Abstract
MYC oncogene is a transcription factor with a wide array of functions affecting cellular activities such as cell cycle, apoptosis, DNA damage response, and hematopoiesis. Due to the multi-functionality of MYC, its expression is regulated at multiple levels. Deregulation of this oncogene can give rise to a variety of cancers. In this review, MYC regulation and the mechanisms by which MYC adjusts cellular functions and its implication in hematologic malignancies are summarized. Further, we also discuss potential inhibitors of MYC that could be beneficial for treating hematologic malignancies.
Keywords: MYC, Oncogene, Regulation, Cell cycle, Apoptosis, DNA damage response, Prognostic importance, Therapeutic implications, Hematological malignancies
Introduction
MYC (mostly referred to as c-Myc) is a super-transcription factor encoded by the MYC gene located at chromosome 8 q24.21 [1]. The MYC oncoproteins (C-myc, N-myc, and L-myc) controls the transcription of nearly 15% of expressed genes [2]. MYC's main downstream mediators, including those participating in ribosome biogenesis, mRNA translation, cell-cycle regulation, and stress responses, impact a vast range of biological events, such as proliferation, differentiation, survival, programmed cell death, and immune regulation [2, 3].
There is a high level of architectural homology in the motifs at the flanked domains of the MYC family members, including the basic-region (BR), helix-loop-helix (HLH), and leucine-zipper (LZ) in C-terminal, and three extremely conserved regions called MYC boxes 1–3 (MB 1–3) at the N-terminal [3–5]. MYC creates a heterodimer with its co-factor, Max (MYC/Max), via BR, HLH, and LZ motifs requisite for DNA–protein interactions (Fig. 1) [3–5]. The chromatin-modifying complex consisting of TIP60, TRRAP, TIP48, and GCN5 recruited by MYC/Max heterodimer propels transcription through binding to the E-box DNA region (CACGTG) within the regulatory domain of target genes [3–5]. Accumulation of MYC at the promoter sequences of target genes can also augment the transcriptional activity of genes (Fig. 2) [6].
Fig. 1.

Crystal structure of MYC/MAX heterodimer. MYC usually forms as a heterodimer with MAX (MYC/MAX) to bind to DNA in E-box region (CACGTG). This structure mainly contains the basic-region (BR), helix-loop-helix (HLH), and leucine-zipper (LZ), which are required for DNA binding
Fig. 2.
Schematics of MYC protein and its transcriptional activity. A: MYC gene on chromosome 8 alongside MYC protein (439 aa) that mainly contains the basic-region (BR), helix-loop-helix (HLH), and leucine-zipper (LZ) at C-terminal, and three extremely conserved regions called MYC boxes 1–3 (MB 1–3) at the N-terminal. B: The chromatin-modifying complex consisting of TIP60, TRRAP, TIP48, and GCN5 recruited by MYC/Max heterodimer propels transcription through binding to the E-box DNA region (CACGTG) within the regulatory domain of target genes. C: the accumulation of MYC at the promoter sequences of target genes increases the transcriptional activity. NLS nuclear localization sequence
The MYC expression pattern is tightly regulated in normal conditions, though MYC is often dysregulated in cancers. Retroviral integration, chromosomal rearrangements, activation of super-enhancers of its gene, and mutations in signaling pathways related to MYC can promote MYC's instability and overexpression [3]. The MYC expression is highly controlled at several levels, including transcription (initiation and elongation), mRNA stability, translation, and post-translation (protein stability). MYC is a very short-lived protein with a half-life of about 20–30 min because of quick turnover through the ubiquitin–proteasome system [7]. Consequently, the MYC protein level is strongly controlled by ubiquitin–proteasome degradation.
The pivotal role of MYC in the cell cycle regulation and the proliferation rate has been deeply investigated in several studies. Reduced need for growth factors, increased cell division, and size can be seen in response to transfection or transduction with MYC [8–10]. Entering and exiting cell-cycle is achievable by decreasing or increasing MYC expression [11, 12]. After mitogenic stimulation of MYC expression, which is undetectable in quiescent cells, MYC increases rapidly and mediates cell entry to the G1 phase. This is followed by a decrease in MYC mRNA and protein levels [13]. A better understanding of cell-cycle regulation by MYC helps find novel therapeutic approaches to target the MYC.
The role of MYC in cell damage has been investigated in numerous studies. In DNA damage caused by UV irradiation or other agents, MYC levels are decreased through different mechanisms, including alternation in MYC transcription and protein turnover [14–16]. The results of several studies exhibit that decreased levels of MYC are seen as a DNA damage response (DDR) [15, 17, 18]. A decreased MYC levels and accumulation of p53 in DDR is a normal response to regulating cell damage [14]. MYC promotes apoptosis via increasing the p53 levels indirectly, in turn, p53 suppresses MYC expression. DNA repair inhibition, ROS generation, and increased replication stress are among the MYC-induced DDR mechanisms [19]. In cancer however, this fine-tuned interplay between p53 and MYC is mostly deregulated.
The first oncogene reported to induce apoptosis was MYC [20]. A well-known fundamental function of MYC is induction of apoptosis. MYC transcription factor has a dual role in tumor cells. It can activate and repress various downstream pathways that can induce proliferation or apoptosis [6]. Apoptosis has a role in physiological processes, such as embryonic development, tissue morphogenesis cellular hemostasis life. Hence, MYC-induced apoptosis indicates this transcription factor's normal function in controlling cell death [21]. Indeed, MYC exerts a safeguard mechanism by induction of apoptosis. It should be noted that a higher level of MYC is required for apoptosis compared to the concentrations needed to trigger cell proliferation, indicating that under normal conditions, cells are able to proliferate [22].
The MYC is a “global” transcription factor contributing to various cellular processes, one of which is hematopoiesis. In the bone marrow (BM) of adults, 300 million cells are produced every minute [23]. Regulation of hematopoiesis requires cell–cell interactions, cytokines, and coordinated activity of transcription factors. Studies have revealed that MYC has a significant role in nearly every step of the way [23, 24].
Uncontrolled MYC expression is observed in human leukemias and lymphomas. Generally, MYC overexpression does not stem from point mutations in the gene [25–27]. Rather in hematological malignancies such as acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), and myeloid neoplasms, overexpression is mainly due to the gene amplification, chromosomal translocations, and dysregulation at the transcriptional level [28]. Overall, given MYC’s functions, it is not surprising that deregulation and deletion of MYC can contribute to tumorigenesis, particularly in hematological cells.
Aberrant MYC expression usually confers a poor prognosis. Targeting the MYC family, especially MYC, is of utmost significance in identifying treatment options for hematological malignancies [29]. Here, we explain the role of MYC in various cellular functions, including cell cycle, MYC-mediated DDR, and apoptosis, as well as MYC regulatory processes. In particular, different types of hematological malignancies and their association with MYC deregulation have been thoroughly discussed in this review along with the effects of various MYC inhibitors.
MYC regulation
MYC regulation and transcriptional activity are critical to maintaining normal cellular processes such as cell growth, differentiation, and programmed cell death. Deregulation of MYC oncogene has been shown to contribute to more than half of human cancers [4, 30].
The mechanisms that control MYC transcription are complex. Several promoters of MYC such as P0, P1, P2, P3, and initiation regions are involved. Multiple signals, transcription factors, and chromatin components have a role in the regulation of MYC mRNA levels [31, 32]. The nuclear factor of activated T cells (NFAT) family of transcription factors includes four Ca2+-regulated members (NFAT1-NFAT4) initially discovered in T lymphocyte as transcriptional activators of interleukin 2 [33]. Previous studies indicate that NFAT1/2 can regulate MYC gene expression by binding to specific sequence elements within the proximal MYC promoter [34]. Mognol and et al. demonstrated that the Ca2+\calcineurin\NFAT1 signaling pathway in mouse T lymphocyte regulates MYC expression, the difference is that NFAT1 binds to the distal site of the MYC promoter. Since the lack of NFAT1 in the studied cells shows decreased levels of MYC, NFAT1 is known as a positive regulator of MYC expression [35].
In addition to transcriptional regulation, MYC stability and activity are regulated by several post-translational modifications (PTM), such as phosphorylation, acetylation, methylation, ubiquitination, sumoylation, and glycosylation. There are multiple domains in MYC that different proteins interact with. The transactivation domain (TAD), is a 143 amino acid acidic domain localized at the N-terminus. It contains two conserved regions, Myc box (MB) I and II, mainly required for MYC regulation and cofactor recruitment, respectively [36, 37]. MYC contains two phosphorylation sites near its within MB I, Threonine 58 (Thr-58), and Serine 62 (Ser-62), which are highly conserved across all mammalian MYC isoforms [38, 39].
