Abstract
Circulating alloantibodies in transplant recipients are often associated with increased antibody-mediated as well as cellular rejection. We tested the hypothesis that alloantibodies facilitate cellular rejection by functioning as opsonins to enhance T cell activation using a BALB/c to C57BL/6 heart or skin transplant model. Long-term heart and skin survival induced with anti-CD154 alone or in combination with donor-specific transfusion (DST), respectively, was abrogated by the presence of anti-Kd mAbs, and alloreactive T cell-activation as well as acute rejection was observed. The prevention of graft acceptance in the skin model was dependent on anti-Kd binding to and converting DST from tolerigenic to immunogenic. Adoptive transfer of CFSE-labeled TCR-Tg T cells into B6 recipients treated with anti-CD154/DST revealed the ability of anti-Kd to enhance the proliferation of anti-Kd-specific T cells via the indirect pathway, as well as of non-Kd reactive, recipient MHC-restricted CD4+ and CD8+ T cells. Thus alloantibodies with restricted specificity are able to facilitate the indirect presentation as well as the cross-presentation of a larger repertoire of ‘linked’ donor-derived antigens. These observations highlight the ability of alloantibodies to function not only in classical humoral rejection but also as opsonins that facilitate the CD40-CD154-independent activation of alloreactive T cells.
Keywords: rodent, T cells, alloantibodies, transplantation
Introduction
Tolerance induction remains elusive in larger recipients including non-human primates and humans, in spite of success in rodent models. One explanation is that pre-transplant patients have high frequencies of memory alloreactive T cells that are resistant to tolerance induction (1, 2). These allo-reactive T cells can be generated by allo-sensitization as a result of previous pregnancies, blood transfusions, or transplants. In addition, responses to pathogens often result in the sensitization of alloreactive T cells by cross-reactivity, and the cumulative lifetime exposure to pathogens can result in a high frequency of memory alloreactive T cells. Indeed deliberate sensitization or infection of rodent recipients, resulting in high frequencies of memory alloreactive CD4+ and CD8+ T cells, leads to an acquired resistance to tolerance induction (3-6). In addition, we recently reported that memory alloreactive B cells alone, or in combination with alloantibodies, prevented the acquisition tolerance in anti-CD154-treated recipients (7). Thus the presence of memory B cells and alloantibodies, or T cells, can independently resist tolerance induction.
Alloantibodies in the clinical setting are strongly correlated with the occurrence of hyperacute, acute antibody-mediated (AAMR), antibody-mediated cellular and chronic rejection (AACR) (8-11). The importance of antibodies, upstream of complement activation, in precipitating acute rejection was suggested by Wasowska et al. in an experimental model with B-cell deficient recipients that exhibited delayed rejection of allogeneic hearts (12). Acute rejection was restored to normal kinetics by the administration of complement-activating alloreactive mAbs, but not non-complement-activating mAbs (13, 14). The mechanistic basis for these observations were postulated to be alloantibodies binding to vascular endothelium and stimulating the local production chemokines such as monocyte chemotactic protein 1 (MCP-1) and neutrophil chemoattractant growth-related oncogene alpha (KC), which attract effector cells including macrophages, monocytes, basophils, neutrophils and T cells into the graft to mediate acute rejection. These and other similar observations provided the mechanistic basis for the clinical use of C4d deposition as a marker of antibody-deposition and antibody-mediated allograft rejection (15-18)
In addition to the established mechanisms of antibodies binding to graft endothelium to induce allograft rejection, alloantibodies may also be able to contribute to acute allograft rejection by facilitating T cell activation. In particular, we hypothesize that alloantibodies promote allospecific T cell priming through the generation of opsonized donor cells, which are more efficiently taken up and presented by antigen-presenting cells (APC). In this study, we used anti-CD154 and DST to induce long-term allogeneic skin survival and anti-H-2Kd mAbs to test this hypothesis and to define the mechanism by which alloantibodies can prevent the induction of graft acceptance by anti-CD154-based therapies.
MATERIALS AND METHODS
Mice
Male and female C57BL/6 (B6; H-2b), BALB/c (H-2d), and C3H (H-2K) mice, aged 7-9 weeks, were purchased from The Jackson Laboratories (Bar Harbor, ME) and The National Cancer Institute, Frederick Cancer Research and Development Center (Frederick, MD). Rag−/−B6 (B6.129P372-Rag1/Rag2tm1Mnz/J), TCRβδ−/− B6 (B6.129P2-Tcrbtm1MomTcrdtm1Mom/J), Act-OVA B6 (C57BL/6-Tg (CAG-OVA) 916Jen/J), OT-I B6 (C57BL/6-Tg (TcraTcrb) 1100Mjb/ /J), and OT-II B6 (C57BL/6-Tg (TcraTcrb) 425Cbn/J) mice were purchased from The Jackson Laboratories (Bar Harbor, ME). Female C57BL/6Ji-Kbtm1N12 mice (Kb−/−) were purchased from Taconic (Germantown, NY) at 6 weeks of age. Act-OVA BALB/c mice were a generous gift from Dr. Marisa Alegre (The University of Chicago), the TCR75 CD4+ TCR Tg mice were from Dr. R. Pat Bucy (The University of Alabama at Birmingham), the Kd.B6 mice were from Dr. Sasha Chervonsky (The University of Chicago) and the CD8−/− B6 mice were from Dr. Yang-Xin Fu (The University of Chicago). All animals were maintained and bred in the pathogen-free Carlson Barrier animal facility at The University of Chicago. The use of mice for these studies are summarized in Table 1 and have been reviewed and approved by The University of Chicago Institutional Animal Care and Use Committee.
table I.
