Abstract
Objective
The aims of this study were to (i) explore psychotic experiences across the entire amyotrophic lateral sclerosis‐frontotemporal dementia (ALS‐FTD) spectrum from a clinical and genetic perspective, (ii) determine the rate of abnormal perceptual experiences across the five sensory modalities and (iii) explore the neurobiological factors that lead to psychosis vulnerability in ALS‐FTD.
Methods
In a prospective case‐controlled study design, 100 participants were enrolled including ALS (n = 37, 24% satisfied criteria for ALS‐Plus), ALS‐FTD (n = 11), bvFTD (n = 27) and healthy controls (n = 25). Psychotic experiences, perceptual abnormalities and psychosocial factors were determined by means of the clinical interview and carer and patient reports. Voxel‐based morphometry analyses determined atrophy patterns in patients experiencing psychosis‐like experiences and other perceptual abnormalities.
Results
The rates of psychotic experiences and abnormalities of perception in each sensory modality were high across the entire ALS‐FTD continuum. The rate was highest in those with C9orf72 expansions. Rates were also high in patients with pure ALS including psychosis measured by carer‐based reports (18%) and self‐report measures of psychotic‐like experiences (21%). In an ENTER regression model, social anxiety and ACE‐III scores were the best predictors of psychosis proneness, accounting for 44% of the score variance. Psychosis‐like experiences and perceptual abnormalities were associated with a predominantly frontal and temporal pattern of atrophy that extended to the cerebellum and centred on the anterior thalamus.
Interpretation
The model for psychosis proneness in ALS‐FTD likely includes complex interactions between cognitive, social and neurobiological factors that determine vulnerability to psychosis and that may have relevance for individualised patient management.
Introduction
Amyotrophic lateral sclerosis (ALS) overlaps significantly with frontotemporal dementia (FTD) at a cognitive and behavioural level, with up to 15% of ALS patients meeting clinical diagnostic criteria for FTD and up to 40% exhibiting cognitive or behavioural changes similar to those seen in FTD. 1 Likewise, a proportion of patients presenting with FTD develop ALS and a high proportion have subtle motor abnormalities. 2 The C9orf72 genetic expansion is the most common genetic abnormality identified in both familial FTD and familial ALS, further consolidating the link across conditions. 3 , 4 Patients with this expansion exhibit high rates of delusions and hallucinations and may experience psychotic symptoms for years before the onset of motor or cognitive features, which not only causes considerable diagnostic difficulty but also raises the concept of an overlap with neuropsychiatric diseases. 5 Prior to the discovery of the C9orf72 expansion, psychotic symptoms were considered to be relatively rare in FTD. 6 , 7 Major psychiatric symptoms are also regarded as rare in pure ALS, although their prevalence has not been systematically evaluated.
Psychotic symptoms traditionally take the form of delusions or hallucinations, both of which are broadly considered to represent a loss of perception of reality. 8 , 9 Psychotic symptoms in C9orf72 carriers can be variable in both intensity and character. They encompass the spectrum of delusions (including persecutory, somatic, jealous and grandiose) and hallucinations (visual, auditory and tactile) and are usually negative in nature. 10 Somatic delusions are relatively common and may be related to an altered body schema in C9orf72 carriers in relation to atrophy of posterior regions including the parietal lobe and cerebellum. 11 , 12 Although there are no direct studies comparing psychotic symptoms (delusions and hallucinations) between C9orf72 carriers and schizophrenia, it is generally accepted that in schizophrenia the intensity, frequency, severity and functional impact of these symptoms can be more severe, particularly during acute episodes. Subtle disorders of perception, which can precede and accompany frank psychotic features in psychiatric populations, have not been examined across the amyotrophic lateral sclerosis‐frontotemporal dementia (ALS‐FTD) spectrum. 13 The distinction between abnormal perceptual experiences and psychosis is not clear, and both are likely closely linked. Some are advocating that abnormal perceptual experiences have a natural progression to psychotic symptoms when paired with other predisposing factors including social and physical anhedonia. Others, however, have suggested that they form a continuum with the normal population, are part of an individual's personality and, in some cases, improve creativity. 14 , 15 , 16 , 17 Anecdotally, in FTD and ALS, patients often describe abnormal sensory experiences that are difficult to define and do not meet criteria for frank psychosis.
The underlying neurobiological mechanisms associated with the generation of psychosis in FTD and ALS are relatively unknown but may be linked to regional cortical and subcortical atrophy that include the frontal and temporal cortices and thalamus. 7 This paucity of knowledge contrasts with schizophrenia, the archetypal psychotic disorder, where genetic susceptibility, environmental exposure to drugs, social isolation and stress are known risk factors. 18 Neuroimaging studies of first‐episode psychosis and schizophrenia have found associations between psychotic symptoms and atrophy in the frontal and temporal cortices as well subcortical structures and the cerebellum. 19 , 20 Of all the regions implicated across neurodegenerative and neuropsychiatric disorders, the thalamus appears worthy of specific interest. 21 , 22 The thalamus has emerged in parallel with the C9orf72 expansion as a region of interest largely due to findings from C9orf72 carriers, demonstrating significant thalamic atrophy and aberrant connectivity. 23 , 24 In the broader neuropsychiatric discipline, the thalamus has been repeatedly implicated in psychosis with evidence from multiple imaging modalities including both structural and functional MRI, and PET imaging, as well as pathology studies. 25 , 26 , 27 , 28 , 29 In general, a variety of methods have been utilised to consider the thalamic regions involved in neuropsychiatric disorders from connectivity studies to more recently studies that segment and measure specific thalamic nuclei volume. Across disorders, the pulvinar and mediodorsal nuclei have been most consistently implicated in studies of schizophrenia and first‐episode psychosis with additional evidence for ventrolateral nuclei involvement. 25 , 30 , 31 Both the mediodorsal and ventrolateral nuclei mediate extensive white matter connections to the frontal and prefrontal cortex. The role of the cerebellum also deserves some consideration. The cerebellum was traditionally considered to be concerned exclusively with motor control, but evidence has now emerged suggesting a role in a range of cognitive abilities including memory, emotion and language. 32 The cerebellum is linked to the prefrontal cortex by projections through the thalamus, known as the thalamo‐cortico‐cerebellar network. 33
In ALS and FTD, certain patients seem to be more vulnerable than others. This suggests that factors beyond brain degeneration may be at play and that these may include psychosocial factors. The current study aimed to determine the rate of psychotic features and disordered perception in a clinically and genetically well‐characterised cohort of patients from across the ALS‐FTD spectrum. A secondary aim was to determine the psychosocial factors associated with psychosis vulnerability to determine the interplay between these variables and identify any potential modifiable factors. A final aim was to examine the neural basis of psychotic‐like and abnormal perceptual experiences. We hypothesised that these experiences would be common across the entire spectrum and that psychosocial and cognitive factors might render patients more vulnerable. Based on our previous studies investigating the neural substrates of psychosis in FTD, we hypothesised the involvement of a distributed temporo‐frontal network of brain structures including the orbitofrontal cortex, insula, amygdala, dorsal and medial frontal cortex, and superior temporal gyrus, extending to key modulatory brain structures including the thalamus, and cerebellum. 7
Material and Methods
Participants
In total, 100 consecutive participants were enrolled including patients with ALS (37%; of these 24% satisfied criteria for ALS‐Plus; see below); ALS‐FTD (11%), bvFTD (27%) and a group of age‐, education‐ and sex‐matched healthy controls (25%). Controls were recruited from a control database at the Brain and Mind Centre, University of Sydney. All participants were assessed at the Brain and Mind Centre, University of Sydney. Patients were included in the study if they satisfied criteria for probable or definite bvFTD according to international consensus criteria or possible, probable or definite ALS according to El Escorial criteria and the recently proposed diagnostic criteria for ALS using standard clinical assessment criteria. 34 , 35 , 36 , 37 Patients with FTD and concurrent ALS (ALS‐FTD) were included in the study. Participants were excluded from the study if they were consumers of cannabis or other hallucinogenic drugs either in the past or at present. Participants were also excluded if they were prescribed medications known to cause psychotic symptoms or if they were diagnosed with a primary psychotic disorder more than 10 years prior to FTD or ALS symptom onset. Prescribed medications that could cause psychotic symptoms included corticosteroids, Parkinsonian medications, the anti‐epileptic drug leviteracetam, opioids, digoxin and the b‐blockers propranolol and metoprolol. There were no patients in our cohort on any of these medications. There were two participants excluded because they did not complete the questionnaires (one from the ALS cohort and one from the bvFTD cohort). Exclusion criteria also included any severe hearing or visual deficits or previously diagnosed taste or smell disorders or reported deficits.
Ethical approval for this study was obtained from the University of Sydney, the University of New South Wales and the South Eastern Area Health Service ethics committees. Participants, or their person responsible, provided informed written consent in accordance with the Declaration of Helsinki.