Phosphorylation of Ser-62 MYC by extracellular receptor kinase (ERK) and cyclin-dependent protein kinase 2 (CDK2) lead to stabilizing MYC whereas Thr-58 phosphorylation by glycogen synthase kinase (GSK-3β) results in degradation of MYC through the ubiquitin–proteasome pathway [40]. It has been shown that both Raf\MEK\ERK kinase cascade and the phosphoinositide 3-kinases (PI3K) \Akt signaling pathway significantly elevate the half-life of MYC through negative feedback. Mitogenic stimulation can promote production and stability of Myc and activation of Ras. Ras increases MYC protein stability by ERK-mediated phosphorylation of Ser-62 [40, 41]. Ras induces activation of PI3K\Akt cascade that leads to preventing phosphorylation of Thr-58 by suppressing GSK-3β and stabilizing and elevating MYC protein levels. During the late G1 phase of the cell cycle, reduced Ras activity, leads to Akt signaling downregulation, which results in destabilization and degradation of MYC [42]. Studies show that interaction between Ser-62 and Thr-58 play a vital role in regulating MYC expression during induced cell proliferation.
Bromodomain protein 4 (BRD4) is an epigenetic and transcriptional regulator with intrinsic histone acetyltransferase (HAT) and\or kinase activities localized at its carboxy-terminal and amino-terminal domains, respectively [43]. Similar to GSK-3β, BRD4 directly interacts with Myc and phosphorylates it at Thr-58, resulting in Myc destabilization. GSK-3β is mostly cytoplasmic and translocates to the nucleus in response to inducing extrinsic signaling, but BRD4 is predominantly in nucleus thus, it is more likely that BRD4 plays a more critical role in maintaining hemostatic levels of Myc. Moreover, BRD4, ERK1, and Myc form a trimeric complex and regulator network to sustain hemostatic levels of Myc. On the contrary, Myc can suppress the HAT activity of BRD4 and thereby regulate BRD4 function while ERK1 inhibits the BRD4 kinase activity [44].
The Ras\Raf signaling cascade has an important role in the regulation of the MYC promoter. Small GTP-binding protein Ras promotes MYC expression by inducing the Raf\MAPK\MEK pathway. Platelet-derived growth factor (PDGF) receptors and Src kinase also can augment the activity of Ras proto-oncogene, which results in activating the mitogen-activated protein kinase (MAPK) pathway [45]. However, both PDGF receptors and Src mediate the induction of MYC expression independently of Ras. Indeed, in response to PDGF, Src activates a signaling pathway known as the Src pathway that culminates in the transcription of MYC. Src phosphorylates Vav2 mediator, resulting in the activation of Rho proteins such as Rho, Rac, and cell division complex 42 (cdc42). Evidence shows that activated Rac highly stimulates MYC promoter and increases MYC mRNA levels in NIH3T3 cells. Rho and cdc42 also induce MYC promoter and MYC expression [46].
Protein phosphatase 2A (PP2A) is a major substrate-specific Serine/Threonine phosphatase that regulates MYC protein levels. PP2A is a heterotrimeric protein composed of a scaffold A subunit, catalytic C subunit, and a third highly variable regulatory B subunit [47, 48]. Structural A and catalytic C subunits exist in two isoforms, α or β. Regulatory B subunits fall into more than 23 isoforms belonging to four unrelated families named B\B55, B′/B56, B″, and B‴. B56 subunits include α, β, γ, δ, and ε isoforms. B56α is the only B subunit able to negatively regulates MYC protein stability and function [49, 50]. PP2A complex targeting the MYC protein phosphorylated at Ser-62 and Thr-58, dephosphorylates Ser-62 residue and regulate MYC turnover through ubiquitin-mediated proteasomal degradation [50]. Moreover, PP2A, which contains the B56α subunit, also can activate GSK-3β by dephosphorylating it [51].
The Pin1 prolyl isomerase is an essential controller of the phosphorylation signaling pathway that explicitly recognizes and isomerize the phosphorylated Serine\Threonine-Proline (phospho(p)Ser\Thr-Pro) motifs [52]. Pin1 can also convert the cis conformation to trans. The double-phosphorylated MYC (pThr-58 and pSer-62) is recognized and undergoes isomerization by Pin1, which catalyzes conversion of Pro-63 Myc to the trans conformation. This isomerization at Pro-63 Myc makes it an ideal substrate for PP2A-B56α to remove stabilizing Ser-62 residue and targets pThr-58 Myc for ubiquitin-mediated proteasomal degradation by the E3 ubiquitin ligases [53–55]. Furthermore, the MYC can be a substrate for Pin1 directly. WW phospho-binding domain of Pin1 is required for interaction with MYC, which recognizes phosphorylated sites. Phosphorylation at Thr-58 and Ser-62 residues can affect Pin1 interaction with the MBI site of MYC. The evidence indicates that the role of Thr-58 compared with Ser-62 is more critical for Pin1 binding to MYC [56]. Pin1 also affects pSer-62 MYC through stabilizing Pro-63 in the cis conformation. This results in protecting Ser-62 phosphate from PP2A-B56α-mediated dephosphorylation. This function of Pin1increases Myc stability, prolongs its interaction with DNA, and alters its transcription activity [57]. Thus, Pin1 could have a dual function by catalyzing the conformational change between cis and trans.
The main mechanism for controlling Myc family protein turnover is ubiquitin-mediated degradation by different E3 ubiquitin ligases. MYC is poly-ubiquitinylated by several E3 ubiquitin ligases, including Fbw7, Skp2, TRUSS, HectH9, TRIM32, and CHIP [58–63]. Fbw7 (also named hCdc4 or hSel 10) is a well-known E3 ubiquitin ligase. It is a member of the F-box proteins family that are components of SCF-type (Skp-Cullin-F box) ubiquitin ligase [64]. The Fbw7 human gene encodes three isoforms (Fbw7α, Fbw7β, and Fbw7γ), by alternative splicing. These isoforms are distinct in their subcellular localization (Fbw7α: nucleoplasmic, Fbw7β: cytoplasmic, Fbw7γ: nucleolar). Among them, both the Fbw7α and Fbw7γ isoforms are involved in regulating Myc protein turnover [65, 66]. MYC phosphorylation at Thr-58 and Ser-62 is required for Fbw7 to regulate MYC stability. Fbw7 recognizes the phospho-degron sequence that includes Thr-58 and Ser-62 within MBI. They control the Fbw7-mediated turnover of MYC. When Pin1 and PP2A-B56α dephosphorylate Ser-62, Fbw7 E3 ligase recognizes pThr-58 and mediates degradation of MYC by 26S proteasome [54, 59].
The multi-domain scaffold protein Axin1 stimulates formation of a complex between GSK-3β, PP2A-B56α, Pin1 and MYC. This complex can undergo ubiquitin-mediated degradation to suppress MYC transcriptional activity. Chromatin immunoprecipitation detects Axin1 on Myc promoter along with Fbw7, GSK-3β, PP2A-B56α, Pin1 complex and parts of 26S proteasome [55, 57].
F-box protein Skp2 is another E3 ubiquitin ligase and belongs to the Cullin-RING ligase that is identified for MYC ubiquitination and degradation. Skp2 interacts with two conserved and functionally vital regions of the MYC, basic-helix-loop-helix-leucine zipper (bHLHZ) motif (amino acids 379–418) and MBII (amino acids 129–147). During G1 to S phase transition this stimulates MYC degradation [60, 67]. Unlike Fbw7, which is associated with the MBI domain of MYC, Skp2-mediated ubiquitination is phosphorylation independent [67]. Although Skp2 reduces MYC protein stability and induce its degradation, this complex has the opposite effect on MYC transcriptional activity, which means that Skp2 as a cofactor of MYC promotes its transcriptional activity [60].
In addition to Fwb7 and Skp2 as the two main pathways of ubiquitin-mediated degradation of MYC, the other pathways exist for Myc degradation. Chung and colleagues first reported Romo1 (Reactive oxygen species modulator 1) in 2006 as a protein that enhances cellular ROS levels [68]. Romo1 is located in the mitochondrial membrane and induces ROS release produced by complex III of the mitochondrial electron transport chain into the cytosol [69]. Indeed, Romo1 can cause cytoplasmic translocation of Skp2 and Myc promoting its ubiquitination and degradation. Romo1\ROS\Skp2 is another pathway involved in Myc turnover. Romo1 also can promote Skp2\Myc interaction and Myc ubiquitination. Lee et al. demonstrated in a negative feedback loop, Myc stimulates Romo1 expression to increase cellular ROS levels. ROS in turn enhances cytoplasmic translocation of Skp2, which results in Myc ubiquitination and degradation [70].
