Abstract
Objective
The delivery of healthcare at home has expanded to intravenous infusions of monoclonal antibodies. A recently developed model of care for home infusions of natalizumab for people with relapsing‐remitting multiple sclerosis was evaluated. This pilot study of home infusions of natalizumab and usual care (attendance in a hospital out‐patients’ clinic) compared safety, feasibility, patient satisfaction, effectiveness and costs.
Methods
In this randomised AB/BA crossover trial, 37 adults were randomised to usual care (n = 19) or home infusions (n = 18). After three infusions, patients crossed over to the alternate treatment for another three infusions. Patient safety outcomes and adherence, satisfaction, quality of life, disability and costs were compared.
Results
No adverse events were recorded from 207 infusions from 35 patients across both home and clinic infusions. There was no difference in adherence (p = 0.71) and infection rates (p = 0.84) between home and clinic settings. Satisfaction with “convenience” of home infusions was significantly greater (p = 0.008) but there were no differences in quality of life measures. Excluding pharmacy, costs were A$74 lower per infusion at home, including A$16 of patients” out‐of‐pocket costs.
Interpretation
There were no differences in safety and effectiveness between clinic and home infusions of natalizumab. The home infusions were shown to be feasible, more convenient and less expensive than usual care. Larger scale studies are required to verify these preliminary findings, particularly around safety and management of hypersensitivity adverse events in the home setting and for equivalence of clinical outcomes.
Introduction
Natalizumab (Tysabri™, Biogen Inc, Cambridge, Massachusetts) is routinely delivered as a 1‐h intravenous infusion under physician supervision in hospital clinics, day units and free‐standing infusion centres for people with relapsing‐remitting multiple sclerosis (MS). 1 Natalizumab therapy is generally well tolerated by patients although known adverse drug events include hypersensitivity (e.g. anaphylaxis, urticaria), infections (e.g. urinary tract infections) and milder non‐specific infusion‐related reactions such as fatigue, headache and rash. 2 , 3 Acute hypersensitivity reactions occur in 3%–4% of patients, mostly during the first or second infusion. 4
In the longer term, the main adverse event of concern is opportunistic infection by the John Cunningham Virus (JCV), increasing the risk of progressive multifocal leukoencephalopathy (PML). 5 The presence of anti‐JCV antibodies, natalizumab treatment for more than 2 years and prior immunosuppressant use is associated with an increased risk of PML. 6 Routine monitoring of the anti‐JCV antibody index and magnetic resonance imaging screening may be used to inform a risk‐stratified approach to natalizumab treatment and PML, especially for immunosuppressant‐naïve patients. 7 Aside from PML, the longer term safety of natalizumab is consistent with shorter use safety profiles. 8 , 9
There is a worldwide trend to delivering healthcare in the community rather than hospital, which is partly driven by increased demand for hospital services and benefits to patients. 10 , 11 Such benefits include reduced incidence of nosocomial infection; greater convenience, comfort, choice and flexibility and patient‐centred care; and reduced stress and out‐of‐pocket expenses. 12 , 13 , 14 , 15 Infusion therapy at home began in the 1970s with parenteral nutrition and intravenous immunoglobulin, and has more recently expanded into other diseases and treatments, including cancer and cellulitis. 16 , 17 , 18 A systematic review of home infusion therapy concluded that home infusions were safe and clinically effective, resulted in significantly lower costs and were overwhelmingly preferred by patients because of increased physical and mental wellbeing and decreased disruption of family and personal responsibilities. 19 Although uptake of home infusions of monoclonal antibodies has lagged other forms of treatment such as antibiotic therapy, 19 home infusions of infliximab have been demonstrated to be safe and cost‐effective for children and adults with Crohn’s disease 20 , 21 and, more recently, natalizumab has been delivered by infusion at home for people with MS. 22
A new model of care 23 of home infusions of natalizumab must be demonstrably safe, feasible to deliver, acceptable for patients and healthcare staff, effective in treating symptoms and cost‐effective. As a companion piece to qualitative studies investigating acceptability 24 (Juaton M, Cusack L, Schultz T. Healthcare workers’ experiences of transitioning natalizumab infusions from hospital services to an in‐home setting: A qualitative study. under review), this study is a pilot study of home infusions of natalizumab and usual care (attendance in a hospital out‐patients’ clinic) to compare safety, feasibility, patient satisfaction, effectiveness and costs.
Methods
Trial design
The study design was an AB/BA randomised crossover trial, helping to maximise power from a pilot study. 25 After recruitment, participants were randomised to an AB (i.e. commencing in the clinic) or BA (commencing at home) trial group; after three infusions in treatment period 1 patients crossed over to the alternate model of care for another three infusions in treatment period 2. The washout period between treatment periods was 4 weeks, which is the standard period between infusions required to maintain therapeutic levels of natalizumab. 6
Participant recruitment and randomisation
Commencing in November 2016, patients were recruited from the Royal Adelaide Hospital Ambulatory Care Day Unit during their usual infusions. Recruitment continued until January 2017 using the following inclusion criteria:
ability to understand the purpose and risks of the study and provide signed and dated informed consent and authorisation to use protected health information,
adult MS patients (≥18 years) being prescribed natalizumab,
a minimum of six prior natalizumab infusions in an infusion service unit and assessed as safe for the home infusion program by the prescribing Neurologist and
JCV negative.
Patients not meeting each inclusion criteria, or unable or unwilling to provide informed consent, or living outside of metropolitan Adelaide were excluded.
A 1:1 allocation block randomisation schedule (blocks of 4) was developed using random numbers calculated from MS‐Excel by author TS. Participants were randomised by the recruiter (author MJ) contacting author TS immediately after patients consented. We attempted to recruit all eligible patients but a power analysis was not conducted as the main aim of the study was to test safety, acceptability and feasibility.