Clinical and neuropsychological assessment
Global cognitive function was measured using the Addenbrooke's Cognitive Examination–III (ACE‐III), adapted for patients with motor and bulbar issues. 38 Behavioural changes were assessed with the Cambridge Behavioral Inventory‐Revised (CBI‐R). 39 All participants including controls were assessed by means of the CBI‐R, the ACE‐III, a battery of neuropsychological assessments and MRI Brain. Patients from the ALS clinic were assessed for evidence of cognitive and or behavioural impairment in line with the Strong diagnostic criteria including measures of executive function and language. 40 Patients were deemed to have cognitive or behavioural impairment if they satisfied these criteria and were termed ALS‐Plus (ALS‐P). If they satisfied criteria for both behavioural and cognitive impairment, then ALS‐FTD was diagnosed according to the Strong criteria if they also showed a progressive deterioration and demonstrated symptoms in line with the current consensus diagnostic criteria for behavioural variant frontotemporal dementia. 41
Genetic assessment
DNA was extracted from whole blood collected for genetic screening following informed consent and using protocols approved by the Human Research Ethics Committee of the South Eastern Sydney and Illawarra Area Health Service. The repeat primed PCR technique for the C9orf72 expansion was performed using the procedure described previously, 42 based on the protocol of Renton and colleagues. 3 Patients with a family history were also screened for other common genetic mutations (GRN, MAPT, SOD‐1) by Sanger sequencing of genomic DNAs corresponding to all coding exons. 43 , 44
Psychosis and perceptual abnormalities assessment
The presence of psychosis was first determined by means of the beliefs subscale of the CBI‐R completed by the carer. The CBI‐R is a commonly used tool to measure behavioural change including psychotic symptoms in patients on the ALS‐FTD spectrum. The beliefs subscale includes three questions relating to the previous one month and asks if the patient (1) sees things that are not really there (visual hallucinations), (2) hears things that are not really there (auditory hallucinations and (3) has odd or bizarre ideas that cannot be true (delusions). If the carer endorsed any of these symptoms, then psychosis was considered to be present.
All participants completed the Cambridge Anomalous Perceptions Questionnaire (CAPS), a validated questionnaire developed to probe psychosis proneness and abnormal perceptions (Table S1). 45 The 32‐item questionnaire includes three questions which directly reflect Schneider's first rank symptoms that are strongly suggestive of schizophrenia. These include the following: (1) Do you ever hear your own thoughts repeated or echoed? (2) Do you ever hear your own thoughts spoken aloud in your head, so that someone near might be able to hear them? (3) Do you ever hear voices commenting on what you are thinking or doing?
A factor analysis determined that these three questions and a question of visual hallucinations (Do you ever see things that other people cannot?) form a single ‘clinical psychosis factor’. For the purpose of this study, and in the absence of validated measures in ALS and FTD, this ‘clinical psychosis factor’ was considered a measure of psychosis‐like perceptual abnormalities. This was considered distinct from the CBI‐R subscale that aims to directly measure the presence or absence of psychotic symptoms. An overall ‘psychosis severity score’ was obtained from the clinical psychosis factor of the CAPS by considering the frequency, and how distracting and distressing the symptoms were. Additional items of the CAPS were dichotomized into five sections when they corresponded to the five senses (vision, hearing, touch, taste and smell) and rated as present or absent (Table S1). Question 22 (Do you ever look in the mirror and think that your face seems different from usual?) was not included as it could be related to changes due to the disease process.
Psychological and social factors assessment
Depression and anxiety were assessed by means of a validated self‐report questionnaire, the 42‐item Depression, Anxiety and Stress Scale (DASS). 46 Social isolation and social anxiety were determined by means of subsections 2 and 3 of the self‐report Schizotypal Personality Questionnaire (SPQ). 47
Structural neuroimaging
Imaging acquisition
Participants were scanned at the Brain and Mind Centre using a 3T GE Discovery MR750 scanner with a standard 8‐channel head coil. Whole‐brain T1‐weighted MRI scans were acquired using a MPRAGE sequence (TR/TE = 5.8/2.6 msec; flip angle = 8°, 1 mm isotropic resolution). Patients were scanned on the same day or following day of clinical examination, and diagnosis and all questionnaires were completed within 2 weeks of MRI acquisition.
Imaging analysis
Participant's structural MRI scans were analysed using an optimised voxel‐based morphometry (VBM) approach using FMRIB's Software Library (FSL). Participants were included across all diagnoses including ALS, ALS‐P, ALS‐FTD, bvFTD and also controls. Covariates included age and education status.
An updated preprocessing pipeline was implemented whereby T1‐weighted images were reoriented, cropped and bias‐field corrected, prior to non‐linear registration‐based brain extraction for more accurate skull stripping. Resulting bias corrected images were inspected for intensity abnormalities along cortical edges and brain stem, and quality of brain extraction. Next, tissue segmentation was carried out using FSL‐FAST. 48 Resulting grey matter partial volume images were aligned to MNI152 standard space using a combination of affine and non‐linear registration. 49 A study specific template was generated, to which native participant structural images were non‐linearly registered. Registered grey matter partial volume images were modulated by the Jacobian of the warp field to correct for local expansion or contraction and smoothed with an isotropic Gaussian kernel with a sigma of 3 mm.
Whole brain and region of interest analyses were carried out to determine differences in grey matter integrity associated with the presence of ‘psychosis‐like perceptual experiences’ as endorsed on the CAPS as present or absent and directly compared to those without psychosis‐like perceptual experiences across all participants. Similarly, differences in grey matter integrity associated with the presence of sensory perceptual abnormalities, also endorsed on the CAPS, and including any abnormality across each of the five senses were determined. Based on a priori hypothesis of an underlying temporo‐frontal neural network (thalamus, superior temporal gyrus, fusiform gyrus, inferior frontal gyrus, orbitofrontal cortex, insula, anterior cingulate) that extended to include the cerebellum, underpinning psychosis, a region of interest mask was generated using the Harvard–Oxford cortical and subcortical atlases. Significant differences were assessed using non‐parametric permutation based voxel‐wise testing (5000 permutations) and the threshold‐free cluster enhancement (TFCE) approach. Clusters were corrected for multiple comparisons and considered significant after controlling for family‐wise error at p < 0.05. The significance level for reported uncorrected clusters was set at p < 0.001 with ≥100 contiguous voxels. Anatomical location of reported clusters was defined with reference to the Harvard–Oxford cortical and Subcortical atlases, and the Oxford Thalamic Connectivity atlas.
Behavioral analyses
Data were analysed using SPSS 24.0 statistical package. Continuous variables were analysed using univariate ANOVA, with post hoc analyses comparing differences across groups, using Sidak correction for multiple comparisons, and statistical significance was set at p < 0.05. The independent T test was analysed for instances when only two continuous variables were present. Categorical data were compared with chi‐square tests. For the chi‐square tests, a post hoc p value was set at 0.01 (0.05/number of groups = 5) for the diagnostic category and 0.017 (0.05/3) for the genetic categories. Effect size was calculated for all main effects across continuous and categorical variables. Correlations between scores were assessed by means of Spearman's correlations. A linear regression model was applied to determine the best predictors of psychosis. Finally, to determine the relationship between perceptual disorders and psychotic symptoms in ALS and FTD, a preliminary and exploratory analysis was performed with the available data using chi‐square test of independence and a liberal threshold of p < 0.05.
Results
Demographics, cognitive and behavioural screening, and genetics
Comparison of the diagnostic groups as described in Table 1 and genetic groups in Table S2 revealed a higher ACE‐III score for controls and ALS patients compared to ALS‐FTD and bvFTD (all p values < 0.001); and a higher score for C9orf72 carriers compared to controls and non‐carriers (all p < 0.01). The groups were otherwise matched on demographic data. Of the 75 patients included in the study, 21% tested positive for the C9orf72 expansion. No patients carried GRN, MAPT or SOD‐1 mutations. Patients with bvFTD and ALS‐FTD scored higher on the CBI‐R (indicating higher levels of impairment) than those with ALS and ALS‐P (all p < 0.05).
Table 1.