List of mice used.
| Mouse Strain | Experimental Purpose | Figure/Table Used |
|---|---|---|
| BALB/c | Donor | Figures 1, 2,3,5 |
| C57BL/6 | Recipient | Figures 1, 2,3,4,5 Tables I, II |
| C3H | Donor | Figures 1 Tables I, II |
| TCRβδ−/− | Recipient, test the requirement of T cells | Figure 2 |
| CD8−/− | Recipient, test the requirement of CD8+ T cells | Figure 2 |
| Kd.C57BL/6 (Kd Tg) (Kd.B6) | Used for crossing to other strains | Table I, II |
| C3H x Kd.C57BL/6 F1 (Kd.H-2bxk) | Donor, test the importance of Kd expression on DST | Table I, II |
| C3H x C57BL/6 F1 (H-2bxk) | Donor, test the importance of Kd expression on DST; control | Table I, II |
| TCR75 TCR TG | Used CD4+ T cells for adoptive transfer | Figure 3 |
| Act-Ova C57BL/6 | Donor, expresses ovalbumin as a transmembrane protein under the actin promoter | Figure 4 |
| OT-I C57BL/6 | Used CD8+ T cells for adoptive transfer | Figure 4 |
| OT-II C57BL/6 | Used CD4+ T cells for adoptive transfer | Figure 4 |
Skin Transplantation
Donor tail skin was removed by carefully using sterile forceps, placed in a petri dish containing a PBS-soaked gauze and cut into 2cm2 square pieces. Donor tail skin was placed on the recipient bed and secured with 7-0 nylon sutures, then two ¾”x 3” adhesive band-aids were wrapped around the recipient trunk to preserve graft integrity during healing. In some experiments, 200 μl/mouse of sera from naïve or pre-sensitized B6 mice (immunized with 107 BALB/c spleen cells i.p. at day −14) were injected intra-venously (i.v.) into recipients on days −2, 0 and 2 post-transplantation of BALB/c skin into B6 recipients treated with anti-CD154 and DST. In other groups, 0.5mg anti-Kd IgG2a (ATCC HB159, BioXCell, West Lebanon, NH) or isotype control IgG2a (ATCC C1.18.4, BioXCell, West Lebanon, NH) mAbs were i.v., on the day of transplantation. On day seven post-transplantation, the band-aids were removed and skin grafts monitored. Rejection was defined as >90% scarring of the skin graft. In some experiments, CFSE-labeled TCR75, OTI, and OT II TCR Tg T cells (0.25-1 x 106) were transferred by intravenous (i.v.) injection in B6 recipients, immediately post-skin transplantation. Animals were sacrificed on day five for analysis of CFSE dilution by flow cytometry.
Heterotopic Heart Transplantation
Heart transplantation was performed as previously described (19). Briefly, the donor aorta and pulmonary artery were anastomosed to the recipient abdominal aorta and inferior vena cava respectively. Graft function was monitored by abdominal palpation daily until rejection, which was defined as total cessation of contractions and was confirmed by direct visualization of the allograft.
Costimulatory Blockade Regimen
Donor splenocytes (DST) was prepared by passing whole splenocyte preparations through a sterile 0.2μm filter and resuspending in PBS, and 2 x 107 cells were injected i.v. on the day of transplantation. Anti-CD154 mAbs were purified from protein-free hybridoma medium (Invitrogen; Carlsbad, CA) using 45% ammonium sulfate precipitation and dialyzed for 48 hours in PBS. 1mg of anti-CD154 mAbs/dose/mouse was injected i.v. on days 0, 7 and 14 post-transplantation. Human CTLA4Ig (huCTLA4Ig; 0.5mg /mouse) (Bristol-Myers Squibb; New York City, NY) was administered daily, by intra-peritoneal (i.p.) injection, for 7 days post-skin transplantation.
Flow Cytometry
PE-conjugated anti-mouse Vβ8.3, PE-conjugated anti-mouseVα2, APC-conjugated anti-mouse CD4, APC-conjugated Strepavidin, APC-conjugated anti-mouse CD19, APC-conjugated anti-mouse Gr-1, APC-conjugated anti-mouse CDllc, APC-conjugated anti-mouse CDllb, PerCP-conjugated anti-mouse CD19, Biotin-conjugated anti-mouse IgM, Biotin-conjugated anti-mouse IgG2a, Biotin-conjugated anti-mouse CD45.1, Biotin-conjugated anti-mouse Vβ5, FITC-conjugated anti-mouse IgG, Pe-Cy7-conjugated anti-mouse CD4, and Pe-Cy7-conjugated anti-mouse CD8 were purchased from BD Biosciences (San Jose, CA). Cells from were labeled on ice for 45 min with appropriate antibodies, followed by two washes in 2% FBS/PBS 0.01% Sodium Azide (FACS buffer). Cells were then resuspended in FACS buffer and analyzed on the FACScanto, or LSRII benchtop analyzers (BD Biosciences) using FlowJo flow cytometry analysis software (Tree Star Inc., Ashland, OR).
IFN-γ ELISPOT
Assays were performed as previously described in detail (20). Briefly, ELISPOT plates (Millipore, Bedford, MA) were coated overnight with anti-IFN-γ mAb (BD Biosciences, San Jose, CA), and then blocked with sterile 10% FBS in PBS (PBST). Responder splenocytes were harvested from transplant recipients, and in some experiments T cells were purified by negative selection using a mouse Pan T cell Isolation kit (Miltenyi Biotec, Auburn, CA). Responders (106/well) were incubated with or without anti-CD3 (clone 2C11), C3H, BALB/c, or B/6 stimulators (0.4 x 106/well; γ-irradiated at 1200 Rads for 10 minutes), and then incubated overnight. Biotinylated anti-IFN-γ detection mAb (BD Biosciences, San Jose, CA) was added followed by horseradish peroxidase (HRP)-conjugated anti-biotin (BD Biosciences). Plates were developed, as previously described, and the resulting spots were analyzed using an Immunospot Series 1 Analyzer (Cellular Technology Ltd., Shaker Heights, OH).
Statistical Analysis
Statistical analysis to determine differences between groups were performed using the Student’s t test for equal or unequal variances using Prism 4 for Macintosh (GraphPad, San Diego, CA). Kaplan Meier survival analysis was performed to determine significant differences in median graft survival between groups. A value of p<0.05 was considered statistically significant.
