Abstract
Background: We aimed to evaluate atrial fibrillation occurrence, reasons for healthcare visits, mortality, causes of death and examined patterns by relative deprivation in the UK.
Methods: To study the atrial fibrillation (AF) incidence, mortality and case-fatality, we implemented a prospective cohort study with the linked electronic health records of 5.6 million population in the United Kingdom Clinical Practice Research Datalink from 1998 to 2016. A matched case-control study was used to investigate causes of hospitalisation and death comparing individuals with and without incident AF.
Results: During a median follow-up of 10.3 years, 199,433(3.6%) patients developed incident AF. Increased risk of hospitalisation for heart failure, cardiovascular conditions and infection was present among patients who later developed AF. Following an AF diagnosis, patients were frequently admitted to the hospital for heart failure, lower respiratory tract infection, chronic obstructive pulmonary disease and ischemic heart disease. One in 5 AF patients died during the first year after diagnosis, and the mortality increased to 42.7% at the fifth year. The excess deaths in AF cases compared to controls may result from cardiovascular diseases, infection and metabolic disorders. Individuals from areas with higher deprivation in socioeconomic and living status had both higher AF incidence and fatality.
Interpretation: We observed an elevated risk of hospitalisation for cardiovascular or respiratory diseases among incident AF patients, and the considerable disparity in AF burden by socioeconomic deprivation informs priorities for prevention and provision of patient care.
Funding: The study was supported by the GlaxoSmithKline, University College London Hospital and National Institute for Health Research. The funders did not have any role in study design, data collection, data analysis, interpretation, and writing of the report.
Keywords: Atrial Fibrillation, Electronic Health Records, healthcare contacts, epidemiology, cause of death, health inequality
Research in Context.
We searched PubMed for studies published between March 21, 2001, and March 21, 2021, that included “atrial fibrillation” and “Electronic health records” in their title. References from systematic reviews and clinical practice guidelines and relevant studies recommended by experts were also reviewed. We found no study simultaneously investigating atrial fibrillation (AF) epidemiology, healthcare utilisation and outcomes. Evidence from previous AF epidemiology studies frequently referred to limitations in restricted populations or design.
Added value of this study
The study provides comprehensive evidence for the AF burden on population health and healthcare utilisation. We found approximately two in five AF patients had three or more comorbidities at the time of diagnosis. The leading prevalent comorbidities were hypertension, heart failure and chronic kidney disease, and the main reasons for repeated hospitalisations prior to AF diagnosis were ischaemic heart disease, lower respiratory tract infection and heart failure, whereas patients frequently admitted to the hospital after diagnosis for AF and cardiopulmonary conditions. Excess deaths among AF cases were mainly due to conditions involving the circulatory and digestive system, metabolic disorders, and infection. Our findings are based on population electronic health records derived from routine care in the country. The results may hence be applicable to the general clinical practice in comparable populations.
Implications of all the available evidence
The repeated hospitalisations for ischemic heart disease and heart failure in a given patient should draw the physicians attention to the likelihood of ongoing undiagnosed AF. After AF diagnosis, it is important to manage AF together with the related cardiovascular comorbidities, as these conditions continue to be the main reasons for hospitalisation and the leading cause of death among AF patients. At diagnosis, AF patients from more deprived areas had more comorbidities, despite being younger than those from wealthy areas. We found that individuals from areas with higher deprivation in socioeconomic and living status had both higher AF incidence and case fatality. The discrepancy in AF incidence and mortality by disparity warrants targeted prevention strategies, health-care resource planning and utilisation in AF management of the general population.
Alt-text: Unlabelled box
1. Background
Atrial fibrillation (AF) is the most frequent cardiac arrhythmia worldwide, with 37.6 million cases [1] and an attributable 287,200 deaths in 2017 [2]. In the UK, there are an estimated 1.5 million individuals living with AF [3], similar to the number of people with MI and exceeding those of cerebrovascular diseases and heart failure in 2020 [4, 5]. Despite the high cost of AF management [6, 7], little is known of the healthcare utilisation of newly diagnosed AF.
Insights from the healthcare contacts of AF population may facilitate improvements in disease prevention and treatment strategies, such as early detection and screening [8], risk factor and comorbidities management and prediction of subsequent outcomes. Evidence from previous AF epidemiology studies may be limited due to study design and that the population is based on regional cohorts [[9], [10], [11], [12]], surveys [13, 14], or trials [15, 16]. Population registries have focused on risk factors and subsequent diseases [17, 18]. A comprehensive study investigating AF epidemiology, healthcare utilisation and outcomes remains absent.
Nationwide electronic health records (EHR) provide a unique opportunity to investigate the AF progression and patient journey [19], from clinical predisposition to prognosis, in the general population receiving usual care. Thus, using the linked EHR of primary, secondary care and vital status from 5.6 million individuals in England, we assessed the AF incidence, co-morbidities, reasons for healthcare contacts, mortality and causes of death in AF patients. The study aims to provide evidence based on population data on the state of AF in the population for the development of more comprehensive and effective care strategies.
2. Method
2.1. Data sources
The Clinical Practice Research Datalink (CPRD) was established in 1987 [20] and as of 2018 includes 7,998,501 patients in the UK with linked data of primary care consultation, hospital data (Hospital Episodes Statistics, HES), national cancer registry (National Cancer Intelligence Network) and death registry data (Office for National Statistics, ONS) [21, 22]. The data are generally representative of the age, gender and geographic distribution of the UK population [20, 23]. Previous validation studies of the UK EHR showed high quality and completeness of clinical information recorded in the data [21, 22, 24]. The Medicines and Healthcare products Regulatory Agency Independent Scientific Advisory Committee [17_205], under Section 251 of the National Health Service (NHS) Social Care Act 2006 approved the data use for the present study. The study followed the REporting of studies Conducted using Observational Routinely-collected health Data recommendations [25].
2.2. Study population and design
We implemented two study designs, first a prospective cohort study to assess AF incidence and second a matched case-control study for mortality, cause of death and healthcare contacts.
