Abstract
Patient: Male, 39-year-old
Final Diagnosis: SARS-CoV-2
Symptoms: Fatigue • dyspnea • fever
Medication: Dexamethasone • heparin
Clinical Procedure: C-reactive protein apheresis
Specialty: Immunology
Objective:
Unusual clinical course
Background:
High C-reactive protein (CRP) plasma levels in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are associated with poor prognosis. CRP, by activating the classical complement pathway and interacting with macrophages via Fc gamma receptors, can cause pulmonary inflammation with subsequent fibrosis. Recently, we have reported first-in-man CRP apheresis in a “high-risk” COVID-19 patient. Treatment was unfortunately clinically unsuccessful. Here, we report on successful CRP apheresis treatment in a “lower-risk” COVID-19 patient with respiratory failure.
Case Report:
A 39-year-old male patient suffering from fatigue, dyspnea, and fever for 4 days was referred to us. The patient had to be intubated. Polymerase chain reaction (PCR) analysis of a throat smear revealed SARS-CoV-2 infection. Mutation analysis revealed the VOC B. 1.1.7 variant. CRP levels were 79.2 mg/L and increased to 161.63 mg/L. Procalcitonin (PCT) levels were continuously normal (<0.5 ng/ml). Antibiotic therapy was started to avoid bacterial superinfection. CRP apheresis was performed once via central venous access. CRP levels declined from a maximum of 161.63 mg/L to 32.58 mg/L. No apheresis-associated adverse effects were observed. Subsequently, CRP plasma levels declined day by day and normalized on day 5. The patient was extubated on day 5 and discharged from the Intensive Care Unit (ICU) on day 6. A second low CRP peak (maximum 22.41 mg/L) on day 7 remained clinically inapparent. The patient was discharged in good clinical condition with a CRP level of 6.94 mg/L on day 8.
Conclusions:
SARS-CoV-2 infection can induce an uncontrolled CRP-mediated autoimmune response of ancient immunity. In this patient, the autoimmune response was potently and successfully suppressed by early selective CRP apheresis.
Keywords: Blood Component Removal, C-Reactive Protein, COVID-19
Background
C-reactive protein (CRP), the typical human acute-phase protein, was probably the first antibody-like molecule in the evolution of the immune system [1]. Evolutionarily, CRP appeared earlier than antibodies and was, for example, already expressed in ancient Limulus polyphemes [2]. CRP partly utilizes the same biological structures: like antibodies, the molecule activates complement via C1q [3] and opsonizes biological particles via Fc gamma receptors [4–6]. CRP may be considered as an atavism in the human immune system [1]. For human medicine, it is important to note that CRP, like antibodies, can cause (severe) autoimmune reactions in various human diseases [1,7–10].
Based on these observations, we hypothesized in 2020 that CRP, in SARS-CoV-2 infection, can induce an uncontrolled CRP-mediated autoimmune response of ancient immunity [9,11]. In other words, CRP may be a central effector molecule in this potentially life-threatening disease. This scientific hypothesis is supported (but certainly not proven) by the established strong association between high CRP levels and poor prognosis in COVID-19 disease [12]. Subsequently, other authors have speculated on a causal connection [13].
The only way to prove or disprove such a hypothesis may be to use selective CRP inhibitors in controlled clinical trials [14]. To date, CRP apheresis [15,19] is the only effective (and legally approved) method to selectively eliminate CRP from human plasma. In 2020, we performed the first-in-man CRP apheresis in COVID-19 disease, in a “high-risk” (72-year-old, multimorbid, advanced-stage) patient [11]. Although we observed promising signals, treatment was (for various reasons discussed in the original report [11]) clinically unsuccessful.
Here, we report on the second COVID-19 patient we treated by CRP apheresis. This time, we have successfully treated a “lower-risk” (39-year-old, moderately hypertensive, early-stage) COVID-19 patient with respiratory failure.
