Abstract
Objective
Previous work in Huntington’s disease (HD) has shown that a sense of meaning and purpose (M&P) is positively associated with positive affect and well‐being (PAW); however, it was unknown whether HD‐validated patient‐reported outcomes (PROs) influence this association and how M&P impacts PROs in the future. Our study was designed to examine if HD‐validated PROs moderate the relationship between M&P and PAW and to evaluate if baseline M&P predicts 12‐ and 24‐month changes in HD‐validated PROs.
Methods
This was a longitudinal, multicenter study to develop several PROs (e.g., specific for the physical, emotional, cognitive, and social domains) for people with HD (HDQLIFE). The sample consisted of 322 people with HD (n = 50 prodromal, n = 171 early‐stage manifest, and n = 101 late‐stage manifest HD). A single, multivariate linear mixed‐effects model was performed with PAW as the outcome predicted by main effects for M&P and several moderators (i.e., an HD‐validated PRO) and interactions between M&P and a given PRO. Linear‐mixed models were also used to assess if baseline M&P predicted HD‐validated PROs at 12 and 24 months.
Results
Higher M&P was positively associated with higher PAW regardless of the magnitude of symptom burden, as represented by HD‐validated PROs, and independent of disease stage. In our primary analysis, baseline M&P predicted increased PAW and decreased depression, anxiety, anger, emotional/behavioral disruptions, and cognitive decline at 12 and 24 months across all disease stages.
Interpretation
These findings parallel those seen in the oncology population and have implications for adapting and developing psychotherapeutic and palliative HD interventions.
Introduction
Huntington’s disease (HD) is an autosomal dominant, progressive neurodegenerative disease. There is currently no available cure or disease‐modifying agent. 1 HD affects approximately 30,000 people in the United States, with another 150,000 living at‐risk. 2 Symptoms typically manifest between ages 30 and 50 with the triad of motor, cognitive, and emotional/behavioral impairments. There is a marked scarcity of evidence‐based treatments. 3 Suicide is a leading cause of death. 4
As part of a national initiative to improve the measurement of health‐related quality of life (HRQOL) in HD, several stakeholders (e.g., clinicians, informal care partners, people with the HD genetic mutation) convened and identified several symptoms significant within the HD triad (i.e., motor, cognitive, and emotional/behavioral disturbances). 5 This resulted in the formation of several HD‐validated patient‐reported outcomes (PROs). These PROs have subsequently undergone rigorous psychometric and clinometric testing among people with HD genetic mutation across various disease stages. 6 The PROs include outcomes, such as stigma, anxiety, anger, satisfaction with social roles, chorea, speech, and swallowing difficulties, concerns with death and dying, end of life planning, meaning and purpose, positive affect and well‐being, cognition, and emotional/behavioral disruptions.
Despite the multitude of PROs, the non‐motor ones (e.g., cognition or depression) seem to be more potent contributors to HRQOL scores than do the motor impairments, and perhaps more so in the earlier disease stages. 7 , 8 , 9 Additionally, there is a high prevalence of spiritual and existential distress (hopelessness, concern with death and dying, suicidality), 10 , 11 which is more salient than in other neurological diseases. 12 However, there are no evidence‐based palliative care models or psychotherapeutic interventions that can ameliorate these distressing symptoms among people with the HD gene mutation. 9 , 13 , 14
Work within oncology has suggested that a sense of meaning and purpose may buffer against depression and suicidality. 15 , 16 , 17 , 18 , 19 Work within palliative care and psycho‐oncology have further substantiated these observations. 20 , 21 , 22 , 23 , 24 , 25 , 26 Meaning‐centered interventions that allow people with advanced cancer to connect to various forms of meaning are associated with reductions in spiritual and existential distress and improvements in HRQOL. 27 , 28 , 29 , 30 , 31 Previous reports among people with the HD genetic mutation have suggested that the most robust positive association with meaning and purpose (M&P) is positive affect and well‐being (PAW). 32 However, it is unclear if this relationship is maintained when considering the magnitude of symptom burden as conceptualized with HD‐validated PROs (e.g., chorea, difficulties with speech and swallowing, depression, etcetera). Furthermore, it was unknown whether M&P may predict longitudinal changes in HD‐validated PROs.
Therefore, our overall goal of the study was to establish whether “meaning” represents a rational therapeutic target for intervention development to alleviate spiritual and existential suffering among people with HD gene mutation. Our objectives were twofold. Our first objective was to determine if an HD PRO, M&P, as embodied by the HDQLIFE M&P questionnaire, 11 will remain positively associated with joy, life contentment, and happiness, as measured by the NeuroQOL PAW questionnaire, 33 despite the burden of a variety of physical (e.g., chorea), emotional (e.g., depression), or social (e.g., ability to participate in activities) symptoms, as characterized by several NeuroQOL/PROMIS and HDQLIFE validated PROs for this population. We hypothesized that regardless of the disease stage, people with either premanifest or manifest HD and higher M&P would express higher levels of PAW, even in the face of severe symptomatology (Hypothesis 1). Our second objective was to ascertain if M&P at baseline predicted longitudinal HRQOL outcomes among a cohort of people with pre‐, early‐, and late‐stage manifest HD at 12 and 24 months. We hypothesized that baseline M&P would predict future improvements in PAW and reductions in negative emotional PROs (Hypothesis 2).
Methods
Participants
This analysis included 322 people with the gene mutation for HD (n = 50 prodromal, n = 171 early‐stage manifest, and n = 101 late‐stage manifest HD). Participants were part of a larger study, conducted between 2012 and 2016, designed to develop and validate new measures of HRQOL; full details of that study are reported elsewhere. 6 Eligibility criteria included a positive gene test and/or a clinical diagnosis of HD, ≥18 years of age, and the ability to provide informed consent (cognitive status was confirmed using a standard assessment when warranted 34 ). Study participants were recruited through established movement disorders clinics, support groups, nursing homes with HD‐specific units, the National Research Roster for Huntington’s Disease, existing research registries, online medical record data capture systems, 35 and through articles/advertisements targeting the HD community. A portion of the sample was collected in conjunction with the Predict‐HD study, a longitudinal, global cohort study. 36
Participant characterization and clinician‐administered measure of functioning
The Problem Behaviors Assessment is a clinician‐rated tool based on a semi‐structured interview; one of the domains is suicidality (PBA‐s). A score of “0” means no symptoms and “4” means symptoms are occurring daily. Eighty‐four percent of our cohort had a PBA‐s score of 0; the remaining were >0. The Total Functional Capacity (TFC) from the Unified Huntington’s Disease Ratings Scales (UHDRS) 37 was used to characterize people's functional capacity with the HD gene mutation in this sample. TFC provides an assessment of an individual’s ability to work, manage finances, do chores, live independently, perform daily living activities, and determine the HD stage for those with manifest HD. Total scores range from 0 to 13, with higher scores indicating better ability. Early‐stage manifest HD was defined as TFC scores of 7–13, and late‐stage manifest HD was defined as TFC scores of 0–6. 38 In addition, the final question on the UHDRS was used to determine manifest versus premanifest HD. Specifically, this question asks the clinician to rate their confidence from 1 (0% confidence) to 4 (>99% confidence) that the participant has motor manifest HD. Participants who received a rating of 3 or less were classified as having prodromal HD.