2.2.1. Population-wide cohort for incidence, case-fatality, risk factors and comorbidities
In the population-wide prospective cohort, we identified individuals aged 18 years or older that had been registered in the current primary care practice for at least one year. The study period was between 1 January 1998 and 31 May 2016, and individuals were excluded if they had a prior history of AF before study entry. Follow-up ceased for the following reasons: death, the end date of registration with the practice, last day of the general practice data collection or the end of the study period (31 May 2016).
2.3. Incidence
Atrial fibrillation was defined from the International Classification of Diseases (ICD), tenth revision as I48 from HES and Read codes G573400, G573500, 3272.00, G573000, G573300, G573.00, G573z00 from CPRD. Based on the definition, AF cases included a minor proportion of atrial flutter [26, 27]. The definition was developed and tested in cardiovascular disease research using linked bespoke studies and electronic health records platform (https://caliberresearch.org/portal) [28]. Previous research has shown high validity and completeness of the disease definition in AF [[29], [30], [31]] and other conditions [30]. Age-specific and sex-specific cumulative incidence were obtained by dividing the number of incident cases by disease-free eligible individuals in the cohort. Alternatively, in survival analyses, the incidence rate was defined as time to initial diagnosis of AF.
2.4. Risk factors
We used the Index of Multiple Deprivation (IMD) 2015 quintile to describe socioeconomic status, with a higher quintile representing the more deprived areas [32]. For incident AF cases, we reported 13 common chronic conditions associated with AF reported in the literature [14, [33], [34], [35], [36]], or with high prevalence observed in the study cohort: hypertension, diabetes, valvular disease, hyperthyroidism, asthma, angina, ischemic heart disease (including unstable angina and acute myocardial infarction), heart failure, stroke, cancer, chronic kidney disease, chronic obstructive pulmonary disease and dementia. Patients without a diagnosis were assumed to be free from that condition. We reported the proportion of individuals with a diagnosis recorded in their primary care or hospital admissions, before their initial diagnosis of AF. CHA₂DS₂-VASc Score was calculated [37]. Diagnosis code lists for each condition were adapted from the CALIBER code repository [28] (Supplementary table S1).
2.4.1. Matched case-control for reasons for clinical visits, mortality risk and cause of death
For each incident AF patient, a matched control was selected according to the sex and age at the index date, which was defined as cohort entry date for controls and the initial AF diagnosis for individuals with AF.
2.5. Cause of healthcare contacts
We investigated the primary diagnoses at general practice (GP) consultation and hospitalisation within five years before and after the diagnosis date in individuals with AF and their age and sex-matched controls. For each person, the most frequent GP visits recorded in CPRD and primary diagnosis for hospital admissions documented in HES were identified at year five, bi-yearly intervals from year two to five, and yearly intervals. Subsequently, we summarised the top reasons for clinical visits pre and post index date.
2.6. Mortality
We identified death, date of death and causes of death from the Office of National Statistics (ONS) records. The cumulative case-fatality proportion was defined as the percent of deaths among all incidence AF cases. Rates of death among AF patients and controls were compared at 30-day, 1-year and 5-year.
2.7. Statistical analyses
Baseline characteristics were presented among AF patients and matched controls. We reported frequencies (%) for categorical data and means with standard deviation for continuous data, and chi- square and t tests were used to examine the difference between sex and socioeconomic categories. The observed incidence and cumulative case-fatality were reported and then standardised to the 2013 European Standard Population [31] by five-year age bands and sex. The difference in the rate of death was compared by Kaplan-Meier curves between AF cases and controls at 30-day, the first year and fifth year, and by a priori population subgroups (categories of age, gender, multiple deprivation, valvular disease and CHA₂DS₂-VASc score categories) to control confounding in mortality analyses. For subgroup analyses by multiple deprivation status at baseline, we used Kaplan-Meier estimation to evaluate the proportional hazard assumption. When appropriate, Cox regression model was applied to estimate the AF incidence and mortality rate, adjusted by age and sex. Primary cause of death within the fifth year of follow-up was compared by disease and by ICD chapters.
We performed the analyses in the secured Data Safe Haven, meeting the data safety and information governance requirements by University College London, NHS Digital and ONS. Analyses were performed in Statistical Analysis System (version 9.4) and R (version 3.6.1). The funders did not have any role in study design, data collection, data analysis, interpretation, and writing of the report.
3. Results
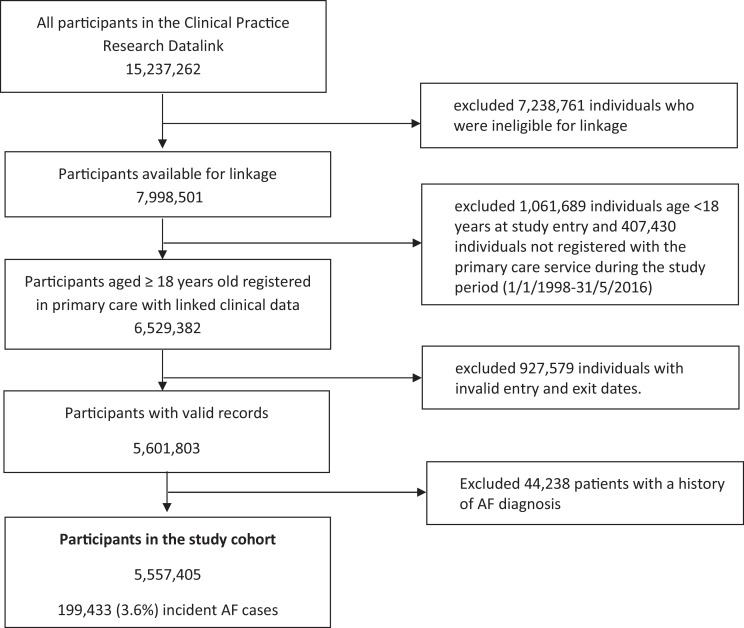
We identified 5,557,405 eligible individuals in the UK contributed clinical information between 1 January 1998 and 31 May 2016 in the study (Fig. 1). There were 199,433 (3.6%) incident diagnoses of AF over a median of 10.3 (interquartile range: 4.8-15.0) years of follow-up. The median follow-up among AF patients after the initial diagnosis was 2.7 (0.7-6.0) years.