Case Report
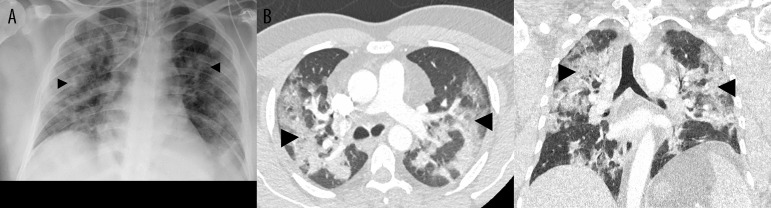
A 39-year-old man suffering from cough, breathlessness, and fever for 4 days was referred to the Emergency Unit of Immenstadt Hospital, Clinic Association Allgaeu, Germany. His history of concomitant diseases only included moderate arterial hypertension. Rapid antigen testing revealed SARS-CoV-2 positivity. Arterial oxygen saturation (SO2) at admission was 88% with 10 L/min oxygen supply. Laboratory tests showed increased CRP plasma levels (79.2 mg/L, reference range 0.00-5.00 mg/L), but no leukocytosis; instead, there was lymphopenia of 14.9% (reference range 17–47%). Imaging, including chest X-ray and thoracic computed tomography (CT), revealed typical streaky infiltrates on both sides (Figure 1). D-dimers were slightly elevated (0.93 µg/mL, reference range 0.00–0.50 µg/mL). Pulmonary embolism, however, was definitively excluded by CT angiography.
Figure 1.
Supine chest X-ray immediately after intubation and computed tomography (axial/coronal) on admission (A, B) showing typical bilateral infiltrates and beginning of fibrosing alveolitis (black arrows).
Three sequential blood cultures taken over the following days revealed 1 positive culture only (Micrococcus luteus, considered as contamination). PCR analysis of a throat smear for SARS-CoV-2 was initiated. High-flow oxygen therapy (HFOT)/80%O2 and non-invasive ventilation (NIV)/70%O2 achieved a PaO2 of 60% only. The patient was rapidly transferred to the ICU and intubated. PCR results revealed SARSCoV-2 positivity. Mutation analysis of combined deletion H69-V70 and mutation N501Y revealed the VOC B. 1.1.7 variant. Empiric antibiotic therapy with piperacillin/tazobactam was started immediately after transfer to the ICU on day 0 to avoid bacterial superinfection.
A sharp increase in CRP plasma levels over the next day and continuous respiratory worsening led us to start CRP apheresis in addition to continuous standard therapy for SARS-CoV-2 infection. The latter included lung-protective ventilation with low tidal volumes, best positive end-expiratory pressure (after PEEP-trial), i.v. dexamethasone 6 mg/d, anti-coagulation with heparin, intermittent prone positioning of 16 to 20 h, and, finally, antibiotic treatment to avoid potential bacterial super-infection. Written informed consent for CRP apheresis was obtained from the patient`s relatives after a detailed informative discussion. Discussion with the Ethics Committee of Ulm University had been conducted previously [11].
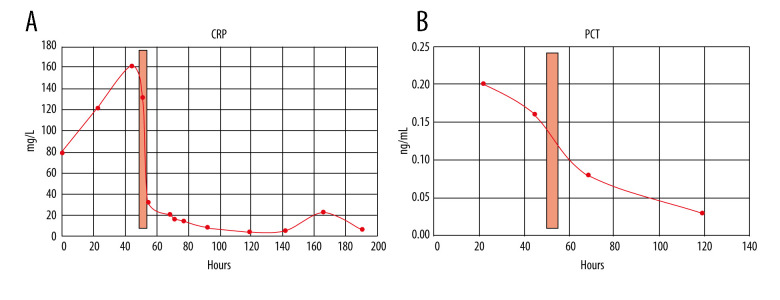
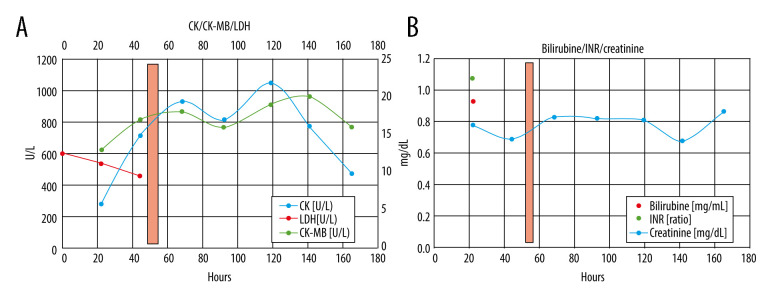
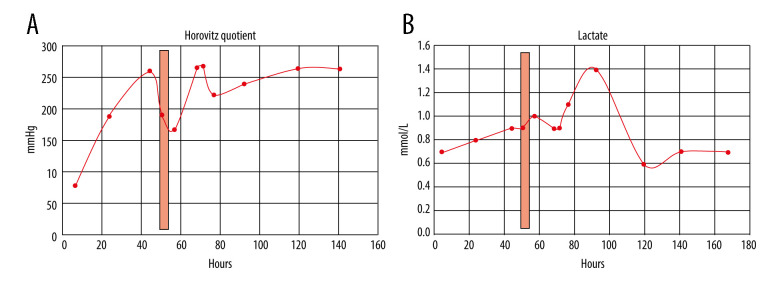
