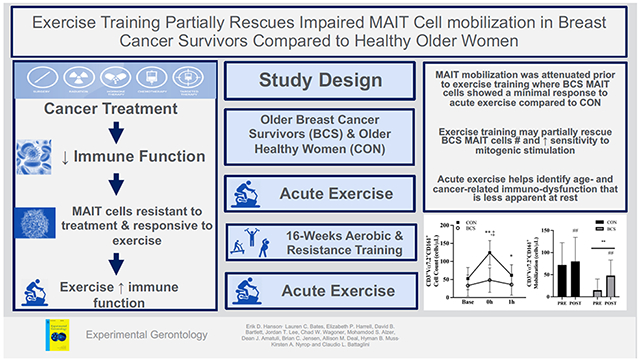
Abstract
Exercise may attenuate immunosenescence with aging that appears to be accelerated following breast cancer treatment, although limited data on specific cell types exists and acute and chronic exercise have been investigated independently in older adults.
Purpose:
To determine the mucosal associated invariant T (MAIT) cell response to acute exercise before (PRE) and after (POST) 16 weeks of exercise training in breast cancer survivors (BCS) and healthy older women (CON).
Methods:
Age-matched BCS and CON performed 45 minutes of intermittent cycling at 60% peak power output wattage. Blood samples were obtained at rest, immediately (0h) and 1h after exercise to determine MAIT cell counts, frequency, and intracellular cytokine expression.
Results:
At PRE, MAIT cell counts were greater in CON (137%) than BCS at 0h (46%, p<0.001), with increased MAIT cell frequency in CON but not BCS. TNFα+ and IFNγ+ MAIT cell counts increased at 0h by ~120% in CON (p<0.001), while BCS counts and frequencies were unchanged. Similar deficits were observed in CD3+ and CD3+ CD8+ cells. At POST, exercise-induced mobilization and egress of MAIT cell counts and frequency showed trends towards improvement in BCS that approached levels in CON. Independent of group, TNFα frequency trended to improve (p=0.053).
Conclusions:
MAIT mobilization in older BCS following acute exercise was attenuated; however, exercise training may partially rescue these initial deficits, including greater sensitivity to mitogenic stimulation. Using acute exercise before and after interventions provides a unique approach to identify age- and cancer-related immuno-dysfunction that is less apparent at rest.
Keywords: exercise oncology, exercise immunology, immune, geriatric oncology
Graphical abstract
1. INTRODUCTION
Breast cancer (BC) is the most commonly diagnosed cancer in women world-wide (Siegel et al., 2020). Five year survival rates for localized disease approach 90%, with more women surviving BC than ever before (Siegel et al., 2020). However, BC treatments may accelerate age-related declines, including alterations in body composition, fatigue, chronic inflammation and physical function that all contribute to reduced quality of life (Hanson and Hurley, 2011; Nyrop et al., 2019; Schmitz et al., 2007). One side effect that has received less attention, but pertains to both recurrence and secondary disease prevention, is immune system dysfunction.
Radiation and chemotherapy have detrimental effects on the immune system, including decreased cell counts and cytokine production (e.g. TNFα and IFNγ) in T cells and NK cells (Dusseaux et al., 2011; Verronèse et al., 2016; Won et al., 2016) that may hasten age-related immunosenescence (Simpson et al., 2012). Regenerative capacity of naïve cells is also reduced (Gustafson et al., 2020), potentially affecting tissue homeostasis, repair, and infection control (Tao et al., 2015). Moreover, delayed recovery to pre-treatment levels is associated with greater rates of metastatic BC (Kang et al., 2010). Exercise training during BC has been used to target classical immune populations, with evidence of improved function without changes in natural killer (NK), B or conventional T cell counts or frequencies (Fairey et al., 2005; Hagstrom et al., 2016; Hutnick et al., 2005; Nieman et al., 1995). In addition, unconventional T cells, including natural killer T (NKT) cells, gamma delta T cells, and mucosal associated invariant T (MAIT) cells, have anti-cancer properties and respond to acute and chronic exercise (Hanson et al., 2021).
Residing primarily in the lungs, liver, and gastrointestinal tract (Dusseaux et al., 2011; Hinks and Zhang, 2020), MAIT cells are identified via a semi-invariant Vα7.2-Jα33/12/20 T cell receptor and high CD161 expression (Billerbeck et al., 2010). MAIT cell function has been well-characterized (Hinks and Zhang, 2020). Activation of these cells relies on antigen presentation via the major histocompatibility complex class I-related (MR1) molecule and costimulatory factors such as IL-7, tumor necrosis factor (TNF), and type I interferons (IFN) that occurs in both T cell receptor dependent and independent mechanisms. Upon activation, MAIT cells are involved in the host defense response to bacteria by rapidly producing Th1/Th17 cytokines, including TNFα, IFNγ and IL-17 (Dusseaux et al., 2011), but also have been implicated in viral infections (Hinks and Zhang, 2020) and cancer as effector cells within the tumor-infiltrating lymphocyte subsets (Sundström et al., 2019; Zumwalde et al., 2018).
To our knowledge, the only studies examining MAIT cells and acute exercise are in young healthy men. After submaximal and maximal aerobic exercise, both MAIT cell counts and frequency increased (Hanson et al., 2019, 2017), with counts but not frequency returning to baseline after 1h of recovery. TNFα and IFNγ frequency also increased with acute exercise, suggesting greater responsiveness to mitogenic stimulation (Hanson et al., 2019). Curiously, MAIT cells appear resistant to chemotherapy-induced declines that plague conventional T cells (Dusseaux et al., 2011; Won et al., 2016). This feature, in conjunction with the enhanced cytokine response to acute exercise, makes MAIT cells an intriguing target to mitigate cancer recurrence and immunosenescence during survivorship. However, while regular moderate intensity exercise may enhance natural immunity (Schmidt et al., 2017) and reduce susceptibility to cancer via enhanced immune system function (Koelwyn et al., 2015), the ability of exercise training to alter MAIT cell counts, frequency or function remains unknown.
Exercise interventions in older women with BC have mostly examined only basic immunological parameters (e.g. complete blood counts, circulating cytokines), with limited data on specific cells or functional outcomes (Fairey et al., 2005; Khosravi et al., 2019). Moreover, immune modulations using acute or chronic exercise have only been performed separately (Khosravi et al., 2019). This approach fails to consider if the acute response changes after training. Modulations in hormone, inflammatory and immune factors play critical roles in cancer progression (Dethlefsen et al., 2017; Khosravi et al., 2019) therefore investigating adaptations of these parameters to acute exercise after exercise training is critical. Finally, training studies have compared immune outcomes relative to usual care (Fairey et al., 2005; Hagstrom et al., 2016; Hutnick et al., 2005; Nieman et al., 1994), a logical approach although limited in its inability to make comparisons to the normal response in older adults. Presently, it is unclear if the training approaches (e.g., volume, intensity, duration) are insufficient or if the immune system is incapable of responding due to persistent immune dysfunction. The use of an acute exercise stimulus before and after exercise training may provide important insight into the magnitude of immune system activation in a unique population of immune cells.
Therefore, the purpose of this study was to determine the effects of acute exercise in breast cancer survivors (BCS) and healthy older women (CON) on MAIT cell counts, frequency, and intracellular cytokine expression before and after a 16 week exercise intervention designed to meet current exercise oncology guidelines (Campbell et al., 2019).
2. METHODOLOGY
2.1. Participants.
Participants in this trial were a sub-set from larger investigation (NCT03760536). BCS (n=13) diagnosed with stage I-III breast cancer who were no more than 1 year since final treatment received, along with age-matched CON (n=13) with no cancer history were recruited. Participants were inactive, defined as exercising <30 min at a moderate intensity 2x/week that failed to meet guidelines established by the American College of Sports Medicine. The study was approved by the Protocol Review Committee and the Institutional Review Board at the University of North Carolina at Chapel Hill. Participants provided written informed consent prior to enrolling in the study.
2.2. Study Design.
Using a non-randomized, parallel groups design, BCS and CON completed 3 baseline (PRE) testing visits over 2 weeks before starting a 16 week community-based exercise program administered in a small group setting. At the completion of training, follow up testing (POST) was the same as baseline (Figure 1).
