified as monomicrobial infection, polymicrobial infection, and no growth, and clinical outcomes were reviewed for each patient according to the microbiological findings. Differences in mortality, chronic illness, related events and clinical courses were compared between the death and survival groups.
Clinical assessment of Vibrio vulnificus and MRSA patients
Further, we enrolled these patients with monomicrobial infection of V. vulnificus and MRSA, and categorized them into two subgroups. Differences in age, gender, presence of comorbidities, the interval between contact or injury and admission to ER, the interval between diagnosis and first surgery, affected sites, mortality, laboratory findings at the time of admission, the LRINEC score, and clinical outcomes were compared between the two subgroups.
Statistical analysis
Statistical analyses were performed with the use of Statistical Product and Service Solutions (SPSS) Version 18.0 statistical software (SPSS, Chicago, Illinois). We used the two-tailed t-test for continuous variables and the Fisher exact test for categorical variables to examine significant relationships between risk factors and outcomes between Vibrio vulnificus and MRSA groups. A value of p < 0.05 was considered significant.
Results
Patient characteristics in the death and survival groups
Twenty patients died, resulting in a mortality rate of 10.9%. Thirteen patients had received amputation with an amputation rate of 7%. Sixty-five patients had reported to have a history of seawater or seafood contact, and 28 patients recalled to have a farm injury or a dirty-water contact. The time interval from symptom to presentation at the emergency room ranged from one to four days (mean, 2.2 days) prior to admission. The mean time-interval from admission in the emergency room to the first operation ranged from 2 to 12 h (mean 5.85 h).
Age, gender, interval between symptom and admission, interval between diagnosis of necrotizing fasciitis and first surgery, infective regions, nature of first surgery, and hospital days did not differ significantly between the death and survival groups. Ninety-three patients (50.5%) had associated with a history of hepatic dysfunction, such as liver cirrhosis, hepatitis B or C, hepatocellular carcinoma, or alcoholic liver disease. Seventy-four patients (40.2%) had associated with a history of diabetes mellitus. NF patients with both hepatic dysfunction and diabetes mellitus have higher mortality than did those with hepatic dysfunction or diabetes mellitus alone (p = 0.037) (Table 1). The death group had a significantly higher incidence of shock at ER (p = 0.0002) and bacteremia (p = 0.0004) than did the survival group (Table 2).
Table 1.
Comparison between the deaths and survivors for charcteristics at first consultation in the ER and ward
| Deaths | Survivors | |||
|---|---|---|---|---|
| N = 20 | N = 164 | P value | ||
| Age (years) | Mean | 66.6 | 66.1 | 0.55 |
| Gender (%) | ||||
| Male | 9 (45) | 111 (67.7) | ||
| Female | 11 (55) | 53 (32.3) | ||
| Interval from contact or injury to presentation at ER (days) | Mean | 2.2 | 3.4 | 0.32 |
| Interval from diagnosis at ER to first operation (hours) | Mean | 4.3 | 5.8 | 0.22 |
| Underlying chronic disease (%) | ||||
| Hepatic dysfunction and DM | 8 (40) | 30 (18.3) | 0.037* | |
| Hepatic dysfunction alone | 7 (35) | 48 (29.3) | 0.61 | |
| Diabetes Mellitus alone or with others | 1 (5) | 35 (21.3) | 0.13 | |
| Chronic renal insufficiency | 1 (5) | 8 (4.9) | ||
| Heart disease | 2 (10) | 19 (11.6) | ||
| Cancer | 1 (5) | 8 (4.9) | ||
| Gout | 0 | 4 (2.4) | ||
| Nil | 0 | 12 (7.3) | ||
| Wound location (%) | 0.47 | |||
| Upper extremity | 10 (50) | 93 (56.7) | ||
| Lower extremity | 10 (50) | 61 (43.3) | ||
| First operation | 0.29 | |||
| Fasciotomy | 19 (95) | 162 (98.7) | ||
| Amputation | 1 (5) | 2 (1.3) | ||
| Final operation after first fasciotomy (%) | 19 | 162 | ||
| Amputation | 2 (10.5) | 8 (4.9) | ||
| Split-thickness skin graft | 0 | 80 (49.4) | ||
| Flap | 0 | 6 (3.7) | ||
| Debridement | 8 (42.1) | 46 (28.4) | ||
| Without secondary operation | 9 (47.4) | 22 (13.6) | ||
| Hospital days | Mean | 34.25 | 35.21 | 0.08 |
Hepatic dysfunction included liver cirrhosis, hepatitis B or C, hepatocellular carcinoma, and alcoholic liver disease
*Mean p < 0.05 and the difference was significant
Table 2.
