Abstract
Objectives
Preclinical Alzheimer’s disease (AD) clinical trials screen cognitively unimpaired older adults for biomarker criteria and disclose their results. We examined whether participants in the Anti‐Amyloid Treatment in Asymptomatic Alzheimer’s disease Study with “elevated” and “not elevated” amyloid differed in scores on the “Views and Perceptions of Amyloid Imaging” questionnaire. We hypothesized that, prior to disclosure, those with elevated amyloid would score higher than those with not elevated amyloid. We also quantified how responses changed after result disclosure.
Methods
We assessed data from 4327 individuals who completed the questionnaire at screening visit 1 and after amyloid disclosure. We used linear regression models to assess the relationship between questionnaire category scores and amyloid status. We also quantified the relationship between category score changes and amyloid status.
Results
Overall, participants scored altruism and contribution to research as the strongest motivations for undergoing amyloid imaging. Those with elevated amyloid scored 0.23 points higher in the Perceived Risk category, on average, than those who had not elevated amyloid prior to disclosure; this effect attenuated towards zero after adjusting for Cognitive Function Instrument score. After disclosure, participants with elevated amyloid demonstrated less within‐subject change in Perceived Risk, on average, compared to those with similar pre‐disclosure scores who had not elevated amyloid, while demonstrating greater changes in the altruism and planning categories.
Interpretation
Altruism and learning disease risk motivated enrollment in this preclinical AD trial. Participants with elevated amyloid differed from their not elevated counterparts in their perceptions of amyloid imaging, even before undergoing the procedure.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by gradual cognitive and functional decline, eventually resulting in dementia and death. While clinical symptoms of sporadic AD typically begin after age 65, 1 brain changes associated with disease, such as accumulation of amyloid plaques in the brain, may begin as early as 20 years before symptom onset. 2 In an effort to intervene earlier in the disease process, researchers have begun performing clinical trials that enroll patients who show no clinical symptoms of AD but who demonstrate biomarker evidence of the disease such as elevated brain amyloid, which can be observed through the use of neuroimaging. This construct has been named “preclinical AD.” 3
The Anti‐Amyloid Treatment in Asymptomatic Alzheimer’s disease (A4) Study is a fully enrolled ongoing phase 3 preclinical AD clinical trial of the investigational drug solanezumab. 4 Cognitively unimpaired older adults were screened in A4 to see if they demonstrated elevated brain amyloid on positron emission tomography (PET) imaging and were therefore eligible for randomization. The screening phase of A4 incorporated the Views and Perceptions of Amyloid Imaging questionnaire. The questionnaire asks participants to score how strongly they identify or agree with a series of reasons for undergoing amyloid imaging as personal motivating factors. Participants were administered the questionnaire before and after undergoing amyloid PET during screening.
Knowledge of why participants undergo amyloid imaging in preclinical AD trials could help researchers develop targeted recruitment strategies and improve biomarker disclosure processes for future studies. Improved recruitment of underrepresented populations 5 and assurance of sensitive disclosure of biomarker results among differing cultures 6 are particular areas of need. We therefore sought to characterize responses to the Views and Perceptions of Amyloid Imaging scale in the A4 Study and determine if participants with elevated and not elevated amyloid differed from each other. Since A4 participants have shown other differences prior to their amyloid result, 4 we hypothesized that, prior to learning their biomarker status, those with elevated amyloid would identify with presented motivations more strongly than those with not elevated amyloid. In particular, we expected that differences would be apparent in Perceived Risk components, due to known associations with higher brain amyloid levels such as family history of disease and subjective cognitive complaints. 4 , 7 We further hypothesized that this association would manifest, at least in part, via participant responses to the Cognitive Function Instrument (CFI), 8 a subjective cognitive performance scale. Finally, we sought to quantify how responses to the Views and Perceptions of Amyloid Imaging questionnaire changed after participants were told their amyloid result. Since learning amyloid status impacts perception of AD risk and self, 9 we hypothesized that those with elevated amyloid would have greater within‐subject score change than those with not elevated amyloid.
Materials and Methods
Data collection
The A4 Study screened individuals from 67 clinical trial sites in the US, Canada, Australia, and Japan. 4 Screening eligibility criteria for A4 were that participants were between age 65 and 85 years, were assessed to be cognitively unimpaired, were living independently, and had a study partner capable of serving annually to complete informant‐based assessments of participant cognition and function. Six screening visits (see Fig. 1) were used to determine whether participants met inclusion criteria for the A4 Study’s randomization phase. The analyses performed in our study used a subset of the data collected from the A4 screening visits. Criteria for our analyses were that screened participants had an available PET standardized uptake value ratio (SUVr) observation and had completed the Views and Perceptions of Amyloid Imaging questionnaire both at screening visit 1 and after amyloid result disclosure (screening visit 3; see Fig. 1).
Figure 1.