Demographics, cognitive and behavioural screening across the ALS‐FTD spectrum.
| ALS (n = 28) | ALS‐Plus (n = 9) | ALS‐FTD (n = 11) | bvFTD (n = 27) | Controls (n = 25) | F value | p value | Effect size | Post hoc | p value | Confidence intervals | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C9orf72 Carriers (%) | 7 | 11 | 64 | 22 | 0 | ||||||
| Sex (M:F) | 22:6 | 7:2 | 10:1 | 17:10 | 13:12 | — | 0.076 | — | |||
| Age (years) | 63.2 ± 10.2 | 61.6 ± 13.4 | 60.6 ± 8.2 | 63.7 ± 8.5 | 60 ± 10.5 | 0.303 | 0.846 | 0.017 | |||
| Education (years) | 13 ± 3.4 | 12.9 ± 3.6 | 12.7 ± 2.1 | 12.5 ± 2.6 | 13.6 ± 2.1 | 0.369 | 0.83 | 0.023 | |||
| Disease duration (years) | 2 ± 1.9 | 3 ± 3.5 | 4.5 ± 4.6 | 6.2 ± 4.3 | — | 5.721 | 0.002 | 0.222 | bvFTD > ALS | 0.001 | 1.35–7 |
| ACE‐III (max 100) | 94.7 ± 3.2 | 88.6 ± 8.2 | 77.5 ± 9.6 | 78.2 ± 11.4 | 96.6 ± 2.5 | 18.458 | <0.001 | 0.497 | ALS > bvFTD | <0.001 | −23.1 to −9.9 |
| ALS‐P > FTD | 0.01 | −19.1 to −1.6 | |||||||||
| HC > bvFTD | <0.001 | −25.9 to −10.3 | |||||||||
| ALS > ALS‐FTD | <0.001 | 8.5–25.9 | |||||||||
| HC > ALS‐ FTD | <0.001 | −28.4 to −9.2 | |||||||||
| ALS‐P>ALS‐FTD | 0.03 | −21.4 to −0.7 | |||||||||
| ALSFRS | 39.7 ± 10 | 40.1 ± 7.1 | 39.5 ± 3.5 | — | — | 0.783 | |||||
| CBI total score | 15.4 ± 9.9 | 32.1 ± 16.3 | 60 ± 28.1 | 63.4 ± 25.6 | — | 29.308 | <0.001 | 0.647 | FTD > ALS | <0.001 | 29.8–66.2 |
| FTD > ALS‐P | <0.001 | 9.3–53.2 | |||||||||
| ALS‐FTD > ALS | <0.001 | −68.8 to −20.4 | |||||||||
| ALS‐FTD > ALS‐P | 0.04 | −55 to −0.75 | |||||||||
| ALS onset (limb:bulbar) | 20:8 | 6:3 | 6:5 | — | — | — | 0.54 | — |
Values are expressed as mean ± standard deviation. Significant values are highlighted in bold. Effect size measured as Eta2.
Psychosis in the ALS‐FTD spectrum
As shown in Table 2, rates of psychotic features according to the CBI‐R were high across all patient groups (ALS [18%], ALS‐P (22%), bvFTD [39%] and ALS‐FTD [55%)]). The rates of psychosis‐like perceptual experiences measured by the CAPS were also high and paralleled the pattern seen for psychotic features as measured by the CBI‐R. The severity of perceptual experiences related to psychosis (psychosis severity score) also differed significantly across ALS (M = 7.5, SD = 3.9), ALS‐P (M = 6.7, SD = 2.9), ALS‐FTD (M = 28, SD = 12), bvFTD (M = 23.4, SD = 12.7) and controls (all scored 0; F = 5.773, p = 0.005, Eta 2 = 0.464). Participants with FTD and ALS‐FTD scored higher than patients with ALS (p = 0.04, 95%CI = 0.39–31.4 and p = 0.02, 95% CI = −38.7 to −2.3, respectively).
Table 2.
Psychosis and perceptual abnormalities across the spectrum of ALS‐FTD.
| ALS (n = 28) | ALS‐P (n = 9) | ALS‐FTD (n = 11) | bvFTD (n = 27) | Controls (n = 25) | X 2 | p value | Effect size | Post hoc | X 2 | p value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Psychosis present on CBI‐R (%) | 18 | 22 | 55 | 39 | — | 5.36 | 0.15 | 0.271 | ||||
| CAPS ‐ Clinical Psychosis Factor | ||||||||||||
| Symptom endorsed (%) | 21.4 | 33.3 | 45.5 | 37 | 0 | 13.7 | 0.008 | 0.370 | bvFTD > HC | 11.464 | 0.001 | |
| ALS‐FTD > HC | 13.196 | <0.001 | ||||||||||
| ALS‐P > HC | 9.140 | 0.003 | ||||||||||
| ALS > HC | 6.04 | 0.01 | ||||||||||
| Thought broadcast (%) | 18 | 22 | 27 | 22 | 0 | 9.33 | 0.05 | 0.305 | ||||
| Thoughts repeated (%) | 18 | 0 | 18 | 22 | 0 | 6.91 | 0.14 | 0.263 | ||||
| Third person voices (%) | 7 | 11 | 27 | 11 | 0 | 6.25 | 0.17 | 0.254 | ||||
| Visual hallucinations (%) | 11 | 0 | 18 | 22 | 0 | 8.28 | 0.09 | 0.287 | ||||
| CAPS – Perceptual Abnormality Domains | ||||||||||||
| Auditory (%) | 25 | 33 | 73 | 41 | 8 | 14.77 | 0.005 | 0.392 | ALS‐FTD > ALS | 7.6 | 0.006 | |
| ALS‐FTD > HC | 13.42 | <0.001 | ||||||||||
| Visual (%) | 14 | 22 | 55 | 26 | 4 | 13.06 | 0.011 | 0.363 | ALS‐FTD > HC | 11.97 | <0.001 | |
| ALS‐FTD > ALS | 6.71 | 0.01 | ||||||||||
| Taste (%) | 32 | 33 | 54 | 33 | 0 | 14.13 | 0.007 | 0.378 | bvFTD > HC | 9.71 | 0.002 | |
| ALS‐FTD > HC | 15.8 | <0.001 | ||||||||||
| ALS > HC | 9.33 | 0.002 | ||||||||||
| Smell (%) | 21.4 | 33 | 73 | 22 | 0 | 22.75 | <0.001 | 0.479 | ALS‐FTD > HC | 21.874 | <0.001 | |
| ALS‐P>HC | 8.46 | 0.004 | ||||||||||
| ALS‐FTD>bvFTD | 8.57 | 0.003 | ||||||||||
| ALS‐FTD>ALS | 9.03 | 0.003 | ||||||||||
| Touch (%) | 46 | 56 | 82 | 33 | 4 | 23.7 | <0.001 | 0.487 | ALS‐FTD>bvFTD | 7.37 | 0.007 | |
| ALS‐FTD>HC | 23.06 | <0.001 | ||||||||||
| bvFTD>HC | 7.2 | 0.007 | ||||||||||
| ALS‐P>HC | 12.1 | 0.001 | ||||||||||
| ALS>HC | 12.23 | <0.001 | ||||||||||
| Psychiatric (%) | 4 | 22 | 36 | 33 | 0 | 17.7 | 0.001 | 0.42 | bvFTD>ALS | 8.19 | 0.004 | |
| ALS‐FTD>ALS | 7.6 | 0.006 | ||||||||||
| bvFTD>HC | 10.08 | 0.002 | ||||||||||
| ALS‐FTD>HC | 10.28 | 0.001 | ||||||||||
| Neuropathy (%) | 43 | 33 | 64 | 19 | 4 | 18.5 | 0.001 | 0.43 | ALS‐FTD>bvFTD | 7.36 | 0.007 | |
| ALS‐FTD>HC | 15.72 | <0.001 | ||||||||||
| ALS>HC | 10.77 | 0.001 | ||||||||||
Values are expressed as percentages of the cohort experiencing at least one psychotic symptom and also each separate question for the CAPS – clinical psychosis factor. Values are also expressed as percentages of the cohort experiencing at least one perceptual abnormality for each CAPS perceptual abnormality domain. Significant values are highlighted in bold. X 2 represents the chi‐square value and effect size is measured as Kramer's V.
Among these symptoms, 18% of patients with ALS reported thought broadcasting and thought repetition, 11% reported hallucinations while only 7% reported hearing voices in the third person. In contrast, 27% of ALS‐FTD patients reported hearing voices in the third person, and 18% reported visual hallucinations. Visual hallucinations were also common in patients with bvFTD cohort (22%) as was thought broadcasting and repetition (both 22%).
Psychosis in C9orf72 expansion carriers
According to the CBI‐R, carriers were significantly more likely to experience psychotic symptoms compared to non‐carriers (63% vs. 22%; p ≤ 0.001; Table 3). The psychosis severity score was significantly different across groups including carriers (M = 15.6, SD = 18.6), non‐carriers (M = 3.2, SD = 6.4) and controls (F = 18.97, p = 0.018, Eta 2 = 0.206). C9orf72 carriers scored significantly higher than non‐carriers and controls (both p < 0.001, 95% CI = 6.41–8.41 and 8.8–22.45, respectively).
Table 3.
