Abstract
Breast cancer (BC) accounts for significant morbidity and mortality among women worldwide. About one in three patients with breast cancer present with lymph node (LN) metastasis and LN status is one of the most important prognostic predictors in patients with BC. In addition to their prognostic value, LNs initiate adaptive immunity against BC. Yet, BC cells often avoid immune-mediated destruction in LNs. This review provides an overview of the ways by which BC cells modulate LN stromal and hematopoietic cells to promote metastasis and immune evasion.
The leading cause of mortality in patients diagnosed with breast cancer (BC; and other cancers) is metastatic outgrowth.1 Dissemination of cancer cells from the site of tumor origin to locoregional and distant organs is a multistep process. Cancer cells that detach from primary tumors may enter blood vessels or lymphatic vessels. Lymphatic vessel invasion is associated with shorter disease-free survival and overall survival.2 Most breast cancer cells that enter lymphatic vessels travel to regional tumor-draining lymph nodes (TDLNs) in the axilla.2 The propensity of BC cells to metastasize to lymph nodes (LNs) is associated with molecular subtype in addition to other clinicopathologic variables such as age, sex, primary tumor size, and histologic grade.
Biopsy of the sentinel TDLN [the first lymph node(s) to receive lymph drainage from tumors] is commonly performed for BC staging and treatment decisions, and LN metastasis is generally associated with worse survival than node-negative patients.3 One explanation for poorer survival, according to the spectrum theory of metastasis, is that LNs are prognostic, as they are indicative of the metastatic potential of cancer cells, and that nodal metastases may seed distant metastases.4 Indeed, recent studies support the hypothesis that BC metastasis to distant organs, such as lung and bone marrow, can be seeded from LN metastases.5, 6, 7
The primary functions of LNs in normal physiology are to filter and concentrate antigen and to activate T and B cells. The centralization of antigen and immune cells within LNs supports the initiation of adaptive immunity, including anti-tumor immunity. BC cells, by avoiding immune detection and through production of immunosuppressive factors, limit the generation of anti-tumor T cells. Insufficient anti-tumor T cell output from LNs coupled with immune suppression within tumors likely prevent the eradication of BC cells at the primary site. As a consequence, BC cells with metastatic properties colonize LNs (Figure 1) that are permissive for metastatic outgrowth and further metastasis of BC cells. The aim of this review is to summarize recent progress on how breast cancers subvert LN function to enable local and distant metastasis.
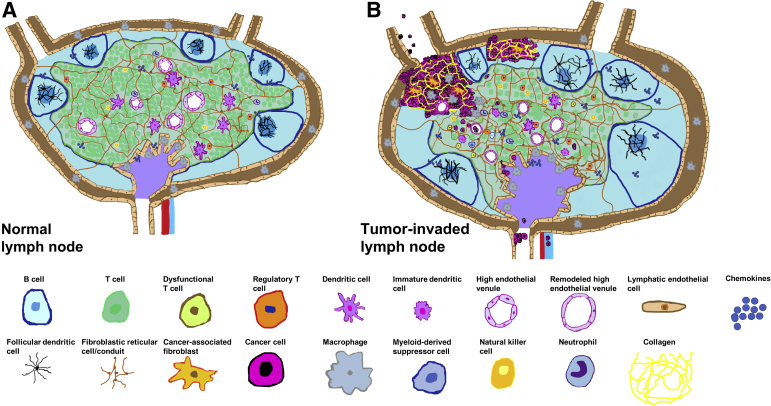
Figure 1.
Cellular changes in lymph nodes associated with breast cancer metastases. A: Graphical representation of a normal lymph node. The subcapsular sinus (brown) contains macrophages and is lined by lymphatic endothelial cells. The cortex (blue) contains follicles that consist of B cells and follicular dendritic cells. Fibroblastic reticular cells/conduits are depicted in the cortex and paracortex (green). The paracortex contains T cells, dendritic cells, and high endothelial venules. Macrophages are depicted in the medulla (purple). B: Enlargement of tumor-involved lymph node is seen with the invasion and growth of cancer cells. B cells accumulate in tumor-involved lymph nodes (germinal centers are depicted as darker blue), whereas the overall T cell compartment contracts. Of note, the regulatory T cell population has been shown to expand in tumor-involved lymph nodes. Remodeling of lymph nodes and enhanced collagen production by fibroblastic reticular cells and recruited cancer-associated fibroblasts have been observed in tumor-involved lymph nodes. High endothelial venules are remodeled and show an increased lumen diameter and thinner endothelial layer. In tumor-involved lymph nodes, the lymphatic vasculature is expanded, many dendritic cells are immature, and subsets of functionally impaired natural killer cells and T cells are present. Macrophages, neutrophils,8 and myeloid-derived suppressor cells are recruited to tumor-involved lymph nodes and exhibit a protumor phenotype. Cancer cells can gain access to lymphatic and blood vasculatures of lymph nodes to further metastasize.
Conditioning Lymph Nodes for Metastasis
Nascent blood vessels in tumors are leaky, resulting in increased interstitial fluid pressure, leading to dilated lymphatic vessels and increased lymph transport of proteins and BC cells.9 Lymph enters TDLNs through afferent lymphatic vessels in conjunction with either antigen in soluble form or antigen-presenting cells. Proteomic profiling of lymph from afferent lymphatic vessels, leading to TDLNs, revealed proteins associated with host immunomodulation, angiogenesis, and lymphangiogenesis.10 In addition, proteins that support BC migration, invasion, and metastasis are enriched in afferent lymph of animals with metastatic breast tumors compared with those with nonmetastatic breast tumors.10 Some proteins identified in lymph are associated with exosomes, which have been shown to condition other premetastatic BC niches.11
Lymphatic Metastasis of BC Cells
BC cells undergo biochemical and morphologic changes to facilitate metastatic spread through lymphatics, most notably through an epithelial-to-mesenchymal transition.12 BC cells within lymph aggregate into clusters and express both epithelial and mesenchymal markers,13 indicative of a hybrid cellular state associated with stemness. These properties of cancer cells circulating in lymph may enhance their ability to form secondary tumors in LNs.
Although the role of primary tumor lymphangiogenesis in facilitating lymphatic metastasis of BC cells (and other cancer cell types) has been studied extensively, lymphangiogenesis may also occur in premetastatic TDLNs. In this case nodal lymphatic vessels may be sufficient for LN metastasis, independent of primary tumor lymphangiogenesis.14 Multiple studies have revealed that chemokines and chemotactic signals expressed in LNs can actively direct BC migration to this organ (Figure 2). The chemokine receptors CXCR4 and CCR7 are both highly expressed on human BC cells.15 Expression of CXCL12, the ligand for CXCR4 and CCR7, is highest in normal human LNs compared with other organs that develop BC metastases. Blocking the CXCR4/CXCL12 interaction reduces the incidence of LN metastasis in murine studies. Lymphatic endothelial cells in TDLNs have been identified as a source of other chemotactic signals that promote the migration and entry of BC cells. For instance, IL-6 from human BC cells up-regulated the expression of chemokine (C-C motif) ligand (CCL) 5 in lymphatic endothelial cells.16 The formation of this chemotactic gradient promotes the migration of CCR5-expressing BC cells from primary tumors to LNs, which is blocked with maraviroc, a CCR5 inhibitor.16 Other inflammatory stimuli, including IL-1β and tumor necrosis factor-α, induce the expression of the CCL1 chemokine on lymphatic endothelial cells17 and promote the entry of CCR8-expressing BC cells into LNs. Disrupting the CCL1-CCR8 axis blocks the entry and formation of LN metastasis. Lymphatic endothelial cells in TDLNs of patients with BC have elevated expression of integrin α4β1, which may bind vascular cell adhesion molecule 1 on BC cells to promote their entry into TDLNs.14
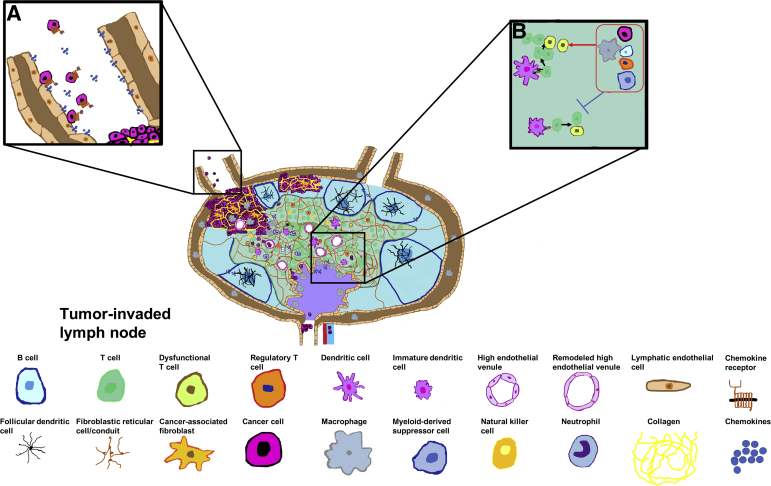
Figure 2.