RESULTS
I. Alloantibodies elicit allograft rejection in anti-CD154 treated recipients.
Our previous findings that low-levels of circulating alloantibodies can synergize with memory B cells to mediate CD154-independent heart allograft rejection (7) prompted us to test whether a higher levels of circulating alloantibodies alone have the same effect. We observed a single dose of 0.5mg anti-Kd mAbs injected on the day of BALB/c cardiac allograft transplantation prevented the induction of long-term acceptance in anti-CD154-treated recipients (Figure 1A). Because cardiac allografts are vascularized with recipient endothelium and are therefore susceptible to antibody-mediated rejection (21), it is possible that the observations of rejection are due, at least in part to antibodies binding to the cardiac allograft. We therefore also tested whether allogeneic skin grafts are similarly affected by anti-Kd mAbs. Untreated B6 recipients rejected BALB/c skin grafts while recipients treated with anti-CD154/DST accepted their grafts long term (MST=60 days) (Figure 1B). Administration of anti-Kd mAbs, but not isotype control mAbs, to anti-CD154/DST-treated recipients on the day of skin transplantation resulted in the acute rejection of the skin allografts (MST=12 days; p=0.0013) (22). To confirm that the rejection was not due to the presence of contaminating endotoxin (23-25), anti-Kd mAbs were denatured by heating for 10 minutes at 100°C and then injected into anti-CD154/DST-treated B6 recipients. Additionally, anti-Kd mAbs injected into B6 recipients of C3H skin grafts (H-2k) treated with anti-CD154/C3H-DST. Under both conditions, all of the skin allografts were accepted long term (MST=60 days). These experiments demonstrate that anti-Kd mAbs can also mediate acute skin allograft rejection in anti-CD154/DST-treated recipients, based on the specific recognition/binding of H-2Kd on donor graft and/or DST, and not through non-antigen-specific effects. Finally we confirmed that the transfer of serum from pre-sensitized mice (200 μl/mouse; day −2, 0 and 2 post-transplantation) but not from naïve mice also prevented BALB/c skin graft acceptance in B6 mice treated with anti-CD154/DST (Figure 1C).
Figure 1.

Anti-Kd mAbs elicits CD154-independent cardiac and skin allograft rejection. a. Rejection of BALB/c heart grafts by B6 recipients treated with anti-CD154 and anti-Kd mAbs (N = 4) ( closed square) compared to B6 recipients treated with anti-CD154 alone (N = 15) (closed triangle) or untreated recipients (N = 4) (open circle). b. Rejection of BALB/c skin grafts by B6 recipients treated with anti-Kd mAbs plus anti-CD154/DST (N = 20) (closed triangle) in comparison to long-term graft survival of BALB/c grafts by B6 recipients treated with anti-CD154/DST (N = 11) (open diamond) and rejecting untreated B6 recipients of BALB/c grafts (N = 9) (closed square). Acceptance of BALB/c grafts by B6 recipients treated with anti-CD154/DST and denatured anti-Kd mAbs (N = 5) (closed circle), or mouse IgG isotype control (N = 4) (asterisk), and C3H grafts by B/6 recipients treated with anti-Kd mAbs and anti-CD154/DST (N = 4) (half closed downward triangle). c. The transfer of serum from pre-sensitized mice (200 μl/mouse; day −2, 0 and 2 post-transplantation; Pre-Sen), but not from naïve mice, prevented BALB/c skin graft acceptance in B6 mice treated with anti-CD154/DST (N=4/group).
II. Alloantibody-mediated rejection is T cell dependent.
The observations that anti-Kd mAbs could override the tolerigenic effects of anti-CD154 ± DST to elicit acute graft rejection in both heart and skin models were consistent with the hypothesis that anti-Kd mAbs can function as opsonins to enhance alloreactive T cell activation and T cell-dependent rejection. Such a hypothesis would predict increased frequencies of alloreactive IFNγ-producing cells in B6 recipients treated with anti-CD154/DST and anti-Kd mAbs compared to those not receiving anti-Kd mAbs. Indeed, we observed B6 cardiac or skin recipients treated anti-CD154±DST and anti-Kd mAbs had an increased frequency of primed BALB/c-specific IFN-γ-producing cells compared to recipients that did not receive anti-Kd mAbs (Figure 2A & 2B). These data collectively support the conclusion that anti-Kd mAbs are able to override the immunosuppressive effects of anti-CD154 ± DST to facilitate the priming of allospecific T cells that mediate allograft rejection.
Figure 2.
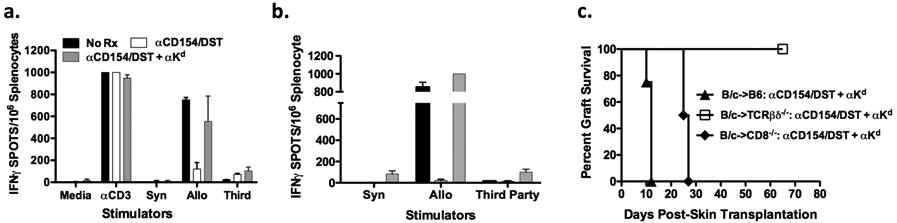
Role of T cells in anti-Kd mAb-dependent graft rejection a. Frequencies of alloreactive IFN-γ producing cells in untreated B6 recipients (N = 3) (black), or in recipients of cardiac transplants treated with anti-CD154/DST (N = 4) (white), or anti-CD154/DST with anti-Kd (N = 4) (grey). Splenocytes were stimulated in vitro with irradiated B6 (Syn), BALB/c (Allo), C3H (Third-Party) stimulators, and controls were anti-CD3 or media. Data are presented as mean ± standard error. b. The frequency of alloreactive T cells secreting IFN-γ were measured in untreated B6 recipients (N = 3) (black), anti-CD154/DST treated B6 recipients (N = 3) (white), and anti-CD154/DST with anti-Kd mAbs (N = 4) (grey). c. Acceptance of BALB/c grafts by TCRβδ−/− recipients treated with anti-CD154/DST and anti-Kd mAbs (N = 6) (open square) compared to rejection by similarly treated B6 recipients (N = 20; p = 0.0003) (closed triangle). Comparably treated B6.CD8−/− recipients exhibited delayed rejection (N = 4; p = 0.0027) (closed diamond).
To test the necessity of T cells, TCRβδ−/− mice that lack both αβ and γδ T cell subsets, as well as CD8−/− mice, were used as recipients of BALB/c skin grafts. Long-term graft survival (>80 days) was achieved in the TCRβδ◻/− recipients receiving anti-CD154/DST and anti-Kd mAbs (p=0.0003) (Figure 2C), confirming that the rejection observed in B6 mice was T cell-dependent and that anti-Kd mAb binding to the skin allograft was not sufficient to cause acute rejection in the absence of T cells. Graft rejection was significantly delayed (MST=26 days) (p=0.0027) in CD8−/− recipients compared to WT B6 recipients, suggesting that both CD4+ and CD8+ T cells contribute to anti-Kd-mediated graft rejection. Experiments were not performed in CD4−/− recipients because the presence of CD4+ T cells was essential for anti-CD154-mediated graft acceptance.
III. Anti-Kd mAb recognition of H-2Kd on DST is necessary and sufficient to mediate skin allograft rejection in anti-CD154/DST-treated recipients.