Li et al. studies indicate the 11S proteasome activator REGγ as a novel ubiquitination-independent pathway to promote MYC turnover [71]. Unlike the other two isoforms of REG (REGα, REG β) that are predominantly localized in the cytoplasm, REGγ is mainly located in the nucleus and related to the 20S proteasome [72]. REGγ can interact with MYC. The C-terminal domain of MYC is responsible for this interaction between REGγ and MYC. Ectopic expression of REGγ suppresses MYC transcriptional activity and promotes the degradation of MYC. This study showed that the knockdown of REGγ significantly elevates the stability of the MYC protein. REGγ also negatively regulates MYC-mediated gene expression and cell growth [71].
Pirh2 (p53-induced RING-H2 protein), also called Rchy1, has an important role in tumorigenesis with ubiquitin ligase activity [73]. As shown in the studies of Hakem et al., Pirh2 can control MYC stability through polyubiquitination and proteolysis of MYC. Of note, Skp2 interacts with MBII and C-terminal domain of MYC and N- and C-terminal domain of Pirh2. It was also shown that in Pirh2-knocked down human RKO cells and Pirh2 deficient murine cells, the level of MYC protein significantly increased. This shows that Fbw7, Skp2 and Pirh2 play a critical role in MYC turnover [74].
The transcription factor PLZF (promyelocytic leukemia zinc finger), also known as zbtb16, belongs to the POZ-Krüppel (POK) family that binds to a specific DNA sequence and regulate various biological process including cell proliferation, differentiation, and organ development [75]. Wild type (wt) PLZF directly binds to the MYC promoter, which mediates repression of the MYC promoter and reduces the level of MYC mRNA and phosphorylation [76]. PLZF can regulate MYC post-transcriptionally, through its impact on the Akt\MAPK pathway. Indeed, PLZF modulates the MAPK pathway, decreasing phosphorylation of MYC at Ser-62. As well, it reduces phosphorylation of Thr-58, resulting in increased MYC stability whereas reducing its transcriptional activity [77].
In recent decades, the importance of microRNAs (miRNAs) as oncogene\tumor suppressors has been recognized. miRNAs are short non-coding RNA molecules ranging from 21 to 25 nucleotides in length, which bind to a target sequence within the untranslated region (3'-UTR) of an mRNA [78, 79]. miRNAs can regulate gene expression in a post-transcriptional manner. miR-34c is a member of the miR-34 family that targets MYC during DNA damage. To restrict Myc-induced DNA synthesis, repression of MYC by miR-34c is a crucial event in response to DNA damage. It inhibits continuous DNA synthesis and proliferation in the face of damaged DNA [80, 81].
The role of MYC in cell cycle regulation
The pivotal role of MYC in the cell cycle regulation and the proliferation rate has been assessed in several studies (Figs. 3, 4) [8–10]. Entering and exiting cell cycle in quiescent cells is achievable by MYC regulation [11, 12]. MYC has an important role in entering the G1 phase. This phase is longer in MYC deficient rat fibroblasts in comparison to the wildtype-cells [82]. The human ubiquitin ligase HectH9 contributes in MYC-mediated cell cycle progression and activation of target genes. In human tumor cell lines lacking HectH9, cells cannot progress beyond the G1 phase of cell cycle [58]. The stability of MYC is regulated by the Raf-MEK-ERK and the PI3K-Akt cascades. ERK-mediated MYC phosphorylation at Ser26 protects it from degradation, while GSK-3β phosphorylates MYC at Thr58 and exposes it to ubiquitin–proteasome mediated degradation. In the early G1 phase, Ras-induced ERK activation leads to GSK-3β inhibition, but in the end of G1 phase, GSK-3β is activated upon decreased function of Ras-PI3K-Akt pathway [40, 83, 84].
Fig. 3.
A brief overview of MYC regulation. Multiple regulators from different classes are involved in MYC regulation. Red and green arrows point to the negative and positive regulatory effects of each factor on MYC, respectively
Fig. 4.
Diverse mechanisms are considered for MYC collaboration in cell cycle progression. The positive regulators of cell cycle are induced or activated by MYC. Through multiple mechanism, MYC blocks the activity of cell cycle regulators
Ectopic MYC expression induces cells to enter S-phase and mediates mitosis in the absence of growth factors [85]. Schuhmacher et al. studied the effects of fine-tuned MYC protein on proliferation rate and cell cycle distribution in human lymphoblastoid p493-6 cell line. They observed that the steady increase in the fraction of cells in the S- and G2/M- phase, increased proliferation rate, and cell size relies on high levels of MYC in a dose-dependent manner, [86, 87]. Using MYC antisense oligonucleotides in human lymphoid and myeloid cells prevents entry into S-phase [88, 89]. Depletion of MYC in 23 cell lines with short-hairpin in a systematic study led to cell cycle arrest in G0/G1 in normal and some tumor-derived cell lines, whereas G2/M arrest happened in other tumor-derived cell lines [90]. Cell proliferation is hampered by the action of Mxd proteins that antagonize MYC transcriptional activity [37, 91, 92]. Expression of MadMYC a dominant negative MYC mutant containing the DNA binding and dimerization domains of MYC and the trans-repression domain of Mxd1 (also called Mad1), can inhibit the cell cycle arrest [93, 94]. The expression of this mutant blocks CCNB1 (cyclin B1) upregulation following stimulation of starved cells with serum [95]. In response to moderate hypoxia, HIF-1α inhibits MYC causing cell cycle arrest, but HIF-2α can reverse it [96, 97]. The results of these studies reveal that MYC regulates cell cycle progression.
A family of serine-threonine protein kinases consisting of a regulatory subunit, cyclin, and a catalytic subunit or CDK [98, 99]. The levels of cyclin oscillate throughout the cell cycle, resulting in CDK activation. In contrast, the activity of CDKs remains stable during the cell cycle (CDK1, 2, 4, and CDK6 for G1 phase, CDK2 for S phase, and CDK1 for G2 and M phases) [100]. Decreased activity of CDK4, 6 and CDK2 as well as prolonged G1- and G2-phase were seen in MYC-deficient cells [101]. Hypophosphorylated Rb sequesters and forms a complex with E2F1 transcription factor to suppress transcription of genes related to S-phase [102, 103]. At the early G1 phase, Rb is phosphorylated and E2F transcription factors are released upon the activation of CDK4/6 by D-type cyclins [104, 105]. G1/S transition relies on the CDK2 activation by cyclin E. Transition to S phase requires the expression of E2F target genes, which is dependent on further Rb phosphorylation by the action of cyclin E1/2-CDK2 in the end of G1 phase [106]. Cyclin E is degraded and replaced by cyclin A that is required for DNA replication and transition from S to M phase [107, 108]. CDK1 activation by B-type cyclins promotes transition into M phase [98, 109].
CDK inhibitory proteins (CKIs) comprising of the INK4 and the CIP/KIP (CDK interacting protein/kinase inhibitory protein) families downregulate CDKs and [110, 111]. The members of INK4 family including p15, p16, p18, and p19 bind to CDK4/6 to hamper their kinase activity and impair the CDK4/6-cyclin D interaction [110, 111]. p15 and p16 impede Rb phosphorylation and S-phase entry [112]. In response to high levels of p15, cell proliferation as the consequence of p27 redistribution from cyclin D-CDK4/6 to cyclin E-CDK2 is blocked [113].
ARF (alternative reading frame) gene is located in the INK4A/ARF/INK4B locus on chromosome 9p21 and shares the exon 2 and 3 with p16. ARF causes cell cycle arrest in G1 and G2 and favors MYC-mediated apoptosis via both p53- dependent and -independent pathways [114–116]. ARF sequesters MDM2 from p53, which is followed by p53 stabilization and activation and after that induction of p21 and other proteins triggers apoptosis in a p53-dependent pathway [117]. Moreover, ARF interaction with MYC has been shown in several studies [118, 119]. Following the elevation of MYC levels, ARF binds with MYC and prevents its transactivation ability to induce hyperproliferation and transformation; albeit, ARF cannot prevent MYC-induced apoptosis. The underlying mechanism is possibly due to the transrepression of particular anti-apoptotic genes by MYC [120–123].
