Abstract
The objective of this pilot study was to assess a 2‐year change in innate immune burden in 15 progressive multiple sclerosis (MS) patients using PK11195‐PET. Sixteen age‐matched healthy controls (HC) were included for baseline comparison. PK11195 uptake was higher in MS patients compared to HC within normal‐appearing white matter (NAWM) and multiple gray matter regions. In patients, PK11195 uptake increased in NAWM (p = 0.01), cortex (p = 0.04), thalamus (p = 0.04), and putamen (p = 0.02) at 12 months. Among patients remaining at 24 months, there was no further increase in PK11195. Our data suggest that innate immune activity may increase over time in patients with progressive MS.
Introduction
Focal inflammatory demyelinating lesions are the predominant pathological findings in the patients with relapsing‐remitting multiple sclerosis (MS) whereas diffuse and widespread axonal injury with microglial activation can be found in post‐mortem studies of progressive disease. 1 The primary innate immune cells in MS consist of infiltrating macrophages/monocytes and resident microglia, which can function to cause central nervous system (CNS) injury in MS both through direct effects on neighboring cells, such as oligodendrocytes, and through the generation of soluble proinflammatory mediators that have distant effects on cells, such as neurons. 2
Positron emission tomography imaging (PET) in combination with the ligand 11C‐PK11195 (PKPET) binds to the 18 kDa translocator protein (TSPO) is used to evaluate activated microglia/macrophages in vivo. 3 In this study, we will utilize PKPET to quantify the change in innate immune inflammatory burden in a cohort of progressive multiple sclerosis patients and hypothesize this activity increases over time.
Methods
This was a pilot study designed to determine baseline and longitudinal regional‐specific change in the uptake of PKPET in patients with progressive patients. Eighteen patients with either secondary progressive (SP) or primary progressive (PP) MS and were on stable disease‐modifying therapy (DMT) for at least 6 months were enrolled in the 2‐year longitudinal PKPET study. The design of the study included MRI, PKPET, and expanded disability status score (EDSS) at baseline, 6, 12, and 24 months (mo). Three patients withdrew consent prior to baseline PET scans. Full details regarding the demographics and clinical characteristics of the 15 subjects that remained in the study and considered in this analysis are provided in the Supplemental Table S1. Nine patients had SPMS and six had PPMS. The mean age of the patients was 55 ± 11 years, mean disease duration was 18.24 ± 11.85 years, and mean baseline EDSS score is 5.1 ± 1.9. Sixteen age‐matched healthy controls (HC) were imaged to compare to patients’ baseline PKPET scans. This study was approved by our local institutional review board and written informed consent was obtained.
Radiopharmaceutical
(R)‐[N‐methyl‐11C]PK11195 was prepared by modifying previously reported procedures. 4 All doses were diluted in saline for a final volume of 10 mL and infused over 60 s with an automated pump in a “slow bolus” paradigm.
Image acquisition and processing
All PET images were acquired with the same LSO time‐of‐flight whole‐body PET scanner (mCT, Siemens/CTI). PET images were corrected for photon absorption and scatter using an in‐line CT scanner set at 120 kV, a pitch of 1.5, and 30 mA, and for random using the delayed coincidence window subtraction method. PET data were reconstructed in a 400 × 400 matrix with a voxel size of 1.082 × 1.082 × 2.025 mm3 using a zoom of 2.0 and an iterative‐plus‐time of flight (+ TOF) list‐mode reconstruction algorithm provided by the manufacturer using OSEM methods with 4 iterations and 21 ordered subsets. Tissue concentrations were estimated by reconstructing data into 22 frames (4 frames of 15 s (s) each, then 4 × 30 s, 3 × 60 s, 2 × 120 s, 8 × 300 s, and 1 × 600 s) for a total scan time of 60 min.