Prometastatic and immune evasion mechanisms in lymph nodes. Inset A: Lymphatic endothelial cells actively recruit breast cancer cells to lymph nodes through chemokines that interact with chemokine receptors on breast cancer cells. Inset B: Cancer cells arrest dendritic cell maturation to limit the priming of antigen-specific T cells in tumor-draining lymph nodes. Inset B: Macrophages, regulatory T cells, myeloid-derived suppressor cells, cancer cells, and B cells (grouped in red boxed area) utilize different mechanisms to likely inhibit (blue symbol) T cell activation and promote T cell and natural killer cell dysfunction (red arrow points to dysfunctional T cells).
Initiation of Lymph Node Metastasis
The highly organized LN consists of three major sections surrounded by a fibrous capsule: the cortex, paracortex, and medulla (Figure 1). Afferent lymph enters the subcapsular sinus (SCS), a double layer of lymphatic endothelial cells that surrounds the LN cortex. Lymph from the SCS and cortical sinuses drains into the medulla, where it is emptied into the efferent lymphatic vessel. BC cells may passively drain through this route and exit to nearby LNs. However, some BC cells arriving in the afferent lymph may successfully colonize LNs. Sinusoidal macrophages in the SCS and medulla play a role in protection against metastatic colonization because a low frequency of sinusoidal macrophages is associated with the presence of BC metastasis in TDLNs.18 Furthermore, depletion of sinusoidal macrophages with anti–colony stimulation factor 1 receptor therapy enhances metastatic burden in LNs.19 The elevated expression of proteolytic enzymes in LNs with BC metastases may promote LN progression through the degradation of extracellular matrix,20 allowing BC cells to break through the SCS and penetrate deeper into the LN.
Modulation of Immune and Stromal Cells in Lymph Nodes
B cells
Beneath the SCS are B cells and follicular dendritic cells (DCs) within follicles. Although little is known about follicular DCs in TDLNs, it has been known for several decades that B cells accumulate in breast TDLNs.21 However, their role in the immune response to BC remains controversial. B cells have the capacity to play a critical role in anti-breast tumor immunity through their ability to present antigen and produce cytokines that shape T cell activation and differentiation.22 In addition, B cells secrete antibodies that recognize tumor-associated antigen.23 Yet, the infiltration of B cells into primary breast tumors has been associated with both positive and negative clinical outcomes.24 The protumor and anti-tumor impact of B cells in BC progression may be explained by subset differences; regulatory B cells predominately suppress anti-tumor T cells, whereas effector B cells act to limit cancer progression.
In clinical studies, the number of B cells in sentinel TDLNs of patients with BC was found to have prognostic significance. Increased B cell density was associated with improved disease-free survival25 and thus had a positive role in controlling cancer.
In murine studies, adoptive transfer of activated B cells from breast TDLNs indirectly reduces the formation of lung metastases in tumor-bearing hosts by stimulating anti-tumor T cell responses.26 In addition, antibodies from tumor-educated B cells are able to lyse BC cells through a complement-dependent mechanism.26 Moreover, activated B cells expressing FasL can directly engage its receptor Fas on BC cells to mediate killing.27
Compelling evidence suggests that B cells can, in contrast, exert a protumor effect by suppressing anti-tumor T cell responses. B cells promote the conversion of CD4 T cells into regulatory T cells (Tregs)28 ex vivo, and depletion of B cells decreases the number of Tregs in organs, including TDLNs.29 Together, these studies suggest that B cells expand Tregs, which, in turn, limit anti-tumor immunity.
The expression of granzyme B, a serine protease expressed by cytotoxic T cells and natural killer (NK) cells, has also been found in B cells within breast tumors30 and TDLNs.31 IL-21 up-regulates granzyme B in B cells from peripheral blood mononuclear cells, which limits T cell proliferation by degrading the T cell receptor ζ-chain, essential for effective signaling trough the T cell receptor.30 Similarly, B cells isolated from LNs of breast tumor–bearing mice strongly suppress the proliferation of CD4 and CD8 T cells.32 This suppression is more pronounced after enriching regulatory B cells in tumor-bearing animals with an anti-CD20 antibody.32 Together, these findings suggest that B cells may directly limit anti-tumor T cell proliferation.
Although B cells from TDLNs have been shown to produce antibodies against BC cell antigen,23,33 contrasting roles for such antibodies have been reported. As mentioned earlier, antibodies can lead to lysis of BC cells.26 Antibodies from murine nodal B cells directly target BC cells but surprisingly exert protumor effects.23 These tumor-promoting antibodies activate the heat shock protein family A member 4 glycoprotein on BC cells, leading to the up-regulation of the chemokine receptor CXCR4 in BC cells. In response to CXCL12 produced by LN stromal cells, CXCR4+ BC cells migrate to LNs. High levels of tumor and blood heat shock protein family A member 4 expressions are associated with increased LN metastasis and poor prognosis of patients with BC.23
Dendritic Cells
Conventional DCs migrate through lymphatic vessels and into the LN paracortex (Figure 1), where they, along with LN-resident DCs, orchestrate adaptive anti-tumor immunity through the presentation of tumor antigen to T cells. Pathologic and cellular analyses have shown an overall decline in the total number of DCs in sentinel and nonsentinel tumor-involved TDLNs.34,35 Independent of metastasis, the number of DCs in TDLNs is a strong predictor of disease-free survival. Furthermore, nodal metastases impair the antigen-presenting functions of DCs in LNs by arresting their maturation.36 Compared with noninvaded sentinel TDLNs and sentinel TDLNs with micrometastases, fewer CD208+ mature DCs are observed in the paracortex of sentinel TDLNs with breast macrometastases.37 Chang et al38 found fewer aggregates of DCs in tumor-involved TDLNs compared with healthy control LNs. Within the aggregates, only 30% of DCs were mature, as determined by CD83 expression, compared with 80% of DCs displaying maturation markers in healthy counterparts.38 Significantly fewer T cells are associated with immature DCs in tumor-involved TDLNs, suggesting that T cell activation may be impaired. More importantly, DC maturation in TDLNs correlates with the duration of disease-free survival in patients with BC.38
Van Pul et al35 found fewer activated conventional and plasmacytoid LN-resident DCs in TDLNs compared with migratory DCs from the same nodes. The presence of LN metastases caused a further decline in LN-resident DC activation markers. Interestingly, DC activation was more suppressed in patients with hormone receptor–negative tumors compared with those with hormone receptor–positive tumors.
T Cells
Naïve and central memory lymphocytes enter the LN paracortex across high endothelial venules (HEVs). The proximity of DCs and lymphocytes within the paracortex increases the probability of rare antigen-specific lymphocytes (eg, BC-specific T cells) finding their cognate antigen and becoming activated. Once activated, antigen-specific T cells exit LNs through the efferent lymphatic vessel and travel to sites of inflammation, such as primary breast tumors. Presence of CD8 T cells in certain molecular subtypes of primary breast tumors is associated with a good prognosis,39 likely because of their ability to control tumor growth and limit metastasis. Wang et al40 found that a small number of cancer cell mutations and low neoantigen burden in breast tumors correlated with lymphatic metastasis. Conversely, the absence of LN metastasis was associated with a higher mutation burden that corresponded to an enhanced innate and adaptive immune response across different BC subtypes. T cell receptor sequencing revealed higher frequencies of tumor-specific T cell clones in primary tumors from patients with breast cancer relative to matched LNs, providing genetic evidence that T cells migrate from TDLNs to primary tumors, where they expand. Relative to noninvolved LNs,41 LNs with metastases showed significantly more T cell receptor overlap with T cell clones found in primary tumors. The presence of tumor antigens derived from LN metastases may lead to an expansion of tumor-specific T cell clones in LNs. Alternatively, T cell clones expanded in the primary tumor may recirculate to LNs.