Demographic and clinical presentations of deaths and survivors
| Characteristics (%) | Deaths (N-20) |
Survivors (N-164) |
p-value |
|---|---|---|---|
| Related events | |||
| Seawater or seafood contact | 4 (20) | 61 (37.2) | |
| Farm/dirty-water contact | 1 (5) | 27 (16.5) | |
| Vital signs | |||
| Body temperature > 38.5 °C | 2 (10) | 28 (17) | 0.53 |
| Heart rate ≥ 100 | 13 (65) | 81 (49.4) | 0.24 |
| Respiratory rate > 20 | 13 (65) | 87 (53) | 0.35 |
| Systolic blood pressure < 90 | 10 (50) | 20 (12.2) | 0.0002* |
| Cultures | |||
| Blood (P) Wound (P) | 12 (60) | 23 (14) | |
| Blood (P) Wound (N) | 1 (5) | 17 (10.4) | |
| Blood (N) Wound (P) | 3 (15) | 74 (45.1) | |
| Blood (N) Wound (N) | 4 (20) | 50 (30.5) | |
| Presence of bacteremia | 13 (65) | 40 (24.4) | 0.0004* |
| Vibrio vulnificus | 5 (38.5) | 22 (55) | |
| Aeromonas spp. | 4 (30.7) | 5 (12.5) | |
| Pseudomonas aeruginosa | 1 (7.7) | 1 (2.5) | |
| E. coli | 0 | 1 (2.5) | |
| MRSA | 1 (7.7) | 1 (2.5) | |
| MSSA | 0 | 1 (2.5) | |
| CONS | 0 | 2 (5) | |
| β-hemolytic streptococcus | 1 (7.7) | 6 (15) | |
| Viridans streptococci | 0 | 1 (2.5) | |
| Polymicrobial | 1 (7.7) | 0 |
Blood (P), culture-positive blood sample; Blood (N), culture-negative blood sample; Wound (P), culture-positive surgical wound sample; Wound (N), culture-negative surgical wound sample; CONS, Coagulase negative Staphylococcus
*Mean p < 0.05 and the difference was significant
Microbiological findings in NF patients
Instead of polymicrobial infection, most NF samples were found to have monomicrobial infection (58.7%). Among bacterial species identified, V. vulnificus was the most dominant pathogen (21.7%), followed by S. aureus (16.8%), β-hemolytic Streptococcus (6.5%), Aeromonas spp. (6%), and Pseudomonas aeruginosa (2.7%). The patients with monomicrobial Aeromonas spp and monomicrobial Group non-ABD β-hemolytic Streptococcus infections had the highest mortality rate of 45.5 and 33.3% respectively. Fifty-three patients had bacteremia, and V. vulnificus patients had a significantly higher incidence of bacteremia (50.9%). Thirteen patients with bacteremia were obtained in the death group, from the blood in one V. vulnificus patient and from both blood and wounds in 12 patients. There were four V. vulnificus, four Aeromonas spp., one P. aeruginosa, one MRSA, one Group non-ABD β-hemolytic Streptococcus and one polymicrobial specimens revealed positive in both blood and wound cultures. The mortality rate of Gram-negative pathogens was 16.7% (10/60), which was higher than that of Gram-positive pathogens (6.25%, 3/48) (Table 3).
Table 3.