The members of CIP/KIP family (p21, p27, and 57) are involved in the suppression of cyclinA, E, D/CDK complex catalytic activity [124–126]. MYC engages in cell cycle regulation not only by the upregulating of genes necessary for cell cycle progression but also by impairing the negative regulators of cell cycle [127, 128]. Different mechanisms are considered to explain MYC participation in cell cycle regulation. MYC- mediated E2F family induction by binding to E box in their promoter leads to S-phase progression [129–132]. In addition, RB phosphorylation by cyclin/CDK complexes rescues E2F transcription factors from inhibitory interaction with Rb and mediates the expression of E2F target genes implicated in cell cycle promotion [104, 105, 127]. The increased levels of cyclin/CDK complexes by MYC are mediated through different mechanisms, whether by inducing gene expression or by regulating phosphorylation and dephosphorylation of diverse residues of CDK proteins [128]. Moreover, MYC induces miR-221, which modulate Rb mRNA [133, 134].
It was demonstrated that MYC induces the CDK genes including CDK4 [135] and CDK6 [136, 137], but there is controversy with regards to CDK2. Yap et al. observed both mRNA and protein levels of CDK2 were induced upon MYC overexpression [137], but another experiment shows a different role for this gene [138]. Based on ChIP assay, MYC binds to CDK1 promoter [139], but other proteins such as Ras [140] or cyclin C [141] cooperate with MYC to augment the expression of CDK1. Increased activity of CAK (CDK activating kinase) by MYC is also required for complete activation of cyclin/CDK complexes, since CAK carries out the activating phosphorylation of CDK T loop [142–145]. Moreover, MYC counteracts the inhibitory phosphorylation of CDKs either by targeting Wee1 through miR-221 induction or provoking Cdc25 phosphatase [133, 134, 142, 143, 146, 147]. Mir-221 also targets mRNAs of p27, p57, and Rb [133, 148].
In addition to inducing CDK genes, MYC also regulates the expression of cyclins. MYC has conflicting roles in cyclin D1 regulation. Depending on the cell types, MYC can increase, suppress or not affect the expression of cyclin D1 [138, 149–152]. On the other hand, MYC induces the expression of cyclin D2 [153, 154], D3 [155], E1 [156, 157], E2 [157, 158], A [138, 159–161], and B1 [95, 162, 163]. MYC recruits TRRAP to induce histone acetylation and subsequently cyclin D2 expression [154]. MYC mediates cyclin E1 induction either directly or by inducing E2F transcription factors [164]. Serial analysis of gene expression was done by Menssen et al. to identify MYC target genes. CDC2-L1, cyclin B1, and cyclin E binding protein 1 are among the MYC-induced cell cycle regulators involved not only in G1/S transition but also in G2 progression [95].
Apart from inducing positive cell cycle regulators, MYC also represses the activity of cell cycle inhibitors [127]. TGF-β signaling inhibits MYC and induces p15 and p21 to mediate cell cycle arrest in G1 phase. TGF-β-induced p21 is abolished through AP4 transactivation by MYC [165]. TGF-β treatment in lung epithelial cells downregulates MYC rapidly and induces p15 expression. Exogenous MYC expression blocks TGF-β -induced p15 expression [166]. After TGF-β treatment, Miz-1 binds to transcriptional initiator site (Inr) within the proximal region of the p15 promoter to augment p15 activity [167, 168]. MYC collaborates with other proteins, including Miz-1, SP1, and SMAD to block p15 induction. MYC and SP1 switch from transcriptional activator to transcriptional repressor upon their interaction with MYC and following their co-activators replacement [167–170]. Miz-1-mediated p300 recruitment and p15 induction are at the mercy of Miz-1 interaction with MYC [167, 168]. MYC forms an inhibitory complex with SP1 and SMAD to repress p15 upon TGF-β treatment [170].
Induction of E2F1 transcription factor, which induces ARF expression, and counteracts ARF ubiquitination and degradation by ULF ubiquitin ligase has been considered for MYC-induced ARF upregulation [171–174].
MYC regulates p21 in different ways. It overrides p21 induction by p53 and paves the way for p53-induced apoptosis [175]. Cell cycle cessation upon DNA damage is thwarted by MYC counteraction with p53-induced p21 and GADD45 [176–180]. Nuclear localization of Cyclin B1 is reduced by the action of GADD45, yielding Cdc2 kinase activity inhibition [181]. The binding of MYC and Miz-1 is one of the mechanisms that either directly inhibits p21 expression or indirectly via recruiting the DNA methyltransferase DNMT3a [182–184]. The ability of MYC to form a ternary complex with histone demethylase KDM5B and the transcriptional factor TFAP2C conflicts with p21 induction [185]. Besides, MYC interference with SP1 and Ras-mediated p21 induction [186, 187], and MYC-induced transcription factor AP4 [188] and miR-17-92 [189, 190] result in p21 suppression.
The expression of p27 and MYC shows an opposite pattern in several studies, as high levels of MYC are associated with low levels of p27 [191–194]. MYC represses p27 at both transcriptional and post-transcriptional levels. p27 regulation at the transcriptional level is mediated by MYC interaction with Inr element within the p27 promoter or its interaction and inhibitory effect on Foxo3a, a transcription factor required for p27 upregulation [195, 196]. MYC-mediated mir-221 and miR-222 upregulation repress 27 at the post-transcriptional level [134, 148]. MYC-induced cyclin E transcription directly or through E2F transcriptional induction bypasses G1/S arrest and antagonizes p27 [156, 197, 198]. MYC sequesters p27 from cyclin E-CDK2 complex by inducing the expression of D-type cyclin and CDK4 and CDK6 [153, 199, 200]. MYC-mediated cyclin E induction also stimulates p27 phosphorylation at Thr-187 and makes it more prone to be recognized and degraded by the SCFSKP2 ubiquitin ligase complex. Moreover, the expression of several subunits of SCFSKP2 ubiquitin ligase complex are induced by MYC to promote p27 proteasome degradation [201–206].
The transcription factor FoxM1 is a MYC target gene and controls G2/M promotion [207]. MYC targets several genes related to DNA replication and mitosis. It promotes DNA replication in both transcriptional-dependent and -independent manner. A number of origin recognition complex (ORC) genes, including ORC1, ORC2, ORC4, and ORC5 are among MYC target genes [137, 157]. Ctd1 [208], the main component of the pre-replicative complex, cdc6 [136, 157] and MCM proteins [157, 209, 210] as MCM3, MCM4, MCM5, and MCM6, proteins required for initiation and elongation of DNA replication, are induced by MYC. MYC also interacts with pre-replicative complex to increase replication origin activity [211]. In addition to CDK1, MYC regulates other genes encoding proteins involved in mitosis. MYC mediates mitosis progression by provoking subunits of anaphase promoting complex/cyclosome (APC/C), including Anapc5, cdc16, and cdc23, to increase the degradation of cyclin B1 and securin, which controls the transition of metaphase-anaphase transition [137, 212, 213]. On the other hand, it was shown that MYC has a different role as Anapc2. As well as securin degradation, MYC represses securin gene expression (PTTG1) [137]. Cells overexpressing MYC showed delayed anaphase onset through transactivation of MAD2 (mitotic arrest deficient) and BubR1 [214].
A study reported that in response to anti-mitotic drugs, such as taxol, nocodazole, Eg5 inhibitor, and other drugs disrubpting mitosis, MYC augments apoptosis [215]. Following treatment with the aforementioned agents, cells with low levels of MYC showcased less apoptosis compared to cells having high levels of MYC. Besides, cells overexpressing MYC exhibited more anomalies, since MYC exacerbates drug-induced micronuclei formation, a hallmark of chromosome instability [215]. Although normal mitosis took place in both high- and low-levels of MYC, mitotic timing and spindle morphology were under the control of MYC levels. In cells having high level of MYC, the spindle length and metaphase plate width were reduced and increased, respectively. Furthermore, acceleration of nuclear envelope breakdown (NEBD) to metaphase and delayed anaphase was seen in cells with high level of MYC. MYC modulates mitosis by controlling mitosis related events, including centriole biogenesis, kinetochore assembly, proteolysis,abscission, and cytokinesis [215].