Figure 1.
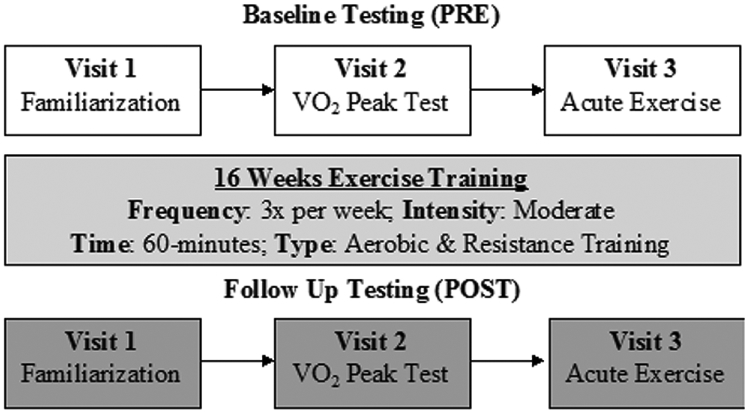
Study overview showing the 3 baseline (PRE) and 3 follow up (POST) testing sessions completed before and after 16 weeks of combined exercise training.
2.2.1. Visit 1
Participants completed a medical history and 12-lead electrocardiogram that was approved by the study cardiologist prior to future visits. Participants were familiarized an electronically braked cycle ergometer and the mask used during the cardiopulmonary exercise test (CPET). Participants cycled for 2 min with no resistance, followed by 3 min at 20 watts, before initiating a continuous ramp cycling protocol with wattage increasing approximately 1 Watt every 4 seconds (15 W/min protocol). Rating of Perceived Exertion (RPE) and heart rate (Polar Electro Inc., Lake Success, NY) were continually monitored. CPET familiarization was terminated at 75% of heart rate reserve.
2.2.2. Visit 2
Body Composition (total fat and lean mass, % fat) was measured using Dual X-ray Absorptiometry (Discovery W, Hologic, Inc., Bedford, MA). The machine was calibrated prior to each use, and all scans were analyzed by the same technician at PRE and POST. A maximal effort CPET was then used to assess cardiorespiratory function using the same procedures as visit 1 except the test terminated at volitional exhaustion. Expired respiratory gases were analyzed using a metabolic system (TrueMax 2400, Parvo Medics, Salt Lake City, UT) to determine peak oxygen uptake (VO2peak), defined as the average of the 3 highest recordings in the final minute. Peak power output was recorded as the maximal wattage completed in the last minute prior to test termination.
2.2.3. Visit 3
The last testing session occurred 1-7 days after visit 2. Participants reported to the laboratory between 0700 and 1000 in a hydrated state and having refrained from caffeine and alcohol consumption over the previous 8 and 24h, respectively. After 10 min of rest, an indwelling venous catheter was inserted into the antecubital vein of the forearm and a resting blood sample was obtained. The catheter was periodically flushed with 0.9% sodium chloride saline.
To induce an immune response, participants completed an acute, intermittent cycling bout. Participants completed a 1 min warm up at 0% and 30% of peak power output, followed by 10 intervals of 3 min of cycling at 60% of peak power from the CPET followed by 1.5 min of passive recovery. This protocol has previously been well-tolerated by both breast (Evans et al., 2015) and prostate (Hanson et al., 2020, 2018) cancer survivors. Heart rate and RPE were determined in the last 30 s of each stage. Additional blood samples were obtained immediately following exercise (0h) and after 1h of seated recovery with ad libitum access to water.
2.3. Exercise Training Intervention.
The 16 week exercise intervention was a community-based program that included progressive aerobic and resistance exercise within each session. Trained exercise specialists administered the program in small groups 3x/week for ~1h (sTable 1). Aerobic exercise was completed first, with modality being self-selected by participants, and included cycling, walking, jogging, elliptical, rowing, or seated stepper. For the first 2 weeks, exercise volume and intensities started at 10-15 min of low intensity exercise (RPE of 8-11). For weeks 3-7, exercise intensity remained constant but duration increased to 10-30 min. By week 8, the goal was for participants to exercise for 30 min at moderate intensity (RPE of 12-14), which was sustained over the rest of the intervention.
Immediately following aerobic exercise, resistance training included 6 exercises per session targeting the major muscle groups using body weight, bands, dumbbells or machines. Specific exercises varied with each session but included both upper and lower body movements and at least one core exercise performed in a circuit lasting 30 min. In the first 2 weeks, 1 set of 15 repetitions for each exercise was performed at light to moderate intensity (RPE 7-13). For weeks 3-5, 2 sets of 10-15 repetitions were completed. From week 6 onwards, 2 sets for 10 repetitions were completed at an RPE of 14-15. Modifications were made for comfort and safety related to any surgery limitations.
Participants were monitored and supervised at all times by training staff. Exercise adherence was the percentage of days attended vs. days prescribed (48 total). Exercise compliance was determined for each mode separately, with an aerobic compliant day defined as completing >80% of the prescribed time (min, for aerobic) or volume (sets x reps, strength) at the prescribed intensity (RPE) vs. total days prescribed.
2.4. Hematology Analysis.
Complete blood counts were performed in duplicate using an automated hematology analyzer (Sysmex XP-300, Kobe, Japan) and averaged with a maximal white blood cell difference of 0.1 cells/μL (Hanson et al., 2020, 2017). Hematocrit and hemoglobin were used to estimate plasma volume shifts following exercise (Dill and Costill, 1974).
Peripheral blood mononuclear cells (PBMC) were isolated using density gradient centrifugation as described previously (Hanson et al., 2020, 2017). PBMCs were counted (TC-20, Bio Rad, Hercules CA) and then cryopreserved in 90% fetal bovine serum (FBS) and 10% dimethyl sulfoxide until the end of the study.
2.5. PBMC Stimulation and Immunofluorescence.
Cryopreserved PBMCs were thawed in a 37°C water bath and washed in complete media (10% FBS, 1% Penicillin-Streptomycin in RPMI). Cells were counted and viability (majority >90%) was determined using an automated counter (TC-20, Bio Rad, CA, USA) before being allowed to rest for 2 h at 37°C and 5% CO2. Following the rest, cell viability remained consistent (within 5%) and PBMCs were stimulated with 2 ng/mL phorbol 12-myristate 13-acetate (PMA) and 1 ng/mL ionomycin or vehicle controls for 4 h at 37°C and 5% CO2. Cells were then washed and resuspended in 100 μL of cell staining buffer (BioLegend, San Diego, CA) containing the following mouse anti-human monoclonal antibodies (BioLegend, San Diego, CA): CD3 (APC-Cy7), CD4 (Amycan), CD8 (AF700), TCR Vα7.2 (PE), and CD161 (BV605) for 15 minutes in the dark at 4°C. Next, cells were washed twice in PBS before 200 μL of fixation and permeabilization buffer was added (Cytofix/Cytoperm kit, BD Biosciences, San Jose, CA) in accordance with manufacturer instructions Intracellular IFNγ (Pacific Blue) and TNFα (BV650) were stained in 100 μL of 1x perm/wash buffer at 4°C in the dark for 30 min. After washing, cells were resuspended in 300 μL of cell staining buffer for flow cytometry analysis. Titrations were performed to determine optimal antibody concentrations.
2.6. Flow Cytometry.
Cellular events were analyzed using a LSRII flow cytometer running FACSDiva 8.0.1 Software (BD Biosciences, CA USA). Our gating strategy has been shown elsewhere (Hanson et al., 2017). From the CD3+ population, Vα7.2 and CD161 bright cells were determined MAIT cells, with intracellular cytokine levels. Unlabeled cells and single-color AbC Total Antibody Compensation Beads (Thermo Fisher Scientific, Hampton, NH) were used in addition to fluorescence minus one controls for PE as this channel was used to detect Vα7.2. Gating analyses were completed using FlowJo version 10.7 (Ashland, OR). MAIT cell counts (cells/μL) were determined by multiplying the percentage of each population with the hematology lymphocyte counts.
