Abstract
Despite being the subject of extensive research and clinical trials, neuroblastoma remains a major therapeutic challenge in pediatric oncology. The p53 protein is a central safeguard that protects cells against genome instability and malignant transformation. Mutated TP53 (the gene encoding p53) is implicated in many human cancers, but the majority of neuroblastomas have wild type p53 with intact transcriptional function. In fact, the TP53 mutation rate does not exceed 1–2% in neuroblastomas. However, overexpression of the murine double minute 2 (MDM2) gene in neuroblastoma is relatively common, and leads to inhibition of p53. It is also associated with other non-canonical p53-independent functions, including drug resistance and increased translation of MYCN and VEGF mRNA. The p53-MDM2 pathway in neuroblastoma is also modulated at several different molecular levels, including via interactions with other proteins (MYCN, p14ARF). In addition, the overexpression of MDM2 in tumors is linked to a poorer prognosis for cancer patients. Thus, restoring p53 function by inhibiting its interaction with MDM2 is a potential therapeutic strategy for neuroblastoma. A number of p53-MDM2 antagonists have been designed and studied for this purpose. This review summarizes the current understanding of p53 biology and the p53-dependent and -independent oncogenic functions of MDM2 in neuroblastoma, and also the regulation of the p53-MDM2 axis in neuroblastoma. This review also highlights the use of MDM2 as a molecular target for the disease, and describes the MDM2 inhibitors currently being investigated in preclinical and clinical studies. We also briefly explain the various strategies that have been used and future directions to take in the development of effective MDM2 inhibitors for neuroblastoma.
Keywords: neuroblastoma, p53, MDM2, inhibitors, targeted therapy
1. Introduction
Neuroblastoma (NB) is a tumor of the sympathetic nervous system and one of the most common tumors found in children [1–3]. Statistically, the incidence rate of neuroblastoma is 1.2 cases per 100,000, and it is responsible for approximately 15% of all cancer deaths in children [4–11]. The current survival rate for NB is less than 50%, as most patients develop resistance to therapy and suffer relapse [4–11]. In addition, NB survivors are at risk of severe illness due to therapy-related toxicities and the development of secondary tumors [12, 13]. Further, the heterogeneity of NB limits the development of a successful targeted therapy for these tumors [14–16]. Hence, there is an urgent need to better understand the biology of NB and to develop more effective and safer therapies for NB.
The etiology of neuroblastoma remains largely unknown, and it is only recently that the tools have become available to investigate the cellular changes that occur at the genetic and epigenetic levels during cancer development and progression. Whole genome sequencing has identified several genetic aberrations, including mutations, deletions, amplifications, and rearrangements of genes that lead to NB development [17, 18]. Oncogenes and tumor suppressor genes are being extensively studied to tease apart their pivotal role in neuroblastoma tumorigenesis [19].
The p53 tumor suppressor is a critical protein responsible for inducing apoptosis or cell cycle arrest in response to DNA damage and cellular stress [20, 21]. More than 50% of human cancers harbor TP53 mutations, mostly within the p53 DNA binding domain, and this impairs its transcriptional activity [22, 23]. Although there is evidence that p53 activity is lacking in many neuroblastoma tumors, TP53 is rarely mutated in human neuroblastoma, with the reported mutation rate not exceeding 2% [24]. In addition, studies have indicated that p53 signal transduction is functional in neuroblastoma [25–27]. This suggests that p53 is being negatively impact via other mechanisms. The murine double minute 2 (MDM2) oncogene is amplified in numerous human malignancies, including neuroblastoma [28]. MDM2 protein overexpression in neuroblastoma cells can also occur without MDM2 gene amplification [29]. The overexpression of MDM2 in tumors is linked to a poorer prognosis for cancer patients [30]. Although MDM2 was initially discovered as a negative regulator of p53 [31, 32], it exerts a variety of oncogenic effects through diverse mechanisms. For example, the N-terminus of MDM2 binds p53 and represses p53-mediated transcription [31], while the C-terminus acts as an E3 ubiquitin ligase that mediates p53’s degradation [33]. Numerous studies have shown that inhibiting the p53-MDM2 interaction via MDM2 antagonists can activate p53-induced apoptotic signaling in neuroblastoma [34, 35]. The pharmacologic inhibition of the p53-MDM2 interaction appears to represent an effective approach for the treatment of neuroblastoma. In addition to its effects on p53, there are numerous p53-independent oncogenic functions of MDM2 in cancer cells. For instance, MDM2’s C-terminal RING finger domain has been found to bind X-linked inhibitor of apoptosis protein (XIAP) mRNA, which regulates the translation of XIAP, leading to the development of resistance to anticancer treatments [36]. MDM2 has also been found to interact with TAp73, and negatively controls its transcriptional activity [37]. A study by Shi et al. showed that HDM2, the human homolog of MDM2, inhibits p73 binding to the Noxa promoter and represses Noxa transcription in neuroblastoma cells [38]. Thus, in the presence of high levels of MDM2, the transcriptional activity of TAp73 is inhibited, enhancing the survival and chemoresistance of cancer cells [39]. These characteristics make MDM2 a promising therapeutic target for human cancers, regardless of the p53 status.
This review summarizes the current understanding of p53 biology, particularly non-mutational p53 inactivation (cytoplasmic sequestration) and the functional status of p53 in neuroblastoma. It also sheds light on the p53-dependent and –independent oncogenic functions of MDM2 in neuroblastoma, and discusses the regulation of the p53-MDM2 axis in such cells. Because MDM2 is also frequently amplified or overexpressed in neuroblastomas with apparently normal p53, we also discuss the use of MDM2 itself as a molecular target in neuroblastoma, and describe the MDM2 inhibitors currently in development at the preclinical and clinical levels. A special emphasis has also been placed on combination therapy using MDM2 antagonists with other molecules for the treatment of neuroblastoma. We also explain various future strategies that might be used to augment the efficacy of the current MDM2 inhibitors for neuroblastoma treatment.
2. Role of p53 pathway in neuroblastoma: basic biology and clinical relevance
The tumor suppressor p53, the so-called the guardian of the genome, has been reported to be mutated in more than 50% of human cancers [40]. However, mutations have only been found in ~ 2% of cases of relapsed or refractory neuroblastoma [27]. Despite possessing the wild-type gene, the accumulation of p53 with enhanced stability has been observed in neuroblastoma [27]. One possible reason for this accumulation is the embryonic nature of these tumors, as the precursor cells fail to mature [41]. Another reason for p53 accumulation is the cytoplasmic sequestration of wild-type p53 in undifferentiated neuroblastomas [42]. This is one non-mutational mechanism that may lead to p53 inactivation in neuroblastoma [43]. In fact, the cytoplasmically-sequestered p53 in neuroblastoma is associated with a failure of cells to undergo G1 arrest following DNA damage [43].
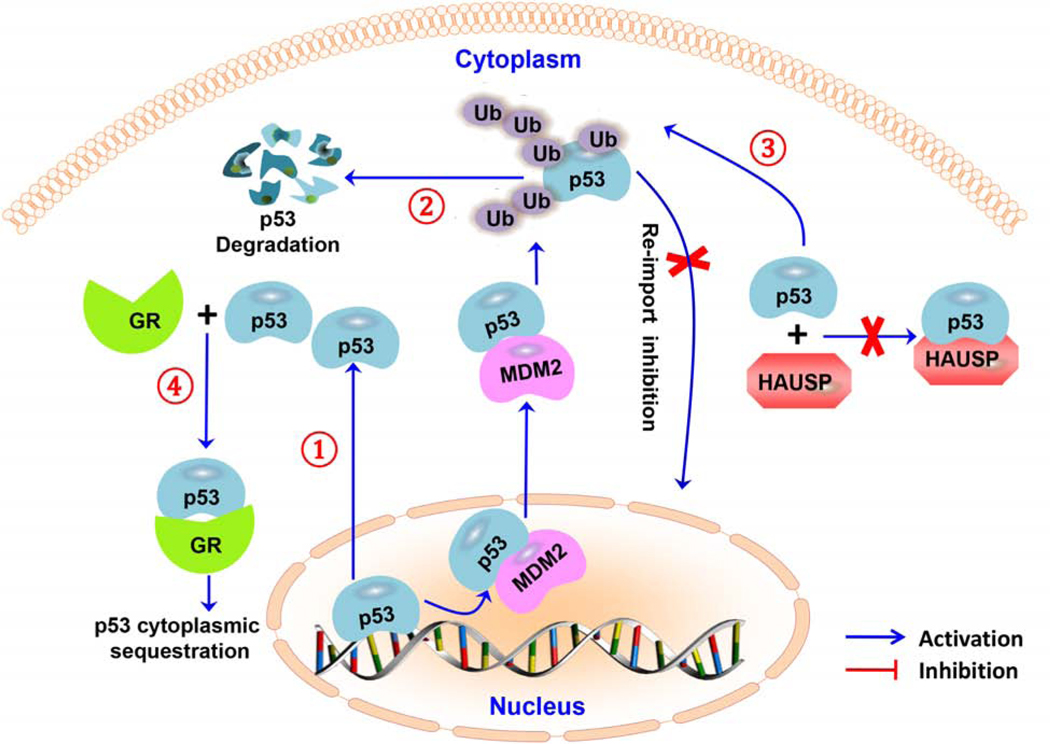
Various mechanisms have been proposed to underlie the cytoplasmic sequestration of p53 in neuroblastoma cells (Fig. 1). For instance, there may be hyperactive nuclear export of p53 [44]. Interestingly, a study by Zaika et al. showed that the p53 cytoplasmic sequestration phenotype in neuroblastoma cells is due to a profound resistance toward MDM2-directed degradation [45]. The same study suggested that degradation-resistant p53 is associated with altered p53 posttranslational modification, which is characterized by a loss of positively-charged residues and/or gain of negatively-charged residues in the amino acid 372–382 region of p53 [45]. A study by Becker et al. described another mechanism of p53 cytoplasmic sequestration, which involves aberrant p53 hyperubiquitylation that makes p53 unable to shuttle to the nucleus [46]. The hyperubiquitylation of p53 in neuroblastoma proposed in this model is not caused by MDM2 (E3 ligase) overexpression and/or herpesvirus-associated ubiquitin-specific protease (HAUSP) (p53-deubiquitylating enzyme) downregulation, but due to an impaired p53-HAUSP interaction [46]. As a result, the non-ubiquitylated p53 pool is decreased compared to ubiquitylated p53 species. Moreover, only the polyubiquitylated species are degraded, while the multi (and mono) ubiquitylated species accumulate and remain stable in the cytoplasm [46]. A study by Sengupta et al. also demonstrated that the glucocorticoid receptor (GR) forms a complex with p53 and leads to wild-type p53 cytoplasmic retention and inactivation in neuroblastoma cells [47].
Figure 1. Molecular mechanisms responsible for the cytoplasmic sequestration of p53 in neuroblastoma cells.
(1) hyperactive nuclear export; (2) resistance to p53 degradation; (3) p53 hyperubiquitylation; (4) binding of a glucocorticoid receptor complex with p53 protein. Abbreviations: herpesvirus-associated ubiquitin-specific protease (HAUSP); ubiquitin (Ub), and glucocorticoid receptor (GR).
Research has been conducted to investigate the function and localization of p53 in neuroblastoma in relation to the tumor cell differentiation status. Active p53 is present in both differentiated and undifferentiated NB69 and NBLW neuroblastoma cell lines [25]. The same study showed that p53-responsive genes, such as MDM2 and p21WAF1, are upregulated after irradiation-induced DNA damage in both differentiated and undifferentiated neuroblastoma cells, and the cleavage of Caspase 3 and poly ADP (adenosine diphosphate)-ribose polymerase (PARP) was reduced in cells with retinoic acid-induced differentiation [25]. This suggests that intact transcriptional function of p53 may coexist with resistance to apoptosis in differentiated neuroblastoma cells [25]. Tweddle et al. performed a cell cycle analysis of neuroblastoma cells lines after 4 Gy irradiation and found that such cells fail to undergo G1 arrest after DNA damage, in spite of the presence of functionally active p53 [48]. In neuroblastomas, the increased p53 activity due to this cytoplasmic sequestration is more inclined towards apoptotic pathways than DNA repair pathways, suggesting that it may be possible to increase p53 activity to induce apoptosis in such tumors [49].
As noted above, while p53 mutations in neuroblastoma are rare, they may be found in relapsed or progressive tumors [50]. Various cell lines such as LAN-1 (Codon 182, Cys to Stop, [50]), NMB (Codon 245, Gly to Ser, [50]), SJNB-4 (Codon 176, Cys to Phe, [50]), CHLA-119 (Codon 342, Arg to Leu, [51]), CHLA-90 (Codon 286, Glu to Lys, [51]), NB-6 (Codon 282, Arg to Trp) [52], and IGR-N-91 (TP53 has duplication of exons 7–8-9, resulting in duplication spanning amino acids 225–331 [53, 54]) have been derived from tumors after treatment [27]. These cell lines are more chemoresistant than p53 wild-type neuroblastoma cell lines [27]. Studies have shown a variety of other mutations in neuroblastoma cell lines, which include substitutions of cysteine to phenylalanine at codon 135 (135Phe) and cysteine to tyrosine at codon 135 (135Tyr) from patients with relapsed disease [50]. In addition, a 135Phe mutation has also been detected in heavy metal-selected multidrug-resistant neuroblastoma IMR-32 cells [50]. The correlation of p53 mutations and drug resistance in neuroblastoma cells has been confirmed by various transfection experiments with mutant p53. For instance, SH-EP neuroblastoma cells were transfected with the 135Phe mutant and treated with doxorubicin and teniposide, and showed 4.0-fold and 4.2-fold higher resistance to treatment in comparison to control cells transfected with the empty vector [27]. In addition to p53 inactivation via mutation, there are other non-mutational mechanisms which can inactivate p53 in neuroblastoma cells. For instance, neuroblastoma cell lines derived from tumors at relapse may have MDM2 amplification, and such cells are more chemoresistant and have reduced p53 function compared to normal cell lines [27]. Thus, chemotherapeutic drugs that can exert their effects independent of functional p53 should be used against neuroblastoma, especially in patients with relapsed or progressive tumors.