Volumes of interests (VOIs)
Automated brain segmentation was performed on magnetization‐prepared rapid gradient‐echo (MPRAGE), acquired on 3.0T Siemens Skyra, using FreeSurfer v8.0 (Martinos Center for Biomedical Imaging, Charlestown, Massachusetts). Summed PET images were coregistered to their corresponding baseline MRI scans using rigid registration with mutual information in PMOD® (PMOD Technologies Ltd., Zurich Switzerland). Standard FreeSurfer templates for cerebral VOIs, including cortical gray matter (GM), thalamus (TH), caudate (CAU), putamen (PU), hippocampus (HIPP), and amygdala (AMG) were coregistered to the PET images. Additional VOIs included normal‐appearing white matter (NAWM), whole lesion masks were also coregistered to the PET images.
The distribution volume ratio (DVR) was calculated using the reference Logan graphical model (RLGM). 5 A computational supervised clustering methodology (SuperPK) was used for extracting a reference curve on PK‐PET. 6
Statistical analysis
ANCOVA was used to estimate the mean change in DVR from baseline to HC. A separate two‐way repeated‐measures ANOVA test was used to compare the mean change in DVR from baseline to each longitudinal time point within various VOIs, controlling age. p < 0.05 was considered statistically significant.
Results
Fifteen patients with progressive MS completed baseline and 6‐month PKPET and 14 patients completed a third PKPET scan at 12 months. Only 10 patients completed the full study duration of 24 months. The reasons for discontinuation included patient preference, lost to follow‐up, interruption of research activities due to the COVID‐19 pandemic, and lastly due to PET data analysis failure (one subject).
The table presents the DVR for patients as compared to age‐matched HC and the longitudinal change among MS patients. Multiple VOIs demonstrated an increase in PK11195 uptake among all MS patients, which included NAWM (p = 0.0417), thalamus (p = 0.021), putamen (p = 0.005), hippocampus (p = 0.005), and amygdala (p = 0.0001), as compared to age‐matched HC at baseline (Fig. 1). The lesioned white matter showed the lowest uptake and was significantly lower than HC white matter. PK uptake in NAWM within PPMS patients was higher than HC but was not significant (p = 0.14) and lesion uptake for SPMS was similar to HC white matter (p = 0.19). Although there was no significant change in PK11195 uptake at 6 months, over the course of 12 months, the PK11195 uptake significantly increased in NAWM (p = 0.01), cortex (p = 0.04), thalamus (p = 0.04), and the putamen (p = 0.02) (Table 1 and Fig. 2). The increase in PK11195 appeared to plateau at 24 months, although the sample size had significantly decreased. There was no significant change in EDSS at 12 months (p = 0.66) or 24 months (p = 0.86) from baseline.
Figure 1.
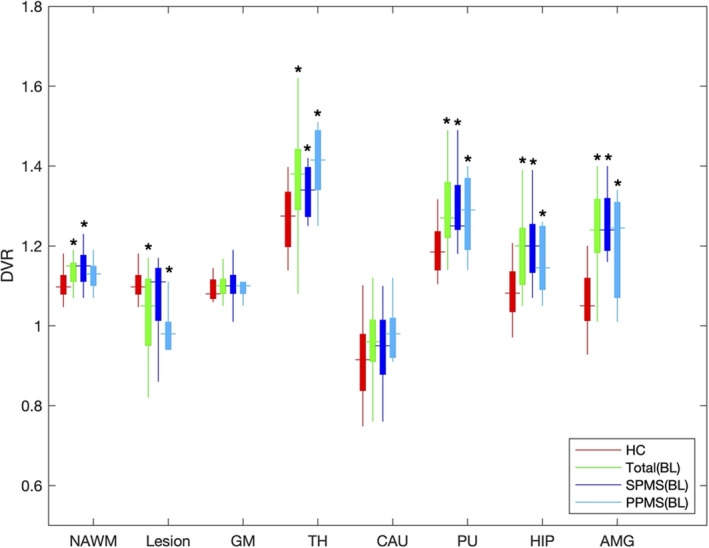
Comparison with HC (n = 16) and MS baseline (n = 15) in DVR. PK11195 PET DVR among in VOIs in healthy control (HC) as compare to MS patients at baseline scan. Nine secondary progressive (SP) and six primary progressive (PP) patients included. VOIs included normal‐appearing white matter (NAWM), whole lesion mask (Lesion), cortical gray matter (GM), thalamus (TH), caudate (CAU), putamen (PU), hippocampus (HIPP), and amygdala (AMG). *p < 0.05.