Fig. 1.
Flow chart of the study population.
The mean age at AF diagnosis was 75.8 years (Standard Deviation (SD): 12.7 years). Half of the incident AF cases were women, whereas men developed AF at a younger age (72.3 (13.1) years) than women (78.9 (11.5) years) (Table 1). Four in every five (84%) incident AF cases had at least one comorbidity. The leading prevalent comorbid conditions were hypertension, heart failure and chronic kidney disease; 60% had 2 or more comorbidities at the time of diagnosis. One in ten AF patients were prescribed with oral anticoagulants at the time of diagnosis, and the percentage increased to one in three during study follow-up. Individuals with AF living in the most deprived areas had a higher prevalence of cardiovascular and non-cardiovascular conditions, also developing AF at a younger age than the least deprived (Table 1).
Table 1.
Characteristics of controls and individuals with incident atrial fibrillation at study entry
| Individuals with incident atrial fibrillation |
||||||
| By sex | By socioeconomic status | |||||
| Comparisons | All AF | Male | Female | Quintile 1 | Quintile 5 | |
| Age category at diagnosis (years) | (n=199 433) | (n=199 433) | (n=99 281) | (n=100 152) | (n=33 632) | (n=44 466) |
| Age at diagnosis (years) | 75.7 (12.7) | 75.8 (12.7) | 72.7 (13.1) | 78.9 (11.5) | 76.3 (12.8) | 75.0 (12.8) |
| Women | 100152 (50.2%) | 100152 (50.2%) | •• | •• | 16797 (49.9%) | 22241 (50%) |
| Socioeconomic status quintile | ||||||
| Quintile 1 (least deprived) | 33360 (16.7%) | 33632 (16.9%) | 16835 (17%) | 16797 (16.8%) | •• | •• |
| Quintile 5 (most deprived) | 40915 (20.5%) | 44466 (22.3%) | 22225 (22.4%) | 22241 (22.2%) | •• | •• |
| Smoking | 32130 (16.1%) | 86207 (43.2%) | 53004 (53.4%) | 33203 (33.2%) | 13026 (38.7%) | 21902 (49.3%) |
| CHA₂DS₂-VASc ≥ 1 for men or ≥2 for women | 171889 (86.2%) | 173354 (86.9%) | 82484 (83.1%) | 90870 (90.7%) | 28765 (86.5%) | 38875 (87.4%) |
| Comorbidities | ||||||
| Hypertension | 45182 (22.7%) | 99644 (50.0%) | 46309 (46.6%) | 53335 (53.3%) | 16224 (48.2%) | 22886 (51.5%) |
| Diabetes | 12487 (6.3%) | 31099 (15.5%) | 17064 (17.2%) | 13945 (13.9%) | 4471 (13.3%) | 7704 (17.3%) |
| Valvular disease | 1941 (1%) | 20467 (10.3%) | 9870 (9.9%) | 10597 (10.6%) | 3656 (10.9%) | 4417 (9.9%) |
| Angina | 14858 (7.5%) | 37846 (19.0%) | 20854 (21.0%) | 16992 (17.0%) | 5657 (16.8%) | 9810 (22.1%) |
| Ischemic heart disease | 10349 (5.2%) | 33358 (16.7%) | 19897 (20.0%) | 13461 (13.4%) | 5289 (15.7%) | 7961 (17.9%) |
| Heart failure | 791 (0.4%) | 41925 (21.0%) | 20236 (20.4%) | 21689 (21.7%) | 6254 (18.6%) | 10297 (23.2%) |
| Stroke | 7363 (3.7%) | 23208 (11.6%) | 10897 (11.0%) | 12311 (12.3%) | 3715 (11.0%) | 5290 (11.9%) |
| Asthma | 12828 (6.4%) | 30252 (15.2%) | 14059 (14.2%) | 16193 (16.2%) | 4908 (14.6%) | 7425 (16.7%) |
| Hyperthyroidism | 1932 (1%) | 4825 (2.4%) | 1028 (1.0%) | 3797 (3.8%) | 730 (2.2%) | 1178 (2.6%) |
| Cancer | 20306 (10.2%) | 38976 (19.5%) | 20263 (20.4%) | 18713 (18.7%) | 6805 (20.2%) | 8354 (18.8%) |
| Chronic kidney disease | 4121 (2.1%) | 40362 (20.2%) | 18985 (19.1%) | 21377 (21.3%) | 6222 (18.5%) | 9750 (21.9%) |
| Chronic obstructive pulmonary disease | 8207 (4.1%) | 25795 (12.9%) | 14182 (14.3%) | 11613 (11.6%) | 3377 (10.0%) | 7306 (16.4%) |