Psychosis and perceptual abnormalities in C9orf72 carriers, non‐carriers and controls.
| C9orf72 carriers (n = 16) | C9orf72 non‐carriers (n = 59) | Controls (n = 25) | X 2 | p value | Effect size | Post hoc | X 2 | p value | |
|---|---|---|---|---|---|---|---|---|---|
| Psychosis present on CBI‐R | 63 (10) | 22 (13) | — | 118.57 | <0.001 | 0.751 | |||
| CAPS – clinical psychosis factor | |||||||||
| Symptom endorsed | 50 (8) | 27 (16) | 0 | 14.14 | <0.001 | 0.376 | Carrier > HC | 18.24 | <0.001 |
| Non‐carrier > HC | 7.9 | 0.005 | |||||||
| Thought broadcast | 19 (3) | 10 (6) | 0 | 4.37 | 0.113 | 0.208 | |||
| Thoughts repeated | 31 (5) | 17 (10) | 0 | 7.84 | 0.02 | 0.28 | |||
| Third person voices | 25 (4) | 9 (5) | 0 | 7.24 | 0.02 | 0.279 | |||
| Visual hallucinations | 37.5 (6) | 9 (5) | 0 | 14.77 | 0.001 | 0.386 | Carrier > HC | 11.38 | 0.003 |
| Carrier > non‐carrier | 8.27 | 0.004 | |||||||
| CAPS – perceptual abnormality domains | |||||||||
| Auditory | 75 (12) | 31 (18) | 8 (2) | 18.65 | <0.001 | 0.44 | Carrier > HC | 16.55 | <0.001 |
| Carrier > non‐carrier | 11.32 | 0.001 | |||||||
| Visual | 56 (9) | 17 (10) | 4 (1) | 17.11 | <0.001 | 0.416 | Carrier > HC | 13.89 | <0.001 |
| Carrier > non‐carrier | 10.28 | 0.001 | |||||||
| Taste | 56 (9) | 31 (18) | 0 | 16.08 | <0.001 | 0.403 | Carrier > HC | 17.42 | <0.001 |
| Non‐carrier > HC | 9.35 | <0.001 | |||||||
| Smell | 63 (10) | 22 (13) | 0 | 20.69 | <0.001 | 0.459 | Carrier > HC | 19.33 | <0.001 |
| Carrier > non‐carrier | 9.69 | 0.002 | |||||||
| Non‐carrier > HC | 6.02 | 0.014 | |||||||
| Touch | 81.25 (13) | 39 (23) | 4 | 25.22 | <0.001 | 0.502 | Carrier > HC | 25.89 | <0.001 |
| Carrier > non‐carrier | 9.01 | 0.003 | |||||||
| Non‐carrier > HC | 10.53 | 0.001 | |||||||
| Psychiatric | 56 (9) | 12 (7) | 0 | 24.8 | <0.001 | 0.498 | Carrier > HC | 18.02 | <0.001 |
| Carrier > non‐carrier | 14.78 | <0.001 | |||||||
| Neuropathy | 56 (9) | 31 (18) | 4 | 13.66 | 0.001 | 0.37 | Carrier > HC | 14.44 | <0.001 |
| Non‐carrier > HC | 7.05 | 0.008 | |||||||
Values are expressed as percentages of the cohort experiencing at least one psychotic symptom, and also each separate question for the CAPS‐ clinical psychosis factor. Values are also expressed as percentages (exact number in brackets) of the cohort experiencing at least one perceptual abnormality for each CAPS perceptual abnormality domain. Significant values are highlighted in bold. X 2 represents the chi‐square value and effect size is measured as Kramer's V.
Disorders of sensory perception in the ALS‐FTD spectrum
All types of perceptual abnormalities were more common in patient groups than in controls (Table 2). Disorders of auditory perception were present in 73% of ALS‐FTD patients which was significantly higher than in ALS (25%; p = 0.006) and controls (8%, p = 0.001).
Within the visual system, a significant difference was present across groups (p = 0.011) with ALS‐FTD reporting abnormalities more frequently (55%) than ALS (14%; p = 0.01) and controls (4%; p = 0.001).
A similar pattern of disordered perception was noted for smell and taste abnormalities which were frequently reported by ALS‐FTD patients (73% and 55%, respectively) while being reported with similar frequencies in ALS (21% and 32%, respectively) and bvFTD (22% and 33%, respectively). There was a significant difference across groups for perception of taste (p = 0.007) with ALS‐FTD, bvFTD and ALS all more likely to report disordered taste than controls (all p < 0.005).
Disorders of touch were subgrouped into those that could be considered to be due to an underlying neurological aetiology (sensation of burning and skin being more sensitive than usual) and those in which a psychiatric basis was more likely (being aware of the presence of another being and of being touched). The symptoms considered to have a psychiatric basis were more frequent in ALS‐FTD (36%) and bvFTD (33%) than ALS (4%, both p < 0.01) and controls (0%, both p < 0.005) with a trend for a higher rate in ALS‐P (22%, p = 0.015). In contrast, symptoms suggestive of a neurological aetiology were frequent in ALS‐FTD (64%) and ALS (43%).
Disorders of sensory perception in C9orf72 expansion carriers
Disorders of auditory and visual perception were present in 75% and 56% of C9orf72 carriers and 29% and 17% of non‐carriers respectively with differences across and between groups as outlined in Table 3. Similarly disordered taste and smell differed across the genetically assigned cohorts and controls with C9orf72 carriers (63%) reporting abnormal smell and taste (63% and 56%) more frequently than non‐carriers (22% and 31%) and controls (0%; all p < 0.015). Abnormalities of touch were significantly different across groups, and those with a presumed psychiatric aetiology were more frequently reported in C9orf72 carriers (56%) than non‐carriers (12%, p < 0.001) and controls (0% p < 0.001).
Factors associated with psychosis‐like perceptual experiences
A significant difference was identified across patient groups and controls for depression, anxiety and social isolation (all p < 0.005; Table 4). Significant differences were also noted in these areas, in addition to social anxiety, in the genetically assigned cohort (all p < 0.005; Table S3).
Table 4.
Psychosocial factors across the spectrum of ALS‐FTD.
| ALS (n = 28) | ALS‐Plus (n = 9) | ALS‐FTD (n = 11) | bvFTD (n = 27) | Controls (n = 25) | F value | p value | Effect size | Post hoc | p value | 95% Confidence intervals | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Depression | 4.4 ± 5.0 | 6.7 ± 7.3 | 8.7 ± 7.6 | 8.9 ± 10.4 | 0.6 ± 1.4 | 4.89 | 0.001 | 0.19 | bvFTD > HC | 0.001 | 2.37–14.13 |
| ALS‐FTD > HC | 0.02 | 0.75–15.3 | |||||||||
| Anxiety | 3.4 ± 2.8 | 6.3 ± 6.3 | 7.3 ± 7.0 | 6.6 ± 8.8 | 0.2 ± 0.6 | 4.92 | 0.001 | 0.19 | bvFTD > HC | 0.003 | (−11.28) to (−1.58) |
| ALS‐FTD > HC | 0.01 | (−13.16) to (−1.14) | |||||||||
| Social isolation | 1.4 ± 2.1 | 1.6 ± 1.9 | 4.3 ± 3.2 | 2.9 ± 3.0 | 0.6 ± 1.2 | 5.94 | <0.001 | 0.22 | bvFTD > HC | 0.02 | 0.26–4.37 |
| ALS‐FTD > ALS | 0.007 | 0.53–5.23 | |||||||||
| ALS‐FTD > HC | 0.001 | −6.13 to −1.17 | |||||||||
| Social anxiety | 1.4 ± 1.6 | 1.2 ± 1.5 | 2.0 ± 2.2 | 1.8 ± 2.4 | 0.6 ± 1.3 | 1.7 | 0.1 | 0.07 |
Values are expressed as mean ± standard deviation. Significant values are highlighted in bold. Effect size measured as Eta2.
For the entire cohort, higher scores on the ‘psychosis severity score’ correlated with social isolation (r = 0.293, p = 0.003), social anxiety (r = 0.473, p < 0.001), depression (r = 0.384, p < 0.001), anxiety (r = 0.362, p < 0.001), ACE‐III score (r = −0.3, p = 0.009) and total CBI score (r = 0.296, p = 0.01). There was no correlation between the psychosis score and education (r = 0.294, p = 0.08) or disease duration (r = 0.81, p = 0.53). In an ENTER regression model, social anxiety (β = 0.51, p < 0.001) and ACE‐III score (β = −0.34, p ≤ 0.001) were the best predictors of psychosis score accounting for 44% of the score variance.
Association between Psychosis and Sensory Perceptual Abnormalities
Finally, an exploratory analysis identified an association between psychosis as measured by the CBI‐R and auditory perceptual experiences (X 2 = 8.5, p = 0.004, Cramer's V = 0.34); psychosis and visual perceptual experiences (X 2 = 6.2, p = 0.013, Cramer's V = 0.29); psychosis and abnormal smell perception (X 2 = 4.99, p = 0.025, Cramer's V = 0.26); psychosis and abnormal touch related to perceptual abnormalities ((X 2 = 4.8, p = 0.03, Cramer’zs V = 0.26). There was no association between psychosis and abnormal touch related to sensory deficits (X 2 = 1.4, p = 0.25, Cramer's V = 0.13) or psychosis and taste (X 2 = 0.208, p = 0.65, Cramer's V = 0.05).