3. The oncogenic role of MDM2 in neuroblastoma: Basics and clinical relevance
As an E3 ubiquitin ligase, MDM2 targets p53 for proteasomal degradation, and generally functions as a negative regulator of p53 [33, 55]. A study by Zaika et al. showed that steady state levels of MDM2 are observed in neuroblastoma cell lines [45]. Even in the absence of MDM2 amplification, high levels of MDM2 expression are observed in neuroblastomas [29]. This is sometimes due to single nucleotide polymorphisms in the promoter of MDM2 [29]. Recent evidence has shown that a T to G single nucleotide polymorphism (SNP) may be present in the promoter region of MDM2 (SNP309; rs2279744), and this SNP may underlie the increase in MDM2 activity associated with the development of some neuroblastomas, or with their malignant behavior [56]. Importantly, this SNP in the MDM2 promotor region enhances the binding affinity of its transcriptional activator, sp1 [56]. As a result, there is enhanced MDM2 transcription, resulting in MDM2 protein overexpression, p53 pathway attenuation, and accelerated tumor formation (Fig. 2). Studies have also shown that the transcriptional activation of MDM2 expression by MYCN contributes to the decreased p53 activity in neuroblastoma [57].
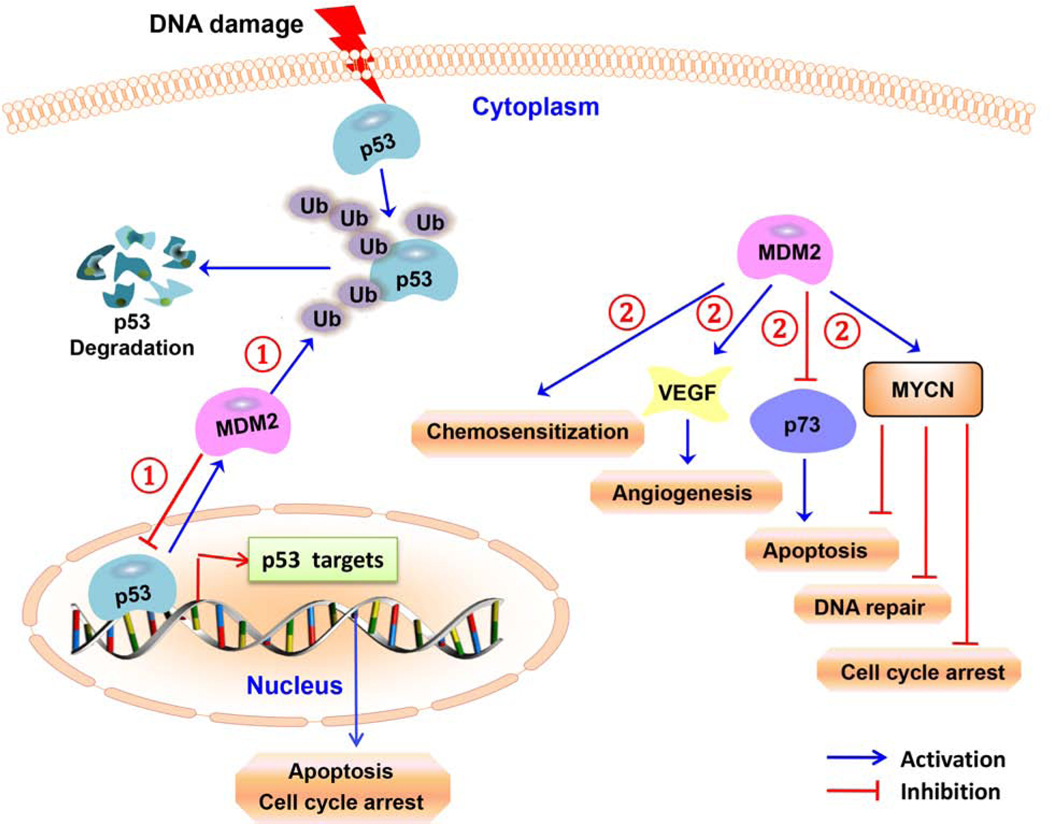
Figure 2. p53-dependent and –independent oncogenic roles of MDM2 in neuroblastoma.
(1) The MDM2 protein attenuates the transcriptional activity of p53 and contributes to tumor formation. MDM2 also catalyzes the ubiquitylation of p53 in neuroblastoma cells. (2) MDM2 increases the translation of VEGF (thereby increasing angiogenesis) and MYCN, leading to neuroblastoma growth. MDM2 expression leads to the development of multidrug resistance and also inhibits the transcriptional activity of p73 in neuroblastoma cells.
More recently, there have been reports of various non-canonical p53-independent oncogenic functions of MDM2 in neuroblastoma (Fig. 2) [51, 58, 59]. For example, under hypoxic conditions, MDM2 is translocated from the nucleus to the cytoplasm, where it binds directly to the VEGF 3’ UTR and stabilizes vascular endothelial growth factor (VEGF) mRNA and subsequently increases VEGF translation, leading to neuroblastoma tumor growth [58]. Further research has identified another p53-independent function of MDM2, wherein the MDM2 RING domain binds to the MYCN mRNA adenylate/uridylate-rich elements (AREs) within the 3’ UTR, increasing the MYCN mRNA stability and translation in neuroblastoma cells [59]. The same study also showed that specific siRNA-mediated MDM2 silencing renders the MYCN mRNA unstable, which leads to a marked inhibition of neuroblastoma cell growth [59]. A study by Keshelava et al. suggested that elevated MDM2 expression is responsible for multidrug resistance in some neuroblastoma cells [51]. In addition, an increased frequency of MDM2 amplification has been found in relapsed neuroblastoma [28]. Overall, the above studies provide evidence that MDM2 represents a target for anti-cancer therapy in neuroblastoma.
4. The p53-MDM2 pathway and its crosstalk with other proteins in neuroblastoma: More complicated than previously thought
It is widely established that MDM2 is an important negative regulator of p53. Mechanistically, MDM2 binds to the transactivation domain of p53 and inactivates it, and also ubiquitylates the protein, targeting it for degradation [40]. A study by Zaika et al. showed that MDM2 interacts with p53 in neuroblastomas, ruling out the possibility that the abnormal p53 stability in neuroblastoma cells is due to the absence of the p53-MDM2 interaction [45]. The in vivo ubiquitylation of p53 in neuroblastoma cells is also functional and still catalyzed by MDM2 in these cells [45].
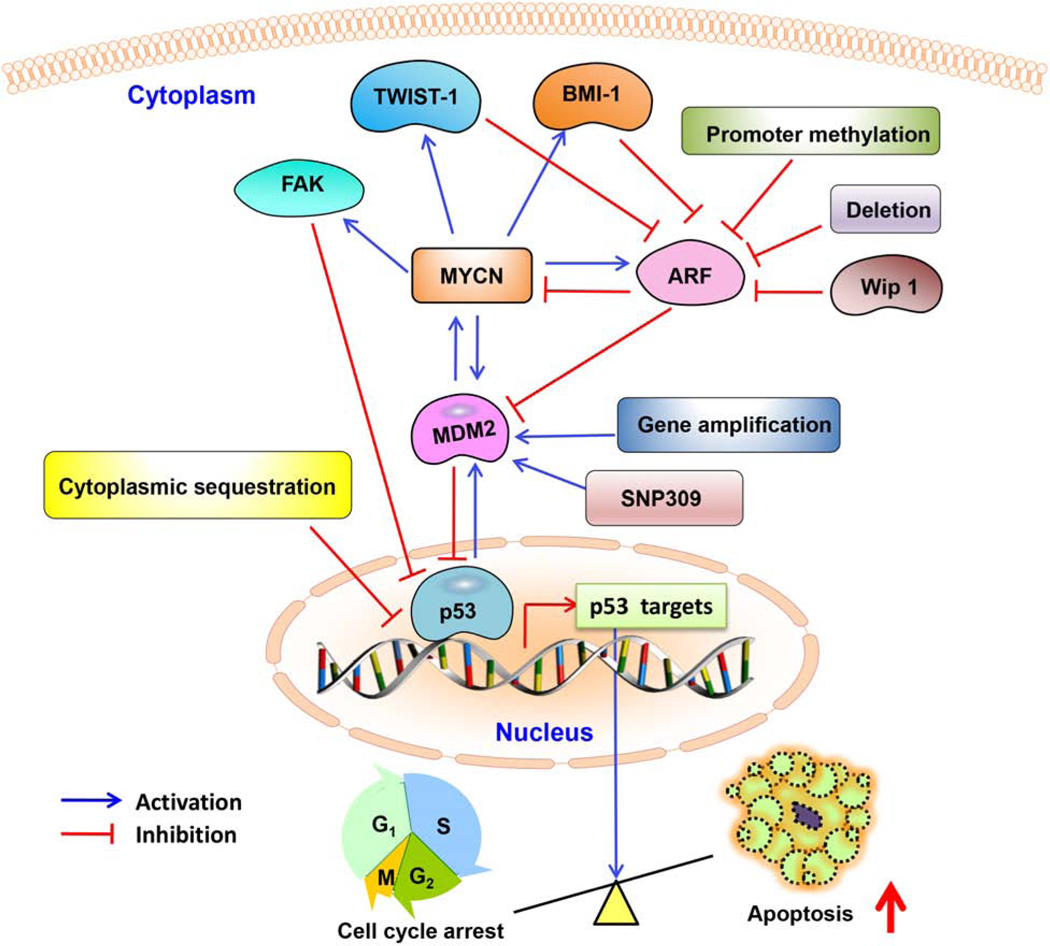
The p53-MDM2 pathway in neuroblastoma is modulated at several different molecular levels, including by direct interactions with other proteins (Fig. 3). In general, p14ARF directly binds MDM2 and impairs MDM2’s regulation of p53 [60]. In neuroblastoma, ARF may be inactivated due to deletions or promoter methylation, resulting in increased MDM2 [28, 61]. According to estimates, approximately half of all neuroblastoma cells lines established from patient tumors after relapse have genetic or epigenetic lesions of the p14ARF or MDM2 locus [62]. In addition, there is increased expression of homeobox protein B cell-specific Moloney murine leukemia virus integration site 1 (BMI-1) and twist family BHLH transcription factor 1 (TWIST-1) in neuroblastoma, both of which contribute to the inhibition of ARF activity [63]. A study by Selmi et al. indicated that N-Myc binds to and activates the TWIST1 promoter, causing the upregulation of TWIST1 in neuroblastoma cells [64]. Likewise, it has been demonstrated that BMI1 is a direct target gene of N-Myc in neuroblastoma cells [65, 66]. Furthermore, the escape from p53-mediated tumor surveillance in neuroblastoma cells has also been attributed to the loss of chromatin remodelling proteins involved in the transcriptional regulation of the cyclin-dependent kinase inhibitor 2A (CDKN2A) (p16INK4a/p14ARF) locus [67]. An amplification or gain of genetic material on the chromosome 17 long arm is the most frequently observed alteration in neuroblastoma [67]. A gene at 17q23.2, encoding protein phosphatase, Mg2+/Mn2+ dependent 1D (PPM1D) (Wip1; a key negative regulator of p53) is overexpressed in neuroblastoma cell lines with a 17q gain/amplification [67]. Overexpression of PPM1D in neuroblastoma provides another mechanism resulting in p14ARF-MDM2-p53 axis deregulation [67].
Figure 3. p53-MDM2 pathway regulation in neuroblastoma cells.
The p53-MDM2 pathway is regulated by several proteins including MYCN, focal adhesion kinase (FAK), ARF, B cell-specific Moloney murine leukemia virus integration site 1 (BMI-1), twist family BHLH transcription factor 1 (TWIST-1), and wild-type p53-induced phosphatase 1 (WIP-1).
A study by Amente et al. has indicated that p14ARF overexpression inhibits the transcriptional activity of N-Myc in human Tet21N neuroblastoma cells [68]. The same study has demonstrated that p14ARF co-immunoprecipitated with N-Myc, suggesting that p14ARF interacts with N-Myc in vivo, and that there is co-localization of p14ARF and N-Myc in neuroblastoma cells [68]. These findings suggest that there is an interaction between p14ARF and N-Myc in neuroblastoma cells, and also that p14ARF inhibits the transcriptional activity of N-Myc in neuroblastoma cells [68]. Future research is needed to experimentally validate the endogenous interaction of p14ARF with N-Myc and to understand the exact mechanism by which p14ARF inhibits the N-Myc function in neuroblastoma cells. However, the research by Amente et al. described above has shown that the ARF protein inhibits MYCN function [68], and a study by Kim and Shohet demonstrated that ARF is, in turn, activated by the N-Myc protein [49, 68]. Thus, there is feedback regulation of the ARF-MDM2-TP53 axis that controls p53 expression in neuroblastoma cells. It is expected that several other mechanisms exist for p53 suppression in chemoresistant neuroblastoma, and it is prudent to investigate the detailed mechanisms of all of these to facilitate the development of an effective therapy for neuroblastoma patients.
It has recently been reported that focal adhesion kinase (FAK) directly interacts with the p53 protein in neuroblastoma cells, and disruption of the FAK-p53 interaction via a specific peptide (RP-TAT) results in inhibited neuroblastoma cell viability [69]. It was also found that there is an inverse correlation between FAK and p53 in neuroblastoma cells [69]. A study by Slack et al. demonstrated that MDM2 is a transcriptional target of MYCN in MYCN-amplified IMR-32 neuroblastoma cells, and the MYCN inhibition results in reduced MDM2 mRNA expression, increased p53 stability, and apoptosis induction [57]. As mentioned above, MDM2 also binds MYCN mRNA and increases its translation [59]. MYCN binds the promoter of FAK and induces FAK expression in neuroblastoma cells [70]. Although the detailed mechanisms are still being elucidated, these findings suggest that there is a FAK-MYCN-MDM2-p53 axis that may be responsible for p53 stabilization and apoptosis induction in neuroblastoma cells.