2.7. Statistical Analysis.
Data were analyzed with SPSS Statistics v25.0 (SPSS, IN., Durham, NC, USA) and Jamovi Statistics v1.2.26 (Sydney, Australia). Figures were created in GraphPad Prism v9 (La Jolla, CA, USA). Participant characteristics at PRE were determined using independent samples t-tests and changes with training were evaluated using a group (BCS vs. CON) x training (PRE vs. POST) repeated-measures ANOVA with Bonferroni post-hoc analysis. All acute immune outcomes at PRE were analyzed using a linear mixed model, with group (BCS vs. CON) and time (Base, 0h, 1h) as fixed factors and subjects as a random effect (Hanson et al., 2020). Group x time interactions were resolved using simple effects to examine group responses at each time point.
For exercise training, immune analyses were performed separately for mobilization and egress. Mobilization was calculated as the change from baseline to 0h. Egress was the change from the 0h to 1h. Within each separate linear mixed model, the fixed factors were group (BCS vs. CON) and training (PRE vs. POST) with subjects as a random effect. Group x training interactions were again resolved using simple effects. Data are presented as mean (SD) with model estimates expressed relative to baseline (time effects) or CON (group effects) and include 95% confidence intervals. Statistical significance was p<0.05, with borderline significant interactions (p<0.1) being examined in detail as part of a pre-planned analyses. Effects sizes were determined using Cohen’s D (d) such that 0.2, 0.5 and 0.8 represent small, medium, and large differences, respectively.
3. RESULTS
3.1. Participants.
At PRE, BCS and CON were age-matched with no differences in lean mass or body fat % (Table 1). At POST, participant characteristics were mostly unchanged except for a group x time interaction for lean mass % (p=0.030) where there was an increase in BCS (p=0.019) and a non-significant decrease in CON (p=0.825). Borderline significant interactions were present for body fat % (p=0.053) and lean mass (p=0.083). BCS were mostly early-stage (Table 2) and all underwent surgery prior to initiating secondary treatments [radiation (46%), chemotherapy (23%) or both (31%)]. At enrollment, BCS were ~3 months since completing primary treatments, with the majority being post-menopausal. There were no differences in baseline measures between pre and post-menopausal women in either BCS or CON.
Table 1.
Participant characteristics before (PRE) and after (POST) exercise training.
| BCS (n=13) | CON (n=13) | P values | |||||
|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | G x T | Group | Time | |
| Age (y) | 57 (9) | 58 (7) | 0.658 | ||||
| Height (cm) | 164.5 (9.0) | 162.7 (5.5) | 0.724 | ||||
| Body Mass (kg) | 69.8 (8.6) | 70.0 (8.3) | 72.3 (19.5) | 72.7 (20.8) | 0.737 | 0.751 | 0.549 |
| Lean Mass (kg) | 38.7 (3.7) | 40.0 (3.0) | 41.2 (9.4) | 40.7 (10.6) | 0.083 | 0.680 | 0.388 |
| Body Fat (%) | 40.9 (7.2) | 39.2 (6.4) | 39.2 (4.4) | 40.4 (5.2) | 0.053 | 0.949 | 0.546 |
| Lean Mass (%) | 55.9 (6.8) | 57.7 (6.0) | 57.6 (3.9) | 56.5 (4.7) | 0.030 | 0.947 | 0.625 |
Data reported as mean (SD). Abbreviations: BCS, Breast Cancer Survivor; CON, Healthy Control; PRE, Pre-Training; POST, Post-Training; G X T, group x time interaction
Table 2.
Clinical characteristics of breast cancer survivors (BCS).
| BCS (n=13) | |
|---|---|
| Time from last treatment (d) | 82 (90) |
| Time from surgery (d) | 224 (115) |
| Time from radiation (d) | 62 (78) |
| Time from chemotherapy (d) | 152 (100) |
| Stage | |
| I (%) | 5 (38) |
| II (%) | 6 (46) |
| III (%) | 2 (15) |
| Tumor Status | |
| ER+ Positive (%) | 12 (92) |
| PR+ Positive (%) | 12 (92) |
| HER2+ Positive (%) | 4 (31) |
| ER−/PR−/HER2− (%) | 1 (8) |
| Pre-Menopausal (%) | 5 (38) |
| Post-Menopausal (%) | 8 (62) |
Data are mean (SD) or (%). Abbreviations: BCS, Breast Cancer Survivor; ER, Estrogen-Receptor; PR, Progesterone-Receptor; HER2, Human Epidermal Growth Factor Receptor 2.
3.2. Exercise Testing
The physiological responses to the CPET (Visit 2) and the acute intermittent exercise trial (Visit 3) are in Table 3. Average HR and RPE during acute exercise were not different between groups or after training. During acute exercise, HR in BCS was at 85% of peak for both PRE and POST while CON was at 86% and 92%, respectively.
Table 3.
Physiological responses to VO2 Peak Test and acute, intermittent exercise before and after training
| BCS (n=13) | CON (n=13) | P values | |||||
|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | G x T | Group | Time | |
| CPET | |||||||
| VO2Peak (mL/kg/min) | 21.4 (6.6) | 22.3 (5.6) | 22.5 (2.8) | 23.6 (4.5) | 0.860 | 0.523 | 0.164 |
| Peak HR (bpm) | 160 (18) | 160 (17) | 157 (10) | 158 (12) | 0.830 | 0.709 | 0.910 |
| Peak Power Output (W) | 119 (34) | 137 (40) | 129 (21) | 136 (24) | 0.050 | 0.713 | <0.001 |
| Duration (min:sec) | 9:56 (2:07) | 11:09 (2:22) | 10:17 (1:25) | 10:47 (2:34) | 0.236 | 0.928 | 0.006 |
| Acute Exercise Trial | |||||||
| Resistance (W) | 74 (20) | 83 (24) | 77 (16) | 82 (82) | 0.194 | 0.848 | <0.001 |
| Average RPE | 14 (3) | 13 (1) | 13 (1) | 14 (1) | 0.070 | 0.709 | 0.588 |
| Average HR (bpm) | 136 (8) | 136 (7) | 135 (8) | 145 (9) | 0.139 | 0.166 | 0.149 |
Data are mean (SD). Abbreviations: BCS, Breast Cancer Survivor; CON, Healthy Control; PRE, Pre-Training; POST, Post-Training; CPET, cardiopulmonary exercise test; VO2peak, peak oxygen uptake; G X T, group x time interaction
Acute Exercise
3.3. Hematological Response.
The acute response to exercise was assessed prior to starting the exercise intervention. There were no interactions for any variable. Leukocyte counts increased by 42% at 0h (2x103/μL cells, 95% CI 1.4, 2.6, d=1.2, p<0.001, sTable 2) and remained slightly elevated (9%) at 1h (0.5x103/μL cells, 95% CI −0.1, 1.0, d=0.3, p<0.001). Exercise and recovery patterns of lymphocyte counts were largely similar to leukocytes, except CON lymphocyte count was 42% higher (p=0.015).
3.4. MAIT Cell Counts and Frequencies.
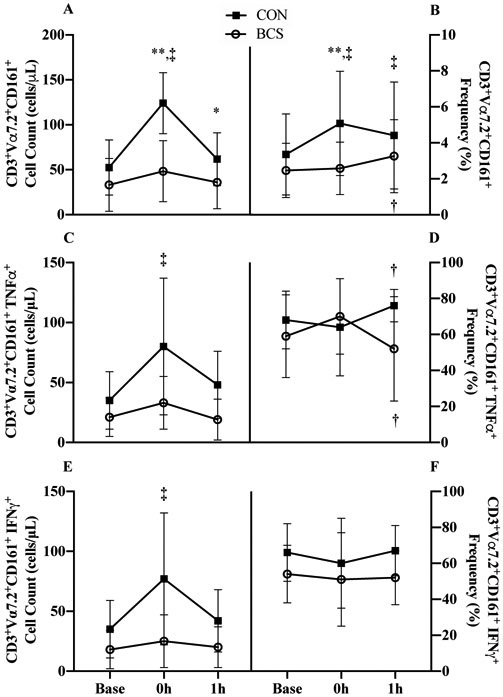
A group x time interaction was observed for MAIT cell counts (p<0.001, Figure 2A). At 0h, CON increased by 137% (72 cells/μL, 95% CI 55, 89; d=1.4, p<0.001) and BCS showed a tendency to increase by 46% (15 cells/μL, 95% CI −2, 33; d=0.5; p=0.086), with the increase at 0h being larger in CON (57 cells/μL, 95% CI 33, 81; p<0.001). At baseline, BCS tended to have lower MAIT cell counts (−37%, d=0.6; p=0.057) that were more pronounced at 0h (61%, p<0.001) as a result of the interaction.