Intervention
Prior to the trial commencing, we developed a model of care 23 for delivery of natalizumab infusions in the home by nurses. Prioritising the delivery of patient‐centred care safely at home, the model of care was responsive to patient needs and prioritised the nurse–patient therapeutic relationship, while incorporating relevant National Standards and factors such as handing over patients between settings and protection of cold chain using medical couriers. 26 As per usual care protocols in the clinic, before every infusion patients in both the clinic and home infusion settings completed a pre‐infusion questionnaire to assess for new symptoms which may suggest PML. In the presence of new PML symptoms, no infusions occur until the patient has been medically reviewed and cleared of concern for PML. This screening is coupled with bi‐annual JCV antibody testing and annual magnetic resonance imaging.
Study outcomes
In addition to demographics and treatment adherence (the proportion of treatments within 3 days of the recommended frequency (either 4‐weekly, or 6‐weekly for one patient who had received extended interval dosing for 12 months prior to the study) from the total number of eligible treatments), four types of patient outcomes were measured.
Patient safety outcomes
Patient safety outcomes included adverse events (AEs) (number, severity, treatment patterns and patient outcomes) and infections (number, type, including whether hospital acquired and treatment) collected by unblinded nurses administering natalizumab after each cycle of treatment. Anaphylaxis, common AEs leading to discontinuation if not previously experienced and general AEs were recorded.
Patient satisfaction
Patient satisfaction with their previous three infusions was measured at the end of both treatment periods 1 and 2. Unblinded nurses administered the “Treatment Satisfaction Questionnaire for Medication (TSQM)”, 27 which has been recently used to measure treatment satisfaction for people with MS. 28 The TSQM consists of 14 items scaled on a 5‐ to 7‐point bipolar scale. Items are combined into four summary scores: effectiveness, side effects, convenience and overall satisfaction; higher scores imply higher levels of satisfaction.
Quality of life
The 81‐item MS Quality of Life Inventory (MSQLI) was administered at the start of treatment period 1 and end of treatment period 2 by unblinded nurses. It consists of 10 individual scales providing a quality of life measure that is both generic and MS‐specific. 29 The MSQLI includes the SF‐36, one of the most widely used generic health status measures, which includes two summary scales (Physical Components Scale and Mental Components Scale) presented in t‐score format, in which a score of 50 is equivalent to the mean for the US population, a score of 60 is one standard deviation (SD) above the mean and a score of 40 is one SD below the mean. Similarly, scores of 70 and 30 are two SDs above and below, respectively, the mean. Other SF‐36 subscales are derived from 2 to 10 questionnaire items and are scored out of a total of 100. In all subscales, a higher score is indicative of a better outcome for patients.
The nine other scales of the MSQLI are as follows: MFIS‐5, Modified Fatigue Impact Scale; PES, MOS Pain Effects Scale; SSS, Sexual Satisfaction Scale; BLCS, Bladder Control Scale; BWCS, Bowel Control Scale; VIS, Impact of Visual Impairment Scale; PDQ, Perceived Deficits Questionnaire; MHI, Mental Health Inventory; MSSS, MOS Modified Social Support Survey. Short‐form versions of scales were used when available (e.g. use of the 5‐item Modified Fatigue Impact Scale).
Expanded disability status scale
The expanded disability status scale is used to quantify disability in people with multiple sclerosis and monitor changes in the level of disability over time. 30 , 31 As per the MSQLI, it was administered at the start of treatment period 1 and end of treatment period 2 by unblinded author KW, who had undergone training, and measured EDSS under the supervision of author JR.
Costs
The costs to deliver infusions (including direct costs such as nursing and consumables, and indirect costs such as overheads and facility management) were derived from the clinic’s Casemix Clinical Costing System and the home nursing provider’s own costings, which also included additional costs such as medical couriers and professional indemnity insurance. Additional financial (including travel, parking) and human resources (time, being accompanied to infusions, use of carers), and healthcare utilisation by patients (visiting a general practitioner or the emergency department, being admitted to hospital or seeing a carer) between infusions were recorded by nurses surveying patients after each infusion.
Analyses
Descriptive statistics for continuous and categorical demographic variables were calculated, and chi‐square and t‐tests (for normally distributed data) were used for simple inferential tests. For healthcare utilisation, each participant was categorised on a 2 × 2 matrix of utilisation at the clinic and home during their three infusions; differences between matched pairs were tested using McNemar’s test. 32 For multivariate analysis, assumptions of a linear regression model were tested by assessing scatter plots and histograms for normality of residuals and random scatter of variance. Linear mixed‐effects models were performed for SF‐36 and MSQLI variables versus the interaction of group (AB and BA) and treatment periods(1 and 2), controlling for repeated measurements over time. When assumptions of a linear model were not met (e.g. TSQM, some MSQLI subscales), ordinal logistic generalised estimating equation (GEE) models were performed versus the interaction of intervention group (AB and BA) and treatment periods (1 and 2), controlling for repeated measurements over time.
The analyses were conducted using SAS by a statistician blinded to the allocation of treatment.
Ethical considerations
The study was approved by the Royal Adelaide Hospital Human Research Ethics Committee (HREC/16/RAH/192). Prior to performing any study‐related activities, written informed consent was obtained from the participant. Prior to obtaining informed consent, all potential participants were provided with an information sheet relevant to the subject’s participation and presented in an easy to understand form. The study protocol was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR CT‐2016‐CTN‐02067‐1).