Extensive research has been conducted to demonstrate the crosstalk of the MDM2-p53 pathway with MYCN. One such study was conducted by He et al., and showed that MDM2 overexpression inhibits p53 expression in non-MYCN-amplified neuroblastoma cells, while MDM2 overexpression fails to affect the p53 expression in MYCN-amplified neuroblastoma cells [71]. This provides clear evidence that the regulation of p53 by MDM2 is dependent on the MYCN status in neuroblastoma cells. The same study also provided evidence that MDM2 and MYCN reciprocally regulate p53, and the p53 function and expression remain constant in response to MDM2 modification in MYCN-amplified neuroblastoma [71].
5. Targeting the p53-MDM2 pathway in neuroblastoma: A valid therapeutic target?
As mentioned above, the p53-MDM2 pathway has recently emerged as a potential therapeutic target for numerous cancers, including neuroblastoma. Since most neuroblastomas have functional, wild-type p53, it is prudent to either develop a direct MDM2 inhibitor or target protein modulators to inhibit MDM2, thus increasing the functional activity of p53. On the other hand, considering its p53-independent activity, an ideal MDM2 inhibitor should also exert anticancer activity independent of wide-type p53.
Our group is among the first to propose targeting MDM2 for cancer therapy and prevention [30, 40]. We and others have proposed several novel strategies to target MDM2 for cancer therapy [40]. For instance, the discovery and development of oligonucleotide therapeutics targeting MDM2 represented a novel approach to activating p53 [72–74]. The identification of several p53-independent functions of MDM2 and the determination of novel cellular molecules that interact with MDM2 and/or the MDM2-p53 pathways (p21 [75], E2F transcription factor 1 (E2F1) [76], PA28gamma [77], RING1 and YY1 binding protein (RYBP) [78], ribosomal protein S7 (RPS7) [79], RPS25 [80], and nuclear factor of activated T cells 1 (NFAT1) [81]) has broadened the scope of potential MDM2 inhibitors. The identification of natural product-based MDM2 inhibitors (genistein [82], curcumin [83], ginsenosides [84–91], artemisinin [92–94], and sesquiterpenoids [95–99]), and discovery of synthetic small molecule MDM2 inhibitors (synthetic iminoquinones [100–105] and SP series [106–111]) have shown that both p53-dependent and –independent activities can be targeted by MDM2 inhibitors. It is estimated that, thus far, there have been over 200 MDM2 inhibitors reported of various chemical classes and that function by different mechanisms of action [112–116]. In the upcoming section, we discuss the MDM2 inhibitors currently being investigated in preclinical and clinical studies of neuroblastoma.
5.1. Preclinical studies of MDM2 inhibitors in neuroblastoma
Many MDM2 inhibitors have been reported to have in vitro and in vivo anticancer activities in preclinical studies [113, 115], and some of them have entered clinical trials [117, 118]. When developing an MDM2 inhibitor, it is important that the following desirable drug-like properties be kept in mind when selecting a clinical candidate: (1) high binding affinity and specificity towards MDM2; (2) high cytotoxicity to neuroblastoma cells with both wild type and dysfunctional p53; and (3) a desirable pharmacokinetic profile with low systemic toxicity.
5.1.1. Nutlins (cis-imidazolines)
As shown in Figure 4, Nutlin-3, a p53/MDM2 antagonist, has been shown to be effective against the variety of cancers with wild-type p53 protein, including neuroblastoma [119]. An in vitro study showed that nutlin-3 treatment targets the MDM2 and p53 interaction and activates the p53 pathway in both chemosensitive and chemoresistant wild-type p53 neuroblastoma cells [119]. The study also demonstrated that oral administration of Nutlin-3 led to primary tumor growth inhibition, p53 activation, and reduced metastasis in mice bearing chemoresistant/p53 wild type neuroblastoma xenografts [119]. In addition, a study by Barbieri et al. showed that Nutlin-3a increases the anticancer effects of chemotherapeutic drugs (such as etoposide, cisplatin, and camptothecin) and mediates apoptosis through rapid stabilization of p53 in neuroblastoma cells [120]. Nutlin-3a interferes with p53 hyperubiquitylation and results in the relocalization of p53 from the cytoplasm to the nucleus, and also reactivates p53’s transcription function and induction of apoptosis in neuroblastoma cells [46]. Furthermore, Nutlin-3 uncouples p53 from MDM2 and induces p53 and HIPK2 accumulation, consequently triggering apoptosis in MYCN-amplified (MNA) neuroblastoma cells [63]. This suggests that Nutlin-3 not only uncouples p53 from MDM2 inhibition, but also causes HIPK2 induction and apoptosis. In a subsequent study, Nutlin-3 was found to induce cell death, and works in cooperation with clastogenic drugs (cisplatin, doxorubicin, and bleomycin) by downregulating galectin-3 in MNA neuroblastoma cells [121]. Additionally, another study showed that Nutlin-3a treatment induces p53 accumulation and apoptosis in MYCN-amplified neuroblastoma cell lines, which are more sensitive to apoptosis compared to non-MYCN-amplified neuroblastoma cell lines [122]. A recent study by Merugu et al. provided the first evidence that ex vivo Nutlin-3 treatment of blood and bone marrow increases p53 and p21 expression in circulating tumor cells (CTCs) and disseminated tumor cells (DTCs) from neuroblastoma patients [123]. However, in spite of the potent efficacy observed in in vitro and in vivo studies, Nutlin-3 has poor bioavailability, making it sub-optimal for clinical use. Thus, more research is needed to develop effective MDM2 inhibitors with both high potency and acceptable bioavailability and pharmacological properties.
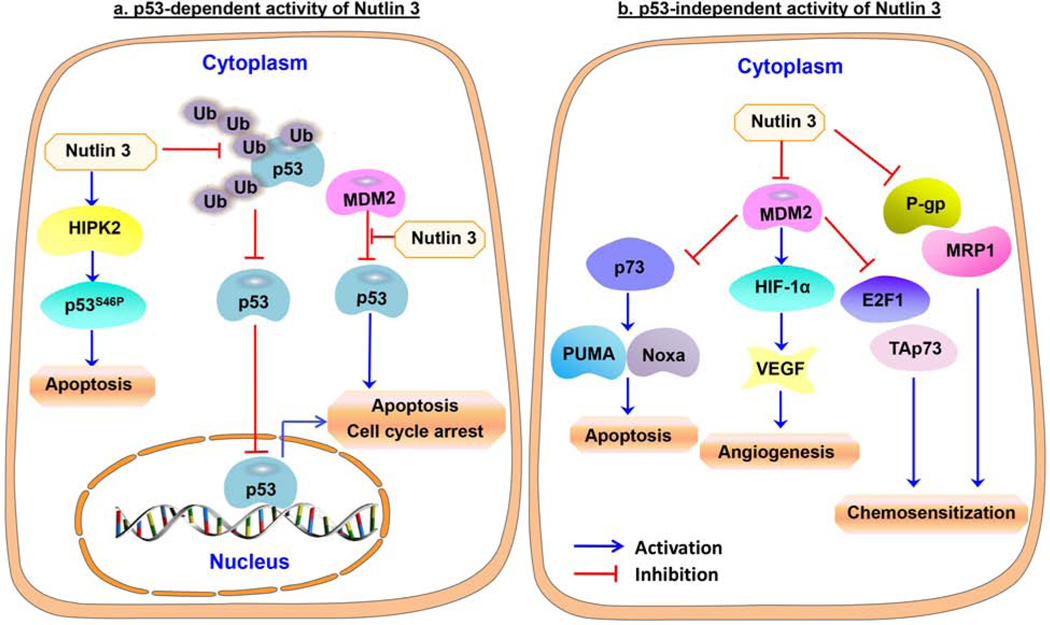
Figure 4. Molecular mechanisms of action of Nutlins in neuroblastoma cells.
(a) The p53-dependent activity of Nutlins 3 and 3a. Nutlins bind to the p53 binding pocket in MDM2 and inhibit the p53-MDM2 interaction. In addition, Nutlin-3a also inhibits p53 hyperubiquitylation, allowing the re-import of p53 into the nucleus to activate apoptosis. In neuroblastoma cells, Nutlin-3 also activates HIPK2 to increase the phosphorylation of p53 at serine 46, resulting in apoptosis. (b) The p53-independent activity of Nutlins. Nutlin-3 inhibits P-glycoprotein (P-gp) and multidrug resistance protein 1 (MRP1) and increases the sensitivity of cancer cells to chemotherapeutic drugs. Nutlin treatment also decreases VEGF production and angiogenesis in neuroblastoma cells, and blocks the interaction of MDM2 with p73, resulting in increased expression of p73 and PUMA/Noxa to induce apoptosis in neuroblastoma cells.
Evidence has shown that Nutlin-3 activates TAp73 and E2F1 and potentiates the ability of doxorubicin to inhibit cell proliferation and induce apoptosis in doxorubicin-resistant p53-null neuroblastoma cells [124]. In addition, another study showed that Nutlin-3a disrupts the MDM2-p73 interaction and then activates and stabilizes pro-apoptotic p73 in neuroblastoma cells [125]. It has also been found that Nutlin-3a competitively inhibits P-glycoprotein (P-gp) and multi-drug resistant protein 1 (MRP1) and sensitizes chemotherapy-resistant neuroblastoma cells to treatment [126]. A study by Patterson et al. demonstrated that MDM2 inhibition by Nutlin-3a inhibits hypoxia-inducible factor 1 alpha (HIF-1α) expression and downstream VEGF expression in neuroblastoma, and decreases the metastatic burden in mice bearing tumor xenografts [127].
In addition, various p53-dependent and -independent activities have been proposed for the Nutlins, particularly effects on deregulated metabolism, genomic instability, inflammation, and immune evasion [40, 57], and these warrant further investigation. In spite of the extensive use of Nutlin-3 for the activation of p53 in cancer cells, the non-specific, off-target activity of Nutlin-3 has been emerged as a challenge to its further use. A report by Michaelis et al. indicated that continuous exposure of p53 wild-type neuroblastoma cells to Nutlin-3 leads to the establishment of p53-mutated, multidrug-resistant sublines [128]. Thus, cancer patients with Nutlin-3 treatment should be carefully monitored for the emergence of p53 mutations and drug resistance. Interestingly, neuroblastoma cells that have adapted to treatment with RITA (a small molecule that prevents MDM2 from binding to p53) remain sensitive to Nutlin-3 treatment and also display a lower resistance phenotype and radiation resistance compared to Nutlin-3-adapted neuroblastoma cells [129]. Thus, there may be some rationale for combining different MDM2/p53 inhibitors. Nevertheless, new MDM2 inhibitors should be designed with higher binding affinity towards MDM2 in cancer cells, while avoiding off-target effects in normal cells.
5.1.2. SAR405838 (MI-77301)
SAR405838 (MI-77301) is a potent and orally-active spirooxindole MDM2 antagonist that inhibits the interaction between MDM2 and p53. Recently, Lu et al. demonstrated that this molecule induces p53-mediated apoptosis in p53 wild-type neuroblastoma cells by stabilizing p53 both in vitro and in vivo [130]. The same study also showed that SAR405838 has similar activity to Nutlin-3 in p53 wild-type IMR-32 neuroblastoma cells [130]. Moreover, it has been found that the addition of SAR405838 to doxorubicin in p53 wild-type chemo-sensitive neuroblastoma IMR-32 and SH-SY5Y cells lines led to more potent effects compared to treatment with doxorubicin alone [130]. SAR405838 also augmented the doxorubicin-induced apoptosis in chemoresistant neuroblastoma LA-N-6 cancer cells, suggesting that it overcomes the chemoresistance in neuroblastoma cells [130].
5.1.3. MK-8242
MK-8242 is a potent small molecule inhibitor of MDM2 that stabilizes p53 and induces apoptosis or cell growth arrest. In the Pediatric Preclinical Testing Consortium (PPTC), in vitro testing of the compound showed that it exerted anticancer effects against wild type TP53 neuroblastoma cells lines, with an IC50 value less than 0.2 μM, whereas the IC50 for a cell line with mutant TP53 was greater than 10 μM [131].
5.1.4. MI-63
MI-63 is a non-peptide spirooxindole that inhibits the p53-MDM2 interaction. A study by Gamble et al. indicated that MI-63 results in more extensive apoptosis in MYCN-amplified cells compared to non-MYCN-amplified neuroblastoma cell lines [122]. Treatment of neuroblastoma cells with MI-63 leads to slight G1 arrest, but knocking down MYCN does not affect the cell cycle response to MI-63 in these cells, suggesting that the effects on apoptosis and cell cycle progression occur via different pathways [122].
5.1.5. RG7388 (RO5503781 or idasanutlin)
RG7388 is a second-generation Nutlin developed to improve the efficacy and toxicity profile of the Nutlins. RG7388 treatment induced functional activation of the p53 pathway and apoptosis in p53 wild-type neuroblastoma cell lines. However, it caused a decrease in p53 expression in all p53 mutant neuroblastoma cell lines studied [132]. Chen et al. also highlighted that when RG7388 is used in combination with chemotherapeutic drugs, it leads to increased apoptosis in p53 wild-type neuroblastoma cells [133].
5.1.6. RG7112 (RO5045337)
RG7112 is derived from the structural modification of Nutlin-3a. RG7112 has been found to inhibit the p53-MDM2 interaction and restore the physiological activity of p53 in different malignancies including ovarian cancer, glioblastoma, leukemia, and soft tissue sarcoma. Recently, it has been observed that RG7112 reduces the viability and enhances the p52/p21 protein expression in wild-type p53 neuroblastoma cells [134]. Interestingly, the combination of RG7112 and navitoclax (a B-cell lymphoma 2 (Bcl2)/B-cell lymphoma-extra large (Bcl-xL) inhibitor) may exert synergistic activity in neuroblastoma cells [134].