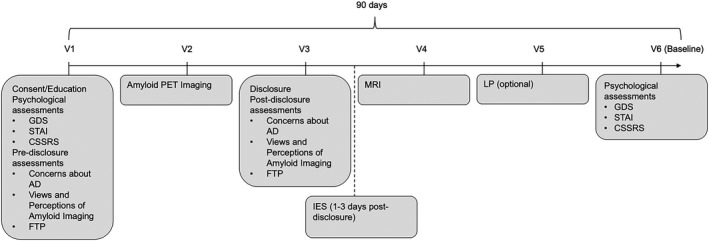
Screening timeline for the A4 Study. Screening required 4 to 5 visits depending on whether the participant completed an optional lumbar puncture (LP). Education, informed consent, and psychological and pre‐disclosure assessments were completed at screening visit 1. Amyloid results were disclosed at visit 3. The Views and Perceptions of Amyloid Imaging questionnaire was completed at screening visits 1 and 3. CSSRS indicates Columbia Suicide Severity Rating Scale; FTP, future time perspective; GDS, Geriatric Depression Scale; IES, Impact of Events Scale; MRI, magnetic resonance imaging; PET, positron emission tomography; STAI, State‐Trait Anxiety Scale; V, visit.
Amyloid levels
In the A4 Study, participants were told they had “elevated” or “not elevated” brain amyloid levels based on their PET result. Participants with PET SUVr greater than 1.15 were categorized as elevated amyloid. Participants with PET SUVr less than 1.10 were categorized as not elevated amyloid. Participants with PET SUVr between 1.10 and 1.15 required an additional visual read to determine amyloid eligibility in A4, with those with at least a two‐reader consensus being included in the elevated amyloid group (i.e., eligible for further screening and randomization). 4
Views and perceptions of amyloid imaging
The primary outcomes of interest in this analysis were item scores in the Views and Perceptions of Amyloid Imaging questionnaire. The questionnaire was adapted from previous work 10 for the A4 Study. Thus, these are among the first data using this instrument, to our knowledge. Participants scored how strongly they identified with each of nine reasons for undergoing amyloid imaging on 5‐point Likert scales, with a score of 1 indicating the participant identifies with the presented motivation “Not at all” and a score of 5 indicating the participant “Extremely” identifies with the presented motivation. The questionnaire also has a tenth open‐response question that we did not include in this analysis.
We a priori grouped the items from the questionnaire into four thematic categories (see Table 1): Perceived Risk (Items 2, 7; category score range: 2–10), Altruism/Contribute to Research (Items 4, 5; category score range: 2–10), Plan/Prepare (Items 1, 6, 8; category score range: 3–15), and Curiosity (Items 3, 9; category score range: 2–10). Individual item scores in each category were summed to create total category scores. For example, the Curiosity category combines a participant’s scores from Items 3 and 9; the lowest score one can give each item is 1, meaning the minimum score the category as a whole can receive is 2; likewise, 5 is the highest score one can give each item, creating a category maximum of 10. Thematic categories grouping more items (i.e., Plan/Prepare) subsequently had larger category minimums and maximums. Changes in scores were calculated by subtracting the score at screening visit 1 from the post‐disclosure score. For summary tables and statistical models, we used the raw category scores; for plotting purposes, we transformed category scores to normalized Z‐scores by subtracting the average category score across visits from a participant’s category score and then dividing by the category standard error.
Table 1.
A priori grouping of Views and Perceptions of Amyloid Imaging questionnaire items into thematic categories.
| Category | Questionnaire Item (Numbering from original scale) |
|---|---|
| Perceived risk (category score range: 2–10) | 2. To put my mind at ease if I found out I do not have elevated amyloid on my PET scan |
| 7. To confirm the feeling that I might already be developing symptoms of Alzheimer’s disease dementia | |
| Altruism/contribute to research (category score range: 2–10) | 4. To be able to participate in anti‐amyloid clinical trials (such as the A4 trial) |
| 5. The desire to contribute to research on Alzheimer’s disease | |
| Plan/prepare (category score range: 3–15) | 1. To seek information on preventative measures (e.g., change diet, exercise, or other lifestyle changes) |
| 6. To arrange my personal affairs | |
| 8. To prepare my family for my possible illness in the future | |
| Curiosity (category score range: 2–10) | 3. To know more about my risk of developing Alzheimer’s disease dementia |
| 9. Curiosity |
Item scores in each category were summed to create total category scores.
Statistical analyses
For analysis purposes, participants who selected their race as Native American/Alaska Native, Native Hawaiian/Pacific Islander, Other, a combination of two or more races, or selected “refused to answer” for either race or ethnicity were categorized as “Other.” Mutually exclusive ethnoracial categories (non‐Hispanic [NH] white, NH Black, NH Asian, Hispanic/Latino, Other) were created by combining race and ethnicity information for the remaining participants.