Bacterial species from necrotizing fasciitis patients
| Isolated bacteria | No. of patients (%) | Dead patients | Survival patients | Mortality rate of pathogen (%) |
|---|---|---|---|---|
| Variable (%) | (N = 184) | |||
| Monomicrobial infection | 108 (58.7%) | 13 | 95 | 12 |
| Gram-positive aerobic pathogens | 48 (44.4%) | 3 | 45 | 6.25 |
| Staphylococcus aureus | 31 (64.6) | 2 | 29 | 6.5 |
| MRSA | 16 | 1 | 15 | 6.3 |
| MSSA | 15 | 1 | 14 | 6.7 |
| Viridans streptococci | 2 (4.2) | 0 | 2 | 0 |
| Coagulase negative Staphylococcus | 3 (6.2) | 0 | 3 | 0 |
| β-hemolytic streptococcus | 12 (25) | 1 | 11 | 8.3 |
| Group non-ABD | 4 | 1 | 3 | 25 |
| Group B | 4 | 0 | 4 | 0 |
| Group A | 2 | 0 | 2 | 0 |
| S. dysgalactiae | 1 | 0 | 1 | 0 |
| S. equisimilis | 1 | 0 | 1 | 0 |
| Gram-negative aerobic pathogens | 60 (55.6%) | 10 | 50 | 16.7 |
| Vibrio vulnificus | 40 (66.6) | 4 | 36 | 10 |
| Aeromonas spp | 11 (18.3) | 5 | 6 | 45.5 |
| Pseudomonas aeruginosa | 5 (8.3) | 1 | 4 | 20 |
| Enterobacter cloacae | 1 (1.7) | 0 | 1 | 0 |
| E. coli | 1 (1.7) | 0 | 1 | 0 |
| Klebsiella pneumoniae | 1 (1.7) | 0 | 1 | 0 |
| Shewanella putrefaciens | 1 (1.7) | 0 | 1 | 0 |
| Polymicrobial infection | 22 (12.0%) | 3 | 19 | 13.6 |
| No growth | 54 (29.3%) | 4 | 50 | 7.4 |
| Total | 184 | 20 | 164 | 10.9 |
Those pathogens identified in the polymicrobial cultures were S. aureus, Streptococcus spp., Escherichia coli, Enterobacter cloacae, Pseudomonas aeruginosa, Enterococcus faecalis, Morganella morganii, Proteus, Peptostreptococcus anaerobius, Acinetobacter spp. and Bacteroides fragilis. The mortality rate of polymicrobial NF was 13.6%. Bacterial growth was absent in 54 patients, and 4 patients died, representing a mortality rate of 7.4%.
Clinical features and laboratory findings difference between MRSA-NF and V. vulnificus-NF groups
The mortality rate among the patients infected with MRSA and V. vulnificus were 6.25% (1/16) and 10% (4/40), respectively. Age, sex, wound location, vital signs, and outcomes did not differ between the two groups. In the MRSA group, One patient who had liver cirrhosis with diabetes mellitus died. One patient had liver cirrhosis with hepatitis C, one had liver cirrhosis, one had hepatitis C alone, and one had alcoholic liver disease.
In the V. vulnificus group, ten patients had hepatitis B or C with diabetes mellitus, and one died. Six patients had liver cirrhosis with/without hepatitis B or C, and one died. One patient who had hepatocellular carcinoma died. Six patients had a history of hepatic hepatitis B and C, and one died. One patients had liver cirrhosis and diabetes mellitus, and one patient alcoholic liver disease. Pre-existing hepatic dysfunction, the interval between contact and admission to ER, hypotensive shock in ER, and the event with seawater or seafood contact were associated with V. vulnificus NF. There were 15 V. vulnificus NF patients with blood and tissue culture-positive samples, and 11 patients with blood culture-positive samples. Twenty-six patients with Vibrio-NF (65%) were found with bacteremia, while only 3 patients with MRSA infection (18.7%) had positive blood and tissue cultures (p = 0.002) (Table 4).
Table 4.
Demographic and clinical presentations of MRSA and V. vulnificus associated necrotizing fasciitis patients
| Characteristics | MRSA (N-16) |
Vibrio vulnificus (N-40) |
p-value |
|---|---|---|---|
| Age (years) | |||
| Mean | 62.8 | 71.2 | 0.35 |
| Gender | |||