Table 1.
Baseline and longitudinal PK11195 uptake.
|
DVR values (mean ± SD) |
NAWM | Lesion | GM | TH | CAU | PU | HIPP | AMG |
|---|---|---|---|---|---|---|---|---|
|
BL (n = 15) |
1.15 ± 0.06 | 1.03 ± 0.11 | 1.10 ± 0.04 | 1.37 ± 0.13 | 0.96 ± 0.10 | 1.29 ± 010 | 1.19 ± 0.10 | 1.23 ± 0.10 |
|
6 mo (n = 15) |
1.15 ± 0.07 | 0.99 ± 0.19 | 1.11 ± 0.04 | 1.39 ± 0.11 | 0.95 ± 0.13 | 1.30 ± 0.10 | 1.20 ± 0.11 | 1.22 ± 0.11 |
| 12 mo (n = 14) | 1.16 ± 0.06 | 1.01 ± 0.13 | 1.12 ± 0.03 | 1.41 ± 0.11 | 0.96 ± 0.13 | 1.30 ± 0.09 | 1.20 ± 0.10 | 1.23 ± 0.11 |
| 24 mo (n = 10) | 1.16 ± 0.07 | 1.01 ± 0.14 | 1.12 ± 0.05 | 1.39 ± 0.14 | 0.97 ± 0.11 | 1.30 ± 0.11 | 1.21 ± 0.12 | 1.23 ± 0.11 |
|
HC (n = 16) |
1.1 ± 0.04 | N/A | 1.07 ± 0.11 | 1.27 ± 0.08 | 0.91 ± 0.10 | 1.19 ± 0.07 | 1.09 ± 0.07 | 1.07 ± 0.08 |
| P values | ||||||||
| BL versus HC | 0.05 | 0.01 | 0.41 | 0.03 | 0.11 | 0.01 | 0.01 | 0.00 |
| BL versus 6 mo | 0.55 | 0.13 | 0.40 | 0.27 | 0.36 | 0.56 | 0.80 | 0.37 |
| BL versus 12 mo | 0.01 | 0.48 | 0.04 | 0.04 | 0.53 | 0.02 | 0.36 | 0.89 |
| BL versus 24 mo | 0.49 | 0.88 | 0.21 | 0.43 | 0.96 | 0.53 | 0.34 | 0.50 |
| 12mo versus 24 mo | 0.10 | 0.43 | 0.70 | 0.72 | 0.45 | 0.89 | 0.52 |
0.97 |
Abbreviations: DVR, distribution volume ratio, BL, baseline for MS patients, mo, month, SD, standard deviation, HC, healthy control, NAWM, normal‐appearing white matter, Lesion, whole lesion mask, GM, cortical gray matter, TH, thalamus, CAU, caudate, PU, putamen, HIPP, hippocampus, AMG, amygdala.
Figure 2.