In the primary analysis, we used linear regression models to assess the relationship between questionnaire category scores recorded at screening visit 1 as the response and amyloid status as the independent variable of interest. Participant age, years of education, sex, ethnoracial group, and family history of AD were adjusted for in all models as potential confounding variables. Age and education were treated as continuous, while NH white was used as an ethnoracial reference group for comparisons. Previous research has shown that participants with spousal study partners make clinical trial enrollment decisions largely in tandem, particularly when biomarker testing is necessary. 11 , 12 , 13 This dynamic may impact participants’ views and perceptions of undergoing amyloid imaging, 14 thus study partner type (dichotomized as spouse or non‐spouse) was also adjusted for in all models as a potential confounding variable. CFI scores were adjusted for as a potential mediator in follow‐up analyses to determine if the relationship between amyloid status and first‐visit category scores was independent of subjective memory concerns. In secondary analyses, we used similar linear regression models to assess the relationship between changes in category scores and amyloid status. Potential confounding variables similar to those in the primary analyses were adjusted for in the secondary models, with the addition of first‐visit category score. We quantified uncertainty in all analyses with 95% confidence intervals. Statistical analyses were conducted using R version 3.6.2. 15
Results
Participants
Table 2 describes the demographics of 4327 individuals from the screening phase of the A4 Study used in this analysis. Just under a third of participants (29.6%; n = 1280) were categorized as elevated amyloid, while more than two‐thirds (70.4%; n = 3047) were categorized as not elevated amyloid. Overall, the sample was mostly female (59.3%; n = 2566) and NH white (88.8%; n = 3807).
Table 2.
Characteristics of analysis participants.
| Elevated amyloid (n = 1280) | Not elevated amyloid (n = 3047) | Total (n = 4327) | |
|---|---|---|---|
| Male | 530 (41.4%) | 1231 (40.4%) | 1761 (40.7%) |
| Family history of AD | 626 (48.9%) | 1228 (40.3%) | 1854 (42.9%) |
| Age | 72.04 (4.87) | 70.93 (4.51) | 71.26 (4.65) |
| Years of education | 16.54 (2.81) | 16.59 (2.85) | 16.57 (2.84) |
| Ethnoracial group | |||
| NH white | 1159 (91.6%) | 2648 (87.6%) | 3807 (88.8%) |
| NH Black | 30 (2.4%) | 117 (3.9%) | 147 (3.4%) |
| NH Asian | 29 (2.3%) | 138 (4.6%) | 167 (3.9%) |
| Hispanic | 37 (2.9%) | 96 (3.2%) | 132 (3.1%) |
| Other | 11 (0.9%) | 24 (0.8%) | 35 (0.8%) |
| Missing | 14 (1.1%) | 24 (0.8%) | 38 (0.9%) |
| APOE ε4 carrier | 738 (58.2%) | 762 (25.3%) | 1500 (35.0%) |
| CFI | 2.24 (2.12) | 1.80 (1.97) | 1.93 (2.02) |
Continuous variables are summarized as means (SD), while discrete variables are summarized as counts (%).
As has been reported elsewhere, 4 participants who had elevated amyloid were observed to be less often NH Black (2.4%) or NH Asian (2.3%) than those with not elevated amyloid (3.9% and 4.6%, respectively). They were also observed to be slightly older and to more often have a family history of AD (48.9% vs. 40.3%).
Perceptions of amyloid imaging prior to disclosure
Table 3 describes the Views and Perceptions of Amyloid Imaging category scores by amyloid group. The Plan/Prepare and Altruism/Contribute to Research categories had the highest average visit 1 scores in both amyloid groups as well as overall, though the Plan/Prepare category had a larger maximum score of 15 (instead of 10). The Altruism/Contribute to Research category achieved the highest average normalized Z‐scores (Fig. 2A).
Table 3.
Views and Perceptions of Amyloid Imaging category scores by amyloid elevation group summarized as mean (SD).
| Elevated amyloid (n = 1280) | Not elevated amyloid (n = 3047) | Total (n = 4327) | |
|---|---|---|---|
| Perceived risk | |||
| Visit 1 | 6.19 (2.10) | 5.97 (2.14) | 6.03 (2.13) |
| Post‐disclosure | 6.24 (2.16) | 6.47 (2.19) | 6.40 (2.18) |
| Altruism/contribute to research | |||
| Visit 1 | 8.52 (1.35) | 8.45 (1.47) | 8.47 (1.44) |
| Post‐disclosure | 8.67 (1.37) | 8.52 (1.47) | 8.56 (1.45) |
| Plan/prepare | |||
| Visit 1 | 9.28 (3.13) | 9.21 (3.27) | 9.23 (3.23) |
| Post‐disclosure | 9.80 (3.10) | 9.59 (3.38) | 9.65 (3.30) |
| Curiosity | |||
| Visit 1 | 7.16 (1.77) | 7.28 (1.80) | 7.25 (1.79) |
| Post‐disclosure | 7.28 (1.80) | 7.39 (1.82) | 7.36 (1.81) |
Figure 2.