Neuroimaging correlates of psychosis‐like and sensory perceptual abnormalities
Temporo‐frontal grey matter, subcortical and cerebellar abnormality was assessed in patients using VBM (Table 5). Patients with psychosis‐like perceptual experiences showed significantly reduced grey matter integrity predominantly in the prefrontal cortex, bilateral superior temporal gyrus and inferior frontal gyrus, compared to patients without. In addition, there was significant atrophy in cerebellar regions that included lobules VI, VIII and IX. Patients with sensory perceptual abnormalities showed predominantly reduced grey matter integrity in the bilateral orbitofrontal cortices and anterior thalamus. Cerebellar lobules V, VI, VIII also showed significant atrophy. Overlapping clusters of grey matter abnormality across both conditions was observed in the left temporo‐occipital fusiform gyrus, and bilateral superior temporal gyrus and anterior thalamus (Fig. 1). Analysis of brain structures implicated in the temporo‐frontal neural network indicated significant grey matter thalamic abnormality primarily involving subregions associated with frontal structural connectivity that include anterior and medial thalamic regions 50 (Fig. 2). Regions of cerebellar atrophy predominantly corresponded to lobules associated with sensorimotor functioning. 51
Table 5.
Voxel‐based morphometry results showing regions of increased grey matter atrophy associated with presence of (A) sensory perceptual abnormalities and (B) psychosis‐like experiences across patient groups
| Region | Hemisphere | Size (Voxel) | Peak voxel (MNI) | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| (A) Sensory Perceptual Abnormalities | |||||
| Medial/Dorsolateral Frontal Lobe, Orbitofrontal Cortex, Ant. Cingulate Gyrus | L/R | 5783 | 8 | 36 | −26 |
| Precentral Gyrus, Insula Cortex | L | 4075 | −26 | −6 | 44 |
| Lingual Gyrus, Temporal‐occipital Fusiform Cortex | L/R | 2786 | −24 | −62 | −6 |
| Sup. Frontal Gyrus | R | 1663 | 22 | 22 | 42 |
| Cerebellum (VI) | L | 1062 | −32 | −53 | −27 |
| Inf. Temporal Gyrus | L | 813 | −60 | −22 | −26 |
| Sup. Temporal Gyrus | R | 638 | 56 | −28 | 8 |
| Cerebellum (VIII, IX) | L/R | 598 | 8 | −53 | −45 |
| Inf. Temporal Gyrus, Fusiform gyrus | R | 512 | 40 | −20 | −32 |
| Temporal‐occipital Fusiform Cortex | L | 470 | −36 | −54 | −14 |
| Postcentral Gyrus | L | 460 | −42 | −38 | 52 |
| Insular Cortex | R | 280 | 38 | 2 | −18 |
| Lateral Occipital Cortex | R | 252 | 40 | −62 | 10 |
| Lateral Occipital Cortex | L | 179 | −40 | −58 | 30 |
| Brain Stem (Medulla) | — | 119 | −8 | −38 | −42 |
| (B) Psychosis‐like Experiences | |||||
| Dorsolateral Frontal Lobe, Inf. Temporal Gyrus, Temporal‐occipital Fusiform Cortex | R | 6108 | 46 | 36 | ‐10 |
| Cerebellum (V, VI, VIII) | L/R | 5946 | 6 | −62 | −13 |
| Sup. Frontal Gyrus, Precentral Gyrus | L | 3528 | −18 | −4 | 70 |
| Inf. frontal gyrus, Sup. Temporal Gyrus | L | 3009 | −48 | 36 | −2 |
| Temporal‐occipital Fusiform Cortex | L | 2020 | −42 | −60 | −14 |
| Inf. Temporal Gyrus | L | 1255 | −44 | −26 | −26 |
Results are displayed as peak voxels of clusters at p < 0.001, uncorrected, ≥100 contiguous voxels.
Figure 1.
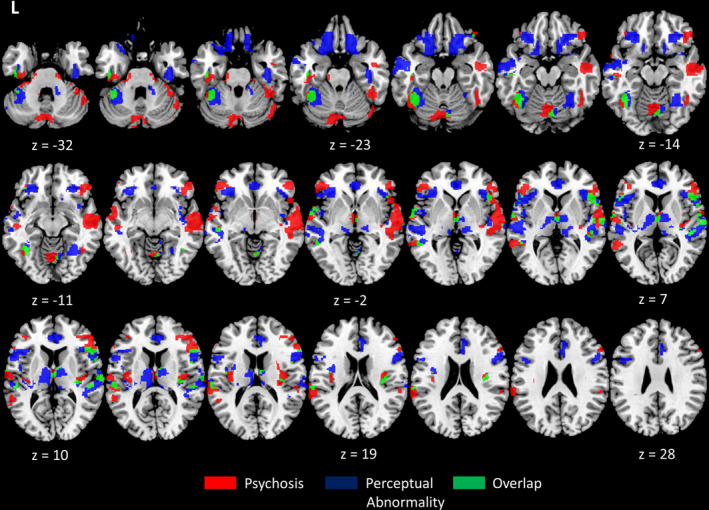
Reduced grey matter integrity associated with psychosis‐like experiences and sensory perceptual abnormalities in patients. Legend: Axial slices of regions of increased grey matter atrophy in patients with psychosis‐like experiences (red), sensory perceptual abnormalities (blue) and their overlap (green), compared to patients without. Clusters are overlaid on the MNI standard brain and significant at p < 0.001, uncorrected, ≥100 contiguous voxels.
Figure 2.
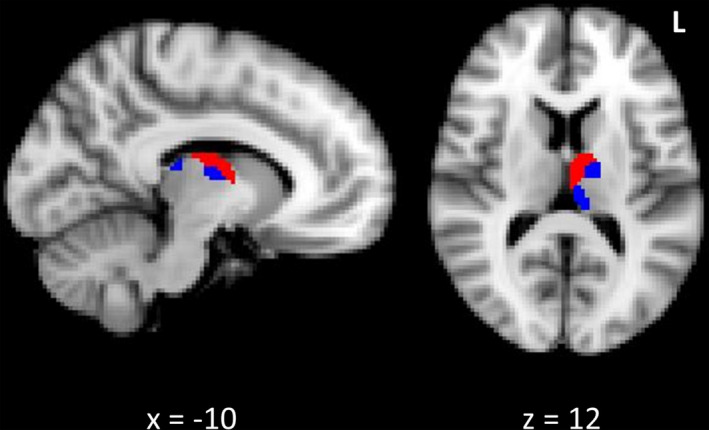
Thalamic atrophy associated with psychosis and perceptual abnormalities in patients. Legend: Reduced grey matter in the anterior and medial regions of the left thalamus in patients with psychosis‐like experiences (red) and sensory perceptual abnormalities (blue), compared to patients without. Clusters are overlaid on the MNI standard brain and significant at p < 0.05, corrected for multiple comparisons.
Discussion
This is the first study to measure psychotic symptoms and abnormal perceptual experiences across the spectrum of ALS and FTD. The most striking finding was the presence of psychotic symptoms in almost 20% of patients with pure ALS, as measured by means of a carer‐based assessment tool, as up until now these symptoms were considered rare in ALS without cognitive or behavioural features. Psychotic symptoms were also present in more than half of those with ALS‐FTD and a third with bvFTD. This study identified cognitive and psychosocial factors, namely, depression, increased social anxiety and isolation, as well as deficits of global cognitive function, that are associated with the presence and severity of psychosis. This lays the groundwork to study the issue of individual vulnerability to psychosis in ALS and FTD that ultimately may pave the way towards behavioural and psychological interventions for these symptoms. Abnormal sensory perceptual experiences were common across FTD and ALS. Patients with psychosis‐like experiences and perceptual abnormalities exhibited a similar pattern of brain atrophy, centred on the anterior thalamus, cerebellum and frontal and temporal regions with involvement of more distributed areas including the precentral gyrus, in line with previous findings. 7
In the last few decades, psychotic symptoms have been reported in ALS but usually in association with dementia and more recently in the setting of the C9orf72 expansion. 52 , 53 This study has now shown that patients with pure ALS are also vulnerable to these symptoms. It is likely that such symptoms are overlooked or go unnoticed because of the more obvious progressive physical disability. 54 , 55 Within this study, only 7% of patients with pure ALS carried the C9orf72 expansion suggesting that psychotic symptoms and perceptual experiences are not entirely related to this genetic abnormality. Psychotic symptoms were reported in almost 25% of non‐carriers suggesting that they may be a common feature of the neurodegenerative process in FTD and ALS but for unknown reasons are amplified by the C9orf72 expansion. 7 There remains controversy as to the best method to determine the presence of psychotic symptoms in the ALS‐FTD spectrum. Various methods have been used in the past, likely accounting in part for the variability in reported rates. This study approached the measurement of symptoms related to psychosis from two perspectives. Firstly, the more traditional measure of carer‐based assessments determined the presence of delusions and hallucinations and then a self‐report measure probed psychosis proneness. The results from each modality showed a similar pattern of endorsements across the spectrum. Both carer‐ and patient‐based questionnaires have potential for significant bias—and perhaps in ALS, a carer‐based approach, such as the CBI, may not be the most sensitive means of determining behavioural change of any type, particularly in the early stages of a terminal disease that tends to focus on physical disability. Self‐report measures on the other hand also may present issues particularly in the presence of cognitive deficits and when insight may be impaired. The measure used in this study to determine perceptual abnormalities, the CAPS, was designed for a non‐clinical sample. The decision was made to use this tool as it closely addressed the research question, and although our cohort was a clinical one, it was not a traditionally psychiatric or psychotic one. Together, these issues highlight the lack of gold standard measurements for psychotic symptoms in neurodegenerative disorders.