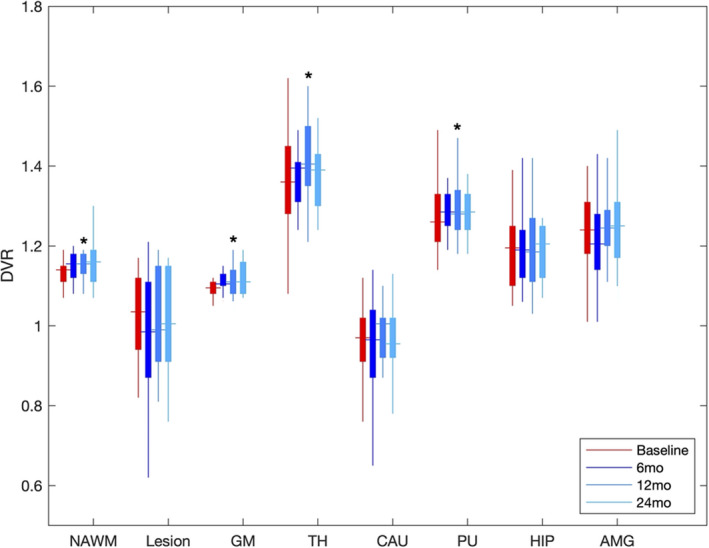
Longitudinal change in PK11195 in progressive MS patients. Longitudinal change in PK11195 PET DVR over the course of 6 (n = 15), and 12 months (n = 14) and 24 months (n = 10). VOIs include normal‐appearing white matter (NAWM), whole lesion mask (Lesion), cortical gray matter (GM), thalamus (TH), caudate (CAU), putamen (PU), hippocampus (HIPP), and amygdala (AMG). *p < 0.05.
Discussion
In this pilot study of progressive MS patients, we were able to demonstrate multiple regions with a significant increase in PK11195 uptake as compared to age‐matched HC. These results confirm previously published studies that demonstrate an increased TSPO PET uptake in NAWM and thalamus. 7 This study demonstrates for the first time that an increase in TSPO uptake, representing increased microglia/macrophage activity, occurred within NAWM, thalamus, cortical GM, and within the putamen over the course of 12 months.
Diffuse neuronal loss predominates the progressive stages of MS, 8 however, evidence exists that this process occurs early on in the disease, and generally follows a pattern, starting with thalamic volume loss followed by cortical neuronal loss. The main pathological mechanism, driving neuronal degeneration, is felt to be secondary to chronically demyelinated axons and leads to axonal instability. 9 , 10 Understanding the extent that diffuse innate immune activity drives neuronal degeneration remains undefined. PK11195‐PET imaging is a well‐established method to assess CNS inflammation and has been used to investigate changes in the innate immune system in the cortex of MS patients, 11 deep gray matter (i.e. thalamus) 7 as well as in NAWM of patients with clinically isolated syndrome. 12 These studies describe cross‐sectional findings, but longitudinal analyses of inflammatory changes in progressive MS patients have yet to be reported. Importantly, none of these studies, including the current study, reach a sample size to explore the relationship of innate immune activity with a structural loss on MRI, such as cortical thinning or thalamic atrophy.
In a recent study of a relatively large cohort of both relapsing and secondary progressive MS patients, it was demonstrated that baseline TSPO uptake in NAWM was predictive of disease progression at 4 years. 13 Moreover, it was found that perilesional white matter PK uptake related to disease progression in patients without relapses. Although our cohort did not experience disease progression over the course of 12 months, our results suggest a diffuse and ongoing process in the white matter. Taken together, this suggests that targeting a reduction in diffuse inflammation may provide a novel therapeutic strategy to decrease disease progression.
This is a pilot study and has significant limitations. In our cohort, we were unable to extract a reference region curve for one subject using SuperPK. Although the reason for the failure of the clustering algorithm was unclear, we hypothesize that the kinetic class used for the reference region was ill‐defined; this may occur if there is widespread activation, wherein fewer and fewer voxels are included in the reference region time–activity curve and statistical variation increases. In addition, the increase in PKPET uptake over the course of 24 months did not reach significance, we feel that this is related to the loss of patients. Poor retention in our study was related to various factors, however, given the disability of the cohort, traveling for additional imaging studies is a likely contributing factor, thus minimizing the number of scans of should be considered in designing future PET studies of progressive patients. Last, our lesion analysis was not designed to evaluate the presence of inflammation among individual lesions. Given the lower resolution of PET, accurate detection of PK uptake among smaller lesions is difficult, and unfortunately, a whole lesion approach likely dilutes the potential presence of innate immune activity within individual lesions.