Figure 2.
The response to acute exercise before (PRE) exercise training for A) MAIT cell counts and B) proportions; MAIT cell TNFα+ C) cell counts and D) proportions; and MAIT cell IFNγ+ E) counts and F) proportions. Data are mean (SD). Abbreviations: Base, baseline; 0h immediately following exercise; 1h: 1 hour after exercise; BCS, breast cancer survivor; CON, healthy control.
*P<0.05 and ** P<0.01 vs. CON
† P<0.05 and ‡ P<0.01 vs. Base
A group x time interaction was also present for MAIT cell frequency (p<0.001, Figure 2B). At 0h, CON proportions increased (1.7%, 95% CI 1.1, 2.4; d=0.7, p<0.001) with only a trend for BCS (0.7%, 95% CI 1.7, 3.3, d=0.1 p=0.068) such that difference between groups was 2.5% (p=0.005). At 1h, both groups were elevated above baseline (CON: 1.1%, 95% CI 0.4, 1.7; p<0.001; BCS: 0.8%, 95% CI 0.2, 1.4; p=0.012) but no group differences were observed.
3.5. MAIT Cell Cytokine Counts and Frequencies.
A group x time interaction was present in MAIT cell TNFα counts (p=0.017, Figure 2C). At 0h, CON increased by 128% (45 cells/μL, 95% CI 29, 60; p<0.001) where BCS was unchanged with both groups returning to baseline by 1h.
A group x time interaction was also present in MAIT cell TNFα frequency (p=0.019, Figure 2D). There were no changes initially at 0h. At 1h, BCS decreased while CON increased such that the group difference for TNFα proportion emerged (−30.1%, 95% CI −50.2, −10.0; p=0.005).
A group x time interaction for MAIT cell IFNγ counts (p=0.002, Figure 2E) revealed that at 0h, CON increased by 119% (42 cells/μL, 95% CI 28, 56; d=1.0; p<.001) where BCS was unchanged. Both groups returned to baseline levels by 1h.
There was a trend of BCS tended to have lower IFNγ frequency overall (11.9%, 95% CI - 0.7, 24.4; d=0.9; p=0.055, Figure 2F). There was no interaction or time effect.
3.6. Conventional T Cell Counts and Frequencies.
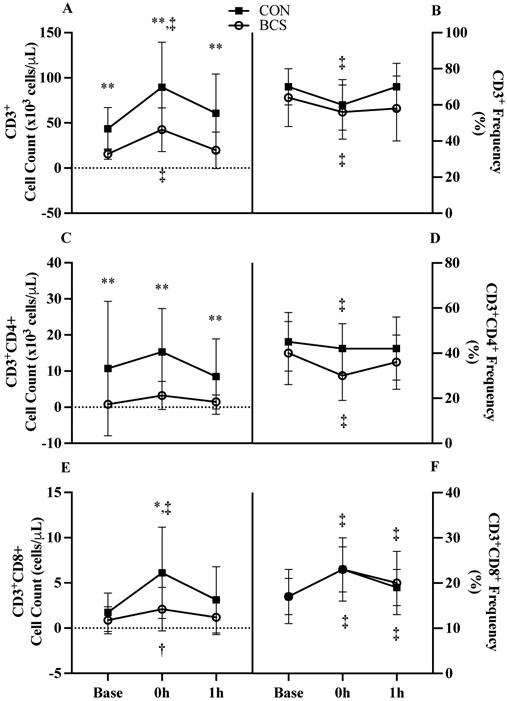
Overall, CD3+ cell counts increased by 123% (36.4 x103 cells/μL, 95% CI 20.7, 52.1; p<0.001, Figure 3A) at 0h before returning to baseline at 1h. CD3+ counts were also 60% lower in BCS (38.6 x103 cells/μL, 95% CI 22.5, 54.7; p<0.001).
Figure 3.
Conventional T cell response to acute exercise for CD3+ A) counts and B) proportions, CD3+CD4+ C) counts and D) proportions, and CD3+CD8+ E) counts and F) proportions. Data are mean (SD). Abbreviations: BCS, breast cancer survivor; CON, healthy control.
*P<0.05 and ** P<0.01 vs. CON
† P<0.05 and ‡ P<0.01 vs. Base
Across time, CD3+ frequency decreased at 0h (−8.7%, 95% CI −11.7, −5.7; p<0.001) but returned to baseline values by 1h. CD3+ frequency showed a borderline significant group x time interaction (p=0.069, Figure 3B) with CON returning to baseline by 1h while BCS remained suppressed (−5.4%, 95% CI −0.6, 11.4; d=0.7).
CD3+CD4+ cell counts were 84% lower in BCS (9.7 x103 cells/μL, 95% CI 3.5, 15.8; p=0.005, Figure 3C) with no interaction or changes over time. CD3+CD4+ frequency was reduced 0h only (−6.5%, 95% CI −10.1, −2.8; p<0.001, Figure 3D).
A group x time interaction was present for CD3+CD8+ counts (p<0.001, Figure 3E). At 0h, CON increased by 2.5 fold (4.4 x103 cells/μL, 95% CI 3.2, 5.5; d=1.1; p<0.001) and BCS by 1.4-fold (1.2 x103 cells/μL, 95% CI 89, 2,356; d=0.6; p=0.035) with the BCS CD3+CD8+ mobilization being 66% smaller (3.1 x103 cells/μL, 95% CI 2.1, 4.2; p=0.007). CD3+CD8+ frequency increased at 0h (5.8%, 95% CI 4.8, 6.8; p<0.001) and remained elevated at 1h (2.5% 95% CI 1.5, 3.5; p<0.001, Figure 3F).
Exercise Training
At POST, CPET test duration increased by 8.5% (p=0.006, Table 3) for both groups. Additionally, there was a 15% increase in peak wattage for BCS that was greater than CON (11 watts, 95% CI 9, 13, d=2.3, p=0.05). VO2 peak and peak HR did not change. Absolute wattage for the acute exercise trial (Visit 2) increased by 9% at POST (p<0.001).
3.7. Exercise Adherence and Compliance:
Of the 48 prescribed training days, BCS adherence was 72% (19) and CON was 69% (19) with no group differences. Exercise compliance was greater in CON for both aerobic (CON: 67% (19), BCS: 54% (21), p=0.027) and strength training (CON: 38% (6), BCS: 29% (10), p=0.019).
3.8. Hematological Response:
For all immune parameters, mobilization was defined as the change from baseline to 0h and egress was defined as the change 0h to 1h and are shown as change scores before (PRE) and after (POST) training for both groups. There was no group x training interaction or main effects for lymphocyte mobilization or egress (sFigure 1A and 1B).
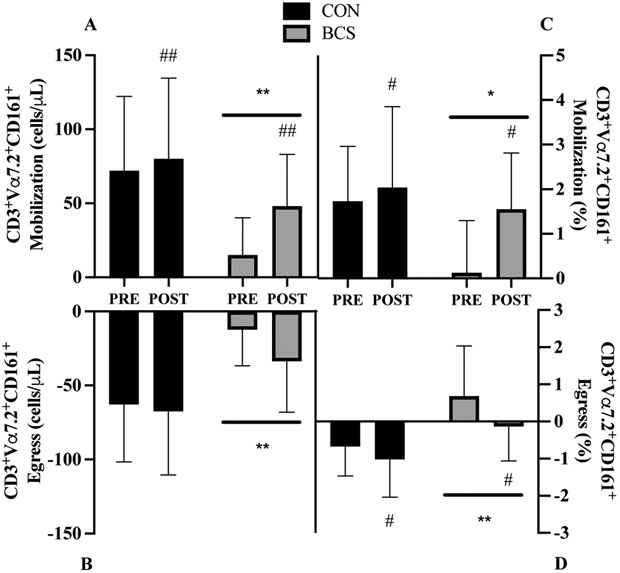
3.9. MAIT Cell Count Mobilization and Egress:
There was a borderline significant group x training interaction (p=0.095, Figure 4A), with BCS demonstrating a 2-fold increase at POST (33 cells/μL, 95% CI 27, 61; d=1.1) while CON was unchanged (8 cells/μL, 95% CI −13, 29; d=0.2). Overall, training increased mobilization by 46% at POST (20 cells/μL, 95% CI 6, 35; p=0.009). BCS mobilization was also 58% lower than CON (45 cells/μL, 95% CI 15,74; d=1.0; p=0.007). There was a trend for a 34% greater egress at POST (−13 cells/μL, 95% CI −26, 0; d=0.4; p=0.056, Figure 4B). BCS cell egress was 65% lower overall (42 cells/μL, 95% CI −67, - 18; d=1.2; p=0.002).
