Summary
The neural circuits underlying the distinct endpoints that define general anesthesia remain incompletely understood. It is becoming increasingly evident, however, that distinct pathways in the brain that mediate arousal and pain are involved in various endpoints of general anesthesia. To critically evaluate this growing body of literature, familiarity with modern tools and techniques used to study neural circuits is essential. This Reader’s Toolbox article describes four such techniques: 1) electrical stimulation, 2) local pharmacology, 3) optogenetics, and 4) chemogenetics. Each technique is explained, including the advantages, disadvantages, and other issues that must be considered when interpreting experimental results. Examples are provided of studies that probe mechanisms of anesthesia using each technique. This information will aid researchers and clinicians alike in interpreting the literature and in evaluating the utility of these techniques in their own research programs.
Introduction
General anesthesia is characterized by reversible loss of consciousness, amnesia, analgesia and lack of movement in response to noxious stimulation. Thousands of people undergo general anesthesia each day, and significant progress has been made in identifying the molecular targets of general anesthetics.1 However, the neural circuits underlying the distinct endpoints that define general anesthesia remain incompletely understood. It is becoming increasingly evident that distinct pathways in the brain that mediate arousal and pain are involved in various endpoints of general anesthesia. Arousal nuclei that play roles in emergence include adrenergic projections from the locus coeruleus,2 dopaminergic projections from the ventral tegmental area,3,4 and cholinergic projections from the lateral dorsal tegmental nucleus,5,6 the pedunculopontine tegmental nucleus, and the basal forebrain.1 Hypothalamic preoptic area neurons are active during sleep and inhibit arousal centers in the midbrain, upper pons and hypothalamus.7 Gamma-aminobutyric acid type A (GABAA) receptor agonists that produce sedation are thought to enhance these pathways, suppressing wakefulness.8 Other arousal centers studied in the context of general anesthesia include histaminergic neurons in the tuberomammillary nucleus,9 orexinergic neurons in the lateral hypothalamus,10 and glutamatergic neurons in the parabrachial nucleus.11
Many laboratories are now conducting studies in rodents that involve the manipulation of neural circuits to investigate specific behavioral endpoints such as unconsciousness and analgesia, as well as the electroencephalographic activity of the anesthetized brain. In this Toolbox article, four techniques that are commonly used to manipulate neural circuits will be reviewed: 1) electrical stimulation, 2) local pharmacology, 3) optogenetics, and 4) chemogenetics (see fig. 1). The following sections will briefly describe each technique and review their respective strengths and weaknesses, as well as examples of how the technique has been applied in anesthesia research. This article is designed to provide an overview of these approaches; for more in-depth information about anesthesia research techniques, other recent reviews will also be helpful.12–14 The goals of this article are to provide readers with the essential background to critically evaluate such studies and for scientists to consider the merits of adding these techniques to their own research.
Figure 1. Four different techniques for manipulating neural activity.
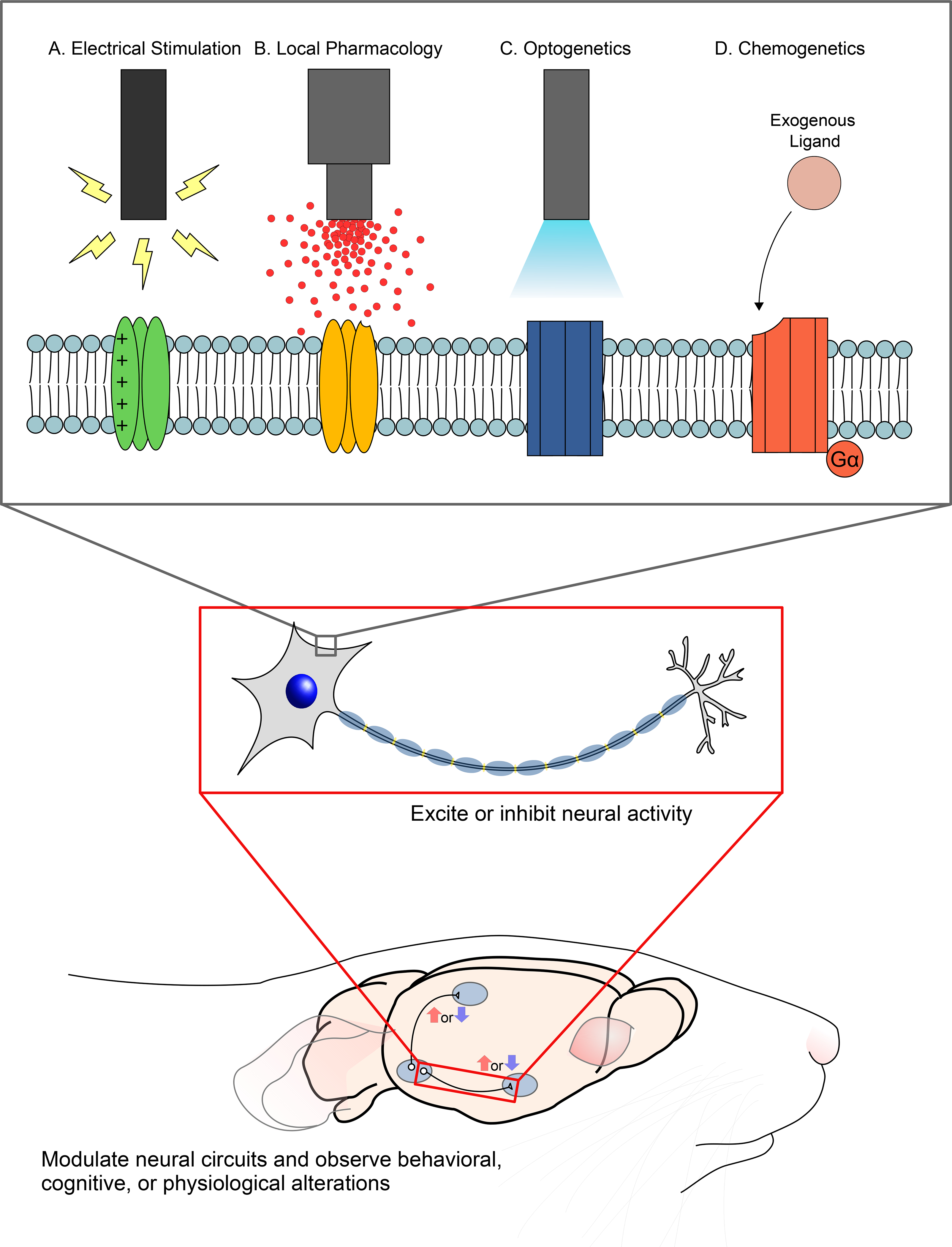
Each of the techniques described in this Reader’s Toolbox excite or inhibit neural activity via their effects on membrane-bound proteins, including (A) voltage-gated proteins, (B) ion channels or other receptors (C) opsins, and (D) modified receptors that respond to an exogenous ligand instead of their endogenous ligand. Because of their different molecular mechanisms, these four techniques exhibit varying degrees of spatial range, temporal dynamics, and neuron-type specificity. Researchers use these techniques for manipulating neural activity in order to study the role of neural circuits in a range of physiological, behavioral, and cognitive states.
Electrical Stimulation
Neural electrical stimulation is a classic research technique used to directly activate or inhibit certain brain areas to test whether they mediate specific behavioral and neurophysiological endpoints. Of note, electrical stimulation is the only method presented in this article that is currently used in humans for therapeutic purposes (e.g. deep brain stimulation for Parkinson’s disease and other neurological disorders).15
In neural circuit research, the electrical stimulation method involves surgical implantation of electrodes into a target brain region using a stereotaxic frame. A signal generator is then connected to the electrode, either via a tethered wire or as a wireless unit. Neural stimulation occurs when an electrical current is passed through the electrode. The current affects the membrane potential of nearby neurons, leading to the excitation or inhibition of neural activity. The electrode type (monopolar or bipolar) and the current waveform, polarity, amplitude, frequency, and duration determine whether the stimulation leads to neural excitation or inhibition.16,17 By increasing the magnitude of current, it is possible to increase the number of neurons recruited in the target area (fig. 2A), often leading to increased vigor of the induced behavior.