Results
Patient characteristics and adherence
Of the 37 randomised participants, 35 received the allocated intervention (Fig. 1). There were no demographic differences between the groups at treatment period 1 (Table 1). Most participants were female, were employed or conducting home duties, and had not experienced a relapse since being on natalizumab over a mean of 4.7 ± 2.4 (SD) years. The mean age of participants was 39.6 ± 13.7 years and age at diagnosis was 31.1 ± 10.0 years. Participants had attended the clinic for most (4.5 years) of the 4.7 years of their natalizumab use (Table 1). All were diagnosed as relapsing‐remitting MS.
Figure 1.
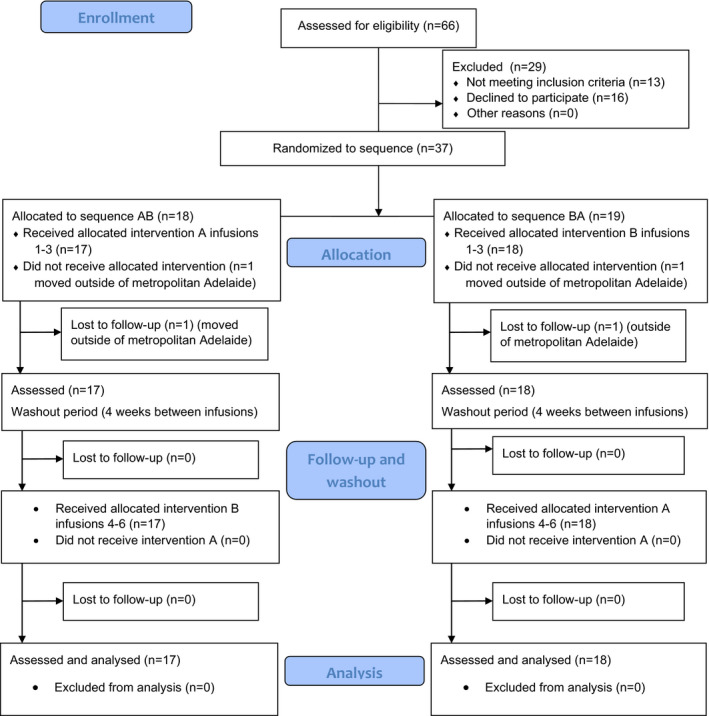
CONSORT (2010) Flow diagram of participants in the crossover trial (A‐Infusion clinic, B‐Home infusions; CONSORT=Consolidated Standards of Reporting Trials).
Table 1.
Summary of demographics for 35 participants at treatment period 1.
| Group AB | Group BA | Totals | p value* | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Gender | |||||||
| Male | 3 | 16.7 | 5 | 29.4 | 8 | 22.9 | 0.4430 |
| Female | 15 | 83.3 | 12 | 70.6 | 27 | 77.1 | |
| Language spoken at home | |||||||
| English | 17 | 100.0 | 16 | 94.1 | 33 | 97.1 | 1.000 |
| Non‐English | 0 | 0.0 | 1 | 5.9 | 1 | 2.9 | |
| Marital status | |||||||
| Single | 7 | 38.9 | 5 | 29.4 | 12 | 34.3 | 0.8055 |
| Married/de Facto | 9 | 50.0 | 9 | 52.9 | 18 | 51.4 | |
| Divorced | 1 | 5.6 | 2 | 11.8 | 3 | 8.6 | |
| Widowed | 1 | 5.6 | 0 | 0.0 | 1 | 2.9 | |
| Other | 0 | 0.0 | 1 | 5.9 | 1 | 2.9 | |
| Your work status | |||||||
| Employed full‐time | 7 | 38.9 | 3 | 17.6 | 10 | 28.6 | 0.1875 |
| Employed part‐time | 2 | 11.1 | 5 | 29.4 | 7 | 20.0 | |
| Home duties | 6 | 33.3 | 4 | 23.5 | 10 | 28.6 | |
| Retired | 1 | 5.6 | 0 | 0.0 | 1 | 2.9 | |
| Student | 1 | 5.6 | 0 | 0.0 | 1 | 2.9 | |
| Unemployed | 0 | 0.0 | 3 | 17.6 | 3 | 8.6 | |
| Other – pension | 1 | 5.6 | 2 | 11.8 | 3 | 8.6 | |
| Partner's work status | |||||||
| Employed full‐time | 7 | 77.8 | 9 | 81.8 | 16 | 80.0 | 0.8532 |
| Employed part‐time | 0 | 0.0 | 1 | 9.1 | 1 | 5.0 | |
| Home duties | 1 | 11.1 | 0 | 0.0 | 1 | 5.0 | |
| Retired | 1 | 11.1 | 1 | 9.1 | 2 | 10.0 | |
| Student | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Unemployed | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Work role | |||||||
| Manager | 2 | 16.7 | 1 | 10.0 | 3 | 13.6 | 1.000 |
| Professional | 3 | 25.0 | 2 | 20.0 | 5 | 22.7 | |
| Technician/trades | 2 | 16.7 | 1 | 10.0 | 3 | 13.6 | |
| Community/personal Services | 1 | 8.3 | 2 | 20.0 | 3 | 13.6 | |
| Clerical/admin | 3 | 25.0 | 2 | 20.0 | 5 | 22.7 | |
| Sales | 0 | 0.0 | 1 | 10.0 | 1 | 4.5 | |
| Machinery/driver | 1 | 8.3 | 0 | 0.0 | 1 | 4.5 | |
| Labourer | 0 | 0.0 | 1 | 10.0 | 1 | 4.5 | |
| Highest education | |||||||
| Postgraduate degree | 1 | 5.9 | 0 | 0.0 | 1 | 2.9 | 0.8432 |
| Grad dipl/grad cert | 0 | 0.0 | 1 | 5.9 | 1 | 2.9 | |
| Bachelor | 3 | 17.6 | 4 | 23.5 | 7 | 20.6 | |
| Advanced dipl/dipl | 2 | 11.8 | 2 | 11.8 | 4 | 11.8 | |
| Cert III/IV | 3 | 17.6 | 4 | 23.5 | 7 | 20.6 | |
| Year 12 | 3 | 17.6 | 4 | 23.5 | 7 | 20.6 | |
| Year 11 or below | 5 | 29.4 | 2 | 11.8 | 7 | 20.6 | |
| Ever experienced a relapse on natalizumab | |||||||
| Yes | 3 | 16.7 | 1 | 6.3 | 4 | 11.8 | 0.6041 |
| No | 15 | 83.3 | 15 | 93.8 | 30 | 88.2 | |
| Mean | SD | Mean | SD | Mean | SD | p value † | |
|---|---|---|---|---|---|---|---|
| Age (years) | 40.2 | 15.6 | 38.9 | 11.9 | 39.6 | 13.7 | 0.78 |
| # Dependents | 0.9 | 1.6 | 0.8 | 1.0 | 0.9 | 1.3 | 0.79 |
| Age at diagnosis (years) | 32.8 | 11.4 | 29.3 | 8.2 | 31.1 | 10.0 | 0.32 |
| Years receiving natalizumab | 5.0 | 2.8 | 4.2 | 1.7 | 4.7 | 2.4 | 0.32 |
| Years at RAH | 4.9 | 2.6 | 4.0 | 1.8 | 4.5 | 2.3 | 0.31 |
AB, start at clinic; BA, start at home.