Figure 4.
MAIT cell A) mobilization and B) egress for absolute cell count and C) mobilization and D) egress for relative frequency before (PRE) and after (POST) exercise training. Mobilization was the change from baseline to 0h. Egress was the change from the 0h to 1h. Data are mean (SD). Abbreviations: BCS, breast cancer survivor, CON, healthy control.
*P<0.05 and ** P<0.01 vs. CON
# P<0.05 and ## P<0.01 vs. PRE
3.10. MAIT Cell Frequency Mobilization and Egress:
There was a borderline significant interaction trend (p=0.089, Figure 4C) for mobilization, with MAIT cell frequency increasing in BCS (1.4%, 95% CI 0.5, 2.3; d=1.0) while CON did not (0.3%, 95% CI −0.6, 1.2; d=0.2). Independent of group, mobilization increased at POST (0.9%, 95% CI 0.04, 2.3; d=0.9; p=0.011) while BCS mobilization was lower than CON (−1.0%, 95% CI 0.2, 1.9; d=0.8; p=0.028). Greater egress was observed at POST (−0.6%, 95% CI −0.1, −0.01; d=0.6; p=0.05, Figure 4D) and between groups (−1.1%, 95% CI −1.7, −0.6; d=1.1; p<0.001).
MAIT Cell Intracellular Cytokine Mobilization and Egress
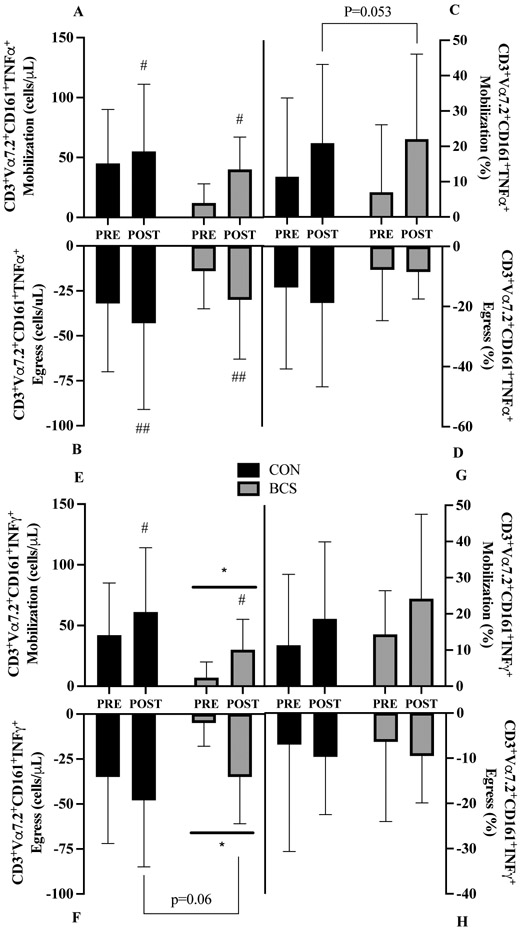
3.11. TNFα Cell Counts:
A borderline significant group x training interaction (p=0.094) for TNFα cell mobilization was present where BCS increased by 75% at POST (28 cells/μL, 95% CI −58, 3; d=1.4; Figure 5A) while CON was unchanged from PRE. There was a main effect of training, with 83% greater mobilization at POST (23 cells/μL, 95% CI 7, 38; p=0.007). For egress, there was nearly a 2-fold improvement at POST (41 cells/μL, 95% CI 19, 63; p<.001, Figure 5B) with no group effects or interaction.
Figure 5.
MAIT cell intracellular TNFα counts for A) mobilization and B) egress and TNFα frequency for C) mobilization and D) egress and intracellular IFNγ E) mobilization and F) egress and TNFα frequency for G) mobilization and H) egress before (PRE) and after (POST) exercise training. Mobilization was the change from baseline to 0h. Egress was the change from the 0h to 1h. Data are mean (SD). Abbreviations: BCS, breast cancer survivor, CON, healthy control.
*P<0.05 and ** P<0.01 vs. CON
# P<0.05 and ## P<0.01 vs. Pre
3.12. TNFα Cell Frequency:
At POST, TNFα proportions mobilization tended to be higher (12%, 95% CI 0.15, 24.4; d=1.0; p=0.053, Figure 5C) with no other effects noted. There were no interaction or main effects present for TNFα egress (Figure 5D).
3.13. IFNγ Cell Counts:
Overall, MAIT cell IFNγ mobilization increased by 88% at POST (21 cells/μL, 95% CI 10, 32; p<.001, Figure 5E). Independent of training, BCS mobilization was 65% lower (33 cells/μL, 95% CI 7, 59; p=0.020). For IFNγ egress, BCS was 70% lower (−29 cells/μL, 95% CI −47, −11; p=0.005, Figure 5F). Egress following training revealed a borderline significant increase at POST (−14 cells/μL, 95% CI −27, −1; d=0.5 p=0.060) with no group x training interaction.
3.14. IFNγ Cell Frequency:
There were no interactions or main effects present for MAIT cell IFNγ mobilization (Figure 5G) or egress (Figure 5H).
4. DISCUSSION
More women are now surviving early stage BC and with a greater life expectancy, these patients must live longer with the adverse effects of treatment that accelerate age-related declines. Exercise training reduces many of the side effects of treatments, although less is known on how the immune system responds to acute and chronic exercise. For the first time, the current study examines MAIT cell counts, percentages, and intracellular cytokine response to acute exercise in BCS relative to CON before and after 16 weeks of exercise training. At PRE, BCS MAIT cell counts and frequency following acute exercise were attenuated. The number, but not frequency, of cells expressing TNFα was also reduced in BCS due to lower circulating MAIT cell levels. At POST, exercise-induced mobilization and egress of MAIT cell counts and frequency showed trends to improve in BCS and approached levels in CON, suggesting that the immune system of BCS may respond better to an acute stimulus after training.
To contextualize our findings, we present the limitations and strengths of this study. Attendance was ~70% of sessions, leaving participants under physical activity guidelines (>150 min/week) for cancer survivors (Campbell et al., 2019) but was consistent with other supervised exercise interventions during BC (Kirkham et al., 2018; Winters-Stone et al., 2012). Exercise compliance was higher in CON and could have contributed to group differences, although improved mobilization and egress in BCS at POST suggest otherwise. Time since treatment was a confounding factor, as we did not have non-exercising BCS. However, the average time since the end of treatment was 3 months, with no correlation between days since last treatment and MAIT cell counts and frequency at baseline (data not shown). Finally, only a subset of the larger study completed the immune (day 3) trial, due to scheduling conflicts. As such, the study was likely underpowered to detect training induced changes, as evidenced by the multiple borderline significant group x training interactions with large effect sizes. Our study also had numerous strengths. We used a novel approach of combining acute and chronic exercise within the same study, whereas previously these factors have been examined independently. We report MAIT cell counts and frequencies, along with functional parameters and complete blood counts for comparisons with other studies, in accordance with current recommendations (Khosravi et al., 2019). We also included a non-cancer control group, which removed any ethical issues with randomizing BCS to usual care, that represents a normal response in older women for comparison.