Figure 2. Electrical stimulation method of circuit manipulation.
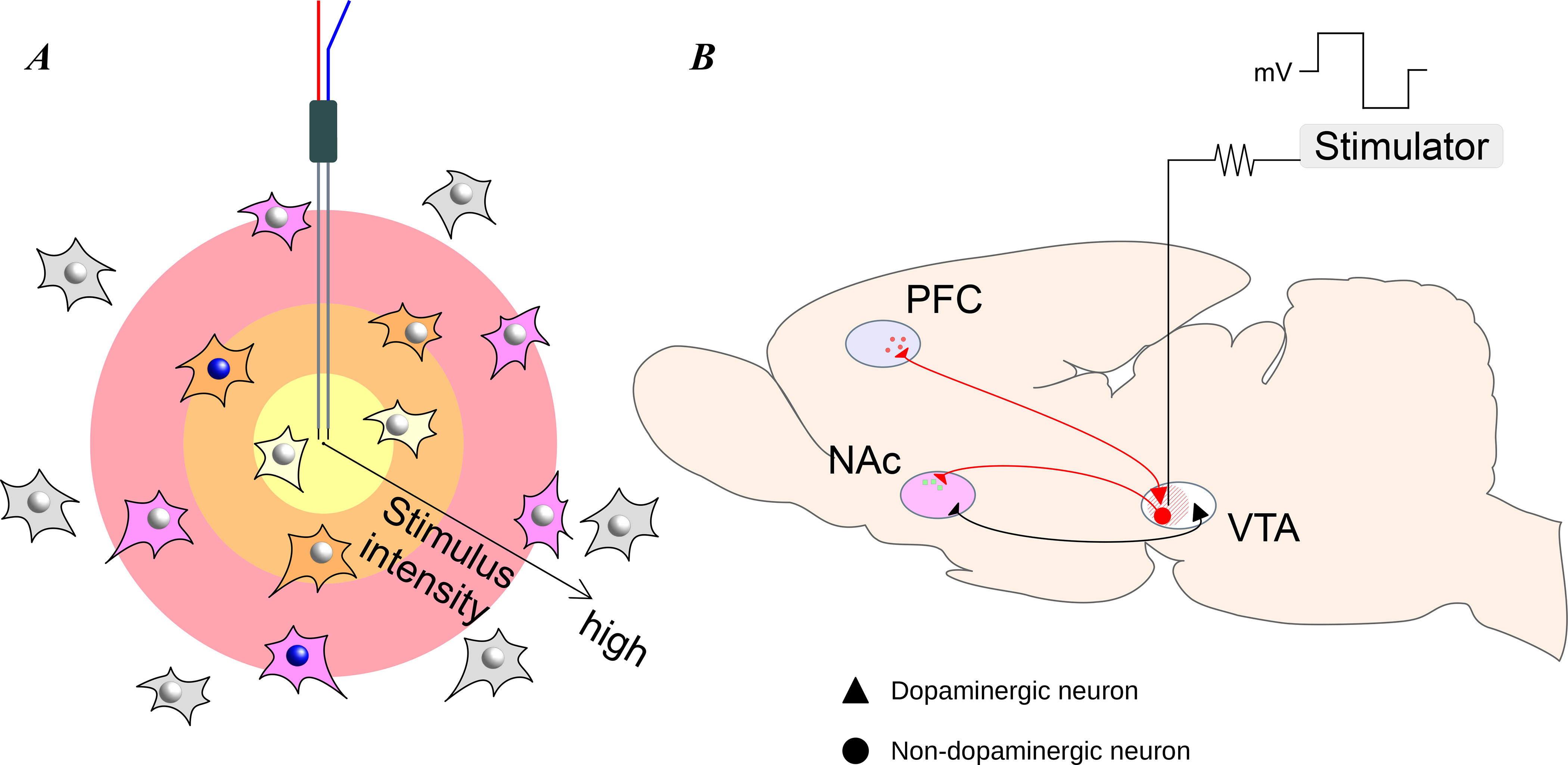
Electrical stimulation is used to manipulate neural activity near the stimulation electrode. (A) As the stimulus intensity increases, it affects neurons in a larger area of neural tissue, agnostic to the neuron type. (B) As an example, electrical stimulation of the ventral tegmental area (VTA) can be used to study its downstream effects on the prefrontal cortex (PFC) and nucleus accumbens (NAc). In this example, the strength and location of the electrical stimulation determines the extent of the effects on the downstream regions. Throughout the figures in this article, triangles indicate dopaminergic neurons, circles indicate non-dopaminergic neurons, red indicates activation of the neurons and black indicates no change in neuronal activity.
In order to demonstrate that the effects produced by electrical stimulation are region-specific, researchers must validate their results by employing appropriate experimental controls. This may include stimulating nearby off-target regions in a separate group of control animals where identical stimulation parameters do not produce the same effect. Following completion of electrical stimulation experiments, histological analysis is used to ensure correct placement of the stimulation electrode and confirm that there is no evidence of heat damage to the stimulated brain tissue. Similar histological analyses are necessary for all the techniques described in this article to verify accurate stereotaxic targeting of the brain region of interest.
Electrical stimulation has been employed to demonstrate that activation of specific arousal-promoting nuclei can induce emergence from general anesthesia. After methylphenidate (a reuptake inhibitor for dopamine) was shown to induce emergence from continuous isoflurane and propofol anesthesia,18,19 electrical stimulation studies in rats identified the ventral tegmental area as a possible site of action underlying this effect. Although dopamine neurons are present in the ventral tegmental area and the neighboring substantia nigra, only stimulation of the ventral tegmental area induced emergence from isoflurane and propofol anesthesia.20 The arousal response was enhanced as the current intensity was increased, and it was correlated with electroencephalogram changes consistent with wakefulness. Similarly, a study in mice showed that electrical stimulation of the parabrachial nucleus, a glutamatergic arousal center in the pons, induced emergence from isoflurane anesthesia.21 It has been reported that electrical stimulation of the central thalamus improved behavioral responsiveness in a minimally conscious patient who suffered from a severe traumatic brain injury,22 suggesting that studies of anesthetic reversal with electrical stimulation may find translational applications to treat patients with disorders of consciousness.
Compared to the other techniques described in this article, electrical stimulation has the advantage of not requiring intracranial injections of drugs or viruses. Also, the stimulus intensity can be precisely controlled to recruit more or fewer neurons. However, there are several important limitations. First, a high current delivered over long periods can result in heat injury to the tissue surrounding the electrode. This can be prevented by lowering the duration and intensity of the stimulation. (Of note, many electrical stimulation paradigms in rodents use higher levels of current compared to those used in humans.)17 Second, electrical stimulation is a non-specific form of neural modulation;23 it will stimulate all the neurons in the vicinity of the electrode, regardless of neuron type and including neurons with axons passing through the area of stimulation (fig. 2B). To avoid unintentional off-target stimulation, the neuroanatomy of the region of interest should be examined for potential axon bundles passing through it, and the stimulation parameters should be held consistent across experiments to minimize variability in the volume of tissue being affected. Finally, electrical artifacts from stimulation can interfere with simultaneous neurophysiological recordings, especially when the stimulation and recording electrodes are in close proximity. This makes it challenging to determine the exact effects of electrical stimulation on membrane potential and firing patterns of a stimulated neuron. Even when inputs are known to be depolarizing, neural inhibition may be the result, if the inputs are large and continuous enough to cause depolarization block. Thus, care must be taken when attributing behavioral effects to excitation or inhibition of neurons in the stimulated area. The other methods discussed in this article (microinjection/microdialysis, optogenetics, and chemogenetics) provide advantages for precisely exciting or inhibiting specific neuronal circuits. Despite these limitations, however, electrical stimulation remains a valuable method with significant potential for translational applications in humans.