Fisher’s exact test.
Student’s t‐test.
In terms of treatment adherence, of the 207 infusions across interventions A and B, 170 (82.1%) were delivered within 3 days of the recommended date (i.e. for 34 of 35 patients within 31 days of the previous infusion, for one patient on extended interval dosing within 45 days). There was no difference between clinic (A) and home (B) in the treatment adherence rate (84/103, 81.6% at clinic, 86/104, 82.7% at home) (χ 2 = 0.0, p = 1.0).
Patient safety outcomes
Nil AEs were reported during either the home infusion or usual clinic‐based care. Infections were reported for 11 patients on a total of 18 occasions. There was no difference in the number of infections during home care (8) compared to clinic (10) (χ 2 = 0.04, p = 0.84). Respiratory infection was the most commonly reported infection overall (six in the clinic compared to one at home). Urinary tract infection was the most commonly reported infection at home (n = 3) compared to the clinic (n = 2); all of these cases were specific to one patient, persistent and related to self‐catheterisation. Two skin infections (n = 2) were reported during home care and one was reported during clinic care.
Patient satisfaction
Satisfaction measures improved from treatment period 1 to 2 for group AB, which started at the clinic, and tended to decrease between treatment periods in group BA, which started at home (Fig. 2A) (Table S1).
Figure 2.
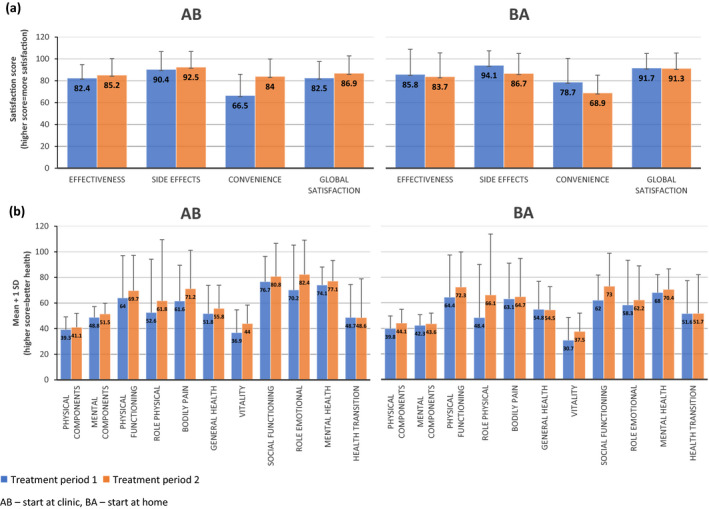
Mean ± 1 SD for AB (n = 17) and BA (n = 18) for (A) four Treatment Satisfaction Questionnaire for Medication (TSQM) domains (transformed scores, range from 0 to 100) and (B) eleven subscales of the SF‐36.
Table 2 presents odds of a poor outcome (i.e. decreased satisfaction) in group AB divided by odds of a poor outcome in BA to give an Odds Ratio, 95% confidence interval and interaction p value. There was a statistically significant interaction for “Convenience” (interaction p value = 0.001). For Group AB, the odds of a low (poor) convenience score at treatment period 2 by treatment period 1 are 94% less than the odds of low (poor) convenience score at treatment period 2 by treatment period 1 for Group BA (Odds Ratio = 0.06, 95% CI 0.01, 0.31). In other words, participants who recently had home infusions were significantly more satisfied with the convenience of their treatment than those who most recently had infusions in the clinic. The direction of odds ratios (<1) for other satisfaction measures showed positive, though not statistically significant, trends in favour of home infusions (Table 2).
Table 2.