4.1. MAIT Cells and Acute Exercise
We first examined the acute response to exercise in MAIT cells from BCS and CON prior to the 16 week intervention. While both groups demonstrated increased absolute MAIT cells counts, the increase in CON was nearly 5-fold greater (72 vs. 15 cells/uL) and suggets a mobilization deficit in BCS. The robust increase in MAIT cell numbers was consistent with observations from our CD3+CD8+ cytotoxic T cell counts, from which the majority of MAIT cells are derived (Hanson et al., 2017). During recovery, MAIT cell counts returned to resting levels but cell frequency remained elevated for both CON and BC and supports previous work from our laboratory (Hanson et al., 2019).
As comparisons within MAIT cells and acute exercise were not possible in BC or aging populations, other immune parameters and populations were evaluated. Although the MAIT cell and acute exercise have only been examined in healthy young men (Hanson et al., 2019, 2017), the response in CON was remarkably consistent with this previous work despite a number of key study differences, including an age gap of 35 years. While aging decreases resting MAIT cells (Lee et al., 2014; Novak et al., 2014), it does not appear to impact mobilization with exercise. The intermittent stimuli used in the current study increased absolute and relative MAIT cell counts and freqencies similarly to submaximal and maximal continuous exercise (Hanson et al., 2019, 2017). The attenuated MAIT cell response in BCS is consistent with other cell types, as women with BC demonstrated ~60% lower NK cell counts compared to healthy older women (Evans et al., 2015) that provides additional indirect support of reduced immune cell mobilization following acute exercise.
There are several possible explanations for the discrepancies between CON and BCS with acute exerise. Immune cell mobilization from the marginal pools results from increased in hemodynamic shear forces and catecholamines binding to their β-adrenergic receptors (Simpson et al., 2016). Shear stress was unlikely to contribute to group differences, as VO2peak and peak power output were comparable and intermittent exercise was performed using the same relative intensity. However, the exercise-induced rises in epinephrine are attenuated in women with BC (Evans et al., 2016). As MAIT cells express adrenergic receptors (Fergusson et al., 2014), a diminished epinepherine response may reduce detachment from the endothelial cells to allow MAIT cells to enter circulation. Chronic, non-resolving inflammation is commonly seen with BC treatment (Bower et al., 2011) and may permit enhanced tumorigenesis (Koelwyn et al., 2015) that reduces the immune response, potentially suppressing circulating numbers which may include MAIT cells.
4.2. MAIT Cells and Exercise Training
Next, we examined the response to acute exercise after 16 weeks of training. There were borderline significant interactions that suggests mobilization of MAIT cells improved in BCS at POST that approached levels in CON. As such, the deficits in BCS observed at PRE were partially rescued. Looking at the main effects, both group and time differences are present. However, it is evident that changes from PRE to POST are driven primarily by improvements in BCS. For MAIT cell frequency, the group x training trend suggests preferential deployment of MAIT cells occurs in BCS only at POST and reflects the greater number of cells in circulation. With a lack of MAIT cell changes with training for comparison, conventional T cells were examined. In BC survivors vs. usual care, no differences were reported after training for resting CD3+, CD4+ or CD8+ cells (Fairey et al., 2005; Hutnick et al., 2005; Nieman et al., 1994), although immune cells were suppressed compared to reference values (Hutnick et al., 2005). While it is possible that MAIT cells respond differently than conventional T cells, the combination of an acute stressor with chronic exercise may be an imporant consideration moving forward.
Compared to mobilization, MAIT cell egress was notably smaller. As a result, MAIT cells counts and frequency remained slightly elevated at 1h, although the implications of this are not yet clear. On one hand, greater cell counts in the blood potentially provides additional protection against infection. As MAIT cells subsist better following chemotherapy (Dusseaux et al., 2011; Won et al., 2016), their importance in T cell immunity is likely to rise following anthracycline treatments that suppress conventional T cells. In contrast, tissue homing receptor proportions on MAIT cells does not changed with acute exercise (Hanson et al., 2019). While not part of the current study, greater MAIT cell numbers combined with constant homing receptor expression after exercise theoretically permits more MAIT cells to traffic into the lungs, gastrointestinal track, and liver, although the attenuated egress observed suggests otherwise.
Greater cell numbers with acute and chronic exercise is promising yet incomplete in the absence of functional capacity. Upon MR1 activation, MAIT cells produce TNFα and IFNγ to combat bacterial and viral infections (Sundström et al., 2015). In the current study, TNFα+ and IFNγ+ MAIT cell counts at PRE increased at 0h but are likely a function of greater MAIT cell numbers alone, as the frequency of cells expressing these intracellular cytokines was unchanged. This contrasts our hypothesis that acute exercise increases sensitivity to mitogenic stimulation. Interestingly, at POST there were higher TNFα+ counts but also greater frequency, the latter being indicative of a greater response to stimulation. As BCS and CON demonstrate higher TNFα proportions only after the exercise intervention and trained men show a similar rise with acute exercise (Hanson et al., 2019), we hypothesize that improved sensitivity to stimulation partly depends on training status.
MAIT cell activation occurs either via MR1 or circulating cytokines (Hinks and Zhang, 2020; Shey et al., 2018). Following vigorous skeletal muscle contractions, numerous myokines are released in substantial quantities (Pedersen and Febbraio, 2012). Among them, IL-6 appears critical to the mobilization of select immune cells, with receptor blockade diminishing the NK but not the total T cell response to acute exercise (Bay et al., 2020). Our laboratory recently assessed IL-6 changes following acute exercise, where levels were ~33% higher breast cancer survivors and ~60% higher in healthy women but did not differ between groups (Bartlett et al., unpublished data). As the acute IL-6 response did not improve with training, it seems unlikely that IL-6 directly influenced the greater MAIT cell mobilization in BCS at POST. However, exercise training reduces pro-inflammatory biomarkers in cancer survivors (Khosravi et al., 2019), including C-reactive protein and TNFα in women with BC completing combined training interventions. As IL-6 attenuates endotoxin-induced TNFα production (Starkie et al., 2003) regular exposure to myokines appears to be one means of reducing inflammation in cancer survivors and thereby improving immune function (Koelwyn et al., 2015). IL-7 is another myokine known for its role in T cell development and has both direct and indirect anti-tumor functions (Lin et al., 2017; Melchionda et al., 2005; Rosenberg et al., 2006). Additionally, IL-7 stimulates MAIT cell IL-17 production (Jiang et al., 2018) and increases circulating MAIT cell counts and frequencies during HIV (Sortino et al., 2018). We speculate the repeated bouts of acute exercise with concomitant IL-7 release may contribute to improved MAIT cell mobilization and sensitivity to stimulated cytokine production that may also help boost immunity following anti-cancer therapies.
4.3. Implications
Improvements in MAIT cell numbers and function using exercise have several possible benefits in women recovering from BC but also older adults. While the clinical relevance of MAIT cells in BC still needs to be established, increased MAIT cells may offer a degree of protection against secondary cancer risk following BC treatment, which included mucosal-associated cancers (Silverman et al., 2016). For example, MAIT cells demonstrate direct cytotoxic effects against colon cancer (Sundström et al., 2015), with disease progression being related to circulating MAIT cell deficiency (Won et al., 2016). As MAIT cells have demonstrated chemotherapy resistance (Dusseaux et al., 2011), this makes these cells an intriguing target for improving immunity with exercise during BC, as well as other cancers (Won et al., 2016). More generally, life-long exercise in aged individuals shows numerous benefits to T cells (which likely includes MAIT cells), although longitudinal studies do not consistently support these findings (Simpson et al., 2012). However, the use of acute exercise before and after exercise interventions may provide a unique approach to identify both age- and cancer-related immuno-dysfunction that is less apparent at rest, with the cumulative effects of repeated exercise bouts potentially being critical to controlling BC tumor growth (Dethlefsen et al., 2016).
4.4. Conclusions
In summary, BCS initially demonstrated an attenuated MAIT cell response to acute exercise compared with healthy older women. Despite the exercise dose falling short of current exercise oncology guidelines, 16 weeks of exercise training enhanced the BCS MAIT cell response approaching the response of CON, including greater sensitivity to ex vivo stimulation. With their resistance to chemotherapy, MAIT cells show potential to respond to exercise and may have a greater role in immunity following BC treatment, although this still needs to be established. Future studies should consider acute and chronic exercise in combination while examining additional immune populations to provide confirmation of these findings.
Supplementary Material
HIGHLIGHTS.