To gain insights into the mechanism by which anti-Kd mAbs prevent the induction of long-term skin allograft acceptance by anti-CD154/DST, we tested whether the anti-Kd mAbs recognized H-2Kd expressed on the graft or on the DST to prevent tolerance induction. To this end we generated F1 C3H x Kd.B6 (Kd.H-2bxk) mice to be used as a source of donor tail skin and DST. Positive controls were anti-Kd mAbs mediating the rejection of F1 C3H x Kd.B6 (Kd.H-2bxk) skin allografts when both skin and DST were from Kd.B6 x C3H F1 (Kd.H-2bxk) (Group I). Negative controls were the acceptance of C3H x B6 F1 (H-2bxk) skin allografts in B6 recipients receiving anti-CD154/H-2bxk DST and anti-Kd mAbs (Table II; Group II). When H-2Kd expression was confined to only the Kd·H-2bxk allograft, and the H-2bxk DST lacked Kd expression, the majority of grafts were accepted (MST>55 days) by recipients receiving anti-CD154/DST and anti-Kd mAbs (Group III). In contrast, when H-2Kd was expressed only on DST, but absent on allograft tissue, rejection was observed in the recipients treated with anti-CD154 and anti-Kd mAbs (Group IV). These observations demonstrate that anti-Kd recognition of H-2Kd on DST, but not on the skin graft, is necessary and sufficient to precipitate CD154-independent skin allograft rejection. To address the concern that rejection induced by the anti-Kd mAbs was due to a non-specific elimination of the DST, we increased the amount of DST administered 4-fold (Group V), which normalized for the amount of DST present after 24 hours in the spleens of B/6 recipients receiving anti-Kd mAbs compared to recipients that did not (data not shown). The kinetics of graft rejection in this group was also similar to B6 recipients receiving the normal dose of DST and anti-Kd mAbs (Group I), suggesting that accelerated clearance of DST is unlikely to be the primary cause of the loss of tolerogenicity.
table II.
Anti-Kd mAb recognition of H-2Kd on DST is necessary and sufficient to mediate skin allograft rejection in recipients treated with anti-CD154/DST
| Group | Graft | DST | Mean ± SEM |
|---|---|---|---|
| I. | Kd .H-2bxk | Kd .H-2bxk | 11.5 ± 0.3 (N=6) |
| II. | H-2bxk | H-2bxk | 60.0 ± 0.0 (N=6) |
| III. | Kd .H-2bxk | H-2bxk | 52.3 ± 7.6 (N=6) |
| IV. | H-2bxk | Kd .H-2bxk | 13.2 ± 0.4 (N=6) |
| V. | H-2d | 4X H-2d | 11.5 ± 0.5 (N=4) |
IV. Anti-Kd mAbs prime Kd-specific T cells as well as T cells that recognize “Linked Antigens” on the DST
We hypothesized that the anti-Kd mAbs converted the DST from tolerigenic to immunogenic, thereby facilitating an anti-CD154-independent activation of graft-reactive T cells. To visualize the fate of graft-reactive T cells in the presence or absence of anti-Kd mAbs in vivo, we performed an adoptive transfer assay, initially with TCR75 CD4+ T cells that specifically recognize the Kd 54-68 peptide presented on I-Ab MHC Class II molecules. 0.25 x 106 CFSE-labeled TCR75 T cells transferred into non-transplanted B6 recipients underwent minimal proliferation, while significant cell proliferation was observed when TCR75 T cells were injected into transplanted but untreated B6 recipients (Figure 3). TCR75 T cell proliferation was significantly reduced in anti-CD154/DST treated-B6 recipients, and anti-Kd mAbs was able to restore T cell proliferation (p=0.0067). These studies confirm that anti-Kd mAbs facilitated the anti-CD154-independent proliferation of Kd-specific T cells in vivo.
Figure 3.

Allospecific TCR75 CD4+ T cells proliferate in the presence of anti-Kd mAbs. CFSE-labeled TCR75 TCR-Tg T cells (0.25 x 106) were injected i.v. at the time of skin transplantation. TCR75 T cells were harvested from the draining lymph nodes five days later and proliferation was measured by flow cytometry. Representative histogram of TCR75 T cell proliferation from untransplanted B6 recipients (naïve B6) ( 1st Panel), B6 recipients of BALB/c grafts without treatment (No Rx) (2nd Panel), treated with anti-CD154/DST (αCD154/DST ) (3rd Panel), or with anti-CD154/DST and anti-Kd mAbs (αCD154/DST + αKd) (4th Panel). The percentage of dividing TCR75Tg T cells from individual mice in each group are summarized (N = 3/ group). Significantly higher percentages of proliferating TCR75 Tg T cells were observed in B6 recipients treated with anti-Kd mAbs and anti-CD154/DST compared to recipients treated with anti-CD154/DST (p = 0.0067).
It is well established that during the course of an immune response to self-antigens, increased diversification of autoreactive specificities arises through a process of epitope spreading (26). To test whether anti-Kd antibodies can promote the priming of donor-reactive T cells that recognize donor antigens not recognized by the anti-Kd mAb, 0.8-1 x 106 CFSE-labeled, OVA-specific CD4+ OT-II T cells were transferred into B6 recipients of Act-OVA BALB/c grafts. OT-II T cells injected into naïve B6 recipients had minimal T cell proliferation, while OT-II T cells displayed robust proliferation upon transfer into untreated B6 recipients of Act-OVA BALB/c grafts (Figure 4). OT-II T cell proliferation was reduced in B6 recipients treated with anti-CD154/DST, and it was restored by the addition of anti-Kd mAbs (p= 0.0015).
Figure 4.
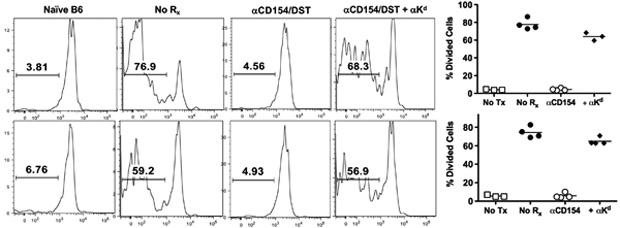
Anti-Kd mAbs primes T cells specific for alloantigens not recognized by anti-Kd mAbs and enhances presentation of exogenously derived alloantigens. All animals were injected with 5uM CFSE labeled 0.8 x 106- 107 OT-II and OT-I TCR Tg T cells at the time of skin transplantation. Actin-OVA BALB/c mice were used as donors for all skin transplants. Flow cytometric analysis of OT-II (top row) and OT-I (bottom row) TCR Tg T cells was measured in the draining lymph nodes five days post-skin transplantation of untransplanted B6 recipients (1st Column), B6 recipients of BALB/c-OVA grafts without treatment (2nd Column), B6 recipients of BALB/c-Ova grafts treated with anti-CD154/DST (3rd Column), and B6 recipients of BALB/c-OVA grafts treated with anti-CD154/DST and anti-Kd mAbs (4th Column). Significantly higher percentages of proliferating cells were observed in B6 recipients treated with anti-Kd mAbs and anti-CD154/DST compared to recipients treated with anti-CD154/DST (for OT-1 p = 0.0001, for OT-II; p = 0.0015).