Ordinal logistic GEE model results (failing assumptions of the linear model) for TSQM and three scales of the MSQLI and linear mixed‐effects model results of outcome versus Group and Stage interaction, adjusting for clustering on patient ID comparing “difference in A minus difference in B”
| Outcome | Scale | Odds ratio* | Lower 95% CL | Upper 95% CL | Interaction p value | |
|---|---|---|---|---|---|---|
| TSQM | Effectiveness | 0.37 | 0.11 | 1.24 | 0.11 | |
| Side effects | 0.64 | 0.10 | 4.05 | 0.64 | ||
| Convenience | 0.06 | 0.01 | 0.31 | 0.001 | ||
| Global satisfaction | 0.53 | 0.13 | 2.15 | 0.37 |
| Estimate | Standard error | Lower 95% CL | Upper 95% CL | Interaction p value | ||
|---|---|---|---|---|---|---|
| SF‐36 | Physical components scale | −2.55 | 5.79 | −14.42 | 9.33 | 0.66 |
| Mental components scale | 1.37 | 4.72 | −8.33 | 11.06 | 0.77 | |
| Physical functioning | −2.16 | 15.03 | −32.91 | 28.58 | 0.89 | |
| Role physical | −8.54 | 21.42 | −52.35 | 35.27 | 0.69 | |
| Bodily pain | 8.06 | 14.93 | −22.48 | 38.59 | 0.59 | |
| General health | 4.21 | 11.38 | −19.03 | 27.45 | 0.71 | |
| Vitality | 0.20 | 7.87 | −15.90 | 16.31 | 0.98 | |
| Social functioning | −6.90 | 12.79 | −33.09 | 19.30 | 0.59 | |
| Role emotional | 8.29 | 18.05 | −28.62 | 45.20 | 0.65 | |
| Mental health | 0.61 | 8.66 | −17.08 | 18.29 | 0.94 | |
| Health transition | −0.18 | 12.90 | −26.52 | 26.16 | 0.99 | |
| MSQLI | MFIS_5 | −0.97 | 2.48 | −6.03 | 4.10 | 0.70 |
| PES | −0.33 | 2.71 | −5.86 | 5.21 | 0.90 | |
| SSS | −0.42 | 3.30 | −7.41 | 6.57 | 0.90 | |
| PDQ | −1.50 | 2.33 | −6.25 | 3.25 | 0.52 | |
| MHI † | 0.24 | 0.99 | −1.79 | 2.27 | 0.81 | |
| MSSS † | 2.32 | 2.33 | −2.45 | 7.08 | 0.33 |
| Odds ratio* | Lower 95% CL | Upper 95% CL | Interaction p value | |||
|---|---|---|---|---|---|---|
| BLCS | 0.91 | 0.30 | 2.78 | 0.87 | ||
| BWCS | 1.09 | 0.33 | 3.61 | 0.88 | ||
| VIS | 2.30 | 0.67 | 7.95 | 0.19 |
CL, Confidence Limits; TSQM, Treatment Satisfaction Questionnaire for Medication; SF‐36, Short Form 36; MFIS‐5, Modified Fatigue Impact Scale; PES, MOS Pain Effects Scale; SSS, Sexual Satisfaction Scale; PDQ, Perceived Deficits Questionnaire; MHI, Mental Health Inventory; MSSS, MOS Modified Social Support Survey; BLCS, Bladder Control Scale; BWCS, Bowel Control Scale; VIS, Impact of Visual Impairment Scale.
Modelling the probability of a poor outcome (e.g. lower convenience) at treatment period 2 by treatment period 1 by dividing odds of a poor outcome in group AB by odds of a poor outcome in BA.
Short‐form scales.
Quality of life
Health status questionnaire (SF‐36)
At the start of treatment period 1, groups AB and BA were about 1 SD below the mean score (for the US population) for Physical Components, and ~1–0.5 SD below the mean score for Mental Components (Fig. 2B and Table S2). There was a small increase in virtually all SF‐36 subscales between treatment periods 1 and 2 for both groups AB and BA, suggesting that all aspects of the general health of participants slightly improved during the trial (Fig. 2B and Table S2A). Linear mixed‐effects models for SF‐36 and subscales showed no statistically significant interactions between group and treatment period for any of the SF‐36 outcomes (Table 2).
Other MSQLI scales
Results for nine MSQLI scales as presented in Figure 3 and Table S3 show similar patterns for both groups. Most of the scales tended to decrease slightly between the treatment periods indicating fewer problems. The opposite patterns in two scales (MHI and MSSS) indicate a slight improvement in these outcomes. Regardless, there were no statistically significant interactions between group and treatment period for any of these MSQLI outcomes (Table 2).
Figure 3.
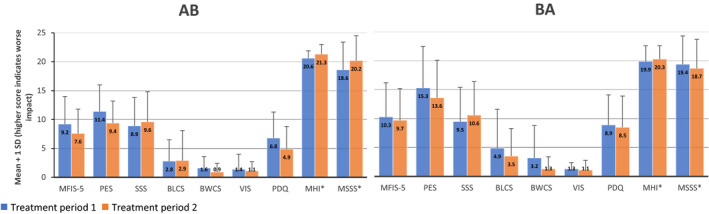
Mean ± 1 SD for AB (n = 17) and BA (n = 18) for nine MSQLI scales.
Expanded disability status scale
Expanded disability status scale (EDSS) scores were measured on all participants at the start of treatment period 1, but due to an unexpected absence of qualified staff and the transition to a new hospital site, only 14 measurements were made in treatment period 2 (Table 3). Under the EDSS criteria, the mean score of 3.0 at treatment period 1 is described as “Moderate disability in one functional system, or mild disability in three or four functional systems. No impairment to walking’. 31 Due to the low numbers of patients in each group, we did not conduct further statistical analysis on this data.
Table 3.
EDSS scores at the start of treatment period 1 and end of treatment period 2.
| Treatment period 1 | Treatment period 2 | |||
|---|---|---|---|---|
| Group AB | Group BA | Group AB | Group BA | |
| n | 18 | 17 | 9 | 5 |
| Mean | 3.0 | 3.0 | 3.1 | 2.1 |
| SD | 1.5 | 1.5 | 2 | 0.7 |
AB, start at clinic; BA, start at home.