PRO administration formats and scoring
All PROs, except M&P and Anger, were administered as computer adaptive tests (CATs) followed by the fixed short‐forms (SFs); M&P and Anger were administered as SFs only. We examined scores from the CAT administrations for all PROs when available (all PROs except for M&P and Anger). The resulting T‐scores (M = 50; SD = 10) are relative to the development population and indicate more of that domain being measured (i.e., higher M&P scores indicate a better sense of purpose—better HRQOL, whereas higher depression scores indicate more sadness—worse HRQOL). The degree of symptom severity was defined based on one standard deviation above and below, which was used to generate groups of high and low people, respectively, on a given PRO. 39
PRO measures
HDQLIFE M&P 6 , 11 assesses an individual’s beliefs about why we do the things we do and make the most out of the time we have; data support its reliability, validity, and responsiveness in HD. 6 , 11 , 40
Neuro‐QoL Depression 41 assesses perceptions of sadness and helplessness; data support its reliability, validity, and responsiveness in HD. 6 , 42 , 43
Neuro‐QoL Anxiety 41 assesses feelings of nervousness and fear; data support its reliability, validity, and responsiveness in HD. 6 , 42 , 43
PROMIS Anger 44 , 45 , 46 assesses feelings of frustration and anger; data support its reliability, validity, and responsiveness in HD. 6 , 42 , 43
Neuro‐QoL PAW 41 assesses feelings of happiness, enjoyment, and contentment; data support its reliability, validity, and responsiveness in HD. 6 , 42 , 43
Neuro‐QoL Stigma 41 assesses perceptions of discrimination toward an individual; data support its reliability, validity, and responsiveness in HD. 6 , 42 , 43
Neuro‐QoL Satisfaction with Social Roles and Activities (SRA) 41 assesses an individual’s perceptions of satisfaction with social roles and activities.; data support its reliability, validity, and responsiveness in HD. 6 , 42 , 43
HDQLIFE Chorea 6 , 47 assesses the impact that chorea (i.e., the dance‐like movements characteristic of HD) has on physical activity and participation; data support its reliability, validity, and responsiveness in HD. 6 , 47 , 48 , 49
HDQLIFE Speech Difficulties 6 , 50 assesses how difficulty with oral expression, language production, and articulation affects communication and well‐being; data support its reliability, validity, and responsiveness in HD. 6 , 48 , 49 , 50
HDQLIFE Swallowing Difficulties 6 , 50 assesses how swallowing and choking problems impact well‐being and eating; data support its reliability, validity, and responsiveness in HD. 6 , 48 , 49 , 50
HDQLIFE Concern with Death and Dying 6 , 11 assesses a person’s thoughts about death and dying 6 , 11 ; data support its reliability, validity, and responsiveness in HD. 6 , 11 , 40
HDQLIFE End of Life Planning 40 , 51 assesses a person’s wishes and preparation about future medical care, including topics related to institutionalization, hospice, and environments desired near death; data support its reliability, validity, and responsiveness in HD. 51
NeuroQOL Cognitive Function 52 assesses a person’s perceived abilities in memory, attention, and other executive functions; data support its reliability, validity, and responsiveness in HD. 43 , 52 , 53
NeuroQOL Emotional and Behavioral Dyscontrol 5 , 43 , 47 assess a person’s impulsivity, lability, and irritability; datasupport its reliability, validity, and responsiveness in HD.
Procedures
Participants completed assessments at baseline, 12 and 24 months. All study visits were approximately 2 h in duration and involved completing an in‐person assessment and an online survey (administered through Assessment CenterSM 54 ). All participants provided informed consent at the baseline visit. At 12 and 24 months, participants who were unable or unwilling to be seen in person were provided a telephone interview option. Local Institutional Review Boards approved data collection.
Statistical analysis plan
PAW at each visit was modeled using linear mixed‐effects repeated measures models with a compound symmetry covariance structure (determined based on the mode of fit). A single model was performed with PAW as the outcome predicted by main effects for M&P and PRO moderators (i.e., depression, anxiety, anger, social participation, chorea, speech, swallow, concern with death and dying, end of life planning, suicidal behaviors, cognition, emotional/behavioral disruptions, and UHDRS/TFC); that is, an interaction between M&P and a moderator—while adjusted for other PROs and other interactions. All variables were treated as continuous except suicidality, which was dichotomized as any versus none. All moderators were tested in a multivariate linear mixed effect model (i.e., depression, anxiety, anger, satisfaction with social roles, chorea, speech, swallowing, concern with death & dying, stigma, end of life planning, suicidal behaviors, cognition, and emotional/behavioral disruptions). The slopes for the relationship between M&P and PAW were reported and plotted for low and high levels of each moderator (low = t‐score 40; high = t‐score 60; for suicidality low = none; high = any) to aid in interpreting interactions. A significant p‐value (<0.05) was interpreted as evidence of effect modification in the relationship between M&P and PAW; a non‐significant p‐value (≥0.05) suggested that the relationship between M&P and PAW was the same across levels of the moderator.
Linear‐mixed models were also used to assess if baseline M&P predicted PAW at follow‐up visits at 12 and 24 months after controlling for baseline PAW. An analogous approach was used to model for PROs and clinician‐administered levels of functioning. We accounted for multiple comparisons by reporting false‐discovery rate‐adjusted p‐values using linear step‐up. 55 Analyses were performed in SAS V9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Data on the number of people eligible, their clinical/demographic data, who completed follow‐up, study attrition, and how missing data was accounted for may be found elsewhere as part of the more extensive HDQLIFE study. 6 Descriptive characteristics for participants in this analysis are shown in Table 1.
Table 1.