| Dementia | 4674 (2.3%) | 12254 (6.1%) | 4354 (4.4%) | 7900 (7.9%) | 2105 (6.3%) | 2647 (6.0%) |
| Two or more comorbidities | 36176 (18.1%) | 118661 (59.5%) | 57925 (58.3%) | 60736 (60.6%) | 18870 (56.1%) | 27877 (62.7%) |
| Oral anticoagulants prescription | ||||||
| At study entry | 2950 (1.5%) | 20093 (10.1%) | 11280 (11.4%) | 8813 (8.8%) | 3414 (10.2%) | 4458 (10.0%) |
| During follow-up | 4705 (2.4%) | 64646 (32.4%) | 35540 (35.8%) | 29106 (29.0%) | 11307 (33.6%) | 14326 (32.2%) |
Data are mean (SD) or n (%). Socioeconomic status refers to Index of Multiple Deprivation 2015 quintile, with SES 1 referring to the most affluent and SES 5 to the most deprived socioeconomic quintile. Number of comorbidities refers to any of the 13 conditions listed. AF patients characteristics between sex difference: p all <0.001 except for socioeconomic status quintile (p=0.30); between the least and most deprivation difference: p all <0.001 except for sex (p=0.84), dementia prevalence (p=0.07), oral anticoagulants prescription at baseline (p=0.56)
3.1. Incidence
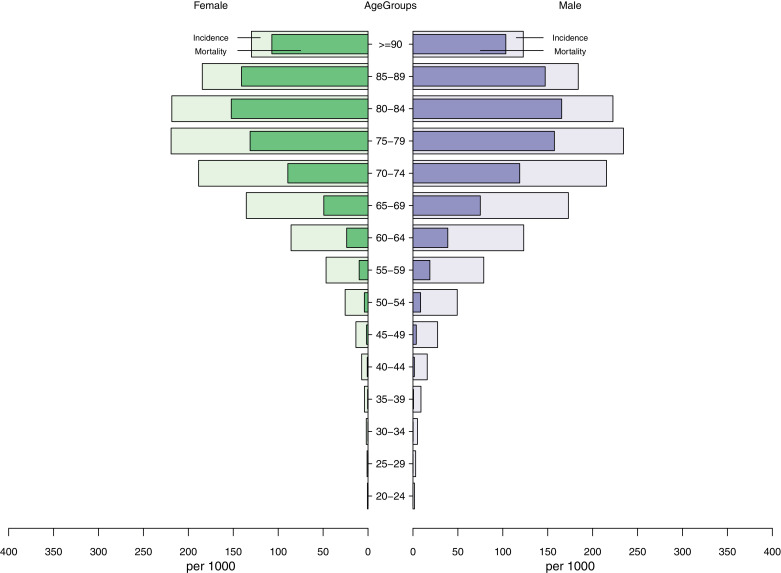
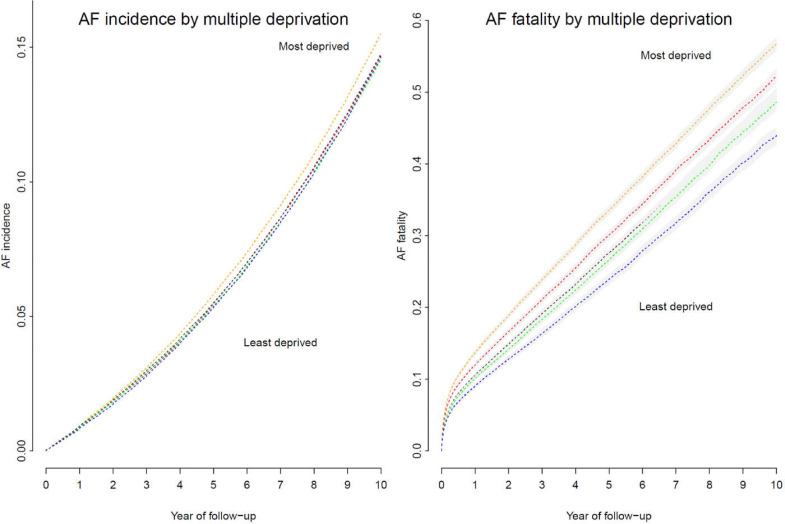
The observed incidence of AF per 1000 persons during the study period ranged from 17.2 (20-24 years) to 234.3 (75-79 years) in men and 0.7 (20-24 years) to 219.2 (75-79 years) in women (Fig. 2). In both sexes, cumulative AF incidence increased with age, peaking in the 70s and declining further with increasing age. The age and sex standardised cumulative AF incidence was 0.07% according to the 2013 European Standard Population. The age-standardised cumulative AF incidence was 0.08% in men and 0.06% in women. Overall, the incidence rates at the first year, fifth year and d year of follow-up were 0.23% (95% confidence interval: 0.22%, 0.23%), 1.28% (1.27%, 1.29%), and 3.12% (3.11%, 3.14%). The age and sex-adjusted incidence rates showed a rising trend of AF incidence with deprivation level (Fig. 3). After accounting for the difference in age and sex, individuals living in the poorest area had a 12% increased risk of developing AF than people living in the wealthiest areas (p: <0.001, Fig. 3).
Fig. 2.
Atrial fibrillation incidence and estimated deaths among incident cases by age groups in men and women
Fig. 3.
Atrial fibrillation incidence and fatality by socioeconomical status
3.2. Healthcare contacts
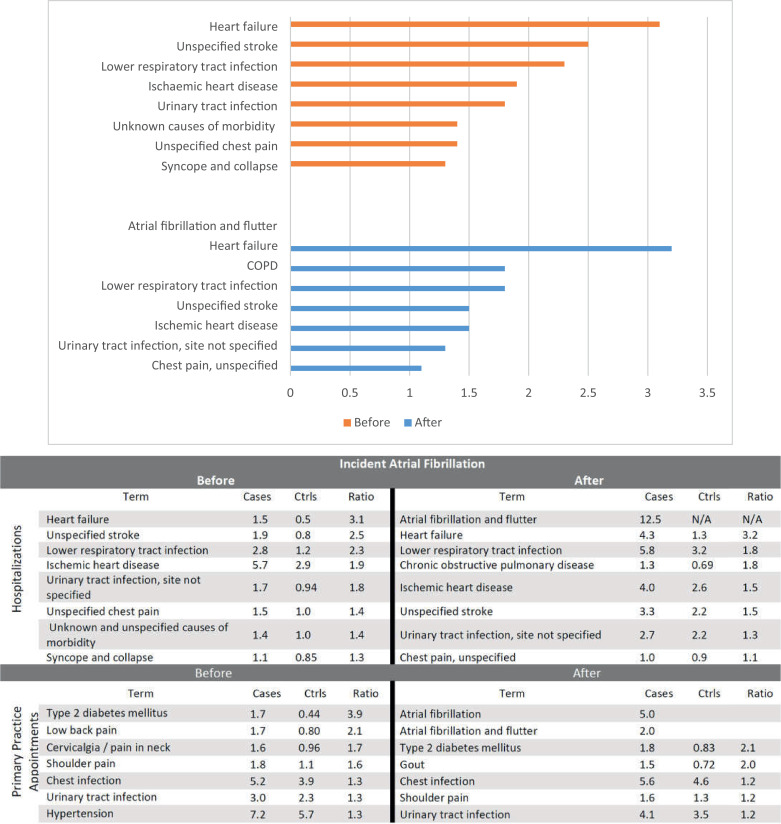
To investigate AF progression, we compared the reason for seeking frequent medical care between AF patients and 199,433 age and sex-matched controls. The mean age was 75.8 years in patients and 75.7 years in controls (Supplementary table S2). The median follow-up among controls after study entry was 6.6 (2.8-11.9) years. The leading causes of hospitalisation among cases than controls prior to the initial AF diagnosis were heart failure, unspecified stroke, lower respiratory tract infection, ischemic heart disease (including acute coronary syndrome and myocardial infarction), urinary tract infection and chest pain. (Fig. 4) Hospitalisation for chronic obstructive pulmonary disease was frequently observed among AF patients the year before diagnosis. (Supplementary Table S3) Following an AF diagnosis, patients were often hospitalised for heart failure, lower respiratory tract infection, chronic obstructive pulmonary disease and ischemic heart disease.