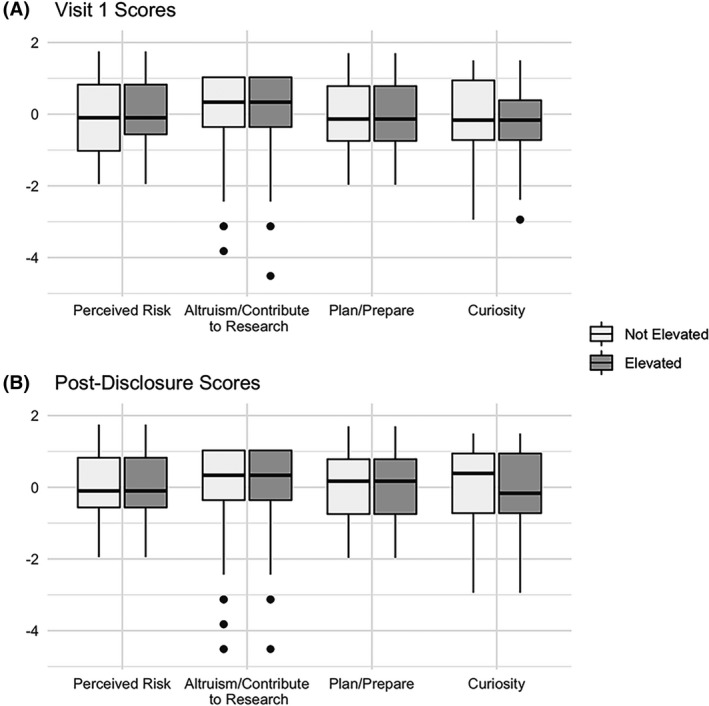
Boxplots of normalized Views and Perceptions of Amyloid Imaging category scores stratified by amyloid elevation status at visits 1 (A) and 3 (B). Scores were transformed into normalized Z‐scores by subtracting the average category score across visits from a participant’s category score, then dividing by the category standard error. Solid dots represent outliers.
In regression models, participants who had elevated amyloid scored 0.23 points higher in the Perceived Risk category, on average, than those with similar demographic characteristics who had not elevated amyloid (95% CI: [0.09, 0.37]) prior to imaging and disclosure (Fig. 3A). No differences between the groups were observed in the Altruism/Contribute to Research category, the Plan/Prepare category, or the Curiosity category (Fig. 3B–D).
Figure 3.
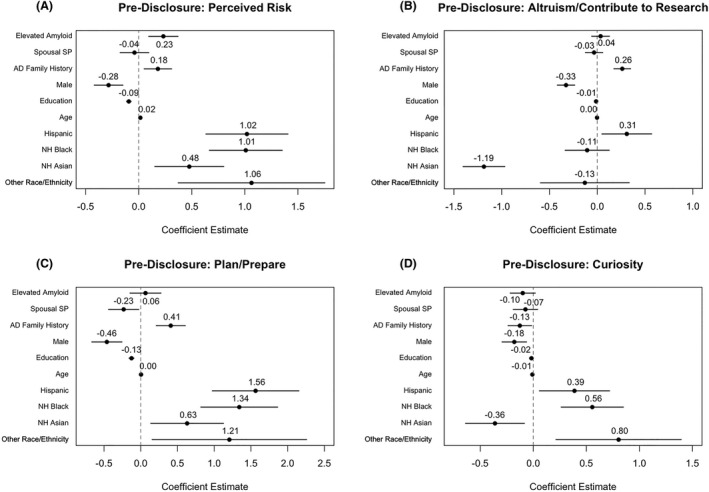
Estimated associations for pre‐disclosure regression model assessing the relationship of amyloid status with perceived risk (A), altruism/controbute to research (B), plan/prepare (C), and curiosity (D) category scores. Points represent estimated score difference and solid lines represent estimated 95% confidence interval.
NH Black and Hispanic participants scored higher compared to NH whites in the Perceived Risk (NH Black: 1.01 points, 95% CI: [0.67, 1.35]; Hispanic: 1.02 points, 95% CI: [0.63, 1.41]; Fig. 3A), Plan/Prepare (NH Black: 1.34 points, 95% CI: [0.82, 1.86]; Hispanic: 1.56 points, 95% CI: [0.97, 2.15]; Fig. 3C), and Curiosity (NH Black: 0.55 points, 95% CI: [0.26, 0.85]; Hispanic: 0.39, 95% CI: [0.06, 0.72]; Fig. 3D) categories. NH Asians scored 0.48 points higher in the Perceived Risk category (95% CI: [0.15, 0.80]; Fig. 3A) and 0.63 points higher in the Plan/Prepare category (95% CI: [0.14, 1.13]; Fig. 3C), while scoring 1.19 points lower in the Altruism/Contribute to Research category (95% CI: [−1.40, −0.97]; Fig. 3B) and 0.36 points lower in the Curiosity category (95% CI: [−0.64, −0.08]; Fig 3D) compared to NH whites. Those with a family history of AD scored between 0.18 and 0.41 higher in every category except Curiosity where they scored 0.13 points lower, compared to those with no family history (Fig. 3A–C).
When CFI scores were added as a potential mediator, the significant effect for both amyloid status and the NH Asian ethnoracial group attenuated towards zero in Perceived Risk category (Elevated Amyloid: 0.12, 95% CI: [−0.02, 0.25]; NH Asian: 0.17, 95% CI: [−0.14, 0.49]). The significant effect for NH Asian also attenuated toward zero in the Plan/Prepare category (0.41; 95% CI: [−0.09, 0.90]). None of the other observed effects shifted significantly with the adjustment of CFI scores, though the effect for amyloid status in the Curiosity category did become statistically significant (−0.13; 95% CI: [−0.24, −0.01]; see Table S1).