CRP apheresis [15,19] was started via Shaldon catheter [11] to allow easy change from dorsal to ventral patient position 50 h after admission. His acute respiratory distress syndrome (ARDS) category [16] changed from severe (PaO2/FIO2 ≤100 mmHg) to moderate (PaO2/FIO2 ≤200 mmHg) after intubation and ventral positioning, and then worsened during CRP apheresis due to dorsal positioning. After CRP apheresis, Horovitz quotient, independent from dorsal or ventral positioning, significantly improved until extubation and hospital discharge. In 1 apheresis session only, ≥7500 mL plasma was treated. CRP plasma levels and other laboratory results during his hospital stay are depicted in Figures 2–4. CRP apheresis efficiently reduced CRP plasma levels (Figure 2) from a maximum of 161.63 mg/L to 32.58 mg/L. Interestingly, CRP levels did not rise again after this first treatment, and instead declined to normal values. Other laboratory parameters, including creatinine and bilirubine plasma levels, l-lactate-dehydrogenase (LDH), and creatinine-kinase (CK), only slightly increased or remained constant (CK-MB) (Figure 3). Sequential Organ Failure Assessment (SOFA) score [17] – creatinine (reference range 0.7–1.2 mg/dL), platelet count (reference range 146 000–328 000) and bilirubine (reference range <1.2 mg/dL) – remained normal until extubation and ICU discharge. Respiratory parameters markedly and quickly improved (Figure 4). Extracorporeal membrane oxygenation was not necessary. The patient was extubated on day 5 after admission and was discharged from the ICU on day 6. A second low CRP peak (22.41 mg/L) on day 7, as reported by others (Esposito F, personal communications), remained clinically inapparent. Control imaging in this young man, if clinically necessary, was recommended in the outpatient setting. He was discharged from our hospital in good clinical condition on day 8 after admission.
Figure 2.
(A) CRP levels (reference range 0.00–5.00 mg/L) during the course of the patient’s hospital stay. CRP levels were elevated on admission (79.2 mg/L) and sharply increased on day 2 (maximum 161.63 mg/L). Due to CRP apheresis (light red columns) via central venous access, CRP levels markedly dropped and further declined later on. A second low CRP peak (22.41 mg/L) on day 7 remained clinically inapparent. (B) PCT levels (reference range <0.5 ng/mL) remained normal during the hospital stay.
Figure 3.
(A) CRP apheresis (light red column) and course of CK/CK-MB and LDH plasma levels. Only CK slightly increased, likely due to positioning of the patient (muscular CK). In contrast, CK-MB levels remained normal. (B) CRP apheresis (light red column), bilirubine, international normalized ratio (INR), and creatinine levels. Normal values for each parameter.
Figure 4.
(A) CRP apheresis (light red column) and course of Horovitz quotient [16]. Horovitz quotient changed from severe (PaO2/FIO2 ≤100 mmHg) to moderate (PaO2/FIO2 ≤200 mmHg) after intubation and ventral positioning, then worsened during CRP apheresis due to dorsal positioning. After CRP apheresis, however, Horovitz quotient, independent from dorsal or ventral positioning, significantly improved until extubation and hospital discharge. (B) CRP apheresis (light red column) and course of lactate (reference range 0.5–1.6 mmol/L). Lactate remained normal during the hospital stay.