Overall, there is a significant decline in the number of T cells in tumor-involved sentinel TDLNs.25 However, BC cell invasion into LNs is associated with an increase in multiple subsets of Tregs.42, 43, 44 The accumulation of Tregs in sentinel TDLNs is an independent predictor of poor prognosis in patients with pathologic undetectable micrometastases42 and with pathologic confirmation of nodal metastases.44 Tregs isolated from both primary tumors and tumor-involved TDLNs maintain their suppressive ability, whereas conventional T cells from primary tumors lose the capacity to proliferate.44 These data suggest that Tregs, relative to conventional T cells, may better retain their functional capacity in the tumor microenvironment. Although multiple studies point to early T cell activation in sentinel TDLNs,35,44 the immune response in LNs shifts from type 1 helper T cell toward a protumor type 2 helper T cell cytokine profile in tumor-involved lymph nodes.35,43 A study found that on invasion of cancer cells into LNs, immune checkpoint expression on T cells remained stable or showed an increase.35 Pathways that regulate tumor progression, T cell anergy, and tolerance were up-regulated in CD45+ immune cells from node-positive patients compared with node-negative patients. CD39+CD8+ exhausted T cells were significantly increased in tumor-involved LNs of patients with BC compared with noninvaded LNs and peripheral blood45 and had an impaired production of tumor necrosis factor-α and IL-2 cytokines that are important for proper T cell effector activity.45
CD45+ cells in the blood and noninvolved LNs of patients with tumor-involved LNs showed down-regulation of immune-related pathways, suggesting that immunomodulatory effects on immune cells are systemic.46 The ability of T cells isolated from tumor-involved LNs to proliferate, produce cytokines, and exhibit cytotoxicity toward target cells ex vivo44,47 suggests the immunosuppressive tumor microenvironment in LNs may neutralize anti-tumor effector T cell function.
NK Cells
NK cells are innate immune cells that possess the cytotoxic capacity to kill cancer cells. Frazao et al,48 through the use of flow cytometry, found significant differences in TDLN NK cells compared with LNs from donors without BC. The number of nodal NK cells was similar between groups, but NK cells in tumor-involved TDLNs expressed higher levels of the inhibitory natural killer group 2 member A receptor, which was enhanced as metastatic disease progressed. Cell degranulation assays showed that NK cell lytic capabilities against BC cells were potentiated by IL-15. Interestingly, NK cells from TDLNs exhibited stronger killing capacity ex vivo than those from donor LNs without BC. Therefore, immunosuppressive molecules in the TDLN microenvironment may inhibit NK cell cytotoxicity, and therapies targeting natural killer group 2 member A49 may enhance NK cell cytotoxicity toward LN metastases.
Fibroblastic Reticular Cells
Fibroblastic reticular cells (FRCs) are specialized myofibroblasts that form a collagen-rich network in LNs.50 Subsets of FRCs exist in each region of the LN to maintain its structural integrity and to provide molecular signals for immune cell migration and survival.50 This complex network transports antigens from lymph to DCs in LNs.50 FRCs also coordinate the travel of lymphocytes and DCs to and within LNs.51
Despite their important functions in LN physiology, there are few reports on FRC involvement in BC LN metastasis. FRCs were significantly expanded in 4T1 murine BC TDLNs.52 Through single-cell RNA sequencing, Li et al53 revealed that the FRCs in mouse mammary tumor virus-polyoma middle tumor-antigen murine BC TDLNs were more metabolically active relative to normal LNs. This metabolic activity may contribute to the development of the premetastatic niche in TDLNs through lymph node remodeling caused by increased lymph flow. In xenograft and experimental BC models, FRCs were proposed to account for the increased collagen density in metastatic LNs,54 along with cancer-associated fibroblasts (CAFs). This finding is consistent with the elevation of collagen levels in metastatic LNs of patients with BC.55 FRCs may sequester BC cells in LNs and as a consequence facilitate the establishment of nodal tumors54 and further direct the movement of cancer cells within LNs, as shown in primary breast tumors.56
Lymph Node Metastases Recruit Additional Hematopoietic and Stromal Cells to Lymph Nodes
Macrophages
Macrophages are a major population of tumor-infiltrating immune cells and key regulators of BC growth and metastasis.57 High numbers of CD68+ tumor-associated macrophages (TAMs) in the primary site are predictive of poor survival in a large cohort of patients with BC.58 A high density of TAMs is also associated with LN metastasis and lymphatic vessel invasion.59 TAMs play an important role in lymphatic metastasis by direct incorporation into the lymphatic vasculature or by stimulating lymphangiogenesis, which provides a route for metastatic dissemination to LNs.60 In addition, TAMs associate with lymphatic endothelial cells to increase lymphatic vessel permeability and promote metastasis.61
Macrophages are also recruited to tumor-involved LNs and have been used as a diagnostic tool for assessing LN metastasis. Near-infrared imaging can distinguish tumor-involved LNs based on the accumulation of CD206+ macrophages.62 On positive axillary LN diagnosis, CD68+ macrophages are increased in noninvolved TDLNs of patients with BC.63
Macrophages in tumor-involved TDLNs become skewed toward a protumor phenotype. Recently, Piao et al64 showed that BC-derived exosomes promote LN metastasis by increasing the number of CD206+ protumor macrophages in the cortex of TDLNs. In addition, macrophages around the subcapsular and medullary sinuses express indoleamine 2,3-dioxygenase; their presence correlates with fewer CD8 T cells in LNs, consistent with the role of indoleamine 2,3-dioxygenase in inhibiting T cell proliferation.65 In an attempt to enhance their anti-tumor activity, sinus macrophages were activated with a toll-like receptor agonist.66 Activated sinus macrophages expressed proinflammatory cytokines, which correlated with increased CD4, CD8, and NK cells in TDLNs66 in a BC model. Although this mechanism of expansion is unclear, sinus macrophage activation is associated with reduced LN and lung metastases and improved survival of animals.66
Myeloid-Derived Suppressor Cells
Heterogeneous myeloid-derived suppressor cells (MDSCs) contribute to LN metastasis and aid in the inhibition of adaptive immune responses.67,68 The production of indoleamine 2,3-dioxygenase by MDSCs in murine primary breast tumors is associated with an increased incidence of LN metastasis.67 The MDSC population is increased in tumor-involved TDLNs of animals and human patients with BC,35,68 and animal studies suggest that CXCR2 is critical for MDSC recruitment to LNs.69 BC cells in close proximity to MDSCs in tumor-involved TDLNs show a mesenchymal-like appearance.68 Co-culture of BC cells with isolated CXCR2+ MDSCs promotes epithelial-to-mesenchymal transition and cancer cell proliferation.68 In vivo, co-injection of CXCR2+ MDSCs and BC cells in mice doubled the rate of LN metastasis and increased the size of nodal lesions compared to that in mice injected with cancer cells alone.68
Elevated MDSCs in tumor-involved TDLNs coincided with an increased number of nodal Tregs,67 impaired activation of LN-resident DC subsets, and T cells with limited effector function.35 Co-culture of MDSCs with T cells led to the up-regulation of immune checkpoint markers on CD4 and CD8 T cells in vitro, which suggests that MDSCs may directly promote T cell dysfunction.68
Cancer-Associated Fibroblasts
CAFs constitute a significant population of nonmalignant cells in primary breast tumors.70 CAF activation, as assessed by immunohistochemical staining with smooth muscle actin and fibroblast activation protein, shows a positive correlation with CD163+ protumor TAMs and LN metastasis in patients with BC.71
CAFs were found in a majority of tumor-involved LNs from a cohort of 43 patients with BC.72 Most studies of CAFs in LN metastases have been limited to immunohistochemical profiling. Histologically, CAFs in primary tumors and matched LN metastases expressed similar biological markers.73 The transcriptional profiles of CAFs from the primary tumor and tumor-involved LNs of patients with BC are also similar.74 RNA sequencing of four CAF subsets found in both primary tumors and tumor-involved LNs also shows transcriptional overlap.75 However, only two of the four CAF subsets identified by Pelon et al75 accumulate in tumor-involved LNs, but play important roles in metastatic progression. The CAF-S1 subset initiates epithelial-to-mesenchymal transition and provided migratory signals for BC cells, whereas the CAF-S4 cells are efficient at matrix remodeling, likely driving invasion of cancer cells into the LN parenchyma. Interestingly, CAF-S4 enrichment in LNs is prognostic for the development of distant metastases in patients with BC.