Descriptive characteristics.
| Characteristic | Prodromal (n = 50) | Early (n = 171) | Late (n = 101) | All (n = 322) | p‐value |
|---|---|---|---|---|---|
| Age (years) | <0.0001 | ||||
| Mean (SD) | 43.4 (11.29) | 51.6 (12.62) | 55.5 (11.71) | 51.6 (12.72) | |
| N | 50 | 171 | 101 | 322 | |
| Gender, n (%) | 0.5942 | ||||
| Female | 23 (46) | 82 (48) | 42 (42) | 147 (46) | |
| Male | 27 (54) | 89 (52) | 59 (58) | 175 (54) | |
| Ethnicity, n (%) | 0.6048 | ||||
| Not Hispanic or Latino | 48 (96) | 159 (93) | 98 (97) | 305 (95) | |
| Hispanic of Latino | 1 (2) | 7 (4) | 1 (1) | 9 (3) | |
| Not provided | 1 (2) | 5 (3) | 2 (2) | 8 (2) | |
| Race, n (%) | 0.0032 | ||||
| Caucasian | 48 (96) | 164 (96) | 93 (92) | 305 (95) | |
| African American | 0 (0) | 2 (1) | 8 (8) | 10 (3) | |
| Other | 1 (2) | 5 (3) | 0 (0) | 6 (2) | |
| Unknown | 1 (2) | 0 (0) | 0 (0) | 1 (0) | |
| Education (years) | 0.0111 | ||||
| Mean (SD) | 15.7 (2.93) | 14.6 (2.70) | 14.2 (2.53) | 14.7 (2.72) | |
| N | 50 | 164 | 98 | 312 | |
| Marital status, n (%) | 0.1216 | ||||
| Single, never married | 5 (10) | 28 (16) | 11 (11) | 44 (14) | |
| Married | 36 (72) | 88 (51) | 63 (62) | 187 (58) | |
| Separated/divorced | 8 (16) | 42 (25) | 23 (23) | 73 (23) | |
| Living with partner | 1 (2) | 7 (4) | 0 (0) | 8 (2) | |
| Widowed | 0 (0) | 6 (4) | 4 (4) | 10 (3) | |
| CAG repeats | 0.0115 | ||||
| Mean (SD) | 41.9 (2.31) | 43.2 (3.98) | 44.8 (7.21) | 43.3 (4.71) | |
| Domain scores, mean (SD) | |||||
| Positive affect & well‐being | 55.1 (8.74) | 54.9 (8.51) | 54.3 (8.46) | 54.8 (8.51) | 0.7249 |
| Meaning & purpose | 49.0 (10.02) | 50.3 (9.75) | 48.7 (8.32) | 49.6 (9.38) | 0.1904 |
| Depression | 49.4 (9.98) | 51.3 (10.80) | 51.3 (11.08) | 51.0 (10.75) | 0.5892 |
| Anxiety | 52.8 (9.68) | 53.4 (10.05) | 54.1 (11.35) | 53.5 (10.39) | 0.8580 |
| Anger | 48.3 (12.11) | 48.4 (12.32) | 47.1 (12.75) | 48.0 (12.40) | 0.7638 |
| Social participation | 50.0 (8.29) | 47.1 (7.87) | 42.8 (7.97) | 46.3 (8.33) | <0.001 |
| Chorea | 42.9 (6.81) | 52.9 (7.32) | 57.4 (7.29) | 52.8 (8.60) | <0.001 |
| Speech | 45.3 (6.84) | 50.7 (7.57) | 55.2 (7.83) | 51.3 (8.19) | <0.001 |
| Swallow | 46.1 (7.16) | 51.6 (8.14) | 56.0 (7.64) | 52.1 (8.45) | <0.001 |
| Concern with death and dying | 50.0 (9.14) | 50.6 (9.58) | 49.8 (11.02) | 50.3 (9.96) | 0.7139 |
| Stigma | 46.1 (7.66) | 51.7 (8.00) | 53.3 (9.67) | 51.3 (8.78) | <0.001 |
| End of life planning | 47.4 (8.23) | 49.8 (9.63) | 53.2 (10.18) | 50.5 (9.78) | <0.001 |
| Suicidal behaviors | 0.6 (2.23) | 0.4 (1.48) | 0.4 (1.45) | 0.4 (1.61) | 0.9277 |
| Cognition | 44.4 (10.33) | 38.3 (8.94) | 28.6 (7.83) | 36.3 (10.43) | <0.001 |
| Emotional/behavioral disruptions | 46.2 (11.06) | 47.4 (10.40) | 46.9 (11.63) | 47.0 (10.86) | 0.6064 |
| UHDRS/TFC | 12.2 (1.58) | 9.9 (1.96) | 3.9 (1.80) | 8.4 (3.65) | <0.001 |
Hypothesis 1. People with the HD gene mutation and high levels of M&P would report high levels of joy, contentment, and happiness even in the face of high levels of symptoms
In a previous bivariate analysis, M&P was moderately correlated with PAW (r = 0.63, p < 0.01). 32 Table 2 includes the results of our linear‐mixed model. The impact of M&P on PAW was moderated by only two other PROs (i.e., depression and chorea). Specifically, there was a positive interaction with depression (p = 0.0258), indicating a stronger relationship among those with higher depression, and negative interaction with chorea (p = 0.0048), indicating a weaker relationship among those with higher chorea. Model estimates were used to calculate strata‐specific slopes for those with low and high levels of each symptom; slopes for the relationship between M&P and PAW are shown in Table 3. For depression, there was a 0.21‐point t‐score increase in PAW per 1 point increase in M&P among those with low depression; among those with high depression, there was a 0.44‐point t‐score increase. For low and high chorea, the M&P slopes were 0.46 and 0.19, respectively, but both were statistically significant. These relationships are plotted as line graphs in Figure 1. For all other PROs in our analysis, the relationship between M&P and PAW remained the same for those with high or low symptoms of a given PRO. There were no significant subgroup differences, either by gender or disease stage.
Table 2.
Linear‐mixed effects model of positive affect & well‐being.
| Variable | Main effects | p‐value | Interactions (with M&P) | |
|---|---|---|---|---|
| Beta [95% CI] | Beta [95% CI] | p‐value | ||
| Intercept | 25.082 | – | – | – |
| Meaning & purpose | 0.814 [−0.112, 1.740] | 0.0847 | – | – |
| Depression | −0.685 [−1.181, −0.189] | 0.0069 | 0.011 [0.001, 0.021] | 0.0258 |
| Anxiety | −0.210 [−0.772, 0.352] | 0.4632 | 0.003 [−0.008, 0.014] | 0.5417 |
| Anger | 0.416 [−0.067, 0.899] | 0.0912 | −0.009 [−0.019, 0.001] | 0.0756 |
| Social participation | 0.472 [0.089, 0.856] | 0.0159 | −0.008 [−0.015, 0.000] | 0.0502 |
| Chorea | 0.624 [0.148, 1.101] | 0.0104 | −0.014 [−0.023, −0.004] | 0.0048 |
| Speech | −0.375 [−0.910, 0.159] | 0.1684 | 0.008 [−0.002, 0.019] | 0.1117 |
| Swallow | −0.212 [−0.667, 0.242] | 0.3594 | 0.005 [−0.004, 0.014] | 0.2460 |
| Concern with death and dying | 0.186 [−0.148, 0.520] | 0.2737 | −0.006 [−0.013, 0.001] | 0.0836 |
| Stigma | 0.150 [−0.320, 0.619] | 0.5314 | −0.005 [−0.014, 0.005] | 0.3517 |
| End of life planning | −0.221 [−0.513, 0.071] | 0.1375 | 0.005 [−0.001, 0.010] | 0.1111 |
| Suicidal behaviors | −1.044 [−9.318, 7.230] | 0.8043 | 0.008 [−0.169, 0.186] | 0.9272 |
| Cognition | 0.084 [−0.263, 0.431] | 0.6348 | −0.001 [−0.008, 0.005] | 0.6663 |
| Emotional/behavioral disruptions | 0.055 [−0.501, 0.611] | 0.8462 | −0.001 [−0.012, 0.010] | 0.8768 |
Table 3.