Local Pharmacology: Microinjection and Microdialysis
Electrical stimulation is useful to characterize the roles of distinct brain regions in producing various endpoints relevant to general anesthesia, but information about specific neurotransmitters and receptors within a localized region is also critically important. Microinjection and microdialysis are related techniques that are used to manipulate systems at the molecular level within a specific brain region.
In studies using microinjection, animals are surgically implanted with a guide cannula over the brain region of interest. Following recovery from the surgery, experiments are performed by inserting an internal cannula through the guide cannula until the tip is in the region of interest. Using a specialized syringe pump that can accurately deliver very small volumes (typically <1 μL), drug-containing solutions are injected directly into the brain (fig. 3A). A slow delivery rate (typically 0.1–2 μL/min) ensures that pressure from the microinjection does not damage or displace the targeted brain tissue. Injected solutions then diffuse away from the tip of the internal cannula, as much as 1 mm away for a 0.5 μL volume.24 Anesthesia research employing this technique often involves microinjection of anesthetics or receptor-specific agonists and antagonists. Following the microinjection, a variety of behavioral and neurophysiological endpoints may be assessed.
Figure 3. Local pharmacological methods of circuit manipulation: microinjection and microdialysis.
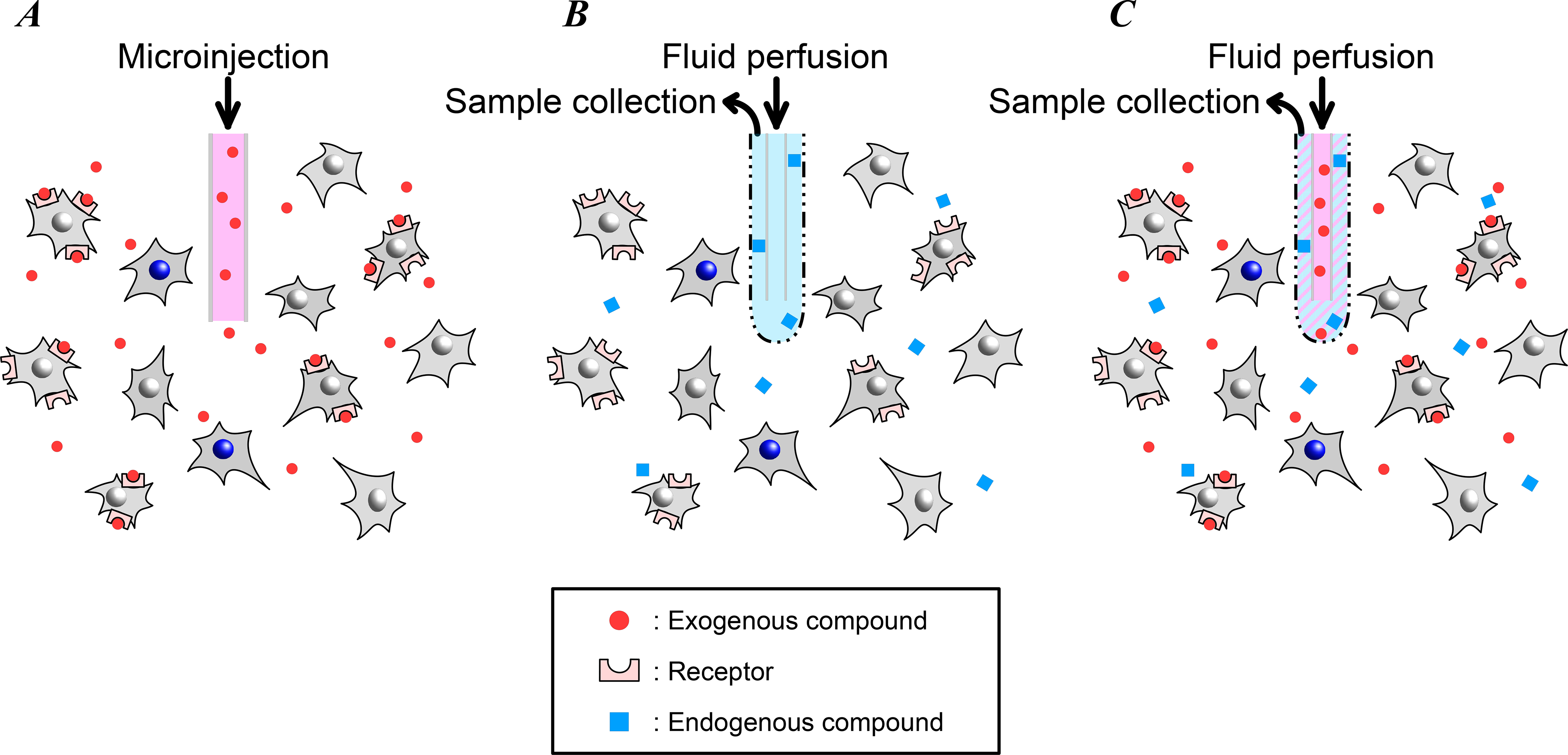
Microinjection and microdialysis are used to manipulate neural activity near the cannula or probe tip by delivering drugs or other substances. In addition, microdialysis can be used to collect samples of substances from the brain for analysis. (A) Drugs and other receptor agonists or antagonists can be delivered to a small volume (typically <1 mm3) of brain tissue using microinjection. (B) Microdialysis is used to collect samples of drugs, neurotransmitters, or other small molecules from the brain to measure concentrations or other analyses. When the perfusate is isosmotic with the surrounding brain tissue, there is no net fluid delivery with microdialysis, in contrast with microinjection. (C) Microdialysis is also used to deliver drugs (often referred to as “reverse microdialysis”). In microdialysis and reverse microdialysis, diffusion of substances into or out of the probe tip depends on the pore size of the semi-permeable membrane.
One study by Minert et al. examined the role of an upper brainstem region, the mesopontine tegmental area, in anesthetic-induced unconsciousness.25 They reported that microinjection of the GABAA-potentiating anesthetics propofol and pentobarbital, as well as the GABAA agonist muscimol, into the mesopontine tegmental area resulted in sustained unconsciousness. In a follow-up study, however, they found that microinjection of the neurosteroid alfaxalone into the mesopontine tegmental area did not have a significant effect.26 Together, these results suggest that specific neural circuits in this region play a role in producing unconsciousness when some, but not all, anesthetics that potentiate GABAA receptors are administered systemically.
Other studies have also used intracranial microinjections to define specific neuromodulator systems. For example, Gamou et al. investigated whether propofol mediates arousal via cholinergic pathways in the brain.27 In this study propofol was microinjected into the perifornical area, which influences cholinergic neuronal activity in the basal forebrain. The authors found that microinjection of propofol in the perifornical area induced sedation and decreased cortical acetylcholine release, similar to the effects of systemic propofol administration. Thus, the effects of propofol, via the perifornical area, on the cholinergic arousal system appear to play an important role in propofol-induced sedation. Dong et al. reported that the orexinergic system can be manipulated to promote recovery from sevoflurane anesthesia.28 By microinjecting orexin receptor agonists into the basal forebrain during continuous inhalation of sevoflurane, they found EEG evidence of arousal and decreased time to emergence. No changes were observed in induction time, indicating that cholinergic and orexinergic arousal systems play roles in anesthetic emergence, but not induction.