| Female | 5 | 14 | 0.52 |
| Male | 11 | 26 | |
| Comorbidities (%) | |||
| Hepatic dysfunction | 5 (31.2) | 25 (62.5) | 0.034* |
| LC alone or with others | 3 (18.75) | 7 (17.5) | 1 |
| Diabetic mellitus | 8 (50) | 12 (20) | 0.136 |
| Cancer | 2 (12.5) | 2 (5) | 0.321 |
| Chronic kidney disease | 4 (25) | 10 (25) | 0.624 |
| Interval from contact or injury | 5.5 | 1.2 | 0.023* |
| to presentation at ER (days) | |||
| Interval from diagnosis at ER | 5.8 | 4.7 | 0.53 |
| to first operation (hours) | |||
| Wound location (%) | |||
| Upper extremity | 8 (50) | 24 (60) | 0.489 |
| Lower extremity | 8 (50) | 16 (40) | |
| Outcome | 6.25% | 10% | |
| Death | 1 | 4 | 0.383 |
| Survivors | 15 | 36 | |
| Related events (%) | |||
| Seawater or seafood contact | 2 (12.5) | 37 (92.5) | 0.001* |
| Farm/dirty-water contacted | 1 (6.25) | 3 (7.5) | 0.43 |
| Vital signs (%) | |||
| Body temperature > 38 °C | 4 (25) | 8 (20) | 0.376 |
| Heart rate ≥ 100 | 10 (62.5) | 22 (55) | 0.607 |
| Respiratory rate > 20 | 7 (43.75) | 23 (57.5) | 0.325 |
| Systolic blood pressure < 90 | 0 | 9 (22.5) | 0.036* |
| Cultures (%) | |||
| Blood (P) Wound (P) | 3 (18.75) | 15 (37.5) | |
| Blood (P) Wound (N) | 0 | 11 (27.5) | |
| Blood (N) Wound (P) | 13 (81.25) | 14 (35) | |
| Presence of bacteremia (%) | 3 (18.75) | 26 (65) | 0.002* |
Blood (P), culture-positive blood sample; Blood (N), culture-negative blood sample; Wound (P), culture-positive surgical wound sample; Wound (N), culture-negative surgical wound sample
Hepatic dysfunction included liver cirrhosis (LC), hepatitis B or C, hepatocellular carcinoma, and alcoholic liver disease
*Mean p < 0.05 and the difference was significant
Analysis of hematology and clinical biochemistry of the NF patients demonstrated no significant difference between these two groups in white blood cell count, segment form neutrophil, band form neutrophil, hemoglobin, albumin, blood sugar, creatinine, and sodium. However, different prevalence patterns between the two groups were observed for C-reactive protein (CRP), mean platelet counts and platelet counts ≤ 1.5 × 105 per mm3 (Table 5).
Table 5.
Blood analysis of MRSA and V. vulnificus necrotiz NF patients
| MRSA (N-16) |
Vibrio vulnificus (N-40) |
p-value | ||
|---|---|---|---|---|
| White cell count (cells/mm3) | Mean | 16,400 ± 7128 | 14,560 ± 10,820 | 0.53 |
| Segmented forms (%) | Mean | 79.6 ± 7.3 | 81.7 ± 10.7 | 0.47 |
| ≦73 | 2 | 7 | 0.494 | |
| > 73 | 14 | 33 | ||
| Band forms (%) | Mean | 2.91 ± 5.17 | 5.54 ± 7.23 | 0.19 |
| = 0 | 7 | 22 | 0.321 | |
| > 0 | 9 | 18 | ||
| Albumin (g/dL) | Mean | 3.47 ± 0.47 | 3.42 ± 0.46 | 0.72 |
| < 3.5 | 9 | 19 | 0.384 | |
| ≥ 3.5 | 7 | 21 | ||
| Platelet counts (per mm3) | Mean | 211,200 ± 114,500 | 139,800 ± 62,940 | 0.004* |
| ≤ 1.5 × 105 | 5 | 26 | 0.023* | |
| > 1.5 × 105 | 11 | 14 | ||
| Blood sugar (mg/dL) | Mean | 206 ± 110 | 169 ± 71 | 0.14 |
| Hemoglobin (g/dL) | Mean | 12.3 ± 1.62 | 13.0 ± 2.11 | 0.19 |
| Creatinine (mg/dL) | Mean | 1.30 ± 0.68 | 1.52 ± 1.25 | 0.52 |
| Sodium (mmol/L) | Mean | 135 ± 2.2 | 136 ± 2.6 | 0.1 |
| C-reactive protein (mg/L) | Mean | 184 ± 132 | 64.5 ± 88.4 | 0.000* |
*Mean p < 0.05 and the difference was significant
The LRINEC score showed a significant statistical difference between the two groups (p = 0.0003), and the numbers of V. vulnificus NF patients with LRINEC score < 6 had a significantly higher than the numbers of patients with MRSA NF group (p = 0.004) (Table 6).
Table 6.