The Lymph Node Vasculature Supports Metastatic Growth and Dissemination
As indicated by a comparison of primary BC tumors and tumor-involved LNs, endothelial cell proliferation and tumor cell proliferation are tightly linked, suggesting that hypoxia-driven angiogenesis may sustain the growth of LN metastases.76 In contrast, neither tumor-involved LNs from animals nor patients with BC show increased blood vessel density compared with noninvolved LNs.77,78 Murine BC cells express markers of hypoxia on initial entry of cancer cells into the SCS.77 BC cells that invade deeper into the LN parenchyma neither express markers of hypoxia, nor have elevated levels of pro-angiogenic molecules relative to noninvolved TDLNs.77 Highly vascularized TDLNs may provide nutrients and oxygen for cancer cells that invade LNs. However, more studies are needed to understand and define the biological cues and mechanisms for the activation of angiogenesis in lymph nodes.
HEVs are specialized post-capillary venules that facilitate the trafficking of immune cells into LNs. The presence of ectopic HEVs in breast tumors is associated with lymphocyte infiltration and a favorable prognosis,79 although tumor-associated blood vessels have traditionally been studied in the context of aiding tumor growth and hematogenous metastasis.60 In TDLNs of patients with BC, HEV endothelial cells are proliferative and HEVs exhibit increased lumen diameter, independent of metastasis.80 A recent study found that HEV remodeling by estrogen receptor–positive, human epidermal growth factor receptor 2– metastatic breast cancer cells was associated with dysregulated FRCs in TDLNs; specifically, the expression of CCL21 was lost in FRCs around dilated HEVs.81 These results suggest that HEV and FRC remodeling may disrupt anti-tumor immunity in lymph nodes.
Imaging studies indicate the mechanism by which nodal breast metastases can further metastasize to distant organs.6,7 BC cells migrate toward HEVs in TDLNs, and their presence within HEVs suggests hematogenous spread from LNs. Interestingly, a doorway system, by which BC cells exit primary tumors and LNs, has been described.82 In this system, direct contact between a BC cell expressing the actin protein Mena, an angiogenic perivascular macrophage, and an endothelial cell facilitates intravasation of tumor cells into the blood circulation.
In patients with BC, lymphangiogenesis in tumor-involved sentinel TDLNs correlates with further metastasis to nonsentinel LNs.83 A BC xenograft model shows that lymphatic vessels develop within nodal lesions and that BC cells invade these intrametastatic lymphatic vessels to gain access to downstream LNs.84 As metastatic lesions progress, increased pressure is measured in sentinel TDLNs of patients with BC. Lymph flow is redirected to nonsentinel LNs when encountering higher pressure in tumor-involved sentinel TDLNs, and this rerouting may also contribute to metastasis beyond sentinel TDLNs.85
Breast Cancer Cells in Lymph Nodes and Beyond
It is unclear whether the microenvironment of LNs dictates the gene expression profile of LN metastases, as studies undertaken to distinguish primary BCs from matched tumor-involved LNs based on genetic signatures have yielded contradictory results. Most of such investigations have found no significant differences in gene expression between primary tumors and their matched tumor-involved LNs.86 Furthermore, the mutational burden of synchronous tumor-involved LNs is similar to that of primary tumors.87 However, some studies found a small number of molecular differences between primary and LN tumors.86,88 Of note, these experiments used bulk tissue to interrogate gene expression. Because of the cellular heterogeneity of tumors at the primary site and in LNs, single-cell RNA sequencing may allow improved resolution of cancer cell gene expression for comparing primary tumor and synchronous LN metastases.
A recent report suggests that metabolic plasticity promotes BC cell survival in LNs. Lee et al89 found that BC cells adapt to LNs by shifting their metabolism to fatty acid oxidation. Treatment with etomoxir, a pharmacologic inhibitor of fatty acid oxidation, suppresses the growth of BC cells in LNs.89
Clonal analysis of murine BC cells shows that multiple clones are present in tumor-involved TDLNs.77,90 BC cells isolated from TDLNs are able to metastasize and colonize distant organs more efficiently than cells from primary tumors, suggesting that aggressive cancer clones are enriched in LNs.91
A phylogenetic study of 16 patients with BC suggests that a quarter of these patients had distant breast cancer cell metastases that likely originated from axillary LN metastases.92 Interestingly, prognosis was worse in patients with distant metastases seeded by LN metastases compared with those seeded by primary tumors. In another phylogenetic analysis of patients with BC, five of seven patients with BC had LN metastases that were phylogenetically closer to at least one distant metastatic tumor.93 Whole exome sequencing revealed that LN metastases can evolve based on copy number alteration.5 In this study, multiple cancer cell clones from LNs were identified in bone marrow metastases.5 In contrast to these studies, Ullah et al94 found no evidence that LN metastases seeded distant metastases in a cohort of 20 patients with BC.
The clinical impact of nodal seeding on distant metastases is difficult to assess. Three randomized clinical trials (American College of Surgeons Oncology Group-Z0011, International Breast Cancer Study Group 23-01, and After Mapping of the Axilla: Radiotherapy Or Surgery)95, 96, 97 show, in patients with BC, that further axillary surgery beyond the sentinel TDLN does not provide additional clinical benefit to patients. Thus, potential residual disease in remaining LNs may be inconsequential to patient survival. However, patients with BC with positive regional LNs have higher rates of systemic metastasis compared with those with node-negative disease.98 Furthermore, patients with BC with LN micrometastasis (<2 mm) have a reduced incidence of distant metastasis compared with patients with macrometastasis.99 Several studies found that patients with extracapsular extension, the growth of cancer cells outside the LN capsule, have poor prognosis100 and thus patients with macroscopic extranodal disease may benefit from axillary LN dissection to prevent locoregional recurrence.
Concluding Remarks
LNs are critical for initiating anti-tumor immunity. Although multiple mechanisms of immune suppression have been described in premetastatic TDLNs, it is becoming more appreciated that lymphatic metastasis, promoted by LNs, induces additional changes within tumor-involved TDLNs. BC cells transform LNs into organs that resemble the microenvironment of primary tumors as the microenvironment of LN metastatic tumors features highly immunomodulatory populations, including myeloid cells, Tregs, and cancer cells themselves. The anti-tumor functions of immune populations in TDLNs appear further suppressed on cancer cell invasion. These data lead to a hypothesis that LNs are converted from a site of anti-tumor immunity to a source of systemic immunosuppression and distant metastasis. Although many similarities between the microenvironment of primary tumors and LN metastases exist, identifying and targeting molecular signals specific to LN metastases may improve the clinical outcome of node-positive patients with BC. Further studies are needed to investigate the cross talk between LN metastases and immune cells within the LN microenvironment and how each cell type in the LN tumor microenvironment may contribute to the metastatic outgrowth in TDLNs.
Because TDLNs remain a critical organ for efficacious immunotherapy during BC progression, reducing metastatic growth may preserve the structure and thus function of LNs to enhance local and systemic host immune responses against BC.
Footnotes
Tumor Microenvironment in Breast Cancer Theme Issue
Supported by National Cancer InstituteK22CA230315 (D.J.), American Cancer Society institutional research grant to Boston University (D.J.), Shamim and Ashraf Dahod Breast Cancer Research Center (D.J.), and METAvivor Early Career Investigator Award (D.J.).
Disclosures: None declared.
This article is a part of a review series on the role of the tumor microenvironment in breast cancer pathogenesis.