The impact of meaning & purpose on positive affect & well‐being by moderator levels based on estimates in Table 2.
| Variable | Levels of moderator | |||
|---|---|---|---|---|
| Low | p‐value | High | p‐value | |
| M&P Beta [95% CI] | M&P Beta [95% CI] | |||
| Depression | 0.21 [0.07, 0.36] | 0.0041 | 0.44 [0.28, 0.60] | <0.0001 |
| Anxiety | 0.29 [0.11, 0.47] | 0.0018 | 0.36 [0.23, 0.49] | <0.0001 |
| Anger | 0.41 [0.26, 0.57] | <0.0001 | 0.24 [0.09, 0.39] | 0.0022 |
| Social participation | 0.40 [0.27, 0.54] | <0.0001 | 0.25 [0.11, 0.39] | 0.0007 |
| Chorea | 0.46 [0.31, 0.61] | <0.0001 | 0.19 [0.04, 0.34] | 0.0136 |
| Speech | 0.24 [0.11, 0.38] | 0.0004 | 0.41 [0.23, 0.58] | <0.0001 |
| Swallow | 0.27 [0.13, 0.42] | 0.0002 | 0.38 [0.23, 0.53] | <0.0001 |
| Concern with death and dying | 0.38 [0.25, 0.52] | <0.0001 | 0.27 [0.13, 0.40] | 0.0001 |
| Stigma | 0.37 [0.23, 0.52] | <0.0001 | 0.28 [0.12, 0.44] | 0.0005 |
| End of life planning | 0.28 [0.15, 0.40] | <0.0001 | 0.37 [0.24, 0.51] | <0.0001 |
| Suicidal behaviors | 0.32 [0.20, 0.44] | <0.0001 | 0.33 [0.14, 0.52] | 0.0006 |
| Cognition | 0.34 [0.26, 0.42] | <0.0001 | 0.31 [0.14, 0.48] | 0.0004 |
| Emotional/behavioral disruptions | 0.33 [0.20, 0.47] | <0.0001 | 0.32 [0.13, 0.50] | 0.0008 |
Figure 1.
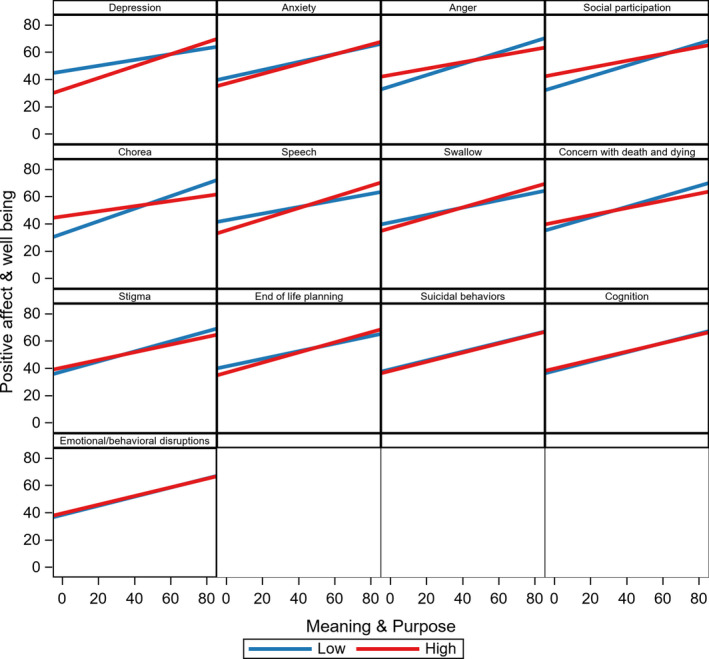
The impact of meaning & purpose on positive affect & well‐being by moderator levels based on estimates in Table 2.
Hypothesis 2. Baseline M&P for people with the HD gene mutation would predict an increased PAW and decreased negative emotional PROs at 12 and 24 months
The predictors are baseline M&P and baseline levels of the outcome. Scores from baseline, 12, and 24 months are modeled—after adjusting for what the levels of each of the outcomes were at baseline (Table 4). M&P at baseline was associated with increased PAW (Beta 95% CI = 0.15 [0.06, 0.24], p = 0.0011), decreased depression (Beta 95% CI = −0.16 [−0.27, −0.06], p = 0.0030), decreased anxiety (Beta 95% CI = −0.12 [−0.22, −0.02], p = 0.0202), decreased anger (Beta 95% CI = −0.20 [−0.33, −0.08], p = 0.0011), decreased emotional/behavioral disruptions (Beta 95% CI = −0.19 [−0.28, −0.09], p = 0.0001), and slowed cognitive decline (Beta 95% CI = 0.10 [0.01, 0.19], p = 0.0329), at both 12 and 24 months. The strongest relationship was seen with anger: a 1 t‐score increase in M&P at baseline is associated with a 0.2 t‐score decrease in anger at follow‐up (regardless of where anger was at baseline). Our analysis that adjusted for multiple comparisons maintained that PAW, depression, anger, and emotional/behavioral disruptions were still significant; however, anxiety and cognition were not. Additionally, no evidence of subgroup variation was seen, except a positive association between baseline M&P and follow‐up social participation, as assessed through NeuroQOL SRA, among the prodromal group (Beta 95% CI = 0.12 [0.01–0.23], p = 0.04) but was not found in other groups.
Table 4.