Ciribilli et al. identified the genetic events associated with cell cycle and apoptosis in MYC transgenic lung tumors [216]. The expression of CDK4 and its related cyclin D1, and transcription factor DP1, a heterodimeric partner of E2Fs, were increased, while p19 was downregulated. CDK1 and cyclin B1 and B2 were overexpressed. In addition, upregulation of several genes such as the serine/threonine kinases Nek6 and Stk6 (Aurora-A kinase), Cks1 (cdc28 protein kinase), Cks2 (cdc28 protein kinase regulator subunit 2), cdc20, regulators of cytokinesis Prk1 (protein 1) and kinesin family members were seen. Moreover, increased ect2 expression, an oncogene required for cytokinesis [217], and downregulation of Lats2 that is a negative regulator of cell cycle [218] were observed. Another transcriptional alteration in lung tumors of MYC transgenic mice models is reversal of p53-induced cell cycle arrest mediated by repression of transcription factors involved in regulation of p53 activity. These include Klf-4 [219], Hey-1 [220], Gas-1 [221], and Hspa9a [222].
In addition to inducing miRNAs that target the negative regulators of cell cycle, MYC also suppresses miRNAs that acting as barriers to cell cycle progression [223]. Let-7 family members, miR-15a/16-1, miR-26a, and miR-34a are among the targets of MYC. Let-7 miRNAs regulate Cdc25a, CDK6, cyclin A, cyclin D1, D2, and D3. MiR-34a negatively regulates expression of CDK4, CDK6, cyclin E2, and E2Fs; miR-15a/16-1 participates in the regulation of CDK6, E2F3, cyclin D1 and D3; and miR-26a represses cyclin D2 and E2 [224–227].
MYC also induces the H19 long-non coding RNA (lncRNA), which silences Rb and promotes proliferation [228–231]. MYC-induced long noncoding RNA (MINCR) is another LncRNA induced by MYC that has a close correlation with its expression. MYC binding to the promoter of selective cell cycle genes is weakened following MINCR knockdown [232].
The role of MYC in DNA damage response
The role of MYC in DNA damage signaling has been investigated in numerous studies. MYC levels are decreased through different mechanisms depending on the extent of DNA damage, including alternation in MYC transcription and protein turnover [14–16]. The results of several studies exhibit that decreased levels of MYC are assumed as a step in DDR [15, 17, 18] (Fig. 5).
Fig. 5.

MYC has multiple effects on the DNA damage response. MYC is essential for NBS1 expression and the activity of ATM and its downstream effectors such as p53. In turn, p53 suppresses MYC expression in a pulsatile pattern. MYC drives cell fate toward apoptosis and overrides cell cycle arrest. MYC also participates in the generation of DNA damage. DNA repair impairment, ROS generation, and increased replication stress are among other MYC-provoked DNA damage response mechanisms
Decreased MYC protein levels and p53 accumulation has been observed in UV-induced DNA damage in which the proteasome-dependent degradation mechanism was implicated for MYC level reduction [14]. Jiang et al. suggested tripchlorolide-induced DNA damage causes proteasome-dependent MYC degradation, resulting in apoptosis induction [15]. Moreover, Fbw7 ubiquitin ligase mediates MYC degradation in response to DNA damage due to the USP28 disassociation from Fbw7 [18], which is required for MYC stability [233]. Exposure of MCF-7 breast tumor cell line to DNA-damaging agents such as topoisomerase II inhibitors VM-26, m-AMSA, and doxorubicin, or ionizing radiation is capable of suppressing MYC mRNA [234–237]. Moreover, continuous treatment of MCF-7 with VM-26 suppresses both mRNA and protein levels of MYC, and its transcriptional activity in response to sustained DNA damage [238]. Treatment of MCF-7 cells with camptothecin at the concentrations, causing DNA damage results in MYC suppression, while in lower concentrations, this attenuation in MYC expression vanishes due to the absence of detectable DNA damage [239]. In response to DNA damage, pRb is dephosphorylated [240] and in the complex with E2F1 represses MYC. Another factor influencing MYC is a acetyl-transferase called TIP60, which has a co-regulator activity towards MYC. Intriguing, TIP60 obstacles tumor progression by modulating DDR [241], and its low levels in different cancers are associated with tumor progression and inferior survival [242].
MYC-mediated activation or repression of many target genes such as Bax, GADD45A, and ONZIN are involved in DDR [243–245]. Pusapati et al. observed MYC overexpression in a transgenic mouse model causing p53 activation following DNA damage and ATM was required for p53 activation to augment apoptosis and interfere with MYC-mediated tumorigenesis [246]. MYC exists upstream of PI3K related to DDR and augments signal transduction following DNA damage [247]. This oncogene has multiple effects on DDR.
MYC can enhance DDR, as the activation of ATM-dependent checkpoints relies on it. Guerra et al. observed that in response to DNA damage, nuclear foci formation of the Nijmegen breakage syndrome 1 (Nbs1) and subsequently phosphorylation and activity of ATM and its downstream effectors were reduced in the cell line lacking MYC, resulting in impairment in p53stabilization and delayed DDR [248]. A previous study showed Nbs, a member of MRN complex, is a target gene for MYC [249]. Nbs1 senses DNA breaks and is essential for ATM activation in the presence of DNA damage [250, 251]. Therefore, MYC-mediated Nbs1 expression affects DNA damage-induced signal transduction pathways. In unstressed conditions, Miz1 associates with topoisomerase II binding protein1 (TopBP1), but upon UV irradiation, TopBP1 detaches from Miz1 [19]. TopBP1-Miz1 association negates TopBP1 proteasomal degradation that ATR-dependent signal transduction is relied on. MYC has a negative effect on ATR-dependent signal transduction in response to DNA damage. This involves TopBP1-Miz1 disassociation and TopBP1 degradation by HectH9 [252].
Upon DNA damage, p53 induces the expression of p21 [176] and GADD45 [177] to mediate cell cycle arrest. MYC has the opposite role, and it represses these genes [178–180] and attenuates p53-mediated cell cycle arrest [253, 254]. However, it drives p53 functions toward apoptosis induction instead of cell cycle arrest [255]. Upon DNA damage induced by gamma irradiation and daunorubicin, MYC interacts with Miz-1 and downregulates p21 expression to favor apoptotic arm of p53 signaling [255]. MYC forms a transcriptional repressor complex with Miz-1 in order to suppress p21 [19, 126]. Transactivation of AP4 by MYC allows cells to re-enter cell cycle even in the presence of DNA damage. Jung et al. showed that after treatment of MCF-7 cells with etoposide, the protein levels of MYC and AP4 were reduced. To the contrary, p21 and p53 levels were elevated. These results show AP4 abrogates p53-mediated cell cycle arrest by suppressing p21 and favors apoptosis induction, resulting in cells sensitization to DNA-damaging agents [165].
MYC can inhibit both G1/S and G2/M arrest. After irradiation, in human mammary epithelial cells (HMEC) overexpressing MYC, G1/S arrest was impaired due to inappropriate hyperphosphorylation of Rb and subsequent reappearance of cyclin A, which leads to the entry of cells into S phase [256]. Overexpression of MYC also attenuates G2/M arrest by upregulating cyclin B1, and stimulates cells with damaged DNA to enter mitosis [257]. The human ubiquitin ligase HectH9 is essential for MYC-mediated cell cycle progression and activation of target genes. In human tumor cell lines lacking HectH9, cells accumulate in the G1 phase of cell cycle [58]. In the study by Robinson et al. primary human fibroblasts overexpressing MYC exhibited accelerated S phase in contrast to prolonged S phase in the cells lacking MYC. They showed the Werner DNA helicase protein (WRN) is required for the repair of stress-induced DNA damage. WRN depletion resulted in DNA damage accumulation in cells overexpressing MYC [258].
MYC promotes apoptosis by bypassing p53-mediated cell cycle arrest [19, 126, 255] and drives cells with damaged DNA toward S phase [247, 259]. Moreover, MYC suppresses the expression of anti-apoptotic proteins such as BCL-XL and BCL-2 [260]. PER1 can push DDR in favour of apoptosis via upregulating MYC. In response to DNA damage, high levels of MYC and concomitantly p21 reduction were seen in cells overexpressing PER1, highlighting the positive effect of PER1 on apoptosis induction [261]. On the other hand, heat shock protein HSP70 opposes MYC-evoked apoptosis in response to etoposide and camptothecin [262]. BRCA1 stands in the way of apoptosis induction ensuing exposure to DNA damaging agents [263, 264]. Further investigation revealed that BRCA1 cooperates with MYC to suppress psoriasin, resulting in resistance to etoposide [265]. Upon treatment with DNA damaging cytotoxic drugs, the physiological level of MYC is required for strong apoptosis induction through the activation of Bid and Bax and pro-apoptotic enzyme PLKδ. Apoptosis is abrogated in MYC null cells, confirming that apoptosis is dependent on expression of this oncogene [266, 267].