MAIT cell mobilization is attenuated in breast cancer survivors with acute exercise
Following training, MAIT cell mobilization is partially rescued
After training, higher TNF levels suggests greater sensitivity to stimulation
Combining acute and chronic exercise provides a unique way to study MAIT cells
ACKNOWLEDGEMENTS
The authors would like to thank the participants for their efforts during testing and training, Michael Bass, William Evans, Kaileigh Moertl, Cameron Stopforth, and Stephanie Sullivan for assistance with data collection and administrating the exercise intervention, and Janet Dow and Ramiro Diaz for assistance with the flow cytometry experiments.
FUNDING
This study was supported by the Breast Cancer Research Foundation (New York, NY). The UNC Flow Cytometry Core Facility is supported in part by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
None
REFERENCES
- Bay ML, Heywood S, Wedell-Neergaard A-S, Schauer T, Lehrskov LL, Christensen RH, Legård GE, Jensen PØ, Krogh-Madsen R, Ellingsgaard H, 2020. Human immune cell mobilization during exercise: effect of IL-6 receptor blockade. Exp. Physiol n/a. [DOI] [PubMed] [Google Scholar]
- Billerbeck E, Kang Y-H, Walker L, Lockstone H, Grafmueller S, Fleming V, Flint J, Willberg CB, Bengsch B, Seigel B, Ramamurthy N, Zitzmann N, Barnes EJ, Thevanayagam J, Bhagwanani A, Leslie A, Oo YH, Kollnberger S, Bowness P, Drognitz O, Adams DH, Blum HE, Thimme R, Klenerman P, 2010. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc. Natl. Acad. Sci. U. S. A 107, 3006–3011. 10.1073/pnas.0914839107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW, 2011. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J. Clin. Oncol 29, 3517–3522. 10.1200/JCO.2011.36.1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Winters-Stone KM, Wisemann J, May AM, Schwartz AL, Courneya KS, Zucker DS, Matthews CE, Ligibel JA, GERBER LH, MORRIS GS, PATEL AV, HUE TF, PERNA FM, SCHMITZ KH, 2019. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sport. Exerc 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen C, Lillelund C, Midtgaard J, Andersen C, Pedersen BK, Christensen JF, Hojman P, 2016. Exercise regulates breast cancer cell viability: systemic training adaptations versus acute exercise responses. Breast Cancer Res. Treat 159, 469–479. 10.1007/s10549-016-3970-1 [DOI] [PubMed] [Google Scholar]
- Dethlefsen C, Pedersen KS, Hojman P, 2017. Every exercise bout matters: linking systemic exercise responses to breast cancer control. Breast Cancer Res. Treat 162, 399–408. 10.1007/s10549-017-4129-4 [DOI] [PubMed] [Google Scholar]
- Dill DB, Costill DL, 1974. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J. Appl. Physiol 37, 247–248. 10.1152/jappl.1974.37.2.247 [DOI] [PubMed] [Google Scholar]
- Dusseaux M, Martin E, Serriari N, Pϩguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E, Lantz O, 2011. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117, 1250–1259. [DOI] [PubMed] [Google Scholar]
- Evans ES, Hackney AC, McMurray RG, Randell SH, Muss HB, Deal AM, Battaglini CL, 2015. Impact of Acute Intermittent Exercise on Natural Killer Cells in Breast Cancer Survivors. Integr. Cancer Ther 14, 436–445. 10.1177/1534735415580681 [DOI] [PubMed] [Google Scholar]
- Evans ES, Hackney AC, Pebole MM, McMurray RG, Muss HB, Deal AM, Battaglini CL, 2016. Adrenal Hormone and Metabolic Biomarker Responses to 30 min of Intermittent Cycling Exercise in Breast Cancer Survivors. Int. J. Sports Med 37, 921–929. 10.1055/s-0042-110654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairey A, Courneya K, Field C, Bell G, Jones L, Mackey J, 2005. Randomized controlled trial of exercise and blood immune function in postmenopausal breast cancer survivors. J. Appl. Physiol 1534–40. [DOI] [PubMed] [Google Scholar]
- Fergusson JR, Smith KE, Fleming VM, Rajoriya N, Newell EW, Simmons R, Marchi E, Björkander S, Kang Y-H, Swadling L, Kurioka A, Sahgal N, Lockstone H, Baban D, Freeman GJ, Sverremark-Ekström E, Davis MM, Davenport MP, Venturi V, Ussher JE, Willberg CB, Klenerman P, 2014. CD161 defines a transcriptional and functional phenotype across distinct human T cell lineages. Cell Rep. 9, 1075–1088. 10.1016/j.celrep.2014.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson CE, Jadhav R, Cao W, Qi Q, Pegram M, Tian L, Weyand CM, Goronzy JJ, 2020. Immune cell repertoires in breast cancer patients after adjuvant chemotherapy. JCI Insight 5. 10.1172/jci.insight.134569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom AD, Marshall PWM, Lonsdale C, Papalia S, Cheema BS, Toben C, Baune BT, Fiatarone Singh MA, Green S, 2016. The effect of resistance training on markers of immune function and inflammation in previously sedentary women recovering from breast cancer: a randomized controlled trial. Breast Cancer Res. Treat 155, 471–482. 10.1007/s10549-016-3688-0 [DOI] [PubMed] [Google Scholar]
- Hanson ED, Bates LC, Bartlett DB, Campbell JP, 2021. Does exercise attenuate age- and disease-associated dysfunction in unconventional T cells? Shining a light on overlooked cells in exercise immunology. Eur. J. Appl. Physiol 10.1007/s00421-021-04679-4 [DOI] [PubMed] [Google Scholar]
- Hanson ED, Danson E, Evans WS, Wood WA, Battaglini CL, Sakkal S, 2019. Exercise Increases Mucosal-associated Invariant T Cell Cytokine Expression but Not Activation or Homing Markers. Med. Sci. Sports Exerc 51, 379–388. 10.1249/MSS.0000000000001780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson ED, Danson E, Nguyen-Robertson CV, Fyfe JJ, Stepto NK, Bartlett DB, Sakkal S, 2017. Maximal exercise increases mucosal associated invariant T cell frequency and number in healthy young men. Eur. J. Appl. Physiol 117, 2159–2169. 10.1007/s00421-017-3704-z [DOI] [PubMed] [Google Scholar]
- Hanson ED, Hurley BF, 2011. Intervening on the side effects of hormone-dependent cancer treatment: The Role of strength training. J. Aging Res 2011. 10.4061/2011/903291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson ED, Sakkal S, Evans WS, Violet JA, Battaglini CL, McConell GK, Hayes A, 2018. Altered stress hormone response following acute exercise during prostate cancer treatment. Scand. J. Med. Sci. Sports 28, 1925–1933. 10.1111/sms.13199 [DOI] [PubMed] [Google Scholar]
- Hanson ED, Sakkal S, Que S, Cho E, Spielmann G, Kadife E, Violet JA, Battaglini CL, Stoner L, Bartlett DB, 2020. Natural killer cell mobilization and egress following acute exercise in men with prostate cancer. Exp. Physiol [DOI] [PubMed] [Google Scholar]
- Hinks TSC, Zhang X-W, 2020. MAIT Cell Activation and Functions. Front. Immunol, 11, 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutnick N, Williams N, Kraemer W, Orsega-Smith E, Dixon R, Bleznak A, 2005. Exercise and lymphocyte ac- tivation following chemotherapy for breast cancer. Med Sci Sport Exer 1827–35. [DOI] [PubMed] [Google Scholar]
- Jiang X, Lian M, Li Y, Zhang W, Wang Q, Wei Y, Zhang J, Chen W, Xiao X, Miao Q, Bian Z, Qiu D, Fang J, Ansari AA, Leung PSC, Coppel RL, Tang R, Gershwin ME, Ma X, 2018. The immunobiology of mucosal-associated invariant T cell (MAIT) function in primary biliary cholangitis: Regulation by cholic acid-induced Interleukin-7. J. Autoimmun 90, 64–75. 10.1016/j.jaut.2018.01.007 [DOI] [PubMed] [Google Scholar]