The observations that CD8+ T cells contribute to the rejection of skin allografts in anti-CD154/DST-treated recipients receiving anti-Kd mAbs (Figure 2C) prompted us to further test whether anti-Kd mAbs can promote the presentation of exogenously derived donor antigens to CD8+ T cells. To this end, 0.8-1 x 106 CFSE-labeled CD8+ OT-I T cells were adoptively transferred into B6 recipients of Act-OVA BALB/c grafts. Minimal OT-I T cell proliferation was observed in non-transplanted B6 recipients, while robust OT-I T cell proliferation was observed in untreated B6 recipients of Act-OVA BALB/c grafts (Figure 4). Anti-CD154/DST inhibited OT-I T cell proliferation while anti-Kd mAbs were able to restore robust OT-I cell proliferation (p=0.001). These observations confirm the ability of anti-Kd mAbs to enhance cross-presentation of graft-derived antigens and induce the proliferation of CD8+ T cells in recipients treated with anti-CD154/DST.
V. Anti-Kd mAbs facilitate the rejection of allografts lacking Kd but expressing “Linked Antigens”
The observation that anti-Kd mAbs enhanced the activation of T cells specific for donor-antigens not recognized by the anti-Kd mAbs, led us to test whether these observations are functionally significant and capable of eliciting the rejection of a second, non-Kd-expressing skin allograft. The experimental approach involved the transplantation of two adjacent allogeneic skin grafts onto B6 recipients. B6 recipients treated with anti-CD154 and anti-Kd mAbs accepted both skin grafts when the grafts and DST were from H-2bxk (B6 x C3H F1) donors, and rejected both grafts when the grafts and DST were from Kd.H-2bxk donors (Table III; Group I and II). When the DST was from Kd. H-2bxk donors and the grafts were from Kd ·H-2b and H-2bxk donors, both grafts were both rejected (Group III). In contrast, when the grafts were from Kd ·H-2b and H-2bxk donors, and the DST was a combination of cells from both donors, only the Kd-expressing graft was rejected (Group IV). Finally when the grafts were from Kd ·H-2bxk or H-2bxk donors, and the DST was a combination of cells from the same two donors, only the Kd-expressing graft was rejected in recipients treated with anti-CD154 (Group V). These observations confirm that linked antigens on the DST were necessary and sufficient for the priming of non-Kd-specific T cells, and that by-stander effects generated by opsonins comprising anti-Kd mAbs and Kd ·H-2b DST did not result in the sufficient priming of H-2k T cell responses to cause the rejection of non-Kd- but H-2k-expressing allogeneic skin grafts.
table III.
Anti-Kd mAbs promote epitope-spreading and cross-presentation of donor alloantigen to alloreactive T cells in vivo.
| Skin Grafts | DST | MST ± SEM |
|---|---|---|
| I. No Kd on DST or Graft | ||
| H-2bxk | H-2bxk | a57.7 ± 1.6 (N=6) |
| H-2bxk | a57.0 ± 1.6 (N=6) | |
| II. Linked Kd on DST and Graft | ||
| Kd .H-2bxk | Kd .H-2bxk | 11.5 ± 0.3 (N=6) |
| Kd .H-2bxk | 11.5 ± 0.3 (N=6) | |
| III. Linked Kd on DST Only | ||
| Kd | Kd .H-2bxk | 11.5 ± 0.5 (N=8) |
| H-2bxk | 12.8 ± 4.3 (N=8) | |
| IV. Linked Kd on Graft Only | ||
| Kd.H-2bxk | ½ Kd + | 11.3 ± 0.3 (N=11) |
| H-2bxk | ½ H-2bxk | b48.3 ± 5.4 (N=11) |
| V. Unlinked Kd on DST and Graft | ||
| Kd | ½ Kd + | 10.8 ± 0.4 (N=6) |
| H-2bxk | ½ H-2bxk | c53.0 ± 5.5 (N=6) |
VI. Alloantibody-mediates B7-dependent, CD154-independent skin allograft rejection.
We hypothesized that anti-Kd generated opsonins can bind to FcγR and complement receptors on APCs, and that this interaction promoted APC maturation and induced the expression of costimulatory molecules that compensated for the blockade of the CD40-CD154 pathway. To test this hypothesis, we additionally targeted the B7-1/B7-2-CD28 pathway by using the CTLA4Ig fusion protein to block the CD28-B7 pathways (27, 28). B6 recipients of BALB/c skin grafts treated with anti-CD154/DST alone or anti-CD154/DST plus CTLA4Ig displayed long-term graft survival (>60 days; Figure 5) (29). In contrast to the rejection of skin grafts in recipients receiving anti-CD154/DST plus anti-Kd mAbs, the addition of CTLA4Ig resulted in 75% of recipients accepting their skin allografts (Figure 5). We conclude from these observations that anti-Kd mAbs generated opsonins that promoted the B7-dependent activation of alloreactive T cells and the rejection of skin allografts in anti-CD154/DST-treated recipients.
Figure 5.
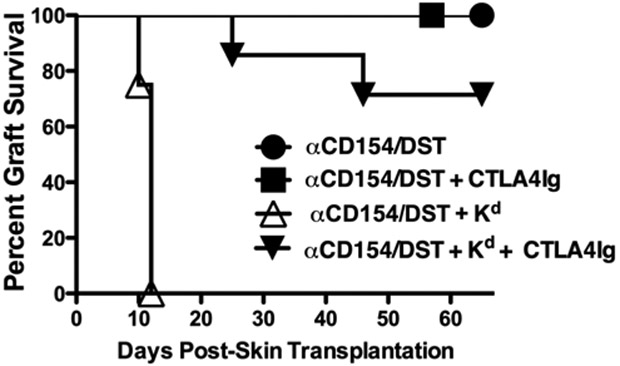
CTLA4Ig overrides the effects of Anti-Kd mAbs and facilitates graft acceptance in anti-CD154/DST-treated recipients. Acceptance of BALB/c skin grafts by B6 recipients treated with anti-CD154/DST + CTLA4Ig (N =4) (closed square) anti-CD154/DST + CTLA4Ig with anti-Kd mAbs (N = 7) (closed downward triangle) but rejection by B6 recipients treated with anti-CD154/DST and anti-Kd mAbs (N = 15) (open upward triangle). B6 recipients treated with anti-CD154/DST (N = 11) (closed circle) also accepted BALB/c skin grafts.