Fig. 4.
Top 10 reasons for hospitalisation in atrial fibrillation patients, compared to controls, within 5 years pre and post incident AF diagnosis.
Compared with controls, type 2 diabetes, osteoarticular pain, chest and urinary tract infection were the leading conditions for frequent GP consultations among AF patients prior to diagnosis. AF patients were more likely to utilise healthcare resources than controls at study entry, yet about 1 in 5 AF patients (41730, 20.9%) did not have any primary care consultation or hospitalisation during the year prior to their AF diagnosis. (Supplementary Table S4) During the first year after the initial diagnosis, almost all AF cases received clinical care. After diagnosis, AF, type 2 diabetes, gout, chest and urinary tract infection were the leading reasons for frequent GP consultations among AF patients than controls.
3.3. Mortality
In all AF cases, the 5-year case fatality increased with age, from 2% (20-25 years) to 84% (≥90 years) in men and from 6% to 82% in women. (Fig. 2) Compared to controls, the rate of death among AF patients surged immediately after diagnosis but reduced over time. (Fig. 5) Among incident AF cases, the mortality at 1 month, the first year and fifth year were 7.8% (95% CI: 7.6%, 7.9%), 19.9% (19.7%, 20.1%), and 42.7% (42.4%, 42.9%), compared to 0.5% (0.5%, 0.57%), 5.8% (5.7%, 5.87%), 26.0% (25.8%, 26.2%) among matched controls. (Supplementary Table S5) Mortality rates are higher among women, those with valvular disease, an elevated CHA₂DS₂-VASc score (≥ 1 for men or ≥2 for women), and individuals from the most deprived areas. (Supplementary table S5) Mortality among AF patients was inversely associated with socioeconomic inequalities (Fig. 2). Compared to AF patients living in the wealthiest neighbourhood, the risk of death rose by 16% and 26% in patients living in the second most and most deprived neighbourhoods, respectively (p <0.001, Fig. 2).
Fig. 5.
Mortality rate in incident atrial fibrillation cases and their matched controls.
The most frequent causes of death in AF patients were ischemic heart disease, stroke, acute myocardial infarction and dementia. (Fig. 6). Contrarily, the top cause of death among non-AF controls was infection (unspecified bronchopneumonia). Ischemic heart disease, stroke and dementia account for a slightly lower proportion of all deaths. The distribution of causes of death showed different patterns in individuals with and without AF (p-value for trend <0.001). A greater number of deaths among individuals with AF than controls were due to conditions such as the circulatory (41.8% and 34.7%, respectively) and digestive system (4.6% and 3.8%), metabolic disorders (1.4% and 1.2%), and infection (1.2% and 0.5%). (Supplementary Figure S1)
Fig. 6.
Leading primary cause of death in AF patients and in age and sex matched control
4. Discussion
The study provides comprehensive evidence for the AF burden on population health and healthcare utilisation. The leading prevalent comorbidities were hypertension, heart failure and chronic kidney disease, and the main reasons for frequent primary care appointments prior to AF diagnosis were type 2 diabetes, pain and infection. We found repeated hospitalisations for ischaemic heart disease, lower respiratory tract infection and heart failure among patients prior to initial AF diagnosis. After diagnosis, patients were admitted to the hospital for AF, lower respiratory tract infection, heart failure, ischaemic heart disease and chronic obstructive pulmonary disease. One in five patients died within the first year of diagnosis, and the mortality doubled in 5 years after diagnosis. Excess deaths among AF cases were mainly due to conditions involving the circulatory and digestive system, metabolic disorders, and infection.
We found approximately three in five AF patients had two or more comorbidities at the time of diagnosis. The high prevalence of multiple morbidities was reported previously in the regional cohort [12] and may be explained by the advanced median age of the incident AF population. In addition, the introduction of the national quality of care initiatives such as the Quality and Outcomes Framework from 2004 [38] and the English NHS Commissioning for Quality and Innovation (CQUIN) scheme from 2009 [39] may have facilitated better case finding and management, subsequently contributing to the high proportion of multi-comorbidities.
Cardiovascular conditions were the main reason for hospitalisation among AF patients. We found that hospitalisations for heart failure were more frequent in patients who later developed AF than their age and sex-matched controls. This observation potentially suggests that these patients may have already presented with atrial myopathy prior to the diagnosis of AF, or have undetected episodes of paroxysmal AF, in addition to AF as a risk factor for heart failure [40] Similarly, increased risk of hospitalisation for stroke was present among cases one year prior to AF diagnosis, having potentially attributed to a higher CVD risk profile or due to sub-clinical or undiagnosed AF.
The study also shows that frequent hospitalisation for lower respiratory tract and urinary infections in individuals with AF are 40% to 60% more than in controls in the year before developing AF, whereas the age and sex distribution for individuals with these conditions were similar in cases and controls. Although other confounders may contribute to the excess hospitalisations for infection, the greater urinary tract or respiratory tract infections in AF patients compared to controls may indicate an underlying inflammatory driver for the development of AF [40], or episodes of paroxysmal AF detected after the hospitalisation [41].