Attitudes after disclosure
Scores generally increased from visit 1 to the post‐disclosure visit 3 across categories and biomarker groups (Table 3). The highest average post‐disclosure scores were in the Plan/Prepare and Altruism/Contribute to Research categories. As seen in Figure 2B, Altruism/Contribute to Research remained the category with the highest average normalized Z‐scores. We also observed that that there was a noticeable disparity in normalized Curiosity scores between the biomarker groups, whereby not elevated participants were observed to rate Curiosity higher than elevated participants.
After disclosure, participants with elevated amyloid demonstrated a lower within‐subject change in score in the Perceived Risk category, on average, compared to those with similar visit 1 scores who had not elevated amyloid (−0.35; 95% CI: [−0.46, −0.24]; Fig. 4A). Participants with elevated amyloid demonstrated greater within‐subject changes in the Altruism/Contribute to Research (0.11; 95% CI: [0.02, 0.19]; Fig. 4B) and Plan/Prepare (0.21; 95% CI: [0.03, 0.38]; Fig. 4C) categories, compared to those with similar visit 1 scores who had not elevated amyloid. There was no difference between the amyloid groups in the change in the Curiosity category.
Figure 4.
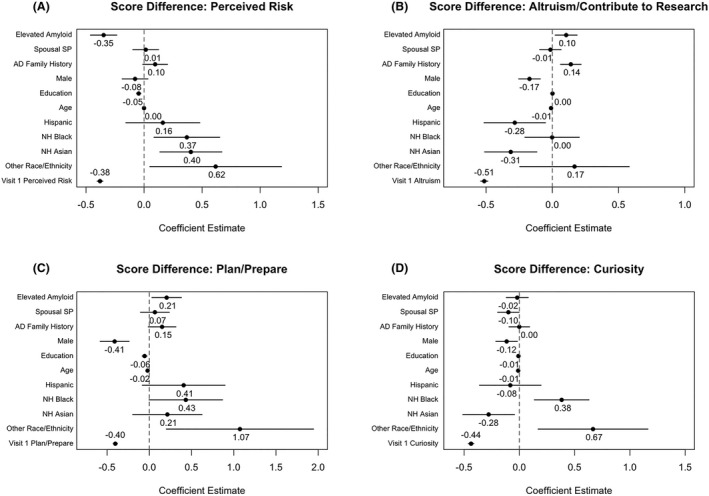
Estimated associations for regression models assessing the relationship of amyloid status with change in perceived risk (A), altruism/controbute to research (B), plan/prepare (C), and curiosity (D) category visit scores. Points represent estimated score change and solid lines represent estimated 95% confidence interval.
Discussion
These are among the first findings to examine participants’ motivations for undergoing amyloid imaging in a preclinical AD trial and how motivations change after biomarker disclosure. Overall, participants in this study rated the Altruism/Contribute to Research category as the most important reasons for undergoing amyloid imaging. Participants with elevated amyloid more strongly rated the Perceived Risk category prior to disclosure compared to their counterparts with not elevated amyloid. Average category scores increased after disclosure of individual amyloid imaging results across all categories and groups, signaling that, after biomarker disclosure, participants as a whole identified more strongly with all categories than they did prior to disclosure. Disclosure of amyloid status produced unique changes in the scale between the amyloid groups. We observed that Perceived Risk increased marginally in both elevated and not elevated amyloid groups but that this change differed between groups; those with not elevated amyloid showed larger within‐subject increases in Perceived Risk than did those with elevated amyloid.
These results may inform future preclinical AD trial recruitment practices. Appealing to altruism may have the broadest utility. Recruitment messages focusing on concerns about perceived risk for AD, such as clinical referrals of “worried well” and campaigns inviting those with memory concerns to screen, however, may more specifically target biomarker eligible participants 16 since participants in the A4 Study with elevated amyloid more strongly identified confirming or assuaging concerns of AD risk as reasons for undergoing amyloid imaging. In particular, this difference was likely due to the perceived cognitive problems of those with elevated amyloid (despite performing normally on objective tests 7 , 8 ), as the effect disappeared after adjusting for the CFI, a subjective cognitive function scale, as we hypothesized.
It is well known that diverse racial and ethnic groups are vastly underrepresented in AD trials. 17 As the analysis models considered in this study were not designed to properly analyze the relationship between ethnoracial groups and amyloid imaging views, these results should be used for the purposes of hypothesis generation and should be confirmed with future studies. Nonetheless, NH Black and Hispanic participants rated the Perceived Risk category, but also the Plan/Prepare category, as more important than NH whites prior to amyloid disclosure, while NH Asians rated the Altruism/Contribute to Research category lower than NH whites. Thus, in addition to well‐described efforts to engage communities and establish trust, 5 , 18 these results may indicate that strategic, culturally specific efforts may be needed to improve diversity within preclinical AD trials. These efforts may need to be combined with larger scale educational programs, given especially that diverse groups have been shown previously to perceive their risk for AD as lower than that for NH Whites. 19 , 20 , 21 , 22
Multiple studies support that disclosing amyloid imaging biomarkers to cognitively unimpaired individuals does not increase anxiety, depression, or suicidal thoughts. 6 , 23 Nevertheless, the current results support the notion that the delivery of amyloid results does differentially impact participants, depending on their specific result. The results may also be important to consider as future similar trials disclose biomarker results to participants.