References
- 1.Redig A.J., McAllister S.S. Breast cancer as a systemic disease: a view of metastasis. J Intern Med. 2013;274:113–126. doi: 10.1111/joim.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ran S., Volk L., Hall K., Flister M.J. Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology. 2010;17:229–251. doi: 10.1016/j.pathophys.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson Y., Bergkvist L., Frisell J., de Boniface J. Long-term breast cancer survival in relation to the metastatic tumor burden in axillary lymph nodes. Breast Cancer Res Treat. 2018;171:359–369. doi: 10.1007/s10549-018-4820-0. [DOI] [PubMed] [Google Scholar]
- 4.Hellman S. Karnofsky Memorial Lecture: natural history of small breast cancers. J Clin Oncol. 1994;12:2229–2234. doi: 10.1200/JCO.1994.12.10.2229. [DOI] [PubMed] [Google Scholar]
- 5.Demeulemeester J., Kumar P., Moller E.K., Nord S., Wedge D.C., Peterson A., Mathiesen R.R., Fjelldal R., Zamani Esteki M., Theunis K., Fernandez Gallardo E., Grundstad A.J., Borgen E., Baumbusch L.O., Borresen-Dale A.L., White K.P., Kristensen V.N., Van Loo P., Voet T., Naume B. Tracing the origin of disseminated tumor cells in breast cancer using single-cell sequencing. Genome Biol. 2016;17:250. doi: 10.1186/s13059-016-1109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira E.R., Kedrin D., Seano G., Gautier O., Meijer E.F.J., Jones D., Chin S.M., Kitahara S., Bouta E.M., Chang J., Beech E., Jeong H.S., Carroll M.C., Taghian A.G., Padera T.P. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science. 2018;359:1403–1407. doi: 10.1126/science.aal3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown M., Assen F.P., Leithner A., Abe J., Schachner H., Asfour G., Bago-Horvath Z., Stein J.V., Uhrin P., Sixt M., Kerjaschki D. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science. 2018;359:1408–1411. doi: 10.1126/science.aal3662. [DOI] [PubMed] [Google Scholar]
- 8.Wculek S.K., Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528:413–417. doi: 10.1038/nature16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoshida T., Isaka N., Hagendoorn J., di Tomaso E., Chen Y.L., Pytowski B., Fukumura D., Padera T.P., Jain R.K. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res. 2006;66:8065–8075. doi: 10.1158/0008-5472.CAN-06-1392. [DOI] [PubMed] [Google Scholar]
- 10.Mohammed S.I., Torres-Luquis O., Zhou W., Lanman N.A., Espina V., Liotta L. Tumor-draining lymph secretome en route to the regional lymph node in breast cancer metastasis. Breast Cancer (Dove Med Press) 2020;12:57–67. doi: 10.2147/BCTT.S236168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia Y., Chen Y., Wang Q., Jayasinghe U., Luo X., Wei Q., Wang J., Xiong H., Chen C., Xu B., Hu W., Wang L., Zhao W., Zhou J. Exosome: emerging biomarker in breast cancer. Oncotarget. 2017;8:41717–41733. doi: 10.18632/oncotarget.16684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pang M.F., Georgoudaki A.M., Lambut L., Johansson J., Tabor V., Hagikura K., Jin Y., Jansson M., Alexander J.S., Nelson C.M., Jakobsson L., Betsholtz C., Sund M., Karlsson M.C., Fuxe J. TGF-beta1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene. 2016;35:748–760. doi: 10.1038/onc.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammed S.I., Torres-Luquis O., Walls E., Lloyd F. Lymph-circulating tumor cells show distinct properties to blood-circulating tumor cells and are efficient metastatic precursors. Mol Oncol. 2019;13:1400–1418. doi: 10.1002/1878-0261.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garmy-Susini B., Avraamides C.J., Desgrosellier J.S., Schmid M.C., Foubert P., Ellies L.G., Lowy A.M., Blair S.L., Vandenberg S.R., Datnow B., Wang H.Y., Cheresh D.A., Varner J. PI3Kalpha activates integrin alpha4beta1 to establish a metastatic niche in lymph nodes. Proc Natl Acad Sci U S A. 2013;110:9042–9047. doi: 10.1073/pnas.1219603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller A., Homey B., Soto H., Ge N., Catron D., Buchanan M.E., McClanahan T., Murphy E., Yuan W., Wagner S.N., Barrera J.L., Mohar A., Verastegui E., Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 16.Lee E., Fertig E.J., Jin K., Sukumar S., Pandey N.B., Popel A.S. Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nat Commun. 2014;5:4715. doi: 10.1038/ncomms5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das S., Sarrou E., Podgrabinska S., Cassella M., Mungamuri S.K., Feirt N., Gordon R., Nagi C.S., Wang Y., Entenberg D., Condeelis J., Skobe M. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J Exp Med. 2013;210:1509–1528. doi: 10.1084/jem.20111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiota T., Miyasato Y., Ohnishi K., Yamamoto-Ibusuki M., Yamamoto Y., Iwase H., Takeya M., Komohara Y. The clinical significance of CD169-positive lymph node macrophage in patients with breast cancer. PLoS One. 2016;11:e0166680. doi: 10.1371/journal.pone.0166680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollmen M., Karaman S., Schwager S., Lisibach A., Christiansen A.J., Maksimow M., Varga Z., Jalkanen S., Detmar M. G-CSF regulates macrophage phenotype and associates with poor overall survival in human triple-negative breast cancer. Oncoimmunology. 2016;5:e1115177. doi: 10.1080/2162402X.2015.1115177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniele A., Zito A.F., Giannelli G., Divella R., Asselti M., Mazzocca A., Paradiso A., Quaranta M. Expression of metalloproteinases MMP-2 and MMP-9 in sentinel lymph node and serum of patients with metastatic and non-metastatic breast cancer. Anticancer Res. 2010;30:3521–3527. [PubMed] [Google Scholar]
- 21.Eremin O., Roberts P., Plumb D., Stephens J.P. Human regional tumour lymph nodes: alterations of micro-architecture and lymphocyte subpopulations. Br J Cancer. 1980;41:62–72. doi: 10.1038/bjc.1980.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garaud S., Buisseret L., Solinas C., Gu-Trantien C., de Wind A., Van den Eynden G., Naveaux C., Lodewyckx J.N., Boisson A., Duvillier H., Craciun L., Ameye L., Veys I., Paesmans M., Larsimont D., Piccart-Gebhart M., Willard-Gallo K. Tumor infiltrating B-cells signal functional humoral immune responses in breast cancer. JCI Insight. 2019;5:e129641. doi: 10.1172/jci.insight.129641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu Y., Liu Y., Fu L., Zhai L., Zhu J., Han Y., Jiang Y., Zhang Y., Zhang P., Jiang Z., Zhang X., Cao X. Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG. Nat Med. 2019;25:312–322. doi: 10.1038/s41591-018-0309-y. [DOI] [PubMed] [Google Scholar]
- 24.Shen M., Wang J., Ren X. New insights into tumor-infiltrating B lymphocytes in breast cancer: clinical impacts and regulatory mechanisms. Front Immunol. 2018;9:470. doi: 10.3389/fimmu.2018.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blenman K.R.M., He T.F., Frankel P.H., Ruel N.H., Schwartz E.J., Krag D.N., Tan L.K., Yim J.H., Mortimer J.E., Yuan Y., Lee P.P. Sentinel lymph node B cells can predict disease-free survival in breast cancer patients. NPJ Breast Cancer. 2018;4:28. doi: 10.1038/s41523-018-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q., Lao X., Pan Q., Ning N., Yet J., Xu Y., Li S., Chang A.E. Adoptive transfer of tumor reactive B cells confers host T-cell immunity and tumor regression. Clin Cancer Res. 2011;17:4987–4995. doi: 10.1158/1078-0432.CCR-11-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao H., Lu L., Xia Y., Dai F., Wang Y., Bao Y., Lundy S.K., Ito F., Pan Q., Zhang X., Zheng F., Shu G., Fang B., Jiang J., Xia J., Huang S., Li Q., Chang A.E. Antitumor effector B cells directly kill tumor cells via the Fas/FasL pathway and are regulated by IL-10. Eur J Immunol. 2015;45:999–1009. doi: 10.1002/eji.201444625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olkhanud P.B., Damdinsuren B., Bodogai M., Gress R.E., Sen R., Wejksza K., Malchinkhuu E., Wersto R.P., Biragyn A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res. 2011;71:3505–3515. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tadmor T., Zhang Y., Cho H.M., Podack E.R., Rosenblatt J.D. The absence of B lymphocytes reduces the number and function of T-regulatory cells and enhances the anti-tumor response in a murine tumor model. Cancer Immunol Immunother. 2011;60:609–619. doi: 10.1007/s00262-011-0972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindner S., Dahlke K., Sontheimer K., Hagn M., Kaltenmeier C., Barth T.F., Beyer T., Reister F., Fabricius D., Lotfi R., Lunov O., Nienhaus G.U., Simmet T., Kreienberg R., Moller P., Schrezenmeier H., Jahrsdorfer B. Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells. Cancer Res. 2013;73:2468–2479. doi: 10.1158/0008-5472.CAN-12-3450. [DOI] [PubMed] [Google Scholar]