It is generally accepted that cognitive deficits may contribute to the risk of psychosis particularly in terms of misinterpretation of anomalous experiences through attention and executive deficits. 56 , 57 Our finding that psychosis‐like experiences were associated with poorer scores on a test of global cognition lends support for a cognitive neuropsychological model for psychosis generation in the ALS‐FTD spectrum. The association identified here with depression may be particularly relevant as ALS patients are known to be at an increased risk for depression. 58 , 59 , 60 Levels of social isolation and social anxiety were strongly correlated with psychosis severity and converges with evidence from the psychosis literature where negative social factors have been well‐documented as risk factors for psychotic disorders. 61 For patients in the ALS‐FTD spectrum, it is possible that a number of elements, including genetic and environmental factors, converge to confer a susceptibility to psychosis in the individual. However, whether psychosocial factors are a consequence of, or contribute to, psychosis vulnerability in ALS‐FTD remains to be determined.
Across the range of sensory modalities, a spectrum of abnormal experiences was identified. Overall, ALS‐FTD patients reported higher rates of abnormalities in each domain than other patient groups. Within the limited scope of the exploratory analysis to determine the relationship between sensory abnormalities and psychotic symptoms, our findings provide circumstantial evidence for a sensory perceptual and psychosis continuum in patients with ALS and FTD. This may in part explain the variability in symptoms described as psychosis in the literature and perhaps also within individuals.
Abnormalities of touch were the most common perceptual changes reported across the spectrum and were particularly common in the ALS phenotypes. Although ALS is considered to spare the sensory system, electrophysiological studies have documented sensory abnormalities in up to 23% of patients. 62 , 63 The literature also suggests subclinical electrophysiological abnormalities in patients with FTD. 2 In the neurodegenerative literature, abnormal perceptual experiences related to vision and the formation of visual hallucinations have been frequently explored in Parkinson's disease. 64 A number of theories exist for the generation of hallucinations in Parkinson's disease that could be relevant here. One theory suggests that loss of bottom‐up inhibition, due to sensory deficits, results in cortical release phenomenon and subsequent abnormal perceptions. 65 Another concept is that disruption of neural networks including the default mode network may be responsible for abnormal salience and visual hallucinations. 66 This may also be a factor in FTD and ALS where disruptions of the default mode and salience networks have also been reported. 67 In FTD and ALS, visual processing has been studied from a neuropsychological viewpoint, while there has been little research in terms of the physiological parameters of vision. In contrast, visual processing has been extensively studied in schizophrenia and has been repeatedly shown to be abnormal. 68 , 69 , 70 Warren and colleagues have undertaken considerable study of auditory processing in FTD, including the emotional salience associated with music, and have shown these processes to be abnormal across FTD subtypes. 71 , 72 At a more fundamental level, they have also found that perception of sound in relation to pleasure/aversion is altered across the FTD subtypes but interestingly is more common in MAPT mutation carriers than C9orf72 carriers. Perhaps most relevant to the study reported here was another seminal paper by this group that identified altered pain and temperature perception that was specifically impaired in C9orf72 carriers that confirms anecdotal reports of abnormal pain syndromes in this patient group, and was linked to atrophy of the posterior thalamus. Taste and smell have also been shown to be abnormal in in FTD and ALS. 73 , 74 , 75 , 76 Together, this evidence suggests that processing deficits across the senses might evoke aberrant perceptual experiences; however, this remains to be determined.
Two previous studies have explored the underlying basis for neuropsychiatric symptoms specific to the C9orf72 expansion using a novel neurophysiological approach and are particularly relevant to this study. Carriers were found to have altered somatosensory body schema processing, and the authors suggested that these deficits might contribute to the development of psychosis generation through alterations in thalamo‐cortico‐cerebellar networks. The findings in the current study of atrophy in cerebellar regions primarily associated with sensorimotor functions converges with this concept and warrants further consideration with regards to the role of the cerebellum in internal monitoring. 20 , 77
The thalamus may also be implicated in both the generation of perceptual abnormalities related to sensory processing and those related to psychotic symptoms. That the anterior and medial thalamic regions, with their connectivity to the frontal cortex, are implicated in FTD and ALS is perhaps not surprising. 78 , 79 While segmentation of the thalamic nuclei is beyond the scope of this study, a recent study showed that the anterior and mediodorsal nuclei of the thalami are involved across FTD syndromes including ALS‐FTD. 80 In schizophrenia, the anterior thalamus, as a nodal link, has been implicated in emotion, memory, learning, conflict monitoring and novelty detection, 81 and the anterior thalamic nuclei have also been shown to be pathologically abnormal. 82 , 83 , 84 As previously discussed, the mediodorsal nuclei have received more attention in the neuropsychiatric literature with strong evidence for a role in psychosis generation. Together with the results from this study, the literature suggests that the thalamus, and perhaps the anterior and medial regions in particular, with specific connections to frontal and temporal regions, might play a modulating role in the generation of psychotic symptoms and perceptual abnormalities in FTD and ALS. The finding of superior temporal gyrus atrophy in our psychosis cohort is notable, as across psychotic disorders, this region has been repeatedly implicated in the generation of auditory hallucinations. 85 , 86 , 87 Outside of areas responsible for speech, the insula, anterior cingulate, posterior cingulate, inferior frontal gyrus, thalamus, cerebellum and precuneus have all been associated with auditory hallucinations, with significant similarities with regions identified in our study. 88 , 89
This study has limitations that should be addressed for future studies. It is possible that the participants who responded to the questionnaires were those who experienced psychotic‐like symptoms and abnormal perceptual experiences, thus potentially biasing the results. To reduce this bias, the sample was unselected, and consecutive patients were given the questionnaires to complete with the assurance that their answers were confidential. It is also possible that in some cases, a psychotic psychiatric disorder was also present. To reduce this risk, patients were excluded if they had a history of a psychotic disorder made more than 10 years prior to the ALS or FTD diagnosis, with the assumption that such psychiatric diagnoses were unrelated, although this is not certain. This illustrates the ongoing need for further understanding of the interactions between psychiatric and neurodegenerative disorders. This study has identified a significantly higher rate of psychotic and perceptual experiences in C9orf72 carriers in line with the stated hypothesis based on previous literature. Although the sample size is consistent with previous studies that focus on the clinical aspects of the expansion, future studies should aim to incorporate a larger genetic sample to specifically confirm the genetic contribution to psychosis vulnerability in ALS‐FTD.
Although psychotic symptoms have been known to occur in FTD patients for many years, only recently has there been a resurgence of interest in this area kindled by the findings in those with the C9orf72 expansion who seem particularly prone to psychosis. This study has demonstrated that these symptoms are not limited to these cases but occur across the spectrum of ALS‐FTD and has laid the foundation for future studies of the underlying mechanisms involved in these processes. The results here do not imply direction of causation but suggest that cognitive, social and neurobiological factors may be relevant to psychosis vulnerability in the ALS‐FTD spectrum and that alterations in thalamo‐cortico‐cerebellar networks may also contribute. Future studies should be prospective and targeted specifically at identifying the mechanisms responsible with an emphasis on the brain network and psychosocial perspective with a view to enabling better understanding and therefore management of these symptoms in the ALS‐FTD continuum.
Author Contribution
Emma M. Devenney conducted the statistical analysis.
Conflict of Interest
The authors have no conflicts to report.
Supporting information
Table S1. Questions related to each sensory domain.
Table S2. Demographic details for C9orf72 carriers, non‐carriers and controls.
Table S3. Psychosocial factors across C9orf72 carriers, non‐carriers and controls.
Acknowledgements
This work was supported by funding to Forefront, a collaborative research group dedicated to the study of frontotemporal dementia and motor neuron disease, from the National Health and Medical research Council of Australia program grant (#1037746) and the Australian Research Council Centre of Excellence in Cognition and its Disorders Memory Program (#CE110001021). E. M. Devenney is supported by a MNDRIA post‐doctoral fellowship. S. Tu is supported by a NHMRC post‐doctoral fellowship (APP1121859). R. M. Ahmed is supported by a NHMRC post‐doctoral fellowship. G. Halliday is supported by a NHMRC Senior Principal Research Fellowship (#1079679). O. Piguet is supported by a NHMRC Senior Research Fellowship (APP1103258).