In addition to revealing how anesthetic drugs affect consciousness in specific brain regions, microinjection is also useful to test mechanisms underlying the hemodynamic side effects of general anesthesia. For example, Hu et al. investigated the role of hyperpolarization-activated cyclic nucleotide-gated channels in the cardiovascular depression induced by propofol.29 Microinjection of propofol into the rostral ventrolateral medulla decreased mean arterial pressure and heart rate. However, when the hyperpolarization-activated cyclic nucleotide-gated channel blocker ZD-7288 was microinjected into the rostral ventrolateral medulla prior to the propofol microinjection, the propofol-induced cardiovascular depression was significantly attenuated. This suggests that hyperpolarization-activated cyclic nucleotide-gated channels in the rostral ventrolateral medulla play an important role in propofol-induced cardiovascular depression, and that the mechanisms underlying propofol-induced unconsciousness and cardiovascular depression may involve different receptor systems in distinct brain regions.
Similar to microinjection, microdialysis is performed by first implanting a guide cannula above a brain region. Instead of drug-containing solutions being delivered directly via an internal cannula, however, a microdialysis probe is inserted through the guide cannula into the region of interest. The microdialysis probe is a concentric tube, with inflow through the center of the probe and outflow through the outer circle. The inner and outer portions of the probe meet at the tip, where the border of the tip is made of a semi-permeable membrane.30 This configuration allows for the delivery of drugs, as well as the collection of neurotransmitters and other small molecules (figs. 3B and 3C). A solution of artificial cerebral spinal fluid or ringers is typically perfused at a rate of 0.3 to 3 μL/min. Substances in the perfused solution diffuse into the brain tissue and substances in the surrounding brain tissue diffuse into the microdialysis probe, depending on their concentration gradient and the pore size of the semi-permeable membrane. Throughout this process, the volume of fluid in the brain remains constant, due to perfusion fluids usually being isosmotic.31 The ability to measure the concentration of extracted substances or to precisely predict the concentrations of delivered substances (using known diffusion properties)32 are distinct advantages of microdialysis over microinjection. Recent analytical chemistry techniques allow investigators to simultaneously measure the concentrations of multiple extracted substances, such as neurotransmitters.33 In addition, drug delivery at a fixed concentration can be maintained for hours with microdialysis, unlike microinjection.
Recently, Pal et al. used microdialysis to demonstrate that delivery of the cholinergic agonist carbachol to the prefrontal cortex restores consciousness in anesthetized rats during continuous inhalation of sevoflurane.5 However, microdialysis delivery of carbachol to the parietal cortex, or norepinephrine to the prefrontal or parietal cortices, did not induce anesthetic emergence. These results suggest that cholinergic modulation of the prefrontal cortex plays an important role in recovery of consciousness from the anesthetized state. Some anesthesia studies have employed a combination of microinjection and microdialysis. For instance, Baghdoyan et al. used microdialysis and found that, surprisingly, GABA levels in the pontine reticular formation decrease during isoflurane anesthesia.34 Then, using microinjections into the pontine reticular formation, they showed that isoflurane induction time increased with a GABA uptake inhibitor and decreased with a GABA synthesis inhibitor. Thus, in this example both microinjection and microdialysis were used to dissect the differential responses of isoflurane on GABAergic neurotransmission in the pontine reticular formation.
Microinjection and microdialysis are useful to investigate the effects of anesthetics in specific brain regions, or to test whether a specific molecular target in a brain region of interest plays a role in producing certain anesthetic endpoints. Using these methods, the effects of systemic administration of a drug can be compared to the effects of applying the drug to a specific region of the brain. In addition, microinjection and microdialysis studies are useful in identifying receptor subtypes and actions through antagonist blocking and concentration-response studies, respectively. However, like electrical stimulation, microinjection and microdialysis have their limitations. With microdialysis, sampling of solutes from the brain usually happens on the order of minutes, limiting the temporal resolution of this technique.31 Because drug spread is determined by diffusion, high drug concentrations are often used with microinjection. Therefore, resulting changes in physiology or behavior may operate through different mechanisms than systemic doses. For example, both Gamou et al. and Minert et al. are examples of studies that used supratherapeutic doses in their microinjections; an awareness of this context is necessary for accurate interpretation of these studies.25,27 (In contrast, due to the constant perfusion of fluid with microdialysis, it is not necessary to use drug concentrations higher than the target concentration.) Related to this issue, the diffusion of drugs away from the delivery point makes it difficult to deliver a uniform concentration in the targeted brain region with microinjection. Thus, it is important to perform control experiments in which only the vehicle is delivered. In addition, histological confirmation of cannula placement and careful interpretation of the results in the context of the concentrations used are essential to arrive at the appropriate conclusions. Despite these caveats, microinjection and microdialysis are effective tools to link molecular pharmacology to specific neural circuits.
Optogenetics
Electrical stimulation, microinjection and microdialysis cannot be used to activate or inhibit specific populations of neurons, as different types of cells in the targeted region will be affected indiscriminately. A single brain region typically contains an array of different neuron types, making it difficult to discern the physiological role of any individual type using these methods. In contrast, optogenetics offers a method to activate or inhibit specific neuronal populations through selective expression of light-activated ion channels, or opsins (fig. 4A).
Figure 4. Optogenetic method of circuit manipulation.
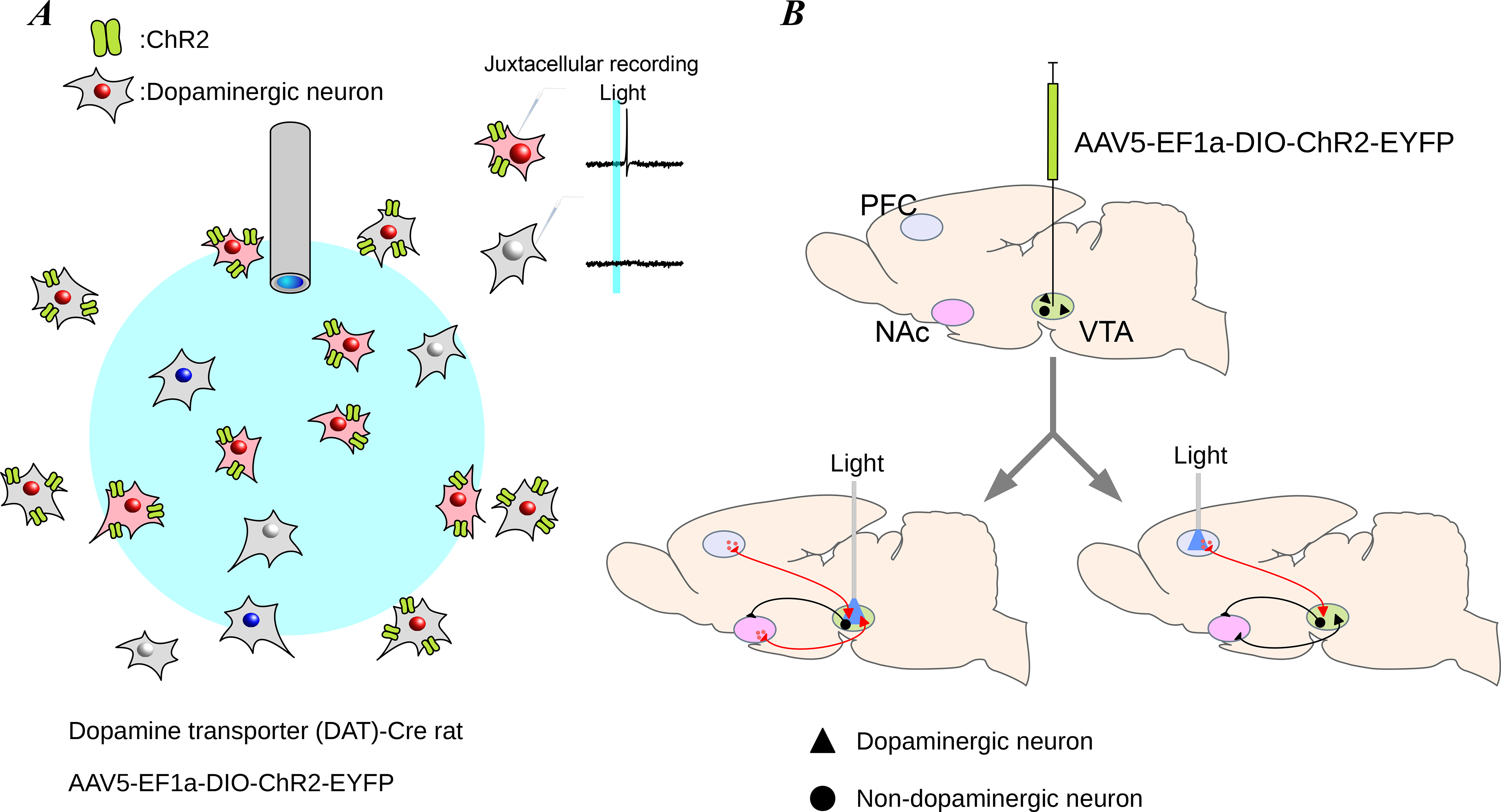
Optogenetic stimulation is used to excite or inhibit targeted neurons that express the opsin, using an optic fiber. (A) In this example, light delivered through an optic fiber is used to excite only neurons that express the opsin, ChR2, in the path of the light. A given light pulse excites an action potential in neurons expressing the opsin (inset top), but not in neurons lacking opsin expression or out of the range of the delivered light (inset bottom). (B) The opsin is typically introduced to the neurons by injecting a virus containing the opsin gene (top). After about 3 weeks to allow for expression of the opsin, neuron bodies (bottom left) or axon terminals (bottom right) can be targeted with light from the optic fiber to determine which downstream targets are affected.