Despite being associated with a marked increase in mortality, AF itself is rarely a cause of death, and the majority of AF patients died of cardiovascular complications, such as ischaemic heart disease, stroke, and myocardial infarction . We observed AF patients compared to controls are more likely to have excess deaths due to cardiovascular diseases, infection and metabolic disorders.
The initial excess risk of death in cases compared to controls plateaus and may be explained by the high short-term fatality in AF with complications of poor prognosis such as sepsis. The excess mortality risk reported in the present study is higher than that in AF patients during a 30-year follow-up in the Olmsted County [11], but similar to the estimates in the Framingham cohort [9].
5. Practice implications and future research
We found that individuals from areas with the highest deprivation in socioeconomic and living status had a 12% greater risk of developing AF and a 26% higher AF fatality than people living in the wealthiest areas. At diagnosis, AF patients from the most deprived areas had more comorbidities, despite being younger than those from wealthy areas. The discrepancy in AF incidence and mortality by deprivation warrants targeted prevention strategies, health-care resource planning and utilisation in AF management of the general population.
The repeated hospitalisations for ischemic heart disease and heart failure in a given patient should draw the physician's attention to the likelihood of ongoing undiagnosed AF. Our findings thus support the need for an in-depth characterisation of AF patients [42]. Where the clinical suspicion is high, further investigation is warranted utilising appropriate electrocardiographic monitoring [43] or an implantable loop recorder [44]. After AF diagnosis, it is important to manage AF together with the related cardiovascular comorbidities, as these conditions continue to be the main reasons for hospitalisation and the leading cause of death among AF patients.
We found one in three AF patients was prescribed oral anticoagulant medication. In individuals with AF, 3.9% of deaths were primarily attributable to stroke, suggesting that anticoagulants, as reinforced in guidelines [7,45] are crucial for the prevention of fatal strokes among AF patients. Respiratory disease is another area that requires clinical attention in the care for AF, as patients were 1.6 to 2 times more likely than AF-free controls to be admitted to hospital for chronic obstructive pulmonary disease and lower respiratory tract infection. Our findings support the current practice guideline [7,45] for an integrated AF care utilising the Atrial fibrillation Better Care pathway for anticoagulation for AF patients with a high risk of subsequent stroke, symptom and rhythm control, and comorbidity management [46].
Future research may be required on utilising patient interactions with the health system to define higher risk groups requiring further investigation for earlier detection of AF. Information on imaging, circulating biomarkers, electrocardiographic features, combined with AF-prone clinical contacts, and novel rhythm monitoring methods [47] may contribute to early identification and possibly prophylactic treatment of AF. Our results also suggest the need for a detailed analysis on multi-comorbidity comparing individuals with and without AF and a comprehensive investigation of the social gradient in atrial fibrillation risk, care and prognosis.
Our study applies a novel approach of integrating epidemiology and patient journey to investigate AF burden and disparity on population health and healthcare utilisation. Major strengths of our research are the power and generalisability based on population electronic health records derived from routine care in the country. The results may hence be applicable to the general clinical practice in comparable populations. With data from 0.2 million AF patients, the scale of our study enables sufficient statistical power for detailed analyses. Second, the study offers an in-depth understanding how AF patients present to the healthcare system both prior to and after diagnosis, and third, the two designs using both prospective cohort and matched case-control study enabled investigations of incidence and prognosis. Fourth, all clinical exposures and outcomes are included in the data, together with socio-demographic information, allowing a comprehensive study on risk factors and comorbidities. However, some limitations exist in our analysis. First, despite its granularity, our data did not have complete information on AF types (e.g. paroxysmal, persistent, permanent) for all AF patients, which may associate with arrhythmia burden and subsequent outcomes [48]. However, as previously noted, the clinical utility of such a temporal pattern/episode-based classification remains inconclusive [49]. Second, some AF cases may have been misclassified due to coding. This limitation was managed with our method following validated AF definition in EHR [24,50], and our study could reproduce results from landmark AF study with clinical adjudication [9]. Finally, as for every observational analysis, there is the risk of unmeasured risk factors or comorbidities. We overcame this limitation by including in study key prognosis factors supported by the previous literature relating to AF and analysing all conditions that participants in the study were seeking care for.
6. Conclusion
We report the epidemiology and healthcare utilisation burden of atrial fibrillation. AF patients had excess hospitalisations for heart failure, cardiovascular conditions, infection and a higher risk of dying from cardiovascular causes. Individuals from areas with the highest deprivation in socioeconomic and living status had a 12% greater risk of developing AF and subsequently a 26% higher fatality than patients living in the wealthiest areas. The needs of early detection of AF, management of comorbidities and considerable disparity inform targeted prevention strategies, healthcare resource planning and utilisation in AF management of the general population.
Contributors
SCC access, analysis, and interpretation of data for the work and draft the manuscript. All authors contributed to the conception and design of the work, further data interpretation, revising it critically for important intellectual content. SCC had final responsibility for the decision to submit for publication.
Declaration of interests
SCC and DA report supported by funding from GlaxoSmithKline for electronic health records research, and the funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Other authors declare no conflict of interest.
Funding
SCC and DA report supported by funding from GlaxoSmithKline for electronic health records research; RS and PDL are supported by the University College London Hospital (UCLH) National Institute for Health Research (NIHR) Biomedical Research Centre. RP is supported by the National Institute for Health Research (NIHR) working with Cochrane Review Groups. LP reported support from Boston Scientific and Abbott.
Data availability statement
Data may be obtained from a third party and are not publicly available. Data used in this study were accessed through NHS Digital that is subject to protocol approval and cannot directly be shared.