5.1.7. RG7775 (RO6839921)
RG7775 inhibits the interaction between MDM2 and p53, leading to stabilization of p53 and subsequent activation of the p53 pathway in a TP53 wild-type orthotopic model of neuroblastoma [135]. Further, the combination of RG7775 with temozolomide induced significantly stronger growth inhibition in comparison to treatment with either agent alone in mouse orthotopic models of SH-SY5Y-Luc and NB1691-Luc tumors [135]. RG7775 also exhibits a favourable pharmacokinetic profile and is well tolerated alone and in combination with various other agents [135].
There are several other small molecule MDM2 inhibitors currently being tested in preclinical models of neuroblastoma. The structures and the known in vitro and in vivo activities of these inhibitors are summarized in Table 1.
Table 1.
Overview of other MDM2 inhibitors and their effects in neuroblastoma
| Compound | Structure | In vitro and in vivo activities |
|---|---|---|
| DS-3032 (Milademetan) [136] | 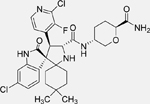 |
• Inhibits growth and migration in TP53 wild-type NB cells • Induces arrest at the G1/S transition, senescence, and apoptosis in TP53 wild-type NB cells • Increases TP53 expression in NB cell lines harbouring wild-type TP53 • Reduces xenograft neuroblastoma tumor growth by activating p53 signaling in vivo |
| Compound 12 [137] | 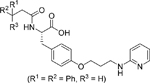 |
• Dual inhibitor of the MDM2/p53 complex and MDM4/p53 complex • Increases p53 protein levels in human NB SH-SY5Y cells • Enhances p53 target gene expression (MDM2, p21, and PUMA) • Inhibits the proliferation of SH-SY5Y cells • Inhibits the growth of cancer stem cells isolated from SH-SY5Y NB cells • Induces apoptosis in SH-SY5Y cells |
| 1-carbaldehyde-3,4-dimethoxyxanth one (LEM2) [138] | 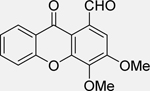 |
• Disrupts the interaction of TAp73 with both MDM2 and mutant p53 in NB cells • Induces G2/M phase arrest • Enhances TAp73 transcriptional activity and apoptosis in p53-null and mutant NB cells • Active against patient-derived NB cells • Synergistic effects with doxorubicin and cisplatin in patient-derived NB cells |
| RITA [139] | • Inhibits growth and induces apoptosis in NB cell lines • Disrupts the interaction between p53 and MDM2/MDMX in NB cells • Induces the expression of p53 targets (PUMA, Noxa, BCL2 Associated X (Bax), and p21) • Induces p53 reactivation and inhibits the expression of MYCN in neuroblastoma cells • Inhibits tumor growth in SKNDZ xenograft in mice, and reduces the expression of N-Myc in xenograft tumors |
|
| Roscovitine (Seliciclib or CYC202) [140] | 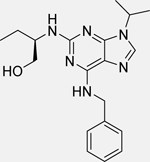 |
• Reduces the viability of NB cells • Induces G2/M cell cycle arrest at 1 μM concentration • Activates p53 and dependent genes (Bax, p21) • Inhibits MDM2, N-Myc, and Akt1 expression • Increases the expression of miR-34a, b c, miR-200b, miR-107, miR-542-5p, and miR-605 that lead to activation of apoptosis in NB cells |
| C2 | 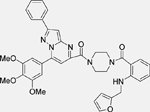 |
|
| C3 | 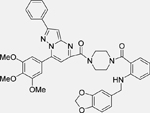 |
|
| SF1126 [141] | 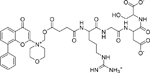 |
• Inhibits proliferation and induces apoptosis in NB cells • Increases sensitivity to doxorubicin in vitro and exhibits synergistic activity in combination with doxorubicin • Reduces the levels pAkt and pMDM2 in a dose- and time course-dependent manner • Inhibits survivin expression, MDM2 activation, and IGF-1-induced Akt phosphorylation • Disrupts the actin cytoskeleton • Inhibits tumor growth in mice bearing NB xenograft tumors |
| Berberine [142] | 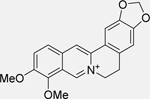 |
• Downregulates death domainassociated protein (DAXX), leading to inhibition of MDM2 • Activates p53 and leads to subsequent apoptosis in NB cells |
| Compound 1 [143] | 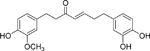 |
• Induces S phase arrest and apoptosis in NB cells • Increases the protein expression levels of ATF2, p53, and p-p53 • Increases ATF3 gene expression, with no significant change in p53 gene expression • Increases the stability of the p53 rotein, and downregulates MDM2 and Aurora A |
5.2. Combination therapy using MDM2 antagonists and other molecules in neuroblastoma
Monotherapy based on MDM2 antagonists has not been able to provide long-lasting anticancer responses. For example, the acquisition of a de novo mutation in the TP53 DNA binding domain leads to the development of resistance to Nutlin-3 in neuroblastoma cells [128]. Thus, using MDM2 antagonists in combination with other drugs or experimental molecules could lead to improved effects and may provide a better therapeutic strategy with lower relapse rates for neuroblastoma patients. Table 2 summarizes the outcomes of previous studies of combination treatment using MDM2 antagonists and other agents in neuroblastoma.
Table 2.
Overview of combination therapy using MDM2 antagonists with other agents in neuroblastoma
| MDM2 antagonist | Combination agent | In vitro and in vivo activities in neuroblastoma |
|---|---|---|
RG7388 (Idasanutlin) [144] 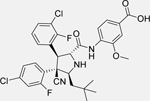
|
Venetoclax (ABT-199) 
|
• The combination of ABT-199 and RG7388 dramatically reduces cell viability compared to treatment with either compound alone • Increases caspase-3 and caspase-7 activity compared to single agent exposure • The combination of idasanutlin and venetoclax inhibits NB tumor growth in vivo significantly more than either treatment alone |
Nutlin-3a or RG7388 [145]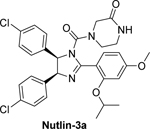
|
Temsirolimus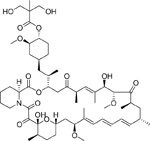
|
• MDM2 inhibition by these antagonists enhances temsirolimusmediated cell growth arrest in all p53 wild-type cell lines • This effect is not observed in p53-mutant or p53-knockdown NB cells • Combination therapy leads to marked induction of apoptosis in p53 wild-type cells; which is abrogated in p53 mutant cells • The combination of temsirolimus and RG7388 strongly potentiates the antitumor activity of both single monotherapies in vivo • Combination treatment profoundly represses survivin expression at both the mRNA and protein levels in p53 wild-type cells, but not in p53 mutant cells |
CGM097 [146]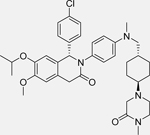
|
Ceritinib 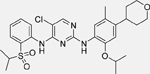
|
• Combination treatment leads to increased antitumor activity in TP53 wild-type NB cell lines harboring ALK aberrations (ALK amplification or mutation) • Combination treatment increases the levels of PARP cleavage (enhanced apoptosis) in TP53 wild-type NB cells • Combination treatment leads to increased antitumor activity in TP53 wild-type neuroblastoma xenograft tumors • CGM097 restores ceritinib sensitivity in NB cells |
Nutlin-3 [147]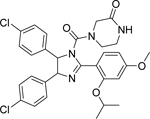
|
YM155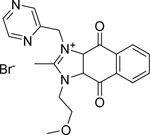
|
• Combination treatment increases the anticancer effects of YM155, p53 signaling (increased p53, p21 levels), and DNA damage (γH2AX) in NB cells |
Nutlin-3 [148] 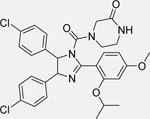
|
Flubendazole 
|
• Combination treatment enhances the anti-cancer effects of flubendazole in p53 wild-type NB cells as determined by cell viability assays |
Nutlin-3 [149]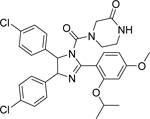
|
Tozasertib 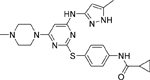
|
• Nutlin-3 increases the effects of tozasertib in p53 wild-type NB cells, as determined by a cell viability assay |
Nutlin-3a [127]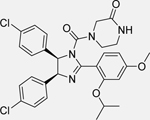
|
Bevacizumab | • Combination treatment leads to significant suppression of the growth of neuroblastoma xenograft tumors compared to control or single agent treatment • Combination treatment leads to significant decreases in the incidence of metastasis in vivo |
Nutlin-3 [150]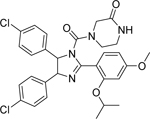
|
Roscovitine (Seliciclib)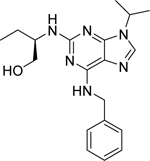
|
• The combination of roscovitine with nutlin-3 synergistically induces apoptosis in NB cells • Combination treatment leads to increased expression of p53, HDM2, and p21, similar to that observed with nutlin-3 alone |
5.3. Clinical studies targeting the p53-MDM2 pathway in neuroblastoma
The preclinical studies above provide evidence that the p53-MDM2 pathway can serve as a critical molecular target for neuroblastoma. Further, these studies have shown that MDM2 inhibitors, such as Nutlin-3, are often effective even in p53-null neuroblastoma cells, highlighting the p53-independent actions of MDM2 inhibitors in neuroblastoma [124]. Despite these promising findings, inhibitors of the MDM2-p53 interaction have not been extensively investigated in clinical trials of neuroblastoma. The details of the limited number of active and terminated clinical trials of MDM2 inhibitors in neuroblastoma are summarized in Table 3.
Table 3.
Clinical trials for neuroblastoma using molecules targeting MDM2 (data obtained from ClinicalTrials.gov)
| Compound | Responsible company | Phase | Status | Clinicaltrial identifier | Other condition(s) targeted in the trial |
|---|---|---|---|---|---|
| RG7388 | Hoffmann-La Roche | Phase I/II | Recruiting | NCT04029688 | • Acute myeloid leukemia (AML) • Acute lymphoblastic leukemia (ALL) • Solid tumors |
| HDM201 | Yael P Mosse, Children’s Hospital of Philadelphia | Phase 1 | Recruiting | NCT02780128 |
There is a current phase I/II clinical study of RG7388 (id: NCT04029688) designed to evaluate the safety, efficacy, and pharmacokinetics of RG7388 (idasanutlin) alone or in combination with either chemotherapy or venetoclax for relapsed/refractory acute leukemia or solid tumors in pediatric and young adult patients. This study is divided into three parts: the first part focuses on identifying the maximum tolerated dose (MTD) and characterizing dose-limiting toxicities (DLTs) of RG7388 in pediatric patients; the second part mainly focuses on evaluating the safety and efficacy of RG7388 in combination with chemotherapy or venetoclax in acute myelocytic leukemia (AML), acute lymphoblastic leukemia (ALL), and neuroblastoma patients; and the last part will be conducted as a further assessment of RG7388 in combination with other agents in AML, ALL, and neuroblastoma patients.
Personalized medicine is considered to be an important strategy to streamline the drug development process and identify molecules that could foster a change in the healthcare system [151–153]. One trial attempting to provide personalized medicine is the Next Generation Personalized Neuroblastoma Therapy (NEPENTHE) study, which involves various treatments/interventions, including HDM201 (a MDM2 inhibitor), next generation sequencing, bone marrow tests, and tumor scans. A clinical study (id: NCT02780128) of this approach is attempting to match genomic aberrations in cells from relapsed tumors with rationally-designed, molecular targeted drug combinations. In the first part of the study, the patient tumors will be subjected to deep sequencing for protocol-specified biomarker identification, and in the second part, participants will be assigned to the tailored therapies based on the genetic changes found in the tumor biopsy.
Even though MDM2 inhibitors are entering into clinical trials for neuroblastoma, major concerns still exist, which include the suggestion that continuous exposure to MDM2 inhibitors may lead to the development of resistance in cancer cells. For instance, continuous exposure to Nutlin-3 can lead to the development of somatic mutations in p53 and the emergence of multi-drug resistant neuroblastoma cells in vitro [128]. Resistance to MDM2 inhibitors has also been reported in other malignancies. In particular, a study by Hoffman-Luca et al. showed that prolonged SAR405838 treatment in a SJSA-1 osteosarcoma cell line resulted in profound resistance due to the development of a mutation of p53 in the DNA binding domain, which prevented it from being activated by SAR405838 [154]. The authors have also highlighted that while SAR405838 treatment led to rapid tumor regression in a SJSA-1 xenograft tumor model, the tumors eventually regrew [154]. In addition, SAR405838 is less effective against in vivo-derived sublines harbouring a single heterozygous C176F p53 mutation, suggesting that cells may be able to develop resistance rapidly and via single mutations [154]. Pharmacological resistance to SAR405838 has also been observed at the clinical level in liposarcoma patients (id: NCT01636479) [155]. Moreover, TP53 mutations often appear in the circulating cell-free DNA of dedifferentiated liposarcoma patients treated with SAR405838, and such mutations increase over time, with a correlation with changes in tumor size, and these are likely to represent the selection of TP53 mutant clones resistant to MDM2 inhibition [155]. Hence, it is important to have a comprehensive understanding of MDM2 amplification in neuroblastoma, as well as its crosstalk with p53 and other proteins, and the impact of mutations of these different molecules should be investigated to facilitate the development of effective MDM2 inhibitors.