Expression of an opsin is confined to a specific neuron type and region within the brain, usually by local injection of a virus containing the opsin gene (see Box 1). There are a variety of opsins, including excitatory (e.g., channelrhodopsin2, or “ChR2”) and inhibitory (e.g., halorhodopsin, or “NpHR”, and archaerhodopsin, or “Arch”) opsins. In order to activate the opsin protein with light, an optic fiber is implanted in the brain, directed at the neuron bodies or associated axons in the region. After allowing 2–3 weeks following virus injection for opsin expression, the targeted neurons can be manipulated by delivering light through the optic fiber. The type of opsin determines the range of wavelengths necessary to activate it. By delivering light in short pulses, neuronal firing can be controlled with millisecond-timescale precision,35 allowing activation or inhibition during both awake and anesthetized conditions.
Box 1. What to Look for in Research Using This Method.
Considerations for evaluating these experimental methods in the literature:
-
Was the proper method used for the scientific question?
Electrical stimulation is ideal for questions where stimulation of all the neurons and axons passing within a given region is desired
Microinjection and microdialysis are ideal for questions regarding specific receptors or analyte levels
Optogenetics is ideal for questions involving excitation or inhibition of distinct neuron types over short time scales (milliseconds to seconds) with high temporal precision
Chemogenetics is ideal for questions involving continuous excitation or inhibition of distinct neuron types over long time scales (minutes to hours) or in multiple locations in the brain
Are there any alternative explanations for the observed effect, such as projections from the stimulated region to unintended targets or secondary drug effects?
Was accurate placement of the electrode, cannula, or genetic modification histologically verified?
- Were proper controls employed? These may include the following:
- Electrical stimulation – Did researchers stimulate a nearby, off-target region to ensure that the observed effect of stimulation was region-specific?
- Microinjection or microdialysis – Did researchers perform separate experiments in which only the vehicle was injected or perfused?
- Optogenetics – Did researchers ensure opsin expression and function by recording neural responses to applied light?
- Chemogenetics – Did researchers ensure that the injected ligand dose was inert via separate experiments in animals lacking chemogenetic receptor expression?
Wang et al. used optogenetics to investigate whether stimulation of glutamatergic neurons in the parabrachial nucleus accelerates recovery from sevoflurane anesthesia.11 Although a related study used electrical stimulation of the parabrachial nucleus to elicit emergence from isoflurane anesthesia,21 the optogenetic tools employed by Wang et al. allowed the authors to examine the specific role of glutamatergic neurons in the parabrachial nucleus. They found that stimulation of parabrachial nucleus glutamatergic neurons alone was sufficient to accelerate recovery from sevoflurane anesthesia. Similarly, Taylor et al. used optogenetic stimulation of dopaminergic neurons in the ventral tegmental area to induce emergence from continuous isoflurane anesthesia.3 After systemic administration of a D1 dopamine receptor antagonist, however, optogenetic stimulation of ventral tegmental area dopaminergic neurons no longer induced an arousal response, demonstrating that the arousal effect was primarily governed by D1 receptors.
In a recent study, Jiang-Xie et al. sought to identify a shared neural circuit affecting both sleep and anesthesia.36 They used optogenetics to excite and inhibit a population of anesthesia-activated neurons in the supraoptic nucleus of the hypothalamus. They discovered that optogenetic stimulation of these neurons using ChR2 increased slow-wave sleep and prolonged recovery from isoflurane anesthesia. On the other hand, optogenetic inhibition of these neurons using Arch led to a shortened recovery from isoflurane, suggesting that these neurons contribute to the maintenance of isoflurane anesthesia.
Optogenetics provides the ability to manipulate specific populations of neurons in a brain region with excellent temporal resolution. In addition, the technique provides the ability to activate neuronal projections selectively with terminal field stimulation. For example, by inducing opsin expression in dopaminergic neurons in the ventral tegmental area but implanting the optic fiber in the nucleus accumbens, the dopaminergic pathway from the ventral tegmental area to the nucleus accumbens can be activated by illuminating the target region (fig. 4B, bottom right). This technique was used to demonstrate that the ventral tegmental area-nucleus accumbens pathway promotes wakefulness in the context of natural sleep.37
Although optogenetics provides a powerful tool to isolate and manipulate specific neural circuits, the method also has limitations. Not every cell within a targeted population will express the opsin, so it can be challenging to determine if an optogenetic manipulation that does not induce a behavioral change is the result of a circuit property or insufficient opsin expression. In addition, many types of neurons exhibit rebound firing in response to inhibition (see, e.g., Figure 1A from Carter et al.), potentially confounding results when optogenetic inhibition is used.38 Like electrical stimulation, optical stimulation involves many parameters such as the intensity and wavelength of the light, the frequency and duration of light pulses, and the total time of stimulation. Prolonged stimulation with light can heat tissue, causing neurophysiological effects independent of opsin activation.39 Because of this, it can be more difficult to inhibit a neural population than to excite it; activating only a subset of neurons may be sufficient to produce a response with excitation, whereas the entire population may need to be inhibited in order to prevent a response. To control for these variables, studies will often confirm functional opsin expression by recording firing rates or membrane voltage from single neurons in vitro, with and without applied light. As with all methods described in this article, histological analysis is vital. For optogenetics, it is necessary to confirm the location of the optic fiber, as well as opsin expression in the targeted region and neuron type via fluorescence imaging and immunolabeling.
Chemogenetics
In addition to optogenetics, which employs light-activated ion channels, a growing number of studies are using chemogenetic techniques, which use engineered ligand-activated receptors40 that are introduced into targeted neurons (Box 1). These receptors are engineered to be activated by specific exogenous ligands that are otherwise inert. This technology has been used to engineer several different protein classes such as G protein-coupled receptors,41–44 ligand-gated ion channels,45,46 kinases,47,48 and non-kinase enzymes.49 Following targeted chemogenetic receptor expression in one or more brain regions, a ligand can be introduced, either locally or systemically, in order to activate the receptor (fig. 5). Because of the ability to introduce the ligand systemically, multiple brain regions can be activated or inhibited simultaneously, without the need for the implanted optic fibers used in optogenetics.