Linear‐mixed effects models of baseline M&P on symptoms at 12 and 24 months. (Suicidal behaviors dichotomized and analyzed using binary logit model). 1
| Outcome (assessed at t1 & t2) | Baseline M&P | p‐value | Baseline symptom | |
|---|---|---|---|---|
| Beta and 95% | Beta and 95% | p‐value | ||
| Positive affect & well being | 0.15 [0.06, 0.24] | 0.0011 | 0.49 [0.38, 0.59] | <0.0001 |
| Depression | −0.16 [−0.27, −0.06] | 0.0030 | 0.54 [0.44, 0.64] | <0.0001 |
| Anxiety | −0.12 [−0.22, −0.02] | 0.0202 | 0.65 [0.55, 0.74] | <0.0001 |
| Anger | −0.20 [−0.33, −0.08] | 0.0011 | 0.52 [0.43, 0.62] | <0.0001 |
| Social participation | 0.01 [−0.07, 0.09] | 0.7156 | 0.45 [0.36, 0.54] | <0.0001 |
| Chorea | −0.03 [−0.08, 0.03] | 0.3522 | 0.76 [0.70, 0.82] | <0.0001 |
| Speech | −0.01 [−0.07, 0.05] | 0.8103 | 0.73 [0.66, 0.80] | <0.0001 |
| Swallow | 0.01 [−0.06, 0.07] | 0.7723 | 0.78 [0.71, 0.85] | <0.0001 |
| Concern with death and dying | −0.03 [−0.12, 0.06] | 0.5022 | 0.55 [0.46, 0.64] | <0.0001 |
| Stigma | −0.00 [−0.08, 0.07] | 0.9006 | 0.64 [0.55, 0.72] | <0.0001 |
| End of life planning | 0.00 [−0.07, 0.08] | 0.9270 | 0.79 [0.72, 0.86] | <0.0001 |
| Suicidal behaviors | −0.02 [−0.05, 0.02] | 0.3550 | 2.10 [1.36, 2.83] | <0.0001 |
| Cognition | 0.10 [0.01, 0.19] | 0.0329 | 0.64 [0.56, 0.72] | <0.0001 |
| Emotional/behavioral disruptions | −0.19 [−0.28, −0.09] | 0.0001 | 0.52 [0.43, 0.60] | <0.0001 |
| UHDRS/TFC | 0.00 [−0.02, 0.02] | 0.6646 | 0.86 [0.81, 0.92] | <0.0001 |
Note: Each line represents an independent linear mixed‐effects model with two covariates: (1) M&P at baseline and (2) levels of outcome domain at baseline.
Discussion
Our results support two key relationships between M&P and HRQOL. First, people with the HD gene mutation who express higher M&P exhibit higher contentment and joy in their lives, independent of the degree of symptom severity experienced. Interestingly, the impact that M&P has on PAW may be more potent even in the face of high depression, suggesting a distinct mechanism by which M&P operates to influence PAW. Namely, the magnitude that M&P has on PAW is greater at high depression than with low depression, suggesting that screenings indicative of high depressive burden could prompt clinicians to refer people with the HD genetic mutation for M&P interventions, which may still significantly influence joy, contentment, and happiness with life. However, the magnitude of M&P and PAW may be mildly attenuated in the face of severe chorea. Therefore, other pharmaco‐ or physio‐ therapies may be necessary to address severe chorea as it may attenuate this relationship. Second, in our primary analysis, baseline M&P was associated with increased PAW and decreased depression, anxiety, impulsivity, cognitive decline, and anger at 12 and 24 months.
Our findings support our first hypothesis that severe non‐motor or motor symptomatology does not negate the positive relationship between M&P and PAW and extends a large body of work in psycho‐oncology. In particular, our data are analogous and extend previous studies from people with advanced cancer, who are more likely to rate high levels of life satisfaction, even in the face of severe symptoms, when they concurrently report high levels of spiritual well‐being. 26 Second, these findings also recapitulate that metaphysical factors beyond clinical depression or anxiety influence PAW, mostly since M&P was undeterred by those moderators. As the most robust correlation in HD to PAW is M&P, 32 clinicians who may conflate existential suffering (i.e., low M&P) with clinical depression might miss an opportunity to intervene using a tailored approach (e.g., integration with chaplaincy). Indeed, our findings build upon data from other serious illnesses, which also observed that factors beyond depression influence negative emotional states, such as hastened death. 23
Data addressing our second hypothesis support that baseline M&P predicts future HRQOL, especially the emotional and cognitive domains, suggesting future investigation areas.
First, while some may consider M&P to be a stable trait since findings suggest that M&P does not change across the different HD stages, 40 interventions have successfully influenced M&P and other reputed state traits. 56 Supporting this claim is that our results follow previous M&P interventions for the advanced cancer population that has shown positive emotional health changes 27 , 28 , 29 , 30 and, therefore, serve as an additional impetus to adapt an M&P intervention to HD.
Second, while the relationship of M&P and cognition did not hold after accounting for multiple comparisons, it is noteworthy that a seminal observational study within Alzheimer’s and other related dementias 57 demonstrated that purpose in life was strongly associated with a lower incidence of Alzheimer’s. A dose–response relationship was also noted, such that a person who scored in the 90th percentile was 2.4 times more likely not to develop dementia as compared to a person in the 10th percentile—and this relationship was even maintained in the face of accounting for depression, chronic diseases, personality factors, and other demographic data. Further, higher purpose in life was associated with a lower risk for mild cognitive impairment development. Exploration of this association deserves further evaluation in HD, especially since around 80% suffer from mild cognitive impairment when motor symptoms manifest. 58
Third, end‐of‐life planning, 59 as represented by HDQLIFE End of Life Planning, is positively associated with M&P. 51 Indeed, previous M&P interventions have also incorporated and positively influenced end‐of‐life planning. 30 The inclusion of this practice to a future, adapted M&P intervention may also be warranted for this cohort, especially given a recent multicenter study of 503 people with HD that demonstrated the remarkably low prevalence of advance directives (38.2%), conversations about death, and dying with loved ones (10.5%), and deciding on a place to die (10.7%). 60
Our analysis is not without limitations. First, we cannot determine causality because there was no experimental condition associated with the observational data. For example, might high positive affect give rise to high M&P? 61 , 62 Second, there are various instruments to measure the existential ideas within M&P. 63 HDQLIFE represents one. However, our findings require verification with additional instrumentation to explore the longitudinal relationship between M&P and our PROs. Third, our models do not consider psychoactive medications, PT/OT utilization, or other exogenous factors, which may bias our analysis.
Despite these limitations, our findings are a compelling first step toward understanding the primary mechanism behind M&P and how it influences HRQOL in people with the HD gene mutation. Notably, M&P influences well‐known suicide risk factors for this population (anger, impulsivity, depression, and anxiety). 64 Indeed, the SI rates in HD are much higher than other neurological diseases 12 , and some reports indicate that the highest suicide rates are before diagnosis (not immediately after) and in stage 2 (e.g., early‐stage manifest), as independence is lost. 64 Models of suicidality suggest that impulsivity may be a necessary but not a sufficient factor in suicide attempts. 65 Thus, a sense of M&P may serve as a resiliency factor for suicide in people with the HD gene mutation in that it can impact factors associated with suicidal ideation (e.g., depression, anxiety) as well as suicidal behaviors (e.g., impulsivity and anger). 66
In conclusion, this study provides a generalization on the value of M&P to people with the HD gene mutation, and future efforts are warranted to adapt and develop meaning‐ and palliative‐ centered interventions to this population. 13
Conflict of Interest
The authors report no potential conflicts of interest related to the research covered in the article.