Laboratory risk indicator for necrotizing fasciitis (LRINEC) score of MRSA and V. vulnificus necrotiz NF
| Patients | ||||
|---|---|---|---|---|
| Variable (%) | Score | MRSA (N-16) | Vibrio vulnificus (N-40) | P value |
| C-Reactive Protein, mg/L | ||||
| < 150 | 0 | 8 (50) | 34 (85) | 0.01* |
| ≧ 150 | 4 | 8 (50) | 6 (15) | |
| White cell count, per mm3 | ||||
| < 15 | 0 | 8 (50) | 24 (60) | |
| 15–25 | 1 | 7 (43.75) | 13 (32.5) | 0.73 |
| > 25 | 2 | 1 (6.25) | 3 (7.5) | |
| Hemoglobin, g/dL | ||||
| > 13.5 | 0 | 3 (18.75) | 18 (45) | |
| 11–3.5 | 1 | 9 (56.25) | 14 (35) | 0.175 |
| < 11 | 2 | 4 (25) | 8 (20) | |
| Sodium, mmol/L | 0.069 | |||
| ≥ 135 | 0 | 8 (50) | 30 (75) | |
| < 135 | 2 | 8 (50) | 10 (25) | |
| Creatinine, umol/L | ||||
| ≦ 141 | 0 | 12 (75) | 32 (80) | 0.467 |
| > 141 | 2 | 4 (25) | 8 (20) | |
| Glucose, mmol/L | ||||
| ≦ 10 | 0 | 9 (56.25) | 26 (65) | 0.376 |
| > 10 | 1 | 7 (43.75) | 14 (35) | |
| Total score | Mean | 6.08 ± 2.86 | 3.12 ± 2.46 | 0.0003* |
| ≥ 6 | 9 (56.25) | 6 (15) | 0.004* | |
| < 6 | 7 (43.75) | 34 (85) |
*Mean P < 0.05 and the difference was significant
Discussion
The Chiayi Chang Gung Memorial Hospital is a tertiary hospital which situated on the western coast of southern Taiwan, and V. vulnificus are the most frequent causative organism of monomicrobial NF. The residents’ occupations were fishermen or farmers who were frequently associated with handling raw seafood, exposure to seawater, and contact with brackish water or soil [6, 16, 16, 17, 19]. However, Vibrio spp. and Aeromonas spp. infections have a relatively high incidence and associated with high mortality in our institution [6, 15–17, 19, 20]. We have identified as hypotensive shock, severe hypoalbuminemia, severe thrombocytopenia, and increased banded leukocyte forms can be considered as clinical and laboratory risk indicators to initiate early surgery for Vibrio and all types of necrotizing fasciitis [6, 15–17, 19–21]. Moreover, we had established a treatment protocol including emergency fasciotomy or amputation, antibiotic therapy with a third-generation cephalosporin and admission to the intensive care unit for suspected Vibrio NF patients, and had successfully decreased the mortality rate of Vibrio NF from 35 to 13% during the 6-year period from 2004 to 2010 [6, 15–17, 19, 20]. In this prospective study, we had diminished the mortality rate of total NF to 10.9% and Vibrio NF to 10% respectively after early suspicion and aggressive traeatment by the team works.
Diabetes mellitus has been reported to be a common underlying disease in NF patients, accounting for 44–72% in the literatures [1–11]. Many literatures had also demostrated that hepatic dysfunction, such as liver cirrhosis, hepatitis B or C, hepatocellular carcinoma, or alcoholic liver disease, was a highly risk factor for developing NF [1–5, 19, 22–24]. Those virulence factors of micro-organisms, such as V. vulnificus, S. aureus, β-hemolytic Streptococcus, Aeromonas spp. and P. aeruginosa, were commonly reported to impair the phagocytic activity of the reticuloendothelial system, and to result in bacterial translocation and bacteremia in patients with hepatic decompensation [13–24]. In this study, we found there were 50.5% of NF patients had associated with a history of hepatic dysfunction, and 40.2% had diabetes mellitus. Most of all, those NF patients with both hepatic dysfunction and diabetes mellitus had a significantly higher mortality. Thus, our finding should alert the clinicans to pay more attention and treat aggressively for those NF patients with a history of hepatic dysfunction and/or diabetes mellitus who may result in fulminant clinical course and mortality in a short time.
Recent literatures have revealed that monomicrobial necrotizing fasciitis caused by Gram-negative pathogens, such as V. vulnificus, Klebsiella pneumoniae, Aeromonas hydrophila, Pseudomonas spp. and Escherichia coli, had persistently increased, and could cause more fulminant clinical courses and higher incidence of mortality rate than Gram-positive pathogens do [19, 25–31]. Monomicrobial Gram-negative NF combined with bloodstream infection was reported to have significantly increased the mortality rate [25–31]. This prospective study indicated that the incidence of monomicrobial necrotizing fasciitis (58.7%) was higher than that of polymicrobial infections (12%). Moreover, we found that the monomicrobial Gram-negative NF patients had a greater proportion (55.6% vs. 44.4%) and revealed higher mortality rates (16.7% vs. 6.25%) than the Gram-positive NF patients. Based on those findings, we should pay more attention to manage Gram-negative NF due to its rapid and fulminant courses with increasing risk of developing bacteremia and poor outcomes.