After disclosure, participants with elevated amyloid increased their endorsement of Altruism/Contribution and Planning/Preparation more than their not elevated counterparts. This may be a positive indication that participants intend to use the results to prepare for a possible future that includes cognitive decline and dementia. 24 The A4 disclosure process included in‐person informed consent and education sessions, where an investigator discussed AD symptoms and the fact that elevated amyloid increases risk but does not guarantee future dementia. 25 The educational materials did not cover risk reduction measures or steps one might take to prepare for the potential onset of dementia later in life such as financial planning, discussing future living arrangements, or purchasing long term care (LTC) insurance. Participants with elevated amyloid reported contemplating these issues in the Study of Knowledge and Reactions to Amyloid Testing (SOKRATES), an ancillary interview study with A4 participants. 9 Including planning and preparation information in the disclosure session or providing informational material for future reference might better support feelings of needing to plan and prepare, which may intensify after disclosure for participants with elevated amyloid. Although participants with not elevated amyloid did not increase their endorsement of Planning/Preparation as much as those with elevated amyloid, they did demonstrate an absolute increase in endorsement of these items. It is possible that this could have been in response to planning for a future that was less likely to include dementia, since it has been shown that these participants also demonstrated an increase in Future Time Perspective (FTP) scale score. 6
Participants with elevated amyloid demonstrated less change in their endorsement of the Perceived Risk category, compared to those with not elevated amyloid, suggesting that they were unsurprised by the result. This may have been driven by differential responses for the two items in this category. Specifically, both amyloid groups demonstrated increased scores for Item 7 ("To confirm the feeling that I might already be developing symptoms of Alzheimer’s disease dementia”), whereas only those with not elevated amyloid demonstrated increased scores in Item 2 ("To put my mind at ease if I found out I do not have elevated amyloid on my PET scan”), while those with elevated amyloid saw an average decrease in score for this item. Previously published results agree with this finding: participants with elevated amyloid similarly demonstrated no change on the FTP scale after biomarker disclosure. 6 In the SOKRATES study, participants learning an elevated amyloid result felt that the result validated their memory concerns, compared to participants with not elevated amyloid who reinterpreted memory concerns as normal aging. 9 These observations serve to endorse the continued need for consistent and sensitive delivery of biomarker results in future preclinical AD trials, especially to participants with elevated amyloid. 25
We note some limitations to this work. While the prescreening sample from the A4 Study was large, sample sizes for diverse racial and ethnic groups were smaller, limiting precision. Paired with the high education levels observed across the sample, it is likely that the A4 Study participants are a unique cohort, potentially impacting the generalizability of our results to more diverse populations. In addition, there are likely many factors that affect a person’s reasoning to undergo amyloid imaging that are not captured by the questionnaire used in this study. We attempted to mitigate this by controlling for plausible confounding factors found in the available data, but it remains possible that some factors may not have been observed or adjusted for. There may have also been additional motivations not covered in the main nine items but addressed in the tenth free‐response item of the questionnaire that was not included in this analysis. Future studies should investigate the themes presented in the free‐response item. We a priori assigned questionnaire items to categories. We assigned Item 4 ("To be able to participate in anti‐amyloid clinical trials [such as the A4 trial]”) to an altruism category since there was no guarantee that one would receive active treatment nor that one would experience benefits if they did receive active treatment in the A4 Study. This, combined with our previous work 26 support the role of altruism and a desire to contribute to research as a main motivation for participation in prevention trials like the A4 Study. We acknowledge that the desire to benefit personally also motivates participation 26 and that this and other alternate assignments of items could have altered results.
Conclusions
Participants in the A4 Study considered altruism and contribution to research the strongest motivations for undergoing amyloid imaging. Prior to disclosure, elevated amyloid participants more strongly endorsed a need to confirm or assuage concerns of AD risk compared to their not elevated counterparts, likely due to their concerns about cognitive impairment. Disclosure of amyloid status produced unique changes on the scale between the amyloid groups, which may be notable for investigators performing future trial disclosures.
Conflict of Interest
Authors M. M. Ryan and D. L. Gillen declare no conflict of interest. J. D. Grill has received consultancy fees from SiteRx, outside the submitted work. No funding was received for the current study. D. L. Gillen and J. D. Grill are supported by NIA 1RF1 AG059407and NIA P30 AG066519. J. D. Grill is also supported by NCATS UL1 TR001414.
Supporting information
Table S1. Estimated associations for pre‐disclosure regression model assessing the relationship of amyloid status with Perceptions of Amyloid Imaging category score, with CFI scores included as a mediator. Model results are reported as coefficient point estimates (95% confidence interval).