DISCUSSION
A consensus is emerging that donor-specific antibodies (DSA) are predictive of poor graft outcome (30, 31), and the activation of complement downstream of DSA binding to graft endothelium comprise the currently accepted paradigm for the mechanistic basis of how DSA effects graft loss (32). The observations in this study challenge the limited scope of this paradigm, and demonstrate the ability of alloantibodies to function as opsonins to facilitate CD40-CD154-independent T cell-activation and the rejection of allogeneic heart and skin grafts. The activation and necessity for allospecific T cells in anti-Kd-mediated allograft rejection is supported by observations of an increased precursor frequency of donor-specific T cells secreting IFN-γ in B6 recipients treated with anti-CD154±DST and anti-Kd mAbs; a robust proliferation of allospecific T cells upon transfer into B6 recipients treated with anti-CD154/DST and anti-Kd mAbs; and the inability of anti-Kd mAbs to mediate skin allograft rejection in TCRβδ−/− recipients.
In the cardiac tolerance model, prevention of long-term graft survival anti-Kd mAbs was associated with an anti-CD154-independent activation of alloreactive T cells. However, it was unclear whether events downstream of anti-Kd binding to the graft endothelium also contributed to and were necessary for rejection. We therefore turned to the skin transplant model, which is less susceptible to allo-antibody-mediated rejection, and where the addition of DST is necessary for the induction of long-term graft survival. In that model, we demonstrate a critical role of both polyclonal graft-reactive antibodies in the sera of pre-sensitized mice or purified anti-Kd mAbs binding to DST to prevent long-term skin graft acceptance in anti-CD154/DST treated recipients. Indeed, if Kd expression was restricted to the skin graft, anti-Kd mAbs had minimal effect on tolerance induction whereas if Kd expression was restricted to the DST, tolerance induction was abrogated. We addressed the concern that the anti-Kd mAbs were simply inducing the rapid elimination of DST, by administered 4-fold more DST to normalize for their more rapid clearance in the presence of anti-Kd mAbs. We observed that anti-Kd mAbs were still able to prevent the induction of long-term graft survival. These observations collectively support a conclusion that anti-Kd mAbs can function as opsonins to enhance alloreactive T cell activation, in addition to their binding to donor endothelial cell binding. In the skin model, anti-Kd mAbs converted the DST from tolerigenic to being immunogenic. Studies are ongoing to visualize the fate of DST in recipients with or without circulating anti-Kd mAbs to provide a more detailed understanding of how DST can be tolerigenic or immunogenic. The ability of alloantibodies to function as opsonins to enhance allograft rejection adds to their recognized role of binding to graft endothelium to elicit humoral rejection. Indeed we speculate that the opsonic activity of alloantibodies may, in part, explain the clinical observations that DSA in the absence of C4d deposition are predictors of graft loss (33), while acknowledging that insensitivity in the detection of C4d may also be a contributory factor. The requirement of C3 for long-term graft survival prevented the use of C3−/− recipients to test the necessity of complement in our model of alloantibody-mediated allograft rejection. However, given that oponins contain complement, we hypothesize that the presence of complement plays an important role in the ability of DST to become immunogenic. Future studies are underway to determine the necessity of complement in alloantibody-mediated allograft rejection.
T cell suppression in transplantation tolerance through linked-recognition is a well-recognized concept that explains infectious tolerance to third-party antigens, if these antigens are linked and presented on the same antigen presenting or donor cell that expresses the tolerized donor antigens (34, 35). We demonstrate a similar phenomenon with regards to the prevention of CD154-induced tolerance by anti-Kd mAbs, which facilitated the proliferation of not only Kd-specific TCR-75 T cells, but also of non-Kd-specific OT-II T cells recognizing OVA antigens expressed on the Act-OVA BALB/c DST and skin grafts. Further we show that the anti-Kd promoted the cross-presentation of DST from Act-OVA BALB/c donors, by demonstrating the proliferation of CD8+ OT-I T cells in recipients treated with anti-CD154/DST. Based on published observations, we infer from these cross-priming observations that CD8+ dendritic cells (DCs) are likely to be the antigen-presenting cell subset targeted by opsonized DST, although we cannot exclude the possibility that other DC subsets may also participate in the activation of alloreactive CD4+ T cells primed by the indirect pathway (36). Again, studies to visualize the fate of opsonized and non-opsonized DST in vivo will provide insights into the APC subset involved in DST uptake and presentation to T cells.
Non-Kd graft-reactive T cells activated in the presence of anti-Kd mAbs and anti-CD154/DST have the ability to reject allografts lacking Kd, as illustrated by the series of two skin allograft transplantations. We also show that this process, which resembles “epitope-spreading’, requires linked-recognition in that the non-Kd antigens have to be co-expressed on the Kd-expressing DST. Further we demonstrate that the administration of a mixture of Kd and non-Kd-expressing third-party allogeneic DST does not result in the priming of the third-party T cells nor the rejection of third-party skin grafts. Thus signals generated by the uptake of opsonized Kd-expressing DST are unable to result in the by-stander activation of APCs presenting third-party antigens. These observations may be explained by anti-Kd mAbs binding to DST, leading to the generation of the antibody- and complement-coated DST which then bind to APCs via FcγR and complement receptors (37-40). This binding should lead to the activation and maturation of APCs which, when under inflammatory conditions, have been shown to undergo major phenotypic and functional modification, including the reduction of further uptake of exogenous antigen (41, 42). While we do not provide a direct demonstration of APC maturation in the presence of anti-Kd, our observations that tolerance can be induced with the combination of anti-CD154/DST and CTLA4Ig but not in the presence of anti-CD154/DST are consistent with increased expression of B7 in DCs matured in the presence of anti-Kd mAbs (42). Studies to track the cells taking up opsonized versus non-opsonized DST are ongoing, and should provide mechanistic insights. The in vivo blockade experiments also suggest a therapeutic strategy for the successful induction of tolerance in the presence of opsonizing DSA. Finally these observations underscore the potential ability of DSA with limited specificities to prime a larger repertoire of alloreactive T cells, which may have significant implications to the clinical scenario of transplanting sensitized recipients with a restricted repertoire of DSA. Our observations suggest that high-titer DSA to a restricted donor antigen repertoire may be comparably detrimental as broadly reactive DSA, with regards to their ability to generate opsonins and prime a broadly reactive alloreactive T cell repertoire.