Costs
Excluding pharmacy costs, the mean costs for an infusion at the clinic (comprising, e.g. nursing, consumables ward costs and non‐clinical) was A$538 ± 74 and the cost to deliver a home infusion (comprising, e.g. nursing time and travel costs, medical courier and insurance) was $58 lower (A$480). The mean “out of pocket” costs to patients were $16.70 ± 15.70 at the clinic and $0.30 ± 1.80 at home. The mean time spent per infusion was 146 ± 15.70 min in the clinic and 53.9 ± 14.6 min at home. In terms of human resources and healthcare utilisation between infusions, across all three infusions in clinic and home settings, most participants were accompanied to the infusion, however, this occurred significantly more often at home than in the clinic (McNemar test, p = 0.037) (Table 4). There were no other differences between clinic and home (Table 4).
Table 4.
Involvement of carers/family members in participants’ infusions
| Home – Yes | Home ‐No | Home ‐ Yes | Home ‐ No | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| Clinic ‐ Yes | Clinic ‐ Yes | Clinic ‐ No | Clinic ‐ No | ||||||
| n | % | n | % | n | % | n | % | ||
| Accompanied by a family member or friend | 7 | 20.0 | 6 | 17.1 | 22 | 62.9 | 0 | 0.0 | 0.0037 |
| Engaged a carer for a family member | 3 | 8.6 | 2 | 5.7 | 0 | 0.0 | 30 | 85.7 | 0.50 |
| Visited a GP | 4 | 11.4 | 4 | 11.4 | 2 | 5.7 | 25 | 71.4 | 0.69 |
| Visited the ED | 0 | 0.0 | 0 | 0.0 | 1 | 2.9 | 34 | 97.1 | 1.0 |
| Admission to a hospital | 0 | 0.0 | 1 | 2.9 | 0 | 0.0 | 34 | 97.1 | 1.0 |
| Seen a carer | 2 | 5.7 | 3 | 8.6 | 3 | 8.6 | 27 | 77.1 | 1.0 |
Discussion
A new model of care for delivering natalizumab infusions in patient’s homes was safe for patients. Indeed, no AEs were reported in any of the 207 infusions conducted in either the clinic or home settings, replicating earlier findings for a similar number of patients over a longer time frame and a total of 494 infusions. 22 Although 1%–2% of natalizumab infusions involve an AE, 6 patients in this study had on average 5 years’ experience with natalizumab and were excluded if they had fewer than six prior infusions when AEs are more likely. 4 Additionally, those more likely to experience an AE (based on past experience) may have been less likely to consent. The small size of these two studies of home infusions of natalizumab suggests that a larger study is required to test the safety and home AE management strategies in the longer term, including ongoing monitoring for PML.
We anticipated that home infusions may result in a lower number of infections from reduced exposure of potentially immunocompromised patients to nosocomial infection in the clinic. We found no obvious differences between home and clinic infection rates, and an overall infection rate of 18/207 (8.7%). Although low numbers and aetiological differences between infection types may have impacted these findings, there is no comparative infection data for other monoclonal antibody home infusion programs. It is nevertheless important to note that infection risks for people with MS on disease‐modifying therapies are elevated compared to the general population, 33 and infection control in home healthcare is a serious problem, with some 3.5% of patients developing a serious infection during home care. 34
Feasibility studies can determine whether an intervention is appropriate for further testing, and may focus on issues including acceptability, expansion of a service into a new setting and limited‐efficacy testing. 35 Acceptability of home infusions of natalizumab for patients and clinicians has been investigated qualitatively. 24 (Juaton M, Cusack L, Schultz T. Healthcare workers’ experiences of transitioning natalizumab infusions from hospital services to an in‐home setting: A qualitative study. under review). Additionally, the evidence from this study supports the feasibility of home infusions of natalizumab, albeit with limitations due to the short‐time frame of three home infusions, limited statistical power from the small number of participants and potential for bias in the unblinded measurement of outcomes such as TSQM and loss to follow‐up in EDSS measures.
The measurement of similar adherence rates between clinic and home also provides supporting evidence that home care is feasible. However, enhanced flexibility at home did not lead to more timely care for people with MS, a finding that may be explained by the clinic’s commitment to ensuring that their patients are adherent to their medication and re‐scheduling infusions when required. Indeed, the frequent re‐scheduling and last‐minute cancellation of clinic appointments were one of the clinic’s reasons for participating in the trial. Although about 18% of infusions are not delivered 4‐weekly as prescribed, extended interval dosing to 5‐ to 7‐week intervals does not increase the risk of relapse. 36 , 37
The finding of greater “Convenience,” as a subscale of Satisfaction, for home infusions is not surprising, given that home care was specifically designed to be patient‐centred. 26 This quantitative finding was strongly reflected in the qualitative interviews of 12 patients, in which “convenience for patients and their families’ was one of three subthemes under the main theme of “patient‐centredness” and contributed significantly to the acceptability of home infusions. 24 Convenience provided patients with autonomy and control over their medical appointments and lifestyle, and freed up time and capacity for family and work. 24 Participants were able to flexibly change the timing of their medical appointment by contacting the infusion nurse; one participant even elected to have an infusion in their workplace.
Infusions at home delivered the same medication at the same dose using the same equipment and the same method of delivery as at the clinic. It was therefore assumed that the home infusions would be equally clinically effective; however, it was important to test this assumption. We found no difference between clinic and home infusions in any of the measures contained in the MSQLI that are commonly used to assess health status for people with MS. Although this study was not powered sufficiently to detect equivalence between the two treatments (which for the main study outcome of AEs would require a sample size of several thousand patients in each group), the similarity in the change from treatment period 1 to 2 in MSQLI, as demonstrated by the linear mixed‐effects and GEE models, does offer support that the clinical effectiveness of the medication at home was similar to that in the clinic.