Acknowledgements
The A4 Study is a secondary prevention trial in preclinical Alzheimer's disease, aiming to slow cognitive decline associated with brain amyloid accumulation in clinically normal older individuals. The A4 Study is funded by a public‐private‐philanthropic partnership, including funding from the National Institutes of Health‐National Institute on Aging, Eli Lilly and Company, Alzheimer's Association, Accelerating Medicines Partnership, GHR Foundation, an anonymous foundation and additional private donors, with in‐kind support from Avid and Cogstate. The companion observational Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN) Study is funded by the Alzheimer's Association and GHR Foundation. The A4 and LEARN Studies are led by Dr Reisa Sperling at Brigham and Women's Hospital, Harvard Medical School and Dr Paul Aisen at the Alzheimer's Therapeutic Research Institute (ATRI), University of Southern California. The A4 and LEARN Studies are coordinated by ATRI at the University of Southern California, and the data are made available through the Laboratory for Neuro Imaging at the University of Southern California. The participants screening for the A4 Study provided permission to share their de‐identified data in order to advance the quest to find a successful treatment for Alzheimer's disease. We would like to acknowledge the dedication of all the participants, the site personnel, and all of the partnership team members who continue to make the A4 and LEARN Studies possible. The complete A4 Study Team list is available on: a4study.org/a4‐study‐team.
Funding Information
The A4 Study is funded by a public‐private‐philanthropic partnership, including funding from the National Institutes of Health‐National Institute on Aging, Eli Lilly and Company, Alzheimer's Association, Accelerating Medicines Partnership, GHR Foundation, an anonymous foundation and additional private donors, with in‐kind support from Avid and Cogstate. The companion observational Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN) Study is funded by the Alzheimer's Association and GHR Foundation. The A4 and LEARN Studies are led by Dr Reisa Sperling at Brigham and Women's Hospital, Harvard Medical School and Dr Paul Aisen at the Alzheimer's Therapeutic Research Institute (ATRI), University of Southern California. The A4 and LEARN Studies are coordinated by ATRI at the University of Southern California, and the data are made available through the Laboratory for Neuro Imaging at the University of Southern California.
Funding Statement
This work was funded by National Center for Advancing Translational Sciences grant UL1 TR001414; National Institute on Aging grants 1RF1 AG059407 and P30 AG066519; National Institutes of Health ; Eli Lilly and Company ; Alzheimer's Association ; Accelerating Medicines Partnership; GHR Foundation; Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN) Study; Alzheimer's Association and GHR Foundation; Brigham and Women's Hospital ; Harvard Medical School ; Alzheimer's Therapeutic Research Institute (ATRI); University of Southern California .
References
- 1. Alzheimer’s Association . 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement 2020;16:391–460. [cited 2020 Jul 20] Available from: https://alz‐journals.onlinelibrary.wiley.com/doi/full/10.1002/alz.12068 [Google Scholar]
- 2. Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimer’s Dement 2016;12:292–323. [cited 2020 Oct 1] Available from: https://alz‐journals.onlinelibrary.wiley.com/doi/abs/10.1016/j.jalz.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science 1992;256:184–185. [cited 2019 Feb 19] Available from: http://science.sciencemag.org/content/256/5054/184 [DOI] [PubMed] [Google Scholar]
- 4. Sperling RA, Donohue MC, Raman R, et al. Association of factors with elevated amyloid burden in clinically normal older individuals. JAMA Neurol 2020;77:735. [cited 2020 Apr 6] Available from: https://jamanetwork.com/journals/jamaneurology/fullarticle/2763540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gilmore‐Bykovskyi AL, Jin Y, Gleason C, et al. Recruitment and retention of underrepresented populations in Alzheimer’s disease research: a systematic review. Alzheimer’s Dement 2019;5:751–770. [cited 2020 Dec 1] Available from: http://www.sciencedirect.com/science/article/pii/S2352873719300794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grill JD, Raman R, Ernstrom K, et al. Short‐term psychological outcomes of disclosing amyloid imaging results to research participants who do not have cognitive impairment. JAMA Neurol 2020;77:1504. [cited 2020 Oct 29] Available from: 10.1001/jamaneurol.2020.2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amariglio RE, Buckley RF, Mormino EC, et al. Amyloid‐associated increases in longitudinal report of subjective cognitive complaints. Alzheimer’s Dement 2018;4:444–449. [cited 2020 Oct 29] Available from: https://alz‐journals.onlinelibrary.wiley.com/doi/abs/10.1016/j.trci.2018.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amariglio RE, Donohue MC, Marshall GA, et al. Tracking early decline in cognitive function in older individuals at risk for Alzheimer disease dementia: the Alzheimer’s disease cooperative study cognitive function instrument [Internet]. JAMA Neurol 2015;72:446–454. [cited 2018 Oct 1] Available from: https://jamanetwork.com/journals/jamaneurology/fullarticle/2110225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Largent EA, Harkins K, van Dyck CH , et al. Cognitively unimpaired adults’ reactions to disclosure of amyloid PET scan results. PLOS One 2020;15:e0229137. [cited 2020 Oct 20] Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0229137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roberts JS, Connell CM. Illness representations among first‐degree relatives of people with Alzheimer disease. Alzheimer Dis Assoc Disord 2000;14:129–136. [cited 2021 May 13] Available from: https://journals.lww.com/alzheimerjournal/Fulltext/2000/07000/Illness_Representations_Among_First_Degree.3.aspx?casa_token=ficzZcu5OD4AAAAA:QJYxpuUkcno0aXtddMHZILPu4X8_QpWjxWvQyl1PF1TcofbUdtJv8N6‐5Gu0‐RrQrDXZcbr5cZGlYR0eHD9ILA [DOI] [PubMed] [Google Scholar]