In summary, our study demonstrates that alloantibodies can function as opsonins to enhance CD154-independent T cell priming and acute allograft rejection in anti-CD154-treated recipients. In the model of skin graft rejection, where long-term graft survival is induced in the presence of DST, antibodies bind to and convert the DST from tolerigenic to immunogenic. Further donor-reactive antibodies binding to DST leads to enhanced indirect presentation of alloantigen, epitope-spreading and cross-presentation of alloantigens. Thus our study provides experimental evidence for a second mechanism by which donor-reactive antibodies can facilitate acute graft rejection, in addition to the well accepted role of mediating complement-dependent humoral- or antibody-mediated rejection.
Acknowledgements
We thank Drs. Yang-Xin Fu, Marisa Alegre, Marcus Clark, Chyung-Ru Wang, Roger Sciammas Emily Ahmed and Tongmin Wang for their helpful discussions and review of this manuscript. We also thank Jing Xu for technical assistance in the mouse heart and skin transplantation studies.
Funding Source:
NIH grant 2R56AI043631 and R01AI083452
Footnotes
Disclosures: The authors have no financial conflict of interest
References
- 1.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, Pearson TC, Ahmed R, and Larsen CP. 2003. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest 111:1887–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sachs DH 2003. Tolerance: of mice and men. J Clin Invest 111:1819–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schenk AD, Nozaki T, Rabant M, Valujskikh A, and Fairchild RL. 2008. Donor-reactive CD8 memory T cells infiltrate cardiac allografts within 24-h posttransplant in naive recipients. Am J Transplant 8:1652–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moxham VF, Karegli J, Phillips RE, Brown KL, Tapmeier TT, Hangartner R, Sacks SH, and Wong W. 2008. Homeostatic proliferation of lymphocytes results in augmented memory-like function and accelerated allograft rejection. J Immunol 180:3910–3918. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Heeger PS, and Valujskikh A. 2004. In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol 172:5456–5466. [DOI] [PubMed] [Google Scholar]
- 6.Gallon L, Gagliardini E, Benigni A, Kaufman D, Waheed A, Noris M, and Remuzzi G. 2006. Immunophenotypic analysis of cellular infiltrate of renal allograft biopsies in patients with acute rejection after induction with alemtuzumab (Campath-1H). Clin J Am Soc Nephrol 1:539–545. [DOI] [PubMed] [Google Scholar]
- 7.Burns AM, Ma L, Li Y, Yin D, Shen J, Xu J, and Chong AS. 2009. Memory alloreactive B cells and alloantibodies prevent anti-CD154-mediated allograft acceptance. J Immunol 182:1314–1324. [DOI] [PubMed] [Google Scholar]
- 8.Qian Z, Jakobs FM, Pfaff-Amesse T, Sanfilippo F, and Baldwin WM 3rd. 1998. Complement contributes to the rejection of complete and class I major histocompatibility complex--incompatible cardiac allografts. J Heart Lung Transplant 17:470–478. [PubMed] [Google Scholar]
- 9.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, and Valente M. 2008. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 8:753–760. [DOI] [PubMed] [Google Scholar]
- 10.Kaczmarek I, Deutsch MA, Kauke T, Beiras-Fernandez A, Schmoeckel M, Vicol C, Sodian R, Reichart B, Spannagl M, and Ueberfuhr P. 2008. Donor-specific HLA alloantibodies: long-term impact on cardiac allograft vasculopathy and mortality after heart transplant. Exp Clin Transplant 6:229–235. [PubMed] [Google Scholar]
- 11.Przybylowski P, Balogna M, Radovancevic B, Frazier OH, Susskind B, Van Buren C, Katz S, Kahan BD, and Kerman R. 1999. The role of flow cytometry-detected IgG and IgM anti-donor antibodies in cardiac allograft recipients. Transplantation 67:258–262. [DOI] [PubMed] [Google Scholar]
- 12.Wasowska BA, Qian Z, Cangello DL, Behrens E, Van Tran K, Layton J, Sanfilippo F, and Baldwin WM 3rd. 2001. Passive transfer of alloantibodies restores acute cardiac rejection in IgKO mice. Transplantation 71:727–736. [DOI] [PubMed] [Google Scholar]
- 13.Murata K, Fox-Talbot K, Qian Z, Takahashi K, Stahl GL, Baldwin WM 3rd, and Wasowska BA. 2007. Synergistic deposition of C4d by complement-activating and non-activating antibodies in cardiac transplants. Am J Transplant 7:2605–2614. [DOI] [PubMed] [Google Scholar]
- 14.Rahimi S, Qian Z, Layton J, Fox-Talbot K, Baldwin WM 3rd, and Wasowska BA. 2004. Non-complement- and complement-activating antibodies synergize to cause rejection of cardiac allografts. Am J Transplant 4:326–334. [DOI] [PubMed] [Google Scholar]
- 15.Behr TM, Feucht HE, Richter K, Reiter C, Spes CH, Pongratz D, Uberfuhr P, Meiser B, Theisen K, and Angermann CE. 1999. Detection of humoral rejection in human cardiac allografts by assessing the capillary deposition of complement fragment C4d in endomyocardial biopsies. J Heart Lung Transplant 18:904–912. [DOI] [PubMed] [Google Scholar]
- 16.Magil AB, and Tinckam K. 2003. Monocytes and peritubular capillary C4d deposition in acute renal allograft rejection. Kidney Int 63:1888–1893. [DOI] [PubMed] [Google Scholar]
- 17.Nickeleit V, Zeiler M, Gudat F, Thiel G, and Mihatsch MJ. 2002. Detection of the complement degradation product C4d in renal allografts: diagnostic and therapeutic implications. J Am Soc Nephrol 13:242–251. [DOI] [PubMed] [Google Scholar]
- 18.Smith RN, Brousaides N, Grazette L, Saidman S, Semigran M, Disalvo T, Madsen J, Dec GW, Perez-Atayde AR, and Collins AB. 2005. C4d deposition in cardiac allografts correlates with alloantibody. J Heart Lung Transplant 24:1202–1210. [DOI] [PubMed] [Google Scholar]