Our previous study had reported that the clinical characteristics of V. vulnificus infection were more rapidly progressive and fulminant than those of the S. aureus infection, either MRSA or MSSA [15]. However, since it was a retrospective study, some medical records of patients did not include accurate descriptions and laboratory data. We conducted this prospective study to investigate the outcomes of early detection and surgery of NF, and the association of the specific characteristics and risk factors on initial examination of V. vulnificus and MRSA NF. We had demonstrated that V. vulnificus NF patients had significant associations with a history of contact with seawater or seafood and MRSA had significant association with a history of diabetes mellitus, previous abrasion injury, pus accumulated in surgical wound, and chronic ulcers [15]. With early diagnosis and emergent surgery, the mortality rates in the patients with V. vulnificus and S. aureus of 18.3% and 13.1% respectively during the 6-year period from 2003 to 2009 were reduced to a mortality rate of 10% and 6.5% respectively during a 3-year period. Moreover, the mortality rate in the patients with MRSA necrotizing fasciitis was reduced from 17.2 to 6.25% [15].
Liver cirrhosis is considered as a risk factor with increasing mortality among NF patient [2–6, 24, 26, 28, 30]. Seven Vibrio NF patients and 3 MRSA NF patients had liver cirrhosis with/without other chronic illness, and one died respectively in this study. We found that one Vibrio NF patient (14.3%) and 3 MRSA NF patients (100%) with liver cirrhosis had CRP bigger than 150 mg/L and LRINEC score > 6. Thus, we considered the lower CRP and LRINEC score were related to rapidly progressive and fulminant course of V. vulnificus NF, not related to liver cirrhosis.
As a diagnostic tool for severe NF, this prospective study indicated that the application of LRINEC score is species dependent due to the higher score for MRSA group, which may be as a result of longer interval from contact to symptom presentation at ER. We confirmed that the LRINEC scoring system is inappropriate for determining the early diagnosis of Vibrio NF patients who revealed fast and fulminant septic status. Otherwise, V. vulnificus group had significant differences in the clinical characteristics and laboratory data, such as hypotension at emergency room, shorter interval from contact or injury to symptom presentation at ER, presence of bacteremia, thrombocytopenia, and lower CRP level than MRSA group. These results demonstrated V. vulnificus NF revealed more rapidly progressive and fulminant than MRSA NF.
This study has several limitations. First, there was 29.3% of microbial cultures of clinical specimens resulting in negative with the mortality rate of 7.4%. Polymerase chain reaction (PCR) can be considered for early detection and accurate confirmation of the pathogens in sterile sites of these suspicious patients for appropriate antibiotics use. The second limitation was that we did not do LRINEC scoring system for all 184 patients. In fact, our emergency department had reported that the Vibrio vulnificus and Aeromonas hydrophila NF patients had average LRINEC score of 3.9 and 3.5, which were lower than the mean of the whole NF group. Thus, LRINEC score not be an accurate tool for necrotizing fasciitis risk stratification and differentiation in the suburban and tertiary coastal hospital [32].
Conclusion
NF patients with both hepatic dysfunction and diabetes mellitus, bacteremia and shock have significantly higher mortality. We should be aware of the increasing incidence of monomicrobial NF and higher mortality rates of Gram-negative pathogens in the warm coastal area. LRINEC score is not a suitable diagnostic indicator for V. vulnificus NF, which is more rapidly progressive and fulminant than MRSA NF. NF needed team works by early suspicion, immediate surgical intervention and aggressive care, which can successfully decrease mortality.
Acknowledgements
None.
Abbreviations
- NF
Necrotizing fasciitis
- ER
Emergency room
- S. aureus
Staphylococcus aureus
- V. vulnificus
Vibrio vulnificus
- MRSA
Methicillin-resistant Staphylococcus aureus
- LRINEC
Laboratory risk indicator for necrotizing fasciitis
- ICU
Intensive care unit
Authors’ contributions
YHT: contributed the conception and design of the study and drafting the article. CJL: contributed acquisition of data. TYH: contributed analysis and interpretation of data. CTH: contributed analysis and interpretation of data. LTK: contributed final approval of the version to be submitted. YHT: participated in its design and coordination. KCH: contributed revising it critically for important intellectual content. All authors read and approved the final manuscript.
Funding
This work did not received any funding.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the author (orma2244@adm.cgmh.org.tw) on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of Chang Gung Medical Foundation (103-2081B). Written informed consent was obtained from all individual patients included in the study for a possible future use of the samples that could be given as an extension of the original research. Informed consent was obtained from all subjects included in this study, and all methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Written informed consent for patient information to be published was provided by each patient.