Acknowledgement
This study was carried out as part of the cardiovascular disease research using linked bespoke studies and electronic health records (CALIBER)© programme (https://www.ucl.ac.uk/health-informatics/caliber), led from the UCL Institute of Health Informatics, is a research resource consisting of anonymised, coded variables extracted from linked electronic health records, methods and tools, specialised infrastructure, and training and support). CALIBER is an open-access research resource consisting of information, tools and phenotyping algorithms available through the CALIBER Portal (https://caliberresearch.org/portal). This study is based in part on data from the Clinical Practice Research Datalink obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data is provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the author/s alone. The OPCS Copyright © 2020, re-used with the permission of The Health & Social Care Information Centre. All rights reserved. Classification of Interventions and Procedures, codes, terms and text is Crown copyright (2016) published by Health and Social Care Information Centre, also known as NHS Digital and licensed under the Open Government Licence available at www.nationalarchives.gov.uk/doc/open-government-licence/open-government-licence.htm The interpretation and conclusions contained in this study are those of the author/s alone.
Footnotes
Clinical trial registration: NCT04786366
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.lanepe.2021.100157.
Contributor Information
Sheng-Chia Chung, Email: s.chung@ucl.ac.uk.
Rui Providencia, Email: r.providencia@ucl.ac.uk.
Appendix. Supplementary materials
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Public Health England. Atrial Fibrillation prevalence estimates. December 2019. Accessed at:https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/879448/AF_prevalence_estimates.xlsx. (27th April 2021).
- 4.Gallagher C, Hendriks JM, Giles L, Karnon J, Pham C, Elliott AD. Increasing trends in hospitalisations due to atrial fibrillation in Australia from 1993 to 2013. Heart. 2019;105(17):1358–1363. doi: 10.1136/heartjnl-2018-314471. [DOI] [PubMed] [Google Scholar]
- 5.The British Heart Foundation. BHF UK Factsheet - Heart and Circulatory Disease Statistics. 2020. Accessed at:https://www.bhf.org.uk/what-we-do/our-research/heart-statistics/heart-statistics-publications/cardiovascular-disease-statistics-2020. (27th April 2021).
- 6.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 7.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2020 [Google Scholar]
- 8.Welton NJ, McAleenan A, Thom HH, Davies P, Hollingworth W, Higgins JP. Screening strategies for atrial fibrillation: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2017;21(29):1–236. doi: 10.3310/hta21290. [DOI] [PubMed] [Google Scholar]
- 9.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386(9989):154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vermond RA, Geelhoed B, Verweij N, Tieleman RG, Van der Harst P, Hillege HL. Incidence of Atrial Fibrillation and Relationship With Cardiovascular Events, Heart Failure, and Mortality: A Community-Based Study From the Netherlands. J Am Coll Cardiol. 2015;66(9):1000–1007. doi: 10.1016/j.jacc.2015.06.1314. [DOI] [PubMed] [Google Scholar]
- 11.Jahangir A, Lee V, Friedman PA, Trusty JM, Hodge DO, Kopecky SL. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation. 2007;115(24):3050–3056. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain AM, Alonso A, Gersh BJ, Manemann SM, Killian JM, Weston SA. Multimorbidity and the risk of hospitalization and death in atrial fibrillation: A population-based study. Am Heart J. 2017;185:74–84. doi: 10.1016/j.ahj.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieuwlaat R, Capucci A, Camm AJ, Olsson SB, Andresen D, Davies DW. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005;26(22):2422–2434. doi: 10.1093/eurheartj/ehi505. [DOI] [PubMed] [Google Scholar]
- 14.Cepelis A, Brumpton BM, Malmo V, Laugsand LE, Loennechen JP, Ellekjaer H. Associations of Asthma and Asthma Control With Atrial Fibrillation Risk: Results From the Nord-Trondelag Health Study (HUNT) JAMA Cardiol. 2018;3(8):721–728. doi: 10.1001/jamacardio.2018.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marijon E, Le Heuzey JY, Connolly S, Yang S, Pogue J, Brueckmann M. Causes of death and influencing factors in patients with atrial fibrillation: a competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation. 2013;128(20):2192–2201. doi: 10.1161/CIRCULATIONAHA.112.000491. [DOI] [PubMed] [Google Scholar]
- 16.DeVore AD, Hellkamp AS, Becker RC, Berkowitz SD, Breithardt G, Hacke W. Hospitalizations in patients with atrial fibrillation: an analysis from ROCKET AF. Europace. 2016;18(8):1135–1142. doi: 10.1093/europace/euv404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friberg L, Benson L, Rosenqvist M, Lip GY. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012;344:e3522. doi: 10.1136/bmj.e3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berntsson J, Li X, Zoller B, Martinsson A, Andell P, Lubitz SA. Risk of Stroke in Patients With Atrial Fibrillation Is Associated With Stroke in Siblings: A Nationwide Study. J Am Heart Assoc. 2020;9(3) doi: 10.1161/JAHA.119.014132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane DA, Skjoth F, Lip GYH, Larsen TB, Kotecha D. Temporal Trends in Incidence, Prevalence, and Mortality of Atrial Fibrillation in Primary Care. J Am Heart Assoc. 2017;6(5) doi: 10.1161/JAHA.116.005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walley T, Mantgani A. The UK General Practice Research Database. Lancet. 1997;350(9084):1097–1099. doi: 10.1016/S0140-6736(97)04248-7. [DOI] [PubMed] [Google Scholar]
- 21.Padmanabhan S, Carty L, Cameron E, Ghosh RE, Williams R, Strongman H. Approach to record linkage of primary care data from Clinical Practice Research Datalink to other health-related patient data: overview and implications. Eur J Epidemiol. 2019;34(1):91–99. doi: 10.1007/s10654-018-0442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallagher AM, Dedman D, Padmanabhan S, Leufkens HGM, de Vries F. The accuracy of date of death recording in the Clinical Practice Research Datalink GOLD database in England compared with the Office for National Statistics death registrations. Pharmacoepidemiol Drug Saf. 2019;28(5):563–569. doi: 10.1002/pds.4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Setakis E PS, Williams TJ, VanStaa T. Representiveness of subset of the general practice research database (GPRD) linked to other data sources. Pharmacoepidemiol Drug Saf. 2018;736:19. [Google Scholar]