In conclusion, our pilot data suggest that diffuse innate immune activity within the CNS may be increasing over time in patients with progressive multiple sclerosis and could play an active role in the pathogenesis of disease progression. Our data support larger studies dedicated to further explore this pathway and evaluate the association with clinical disability.
Conflict of Interest
YK, SP, NM, NZ have nothing to disclose. SG reports grants from Genzyme, during the conduct of the study; grants and personal fees from Genentech, personal fees from Biogen, grants from Mallinckrodt, outside the submitted work.
Supporting information
Table S1. Baseline clinical characteristics of patients.
Acknowledgments
The authors are grateful to the professional staff at the Citigroup Biomedical Imaging Center of Weill Cornell Medical College, including, John W. Babich PhD, Jonathan Dyke PhD, Simon Morim, Amelia Ng, and Nelsie Pastrano‐Redula. The authors would also like to acknowledge the help of Drs. Timothy Vartanian, Nancy Nealon, Ulrike Kaunzner, and Jai Perumal at the Weill Cornell Multiple Sclerosis Center, Department of Neurology, New York, NY. Last, we would like to thank the patients at the MS center. This research was supported by Sanofi‐Genzyme.
Funding Statement
This work was funded by Sanofi‐Genzyme.
References
- 1. Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005;128(Pt 11):2705–2712. [DOI] [PubMed] [Google Scholar]
- 2. Chitnis T, Weiner HL. CNS inflammation and neurodegeneration. J Clin Invest 2017;127(10):3577–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banati RB, Newcombe J, Gunn RN, et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain 2000;123(Pt 11):2321–2337. [DOI] [PubMed] [Google Scholar]
- 4. Kang Y, Schlyer D, Kaunzner UW, et al. Comparison of two different methods of image analysis for the assessment of microglial activation in patients with multiple sclerosis using (R)‐[N‐methyl‐carbon‐11]PK11195. PLoS One 2018;13(8):e0201289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Logan J, Fowler JS, Volkow ND, et al. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 1996;16(5):834–840. [DOI] [PubMed] [Google Scholar]
- 6. Yaqub M, van Berckel BN, Schuitemaker A, et al. Optimization of supervised cluster analysis for extracting reference tissue input curves in (R)‐[(11)C]PK11195 brain PET studies. J Cereb Blood Flow Metab 2012;32(8):1600–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rissanen E, Tuisku J, Rokka J, et al. In vivo detection of diffuse inflammation in secondary progressive multiple sclerosis using PET imaging and the radioligand (1)(1)C‐PK11195. J Nucl Med 2014;55(6):939–944. [DOI] [PubMed] [Google Scholar]
- 8. Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol 2007;17(2):210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schirmer L, Antel JP, Bruck W, Stadelmann C. Axonal loss and neurofilament phosphorylation changes accompany lesion development and clinical progression in multiple sclerosis. Brain Pathol 2011;21(4):428–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998;338(5):278–285. [DOI] [PubMed] [Google Scholar]
- 11. Politis M, Giannetti P, Su P, et al. Increased PK11195 PET binding in the cortex of patients with MS correlates with disability. Neurology 2012;79(6):523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giannetti P, Politis M, Su P, et al. Increased PK11195‐PET binding in normal‐appearing white matter in clinically isolated syndrome. Brain 2015;138(Pt 1):110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sucksdorff M, Matilainen M, Tuisku J, et al. Brain TSPO‐PET predicts later disease progression independent of relapses in multiple sclerosis. Brain 2020;143(11):3318–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline clinical characteristics of patients.