Importantly, MDM2 inhibitors should be tested in experimental models which have more relevance to human disease, because this will make it possible to better predict the effects in clinical trials. Currently, the models which are used for drug safety and efficacy studies and neuroblastoma research include zebrafish, mice, and the chick chorioallantoic membrane (CAM) [156]. These in vivo models include transgenic, xenograft, syngeneic, and humanized animals [157]. Among these, the patient-derived xenograft (PDX) murine model may have the highest relevance to human disease because it retains features of human neuroblastoma, such as the inclusion of some of the human tumor microenvironment (i.e., stromal cells), genetic complexity, histopathology, and the mutational and proteomic profiles [156]. Thus, PDX models can be used to screen and identify effective MDM2 inhibitors for neuroblastoma treatment. One of the aims of the PPTC is to use genomically-characterized patient-derived xenograft lines to test new drugs, providing reliable preclinical in vivo data and identifying agents that can move forward to pediatric clinical trials (NCI PPTC, www.ncipptc.org) [158].
6. Discussion and future directions
Disruption of the p53-MDM2 interaction using MDM2 inhibitors is an appealing therapeutic strategy for neuroblastoma patients that exhibit low numbers of p53 mutations. Many different chemical classes of compounds have been found to inhibit the MDM2-p53 interaction in neuroblastoma cells, including cis-imidazolines (Nutlins), spirooxindoles (MI compounds) and imidazopyrrolidinone. In spite of the fact that MDM2 inhibitors appear to be active against neuroblastoma, treatment-related toxicity and the development of resistance have been demonstrated, so better inhibitors should be designed that can specifically target malignant cells, avoid systemic toxicity, and prevent the development of resistance or mutations. Among the available MDM2 inhibitors, Nutlin-3 has been the extensively used, and has been evaluated in different malignancies, including neuroblastoma [119, 159–161]. Nutlin-3 exhibits both p53-dependent and -independent functions in neuroblastoma cells, and hence can target both p53 wild-type and p53-null neuroblastoma cells [119, 124]. However, Nutlin-3 treatment leads to the development of p53-mutated, multidrug-resistant clones of neuroblastoma cells [128]. Thus, Nutlin-3 is currently being used as a parent compound to synthesize new derivatives which can overcome the development of drug resistance. Such in silico approaches may also help to identify new structural modifications of Nutlin-3 with increased MDM2 binding affinity. The chemoresistance to Nutlin-3 can also be overcome using other agents, such as RITA (another MDM2 antagonist), as it has been found that RITA-adapted neuroblastoma cells are less resistant to chemotherapeutic drugs than Nutlin-3-adapted neuroblastoma cells [129]. In addition, the potential of using a combination of (or sequential treatment with) different MDM2 inhibitors and other small protein inhibitors (such as a Bcl-2 inhibitor, mammalian target of rapamycin (mTOR) inhibitor, ALK inhibitor, survivin inhibitor, aurora inhibitor, VEGF-A inhibitor, and/or cyclin-dependent kinases (CDK) inhibitor) should be implemented to increase the efficacy of MDM2 inhibitors in neuroblastoma cells (see Table 2 for examples). There are several other aspects of development which still need to be addressed to obtain a safe and effective MDM2 inhibitor for neuroblastoma treatment. These include: (1) Is the identified MDM2 inhibitor specific, and does it only activate apoptosis or cell cycle arrest in neuroblastoma cells, sparing the normal host cells? (2) What will be the effect of the MDM2 inhibitor on the p53-MDM2 feedback loop in neuroblastoma cells? (3) Is inhibition of MDM2 via MDM2 antagonists sufficient to cause p53 stabilization and induce apoptosis in neuroblastoma cells?
Although MDM2 interacts with a large number of proteins in addition to p53, MDMX also plays a pivotal role in neuroblastoma cells [162]. In fact, MDMX is required for the inactivation of p53 in neuroblastoma cells [162]. MDMX inhibition by specific shRNA induces the expression of p21 (a target of p53) and reduces the cytoplasmic localization of p53 [162]. The depletion of MDMX decreases the p53 levels in the cytoplasm, and increases the p53 levels in the nuclei of neuroblastoma cells [162]. The research on neuroblastoma has mainly focused on identifying and testing MDM2 inhibitors; very little effort has been made to identify MDMX inhibitors for p53 reactivation in neuroblastoma. Since both MDM2 and MDMX modulate the p53 activity in neuroblastoma cells, identifying dual inhibitors of both MDM2 and MDMX should be implemented to induce additive or synergistic effects. As described in Table 1, Compound 1 has been found to be a dual inhibitor of MDM2/MDM4 (MDMX) and was tested in human neuroblastoma SH-SY5Y cells [143]. Future research should use the scaffold of compound 1 to develop more effective MDM2/MDM4 (MDMX) dual inhibitors with increased activity and bioavailability.
The expression of MDM2 is regulated by several different proteins, including MYCN [57]. A study should therefore be implemented employing a combinatorial regimen of pharmacological inhibition of MYCN along with MDM2 antagonists. In addition, research should also be focused on deciphering other aspects of MDM2 regulation to permit the development of more specific and potent MDM2 inhibitors. The utilization of high-throughput virtual and cell-based screening techniques, natural and synthetic combinatorial libraries, and the use of biochemical and molecular biology approaches, should also be implemented for the development of new MDM2 inhibitors to treat neuroblastoma. Additionally, the use of MDM2 inhibitors should be coupled with laboratory-based monitoring of biomarkers that would help in selecting the patients most likely to benefit from either monotherapy or combination treatment, and to assess the response to treatment.
In summary, although there are many MDM2 inhibitors that have been reported to have effects against various cancers, none of them has been approved for clinical use. There are also some limitations to those currently under clinical investigation. It is hoped that more clinical candidates with better efficacy and safety profiles will enter clinical trials in the near future, and will ultimately affirm that MDM2 is a valid molecular target for the treatment of neuroblastoma.
Highlights.
Targeting p53-MDM2 Pathway is a validated approach for neuroblastoma Therapy;
MDM2 overexpression and constitutive activation are common in neuroblastoma;
MDM2 promotes chemical-induced carcinogenesis;
MDM2 promotes cancer progression by regulating multiple cellular functions; and
MDM2 inhibitors have anticancer activity in neuroblastoma models.
Acknowledgements
We thank the current and former members of our laboratory and our collaborators for their contributions to the publications cited in this review article. Considering that the p53-MDM2 research is a rapidly-growing field, we apologize for not being able to cite many publications due to the limited space allotted for this review.
W.W. and R.Z. were partially supported by National Institutes of Health (NIH)/National Cancer Institute grants (R01CA186662 and R01CA214019). W.W. and R.Z. were also supported by American Cancer Society (ACS) grant RSG-15-009-01-CDD. R.Z. was partially supported by funds for the Robert L. Boblitt Endowed Professor in Drug Discovery and research funds from the College of Pharmacy and University of Houston. J.Z. was supported in part by the John D. Stobo, M.D. Distinguished Chair Endowment Fund, University of Texas Medical Branch. The contents of the article are solely the responsibility of the authors, and do not necessarily represent the official views of the National Institutes of Health or other funding agencies. We thank Dr. Elizabeth Rayburn for excellent assistance in the preparation of this manuscript.
Footnotes
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Nakagawara A, Li Y, Izumi H, Muramori K, Inada H, Nishi M, Neuroblastoma, Japanese journal of clinical oncology, 48 (2018) 214–241. [DOI] [PubMed] [Google Scholar]
- [2].Mallepalli S, Gupta MK, Vadde R, Neuroblastoma: An Updated Review on Biology and Treatment, Current drug metabolism, 20 (2019) 1014–1022. [DOI] [PubMed] [Google Scholar]
- [3].Newman EA, Abdessalam S, Aldrink JH, Austin M, Heaton TE, Bruny J, Ehrlich P, Dasgupta R, Baertschiger RM, Lautz TB, Rhee DS, Langham MR Jr., Malek MM, Meyers RL, Nathan JD, Weil BR, Polites S, Madonna MB, Update on neuroblastoma, Journal of pediatric surgery, 54 (2019) 383–389. [DOI] [PubMed] [Google Scholar]
- [4].Gatta G, Botta L, Rossi S, Aareleid T, Bielska-Lasota M, Clavel J, Dimitrova N, Jakab Z, Kaatsch P, Lacour B, Mallone S, Marcos-Gragera R, Minicozzi P, Sánchez-Pérez MJ, Sant M, Santaquilani M, Stiller C, Tavilla A, Trama A, Visser O, Peris-Bonet R, Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5--a population-based study, The Lancet. Oncology, 15 (2014) 35–47. [DOI] [PubMed] [Google Scholar]
- [5].Lacour B, Goujon S, Guissou S, Guyot-Goubin A, Desmée S, Désandes E, Clavel J, Childhood cancer survival in France, 2000–2008, European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP), 23 (2014) 449–457. [DOI] [PubMed] [Google Scholar]
- [6].Karim-Kos HE, Hackl M, Mann G, Urban C, Woehrer A, Slavc I, Ladenstein R, Trends in incidence, survival and mortality of childhood and adolescent cancer in Austria, 1994–2011, Cancer epidemiology, 42 (2016) 72–81. [DOI] [PubMed] [Google Scholar]
- [7].Ward E, DeSantis C, Robbins A, Kohler B, Jemal A, Childhood and adolescent cancer statistics, 2014, CA: a cancer journal for clinicians, 64 (2014) 83–103. [DOI] [PubMed] [Google Scholar]
- [8].Bidwell SS, Peterson CC, Demanelis K, Zarins KR, Meza R, Sriplung H, Wiangnon S, Chotsampancharoen T, Chitapanarux I, Pongnikorn D, Daoprasert K, Suwanrungruang K, Chansaard W, Rozek LS, Childhood cancer incidence and survival in Thailand: A comprehensive population-based registry analysis, 1990–2011, Pediatric blood & cancer, 66 (2019) e27428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].The Lancet H, Childhood cancer on the agenda, The Lancet. Haematology, 6 (2019) e285. [DOI] [PubMed] [Google Scholar]
- [10].Zheng R, Peng X, Zeng H, Zhang S, Chen T, Wang H, Chen W, Incidence, mortality and survival of childhood cancer in China during 2000–2010 period: A population-based study, Cancer letters, 363 (2015) 176–180. [DOI] [PubMed] [Google Scholar]
- [11].Trama A, Botta L, Foschi R, Ferrari A, Stiller C, Desandes E, Maule MM, Merletti F, Gatta G, Survival of European adolescents and young adults diagnosed with cancer in 2000–07: population-based data from EUROCARE-5, The Lancet. Oncology, 17 (2016) 896–906. [DOI] [PubMed] [Google Scholar]
- [12].Peinemann F, van Dalen EC, Berthold F, Rapid COJEC Induction Therapy for High-risk Neuroblastoma Patients - Cochrane Review, Klinische Padiatrie, 228 (2016) 130–134. [DOI] [PubMed] [Google Scholar]
- [13].Peinemann F, Tushabe DA, van Dalen EC, Berthold F, Rapid COJEC versus standard induction therapies for high-risk neuroblastoma, The Cochrane database of systematic reviews, (2015) Cd010774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ngan ES, Heterogeneity of neuroblastoma, Oncoscience, 2 (2015) 837–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF, Nakagawara A, Berthold F, Schleiermacher G, Park JR, Valteau-Couanet D, Pearson AD, Cohn SL, Advances in Risk Classification and Treatment Strategies for Neuroblastoma, Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 33 (2015) 3008–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tolbert VP, Matthay KK, Neuroblastoma: clinical and biological approach to risk stratification and treatment, Cell and tissue research, 372 (2018) 195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aygun N, Biological and Genetic Features of Neuroblastoma and Their Clinical Importance, Current pediatric reviews, 14 (2018) 73–90. [DOI] [PubMed] [Google Scholar]
- [18].Pugh TJ, Morozova O, Attiyeh EF, Asgharzadeh S, Wei JS, Auclair D, Carter SL, Cibulskis K, Hanna M, Kiezun A, Kim J, Lawrence MS, Lichenstein L, McKenna A, Pedamallu CS, Ramos AH, Shefler E, Sivachenko A, Sougnez C, Stewart C, Ally A, Birol I, Chiu R, Corbett RD, Hirst M, Jackman SD, Kamoh B, Khodabakshi AH, Krzywinski M, Lo A, Moore RA, Mungall KL, Qian J, Tam A, Thiessen N, Zhao Y, Cole KA, Diamond M, Diskin SJ, Mosse YP, Wood AC, Ji L, Sposto R, Badgett T, London WB, Moyer Y, Gastier-Foster JM, Smith MA, Guidry Auvil JM, Gerhard DS, Hogarty MD, Jones SJ, Lander ES, Gabriel SB, Getz G, Seeger RC, Khan J, Marra MA, Meyerson M, Maris JM, The genetic landscape of high-risk neuroblastoma, Nature genetics, 45 (2013) 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nicolai S, Pieraccioli M, Peschiaroli A, Melino G, Raschellà G, Neuroblastoma: oncogenic mechanisms and therapeutic exploitation of necroptosis, Cell death & disease, 6 (2015) e2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bálint EE, Vousden KH, Activation and activities of the p53 tumour suppressor protein, British journal of cancer, 85 (2001) 1813–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Harris SL, Levine AJ, The p53 pathway: positive and negative feedback loops, Oncogene, 24 (2005) 2899–2908. [DOI] [PubMed] [Google Scholar]