Authors’ Contributions
All authors contributed to the conception, organization, execution of the project, and revising and critiquing the manuscript for important intellectual content.
1. Research project: A. Conception, B. Organization, C. Execution;
2. Statistical Analysis: A. Design, B. Execution, C. Review, and Critique;
3. Manuscript Preparation: A. Writing of the first draft, B. Review, and Critique.
LLS: 1A, 1B, 1C, 2A, 2C, 3A, 3B.
JT: 1B, 1C, 2A, 2B, 2C, 3B.
NC: 1A, 1B, 1C, 2A, 2B, 2C, 3B.
B.M.K, A.J.A., J.S.P., D.B., S.F., J.M.H., N.R.B., C.A.D., D.C.: 1A, 1B, 1C, 2C, 3B.
Disclosures
Dr Sokol is an ad‐hoc consultant for Tikvah for Parkinson in the range of $0–$499, ad‐hoc consultant for the American Film Institute on end‐of‐life care/palliative care in the enrage of $500–$999; and receives financial support from the Northwestern PSTP Program in Neurology as well as the R25 NCI 2R25CA190169. Dr Troost has research funding through the University of Michigan with Complexa Inc, Retrophin Inc, and Goldfinch Bio, and the University of Michigan with Vertex Pharmaceuticals and Pfizer Inc. Dr Kluger received research grant support from the National Institute of Aging, National Institute of Nursing Research, and Patient‐Centered Outcomes Research Institute; he has received speaker honoraria from the Parkinson’s Foundation. Dr Applebaum receives financial support from the National Cancer Institute. Dr Paulsen receives support from the NINDS and NIBIB and has received personal compensation in the range of $0–$499 for serving on a Scientific Advisory or Data Safety Monitoring Board for Wave Life Sciences, has received personal compensation in the range of $0–$499 for serving on a Speakers Bureau for HDSA, and has received personal compensation in the range of $0–$499 for serving as a consultant with Acadia. Dr Bega has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Speaker: Teva Pharmaceuticals, Acorda Therapeutics, Neurocrine Biosciences, Adamas Pharmaceuticals Consulting: Biogen Pharmaceuticals, Amgen Pharmaceuticals, Acadia Pharmaceuticals, Genentech, Inc, GE Healthcare, Gerson Lehrman Group, Guidepoint, L.E.K. C., and has received personal compensation in an editorial capacity for Editor: Annals of Clinical & Translational Neurology. Dr Frank has received personal compensation in the range of $500–$4999 for serving as a Consultant for Oscine Therapeutics. Dr Frank has received personal compensation in the range of $500–$4999 for serving as a Consultant for uniQure, has received personal compensation in the range of $500–$4999 for serving as a Consultant for MCG Health. The institution of Dr Frank has received research support from the Huntington’s Disease Society of America. The institution of Dr Frank has received research support from Michael J Fox Foundation. The institution of Dr Frank has received research support from Roche/Genentech. The institution of Dr Frank has received research support from CHDI Foundation. The institution of Dr Frank has received research support from the Huntington Study Group. The institution of Dr Frank has received research support from Triplet Therapeutics. Dr Hauser is supported or has received support from the Coleman Foundation, University of Florida, Instituto Nacional de Caˆncer, American Academy of Hospice and Palliative Medicine, Arnold P. Gold Foundation, National Institute on Aging, Seasons Hospice Foundation, Woodstock, HCSC Insurance Services Company, National Heart, Lung, and Blood Institute, Canadian Patient Safety Institute, Health Research, and Educational Trust, Icahn School of Medicine at Mount Sinai, Centers for Medicare and Medicaid Services, Agency for Healthcare Research and Quality, Children’s Hospitals and Clinics of Minnesota, Instituto Nacional de Caˆncer, Department of Veterans Affairs, National Center for Research Resources, NOVA Research Company, Instituto Nacional de Caˆncer, Lance Armstrong Foundation, Retirement Research Foundation, Society for the Arts in Healthcare, Medical College of Wisconsin, and the National Institute of Nursing Research. Dr Boileau reports no disclosures. Dr Depp receives support from the NIMH, VA, and NCATS. Dr Cella has research funding to his institution from NIH, Abbvie, Amgen, Bayer Healthcare, Bristol‐Myers Squibb, Clovis, Eli Lilly and Company, Glaxo Smith‐Kline, Johnson and Johnson, Novartis, Pfizer, and PledPharma; he has served as a consultant to Abbvie, Bristol‐Myers Squibb, Eli Lilly and Company, Johnson and Johnson, Novartis, and Pfizer. Dr Cella is the president of FACIT.org. Dr Carlozzi reports research grants from the NIH, the Neilsen Foundation, and CHDI, as well as contracts from Goldfinch, LLC, and Health and Human Services – Centers for Medicare & Medicaid Services; she receives honoraria for her role on the CHDI scientific advisory board and is a consultant on the TBI Congressionally mandated study.
Funding Information
Data reported in this manuscript were collected with support from the National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (R01NS077946: PI Carlozzi; R01NS0400068: PI Paulsen), and the National Center for Advancing Translational Sciences (UL1TR000433). Jonathan Troost was supported in part by the National Center for Advancing Translational Sciences (NCATS) for the Michigan Institute for Clinical and Health Research (UL1TR002240).
Funding Statement
This work was funded by National Center for Advancing Translational Sciences grants UL1TR000433 and UL1TR002240; National Institute of Neurological Disorders and Stroke grants R01NS0400068 and R01NS077946; National Institutes of Health ; Michigan Institute for Clinical and Health Research .