Figure 5. Chemogenetic method of circuit manipulation.
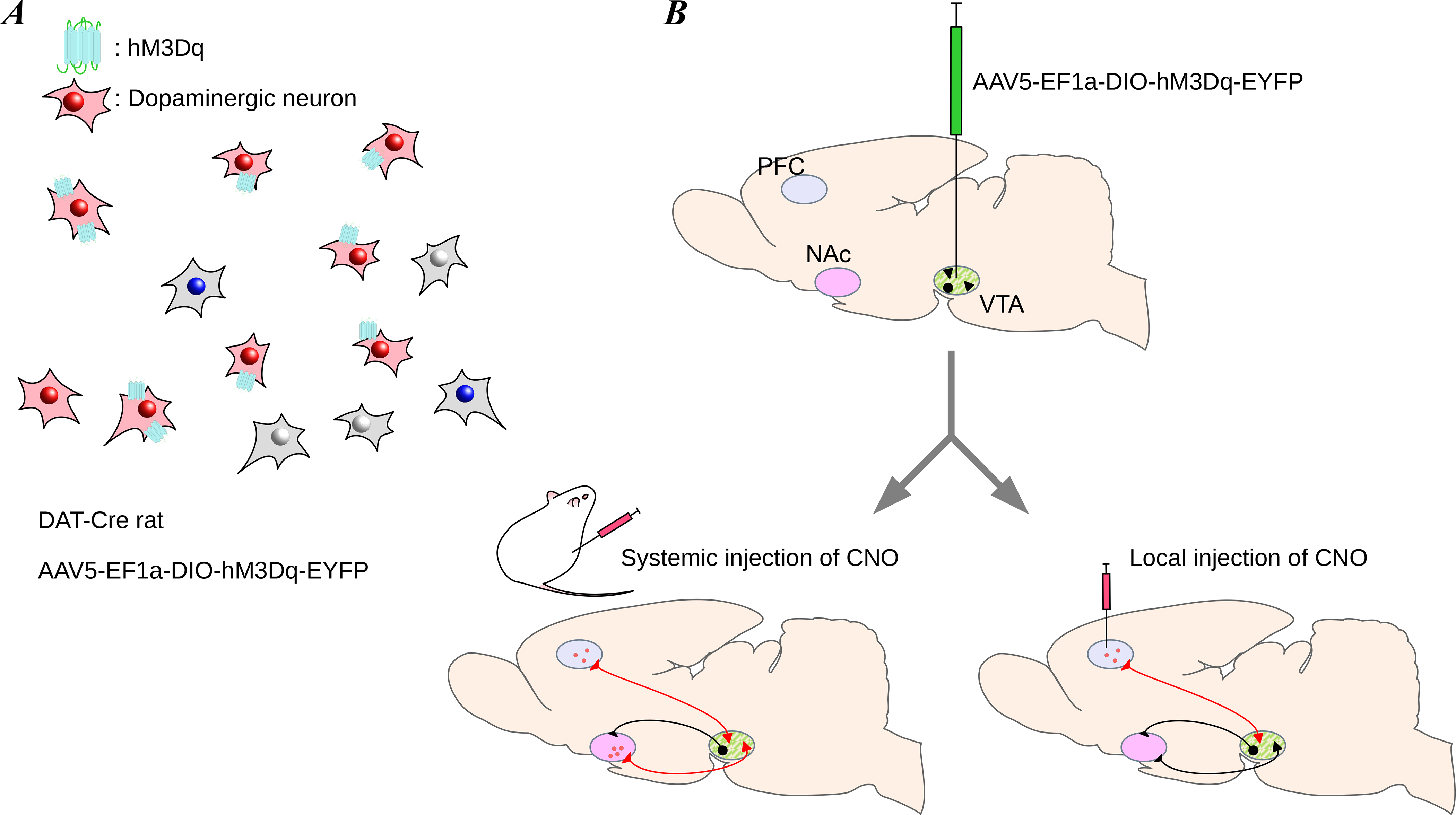
Chemogenetic stimulation can be used to excite or inhibit neural activity by activating receptors on targeted neurons. (A) Only neurons expressing the chemogenetic receptor are affected by their respective exogenous ligand. In this case, the excitatory chemogenetic receptor, hM3Dq, is activated by its ligand, clozapine-N-oxide (CNO). (B) Similar to optogenetics, chemogenetic experiments begin with injection of a virus containing the gene for the chemogenetic receptor (top). After about 3 weeks to allow for expression of the receptors, transfected neurons can be targeted through systemic injection of CNO (bottom left) or local injection of CNO (bottom right; by microinjection). The method of CNO delivery depends on the experiment: systemic injection is ideal for experiments requiring activation of all transfected neurons, whereas local injection is ideal for experiments requiring activation of a small region of transfected neurons.
The most widely used chemogenetic tools are Designer Receptors Exclusively Activated by Designer Drugs (DREADDs).50 DREADDs are modified G protein-coupled receptors derived from human muscarinic receptors (e.g. hM3Dq for excitation and hM4Di for inhibition) that have low affinity for the native ligand acetylcholine, and high affinity for a synthetic ligand such as clozapine-N-oxide (CNO). With excitatory designer receptors such as hM3Dq, binding of CNO ultimately produces neuronal burst firing by increasing intracellular calcium levels. With inhibitory designer receptors such as hM4Di, binding of CNO silences neuronal activity by inhibiting adenylate cyclase. In order to achieve activation and inhibition of the same neuronal populations with different ligands in the same animal, inhibitory kappa opioid receptor-based DREADDs have been introduced.43 Binding of the designer ligand salvinorin B to kappa opioid receptor-based DREADDs also inhibits adenylate cyclase, leading to neuronal inhibition. When kappa opioid receptor-based DREADDs are introduced in combination with excitatory hM3Dq receptors, CNO can be used to excite the same neurons that are inhibited by salvinorin B.
Chemogenetic methods have been used to investigate the roles of neural circuits in general anesthesia. For instance, Luo et al. investigated the role of neurons in the parabrachial nucleus in anesthetic emergence.51 After inducing non-selective expression of hM3Dq excitatory DREADDs in rat parabrachial nucleus neurons, the authors found that activation of these neurons during propofol and isoflurane anesthesia decreased power in the delta band (1–4Hz) of the electroencephalogram and accelerated emergence from anesthesia. However, this intervention did not alter the induction process. In the study by Wang and colleagues mentioned previously, the authors used Vglut2-Cre transgenic mice to induce selective expression of excitatory or inhibitory DREADDs in glutamatergic parabrachial nucleus neurons.11 In contrast to the results reported by Luo et al., Wang et al. discovered that selective activation of glutamatergic parabrachial nucleus neurons prolonged the induction time and decreased the emergence time from sevoflurane anesthesia; inhibition of these neurons slightly increased emergence time. Thus, stimulating neuronal subpopulations within a brain region can yield different results compared to non-selective stimulation.
Chemogenetics allows for selective excitation or inhibition of neuronal populations in specific brain regions with some unique advantages. Because the designer ligands can be delivered systemically, there is no requirement for a permanent intracranial implant such as an electrode, guide cannula or optic fiber. In addition, the designer ligands can provide long-lasting effects up to several hours without the concern for tissue damage in the brain due to electricity or heat. However, chemogenetics also has notable limitations. Although CNO is considered an inert ligand, it is metabolized to the antipsychotic drug clozapine, which has sedative effects and may confound results. This is a particular concern when high doses of CNO (>5 mg/kg) are administered.52 As alternative ligands to CNO, some studies have used perlapine and compound 21; however, perlapine is also sedating and has been used to treat insomnia.50 Thus, it is essential to keep the ligand dose as low as possible and to use appropriate controls in the experimental design, such as ligand administration in a group of control animals lacking DREADDs expression. Second, the on/off kinetics vary by ligand, making it important to select appropriate DREADDs for the given research question. While the effects of CNO peak at 45–50 minutes after i.p. injection and last for about 9 hours,41 the effects of salvinorin B appear shortly after s.c. injection and last for about one hour.43 Finally, when a ligand is administered systemically, any neuron expressing the chemogenetic receptors will be affected. Whereas optogenetic techniques can use the optical fiber placement as a strategy to limit neuronal manipulation to a specific brain region, chemogenetic techniques must rely on limited expression of the receptor by careful virus injection using low volumes.