- Kang D-H, Weaver MT, Park N-J, Smith B, McArdle T, Carpenter J, 2010. Significant Impairment in Immune Recovery Following Cancer Treatment 58, 105–114. 10.1097/NNR.0b013e31818fcecd.Significant [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi N, Stoner L, Farajivafa V, Hanson ED, 2019. Exercise training, circulating cytokine levels and immune function in cancer survivors: A meta-analysis. Brain. Behav. Immun 81, 92–104. 10.1016/J.BBI.2019.08.187 [DOI] [PubMed] [Google Scholar]
- Kirkham AA, Bonsignore A, Bland KA, Mckenzie DC, Gelmon KA, Van Patten CL, Campbell KL, 2018. Exercise prescription and adherence for breast cancer: one size does not FITT all. Med Sci Sport. Exerc 50, 177–186. [DOI] [PubMed] [Google Scholar]
- Koelwyn GJ, Wennerberg E, Demaria S, Jones LW, 2015. Exercise in Regulation of Inflammation-Immune Axis Function in Cancer Initiation and Progression. Oncology (Williston Park). 29, 908–922. [PMC free article] [PubMed] [Google Scholar]
- Lee O-J, Cho Y-N, Kee S-J, Kim M-J, Jin H-M, Lee S-J, Park K-J, Kim T-J, Lee S-S, Kwon Y-S, Kim N, Shin M-G, Shin J-H, Suh S-P, Ryang D-W, Park Y-W, 2014. Circulating mucosal-associated invariant T cell levels and their cytokine levels in healthy adults. Exp. Gerontol 49, 47–54. 10.1016/J.EXGER.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Lin J, Zhu Z, Xiao H, Wakefield MR, Ding VA, Bai Q, Fang Y, 2017. The role of IL-7 in Immunity and Cancer. Anticancer Res. 37, 963–967. [DOI] [PubMed] [Google Scholar]
- Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL, 2005. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J. Clin. Invest 115, 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman DC, Cook VD, Henson DA, Suttles J, Rejeski WJ, Ribisl PM, Fagoaga OR, Nehlsen-Cannarella SL, 1995. Moderate exercise training and natural killer cell cytotoxic activity in breast cancer patients. Int. J. Sports Med 16, 334–337. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Miller AR, Henson DA, Warren BJ, Gusewitch G, Johnson RL, Davis JM, Butterworth DE, Herring JL, Nehlsen-Cannarella SL, 1994. Effect of high-versus moderate-intensity exercise on lymphocyte subpopulations and proliferative response. Int. J. Sports Med 15, 199–206. 10.1055/s-2007-1021047 [DOI] [PubMed] [Google Scholar]
- Novak J, Dobrovolny J, Novakova L, Kozak T, 2014. The Decrease in Number and Change in Phenotype of Mucosal-Associated Invariant T cells in the Elderly and Differences in Men and Women of Reproductive Age. Scand. J. Immunol 80, 271–275. 10.1111/sji.12193 [DOI] [PubMed] [Google Scholar]
- Nyrop KA, Deal AM, Shachar SS, Basch E, Reeve BB, Choi SK, Lee JT, Wood WA, Anders CK, Carey LA, Dees EC, Jolly TA, Reeder-Hayes KE, Kimmick GG, Karuturi MS, Reinbolt RE, Speca JC, Muss HB, 2019. Patient-Reported Toxicities During Chemotherapy Regimens in Current Clinical Practice for Early Breast Cancer. Oncologist 24, 762–771. 10.1634/theoncologist.2018-0590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA, 2012. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol 8, 457–465. 10.1038/nrendo.2012.49 [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Sportès C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, Stetler-Stevenson M, Morton KE, Mavroukakis SA, Morre M, Buffet R, Mackall CL, Gress RE, 2006. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J. Immunother 29, 313–319. 10.1097/01.cji.0000210386.55951.c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T, Van Mackelenbergh M, Wesch D, Mundhenke C, 2017. Physical activity influences the immune system of breast cancer patients. J Can Res Ther 13, 392–398. [DOI] [PubMed] [Google Scholar]
- Schmitz KH, Cappola AR, Stricker CT, Sweeney C, Norman SA, 2007. The Intersection of Cancer and Aging: Establishing the Need for Breast Cancer Rehabilitation. Cancer Epidemiol. Biomarkers & Prev 16, 866 LP – 872. 10.1158/1055-9965.EPI-06-0980 [DOI] [PubMed] [Google Scholar]
- Shey MS, Balfour A, Wilkinson KA, Meintjes G, 2018. Contribution of APCs to mucosal-associated invariant T cell activation in infectious disease and cancer. Innate Immun. 24, 192–202. 10.1177/1753425918768695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A, 2020. Cancer statistics, 2020. CA. Cancer J. Clin 70, 7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- Silverman BG, Lipshitz I, Keinan-Boker L, 2016. Second Primary Cancers After Primary Breast Cancer Diagnosis in Israeli Women, 1992 to 2006. J. Glob. Oncol 3, 135–142. 10.1200/JGO.2016.003699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson RJ, Bigley AB, Spielmann G, LaVoy ECP, Kunz H, Bollard CM, 2016. Human cytomegalovirus infection and the immune response to exercise. Exerc. Immunol. Rev 22, 8–27. [PubMed] [Google Scholar]
- Simpson RJ, Lowder TW, Spielmann G, Bigley AB, LaVoy EC, Kunz H, 2012. Exercise and the aging immune system. Ageing Res. Rev 11, 404–420. [DOI] [PubMed] [Google Scholar]
- Sortino O, Richards E, Dias J, Leeansyah E, Sandberg JK, Sereti I, 2018. IL-7 treatment supports CD8+ mucosa-associated invariant T-cell restoration in HIV-1-infected patients on antiretroviral therapy. AIDS 32, 825–828. 10.1097/QAD.0000000000001760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK, 2003. Exercise and IL- 6 infusion inhibit endotoxin- induced TNF- α production in humans. FASEB J. 17, 1–10. [DOI] [PubMed] [Google Scholar]
- Sundström P, Ahlmanner F, Akéus P, Sundquist M, Alsén S, Yrlid U, Börjesson L, Sjöling Å, Gustavsson B, Wong SBJ, Quiding-Järbrink M, 2015. Human Mucosa-Associated Invariant T Cells Accumulate in Colon Adenocarcinomas but Produce Reduced Amounts of IFN-γ. J. Immunol 195, 3472 LP – 3481. 10.4049/jimmunol.1500258 [DOI] [PubMed] [Google Scholar]
- Sundström P, Szeponik L, Ahlmanner F, Sundquist M, Wong JSB, Lindskog EB, Gustafsson B, Quiding-Järbrink M, 2019. Tumor-infiltrating mucosal-associated invariant T (MAIT) cells retain expression of cytotoxic effector molecules. Oncotarget 10, 2810–2823. 10.18632/oncotarget.26866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao JJ, Visvanathan K, Wolff AC, 2015. Long term side effects of adjuvant chemotherapy in patients with early breast cancer. The Breast 24, S149–S153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verronèse E, Delgado A, Valladeau-Guilemond J, Garin G, Guillemaut S, Tredan O, Ray-Coquard I, Bachelot T, N’Kodia A, Bardin-Dit-Courageot C, Rigal C, Pérol D, Caux C, Ménétrier-Caux C, 2016. Immune cell dysfunctions in breast cancer patients detected through whole blood multi-parametric flow cytometry assay. Oncoimmunology 5, e1100791. 10.1080/2162402X.2015.1100791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters-Stone KM, Dobek J, Bennett JA, Nail LM, Leo MC, Schwartz A, 2012. The effect of resistance training on muscle strength and physical function in older, postmenopausal breast cancer survivors: A randomized controlled trial. J. Cancer Surviv 6, 189–199. 10.1007/s11764-011-0210-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won EJ, Ju JK, Cho Y, Jin H, Park K, Kim T, Kwon Y, Kee HJ, Kim J, Kee S, Park Y, 2016. Clinical relevance of circulating mucosal-associated invariant T cell levels and their anti-cancer activity in patients with mucosal-associated cancer 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumwalde NA, Haag JD, Gould MN, Gumperz JE, 2018. Mucosal associated invariant T cells from human breast ducts mediate a Th17-skewed response to bacterially exposed breast carcinoma cells 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.