Discussion
Although CRP has been known since 1930 [18], its role in the human immune system remains enigmatic. Increasing evidence, however, suggests that CRP is probably the first antibody-like molecule in the evolution of the immune system in general and may be an atavism in the immune system of humans in particular [1]. Assuming that CRP, in COVID-19 disease, can trigger a fulminant autoimmune reaction by CRP-mediated complement and macrophage activation [9–12], we have now treated a “lower-risk” COVID-19 patient with respiratory failure and sharply rising CRP plasma levels by selective and efficient CRP apheresis [11,15,19] through central venous access [11].
Of course, our patient received optimized standard therapy according to the latest medical recommendations. When we observed clinical worsening, we discussed selective CRP apheresis as a bail-out therapy and achieved consensus with his family. Due to successful treatment of cases reported in the media, and due to our long-standing experience with selective CRP apheresis in clinical trials and due to the lack of alternative therapies [11], we finally made the decision to treat our young patient with this novel technology.
SARS-CoV-2 is still a viral infection with high lethality [20–23]. A poorly understood autoimmune response with early and aggressive lung injury and consecutive ARDS seems to be intimately involved in this high lethality. In patients with a poor outcome, CRP levels are usually strongly elevated and are associated with poor prognosis. In contrast, procalcitonin levels are usually not elevated (as was the case in our patient) or are rather moderately elevated, indicating that bacterial or fungal superinfection is not present in the early stages of the disease.
These clinical observations lead to the intriguing question of whether the virus itself or an inadequate, uncontrolled immune response of the ancient immune system (CRP, complement, macrophages) triggered by alveolar hypoxia is the real cause of the fatal clinical course in some patients [9–11,13]. Lowering CRP plasma levels may thus be the key step to avoid immunological self-destruction of the lungs and multi-organ failure. Selective CRP apheresis may be the method of choice to reach this target. In fact, this case report on CRP apheresis in a “lower-risk” COVID-19 patient with respiratory failure further supports our hypothesis for 2 reasons: (1) CRP apheresis in the phase of sharp CRP rise was able to effectively and permanently reduce CRP plasma levels in this patient, and (2) CRP reduction was accompanied by marked clinical improvement, early extubation, and relatively short hospital stay of our patient.
COVID-19 mortality is known to depend on comorbidi-ties [12,21–23]. In comparison to our first-in-man case report on CRP apheresis in COVID-19 disease [11], we have now treated a younger patient with fewer comorbidities. Although the current patient was infected with the B. 1.1.7 variant (considered as being more infectious and potentially more lethal [23]), he definitely suffered from fewer comorbidities than our first patient [11]. Also, he was much younger (39 vs 72 years]. For these reasons, we have clinically rated him as a “lower-risk” COVID-19 patient. The latter may be important for the design of randomized clinical trials, as inclusion of “high-risk” patients could negatively influence the results. Certainly, his younger age and accompanying improved standard therapy may have caused a better outcome. However, the ultimate reason for this favorable outcome may have been the use of early CRP apheresis in combination with fewer comorbidities and improved standard therapy. Perhaps, this early CRP apheresis in the phase of sharp CRP rise suppressed the excessive auto-immune response of the ancient immune system, which may be the real cause of the respiratory failure, multi-organ failure, and death in COVID-19 patients.
In conclusion, this case report underlines the urgent need for a randomized, controlled, multicenter trial comparing CRP apheresis treatment plus standard treatment to standard treatment alone. The latter has been initiated and can markedly improve outcomes in COVID-19 patients with respiratory failure and provide deep and fascinating insights into the role of ancient immunity in humans (https://clinicaltrials.gov/ct2/show/NCT04898062).
Conclusions
SARS-CoV-2 can cause multi-organ failure by triggering an uncontrolled CRP-mediated autoimmune response of ancient immunity. CRP apheresis in the early phase of CRP increase and respiratory worsening may be an effective treatment for patients severely threatened by SARS-CoV-2 infection.
Acknowledgments
We gratefully acknowledge Kerstin Rziha, Medical Care Center Kempten, Germany, and our study nurse, Jacqueline Fiedler, for spontaneously organizing CRP apheresis over the weekend. We acknowledge study nurse Andrea Zimmermann and Dr. Patricia Brunner, Pentracor, for composing the figures. Furthermore, we gratefully acknowledge the doctors and nurses of ICU Hospital Immenstadt, Bavaria, Germany, for treating the patient with all their patience and empathy.