Discussion
Our understanding of anesthetic mechanisms is rapidly expanding beyond receptor pharmacology to neural circuits. To this end, each of the techniques discussed in this article have been used to study the neural circuits underlying specific behavioral and physiological endpoints of anesthesia. Understanding the merits and drawbacks of these methods will allow clinicians and scientists to critically evaluate this expanding area of anesthesia research.
Powerful tools are now available to manipulate various brain regions and neuron types. Some of these tools, such as electrical stimulation, are already available to patients and are being used to treat Parkinson’s disease, epilepsy, sensory loss, and the effects of neural damage.15,53 Clinical trials are also underway to restore vision in human patients using optogenetics.54 Scientists, engineers, and clinicians are actively working to refine these tools, further enhancing our ability to target and probe specific neural circuits. As new techniques emerge, the abilities to address questions about anesthesia and consciousness, as well as treat disease, will continue to advance.
Box 2. Two Methods for Selective Expression of Opsins and Chemogenetic Receptors.
Selective expression of opsins or chemogenetic receptors in a population of neurons requires identification of a unique genetic promoter in the targeted population. Once a promoter is identified, a viral vector encapsulating a plasmid containing the promoter is injected into the brain region of interest. In addition to the promoter, the plasmid contains the gene coding for a light- or ligand-activated ion channel or pump, as well as a gene for a fluorescent protein to confirm expression of the plasmid genes. Two to three weeks after the virus injection are necessary to allow for plasmid gene expression before performing experiments.
In addition to the method described above, transgenic animals are also an effective means for delivering the opsin or chemogenetic receptor genes. This method usually employs Cre–Lox recombination, in which animals with neuron type–specific expression of Cre, an enzyme that causes genetic recombination, are crossed with animals with an inactivated opsin or chemogenetic receptor gene. The Cre recombinase activates the gene, causing only the neurons that express Cre to express the opsin or chemogenetic receptor. Alternatively, the inactivated opsin or chemogenetic receptor gene can be delivered via viral vector. Expression is Cre-dependent and takes approximately 2 to 3 weeks after virus injection.
Box 3. Where to Find More Information on This Topic.
Readers seeking more information about these methods can consult the following resources:
Vahle-Hinz C, Detsch O: What can in vivo electrophysiology in animal models tell us about mechanisms of anesthesia? Br J Anaesth 2002; 89:123–42
Brocker DT, Grill WM: Principles of Electrical Stimulation of Neural Tissue, 1st edition. Amsterdam, Elsevier B.V., 2013
Eckenhoff RG, Dmochowski IJ: Chemical and Biochemical Approaches for the Study of Anesthetic Function: Part A. Methods in Enzymology, 1st edition. London, Academic Press, 2018, Volume 602, pp 2–416
Eckenhoff RG, Dmochowski IJ: Chemical and Biochemical Approaches for the Study of Anesthetic Function: Part B. Methods in Enzymology, 1st edition. London, Academic Press, 2018, Volume 603, pp 2–320
Myers RD: Injection of solutions into cerebral tissue: Relation between volume and diffusion. Physiol Behav 1966; 1:171–4
Chefer VI, Thompson AC, Zapata A, Shippenberg TS: Overview of brain microdialysis. Curr Protoc Neurosci 2009:1–28
Montagni E, Resta F, Mascaro ALA, Pavone FS: Optogenetics in brain research: From a strategy to investigate physiologic function to a therapeutic tool. Photonics 2019; 6:1–19
Kim CK, Adhikari A, Deisseroth K: Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci 2017; 18:222–235
Roth BL: DREADDs for neuroscientists. Neuron 2016; 89:683–94
Funding Statement:
Support was provided by grants P01-GM118269 and R01-GM126155 from the National Institutes of Health, Bethesda, Maryland, and by the Scholar Award from the James S. McDonnell Foundation, Saint Louis, Missouri.
Footnotes
Conflicts of Interest: The authors declare no competing interests.
Clinical trial number: not applicable
Prior Presentations: not applicable
Summary Statement: not applicable
References
- 1.Hemmings HC, Riegelhaupt PM, Kelz MB, Solt K, Eckenhoff RG, Orser BA, Goldstein PA: Towards a Comprehensive Understanding of Anesthetic Mechanisms of Action: A Decade of Discovery. Trends Pharmacol Sci 2019; 40:464–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vazey EM, Aston-Jones G: Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc Natl Acad Sci 2014; 111:3859–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor NE, Van Dort CJ, Kenny JD, Pei J, Guidera JA: Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia 2016. doi: 10.1073/pnas.1614340113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou X, Wang Y, Zhang C, Wang M, Zhang M, Yu L, Yan M: The role of dopaminergic VTA neurons in general anesthesia. PLoS One 2015; 10:4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pal D, Dean JG, Liu T, Li D, Watson CJ, Hudetz AG, Mashour GA: Differential Role of Prefrontal and Parietal Cortices in Controlling Level of Consciousness. Curr Biol 2018; 28:2145–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alkire MT, McReynolds JR, Hahn EL, Trivedi AN: Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology 2007; 107:264–72 [DOI] [PubMed] [Google Scholar]
- 7.Sherin JE, Elmquist JK, Torrealba F, Saper CB: Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci 1998; 18:4705–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown EN, Purdon PL, Van Dort CJ: General Anesthesia and Altered States of Arousal: A Systems Neuroscience Analysis. Annu Rev Neurosci 2011; 34:601–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo T, Leung LS: Basal Forebrain Histaminergic Transmission Modulates Electroencephalographic Activity and Emergence from Isoflurane Anesthesia. Anesthesiology 2009; 111:725–33 [DOI] [PubMed] [Google Scholar]
- 10.Kelz MB, Sun Y, Chen J, Qing CM, Moore JT, Veasey SC, Dixon S, Thornton M, Funato H, Yanagisawa M: An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci U S A 2008; 105:1309–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang TX, Xiong B, Xu W, Wei HH, Qu WM, Hong ZY, Huang ZL: Activation of parabrachial nucleus glutamatergic neurons accelerates reanimation from sevoflurane anesthesia in mice. Anesthesiology 2019; 130:106–18 [DOI] [PubMed] [Google Scholar]
- 12.Vahle-Hinz C, Detsch O: What can in vivo electrophysiology in animal models tell us about mechanisms of anaesthesia? Br J Anaesth 2002; 89:123–42 [DOI] [PubMed] [Google Scholar]
- 13.Eckenhoff RG, Dmochowski IJ: Chemical and Biochemical Approaches for the Study of Anesthetic Function, Part A, Methods in Enzymology, 1st edition. London, Academic Press, 2018, Volume 602, pp 2–416 [Google Scholar]
- 14.Eckenhoff RG, Dmochowski IJ: Chemical and Biochemical Approaches for the Study of Anesthetic Function, Part B, Methods in Enzymology, 1st edition. London, Academic Press, 2018, Volume 603, pp 2–320 [Google Scholar]
- 15.Aum DJ, Tierney TS: Deep brain stimulation: Foundations and future trends. Front Biosci - Landmark 2018; 23:162–82 [DOI] [PubMed] [Google Scholar]
- 16.Brocker DT, Grill WM: Principles of electrical stimulation of neural tissue, 1st edition. Elsevier B.V., 2013 [DOI] [PubMed] [Google Scholar]