- [22].Bullock AN, Fersht AR, Rescuing the function of mutant p53, Nature reviews. Cancer, 1 (2001) 68–76. [DOI] [PubMed] [Google Scholar]
- [23].Hainaut P, Hollstein M, p53 and human cancer: the first ten thousand mutations, Advances in cancer research, 77 (2000) 81–137. [DOI] [PubMed] [Google Scholar]
- [24].Vogan K, Bernstein M, Leclerc JM, Brisson L, Brossard J, Brodeur GM, Pelletier J, Gros P, Absence of p53 gene mutations in primary neuroblastomas, Cancer research, 53 (1993) 5269–5273. [PubMed] [Google Scholar]
- [25].Chen L, Malcolm AJ, Wood KM, Cole M, Variend S, Cullinane C, Pearson AD, Lunec J, Tweddle DA, p53 is nuclear and functional in both undifferentiated and differentiated neuroblastoma, Cell cycle (Georgetown, Tex.), 6 (2007) 2685–2696. [DOI] [PubMed] [Google Scholar]
- [26].Goldman SC, Chen CY, Lansing TJ, Gilmer TM, Kastan MB, The p53 signal transduction pathway is intact in human neuroblastoma despite cytoplasmic localization, The American journal of pathology, 148 (1996) 1381–1385. [PMC free article] [PubMed] [Google Scholar]
- [27].Tweddle DA, Pearson AD, Haber M, Norris MD, Xue C, Flemming C, Lunec J, The p53 pathway and its inactivation in neuroblastoma, Cancer letters, 197 (2003) 93–98. [DOI] [PubMed] [Google Scholar]
- [28].Corvi R, Savelyeva L, Breit S, Wenzel A, Handgretinger R, Barak J, Oren M, Amler L, Schwab M, Non-syntenic amplification of MDM2 and MYCN in human neuroblastoma, Oncogene, 10 (1995) 1081–1086. [PubMed] [Google Scholar]
- [29].Cattelani S, Defferrari R, Marsilio S, Bussolari R, Candini O, Corradini F, Ferrari-Amorotti G, Guerzoni C, Pecorari L, Menin C, Bertorelle R, Altavista P, McDowell HP, Boldrini R, Dominici C, Tonini GP, Raschellà G, Calabretta B, Impact of a single nucleotide polymorphism in the MDM2 gene on neuroblastoma development and aggressiveness: results of a pilot study on 239 patients, Clinical cancer research : an official journal of the American Association for Cancer Research, 14 (2008) 3248–3253. [DOI] [PubMed] [Google Scholar]
- [30].Rayburn E, Zhang R, He J, Wang H, MDM2 and human malignancies: expression, clinical pathology, prognostic markers, and implications for chemotherapy, Current cancer drug targets, 5 (2005) 27–41. [DOI] [PubMed] [Google Scholar]
- [31].Momand J, Zambetti GP, Olson DC, George D, Levine AJ, The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation, Cell, 69 (1992) 1237–1245. [DOI] [PubMed] [Google Scholar]
- [32].Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B, Amplification of a gene encoding a p53-associated protein in human sarcomas, Nature, 358 (1992) 80–83. [DOI] [PubMed] [Google Scholar]
- [33].Haupt Y, Maya R, Kazaz A, Oren M, Mdm2 promotes the rapid degradation of p53, Nature, 387 (1997) 296–299. [DOI] [PubMed] [Google Scholar]
- [34].Van Maerken T, Speleman F, Vermeulen J, Lambertz I, De Clercq S, De Smet E, Yigit N, Coppens V, Philippé J, De Paepe A, Marine JC, Vandesompele J, Small-molecule MDM2 antagonists as a new therapy concept for neuroblastoma, Cancer research, 66 (2006) 9646–9655. [DOI] [PubMed] [Google Scholar]
- [35].Xue C, Haber M, Flemming C, Marshall GM, Lock RB, MacKenzie KL, Gurova KV, Norris MD, Gudkov AV, p53 determines multidrug sensitivity of childhood neuroblastoma, Cancer research, 67 (2007) 10351–10360. [DOI] [PubMed] [Google Scholar]
- [36].Gu L, Zhu N, Zhang H, Durden DL, Feng Y, Zhou M, Regulation of XIAP translation and induction by MDM2 following irradiation, Cancer cell, 15 (2009) 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zeng X, Chen L, Jost CA, Maya R, Keller D, Wang X, Kaelin WG Jr., Oren M, Chen J, Lu H, MDM2 suppresses p73 function without promoting p73 degradation, Molecular and cellular biology, 19 (1999) 3257–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shi Y, Takenobu H, Kurata K, Yamaguchi Y, Yanagisawa R, Ohira M, Koike K, Nakagawara A, Jiang LL, Kamijo T, HDM2 impairs Noxa transcription and affects apoptotic cell death in a p53/p73-dependent manner in neuroblastoma, European journal of cancer (Oxford, England : 1990), 46 (2010) 2324–2334. [DOI] [PubMed] [Google Scholar]
- [39].Rufini A, Agostini M, Grespi F, Tomasini R, Sayan BS, Niklison-Chirou MV, Conforti F, Velletri T, Mastino A, Mak TW, Melino G, Knight RA, p73 in Cancer, Genes & cancer, 2 (2011) 491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nag S, Qin J, Srivenugopal KS, Wang M, Zhang R, The MDM2-p53 pathway revisited, Journal of biomedical research, 27 (2013) 254–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Davidoff AM, Pence JC, Shorter NA, Iglehart JD, Marks JR, Expression of p53 in human neuroblastoma- and neuroepithelioma-derived cell lines, Oncogene, 7 (1992) 127–133. [PubMed] [Google Scholar]
- [42].Moll UM, LaQuaglia M, Bénard J, Riou G, Wild-type p53 protein undergoes cytoplasmic sequestration in undifferentiated neuroblastomas but not in differentiated tumors, Proceedings of the National Academy of Sciences of the United States of America, 92 (1995) 4407–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Moll UM, Ostermeyer AG, Haladay R, Winkfield B, Frazier M, Zambetti G, Cytoplasmic sequestration of wild-type p53 protein impairs the G1 checkpoint after DNA damage, Molecular and cellular biology, 16 (1996) 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Stommel JM, Marchenko ND, Jimenez GS, Moll UM, Hope TJ, Wahl GM, A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking, The EMBO journal, 18 (1999) 1660–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zaika A, Marchenko N, Moll UM, Cytoplasmically “sequestered” wild type p53 protein is resistant to Mdm2-mediated degradation, The Journal of biological chemistry, 274 (1999) 27474–27480. [DOI] [PubMed] [Google Scholar]
- [46].Becker K, Marchenko ND, Maurice M, Moll UM, Hyperubiquitylation of wild-type p53 contributes to cytoplasmic sequestration in neuroblastoma, Cell death and differentiation, 14 (2007) 1350–1360. [DOI] [PubMed] [Google Scholar]
- [47].Sengupta S, Vonesch JL, Waltzinger C, Zheng H, Wasylyk B, Negative cross-talk between p53 and the glucocorticoid receptor and its role in neuroblastoma cells, The EMBO journal, 19 (2000) 6051–6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tweddle DA, Malcolm AJ, Cole M, Pearson AD, Lunec J, p53 cellular localization and function in neuroblastoma: evidence for defective G(1) arrest despite WAF1 induction in MYCN-amplified cells, The American journal of pathology, 158 (2001) 2067–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kim E, Shohet J, Targeted molecular therapy for neuroblastoma: the ARF/MDM2/p53 axis, Journal of the National Cancer Institute, 101 (2009) 1527–1529. [DOI] [PubMed] [Google Scholar]
- [50].Tweddle DA, Malcolm AJ, Bown N, Pearson AD, Lunec J, Evidence for the development of p53 mutations after cytotoxic therapy in a neuroblastoma cell line, Cancer research, 61 (2001) 8–13. [PubMed] [Google Scholar]
- [51].Keshelava N, Zuo JJ, Chen P, Waidyaratne SN, Luna MC, Gomer CJ, Triche TJ, Reynolds CP, Loss of p53 function confers high-level multidrug resistance in neuroblastoma cell lines, Cancer research, 61 (2001) 6185–6193. [PubMed] [Google Scholar]
- [52].Teitz T, Wei T, Liu D, Valentine V, Valentine M, Grenet J, Lahti JM, Kidd VJ, Caspase-9 and Apaf-1 are expressed and functionally active in human neuroblastoma tumor cell lines with 1p36 LOH and amplified MYCN, Oncogene, 21 (2002) 1848–1858. [DOI] [PubMed] [Google Scholar]
- [53].Goldschneider D, Horvilleur E, Plassa LF, Guillaud-Bataille M, Million K, Wittmer-Dupret E, Danglot G, de Thé H, Bénard J, May E, Douc-Rasy S, Expression of C-terminal deleted p53 isoforms in neuroblastoma, Nucleic acids research, 34 (2006) 5603–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Goldschneider D, Blanc E, Raguénez G, Barrois M, Legrand A, Le Roux G, Haddada H, Bénard J, Douc-Rasy S, Differential response of p53 target genes to p73 overexpression in SH-SY5Y neuroblastoma cell line, Journal of cell science, 117 (2004) 293–301. [DOI] [PubMed] [Google Scholar]
- [55].Honda R, Tanaka H, Yasuda H, Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53, FEBS letters, 420 (1997) 25–27. [DOI] [PubMed] [Google Scholar]
- [56].S.F.-A. Cattelani G; Soliera AR; Manzotti G; Raschellà G; Calabretta B, Neuroblastoma: Role of MDM2 and SNP309 as Markers, Pediatric Cancer, 4 19–25 [Google Scholar]
- [57].Slack A, Chen Z, Tonelli R, Pule M, Hunt L, Pession A, Shohet JM, The p53 regulatory gene MDM2 is a direct transcriptional target of MYCN in neuroblastoma, Proceedings of the National Academy of Sciences of the United States of America, 102 (2005) 731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhou S, Gu L, He J, Zhang H, Zhou M, MDM2 regulates vascular endothelial growth factor mRNA stabilization in hypoxia, Molecular and cellular biology, 31 (2011) 4928–4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gu L, Zhang H, He J, Li J, Huang M, Zhou M, MDM2 regulates MYCN mRNA stabilization and translation in human neuroblastoma cells, Oncogene, 31 (2012) 1342–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang Y, Xiong Y, Yarbrough WG, ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways, Cell, 92 (1998) 725–734. [DOI] [PubMed] [Google Scholar]
- [61].Carr-Wilkinson J, O’Toole K, Wood KM, Challen CC, Baker AG, Board JR, Evans L, Cole M, Cheung NK, Boos J, Köhler G, Leuschner I, Pearson AD, Lunec J, Tweddle DA, High Frequency of p53/MDM2/p14ARF Pathway Abnormalities in Relapsed Neuroblastoma, Clinical cancer research : an official journal of the American Association for Cancer Research, 16 (2010) 1108–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Carr J, Bell E, Pearson AD, Kees UR, Beris H, Lunec J, Tweddle DA, Increased frequency of aberrations in the p53/MDM2/p14(ARF) pathway in neuroblastoma cell lines established at relapse, Cancer research, 66 (2006) 2138–2145. [DOI] [PubMed] [Google Scholar]
- [63].Petroni M, Veschi V, Gulino A, Giannini G, Molecular mechanisms of MYCN-dependent apoptosis and the MDM2-p53 pathway: an Achille’s heel to be exploited for the therapy of MYCN-amplified neuroblastoma, Frontiers in oncology, 2 (2012) 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Selmi A, de Saint-Jean M, Jallas AC, Garin E, Hogarty MD, Bénard J, Puisieux A, Marabelle A, Valsesia-Wittmann S, TWIST1 is a direct transcriptional target of MYCN and MYC in neuroblastoma, Cancer letters, 357 (2015) 412–418. [DOI] [PubMed] [Google Scholar]
- [65].Huang R, Cheung NK, Vider J, Cheung IY, Gerald WL, Tickoo SK, Holland EC, Blasberg RG, MYCN and MYC regulate tumor proliferation and tumorigenesis directly through BMI1 in human neuroblastomas, FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 25 (2011) 4138–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ochiai H, Takenobu H, Nakagawa A, Yamaguchi Y, Kimura M, Ohira M, Okimoto Y, Fujimura Y, Koseki H, Kohno Y, Nakagawara A, Kamijo T, Bmi1 is a MYCN target gene that regulates tumorigenesis through repression of KIF1Bbeta and TSLC1 in neuroblastoma, Oncogene, 29 (2010) 2681–2690. [DOI] [PubMed] [Google Scholar]
- [67].Van Maerken T, Vandesompele J, Rihani A, De Paepe A, Speleman F, Escape from p53-mediated tumor surveillance in neuroblastoma: switching off the p14(ARF)-MDM2-p53 axis, Cell death and differentiation, 16 (2009) 1563–1572. [DOI] [PubMed] [Google Scholar]
- [68].Amente S, Gargano B, Diolaiti D, Della Valle G, Lania L, Majello B, p14ARF interacts with N-Myc and inhibits its transcriptional activity, FEBS letters, 581 (2007) 821–825. [DOI] [PubMed] [Google Scholar]
- [69].Gillory LA, Stewart JE, Megison ML, Waters AM, Beierle EA, Focal adhesion kinase and p53 synergistically decrease neuroblastoma cell survival, The Journal of surgical research, 196 (2015) 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Beierle EA, Trujillo A, Nagaram A, Kurenova EV, Finch R, Ma X, Vella J, Cance WG, Golubovskaya VM, N-MYC regulates focal adhesion kinase expression in human neuroblastoma, The Journal of biological chemistry, 282 (2007) 12503–12516. [DOI] [PubMed] [Google Scholar]