Footnotes
Conflicts of Interest
Ahmed Sheriff is founder and shareholder of Pentracor GmbH. Stefan Kayser is an employee of Pentracor GmbH.
Declaration of Figures Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Zimmermann O, Li K, Zaczkiewicz M, et al. C-reactive protein in human atherogenesis: Facts and fiction. Mediators Inflamm. 2014;2014:561428. doi: 10.1155/2014/561428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen NY, Suzuki A, Cheng SM, et al. Isolation and characterization of Limulus C-reactive protein genes. J Biol Chem. 1986;261(22):10450–55. [PubMed] [Google Scholar]
- 3.Kaplan MH, Volanakis JE. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol. 1974;112:2135–47. [PubMed] [Google Scholar]
- 4.Bharadwaj D, Stein MP, Volzer M, et al. The major receptor for C-reactive protein on leukocytes is Fcgamma receptor II. J Exp Med. 1999;190:585–90. doi: 10.1084/jem.190.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manolov DE, Rocker C, Hombach V, et al. Ultrasensitive confocal fluorescence microscopy of C-reactive protein interacting with FcgammaRIIa. Arterioscler Thromb Vasc Biol. 2004;24:2372–77. doi: 10.1161/01.ATV.0000147407.17137.02. [DOI] [PubMed] [Google Scholar]
- 6.Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: Implications for atherosclerosis. Circulation. 2001;103:1194–97. doi: 10.1161/01.cir.103.9.1194. [DOI] [PubMed] [Google Scholar]
- 7.Du Clos TW. Pentraxins: Structure, function, and role in inflammation. ISRN Inflamm. 2013;2013:379040. doi: 10.1155/2013/379040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ries W, Torzewski J, Heigl F, et al. C-reactive protein apheresis as anti-inflammatory therapy in acute myocardial infarction: Results of the CAMI-1 study. Front Cardiovasc Med. 2021;8:591714. doi: 10.3389/fcvm.2021.591714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kayser S, Kunze R, Sheriff A. Selective C-reactive protein apheresis for COVID-19 patients suffering from organ damage. Ther Apher Dial. 2021;25(2):251–52. doi: 10.1111/1744-9987.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheriff A, Kayer S, Brunner P, et al. C-reactive protein triggers cell death in ischemic cells. Front Immunol. 2021;12:630430. doi: 10.3389/fimmu.2021.630430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torzewski J, Heigl F, Zimmermann O, et al. First-in-man: Case report of selective C-reactive protein apheresis in a patient with SARS-CoV-2 infection. Am J Case Rep. 2020;21:e925020. doi: 10.12659/AJCR.925020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smilowitz NR, Kunichoff D, Garshick M, et al. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J. 2021;42:2270–79. doi: 10.1093/eurheartj/ehaa1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pepys MB. C-reactive protein predicts outcome in COVID-19: Is it also a therapeutic target? Eur Heart J. 2021;42:2280–83. doi: 10.1093/eurheartj/ehab169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torzewski J, Fan J, Schunkert H, Szalai A, et al. C-reactive protein and arteriosclerosis. Mediators Inflamm. 2014;2014:646817. doi: 10.1155/2014/646817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheriff A, Schindler R, Vogt B, et al. Selective apheresis of C-reactive protein: A new therapeutic option in myocardial infarction? J Clin Apher. 2015;30:15–21. doi: 10.1002/jca.21344. [DOI] [PubMed] [Google Scholar]
- 16.Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 17.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tillett WS, Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of Pneumococcus. J Exp Med. 1930;52:561–71. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slagman AC, Bock C, Abdel-Aty H, et al. Specific removal of C-reactive protein by apheresis in a porcine cardiac infarction model. Blood Purif. 2011;31:9–17. doi: 10.1159/000320763. [DOI] [PubMed] [Google Scholar]
- 20.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region – case series. N Engl J Med. 2020;382(21):2012–22. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Litvinova M, Wang W, et al. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: A descriptive and modelling study. Lancet Infect Dis. 2020;20:793–802. doi: 10.1016/S1473-3099(20)30230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alpert T, Brito AF, Lasek-Nesselquist E, et al. Early introductions and transmission of SARS-CoV-2 variant B.1.1.7 in the United States. Cell. 2021;184:2595–604.e13. doi: 10.1016/j.cell.2021.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]