We documented a slight improvement in patients’ general health (SF‐36) measures between treatment periods 1 to 2 that was consistent between the two study groups. The median size of the improvement across all 11 SF‐36 subscales was 7.7% in group AB and 6.7% in group BA. The patterns shown in the other scales of the MSQLI are very similar to those demonstrated for the SF‐36. Given the heat sensitivity of people with MS, one possible explanation for this is a seasonal effect of improvement in general health from the heat of summer when the trial commenced in February to the coolness of winter (August) when the trial concluded. Alternatively, it may reflect a placebo effect from participation in the trial.
Our estimate is that home infusions are A$75 less expensive than clinic infusions, with patients' “out‐of‐pocket” expenses contributing almost a quarter of this amount. Time savings for home infusion patients (~90 min) have not been included in costings but likely contribute to the greater convenience of home infusions, which also includes greater accompaniment by a friend or relative. Although the absolute amounts differ due to methodological differences, these estimates of lower costs at home are supported by a recent Australian study of rapid infliximab infusions, which found that infusions were A$10.60 less expensive (median $49.80 vs. $39.20 per infusion, p = 0.20) at home compared to clinic. 38
Strengths and limitations
Our study sample was highly experienced MS patients, having been diagnosed 8–9 years previously on average, and having received natalizumab for nearly 5 years, mostly at the clinic. As such, these “expert patients” 39 are well placed to contribute their skills and insights for the improvement of services.
However, this was a pilot study with a small sample size of 35 participants and is therefore underpowered to test for equivalence in any of the main study outcomes. We limited the delivery of home infusions to the Adelaide metropolitan area. Nearly 30% of eligible participants did not consent to participate in the trial, and we were unable (for ethical reasons) to better understand the reasons for this. Outcomes were collected by unblinded nurses delivering infusions.
Conclusion
This pilot study has demonstrated no differences in safety and effectiveness between clinic and home infusions of natalizumab, and provided strong evidence for feasibility in the latter setting. There was no difference between clinic and home in treatment adherence or quality of life scores. Patients reported that home infusions were more convenient than the clinic, and costs to both the patient and the healthcare system were less at home. Large‐scale studies should be conducted to further test these findings, particularly around the safety and management of hypersensitivity AEs in the home setting and for equivalence of clinical outcomes.
Conflicts of Interest
Ms Thomas is director of Post‐Op Care at Home (Pty Ltd). This organisation was subcontracted to deliver the home care nursing. Therefore, if the outcome of the project is positive and home delivery of natalizumab becomes a new model of care, this could lead to commercial gain for Ms Thomas.
Ms Naidoo is an employee of Biogen, manufacturer of natalizumab. Therefore, if the outcome of the project is positive and home delivery of natalizumab becomes a new model of care, this could lead to commercial gain for Biogen if it leads to more patients receiving natalizumab than would otherwise have been the case. Dr Ravindran received travel support funding for conference attendance from Biogen. All other authors declare that there is no conflict of interest.
Supporting information
Table S1. Four domains of the TSQM showing transformed scores (range from 0 to 100, in which 100 is the highest possible satisfaction) at treatment periods 1 and 2.
Table S2. Summary of 11 subscales of the SF‐36 at treatment periods 1 and 2.
Table S3. Summary of nine scales of the MSQLI at treatment periods 1 and 2.
Acknowledgements
We thank the study participants who agreed to trial the new model of care. We thank the staff of the Ambulatory Care Day Unit at Royal Adelaide Hospital, neurologists at Royal Adelaide Hospital, and staff of Post‐Op Care at Home (Pty Ltd) for their participation in the project. The statistical analyses were conducted by Suzanne Edwards, Adelaide Health Technology Assessment, University of Adelaide.
Funding Information
The project was funded by Biogen Australia and New Zealand as investigator‐initiated research.
Funding Statement
This work was funded by Biogen Australia and New Zealand.