References
- 1. Walker FO. Huntington’s disease. Lancet 2007;369:218–228. [DOI] [PubMed] [Google Scholar]
- 2. Frank S. Treatment of Huntington’s disease. Neurotherapeutics 2014;11:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Killoran A, Biglan KM. Current therapeutic options for Huntington’s disease: good clinical practice versus evidence‐based approaches? Mov Disord 2014;29:1404–1413. [DOI] [PubMed] [Google Scholar]
- 4. Rodrigues FB, Abreu D, Damásio J, et al. Survival, mortality, causes and places of death in a European Huntington’s disease prospective cohort. Mov Disord Clin Pract 2017;4:737–742. Available from http://www.ncbi.nlm.nih.gov/pubmed/30363513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Victorson D, Carlozzi NE, Frank S, et al. Identifying motor, emotional‐behavioral, and cognitive deficits that comprise the triad of HD Symptoms from patient, caregiver, and provider perspectives. Tremor Other Hyperkinet Mov 2014;4:224. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24757585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carlozzi NE, Schilling SG, Lai J‐S, et al. HDQLIFE: development and assessment of health‐related quality of life in Huntington disease (HD). Qual Life Res 2016;13:2441–2455. 10.1007/s11136-016-1386-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ho AK, Gilbert AS, Mason SL, et al. Health‐related quality of life in Huntington’s disease: which factors matter most? Mov Disord 2009;15:574–578. 10.1002/mds.22412 [DOI] [PubMed] [Google Scholar]
- 8. Ho A, Hocaoglu M. Impact of Huntington’s across the entire disease spectrum: the phases and stages of disease from the patient perspective. Clin Genet 2011;80:235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zarotti N, Dale M, Eccles F, Simpson J. Psychological interventions for people with Huntington’s disease: a call to arms. J Huntingtons Dis 2020;9:231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dawson S, Kristjanson LJ, Toye CM, Flett P. Living with Huntington’s disease: need for supportive care. Nurs Health Sci 2004;6:123–130. Available from http://www.ncbi.nlm.nih.gov/pubmed/15130098 [DOI] [PubMed] [Google Scholar]
- 11. Carlozzi NE, Downing NR, McCormack MK, et al. New measures to capture end of life concerns in Huntington disease: meaning and purpose and concern with death and dying from HDQLIFE (a patient‐reported outcomes measurement system). Qual Life Res 2016;8:2403–2415. Available from http://www.ncbi.nlm.nih.gov/pubmed/27393121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erlangsen A, Stenager E, Conwell Y, et al. Association between neurological disorders and death by suicide in Denmark. JAMA 2020;4:444–454. Available from https://jamanetwork.com/journals/jama/fullarticle/2760389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sokol LL, Lum HD, Creutzfeldt CJ, et al. Meaning and dignity therapies for psychoneurology in neuropalliative care: a vision for the future. J Palliat Med 2020;23:1155–1156. Available from http://www.ncbi.nlm.nih.gov/pubmed/32877285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson MO, Frank S, Mendlik M, Casarett D. Utilization of hospice services in a population of patients with Huntington's disease. J Pain Symptom Manage 2018;55:440–443. 10.1016/j.jpainsymman.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 15. Kleiman EM, Beaver JK. A meaningful life is worth living: meaning in life as a suicide resiliency factor. Psychiatry Res 2013;210:934–939. [DOI] [PubMed] [Google Scholar]
- 16. Heisel MJ, Flett GL. Purpose in life, satisfaction with life, and suicide ideation in a clinical sample. J Psychopathol Behav Assess 2004;26:127–135. [Google Scholar]
- 17. Edwards MJ, Holden RR. Coping, meaning in life, and suicidal manifestations: examining gender differences. J Clin Psychol 2001;57:1517–1534. [DOI] [PubMed] [Google Scholar]
- 18. Meier DE, Emmons CA, Wallenstein S, et al. A national survey of physician‐assisted suicide and euthanasia in the United States. N Engl J Med 1998;23:1193–1201. 10.1056/NEJM199804233381706 [DOI] [PubMed] [Google Scholar]
- 19. Chochinov HM, Wilson KG, Enns M, Lander S. Depression, hopelessness, and suicidal ideation in the terminally Ill. Psychosomatics 1998;39:366–370. 10.1016/S0033-3182(98)71325-8 [DOI] [PubMed] [Google Scholar]
- 20. McClain CS, Rosenfeld B, Breitbart W. Effect of spiritual well‐being on end‐of‐life despair in terminally‐ill cancer patients. Lancet 2003;361:1603–1607. [DOI] [PubMed] [Google Scholar]
- 21. Rosenfeld B, Cham H, Pessin H, Breitbart W. Why is Meaning‐Centered Group Psychotherapy (MCGP) effective? Enhanced sense of meaning as the mechanism of change for advanced cancer patients. Psychooncology 2018;27:654–660. 10.1002/pon.4578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosenfeld B, Breitbart W, Galietta M, et al. The schedule of attitudes toward hastened death: measuring desire for death in terminally III cancer patients. Cancer 2000;88:2868–2875. [DOI] [PubMed] [Google Scholar]
- 23. Breitbart W, Rosenfeld B, Gibson C, et al. Impact of treatment for depression on desire for hastened death in patients with advanced AIDS. Psychosomatics 2010;51:98–105. 10.1016/S0033-3182(10)70669-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA 2000;13:2907–2911. 10.1001/jama.284.22.2907 [DOI] [PubMed] [Google Scholar]
- 25. Breitbart W, Heller KS. Reframing hope: meaning‐centered care for patients near the end of life. J Palliat Med 2003;6:979–988. 10.1089/109662103322654901 [DOI] [PubMed] [Google Scholar]
- 26. Brady MJ, Peterman AH, Fitchett G, et al. A case for including spirituality in quality of life measurement in oncology. Psycho‐Oncology 1999;8:417–428. [DOI] [PubMed] [Google Scholar]
- 27. Breitbart W, Pessin H, Rosenfeld B, et al. Individual meaning‐centered psychotherapy for the treatment of psychological and existential distress: a randomized controlled trial in patients with advanced cancer. Cancer 2018;124:3231–3239. Available from http://www.ncbi.nlm.nih.gov/pubmed/29757459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Breitbart W, Poppito S, Rosenfeld B, et al. Pilot randomized controlled trial of individual meaning‐centered psychotherapy for patients with advanced cancer. J Clin Oncol 2012;30:1304–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Breitbart W, Rosenfeld B, Gibson C, et al. Meaning‐centered group psychotherapy for patients with advanced cancer: a pilot randomized controlled trial. Psychooncology 2010;19:21–28. 10.1002/pon.1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodin G, Lo C, Rydall A, et al. Managing Cancer and Living Meaningfully (CALM): a randomized controlled trial of a psychological intervention for patients with advanced cancer. J Clin Oncol 2018;36:2422–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chochinov HM, Hack T, Hassard T, et al. Dignity therapy: a novel psychotherapeutic intervention for patients near the end of life. J Clin Oncol 2005;23:5520–5525. [DOI] [PubMed] [Google Scholar]
- 32. Ready RE, Boileau NR, Barton SK, et al. Positive affect and well‐being in Huntington’s disease moderates the association between functional impairment and HRQOL outcomes. J Huntington's Dis 2019;8:221–232. 10.3233/JHD-180341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salsman JM, Victorson D, Choi SW, et al. Development and validation of the positive affect and well‐being scale for the neurology quality of life (Neuro‐QOL) measurement system. Qual Life Res 2013;22:2569–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jackson WT, Novack TA, Dowler RN. Effective serial measurement of cognitive orientation in rehabilitation: the orientation log. Arch Phys Med Rehabil 1998;79:718–721. [DOI] [PubMed] [Google Scholar]