Funding Information
This work was supported by funding to Forefront, a collaborative research group dedicated to the study of frontotemporal dementia and motor neuron disease, from the National Health and Medical Research Council of Australia program grant (#1037746) and the Australian Research Council Centre of Excellence in Cognition and its Disorders Memory Program (#CE110001021). E. M. Devenney is supported by a MNDRIA post‐doctoral fellowship and a NHMRC post‐doctoral fellowship (APP1178587). S. Tu is supported by a NHMRC post‐doctoral fellowship (APP1121859). R. M. Ahmed is supported by a NHMRC post‐doctoral fellowship. G. Halliday is supported by a NHMRC Senior Principal Research Fellowship (#1079679). O. Piguet is supported by a NHMRC Senior Research Fellowship (APP1103258).
[Corrections added on July 28, 2021 after first online publication: Affiliation information has been updated.]
Funding Statement
This work was funded by National Health and Medical Research Council of Australia grants #1037746, #1079679, and APP1103258.
References
- 1. Devenney E, Vucic S, Hodges JR, Kiernan MC. Motor neuron disease‐frontotemporal dementia: a clinical continuum. Expert Rev Neurother 2015;15:509–522. [DOI] [PubMed] [Google Scholar]
- 2. Burrell JR, Kiernan MC, Vucic S, Hodges JR. Motor neuron dysfunction in frontotemporal dementia. Brain 2011;134(Pt 9):2582–2594. [DOI] [PubMed] [Google Scholar]
- 3. Renton AE, Majounie E, Waite A, et al. A Hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21‐linked ALS‐FTD. Neuron 2011;72:257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeJesus‐Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p‐linked FTD and ALS. Neuron 2011;72:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Devenney E, Hornberger M, Irish M, et al. Frontotemporal dementia associated with the C9ORF72 mutation: a unique clinical profile. JAMA Neurol 2014;71:331–339. [DOI] [PubMed] [Google Scholar]
- 6. Mendez MF, Shapira JS, Woods RJ, et al. Psychotic symptoms in frontotemporal dementia: prevalence and review. Dement Geriatr Cogn Disord 2008;25:206–211. [DOI] [PubMed] [Google Scholar]
- 7. Devenney EM, Landin‐Romero R, Irish M, et al. The neural correlates and clinical characteristics of psychosis in the frontotemporal dementia continuum and the C9orf72 expansion. Neuroimage Clin 2017;13:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Association AP . Diagnostic and statistical manual of mental disorders: DSM‐5. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 9. Organization WH . The ICD‐10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Wkly Epidemiol Rec 1992;67:227. [Google Scholar]
- 10. Hall D, Finger EC. Psychotic symptoms in frontotemporal dementia. Curr Neurol Neurosci Rep 2015;15:1–8. [DOI] [PubMed] [Google Scholar]
- 11. Downey LE, Fletcher PD, Golden HL, et al. Altered body schema processing in frontotemporal dementia with C9ORF72 mutations. J Neurol Neurosurg Psychiatry 2014;85:1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Downey LE, Mahoney CJ, Rossor MN, et al. Impaired self‐other differentiation in frontotemporal dementia due to the C9ORF72 expansion. Alzheimer's Res Ther 2012;4:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yung AR, McGorry PD. The prodromal phase of first‐episode psychosis: past and current conceptualizations. Schizophr Bull 1996;22:353–370. [DOI] [PubMed] [Google Scholar]
- 14. Chapman LJ, Edell WS, Chapman JP. Physical anhedonia, perceptual aberration, and psychosis proneness. Schizophr Bull 1980;6:639. [DOI] [PubMed] [Google Scholar]
- 15. Chapman LJ, Chapman JP. The search for symptoms predictive of schizophrenia. Schizophr Bull 1987;13:497–503. [DOI] [PubMed] [Google Scholar]
- 16. Claridge G. Single indicator of risk for schizophrenia: probable fact or likely myth? Schizophr Bull 1994;20:151–168. [DOI] [PubMed] [Google Scholar]
- 17. Schuldberg D. Six subclinical spectrum traits in normal creativity. Creat Res J 2001;13:5–16. [Google Scholar]
- 18. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature 2010;468:203–212. [DOI] [PubMed] [Google Scholar]
- 19. Glahn DC, Laird AR, Ellison‐Wright I, et al. Meta‐analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiat 2008;64:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fusar‐Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel‐wise meta‐analysis of antipsychotic‐naive VBM studies. Schizophr Bull 2011;38:1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou J, Greicius MD, Gennatas ED, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 2010;133:1352–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schroeter ML, Raczka K, Neumann J, Von Cramon DY. Neural networks in frontotemporal dementia—a meta‐analysis. Neurobiol Aging 2008;29:418–426. [DOI] [PubMed] [Google Scholar]
- 23. Lee SE, Khazenzon AM, Trujillo AJ, et al. Altered network connectivity in frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. Brain 2014;137:3047–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mahoney CJ, Beck J, Rohrer JD, et al. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features Brain 2012;135:736–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ettinger U, Chitnis XA, Kumari V, et al. Magnetic resonance imaging of the thalamus in first‐episode psychosis. Am J Psychiatry 2001;158:116–118. [DOI] [PubMed] [Google Scholar]
- 26. Talvik M, Nordström A‐L, Olsson H, et al. Decreased thalamic D2/D3 receptor binding in drug‐naive patients with schizophrenia: a PET study with [11C] FLB 457. Int J Neuropsychopharmacol 2003;6:361–370. [DOI] [PubMed] [Google Scholar]
- 27. Lang DJ, Khorram B, Goghari VM, et al. Reduced anterior internal capsule and thalamic volumes in first‐episode psychosis. Schizophr Res 2006;87:89–99. [DOI] [PubMed] [Google Scholar]
- 28. Marenco S, Stein JL, Savostyanova AA, et al. Investigation of anatomical thalamo‐cortical connectivity and FMRI activation in schizophrenia. Neuropsychopharmacology 2012;37:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Danos P, Baumann B, Krämer A, et al. Volumes of association thalamic nuclei in schizophrenia: a postmortem study. Schizophr Res 2003;60:141–155. [DOI] [PubMed] [Google Scholar]
- 30. Huang AS, Rogers BP, Sheffield JM, et al. Thalamic nuclei volumes in psychotic disorders and in youths with psychosis spectrum symptoms. Am J Psychiatry 2020;177:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anticevic A, Yang G, Savic A, et al. Mediodorsal and visual thalamic connectivity differ in schizophrenia and bipolar disorder with and without psychosis history. Schizophr Bull 2014;40:1227–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsy Clin Neurosci 2004;16:367–378. [DOI] [PubMed] [Google Scholar]
- 33. Hoshi E, Tremblay L, Féger J, et al. The cerebellum communicates with the basal ganglia. Nat Neurosci 2005;8:1491–1493. [DOI] [PubMed] [Google Scholar]
- 34. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134(Pt 9):2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2000;1:293–299. [DOI] [PubMed] [Google Scholar]
- 36. Shefner JM, Al‐Chalabi A, Baker MR, et al. A proposal for new diagnostic criteria for ALS. Clin Neurophysiol 2020;131:1975–1978. [DOI] [PubMed] [Google Scholar]
- 37. Kiernan MC, Vucic S, Talbot K, et al. Improving clinical trial outcomes in amyotrophic lateral sclerosis. Nat Rev Neurol 2020;17:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hsieh S, Schubert S, Hoon C, et al. Validation of the Addenbrooke's Cognitive Examination III in frontotemporal dementia and Alzheimer's disease. Dement Geriatr Cogn Disord 2013;36:242–250. [DOI] [PubMed] [Google Scholar]
- 39. Wear HJ, Wedderburn CJ, Mioshi E, et al. The Cambridge behavioural inventory revised. Dement Neuropsychol 2008;2:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Strong MJ, Abrahams S, Goldstein LH, et al. Amyotrophic lateral sclerosis‐frontotemporal spectrum disorder (ALS‐FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener 2017;18:153–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Strong MJ, Grace GM, Freedman M, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2009;10:131–146. [DOI] [PubMed] [Google Scholar]
- 42. Dobson‐Stone C, Hallupp M, Bartley L, et al. C9ORF72 repeat expansion in clinical and neuropathologic frontotemporal dementia cohorts. Neurology 2012;79:995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schofield EC, Halliday GM, Kwok J, et al. Low serum progranulin predicts the presence of mutations: a prospective study. J Alzheimers Dis 2010;22:981–984. [DOI] [PubMed] [Google Scholar]
- 44. Stanford PM, Shepherd CE, Halliday GM, et al. Mutations in the tau gene that cause an increase in three repeat tau and frontotemporal dementia. Brain 2003;126:814–826. [DOI] [PubMed] [Google Scholar]