- 24.Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69(1):4–14. doi: 10.1111/j.1365-2125.2009.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10) doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rix TA, Riahi S, Overvad K, Lundbye-Christensen S, Schmidt EB, Joensen AM. Validity of the diagnoses atrial fibrillation and atrial flutter in a Danish patient registry. Scand Cardiovasc J. 2012;46(3):149–153. doi: 10.3109/14017431.2012.673728. [DOI] [PubMed] [Google Scholar]
- 27.Mareedu RK, Abdalrahman IB, Dharmashankar KC, Granada JF, Chyou PH, Sharma PP. Atrial flutter versus atrial fibrillation in a general population: differences in comorbidities associated with their respective onset. Clin Med Res. 2010;8(1):1–6. doi: 10.3121/cmr.2009.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denaxas S, Gonzalez-Izquierdo A, Direk K, Fitzpatrick NK, Fatemifar G, Banerjee A. UK phenomics platform for developing and validating electronic health record phenotypes: CALIBER. J Am Med Inform Assoc. 2019;26(12):1545–1559. doi: 10.1093/jamia/ocz105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruigomez A, Johansson S, Wallander MA, Rodriguez LA. Incidence of chronic atrial fibrillation in general practice and its treatment pattern. J Clin Epidemiol. 2002;55(4):358–363. doi: 10.1016/s0895-4356(01)00478-4. [DOI] [PubMed] [Google Scholar]
- 30.Kuan V, Denaxas S, Gonzalez-Izquierdo A, Direk K, Bhatti O, Husain S. A chronological map of 308 physical and mental health conditions from 4 million individuals in the English National Health Service. Lancet Digit Health. 2019;1(2):e63–e77. doi: 10.1016/S2589-7500(19)30012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morley KI, Wallace J, Denaxas SC, Hunter RJ, Patel RS, Perel P. Defining disease phenotypes using national linked electronic health records: a case study of atrial fibrillation. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0110900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Department for Communities and Local Government (DCLG). The English Index of Multiple Deprivation 2015: guidance. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2015.
- 33.Ellervik C, Roselli C, Christophersen IE, Alonso A, Pietzner M, Sitlani CM. Assessment of the Relationship Between Genetic Determinants of Thyroid Function and Atrial Fibrillation: A Mendelian Randomization Study. JAMA Cardiol. 2019;4(2):144–152. doi: 10.1001/jamacardio.2018.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Agarwal SK, Alonso A, Blecker S, Chamberlain AM, London SJ. Airflow obstruction, lung function, and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2014;129(9):971–980. doi: 10.1161/CIRCULATIONAHA.113.004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. 2016;354:i4482. doi: 10.1136/bmj.i4482. [DOI] [PubMed] [Google Scholar]
- 36.Tung P, Levitzky YS, Wang R, Weng J, Quan SF, Gottlieb DJ. Obstructive and Central Sleep Apnea and the Risk of Incident Atrial Fibrillation in a Community Cohort of Men and Women. J Am Heart Assoc. 2017;6(7) doi: 10.1161/JAHA.116.004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 38.The UK Government. Summary of QOF indicators.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/213226/Summary-of-QOF-indicators.pdf
- 39.Department of Health, Using the Commissioning for Quality and Innovation (CQUIN) payment framework For the NHS in England 2009/10http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_091435.pdf.
- 40.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50(5):e1–e88. doi: 10.1093/ejcts/ezw313. [DOI] [PubMed] [Google Scholar]
- 41.Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Am Coll Cardiol. 2007;50(21):2021–2028. doi: 10.1016/j.jacc.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 42.Potpara TS, Lip GYH, Blomstrom-Lundqvist C, Boriani G, Van Gelder IC, Heidbuchel H. The 4S-AF Scheme (Stroke Risk; Symptoms; Severity of Burden; Substrate): A Novel Approach to In-Depth Characterization (Rather than Classification) of Atrial Fibrillation. Thromb Haemost. 2021;121(3):270–278. doi: 10.1055/s-0040-1716408. [DOI] [PubMed] [Google Scholar]
- 43.Stegmann T, Koehler K, Schulze M, Laufs U, Wachter R, Koehler F. Detection of new-onset atrial fibrillation in heart failure patients using daily non-invasive ECG monitoring in the TIM-HF2 telemedicine trial, European Heart Journal, Volume 41, Issue Supplement_2, November 2020, ehaa946.3467.
- 44.Bloch Thomsen PE, Jons C, Raatikainen MJ, Moerch Joergensen R, Hartikainen J, Virtanen V. Long-term recording of cardiac arrhythmias with an implantable cardiac monitor in patients with reduced ejection fraction after acute myocardial infarction: the Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) study. Circulation. 2010;122(13):1258–1264. doi: 10.1161/CIRCULATIONAHA.109.902148. [DOI] [PubMed] [Google Scholar]
- 45.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC., Jr. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017;14(11):627–628. doi: 10.1038/nrcardio.2017.153. [DOI] [PubMed] [Google Scholar]
- 47.Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N Engl J Med. 2019;381(20):1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogawa H, An Y, Ikeda S, Aono Y, Doi K, Ishii M. Progression From Paroxysmal to Sustained Atrial Fibrillation Is Associated With Increased Adverse Events. Stroke. 2018;49(10):2301–2308. doi: 10.1161/STROKEAHA.118.021396. [DOI] [PubMed] [Google Scholar]
- 49.Hammond-Haley M, Providencia R, Lambiase PD. Temporal pattern/episode duration-based classification of atrial fibrillation as paroxysmal vs. persistent: is it time to develop a more integrated prognostic score to optimize management? Europace. 2018;20(FI_3):f288–ff98. doi: 10.1093/europace/eux178. [DOI] [PubMed] [Google Scholar]
- 50.Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract. 2010;60(572):e128–e136. doi: 10.3399/bjgp10X483562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Data used in this study were accessed through NHS Digital that is subject to protocol approval and cannot directly be shared.