Competing interests
None of the authors reports a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Jt Surg Am. 2003;85-A:1454–1460. doi: 10.2106/00004623-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Fontes RA, Jr, Ogilvie CM, Miclau T. Necrotizing soft-tissue infections. J Am Acad Orthop Surg. 2000;8:151–158. doi: 10.5435/00124635-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Elliott D, Kufera JA, Myers RA. The microbiology of necrotizing soft tissue infections. Am J Surg. 2000;179:361–366. doi: 10.1016/S0002-9610(00)00360-3. [DOI] [PubMed] [Google Scholar]
- 4.Khamnuan P, Chongruksut W, Jearwattanakanok K, Patumanond J, Tantraworasin A. Necrotizing fasciitis: epidemiology and clinical predictors for amputation. Int J Gen Med. 2015;8:195–202. doi: 10.2147/IJGM.S82999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan MS. Diagnosis and management of necrotising fasciitis: a multiparametric approach. J Hosp Infect. 2010;75:249–257. doi: 10.1016/j.jhin.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Tsai YH, Hsu RW, Huang KC, Huang TJ. Laboratory indicators for early detection and surgical treatment of Vibrio necrotizing fasciitis. Clin Orthop Relat Res. 2010;468:2230–2237. doi: 10.1007/s11999-010-1311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Misiakos EP, Bagias G, Patapis P, Sotiropoulos D, Kanavidis P, Machairas A. Current concepts in the management of necrotizing fasciitis. Front Surg. 2014;1:36. doi: 10.3389/fsurg.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnham JP, Kirby JP, Kollef MH. Diagnosis and management of skin and soft tissue infections in the intensive care unit: a review. Intensive Care Med. 2016;42:1899–1911. doi: 10.1007/s00134-016-4576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waldron C, Solon JG, O'Gorman J, Humphreys H, Burke JP, McNamara DA. Necrotizing fasciitis: the need for urgent surgical intervention and the impact of intravenous drug use. Surgeon. 2015;13:194–199. doi: 10.1016/j.surge.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Cheng NC, Tai HC, Chang SC, Chang CH, Lai HS. Necrotizing fasciitis in patients with diabetes mellitus: clinical characteristics and risk factors for mortality. BMC Infect Dis. 2015;15:417. doi: 10.1186/s12879-015-1144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arif N, Yousfi S, Vinnard C. Deaths from necrotizing fasciitis in the United States, 2003–2013. Epidemiol Infect. 2016;144(6):1338–1344. doi: 10.1017/S0950268815002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang JM, Lim HK. Necrotizing fasciitis: eight-year experience and literature review. Braz J Infect Dis. 2014;18:137–143. doi: 10.1016/j.bjid.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee TC, Carrick MM, Scott BG, Hodges JC, Pham HQ. Incidence and clinical characteristics of methicillin-resistant Staphylococcus aureus necrotizing fasciitis in a large urban hospital. Am J Surg. 2007;194:809–812. doi: 10.1016/j.amjsurg.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 14.Changchien CH, Chen YY, Chen SW, Chen WL, Tsay JG, Chu C. Retrospective study of necrotizing fasciitis and characterization of its associated methicillin-resistant Staphylococcus aureus in Taiwan. BMC Infect Dis. 2011;11:297. doi: 10.1186/1471-2334-11-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai YH, Wen-Wei Hsu R, Huang KC, Huang TJ. Comparison of necrotizing fasciitis and sepsis caused by Vibrio vulnificus and Staphylococcus aureus. J Bone Jt Surg Am. 2011;93:274–284. doi: 10.2106/JBJS.I.01679. [DOI] [PubMed] [Google Scholar]
- 16.Hsu JC, Shen SH, Yang TY, Chen PH, Huang KC, Tsai YH. Necrotizing fasciitis and sepsis caused by Vibrio vulnificus and Klebsiella pneumoniae in diabetic patients. Biomed J. 2015;38:136–142. doi: 10.4103/2319-4170.137767. [DOI] [PubMed] [Google Scholar]
- 17.Tsai YH, Hsu RW, Huang KC, Chen CH, Cheng CC, Peng KT, et al. Systemic Vibrio infection presenting as necrotizing fasciitis and sepsis. A series of thirteen cases. J Bone Jt Surg Am. 2004;86-A:2497–2502. doi: 10.2106/00004623-200411000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Lee YC, Hor LI, Chiu HY, Lee JW, Shieh SJ. Prognostic factor of mortality and its clinical implications in patients with necrotizing fasciitis caused by Vibrio vulnificus. Eur J Clin Microbiol Infect Dis. 2014;33:1011–1018. doi: 10.1007/s10096-013-2039-x. [DOI] [PubMed] [Google Scholar]