- 45. Bell V, Halligan PW, Ellis HD. The Cardiff Anomalous Perceptions Scale (CAPS): a new validated measure of anomalous perceptual experience. Schizophr Bull 2006;32:366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Antony MM, Bieling PJ, Cox BJ, et al. Psychometric properties of the 42‐item and 21‐item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychol Assess 1998;10:176. [Google Scholar]
- 47. Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM‐III‐R criteria. Schizophr Bull 1991;17:555. [DOI] [PubMed] [Google Scholar]
- 48. Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation‐maximization algorithm. IEEE Trans Med Imaging 2001;20:45–57. [DOI] [PubMed] [Google Scholar]
- 49. Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal 2001;5:143–156. [DOI] [PubMed] [Google Scholar]
- 50. Johansen‐Berg H, Behrens TEJ, Sillery E, et al. Functional‐anatomical validation and individual variation of diffusion tractography‐based segmentation of the human thalamus. Cereb Cortex 2005;15:31–39. [DOI] [PubMed] [Google Scholar]
- 51. Gellersen HM, Guo CC, O’Callaghan C, et al. Cerebellar atrophy in neurodegeneration—a meta‐analysis. J Neurol Neurosurg Psychiatry 2017;88:780–788. [DOI] [PubMed] [Google Scholar]
- 52. Williams KL, Fifita JA, Vucic S, et al. Pathophysiological insights into ALS with C9ORF72 expansions. J Neurol Neurosurg Psychiatry 2013;84:931–935:jnnp‐2012‐304529. [DOI] [PubMed] [Google Scholar]
- 53. Snowden JS, Harris J, Richardson A, et al. Frontotemporal dementia with amyotrophic lateral sclerosis: a clinical comparison of patients with and without repeat expansions in C9orf72. Amyotroph Lateral Scler Frontotemporal Degener 2013;14:172–176. [DOI] [PubMed] [Google Scholar]
- 54. Mioshi E, Lillo P, Yew B, et al. Cortical atrophy in ALS is critically associated with neuropsychiatric and cognitive changes. Neurology 2013;80:1117–1123. [DOI] [PubMed] [Google Scholar]
- 55. Mioshi E, Caga J, Lillo P, et al. Neuropsychiatric changes precede classic motor symptoms in ALS and do not affect survival. Neurology 2014;82:149–155. [DOI] [PubMed] [Google Scholar]
- 56. Garety PA, Kuipers E, Fowler D, et al. A cognitive model of the positive symptoms of psychosis. Psychol Med 2001;31:189–195. [DOI] [PubMed] [Google Scholar]
- 57. van Hooren S, Versmissen D, Janssen I, et al. Social cognition and neurocognition as independent domains in psychosis. Schizophr Res 2008;103:257–265. [DOI] [PubMed] [Google Scholar]
- 58. Atassi N, Cook A, Pineda CM, et al. Depression in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2011;12:109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Roos E, Mariosa D, Ingre C, et al. Depression in amyotrophic lateral sclerosis. Neurology 2016;86:2271–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Caga J, Ramsey E, Hogden A, et al. A longer diagnostic interval is a risk for depression in amyotrophic lateral sclerosis. Palliat Supportive Care 2015;13:1019–1024. [DOI] [PubMed] [Google Scholar]
- 61. Broome MR, Woolley JB, Tabraham P, et al. What causes the onset of psychosis? Schizophr Res 2005;79:23–34. [DOI] [PubMed] [Google Scholar]
- 62. Kawamura Y, Dyck PJ, Shimono M, et al. Morphometric comparison of the vulnerability of peripheral motor and sensory neurons in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 1981;40:667–675. [DOI] [PubMed] [Google Scholar]
- 63. Pugdahl K, Fuglsang‐Frederiksen A, de Carvalho M, et al. Generalised sensory system abnormalities in amyotrophic lateral sclerosis: a European multicentre study. J Neurol Neurosurg Psychiatry 2007;78:746–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Weil RS, Schrag AE, Warren JD, et al. Visual dysfunction in Parkinson’s disease. Brain 2016;139:2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Diederich NJ, Goetz CG, Raman R, et al. Poor visual discrimination and visual hallucinations in Parkinson's disease. Clin Neuropharmacol 1998;21:289–295. [PubMed] [Google Scholar]
- 66. Shine JM, O’Callaghan C, Halliday GM, Lewis SJ. Tricks of the mind: visual hallucinations as disorders of attention. Prog Neurogibol 2014;116:58–65. [DOI] [PubMed] [Google Scholar]
- 67. Ahmed RM, Devenney EM, Irish M, et al. Neuronal network disintegration: common pathways linking neurodegenerative diseases. J Neurol Neurosurg Psychiatry 2016;87:1234–1241:jnnp‐2014‐308350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Butler PD, Javitt DC. Early‐stage visual processing deficits in schizophrenia. Curr Opin Psychiatry 2005;18:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Butler PD, Zemon V, Schechter I, et al. Early‐stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry 2005;62:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cadenhead KS, Serper Y, Braff DL. Transient versus sustained visual channels in the visual backward masking deficits of schizophrenia patients. Biol Psychiat 1998;43:132–138. [DOI] [PubMed] [Google Scholar]
- 71. Hailstone JC, Ridgway GR, Bartlett JW, et al. Voice processing in dementia: a neuropsychological and neuroanatomical analysis. Brain 2011;134:2535–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fletcher PD, Downey L, Witoonpanich P, Warren J. The brain basis of musicophilia: evidence from frontotemporal lobar degeneration. Front Psychol 2013;4:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Omar R, Mahoney CJ, Buckley AH, Warren JD. Flavour identification in frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry 2013;84:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Luzzi S, Snowden JS, Neary D, et al. Distinct patterns of olfactory impairment in Alzheimer's disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia 2007;45:1823–1831. [DOI] [PubMed] [Google Scholar]
- 75. Rami L, Loy CT, Hailstone J, Warren JD. Odour identification in frontotemporal lobar degeneration. J Neurol 2007;254:431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hawkes C, Shephard B, Geddes J, et al. Olfactory disorder in motor neuron disease. Exp Neurol 1998;150:248–253. [DOI] [PubMed] [Google Scholar]
- 77. McDonald C, Bullmore ED, Sham P, et al. Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: computational morphometry study. Br J Psychiatry 2005;186:369–377. [DOI] [PubMed] [Google Scholar]
- 78. Bocchetta M, Gordon E, Cardoso MJ, et al. Thalamic atrophy in frontotemporal dementia ‐ not just a C9orf72 problem. Neuroimage Clin 2018;18:675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tu S, Menke RAL, Talbot K, et al. Regional thalamic MRI as a marker of widespread cortical pathology and progressive frontotemporal involvement in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2018;89:1250–1258. [DOI] [PubMed] [Google Scholar]
- 80. Bocchetta M, Iglesias JE, Neason M, et al. Thalamic nuclei in frontotemporal dementia: mediodorsal nucleus involvement is universal but pulvinar atrophy is unique to C9orf72. Hum Brain Mapp 2020;41:1006–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Byne W, Hazlett EA, Buchsbaum MS, Kemether E. The thalamus and schizophrenia: current status of research. Acta Neuropathol 2009;117:347. [DOI] [PubMed] [Google Scholar]
- 82. Münkle M, Waldvogel H, Faull R. The distribution of calbindin, calretinin and parvalbumin immunoreactivity in the human thalamus. J Chem Neuroanat 2000;19:155–173. [DOI] [PubMed] [Google Scholar]
- 83. Danos P, Baumann B, Bernstein H‐G, et al. Schizophrenia and anteroventral thalamic nucleus: selective decrease of parvalbumin‐immunoreactive thalamocortical projection neurons. Psychiatry Res 1998;82:1–10. [DOI] [PubMed] [Google Scholar]
- 84. Byne W, Kidkardnee S, Tatusov A, et al. Schizophrenia‐associated reduction of neuronal and oligodendrocyte numbers in the anterior principal thalamic nucleus. Schizophr Res 2006;85:245–253. [DOI] [PubMed] [Google Scholar]
- 85. Shergill SS, Brammer MJ, Williams SC, et al. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry 2000;57:1033–1038. [DOI] [PubMed] [Google Scholar]
- 86. Barta PE, Pearlson GD, Powers RE, et al. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry 1990;147:1457–1462. [DOI] [PubMed] [Google Scholar]
- 87. Modinos G, Costafreda SG, van Tol M‐J, et al. Neuroanatomy of auditory verbal hallucinations in schizophrenia: a quantitative meta‐analysis of voxel‐based morphometry studies. Cortex 2013;49:1046–1055. [DOI] [PubMed] [Google Scholar]
- 88. Allen P, Larøi F, McGuire PK, Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev 2008;32:175–191. [DOI] [PubMed] [Google Scholar]
- 89. Allen P, Modinos G, Hubl D, et al. Neuroimaging auditory hallucinations in schizophrenia: from neuroanatomy to neurochemistry and beyond. Schizophr Bull 2012;38:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Questions related to each sensory domain.
Table S2. Demographic details for C9orf72 carriers, non‐carriers and controls.
Table S3. Psychosocial factors across C9orf72 carriers, non‐carriers and controls.