- 31.Arabpour M., Rasolmali R., Talei A.R., Mehdipour F., Ghaderi A. Granzyme B production by activated B cells derived from breast cancer-draining lymph nodes. Mol Immunol. 2019;114:172–178. doi: 10.1016/j.molimm.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Bodogai M., Lee Chang C., Wejksza K., Lai J., Merino M., Wersto R.P., Gress R.E., Chan A.C., Hesdorffer C., Biragyn A. Anti-CD20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer Res. 2013;73:2127–2138. doi: 10.1158/0008-5472.CAN-12-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDaniel J.R., Pero S.C., Voss W.N., Shukla G.S., Sun Y., Schaetzle S., Lee C.H., Horton A.P., Harlow S., Gollihar J., Ellefson J.W., Krag C.C., Tanno Y., Sidiropoulos N., Georgiou G., Ippolito G.C., Krag D.N. Identification of tumor-reactive B cells and systemic IgG in breast cancer based on clonal frequency in the sentinel lymph node. Cancer Immunol Immunother. 2018;67:729–738. doi: 10.1007/s00262-018-2123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohrt H.E., Nouri N., Nowels K., Johnson D., Holmes S., Lee P.P. Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Med. 2005;2:e284. doi: 10.1371/journal.pmed.0020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Pul K.M., Vuylsteke R., van de Ven R., Te Velde E.A., Rutgers E.J.T., van den Tol P.M., Stockmann H., de Gruijl T.D. Selectively hampered activation of lymph node-resident dendritic cells precedes profound T cell suppression and metastatic spread in the breast cancer sentinel lymph node. J Immunother Cancer. 2019;7:133. doi: 10.1186/s40425-019-0605-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poindexter N.J., Sahin A., Hunt K.K., Grimm E.A. Analysis of dendritic cells in tumor-free and tumor-containing sentinel lymph nodes from patients with breast cancer. Breast Cancer Res. 2004;6:R408–R415. doi: 10.1186/bcr808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mansfield A.S., Heikkila P., von Smitten K., Vakkila J., Leidenius M. Metastasis to sentinel lymph nodes in breast cancer is associated with maturation arrest of dendritic cells and poor co-localization of dendritic cells and CD8+ T cells. Virchows Arch. 2011;459:391–398. doi: 10.1007/s00428-011-1145-3. [DOI] [PubMed] [Google Scholar]
- 38.Chang A.Y., Bhattacharya N., Mu J., Setiadi A.F., Carcamo-Cavazos V., Lee G.H., Simons D.L., Yadegarynia S., Hemati K., Kapelner A., Ming Z., Krag D.N., Schwartz E.J., Chen D.Z., Lee P.P. Spatial organization of dendritic cells within tumor draining lymph nodes impacts clinical outcome in breast cancer patients. J Transl Med. 2013;11:242. doi: 10.1186/1479-5876-11-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu S., Lachapelle J., Leung S., Gao D., Foulkes W.D., Nielsen T.O. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14:R48. doi: 10.1186/bcr3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z., Liu W., Chen C., Yang X., Luo Y., Zhang B. Low mutation and neoantigen burden and fewer effector tumor infiltrating lymphocytes correlate with breast cancer metastasization to lymph nodes. Sci Rep. 2019;9:253. doi: 10.1038/s41598-018-36319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munson D.J., Egelston C.A., Chiotti K.E., Parra Z.E., Bruno T.C., Moore B.L., Nakano T.A., Simons D.L., Jimenez G., Yim J.H., Rozanov D.V., Falta M.T., Fontenot A.P., Reynolds P.R., Leach S.M., Borges V.F., Kappler J.W., Spellman P.T., Lee P.P., Slansky J.E. Identification of shared TCR sequences from T cells in human breast cancer using emulsion RT-PCR. Proc Natl Acad Sci U S A. 2016;113:8272–8277. doi: 10.1073/pnas.1606994113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura R., Sakakibara M., Nagashima T., Sangai T., Arai M., Fujimori T., Takano S., Shida T., Nakatani Y., Miyazaki M. Accumulation of regulatory T cells in sentinel lymph nodes is a prognostic predictor in patients with node-negative breast cancer. Eur J Cancer. 2009;45:2123–2131. doi: 10.1016/j.ejca.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 43.Faghih Z., Erfani N., Haghshenas M.R., Safaei A., Talei A.R., Ghaderi A. Immune profiles of CD4+ lymphocyte subsets in breast cancer tumor draining lymph nodes. Immunol Lett. 2014;158:57–65. doi: 10.1016/j.imlet.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 44.Nunez N.G., Tosello Boari J., Ramos R.N., Richer W., Cagnard N., Anderfuhren C.D., Niborski L.L., Bigot J., Meseure D., De La Rochere P., Milder M., Viel S., Loirat D., Perol L., Vincent-Salomon A., Sastre-Garau X., Burkhard B., Sedlik C., Lantz O., Amigorena S., Piaggio E. Tumor invasion in draining lymph nodes is associated with Treg accumulation in breast cancer patients. Nat Commun. 2020;11:3272. doi: 10.1038/s41467-020-17046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Canale F.P., Ramello M.C., Nunez N., Araujo Furlan C.L., Bossio S.N., Gorosito Serran M., Tosello Boari J., Del Castillo A., Ledesma M., Sedlik C., Piaggio E., Gruppi A., Acosta Rodriguez E.A., Montes C.L. CD39 expression defines cell exhaustion in tumor-infiltrating CD8(+) T cells. Cancer Res. 2018;78:115–128. doi: 10.1158/0008-5472.CAN-16-2684. [DOI] [PubMed] [Google Scholar]
- 46.Zuckerman N.S., Yu H., Simons D.L., Bhattacharya N., Carcamo-Cavazos V., Yan N., Dirbas F.M., Johnson D.L., Schwartz E.J., Lee P.P. Altered local and systemic immune profiles underlie lymph node metastasis in breast cancer patients. Int J Cancer. 2013;132:2537–2547. doi: 10.1002/ijc.27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egelston C.A., Avalos C., Tu T.Y., Simons D.L., Jimenez G., Jung J.Y., Melstrom L., Margolin K., Yim J.H., Kruper L., Mortimer J., Lee P.P. Human breast tumor-infiltrating CD8(+) T cells retain polyfunctionality despite PD-1 expression. Nat Commun. 2018;9:4297. doi: 10.1038/s41467-018-06653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frazao A., Messaoudene M., Nunez N., Dulphy N., Roussin F., Sedlik C., Zitvogel L., Piaggio E., Toubert A., Caignard A. CD16(+)NKG2A(high) natural killer cells infiltrate breast cancer-draining lymph nodes. Cancer Immunol Res. 2019;7:208–218. doi: 10.1158/2326-6066.CIR-18-0085. [DOI] [PubMed] [Google Scholar]