References
- 1. O'Leary S, Beavin J, Bishop C, et al. Practical guidelines for administering natalizumab: a nursing perspective. Int J MS Care 2007;9:1–8. [Google Scholar]
- 2. Fragoso YD, Alves‐Leon SV, Arruda WO, et al. Natalizumab adverse events are rare in patients with multiple sclerosis. Arq Neuropsiquiatr 2013;71:137–141. [DOI] [PubMed] [Google Scholar]
- 3. Hellwig K, Schimrigk S, Fischer M, et al. Allergic and nonallergic delayed infusion reactions during natalizumab therapy. Arch Neurol 2008;65:656–658. [DOI] [PubMed] [Google Scholar]
- 4. Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo‐controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;2:899–910. [DOI] [PubMed] [Google Scholar]
- 5. Torkildsen O, Myhr KM, Bo L. Disease‐modifying treatments for multiple sclerosis ‐ a review of approved medications. Eur J Neurol 2016;23(Suppl 1):18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biogen Australia . Product Information Tysabri. 2016. [cited 2016 1st June]; Available from: https://www.biogen.com.au/content/dam/corporate/en_AU/pdfs/products/TYSABRI/TYSABRI_Product_Information.pdf
- 7. McGuigan C, Craner M, Guadagno J, et al. Stratification and monitoring of natalizumab‐associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J Neurol Neurosurg Psychiatry 2016;87:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Connor P, Goodman A, Kappos L, et al. Long‐term safety and effectiveness of natalizumab redosing and treatment in the STRATA MS Study. Neurology 2014;83:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Butzkueven H, Kappos L, Pellegrini F, et al. Efficacy and safety of natalizumab in multiple sclerosis: interim observational programme results. J Neurol Neurosurg Psychiatry 2014;85:1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Research Council . Health care comes home: the human factors. Committee on the role of human factors in home health care, board on human‐systems integration, division of behavioral and social sciences and education. Washington, DC: The National Academies Press, 2011. [Google Scholar]
- 11. Royal College of Nursing . Moving care to the community: an international perspective. London: Royal College of Nursing, 2014. [Google Scholar]
- 12. Institute of Medicine . Crossing the quality chasm: a new health system for the 21st century. Washington, DC: The National Academies Press, 2001. [PubMed] [Google Scholar]
- 13. Shang J, Ma C, Poghosyan L, et al. The prevalence of infections and patient risk factors in home health care: a systematic review. Am J Inf Control 2014;42:479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gonçalves‐Bradley DC, Iliffe S, Doll HA, et al. Early discharge hospital at home. Cochrane Database Syst Rev 2017;6:CD000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stephens B. Patients' experiences of community IV therapy. Br J Nurs 2013;22(Sup19):S24–S29. [DOI] [PubMed] [Google Scholar]
- 16. Dobson PM. A model for home infusion therapy initiation and maintenance. J Infus Nurs 2001;24:385–394. [DOI] [PubMed] [Google Scholar]
- 17. Fridthjof KS, Kampmann P, Dünweber A, et al. Systematic patient involvement for homebased outpatient administration of complex chemotherapy in acute leukemia and lymphoma. Br J Haematol 2018;181:637–641. [DOI] [PubMed] [Google Scholar]
- 18. Rentala M, Andrews S, Tiberio A, et al. Intravenous home infusion therapy instituted from a 24‐hour clinical decision unit for patients with cellulitis. Am J Emerg Med 2016;34:1273–1275. [DOI] [PubMed] [Google Scholar]
- 19. Polinski JM, Kowal MK, Gagnon M, et al. Home infusion: safe, clinically effective, patient preferred, and cost saving. Healthcare 2017;5:68–80. [DOI] [PubMed] [Google Scholar]
- 20. Condino AA, Fidanza S, Hoffenberg EJ. A home infliximab infusion program. J Pediatr Gastroenterol Nutr 2005;40:67–69. [DOI] [PubMed] [Google Scholar]
- 21. Kuin S, Stolte SB, van den Brink GR , et al. Remicade infusions at home: an alternative setting of infliximab therapy for patients with Crohn's disease. Eur J Gastroenterol Hepatol 2016;28:222–225. [DOI] [PubMed] [Google Scholar]
- 22. Vijayan S, Adams J, Cook L, et al. Establishment of the first at‐home natalizumab infusion service for the treatment of relapsing remitting multiple sclerosis (RMMS). J Neurol Neurosurg Psychiatry 2017;88:e1.80‐e1. [Google Scholar]
- 23. Davidson P, Halcomb E, Hickman L, et al. Beyond the rhetoric: what do we mean by a 'model of care'? Aust J Adv Nurs 2006;23:47–55. [PubMed] [Google Scholar]
- 24. Juaton M, Cusack L, Schultz T. Patients’ experiences of natalizumab treatment in a home environment: a qualitative study. Aust J Adv Nurs 2020;37:14–20. [Google Scholar]
- 25. Mills EJ, Chan AW, Wu P, et al. Design, analysis, and presentation of crossover trials. Trials 2009;10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schultz TJ, Thomas A, Georgiou P, et al. Developing a model of care for home infusions of Natalizumab for people with multiple sclerosis. J Infus Nurs 2019;42:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2004;26:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glanz BI, Musallam A, Rintell DJ, et al. Treatment satisfaction in multiple sclerosis. Int J MS Care 2014;16:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ritvo PG, Fischer JS, Miller DM, et al. MSQLI ‐ multiple sclerosis quality of life inventory: a user's manual. New York: National Multiple Sclerosis Society, 1997. [Google Scholar]
- 30. Kurtzke JF. On the origin of EDSS. Mult Scler Relat Disord 2015;4:95–103. [DOI] [PubMed] [Google Scholar]
- 31. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 32. Fagerland MW, Lydersen S, Laake P. The McNemar test for binary matched‐pairs data: mid‐p and asymptotic are better than exact conditional. BMC Med Res Methodol 2013;13:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luna G, Alping P, Burman J, et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol 2020;77:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shang J, Larson E, Liu J, Stone P. Infection in home health care: results from national outcome and assessment information set data. Am J Inf Control 2015;43:454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bowen DJ, Kreuter M, Spring B, et al. How we design feasibility studies. Am J Prev Med 2009;36:452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bomprezzi R, Pawate S. Extended interval dosing of natalizumab: a two‐center, 7‐year experience. Ther Adv Neurol Disord 2014;7:227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Kempen ZLE , Hoogervorst ELJ, Wattjes MP, et al. Personalized extended interval dosing of natalizumab in MS. Neurology 2020;95:e745. [DOI] [PubMed] [Google Scholar]
- 38. Bohra A, Rizvi Q‐A‐A, Keung CYY, et al. Transitioning patients with inflammatory bowel disease from hospital‐based to rapid home‐based infliximab: a stepwise, safety and patient‐orientated process towards sustainability. World J Gastroenterol 2020;26:5437–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tattersall RL. The expert patient: a new approach to chronic disease management for the twenty‐first century. Clin Med (Lond) 2002;2:227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Four domains of the TSQM showing transformed scores (range from 0 to 100, in which 100 is the highest possible satisfaction) at treatment periods 1 and 2.
Table S2. Summary of 11 subscales of the SF‐36 at treatment periods 1 and 2.
Table S3. Summary of nine scales of the MSQLI at treatment periods 1 and 2.