- 19.Tsai YH, Huang KC, Shen SH, Hsu WH, Peng KT, Huang TJ. Microbiology and surgical indicators of necrotizing fasciitis in a tertiary hospital of southwest Taiwan. Int J Infect Dis. 2012;16:e159–e165. doi: 10.1016/j.ijid.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Hsiao CT, Weng HH, Yuan YD, Chen CT, Chen IC. Predictors of mortality in patients with necrotizing fasciitis. Am J Emerg Med. 2008;26:170–175. doi: 10.1016/j.ajem.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Chang CP, Fann WC, Wu SR, Lin CN, Chen IC, Hsiao CT. Diagnostic performance of initial serum albumin level for predicting in-hospital mortality among necrotizing fasciitis patients. J Clin Med. 2018;7:435. doi: 10.3390/jcm7110435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scher D, Kanlic E, Bader J, Ortiz M, Abdelgawad A. Hepatitis C viral infection as an associated risk factor for necrotizing fasciitis. Orthopedics. 2012;35(4):e510–e513. doi: 10.3928/01477447-20120327-43. [DOI] [PubMed] [Google Scholar]
- 23.Wu CJ, Chen PL, Tang HJ, et al. Incidence of Aeromonas bacteremia in southern Taiwan: Vibrio and Salmonella bacteremia as comparators. J Microbiol Immunol Infect. 2014;47:145–148. doi: 10.1016/j.jmii.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Chuang PY, Yang TY, Huang TW, Tsai YH, Huang KC, Weng HH. Hepatic disease and the risk of mortality of Vibrio vulnificus necrotizing skin and soft tissue infections: a systematic review and meta-analysis. PLoS ONE. 2019;14(10):e0223513. doi: 10.1371/journal.pone.0223513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SY, Yu SN, Lee EJ, Kim T, Jeon MH, Choo EJ, Park S, et al. Monomicrobial gram-negative necrotizing fasciitis: an uncommon but fatal syndrome. Diagn Microbiol Infect Dis. 2019;94:183–187. doi: 10.1016/j.diagmicrobio.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Huang TU, Peng KT, Hsiao CT, Fann WC, Tsai YH, Li YY, et al. Predictors for gram-negative monomicrobial necrotizing fasciitis in southern Taiwan. BMC Infect Dis. 2020;20:60. doi: 10.1186/s12879-020-4796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yahav D, Duskin-Bitan H, Eliakim-Raz N, Ben-Zvi H, Shaked H, Goldberg E, et al. Monomicrobial necrotizing fasciitis in a single center: the emergence of Gram-negative bacteria as a common pathogen. Int J Infect Dis. 2014;28:13–16. doi: 10.1016/j.ijid.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 28.Syue LS, Chen PL, Wu CJ, Lee NY, Lee CC, Li CW, et al. Monomicrobial Aeromonas and Vibrio bacteremia in cirrhotic adults in southern Taiwan-similarities and differences. J Microbiol Immunol Infect. 2016;49(4):509–515. doi: 10.1016/j.jmii.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Lee CY, Kuo LT, Peng KT, Hsu WH, Huang TW, Chou YC. Prognostic factors and monomicrobial necrotizing fasciitis: gram-positive versus gram-negative pathogens. BMC Infect Dis. 2011;11:5. doi: 10.1186/1471-2334-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang CP, Hsiao CT, Fann WC. Risk factors associated with bacteremia correlated with mortality in patients with acute bacterial skin and skin structure infection. Intern Emerg Med. 2019;14(2):259–264. doi: 10.1007/s11739-018-1973-0. [DOI] [PubMed] [Google Scholar]
- 31.Huang TY, Peng KT, Hsu WH, Hung CH, Chuang FY, Tsai YH. Independent predictors of mortality for aeromonas necrotizing fasciitis of limbs: an 18-year retrospective study. Sci Rep. 2020;10:7716. doi: 10.1038/s41598-020-64741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsiao CT, Chang CP, Huang TY, Chen YC, Fann WC. Prospective validation of the laboratory risk indicator for necrotizing fasciitis (LRINEC) score for necrotizing fasciitis of the extremities. PLoS ONE. 2020;15(1):e0227748. doi: 10.1371/journal.pone.0227748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the author (orma2244@adm.cgmh.org.tw) on reasonable request.