- 49.Andre P., Denis C., Soulas C., Bourbon-Caillet C., Lopez J., Arnoux T., Blery M., Bonnafous C., Gauthier L., Morel A., Rossi B., Remark R., Breso V., Bonnet E., Habif G., Guia S., Lalanne A.I., Hoffmann C., Lantz O., Fayette J., Boyer-Chammard A., Zerbib R., Dodion P., Ghadially H., Jure-Kunkel M., Morel Y., Herbst R., Narni-Mancinelli E., Cohen R.B., Vivier E. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175:1731–1743.e13. doi: 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fletcher A.L., Acton S.E., Knoblich K. Lymph node fibroblastic reticular cells in health and disease. Nat Rev Immunol. 2015;15:350–361. doi: 10.1038/nri3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Link A., Vogt T.K., Favre S., Britschgi M.R., Acha-Orbea H., Hinz B., Cyster J.G., Luther S.A. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 52.Commerford C.D., Dieterich L.C., He Y., Hell T., Montoya-Zegarra J.A., Noerrelykke S.F., Russo E., Rocken M., Detmar M. Mechanisms of tumor-induced lymphovascular niche formation in draining lymph nodes. Cell Rep. 2018;25:3554–3563.e4. doi: 10.1016/j.celrep.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y.L., Chen C.H., Chen J.Y., Lai Y.S., Wang S.C., Jiang S.S., Hung W.C. Single-cell analysis reveals immune modulation and metabolic switch in tumor-draining lymph nodes. Oncoimmunology. 2020;9:1830513. doi: 10.1080/2162402X.2020.1830513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rizwan A., Bulte C., Kalaichelvan A., Cheng M., Krishnamachary B., Bhujwalla Z.M., Jiang L., Glunde K. Metastatic breast cancer cells in lymph nodes increase nodal collagen density. Sci Rep. 2015;5:10002. doi: 10.1038/srep10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kakkad S.M., Solaiyappan M., Argani P., Sukumar S., Jacobs L.K., Leibfritz D., Bhujwalla Z.M., Glunde K. Collagen I fiber density increases in lymph node positive breast cancers: pilot study. J Biomed Opt. 2012;17:116017. doi: 10.1117/1.JBO.17.11.116017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Provenzano P.P., Eliceiri K.W., Campbell J.M., Inman D.R., White J.G., Keely P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Condeelis J., Pollard J.W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 58.Mahmoud S.M., Lee A.H., Paish E.C., Macmillan R.D., Ellis I.O., Green A.R. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol. 2012;65:159–163. doi: 10.1136/jclinpath-2011-200355. [DOI] [PubMed] [Google Scholar]
- 59.Yuan Z.Y., Luo R.Z., Peng R.J., Wang S.S., Xue C. High infiltration of tumor-associated macrophages in triple-negative breast cancer is associated with a higher risk of distant metastasis. Onco Targets Ther. 2014;7:1475–1480. doi: 10.2147/OTT.S61838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones D. Parallels of resistance between angiogenesis and lymphangiogenesis inhibition in cancer therapy. Cells. 2020;9:762. doi: 10.3390/cells9030762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Evans R., Flores-Borja F., Nassiri S., Miranda E., Lawler K., Grigoriadis A., Monypenny J., Gillet C., Owen J., Gordon P., Male V., Cheung A., Noor F., Barber P., Marlow R., Francesch-Domenech E., Fruhwirth G., Squadrito M., Vojnovic B., Tutt A., Festy F., De Palma M., Ng T. Integrin-mediated macrophage adhesion promotes lymphovascular dissemination in breast cancer. Cell Rep. 2019;27:1967–1978.e4. doi: 10.1016/j.celrep.2019.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang C., Yu X., Gao L., Zhao Y., Lai J., Lu D., Bao R., Jia B., Zhong L., Wang F., Liu Z. Noninvasive imaging of CD206-positive M2 macrophages as an early biomarker for post-chemotherapy tumor relapse and lymph node metastasis. Theranostics. 2017;7:4276–4288. doi: 10.7150/thno.20999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lopez C., Bosch R., Orero G., Korzynska A., Garcia-Rojo M., Bueno G., Fernandez-Carrobles M.D.M., Gibert-Ramos A., Roszkowiak L., Callau C., Fontoura L., Salvado M.T., Alvaro T., Jaen J., Roso-Llorach A., Llobera M., Gil J., Onyos M., Plancoulaine B., Baucells J., Lejeune M. The immune response in nonmetastatic axillary lymph nodes is associated with the presence of axillary metastasis and breast cancer patient outcome. Am J Pathol. 2020;190:660–673. doi: 10.1016/j.ajpath.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Piao Y.J., Kim H.S., Hwang E.H., Woo J., Zhang M., Moon W.K. Breast cancer cell-derived exosomes and macrophage polarization are associated with lymph node metastasis. Oncotarget. 2018;9:7398–7410. doi: 10.18632/oncotarget.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mansfield A.S., Heikkila P.S., Vaara A.T., von Smitten K.A., Vakkila J.M., Leidenius M.H. Simultaneous Foxp3 and IDO expression is associated with sentinel lymph node metastases in breast cancer. BMC Cancer. 2009;9:231. doi: 10.1186/1471-2407-9-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu J., Xu J., Li M., Zhang Y., Yi H., Chen J., Dong L., Zhang J., Huang Z. Targeting lymph node sinus macrophages to inhibit lymph node metastasis. Mol Ther Nucleic Acids. 2019;16:650–662. doi: 10.1016/j.omtn.2019.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu J., Du W., Yan F., Wang Y., Li H., Cao S., Yu W., Shen C., Liu J., Ren X. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190:3783–3797. doi: 10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
- 68.Zhu H., Gu Y., Xue Y., Yuan M., Cao X., Liu Q. CXCR2(+) MDSCs promote breast cancer progression by inducing EMT and activated T cell exhaustion. Oncotarget. 2017;8:114554–114567. doi: 10.18632/oncotarget.23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen J.Y., Lai Y.S., Chu P.Y., Chan S.H., Wang L.H., Hung W.C. Cancer-derived VEGF-C increases chemokine production in lymphatic endothelial cells to promote CXCR2-dependent cancer invasion and MDSC recruitment. Cancers (Basel) 2019;11:1120. doi: 10.3390/cancers11081120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Houthuijzen J.M., Jonkers J. Cancer-associated fibroblasts as key regulators of the breast cancer tumor microenvironment. Cancer Metastasis Rev. 2018;37:577–597. doi: 10.1007/s10555-018-9768-3. [DOI] [PubMed] [Google Scholar]
- 71.Zhou J., Wang X.H., Zhao Y.X., Chen C., Xu X.Y., Sun Q., Wu H.Y., Chen M., Sang J.F., Su L., Tang X.Q., Shi X.B., Zhang Y., Yu Q., Yao Y.Z., Zhang W.J. Cancer-associated fibroblasts correlate with tumor-associated macrophages infiltration and lymphatic metastasis in triple negative breast cancer patients. J Cancer. 2018;9:4635–4641. doi: 10.7150/jca.28583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rozenchan P.B., Pasini F.S., Roela R.A., Katayama M.L., Mundim F.G., Brentani H., Lyra E.C., Brentani M.M. Specific upregulation of RHOA and RAC1 in cancer-associated fibroblasts found at primary tumor and lymph node metastatic sites in breast cancer. Tumour Biol. 2015;36:9589–9597. doi: 10.1007/s13277-015-3727-1. [DOI] [PubMed] [Google Scholar]
- 73.Mundim F.G., Pasini F.S., Nonogaki S., Rocha R.M., Soares F.A., Brentani M.M., Logullo A.F. Breast carcinoma-associated fibroblasts share similar biomarker profiles in matched lymph node metastasis. Appl Immunohistochem Mol Morphol. 2016;24:712–720. doi: 10.1097/PAI.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 74.Del Valle P.R., Milani C., Brentani M.M., Katayama M.L., de Lyra E.C., Carraro D.M., Brentani H., Puga R., Lima L.A., Rozenchan P.B., Nunes Bdos S., Goes J.C., Azevedo Koike Folgueira M.A. Transcriptional profile of fibroblasts obtained from the primary site, lymph node and bone marrow of breast cancer patients. Genet Mol Biol. 2014;37:480–489. doi: 10.1590/s1415-47572014000400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pelon F., Bourachot B., Kieffer Y., Magagna I., Mermet-Meillon F., Bonnet I., Costa A., Givel A.M., Attieh Y., Barbazan J., Bonneau C., Fuhrmann L., Descroix S., Vignjevic D., Silberzan P., Parrini M.C., Vincent-Salomon A., Mechta-Grigoriou F. Cancer-associated fibroblast heterogeneity in axillary lymph nodes drives metastases in breast cancer through complementary mechanisms. Nat Commun. 2020;11:404. doi: 10.1038/s41467-019-14134-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van den Eynden G.G., Van der Auwera I., Van Laere S.J., Colpaert C.G., Turley H., Harris A.L., van Dam P., Dirix L.Y., Vermeulen P.B., Van Marck E.A. Angiogenesis and hypoxia in lymph node metastases is predicted by the angiogenesis and hypoxia in the primary tumour in patients with breast cancer. Br J Cancer. 2005;93:1128–1136. doi: 10.1038/sj.bjc.6602828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jeong H.S., Jones D., Liao S., Wattson D.A., Cui C.H., Duda D.G., Willett C.G., Jain R.K., Padera T.P. Investigation of the lack of angiogenesis in the formation of lymph node metastases. J Natl Cancer Inst. 2015;107:djv155. doi: 10.1093/jnci/djv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arapandoni-Dadioti P., Giatromanolaki A., Trihia H., Harris A.L., Koukourakis M.I. Angiogenesis in ductal breast carcinoma: comparison of microvessel density between primary tumour and lymph node metastasis. Cancer Lett. 1999;137:145–150. doi: 10.1016/s0304-3835(98)00343-7. [DOI] [PubMed] [Google Scholar]