MYC depletion in colorectal cancer cell lines promotes cell-cycle arrest rather than apoptosis due to the alternation in p53 signaling and its downstream effectors. In fact, p21 is increased, whereas the levels of pro-apoptotic genes such as Bax are decreased [255]. Moreover, under irradiation, fibroblasts undergo apoptosis as a result of MYC function that targets BCL-XL [268]. In colon cancer cell lines overexpressing MYC, camptothecin treatment results in effective apoptosis induction, indicating MYC overexpression contributes to colon cancer cells sensitization to this agent. Following camptothecin treatment, the levels of p53 and its target genes are upregulated, while overexpression of MYC induces p53 and overrides p21 induction [269].
The mechanism underlying chemosensitivity in small cell lung cancer cell lines harboring p53mutations was investigated by Supino and colleagues [270]. They showed upon treatment with doxorubicin, MYC is upregulated to induce apoptosis independent of p53 and renders the cells more prone to chemotherapy. The Y box binding protein (YB1) is a transcription factor with the ability to modulate the outcome of anticancer agents treatment and is responsible for drug resistance [271–273]. p73 interacts with MYC and promotes the formation of MYC-MAX complex to increase the transcriptional activity of MYC, resulting in YB1 upregulation-mediated drug resistance [274].
Etoposide causes DNA damage in S phase and provokes apoptosis in G2 [275]. A study showed MYC is indispensable for apoptosis in G2 phase. The same does not hold true in G1 phase [276]. It was observed that treatment of MYC null cells with etoposide abrogated apoptosis, while cisplatin exposure causes DNA damage irrespective of cell cycle stage.
MYC is capable of activating the transcription of genes involved in DNA repair, including NBS1, KU70, BRCA2, Rad50, and Rad51 [136, 210, 249, 277]. Cui et al. demonstrated that MYC affects the repair of DSB (double strand breaks) caused by ionizing irradiation (IR). The ability to repair DSB was attenuated in MYC-knockdown cells Hela-630 after exposure to IR due to the reduction in DNA damage-induced ATM phosphorylation and DNA-PK kinase activity [278]. Moreover, the upregulation of genes involved in the repair of DSBs through HR and NHEJ is dependent on MYC and HIF2α [279].
It has been suggested that MYC participates in p53 regulation [280–282]. Phesse et al. observed DNA-damaging agents can no longer cause apoptosis when MYC is deleted in the adult murine cells [282]. Tight regulation of MYC levels is essential for precise kinetic apoptosis in response to DNA damage. Treatment of Rat-1 fibroblast cell line with DNA-damaging agent VP-16 demonstrated MYC is required to achieve the optimal apoptotic response [283].
It has been suggested that p53 is involved in MYC modulation. Besides the transactivation of genes involved in cell cycle arrest, p53also represses MYC through a mechanism dependent on histone deacetylation [284]. Following irradiation, the mRNA levels of MYC were reduced in AML-3 cells expressing wild type p53. In K562 cells lacking p53however, there was no reduction [285]. In some studies, the dynamic behavior of p53accounts for its broad function. Several models have been proposed for acquiring p53dynamic response [286–288]. Porter and colleagues showed that MYC and p53have an inverse concentration relationship [289]. In response to DSB, p53induces MYC repression in a pulsatile pattern, which is thought to be due to p53binding to downstream enhancer within a MYC super-enhancer region [290]. This is consistent with previous studies that showed p53 has the potential to suppress MYC [291, 292]. A study stated that MYC, a non-linear transcription factor is capable of universally affecting active genes, but not ones induced priorly to MYC [6, 293]. p53-mediated MYC repression however has different impacts on global transcription. Cell fate is affected by this transcriptional inhibition effect of p53 on MYC. Proper cell cycle arrest and apoptosis prevention are the consequence of p53-mediated MYC repression.
Several miRNAs are involved in the negative regulation of MYC [294–296]. In a study that was done by Cannell and colleagues [80], the levels of p53, p21, and miR-34c were upregulated in HEK293 after treatment with etoposide, while MYC protein levels but not MYC mRNA levels were decreased. p53is a crucial regulator of miR-34 family. These are important mediators of DDR [297–301] that control MYC levels [302, 303]. Nevertheless, it has been shown that p38 MAPK/MK2 pathway also mediates miR-34c induction to prevent aberrant MYC-induced replication even in the absence of p53 [80]. In fact, DNA damage-mediated miR-34c induction gives rise to MYC repression to halt cell cycle at the S phase and counteract DNA synthesis and replication. Li et al. showed that after exposure to UV-induced DNA damage, ribosomal protein L11 is released from the nucleolus to the cytoplasm to promote miR-130a recruitment, resulting in decreased levels of MYC mRNA and protein [304].
Upon DNA damage, bridging integrator 1 (BIN1), a nucleocytoplasmic protein, is activated to mediate apoptosis [305, 306]. Loss of BIN1 attenuates cell response to DNA-damaging agents [306]. This adaptor protein interacts with MYC and perturbs MYC-mediated transactivation of target genes [305, 307, 308]. Pyndiah et al. observed that regardless of TP53 gene status, BIN1 exerts essential roles in enhancing DNA damage caused by cisplatin. The mechanism behind this chemosensitivity is dependent somewhat on BIN1-MYC interaction. BIN1 interaction with PARP1 which is followed by inhibition of the latter is another mechanism that inhibits MYC-induced transactivation, G2/M transition, and sensitizing cells to DNA damage [309]. PARP1 acts as a scaffold, and interacts with proteins involved in the base excision repair (BER) [310–312]. BIN1-mediated PARP1 inhibition also impairs BER pathway and results in chromosomal destabilization. Moreover, BIN1 inhibits indoleamine 2,3-dioxygenase (IDO), resulting in the intracellular NAD reduction and PARP1 activity restriction [313–315]. In addition to PARP1, BIN1 interacts with proteins involved in non-homologous end joining (NHEJ) pathway [316, 317]. It is noteworthy that MYC overexpression restores PARP1 activity by blocking BIN1 activation by Miz-1 to overcome BIN1-mediated PARP1 repression [309].
In another study, the regulatory effects of epigenetic alterations on DDR have been demonstrated [318]. SMAD nuclear interacting protein 1 (SNIP1) has the potential to regulate DDR, apoptosis, and cell cycle [319, 320]. SINP1 interacts with the ten-eleven translocation dioxygenase 2 (TET2). This interaction mediates TET2 binding to several transcription factors such as MYC in order to recruit TET2 to the promoter of MYC target genes and therefore regulates their expression [318].
In addition to being involved in DDR modulation, MYC is also considered an element that causes genomic instability [253, 259, 321–325]. Several mechanisms contribute to MYC-elicited genomic instability. These include inducing DNA damage, gross chromosomal rearrangement, aberrant cell cycle progression, and disrupting DNA repair processes [326]. Moreover, cells with damaged DNA like MYC-induced DSB can re-enter the cell cycle in response to MYC overexpression, resulting in genomic instability [253]. In addition, MYC can promote DNA damage independent of ROS [324]. Increased DNA replication stress is another mechanism that underlies DNA damage induced by MYC [323, 327–329]. Dominguez-Sola et al. noticed the non-transcriptional role for MYC in DNA replication. This oncogene interacts with pre-replicative complex and modulates replication origin activity. They showed MYC overexpression increases replication origin activity, and consequently persuades replication-dependent DNA damage [211]. In addition to non-transcriptional control of DNA replication, MYC activates CTD1 transcription [208], a key component of pre-replicative complex required for origin licensing [330–332]. It was shown Polη, a Y-family translesion synthesis polymerase, relieves MYC-induced replication stress by mediating fork progression to suppress DSB formation [333]. P300 regulates MYC negatively and so counteracts aberrant DNA synthesis [334, 335]. P300 knockdown results in entry into S phase followed by deregulated replication origin activity and DNA synthesis due to MYC induction, leading to the DDR-activated apoptosis [336].
MYC can also play a role in genomic instability through interrupting DSB repair. It has been observed that MYC has the potential to interfere with DNA damage repair [259, 337]. Li et al. showed MYC inhibits the repair of DSBs caused by IR or the action of RAG1/RAG2 during V(D)J recombination [338]. Based on ChIP array studies, there is an association between MYC and the promoter of several DSB repair-related genes including Rad51, Rad51B, Rad51C, XRCC2, Rad50, BRCA1, BRCA2, DNA-PKcs, XRCC4, Ku70, and DNA ligase IV [136, 210, 249, 339]. Song et al. showed that MYC disrupts homologous recombination-mediated DNA repair through upregulating miR-1245 to suppress BRCA1 expression, resulting in hypersensitivity to γ-irradiation [337]. In tyrosine kinase-activated leukemias, elevated generation of ROS, and DNA damage as well as activation of error-prone repair process has been seen [340, 341]. Muvarak et al. [342] proposed MYC overexpression is involved in the activation of alternative NHEJ, an error-prone repair pathway [343, 344], via upregulating the expression of LIG3 and PARP1, which is dependent on expression of FMS-like tyrosine kinase-3 (FLT3)/ITD and BCR-ABL1. Jin et al. showed BCL2 binds to MYC and boosts its transcriptional activity to hindering DNA repair through targeting APE1, a member of the BER pathway [345].