- 35. Hanauer DA, Mei Q, Law J, et al. Supporting information retrieval from electronic health records: a report of University of Michigan’s nine‐year experience in developing and using the Electronic Medical Record Search Engine (EMERSE). J Biomed Inform 2015;55:290–300. 10.1016/j.jbi.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paulsen JS, Langbehn DR, Stout JC, et al. Detection of Huntington’s disease decades before diagnosis: the Predict‐HD study. J Neurol Neurosurg Psychiatry 2008;79:874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kieburtz K, Penney JB, Corno P, et al. Unified Huntington’s disease rating scale: reliability and consistency. Neurology 2001;11:136–142. [Google Scholar]
- 38. Shoulson I, Kurlan R, Rubin AJ, et al. Assessment of functional capacity in neurodegenerative movement disorders: Huntington’s disease as a prototype. Quantif Neurol deficit Bost Butterworths 1989;271–283. [Google Scholar]
- 39. Heaton RK. Revised comprehensive norms for an expanded Halstead‐Reitan Battery: demographically adjusted neuropsychological norms for African American and Caucasian adults, professional manual. Psychol Assess Resour 2004. [Google Scholar]
- 40. Carlozzi NE, Boileau NR, Paulsen JS, et al. End‐of‐life measures in Huntington disease: HDQLIFE meaning and purpose, concern with death and dying, and end of life planning. J Neurol 2019;266:2406–2422. 10.1007/s00415-019-09417-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cella D, Nowinski C, Peterman A, et al. The neurology quality‐of‐life measurement initiative. Arch Phys Med Rehabil 2011;92:S28–S36. 10.1016/j.apmr.2011.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carlozzi NE, Goodnight S, Kratz AL, et al. Validation of Neuro‐QoL and PROMIS mental health patient reported outcome measures in persons with Huntington disease. J Huntingtons Dis 2019;8:467–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carlozzi NE, Boileau NR, Roché MW, et al. Responsiveness to change over time and test‐retest reliability of the PROMIS and Neuro‐QoL mental health measures in persons with Huntington disease (HD). Qual Life Res 2020;29:3419–3439. 10.1007/s11136-020-02596-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pilkonis PA, Choi SW, Reise SP, et al. Item banks for measuring emotional distress from the Patient‐Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment 2011;18:263–283. 10.1177/1073191111411667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cella D, Yount S, Rothrock N, et al. The Patient‐Reported Outcomes Measurement Information System (PROMIS). Med Care 2007;45:S3–S11. 10.1097/01.mlr.0000258615.42478.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cella D, Riley W, Stone A, et al. The patient‐reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self‐reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–1194. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carlozzi NE, Downing NR, Schilling SG, et al. The development of a new computer adaptive test to evaluate chorea in Huntington disease: HDQLIFE Chorea. Qual Life Res 2016;25:2429–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carlozzi NE, Boileau NR, Chou KL, et al. HDQLIFE and neuro‐QoL physical function measures: responsiveness in persons with huntington’s disease. Mov Disord 2020;35:326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carlozzi NE, Ready RE, Frank S, et al. Patient‐reported outcomes in Huntington’s disease: quality of life in neurological disorders (Neuro‐QoL) and Huntington’s disease health‐related quality of life (HDQLIFE) physical function measures. Mov Disord 2017;32:1096–1102. 10.1002/mds.27046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carlozzi NE, Schilling SG, Lai J‐S, et al. HDQLIFE: the development of two new computer adaptive tests for use in Huntington disease, Speech Difficulties, and Swallowing Difficulties. Qual Life Res 2016;25:2417–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carlozzi NE, Hahn EA, Frank SA, et al. A new measure for end of life planning, preparation, and preferences in Huntington disease: HDQLIFE end of life planning. J Neurol 2018;265:98–107. 10.1007/s00415-017-8677-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Carlozzi NE, Boileau NR, Paulsen JS, et al. Psychometric properties and responsiveness of Neuro‐QoL Cognitive Function in persons with Huntington disease (HD). Qual Life Res 2020;29:1393–1403. 10.1007/s11136-019-02391-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lai JS, Goodnight S, Downing NR, et al. Evaluating cognition in individuals with Huntington disease: Neuro‐QoL cognitive functioning measures. Qual Life Res 2018;27:811–822. 10.1007/s11136-017-1755-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gershon RC, Rothrock N, Hanrahan R, et al. The use of PROMIS and assessment center to deliver patient‐reported outcome measures in clinical research. J Appl Meas 2010;14:304–314. 10.1002/mds.27908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 56. Roberts BW, Luo J, Briley DA, et al. A systematic review of personality trait change through intervention. Psychol Bull 2017;143:117–141. 10.1037/bul0000088 [DOI] [PubMed] [Google Scholar]
- 57. Boyle PA, Buchman AS, Barnes LL, Bennett DA. Effect of a purpose in life on risk of incident Alzheimer disease and mild cognitive impairment in community‐dwelling older persons. Arch Gen Psychiatry 2010;67:304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Julayanont P, McFarland NR, Heilman KM. Mild cognitive impairment and dementia in motor manifest Huntington’s disease: classification and prevalence. J Neurol Sci 2020;408:116523. [DOI] [PubMed] [Google Scholar]
- 59. Sudore RL, Lum HD, You JJ, et al. Defining advance care planning for adults: a consensus definition from a multidisciplinary Delphi panel. J Pain Symptom Manage 2017;53:821–832.e1. 10.1016/j.jpainsymman.2016.12.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Downing NR, Goodnight S, Chae S, et al. Factors associated with end‐of‐life planning in Huntington disease. Am J Hosp Palliat Care 2018;28:440–447. 10.1177/1049909117708195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. King LA, Hicks JA, Krull JL, Del Gaiso AK. Positive affect and the experience of meaning in life. J Pers Soc Psychol 2006;90:179–196. [DOI] [PubMed] [Google Scholar]
- 62. Hicks JA, King LA. Meaning in life and seeing the big picture: positive affect and global focus. Cogn Emot 2007;21:1577–1584. [Google Scholar]
- 63. Salsman JM, Schalet BD, Park CL, et al. Assessing meaning & purpose in life: development and validation of an item bank and short forms for the NIH PROMIS®. Qual Life Res 2020;29:2299–2310. 10.1007/s11136-020-02489-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kachian ZR, Cohen‐Zimerman S, Bega D, et al. Suicidal ideation and behavior in Huntington’s disease: systematic review and recommendations. J Affect Disord 2019;250:319–329. 10.1016/j.jad.2019.03.043 [DOI] [PubMed] [Google Scholar]
- 65. Van Orden KA, Witte TK, Cukrowicz KC, et al. The Interpersonal theory of suicide. Psychol Rev 2010;117:575–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Heisel MJ, Flett GL. Does recognition of meaning in life confer resiliency to suicide ideation among community‐residing older adults? A Longitudinal Investigation. Am J Geriatr Psychiatry 2016;24:455–466. 10.1016/j.jagp.2015.08.007 [DOI] [PubMed] [Google Scholar]