- 79.Martinet L., Garrido I., Filleron T., Le Guellec S., Bellard E., Fournie J.J., Rochaix P., Girard J.P. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71:5678–5687. doi: 10.1158/0008-5472.CAN-11-0431. [DOI] [PubMed] [Google Scholar]
- 80.Qian C.N., Berghuis B., Tsarfaty G., Bruch M., Kort E.J., Ditlev J., Tsarfaty I., Hudson E., Jackson D.G., Petillo D., Chen J., Resau J.H., Teh B.T. Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 2006;66:10365–10376. doi: 10.1158/0008-5472.CAN-06-2977. [DOI] [PubMed] [Google Scholar]
- 81.Bekkhus T., Martikainen T., Olofsson A., Franzen Boger M., Vasiliu Bacovia D., Warnberg F., Ulvmar M.H. Remodeling of the lymph node high endothelial venules reflects tumor invasiveness in breast cancer and is associated with dysregulation of perivascular stromal cells. Cancers (Basel) 2021;13:211. doi: 10.3390/cancers13020211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ginter P.S., Karagiannis G.S., Entenberg D., Lin Y., Condeelis J., Jones J.G., Oktay M.H. Tumor microenvironment of metastasis (TMEM) doorways are restricted to the blood vessel endothelium in both primary breast cancers and their lymph node metastases. Cancers (Basel) 2019;11:1507. doi: 10.3390/cancers11101507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van den Eynden G.G., Vandenberghe M.K., van Dam P.J., Colpaert C.G., van Dam P., Dirix L.Y., Vermeulen P.B., Van Marck E.A. Increased sentinel lymph node lymphangiogenesis is associated with nonsentinel axillary lymph node involvement in breast cancer patients with a positive sentinel node. Clin Cancer Res. 2007;13:5391–5397. doi: 10.1158/1078-0432.CCR-07-1230. [DOI] [PubMed] [Google Scholar]
- 84.Kerjaschki D., Bago-Horvath Z., Rudas M., Sexl V., Schneckenleithner C., Wolbank S. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J Clin Invest. 2011;121:2000–2012. doi: 10.1172/JCI44751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nathanson S.D., Mahan M. Sentinel lymph node pressure in breast cancer. Ann Surg Oncol. 2011;18:3791–3796. doi: 10.1245/s10434-011-1796-y. [DOI] [PubMed] [Google Scholar]
- 86.Kroigard A.B., Larsen M.J., Thomassen M., Kruse T.A. Molecular concordance between primary breast cancer and matched metastases. Breast J. 2016;22:420–430. doi: 10.1111/tbj.12596. [DOI] [PubMed] [Google Scholar]
- 87.Yates L.R., Knappskog S., Wedge D., Farmery J.H.R., Gonzalez S., Martincorena I., Alexandrov L.B., Van Loo P., Haugland H.K., Lilleng P.K., Gundem G., Gerstung M., Pappaemmanuil E., Gazinska P., Bhosle S.G., Jones D., Raine K., Mudie L., Latimer C., Sawyer E., Desmedt C., Sotiriou C., Stratton M.R., Sieuwerts A.M., Lynch A.G., Martens J.W., Richardson A.L., Tutt A., Lonning P.E., Campbell P.J. Genomic evolution of breast cancer metastasis and relapse. Cancer Cell. 2017;32:169–184.e7. doi: 10.1016/j.ccell.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Srour M.K., Gao B., Dadmanesh F., Carlson K., Qu Y., Deng N., Cui X., Giuliano A.E. Gene expression comparison between primary triple-negative breast cancer and paired axillary and sentinel lymph node metastasis. Breast J. 2020;26:904–910. doi: 10.1111/tbj.13684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee C.K., Jeong S.H., Jang C., Bae H., Kim Y.H., Park I., Kim S.K., Koh G.Y. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science. 2019;363:644–649. doi: 10.1126/science.aav0173. [DOI] [PubMed] [Google Scholar]
- 90.Wagenblast E., Soto M., Gutierrez-Angel S., Hartl C.A., Gable A.L., Maceli A.R., Erard N., Williams A.M., Kim S.Y., Dickopf S., Harrell J.C., Smith A.D., Perou C.M., Wilkinson J.E., Hannon G.J., Knott S.R. A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature. 2015;520:358–362. doi: 10.1038/nature14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang S.C., Wei P.C., Hwang-Verslues W.W., Kuo W.H., Jeng Y.M., Hu C.M., Shew J.Y., Huang C.S., Chang K.J., Lee E.Y., Lee W.H. TGF-beta1 secreted by Tregs in lymph nodes promotes breast cancer malignancy via up-regulation of IL-17RB. EMBO Mol Med. 2017;9:1660–1680. doi: 10.15252/emmm.201606914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Venet D., Fimereli D., Rothe F., Boeckx B., Maetens M., Majjaj S., Rouas G., Capra M., Bonizzi G., Contaldo F., Galant C., Piccart M., Pruneri G., Larsimont D., Lambrechts D., Desmedt C., Sotiriou C. Phylogenetic reconstruction of breast cancer reveals two routes of metastatic dissemination associated with distinct clinical outcome. EBioMedicine. 2020;56:102793. doi: 10.1016/j.ebiom.2020.102793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leslie P.L., Chao Y.L., Tsai Y.H., Ghosh S.K., Porrello A., Van Swearingen A.E.D., Harrison E.B., Cooley B.C., Parker J.S., Carey L.A., Pecot C.V. Histone deacetylase 11 inhibition promotes breast cancer metastasis from lymph nodes. Nat Commun. 2019;10:4192. doi: 10.1038/s41467-019-12222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ullah I., Karthik G.M., Alkodsi A., Kjallquist U., Stalhammar G., Lovrot J., Martinez N.F., Lagergren J., Hautaniemi S., Hartman J., Bergh J. Evolutionary history of metastatic breast cancer reveals minimal seeding from axillary lymph nodes. J Clin Invest. 2018;128:1355–1370. doi: 10.1172/JCI96149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Olson J.A., Jr., McCall L.M., Beitsch P., Whitworth P.W., Reintgen D.S., Blumencranz P.W., Leitch A.M., Saha S., Hunt K.K., Giuliano A.E., American College of Surgeons Oncology Group Trials Z0010 and Z0011 Impact of immediate versus delayed axillary node dissection on surgical outcomes in breast cancer patients with positive sentinel nodes: results from American College of Surgeons Oncology Group Trials Z0010 and Z0011. J Clin Oncol. 2008;26:3530–3535. doi: 10.1200/JCO.2007.15.5630. [DOI] [PubMed] [Google Scholar]
- 96.Galimberti V., Cole B.F., Zurrida S., Viale G., Luini A., Veronesi P., Baratella P., Chifu C., Sargenti M., Intra M., Gentilini O., Mastropasqua M.G., Mazzarol G., Massarut S., Garbay J.R., Zgajnar J., Galatius H., Recalcati A., Littlejohn D., Bamert M., Colleoni M., Price K.N., Regan M.M., Goldhirsch A., Coates A.S., Gelber R.D., Veronesi U., International Breast Cancer Study Group Trial 23-01 investigators Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14:297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Donker M., van Tienhoven G., Straver M.E., Meijnen P., van de Velde C.J., Mansel R.E., Cataliotti L., Westenberg A.H., Klinkenbijl J.H., Orzalesi L., Bouma W.H., van der Mijle H.C., Nieuwenhuijzen G.A., Veltkamp S.C., Slaets L., Duez N.J., de Graaf P.W., van Dalen T., Marinelli A., Rijna H., Snoj M., Bundred N.J., Merkus J.W., Belkacemi Y., Petignat P., Schinagl D.A., Coens C., Messina C.G., Bogaerts J., Rutgers E.J. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15:1303–1310. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.David Nathanson S., Leonard-Murali S., Burmeister C., Susick L., Baker P. Clinicopathological evaluation of the potential anatomic pathways of systemic metastasis from primary breast cancer suggests an orderly spread through the regional lymph nodes. Ann Surg Oncol. 2020;27:4810–4818. doi: 10.1245/s10434-020-08904-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Veronesi U., Viale G., Paganelli G., Zurrida S., Luini A., Galimberti V., Veronesi P., Intra M., Maisonneuve P., Zucca F., Gatti G., Mazzarol G., De Cicco C., Vezzoli D. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251:595–600. doi: 10.1097/SLA.0b013e3181c0e92a. [DOI] [PubMed] [Google Scholar]
- 100.Yang X., Ma X., Yang W., Shui R. Clinical significance of extranodal extension in sentinel lymph node positive breast cancer. Sci Rep. 2020;10:14684. doi: 10.1038/s41598-020-71594-7. [DOI] [PMC free article] [PubMed] [Google Scholar]