Partlin et al. observed that MYC has the potential to interact with MLH1 mismatch repair protein and inhibits its activity [346]. It was reported that MYC downregulation rendered melanoma cells susceptible to IR-induced apoptosis through inhibition of MLH1 and MSH2, resulting in DNA repair prevention and DNA damage accumulation, followed by induction of apoptosis in a p53-independent manner [347].
MYC and apoptosis
A well-known fundamental function of MYC is the property to sensitize cells to apoptosis. The first oncogene reported inducing apoptosis was MYC [20]. Deregulated MYC expression, along with anti-proliferative signals, can lead to apoptosis [348]. Even in the presence of survival factors, deregulated MYC sensitizes cells to apoptotic stimuli such as irradiation, hypoxia, heat shock, interferons, TNF alpha, and Fas [349]. MYC needs to bind to DNA with its partner Max to induce apoptosis [20].
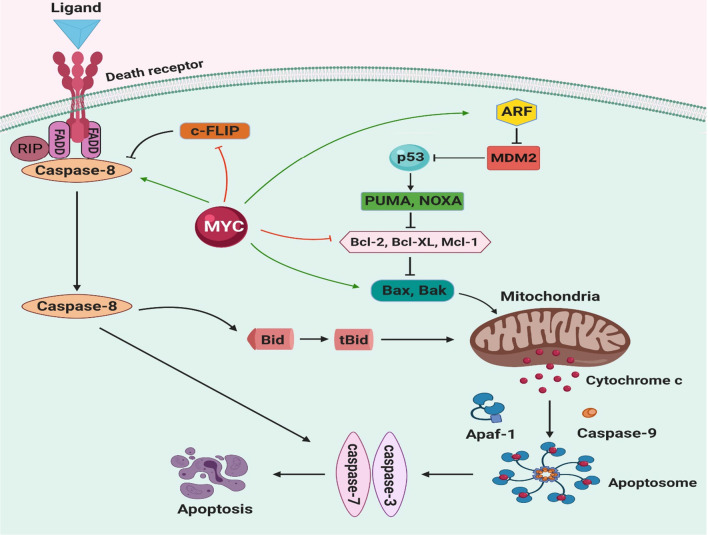
Two main pathways initiate apoptosis, the intrinsic (mitochondrial) and extrinsic pathways. These two pathways have a pivotal role in MYC-induced apoptosis. Different cellular stresses such as oncogene activation, DNA damage, and hypoxia can initiate intrinsic pathway and release of apoptogenic factors, including cytochrome c (cyt c), smac\DIABLO, and apoptosis-inducing factor (AIF) [350]. The release of mitochondrial cyt c into the cytosol facilitates the formation of the apoptosome complex, consisting of cyt c, Apaf-1, and procaspase-9. The apoptosome complex activates caspase-9 to directly cleave and activate effector caspases, caspase-3, and caspase-7. These caspases finally trigger apoptosis [351]. BCL-2 protein family has an essential role in regulating mitochondrial-outer-membrane permeabilization (MOMP), which is required for the release of cyt c. BCL-2 family has three subfamilies based on their functions: (1) anti-apoptotic members (BCL-2, BCL-XL, MCL-1, etc.), (2) BH3-only (pro-apoptotic) proteins (BAD, BID, BIK, BIM, PUMA, NOXA, etc.), (3) pore-formers or ‘executioner’ (pro-apoptotic) proteins (Bax, Bak, Bok) (9). Bax and Bak induce the release of cyt c by oligomerization and forming pores in the mitochondrial outer membrane. Anti-apoptotic proteins such as BCL-2 and BCL-XL inhibit Bax and Bak translocation and oligomerization, resulting in suppression of MOMP and prevention of cyt c release [352, 353]. The balance between pro- and anti-apoptotic members regulates the release of cyt c from mitochondria so that when anti-apoptotic proteins are predominant, they inhibit the release of cyt c [354].
On the other hand, the extrinsic pathway is initiated through death-ligand binding to cell-surface death receptors. Death receptors are a subset of the tumor necrosis factor receptor (TNFR) superfamily that contains eight members: TNFR1, Fas (CD95), DR3, TNF-related apoptosis-inducing ligand receptor 1 (TRAILR1; also called DR4), TRAILR2 (DR5), DR6, ectodysplasin A receptor (EDAR) and nerve growth factor receptor (NGFR). The presence of about 80 amino acid death domain (DD) in the cytoplasmic region of death receptors has an essential role in activating the signaling cascade and induction of apoptosis [355]. Death receptors ligation create a death-inducing signaling complex (DISC), including adaptor molecule FADD, the initiator procaspase-8\10, and an inactive homolog of caspase-8, c-FLIP (cellular FLICE-like inhibitory protein). Interaction between FADD with procaspase-8 promotes homodimerization and autocatalytic cleavage of procaspase-8, leading to the formation of active caspase-8. This active form in turn cleaves and activates downstream caspases such as caspase 3 and 7 that execute cell apoptosis [356–358]. Caspase-8 also cleaves Bid pro-apoptotic protein to truncated Bid (tBid), which can translocate to mitochondria and release cyt c by inducing MOMP. Bid acts as a bridge between extrinsic and intrinsic apoptosis pathways [359, 360]. c-FLIP is a master anti-apoptotic regulator for death receptor-mediating apoptosis, which carries out its function by competing with procaspase-8 to bind to FADD, thus interferes with caspase-8\FADD interaction [361].
When studies show that MYC could provoke translocation of cytochrome c from the mitochondria, it was suggested that MYC could play a role in apoptosis [362]. The activation of Bax and Bak by MYC is an upstream mechanism of cytochrome c release (Fig. 6). Bax is one of the transcriptional targets of MYC and the primary mediator of MYC-dependent apoptosis. Expression of MYC induces Bax upregulation or, indirectly, controls Bax oligomerization [363, 364]. Bax and Bak are required for efficient apoptosis response, and MYC activation alone is not adequate to provoke apoptosis; hence Bax and Bak's deficient cells are significantly resistant to MYC-induced apoptosis [365]. Overexpression of BCL-XL acts as a barrier that inhibits the MYC-induced conformational activation of Bak. Indeed, BCL-XL is a pivotal factor for the mitochondrial apoptosis pathway by its inhibitory effect on Bak activation [366]. Recent studies indicate MYC can induce suppression of both BCL-2 and BCL-XL anti-apoptotic proteins. By blocking MOMP through Bax and Bak inhibition, MYC-dependent apoptosis is prevented [367]. Based on the evidence provided by Muthalagu et al., Bim pro-apoptotic protein is a major mediator of MYC-dependent apoptosis in several solid tissues. MYC appears to stimulate apoptosis through binding to the Bim promoter and elevates Bim expression [22].
Fig. 6.
MYC and apoptotic pathway. Expression of MYC can sensitize cells to a broad range of proapoptotic stimuli such as DNA damage, death receptor, hypoxia, and nutrition deprivation. Through various pathways and possibly by inducing activation of Bax proapoptotic molecule, MYC promote the release of cytochrome c from mitochondria into the cytosol. Activation of Bax forming pores results in mitochondrial outer membrane permeabilization (MOMP). When cytochrome c releases into the cytoplasm, it interacts with APAF-1 and procaspase 9 to form apoptosome. Caspase 9 is activated in the presence of ATP, which in turn cleaves and activates caspase3 and 7, eventually triggering apoptosis. MYC is also involved in the death receptor pathway of apoptosis. Ligand-death receptor binding initiates interaction of adaptor molecules like FADD with death receptor. FADD auto-activates by recruiting procaspase 8. Caspase 8 can directly activate caspase3 and 7. Caspase 8 can also activate BH3-only protein BID, which stimulates MOMP. MYC induces apoptosis by p53dependent and independent mechanisms. Regulation of p53\MDM2\ARF by MYC, can stabilize p53and promote apoptosis
MYC is known as a stimulant that can sensitize cells to several death stimuli such as TNF-α, CD95/Fas, and TRAIL [368, 369]. The molecular mechanism that MYC promotes extrinsic apoptosis pathway is not well established; however, the inhibitory effect of MYC on the NF-kB pathway and suppression of survival genes along with its pro-apoptotic activities has been proposed [370–372]. Klefstrom and colleagues showed that receptor-interactive protein (RIP) is a serine\threonine kinase that initiates programmed cell death by.