- 19.Burdick JF, and Clow LW. 1986. Rejection of murine cardiac allografts. I. Relative roles of major and minor antigens. Transplantation 42:67–72. [DOI] [PubMed] [Google Scholar]
- 20.Valujskikh A, Pantenburg B, and Heeger PS. 2002. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant 2:501–509. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo S, Kurisaki A, Sugino H, Hashimoto I, and Nakanishi H. 2007. Analysis of skin graft survival using green fluorescent protein transgenic mice. J Med Invest 54:267–275. [DOI] [PubMed] [Google Scholar]
- 22.Markees TG, Phillips NE, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, and Rossini AA. 1997. Prolonged survival of mouse skin allografts in recipients treated with donor splenocytes and antibody to CD40 ligand. Transplantation 64:329–335. [DOI] [PubMed] [Google Scholar]
- 23.Chen L, Wang T, Zhou P, Ma L, Yin D, Shen J, Molinero L, Nozaki T, Phillips T, Uematsu S, Akira S, Wang CR, Fairchild RL, Alegre ML, and Chong A. 2006. TLR engagement prevents transplantation tolerance. Am J Transplant 6:2282–2291. [DOI] [PubMed] [Google Scholar]
- 24.Wang T, Chen L, Ahmed E, Ma L, Yin D, Zhou P, Shen J, Xu H, Wang CR, Alegre ML, and Chong AS. 2008. Prevention of allograft tolerance by bacterial infection with Listeria monocytogenes. J Immunol 180:5991–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porrett PM, Yuan X, LaRosa DF, Walsh PT, Yang J, Gao W, Li P, Zhang J, Ansari JM, Hancock WW, Sayegh MH, Koulmanda M, Strom TB, and Turka LA. 2008. Mechanisms underlying blockade of allograft acceptance by TLR ligands. J Immunol 181:1692–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deshmukh US, Kannapell CC, and Fu SM. 2002. Immune responses to small nuclear ribonucleoproteins: antigen-dependent distinct B cell epitope spreading patterns in mice immunized with recombinant polypeptides of small nuclear ribonucleoproteins. J Immunol 168:5326–5332. [DOI] [PubMed] [Google Scholar]
- 27.Sharpe AH, and Freeman GJ. 2002. The B7-CD28 superfamily. Nat Rev Immunol 2:116–126. [DOI] [PubMed] [Google Scholar]
- 28.Chandraker A, Huurman V, Hallett K, Yuan X, Tector AJ, Park CH, Lu E, Zavazava N, and Oaks M. 2005. CTLA-4 is important in maintaining long-term survival of cardiac allografts. Transplantation 79:897–903. [DOI] [PubMed] [Google Scholar]
- 29.Zheng XX, Markees TG, Hancock WW, Li Y, Greiner DL, Li XC, Mordes JP, Sayegh MH, Rossini AA, and Strom TB. 1999. CTLA4 signals are required to optimally induce allograft tolerance with combined donor-specific transfusion and anti-CD154 monoclonal antibody treatment. J Immunol 162:4983–4990. [PubMed] [Google Scholar]
- 30.Toki D, Ishida H, Setoguchi K, Shimizu T, Omoto K, Shirakawa H, Iida S, Horita S, Furusawa M, Ishizuka T, Yamaguchi Y, and Tanabe K. 2009. Acute antibody-mediated rejection in living ABO-incompatible kidney transplantation: long-term impact and risk factors. Am J Transplant 9:567–577. [DOI] [PubMed] [Google Scholar]
- 31.Papassavas AC, Iniotaki-Theodoraki A, Boletis J, Kostakis A, and Stavropoulos-Giokas C. 2000. Epitope analysis of HLA class I donor specific antibodies in sensitized renal transplant recipients. Transplantation 70:323–327. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, and Reed EF. 2009. Effect of antibodies on endothelium. Am J Transplant 9:2459–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haririan A, Kiangkitiwan B, Kukuruga D, Cooper M, Hurley H, Drachenberg C, and Klassen D. 2009. The impact of c4d pattern and donor-specific antibody on graft survival in recipients requiring indication renal allograft biopsy. Am J Transplant 9:2758–2767. [DOI] [PubMed] [Google Scholar]
- 34.Davies JD, Leong LY, Mellor A, Cobbold SP, and Waldmann H. 1996. T cell suppression in transplantation tolerance through linked recognition. J Immunol 156:3602–3607. [PubMed] [Google Scholar]
- 35.Cobbold SP, Adams E, Marshall SE, Davies JD, and Waldmann H. 1996. Mechanisms of peripheral tolerance and suppression induced by monoclonal antibodies to CD4 and CD8. Immunol Rev 149:5–33. [DOI] [PubMed] [Google Scholar]
- 36.Segura E, and Villadangos JA. 2009. Antigen presentation by dendritic cells in vivo. Curr Opin Immunol 21:105–110. [DOI] [PubMed] [Google Scholar]
- 37.Nauta AJ, Castellano G, Xu W, Woltman AM, Borrias MC, Daha MR, van Kooten C, and Roos A. 2004. Opsonization with C1q and mannose-binding lectin targets apoptotic cells to dendritic cells. J Immunol 173:3044–3050. [DOI] [PubMed] [Google Scholar]
- 38.Mevorach D, Mascarenhas JO, Gershov D, and Elkon KB. 1998. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med 188:2313–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morelli AE, Larregina AT, Shufesky WJ, Zahorchak AF, Logar AJ, Papworth GD, Wang Z, Watkins SC, Falo LD Jr., and Thomson AW. 2003. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood 101:611–620. [DOI] [PubMed] [Google Scholar]
- 40.Ronnelid J, Ahlin E, Nilsson B, Nilsson-Ekdahl K, and Mathsson L. 2008. Immune complex-mediated cytokine production is regulated by classical complement activation both in vivo and in vitro. Adv Exp Med Biol 632:187–201. [PubMed] [Google Scholar]
- 41.Garrett WS, Chen LM, Kroschewski R, Ebersold M, Turley S, Trombetta S, Galan JE, and Mellman I. 2000. Developmental control of endocytosis in dendritic cells by Cdc42. Cell 102:325–334. [DOI] [PubMed] [Google Scholar]
- 42.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, and Peach R. 1994. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity 1:793–801. [DOI] [PubMed] [Google Scholar]