- 11. Cox CG, Ryan MM, Gillen DL, Grill JD. A preliminary study of clinical trial enrollment decisions among people with mild cognitive impairment and their study partners. Am J Geriatr Psychiatry 2019;27:322–332. [cited 2019 Oct 15] Available from: http://www.sciencedirect.com/science/article/pii/S1064748118305372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karlawish JHT, Casarett D, Klocinski J, Sankar P. How do AD patients and their caregivers decide whether to enroll in a clinical trial? Neurology 2001;56:789–792. [cited 2021 May 12] Available from: https://n.neurology.org/content/56/6/789 [DOI] [PubMed] [Google Scholar]
- 13. Black BS, Wechsler M, Fogarty L. Decision making for participation in dementia research. Am J Geriatr Psychiatry 2013;21:355–363. [cited 2021 May 12] Available from: https://www.sciencedirect.com/science/article/pii/S1064748112000711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cox CG, Ryan MM, Gillen DL, Grill JD. Is reluctance to share Alzheimer’s disease biomarker status with a study partner a barrier to preclinical trial recruitment? J Prev Alzheimers Dis 2021;8:52–58. [cited 2021 May 13] Available from: 10.14283/jpad.2020.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. R Core Team . R: a language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2017. Available from: https://www.R‐project.org/ [Google Scholar]
- 16. Andersen F, Engstad TA, Straume B, et al. Recruitment methods in Alzheimer’s disease research: general practice versus population based screening by mail. BMC Med Res Methodol 2010;10:35. [cited 2020 Nov 4] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2880123/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Watson JL, Ryan L, Silverberg N, et al. Obstacles and opportunities in Alzheimer’s clinical trial recruitment. Health Affairs 2014;33:574–579. [cited 2020 Mar 31] Available from: https://www.healthaffairs.org/doi/full/10.1377/hlthaff.2013.1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brewster P, Barnes L, Haan M, et al. Progress and future challenges in aging and diversity research in the United States. Alzheimer’s Dement 2019;15:995–1003. [cited 2020 Dec 1] Available from: http://www.sciencedirect.com/science/article/pii/S1552526018334940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Connell CM, Scott Roberts J, McLaughlin SJ. Public opinion about Alzheimer disease among blacks, hispanics, and whites: results from a national survey. Alzheimer Dis Assoc Disord 2007;21:232–240. [cited 2021 Jan 15] Available from: https://journals.lww.com/alzheimerjournal/Fulltext/2007/07000/Public_Opinion_About_Alzheimer_Disease_Among.7.aspx [DOI] [PubMed] [Google Scholar]
- 20. Roberts JS, Connell CM, Cisewski D, et al. Differences between African Americans and whites in their perceptions of Alzheimer disease. Alzheimer Dis Assoc Disord 2003;17:19–26. [cited 2021 Jan 15] Available from: https://journals.lww.com/alzheimerjournal/Fulltext/2003/01000/Differences_Between_African_Americans_and_Whites.3.aspx [DOI] [PubMed] [Google Scholar]
- 21. Akinleye I, Roberts JS, Royal CDM, et al. Differences between African American and White research volunteers in their attitudes, beliefs and knowledge regarding genetic testing for Alzheimer’s disease. J Genet Couns 2011;20:650–659. [cited 2021 Jan 15] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3223287/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ayalon L, Areán PA. Knowledge of Alzheimer’s disease in four ethnic groups of older adults. Int J Geriatr Psychiatry 2004;19:51–57. [cited 2021 Jan 15] Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/gps.1037 [DOI] [PubMed] [Google Scholar]
- 23. Burns JM, Johnson DK, Liebmann EP, et al. Safety of disclosing amyloid status in cognitively normal older adults. Alzheimer’s Dement 2017;13:1024–1030. [cited 2020 Oct 27] Available from: http://www.sciencedirect.com/science/article/pii/S1552526017300481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sheffrin M, Stijacic Cenzer I, Steinman MA. Desire for predictive testing for Alzheimer’s disease and impact on advance care planning: a cross‐sectional study. Alzheimer’s Res Ther 2016;8:55. [cited 2021 Jan 11] Available from: 10.1186/s13195-016-0223-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harkins K, Sankar P, Sperling R, et al. Development of a process to disclose amyloid imaging results to cognitively normal older adult research participants. Alzheimer’s Res Ther 2015;7:26. [cited 2020 Oct 20] Available from: 10.1186/s13195-015-0112-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grill JD, Karlawish J, Elashoff D, Vickrey BG. Risk disclosure and preclinical Alzheimer’s disease clinical trial enrollment. Alzheimer’s Dement 2013;9:356–359.e1. [cited 2019 Jun 3] Available from: http://www.sciencedirect.com/science/article/pii/S1552526012001306 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Estimated associations for pre‐disclosure regression model assessing the relationship of amyloid status with Perceptions of Amyloid Imaging category score, with CFI scores included as a mediator. Model results are reported as coefficient point estimates (95% confidence interval).