- 17.Temel Y: Deep brain stimulation in animal models, 1st edition. Elsevier B.V., 2013 [DOI] [PubMed] [Google Scholar]
- 18.Solt K, Cotten JF, Cimenser A, Wong KFK, Chemali JJ, Brown EN: Methylphenidate actively induces emergence from general anesthesia. Anesthesiology 2011; 115:791–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chemali JJ, Van Dort CJ, Brown EN, Solt K: Active emergence from propofol general anesthesia is induced by methylphenidate. Anesthesiology 2012; 116:998–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solt K, Van Dort CJ, Chemali JJ, Taylor NE, Kenny JD, Brown EN: Electrical stimulation of the ventral tegmental area induces reanimation from general anesthesia. Anesthesiology 2014; 121:311–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muindi F, Kenny JD, Taylor NE, Solt K, Wilson MA, Brown EN, Van Dort CJ: Electrical stimulation of the parabrachial nucleus induces reanimation from isoflurane general anesthesia. Behav Brain Res 2016; 306:20–5 [DOI] [PubMed] [Google Scholar]
- 22.Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, Fritz B, Eisenberg B, O’Connor J, Kobylarz EJ, Farris S, Machado A, McCagg C, Plum F, Fins JJ, Rezai AR: Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature 2007; 448:600–3 [DOI] [PubMed] [Google Scholar]
- 23.Ranck JB: Which elements are excited in electrical stimulation of mammalian central nervous system: A review. Brain Res 1975; 98:417–40 [DOI] [PubMed] [Google Scholar]
- 24.Myers RD: Injection of solutions into cerebral tissue: Relation between volume and diffusion. Physiol Behav 1966; 1:171–4 [Google Scholar]
- 25.Minert A, Yatziv S-L, Devor M: Location of the mesopontine neurons responsible for maintanance of anesthetic loss of consciousness. J Neurosci 2017; 37:0544–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minert A, Baron M, Devor M: Reduced Sensitivity to Anesthetic Agents upon Lesioning the Mesopontine Tegmental Anesthesia Area in Rats Depends on Anesthetic Type. Anesthesiology 2020:1–16 doi: 10.1097/ALN.0000000000003087 [DOI] [PubMed] [Google Scholar]
- 27.Gamou S, Fukuda S, Ogura M, Sakamoto H, Morita S: Microinjection of propofol into the perifornical area induces sedation with decreasing cortical acetylcholine release in rats. Anesth Analg 2010; 111:395–402 [DOI] [PubMed] [Google Scholar]
- 28.Dong H, Niu J, Su B, Zhu Z, Lv Y, Li Y, Xiong L: Activation of orexin signal in basal forebrain facilitates the emergence from sevoflurane anesthesia in rat. Neuropeptides 2009; 43:179–85 [DOI] [PubMed] [Google Scholar]
- 29.Hu Z, Wu Z, Gao J, Jia Q, Li N, Ouyang Y, Yao S, Chen X: Effects of HCN Channels in the Rostral Ventrolateral Medulla Contribute to the Cardiovascular Effects of Propofol. Mol Pharmacol 2018; 94:1280–8 [DOI] [PubMed] [Google Scholar]
- 30.Höcht C, Opezzo JAW, Taira CA: Applicability of reverse microdialysis in pharmacological and toxicological studies. J Pharmacol Toxicol Methods 2007; 55:3–15 [DOI] [PubMed] [Google Scholar]
- 31.Chefer VI, Thompson AC, Zapata A, Shippenberg TS: Overview of brain microdialysis. Curr Protoc Neurosci 2009:1–28 doi: 10.1002/0471142301.ns0701s47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bungay PM, Morrison PF, Dedrick RL: Steady-state Theory for Quantitative Microdialysis of Solutes and Water In Vivo and In Vitro. Life Sci 1989; 46:105–19 [DOI] [PubMed] [Google Scholar]
- 33.Lydic R, Baghdoyan HA, May AL: Neurochemistry of Anesthetic States, 1st edition. Elsevier Inc., 2018 [DOI] [PubMed] [Google Scholar]
- 34.Baghdoyan HA, Vanini G, Watson CJ, Lydic R: γ-Aminobutyric acid-mediated neurotransmission in the pontine reticular formation modulates hypnosis, immobility, and breathing during isoflurane anesthesia. Anesthesiology 2008; 109:978–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K: Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 2005; 8:1263–8 [DOI] [PubMed] [Google Scholar]
- 36.Jiang-Xie L-F, Yin L, Zhao S, Prevosto V, Han B-X, Dzirasa K, Wang F: A Common Neuroendocrine Substrate for Diverse General Anesthetics and Sleep. Neuron 2019; 102:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR, De Lecea L: VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat Neurosci 2016; 19:1356–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, De Lecea L: Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci 2010; 13:1526–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owen SF, Liu MH, Kreitzer AC: Thermal constraints on in vivo optogenetic manipulations. Nat Neurosci 2019. doi: 10.1038/s41593-019-0422-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vlasov K, Van Dort CJ, Solt K: Optogenetics and Chemogenetics, 1st edition. Elsevier Inc., 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL: Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 2009; 63:27–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL: Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand 2007; 104:5163–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vardy E, Robinson JE, Li C, Olsen RHJ, DiBerto JF, Giguere PM, Sassano FM, Huang XP, Zhu H, Urban DJ, White KL, Rittiner JE, Crowley NA, Pleil KE, Mazzone CM, Mosier PD, Song J, Kash TL, Malanga CJ, Krashes MJ, Roth BL: A New DREADD Facilitates the Multiplexed Chemogenetic Interrogation of Behavior. Neuron 2015; 86:936–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Redfern CH, Coward P, Degtyarev MY, Lee EK, Kwa AT, Hennighausen L, Bujard H, Fishman GI, Conklin BR: Conditional expression and signaling of a specifically designed G(i)-coupled receptor in transgenic mice. Nat Biotechnol 1999; 17:165–9 [DOI] [PubMed] [Google Scholar]
- 45.Lerchner W, Xiao C, Nashmi R, Slimko EM, van Trigt L, Lester HA, Anderson DJJ: Reversible Silencing of Neuronal Excitability in Behaving Mice by a Genetically Targeted, Ivermectin-Gated Cl- Channel. Neuron 2007; 54:35–49 [DOI] [PubMed] [Google Scholar]
- 46.Magnus CJ, Lee PH, Atasoy D, Su HH, Looger LL, Sternson SM: Chemical and genetic engineering of selective ion channel-ligand interactions. Science 2011; 333:1292–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD: A chemical-genetic approach to studying neurotrophin signaling. Neuron 2005; 46:13–21 [DOI] [PubMed] [Google Scholar]
- 48.Cohen MS, Zhang C, Shokat KM, Taunton J: Structural bioinformatics-based design of selective, irreversible kinase inhibitors. Science 2005; 308:1318–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Häring D, Distefano MD: Enzymes by design: Chemogenetic assembly of transamination active sites containing lysine residues for covalent catalysis. Bioconjug Chem 2001; 12:385–90 [DOI] [PubMed] [Google Scholar]
- 50.Roth BL: DREADDs for Neuroscientists. Neuron 2016; 89:683–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo T, Yu S, Cai S, Zhang Y, Jiao Y, Yu T, Yu W: Parabrachial Neurons Promote Behavior and Electroencephalographic Arousal from General Anesthesia. Front Mol Neurosci 2018; 11:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M: Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 2017; 357:503–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nag S, Thakor NV.: Implantable neurotechnologies: electrical stimulation and applications. Med Biol Eng Comput 2016; 54:63–76 [DOI] [PubMed] [Google Scholar]
- 54.Simunovic MP, Shen W, Lin JY, Protti DA, Lisowski L, Gillies MC: Optogenetic approaches to vision restoration. Exp Eye Res 2019; 178:15–26 [DOI] [PubMed] [Google Scholar]