- [71].He J, Gu L, Zhang H, Zhou M, Crosstalk between MYCN and MDM2-p53 signal pathways regulates tumor cell growth and apoptosis in neuroblastoma, Cell cycle (Georgetown, Tex.), 10 (2011) 2994–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wang H, Oliver P, Zhang Z, Agrawal S, Zhang R, Chemosensitization and radiosensitization of human cancer by antisense anti-MDM2 oligonucleotides: in vitro and in vivo activities and mechanisms, Annals of the New York Academy of Sciences, 1002 (2003) 217–235. [DOI] [PubMed] [Google Scholar]
- [73].Zhang R, Wang H, Agrawal S, Novel antisense anti-MDM2 mixed-backbone oligonucleotides: proof of principle, in vitro and in vivo activities, and mechanisms, Current cancer drug targets, 5 (2005) 43–49. [DOI] [PubMed] [Google Scholar]
- [74].Zhang Z, Li M, Wang H, Agrawal S, Zhang R, Antisense therapy targeting MDM2 oncogene in prostate cancer: Effects on proliferation, apoptosis, multiple gene expression, and chemotherapy, Proceedings of the National Academy of Sciences of the United States of America, 100 (2003) 11636–11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zhang Z, Wang H, Li M, Agrawal S, Chen X, Zhang R, MDM2 is a negative regulator of p21WAF1/CIP1, independent of p53, The Journal of biological chemistry, 279 (2004) 16000–16006. [DOI] [PubMed] [Google Scholar]
- [76].Zhang Z, Wang H, Li M, Rayburn ER, Agrawal S, Zhang R, Stabilization of E2F1 protein by MDM2 through the E2F1 ubiquitination pathway, Oncogene, 24 (2005) 7238–7247. [DOI] [PubMed] [Google Scholar]
- [77].Zhang Z, Zhang R, Proteasome activator PA28 gamma regulates p53 by enhancing its MDM2-mediated degradation, The EMBO journal, 27 (2008) 852–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chen D, Zhang J, Li M, Rayburn ER, Wang H, Zhang R, RYBP stabilizes p53 by modulating MDM2, EMBO reports, 10 (2009) 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Chen D, Zhang Z, Li M, Wang W, Li Y, Rayburn ER, Hill DL, Wang H, Zhang R, Ribosomal protein S7 as a novel modulator of p53-MDM2 interaction: binding to MDM2, stabilization of p53 protein, and activation of p53 function, Oncogene, 26 (2007) 5029–5037. [DOI] [PubMed] [Google Scholar]
- [80].Zhang X, Wang W, Wang H, Wang MH, Xu W, Zhang R, Identification of ribosomal protein S25 (RPS25)-MDM2-p53 regulatory feedback loop, Oncogene, 32 (2013) 2782–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Zhang X, Zhang Z, Cheng J, Li M, Wang W, Xu W, Wang H, Zhang R, Transcription factor NFAT1 activates the mdm2 oncogene independent of p53, The Journal of biological chemistry, 287 (2012) 30468–30476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Li M, Zhang Z, Hill DL, Chen X, Wang H, Zhang R, Genistein, a dietary isoflavone, down-regulates the MDM2 oncogene at both transcriptional and posttranslational levels, Cancer research, 65 (2005) 8200–8208. [DOI] [PubMed] [Google Scholar]
- [83].Li M, Zhang Z, Hill DL, Wang H, Zhang R, Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway, Cancer research, 67 (2007) 1988–1996. [DOI] [PubMed] [Google Scholar]
- [84].Nag SA, Qin JJ, Wang W, Wang MH, Wang H, Zhang R, Ginsenosides as Anticancer Agents: In vitro and in vivo Activities, Structure-Activity Relationships, and Molecular Mechanisms of Action, Frontiers in pharmacology, 3 (2012) 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Wang W, Qin JJ, Li X, Tao G, Wang Q, Wu X, Zhou J, Zi X, Zhang R, Prevention of prostate cancer by natural product MDM2 inhibitor GS25: in vitro and in vivo activities and molecular mechanisms, Carcinogenesis, 39 (2018) 1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wang W, Rayburn ER, Hang J, Zhao Y, Wang H, Zhang R, Anti-lung cancer effects of novel ginsenoside 25-OCH(3)-PPD, Lung cancer (Amsterdam, Netherlands), 65 (2009) 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wang W, Rayburn ER, Hao M, Zhao Y, Hill DL, Zhang R, Wang H, Experimental therapy of prostate cancer with novel natural product anti-cancer ginsenosides, The Prostate, 68 (2008) 809–819. [DOI] [PubMed] [Google Scholar]
- [88].Wang W, Rayburn ER, Zhao Y, Wang H, Zhang R, Novel ginsenosides 25-OH-PPD and 25-OCH3-PPD as experimental therapy for pancreatic cancer: anticancer activity and mechanisms of action, Cancer letters, 278 (2009) 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wang W, Wang H, Rayburn ER, Zhao Y, Hill DL, Zhang R, 20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol, a novel natural product for prostate cancer therapy: activity in vitro and in vivo and mechanisms of action, British journal of cancer, 98 (2008) 792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Wang W, Zhang X, Qin JJ, Voruganti S, Nag SA, Wang MH, Wang H, Zhang R, Natural product ginsenoside 25-OCH3-PPD inhibits breast cancer growth and metastasis through down-regulating MDM2, PloS one, 7 (2012) e41586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wang W, Zhao Y, Rayburn ER, Hill DL, Wang H, Zhang R, In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng, Cancer chemotherapy and pharmacology, 59 (2007) 589–601. [DOI] [PubMed] [Google Scholar]
- [92].Chen T, Li M, Zhang R, Wang H, Dihydroartemisinin induces apoptosis and sensitizes human ovarian cancer cells to carboplatin therapy, Journal of cellular and molecular medicine, 13 (2009) 1358–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Hou J, Wang D, Zhang R, Wang H, Experimental therapy of hepatoma with artemisinin and its derivatives: in vitro and in vivo activity, chemosensitization, and mechanisms of action, Clinical cancer research : an official journal of the American Association for Cancer Research, 14 (2008) 5519–5530. [DOI] [PubMed] [Google Scholar]
- [94].Li X, Ba Q, Liu Y, Yue Q, Chen P, Li J, Zhang H, Ying H, Ding Q, Song H, Liu H, Zhang R, Wang H, Dihydroartemisinin selectively inhibits PDGFRα-positive ovarian cancer growth and metastasis through inducing degradation of PDGFRα protein, Cell discovery, 3 (2017) 17042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Li X, Yang X, Liu Y, Gong N, Yao W, Chen P, Qin J, Jin H, Li J, Chu R, Shan L, Zhang R, Zhang W, Wang H, Japonicone A suppresses growth of Burkitt lymphoma cells through its effect on NF-κB, Clinical cancer research : an official journal of the American Association for Cancer Research, 19 (2013) 2917–2928. [DOI] [PubMed] [Google Scholar]
- [96].Qin JJ, Sarkar S, Voruganti S, Agarwal R, Wang W, Zhang R, Identification of lineariifolianoid A as a novel dual NFAT1 and MDM2 inhibitor for human cancer therapy, Journal of biomedical research, 30 (2016) 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Qin JJ, Wang W, Sarkar S, Voruganti S, Agarwal R, Zhang R, Inulanolide A as a new dual inhibitor of NFAT1-MDM2 pathway for breast cancer therapy, Oncotarget, 7 (2016) 32566–32578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Qin JJ, Wang W, Voruganti S, Wang H, Zhang WD, Zhang R, Inhibiting NFAT1 for breast cancer therapy: New insights into the mechanism of action of MDM2 inhibitor JapA, Oncotarget, 6 (2015) 33106–33119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Qin JJ, Wang W, Voruganti S, Wang H, Zhang WD, Zhang R, Identification of a new class of natural product MDM2 inhibitor: In vitro and in vivo anti-breast cancer activities and target validation, Oncotarget, 6 (2015) 2623–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Nadkarni DH, Wang F, Wang W, Rayburn ER, Ezell SJ, Murugesan S, Velu SE, Zhang R, Synthesis and in vitro anti-lung cancer activity of novel 1, 3, 4, 8-tetrahydropyrrolo [4, 3, 2-de]quinolin-8(1H)-one alkaloid analogs, Medicinal chemistry (Shariqah (United Arab Emirates)), 5 (2009) 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Wang F, Ezell SJ, Zhang Y, Wang W, Rayburn ER, Nadkarni DH, Murugesan S, Velu SE, Zhang R, FBA-TPQ, a novel marine-derived compound as experimental therapy for prostate cancer, Investigational new drugs, 28 (2010) 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wang W, Cheng JW, Qin JJ, Hu B, Li X, Nijampatnam B, Velu SE, Fan J, Yang XR, Zhang R, MDM2-NFAT1 dual inhibitor, MA242: Effective against hepatocellular carcinoma, independent of p53, Cancer letters, 459 (2019) 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Wang W, Qin JJ, Voruganti S, Nijampatnam B, Velu SE, Ruan KH, Hu M, Zhou J, Zhang R, Discovery and Characterization of Dual Inhibitors of MDM2 and NFAT1 for Pancreatic Cancer Therapy, Cancer research, 78 (2018) 5656–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wang W, Rayburn ER, Velu SE, Chen D, Nadkarni DH, Murugesan S, Chen D, Zhang R, A novel synthetic iminoquinone, BA-TPQ, as an anti-breast cancer agent: in vitro and in vivo activity and mechanisms of action, Breast cancer research and treatment, 123 (2010) 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Wang W, Rayburn ER, Velu SE, Nadkarni DH, Murugesan S, Zhang R, In vitro and in vivo anticancer activity of novel synthetic makaluvamine analogues, Clinical cancer research : an official journal of the American Association for Cancer Research, 15 (2009) 3511–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Deokar H, Deokar M, Wang W, Zhang R, Buolamwini JK, QSAR Studies of New Pyrido[3,4-b]indole Derivatives as Inhibitors of Colon and Pancreatic Cancer Cell Proliferation, Medicinal chemistry research : an international journal for rapid communications on design and mechanisms of action of biologically active agents, 27 (2018) 2466–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Patil SA, Addo JK, Deokar H, Sun S, Wang J, Li W, Suttle DP, Wang W, Zhang R, Buolamwini JK, Synthesis, Biological Evaluation and Modeling Studies of New Pyrido[3,4-b]indole Derivatives as Broad-Spectrum Potent Anticancer Agents, Drug designing : open access, 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Qin JJ, Wang W, Li X, Deokar H, Buolamwini JK, Zhang R, Inhibiting β-Catenin by β-Carboline-Type MDM2 Inhibitor for Pancreatic Cancer Therapy, Frontiers in pharmacology, 9 (2018) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Qin JJ, Wang W, Sarkar S, Zhang R, Oral delivery of anti-MDM2 inhibitor SP141-loaded FcRn-targeted nanoparticles to treat breast cancer and metastasis, Journal of controlled release : official journal of the Controlled Release Society, 237 (2016) 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Wang W, Qin JJ, Voruganti S, Srivenugopal KS, Nag S, Patil S, Sharma H, Wang MH, Wang H, Buolamwini JK, Zhang R, The pyrido[b]indole MDM2 inhibitor SP-141 exerts potent therapeutic effects in breast cancer models, Nature communications, 5 (2014) 5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wang W, Qin JJ, Voruganti S, Wang MH, Sharma H, Patil S, Zhou J, Wang H, Mukhopadhyay D, Buolamwini JK, Zhang R, Identification of a new class of MDM2 inhibitor that inhibits growth of orthotopic pancreatic tumors in mice, Gastroenterology, 147 (2014) 893–902.e892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Espadinha M, Barcherini V, Lopes EA, Santos MMM, An Update on MDMX and Dual MDM2/X Inhibitors, Current topics in medicinal chemistry, 18 (2018) 647–660. [DOI] [PubMed] [Google Scholar]
- [113].Liu Y, Wang X, Wang G, Yang Y, Yuan Y, Ouyang L, The past, present and future of potential small-molecule drugs targeting p53-MDM2/MDMX for cancer therapy, European journal of medicinal chemistry, 176 (2019) 92–104. [DOI] [PubMed] [Google Scholar]
- [114].Qin JJ, Li X, Hunt C, Wang W, Wang H, Zhang R, Natural products targeting the p53-MDM2 pathway and mutant p53: Recent advances and implications in cancer medicine, Genes & diseases, 5 (2018) 204–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Rusiecki R, Witkowski J, Joanna JA, MDM2-p53 Interaction Inhibitors: The Current State-of-Art and Updated Patent Review (2010-Present), Recent patents on anti-cancer drug discovery, 14 (2019) 324–369. [DOI] [PubMed] [Google Scholar]
- [116].Qin JJ, Nag S, Voruganti S, Wang W, Zhang R, Natural product MDM2 inhibitors: anticancer activity and mechanisms of action, Current medicinal chemistry, 19 (2012) 5705–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Burgess A, Chia KM, Haupt S, Thomas D, Haupt Y, Lim E, Clinical Overview of MDM2/X-Targeted Therapies, Frontiers in oncology, 6 (2016) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Tisato V, Voltan R, Gonelli A, Secchiero P, Zauli G, MDM2/X inhibitors under clinical evaluation: perspectives for the management of hematological malignancies and pediatric cancer, Journal of hematology & oncology, 10 (2017) 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Van Maerken T, Ferdinande L, Taildeman J, Lambertz I, Yigit N, Vercruysse L, Rihani A, Michaelis M, Cinatl J Jr., Cuvelier CA, Marine JC, De Paepe A, Bracke M, Speleman F, Vandesompele J, Antitumor activity of the selective MDM2 antagonist nutlin-3 against chemoresistant neuroblastoma with wild-type p53, Journal of the National Cancer Institute, 101 (2009) 1562–1574. [DOI] [PubMed] [Google Scholar]
- [120].Barbieri E, Mehta P, Chen Z, Zhang L, Slack A, Berg S, Shohet JM, MDM2 inhibition sensitizes neuroblastoma to chemotherapy-induced apoptotic cell death, Molecular cancer therapeutics, 5 (2006) 2358–2365. [DOI] [PubMed] [Google Scholar]
- [121].Veschi V, Petroni M, Cardinali B, Dominici C, Screpanti I, Frati L, Bartolazzi A, Gulino A, Giannini G, Galectin-3 impairment of MYCN-dependent apoptosis-sensitive phenotype is antagonized by nutlin-3 in neuroblastoma cells, PloS one, 7 (2012) e49139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Gamble LD, Kees UR, Tweddle DA, Lunec J, MYCN sensitizes neuroblastoma to the MDM2-p53 antagonists Nutlin-3 and MI-63, Oncogene, 31 (2012) 752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Merugu S, Chen L, Gavens E, Gabra H, Brougham M, Makin G, Ng A, Murphy D, Gabriel AS, Robinson ML, Wright JH, Burchill SA, Humphreys A, Bown N, Jamieson D, Tweddle DA, Detection of Circulating and Disseminated Neuroblastoma Cells Using the ImageStream Flow Cytometer for Use as Predictive and Pharmacodynamic Biomarkers, Clinical cancer research : an official journal of the American Association for Cancer Research, 26 (2020) 122–134. [DOI] [PubMed] [Google Scholar]
- [124].Peirce SK, Findley HW, The MDM2 antagonist nutlin-3 sensitizes p53-null neuroblastoma cells to doxorubicin via E2F1 and TAp73, International journal of oncology, 34 (2009) 1395–1402. [PubMed] [Google Scholar]
- [125].Lau LM, Nugent JK, Zhao X, Irwin MS, HDM2 antagonist Nutlin-3 disrupts p73-HDM2 binding and enhances p73 function, Oncogene, 27 (2008) 997–1003. [DOI] [PubMed] [Google Scholar]
- [126].Michaelis M, Rothweiler F, Klassert D, von Deimling A, Weber K, Fehse B, Kammerer B, Doerr HW, Cinatl J Jr., Reversal of P-glycoprotein-mediated multidrug resistance by the murine double minute 2 antagonist nutlin-3, Cancer research, 69 (2009) 416–421. [DOI] [PubMed] [Google Scholar]
- [127].Patterson DM, Gao D, Trahan DN, Johnson BA, Ludwig A, Barbieri E, Chen Z, Diaz-Miron J, Vassilev L, Shohet JM, Kim ES, Effect of MDM2 and vascular endothelial growth factor inhibition on tumor angiogenesis and metastasis in neuroblastoma, Angiogenesis, 14 (2011) 255–266. [DOI] [PubMed] [Google Scholar]
- [128].Michaelis M, Rothweiler F, Barth S, Cinatl J, van Rikxoort M, Löschmann N, Voges Y, Breitling R, von Deimling A, Rödel F, Weber K, Fehse B, Mack E, Stiewe T, Doerr HW, Speidel D, Cinatl J Jr., Adaptation of cancer cells from different entities to the MDM2 inhibitor nutlin-3 results in the emergence of p53-mutated multi-drug-resistant cancer cells, Cell death & disease, 2 (2011) e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Michaelis M, Rothweiler F, Agha B, Barth S, Voges Y, Löschmann N, von Deimling A, Breitling R, Doerr HW, Rödel F, Speidel D, Cinatl J Jr., Human neuroblastoma cells with acquired resistance to the p53 activator RITA retain functional p53 and sensitivity to other p53 activating agents, Cell death & disease, 3 (2012) e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Lu J, Guan S, Zhao Y, Yu Y, Wang Y, Shi Y, Mao X, Yang KL, Sun W, Xu X, Yi JS, Yang T, Yang J, Nuchtern JG, Novel MDM2 inhibitor SAR405838 (MI-773) induces p53-mediated apoptosis in neuroblastoma, Oncotarget, 7 (2016) 82757–82769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Kang MH, Reynolds CP, Kolb EA, Gorlick R, Carol H, Lock R, Keir ST, Maris JM, Wu J, Lyalin D, Kurmasheva RT, Houghton PJ, Smith MA, Initial Testing (Stage 1) of MK-8242-A Novel MDM2 Inhibitor-by the Pediatric Preclinical Testing Program, Pediatric blood & cancer, 63 (2016) 1744–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Lakoma A, Barbieri E, Agarwal S, Jackson J, Chen Z, Kim Y, McVay M, Shohet JM, Kim ES, The MDM2 small-molecule inhibitor RG7388 leads to potent tumor inhibition in p53 wild-type neuroblastoma, Cell death discovery, 1 (2015) 15026-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Chen L, Rousseau RF, Middleton SA, Nichols GL, Newell DR, Lunec J, Tweddle DA, Pre-clinical evaluation of the MDM2-p53 antagonist RG7388 alone and in combination with chemotherapy in neuroblastoma, Oncotarget, 6 (2015) 10207–10221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Al-Ghabkari A, Narendran A, In Vitro Characterization of a Potent p53-MDM2 Inhibitor, RG7112 in Neuroblastoma Cancer Cell Lines, Cancer biotherapy & radiopharmaceuticals, 34 (2019) 252–257. [DOI] [PubMed] [Google Scholar]
- [135].Chen L, Pastorino F, Berry P, Bonner J, Kirk C, Wood KM, Thomas HD, Zhao Y, Daga A, Veal GJ, Lunec J, Newell DR, Ponzoni M, Tweddle DA, Preclinical evaluation of the first intravenous small molecule MDM2 antagonist alone and in combination with temozolomide in neuroblastoma, International journal of cancer, 144 (2019) 3146–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Arnhold V, Schmelz K, Proba J, Winkler A, Wünschel J, Toedling J, Deubzer HE, Künkele A, Eggert A, Schulte JH, Hundsdoerfer P, Reactivating TP53 signaling by the novel MDM2 inhibitor DS-3032b as a therapeutic option for high-risk neuroblastoma, Oncotarget, 9 (2018) 2304–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Giustiniano M, Daniele S, Pelliccia S, La Pietra V, Pietrobono D, Brancaccio D, Cosconati S, Messere A, Giuntini S, Cerofolini L, Fragai M, Luchinat C, Taliani S, La Regina G, Da Settimo F, Silvestri R, Martini C, Novellino E, Marinelli L, Computer-Aided Identification and Lead Optimization of Dual Murine Double Minute 2 and 4 Binders: Structure-Activity Relationship Studies and Pharmacological Activity, Journal of medicinal chemistry, 60 (2017) 8115–8130. [DOI] [PubMed] [Google Scholar]
- [138].Gomes S, Raimundo L, Soares J, Loureiro JB, Leão M, Ramos H, Monteiro MN, Lemos A, Moreira J, Pinto M, Chlapek P, Veselska R, Sousa E, Saraiva L, New inhibitor of the TAp73 interaction with MDM2 and mutant p53 with promising antitumor activity against neuroblastoma, Cancer letters, 446 (2019) 90–102. [DOI] [PubMed] [Google Scholar]
- [139].Burmakin M, Shi Y, Hedström E, Kogner P, Selivanova G, Dual targeting of wild-type and mutant p53 by small molecule RITA results in the inhibition of N-Myc and key survival oncogenes and kills neuroblastoma cells in vivo and in vitro, Clinical cancer research : an official journal of the American Association for Cancer Research, 19 (2013) 5092–5103. [DOI] [PubMed] [Google Scholar]
- [140].Ramaiah MJ, Pushpavalli SN, Lavanya A, Bhadra K, Haritha V, Patel N, Tamboli JR, Kamal A, Bhadra U, Pal-Bhadra M, Novel anthranilamide-pyrazolo[1,5-a]pyrimidine conjugates modulate the expression of p53-MYCN associated micro RNAs in neuroblastoma cells and cause cell cycle arrest and apoptosis, Bioorganic & medicinal chemistry letters, 23 (2013) 5699–5706. [DOI] [PubMed] [Google Scholar]
- [141].Peirce SK, Findley HW, Prince C, Dasgupta A, Cooper T, Durden DL, The PI-3 kinase-Akt-MDM2-survivin signaling axis in high-risk neuroblastoma: a target for PI-3 kinase inhibitor intervention, Cancer chemotherapy and pharmacology, 68 (2011) 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Li J, Gu L, Zhang H, Liu T, Tian D, Zhou M, Zhou S, Berberine represses DAXX gene transcription and induces cancer cell apoptosis, Laboratory investigation; a journal of technical methods and pathology, 93 (2013) 354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Tian Z, An N, Zhou B, Xiao P, Kohane IS, Wu E, Cytotoxic diarylheptanoid induces cell cycle arrest and apoptosis via increasing ATF3 and stabilizing p53 in SH-SY5Y cells, Cancer chemotherapy and pharmacology, 63 (2009) 1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Van Goethem A, Yigit N, Moreno-Smith M, Vasudevan SA, Barbieri E, Speleman F, Shohet J, Vandesompele J, Van Maerken T, Dual targeting of MDM2 and BCL2 as a therapeutic strategy in neuroblastoma, Oncotarget, 8 (2017) 57047–57057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Moreno-Smith M, Lakoma A, Chen Z, Tao L, Scorsone KA, Schild L, Aviles-Padilla K, Nikzad R, Zhang Y, Chakraborty R, Molenaar JJ, Vasudevan SA, Sheehan V, Kim ES, Paust S, Shohet JM, Barbieri E, p53 Nongenotoxic Activation and mTORC1 Inhibition Lead to Effective Combination for Neuroblastoma Therapy, Clinical cancer research : an official journal of the American Association for Cancer Research, 23 (2017) 6629–6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Wang HQ, Halilovic E, Li X, Liang J, Cao Y, Rakiec DP, Ruddy DA, Jeay S, Wuerthner JU, Timple N, Kasibhatla S, Li N, Williams JA, Sellers WR, Huang A, Li F, Combined ALK and MDM2 inhibition increases antitumor activity and overcomes resistance in human ALK mutant neuroblastoma cell lines and xenograft models, eLife, 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Voges Y, Michaelis M, Rothweiler F, Schaller T, Schneider C, Politt K, Mernberger M, Nist A, Stiewe T, Wass MN, Rödel F, Cinatl J, Effects of YM155 on survivin levels and viability in neuroblastoma cells with acquired drug resistance, Cell death & disease, 7 (2016) e2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Michaelis M, Agha B, Rothweiler F, Löschmann N, Voges Y, Mittelbronn M, Starzetz T, Harter PN, Abhari BA, Fulda S, Westermann F, Riecken K, Spek S, Langer K, Wiese M, Dirks WG, Zehner R, Cinatl J, Wass MN, Cinatl J Jr., Identification of flubendazole as potential anti-neuroblastoma compound in a large cell line screen, Scientific reports, 5 (2015) 8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Michaelis M, Selt F, Rothweiler F, Löschmann N, Nüsse B, Dirks WG, Zehner R, Cinatl J Jr., Aurora kinases as targets in drug-resistant neuroblastoma cells, PloS one, 9 (2014) e108758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Ribas J, Boix J, Meijer L, (R)-roscovitine (CYC202, Seliciclib) sensitizes SH-SY5Y neuroblastoma cells to nutlin-3-induced apoptosis, Experimental cell research, 312 (2006) 2394–2400. [DOI] [PubMed] [Google Scholar]
- [151].Vogenberg FR, Isaacson Barash C, Pursel M, Personalized medicine: part 1: evolution and development into theranostics, P & T : a peer-reviewed journal for formulary management, 35 (2010) 560–576. [PMC free article] [PubMed] [Google Scholar]
- [152].Vogenberg FR, Barash CI, Pursel M, Personalized medicine: part 2: ethical, legal, and regulatory issues, P & T : a peer-reviewed journal for formulary management, 35 (2010) 624–642. [PMC free article] [PubMed] [Google Scholar]
- [153].Vogenberg FR, Barash CI, Pursel M, Personalized medicine: part 3: challenges facing health care plans in implementing coverage policies for pharmacogenomic and genetic testing, P & T : a peer-reviewed journal for formulary management, 35 (2010) 670–675. [PMC free article] [PubMed] [Google Scholar]
- [154].Hoffman-Luca CG, Yang CY, Lu J, Ziazadeh D, McEachern D, Debussche L, Wang S, Significant Differences in the Development of Acquired Resistance to the MDM2 Inhibitor SAR405838 between In Vitro and In Vivo Drug Treatment, PloS one, 10 (2015) e0128807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Jung J, Lee JS, Dickson MA, Schwartz GK, Le Cesne A, Varga A, Bahleda R, Wagner AJ, Choy E, de Jonge MJ, Light M, Rowley S, Macé S, Watters J, TP53 mutations emerge with HDM2 inhibitor SAR405838 treatment in de-differentiated liposarcoma, Nature communications, 7 (2016) 12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Nolan JC, Frawley T, Tighe J, Soh H, Curtin C, Piskareva O, Preclinical models for neuroblastoma: Advances and challenges, Cancer letters, 474 (2020) 53–62. [DOI] [PubMed] [Google Scholar]
- [157].Seitz G, Armeanu-Ebinger S, Warmann S, Fuchs J, Animal models of extracranial pediatric solid tumors, Oncology letters, 4 (2012) 859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Houghton PJ, Morton CL, Tucker C, Payne D, Favours E, Cole C, Gorlick R, Kolb EA, Zhang W, Lock R, Carol H, Tajbakhsh M, Reynolds CP, Maris JM, Courtright J, Keir ST, Friedman HS, Stopford C, Zeidner J, Wu J, Liu T, Billups CA, Khan J, Ansher S, Zhang J, Smith MA, The pediatric preclinical testing program: description of models and early testing results, Pediatric blood & cancer, 49 (2007) 928–940. [DOI] [PubMed] [Google Scholar]
- [159].Kojima K, Konopleva M, Samudio IJ, Shikami M, Cabreira-Hansen M, McQueen T, Ruvolo V, Tsao T, Zeng Z, Vassilev LT, Andreeff M, MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy, Blood, 106 (2005) 3150–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, Zhao X, Vu BT, Qing W, Packman K, Myklebost O, Heimbrook DC, Vassilev LT, Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy, Proceedings of the National Academy of Sciences of the United States of America, 103 (2006) 1888–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA, In vivo activation of the p53 pathway by small-molecule antagonists of MDM2, Science (New York, N.Y.), 303 (2004) 844–848. [DOI] [PubMed] [Google Scholar]
- [162].Ohtsubo C, Shiokawa D, Kodama M, Gaiddon C, Nakagama H, Jochemsen AG, Taya Y, Okamoto K, Cytoplasmic tethering is involved in synergistic inhibition of p53 by Mdmx and Mdm2, Cancer science, 100 (2009) 1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]