FADD and caspase-8 dependent pathway. The MYC-mediated promoted expression of RIP can significantly enhance the apoptotic activity via FADD, caspase-8. Both caspase-8 and FADD are crucial for apoptotic synergy between RIP and MYC. [373]. MYC also acts as an inhibitor of c-FLIP expression, which enhances TRAIL-dependent activation of caspase-8 and apoptosis. The ectopic expression of FLIP represents the suppression of MYC-induced apoptosis. Caspase-8 can be increased directly or indirectly through post-translational modification by MYC [374]. Previous studies demonstrated that MYC also induces FasL up-regulation in T-lymphocytes and increases susceptibility to Fas-mediated apoptosis [375].
p53 pathway can become involved in MYC-dependent apoptosis through several mechanisms. p53 accumulates in the nucleus, where it is activated, and promotes growth arrest and/or apoptosis. It stimulates multiple apoptotic genes that have an important role in a different stage of apoptosis by transcription-dependent and independent mechanisms. p53 induces apoptosis via both intrinsic and extrinsic pathways [376, 377]. Post-translational modifications such as phosphorylation, acetylation, ubiquitination, and methylation as well as protein–protein interactions with cooperating factors stabilize and activate p53 [378]. Stable p53 can interact with pro-apoptotic genes such as Puma, Noxa, Apaf-1, and Bax, upregulating their expression. It can also suppress expression of anti-apoptotic proteins like BCL-2, BCL-XL, and MCL-1 [379]. As mentioned above, p53 is an unstable and short-lived protein. under normal conditions, an MDM2 E3 ligase, a primary negative regulator of p53, keeps it at a low level due to continuous degradation. MDM2 inhibits p53 activity by ubiquitination, proteasome-dependent degradation, and promoting its nuclear export [380]. The ARF also known as p14ARF in humans and p19ARF in mouse is a tumor suppressor gene derived from INK4a-ARF locus. ARF inhibits MDM2 and prevents p53 degradation. Ectopic MYC expression upregulates ARF. This inhibits MDM2-mediated degradation of p53 and induces expression of p53 directly, which triggers apoptosis [280, 381]. Activated p53 translocates to the mitochondria, interacting with pro-apoptotic proteins and anti-apoptotic members directly [382, 383]. Several studies show that lack of ARF and p53 attenuate MYC related apoptosis, but some groups suggested an alternative pathway for MYC because even in the absence of both ARF and p53, MYC can induce apoptosis [381, 384]. Death-associated protein kinase (DAPK) is a positive mediator of apoptosis activated by MYC and E2F-1. DAP kinase effect on activating p53 is exerted through an ARF-dependent mechanism, which results in p53-induced apoptosis [385, 386]
According to Maclean et al. MYC can increase gamma irradiation (γ-IR)-induced apoptosis by inhibiting BCL-XL. In the mouse embryo fibroblasts (MEFs) and Eμ-MYC transgenic mice B cells, MYC functions in synergy with γ-IR to sensitize cells and induce apoptosis independent of p53 [268]. Indeed, MYC does not alone induce the DNA damage response in MEFs but stimulates apoptosis in synergy with γ-IR. MYC along with γ-IR suppress BCL-X gene in the B cells of Eμ-MYC transgenic mice. The loss of BCL-X alone, even without BCL-2, is sufficient to sensitize MEFs to γ-IR induced apoptosis. Finally, activation of MYC can cause a decrease in the steady-state levels of BCL-XL protein by reducing BCL-X transcript and suppressing its promoter activity [268].
Reactive oxygen species (ROS) are reactive chemical species containing superoxide, hydroxyl radical, and hydrogen peroxide with a key role in cell signaling and maintaining homeostasis. Cellular processes, such as metabolism and respiration generate ROS. Excessive ROS can induce apoptosis mediated by mitochondria, death receptors, and the endoplasmic reticulum (ER) [387]. The study conducted by Tanaka et al. determined that in serum deprivation circumstances, overexpression of MYC and E2F-1 inhibit NF-kB activity and suppress superoxide dismutase (SOD). Due to the SOD suppression ROS levels elavete, and cells become vulnerable to apoptosis in serum-deprived conditions [388]. Ornithine decarboxylase (ODC) is another downstream transcriptional target of MYC. ODC encodes the rate-limiting enzyme that catalyzes the first step in the polyamine biosynthesis pathway, converting ornithine to putrescine. MYC stimulates ODC activity to elevate synthesis and catabolism of more polyamine storage. In response to excess polyamine accumulation, polyamine oxidase catabolizes polyamine to ROS and finally induces apoptosis [389].
Initially, in murine B cell lymphoma it was found that FOXO is an antagonist of MYC [390]. FOXO3a is a member of the FOXO protein family that plays a key role in modulating MYC stability and mitochondrial gene expression [391]. Mad/Mxd protein family members are important FOXO3a downstream effectors that dimerize with MYC-associated factor X (MAX) and bind to promoter regions of MYC target genes to block MYC function [392]. FOXO3a can also inhibit MYC activity by enhancing the expression of miRNAs that disrupt translation of MYC mRNA [391, 393]. Taken together, it seems that FOXO3a has an integral role in MYC regulation. In a negative feedback loop, MYC can displace FOXO3a from the promoter of its downstream targets such as GADD45 and PUMA, and downregulates FOXO3a activity [394].
FOXO3a activation leads to a decrease in mitochondrial metabolism and gene expression. As well, FOXO3a reduces ROS production in response to stress. In contrast, MYC elevates mitochondrial output and energy production, promoting cells to re-enter cell cycle. Increased ROS levels cause cell damage [395], and FOXO3a counterbalances this by increasing mitochondrial superoxide dismutase (SOD2) and catalase production [391]. FOXO3a also disrupts the MYC-dependent expression of nuclear encoded mitochondrial genes [396]. Furthermore, activation of FOXO3a, independent of SOD2 activation, alters mitochondrial function and decreases cellular ROS [396, 397]. Overall, the interplay between MYC, FOXO3a, and mitochondrial proteins seems to be critical in regulating MYC and ROS-related activities.
Moreover, cell division cycle 25 (cdc25) phosphatase, a dual-specificity protein phosphatase, is composed of three members: cdc25A, cdc25B, cdc25C. while both cdc25B and cdc25C play an important role in promoting G2\M progression, cdc25A plays a more extensive general function [398]. Cdc25A is a direct transcriptional target of MYC, and its activation contributes to MYC-mediated apoptosis [399]. However, inhibition of cdc25A expression does not suppress MYC-mediated apoptosis because other MYC target genes can compensate for the lack of cdc25A. MYC can stimulate the expression of cdc25A through MYC\MAX heterodimer binding to its promoter. MYC activation can increase cdc25A mRNA and protein levels [147, 400]. The Pim-1 oncogene is another partner for MYC in apoptosis induction. Post-translational phosphorylation of MYC by Pim-1 kinase increases the stability of MYC protein and enhances its transcriptional activity [401]. In addition, Pim-1 can phosphorylate cdc25A as a substrate, and regulates its phosphatase activity. Therefore, evidence indicates that cdc25A links Pim-1 to MYC and plays a vital role in apoptosis induction [402].
The role of MYC in hematopoiesis and hematological malignancies
MYC is a “global” transcription factor contributing to various cellular processes, one of which is hematopoiesis. Studies have determined that MYC has a quintessential role in nearly every step of the way [23, 24]. The architecture of hematopoiesis is highly organized. Long-term hematopoietic stem cells (LT-HSCs) differentiate into multipotent progenitors (MPPs) first. Both LT-HSCs and MPPs are LSK (Lin−/Sca1+/c-Kit+) cells, which turn into common myeloid and lymphoid progenitors (CMPs and CLPs) [403–405]. Throughout these steps, the self-renewal potential of LT-HSCs is reduced. MYC expression however stays high, indicating its essential role in regulating differentiation and proliferation. High amounts of MYC can be regulated by a single E3 ubiquitin ligase called Fbw7 [406, 407]. The in vitro MYC-mediated inhibition of hematopoietic cell differentiation was first discovered in the 1980s [408]. Later on, a GFP-fused MYC knock-in mouse model was designed to pave the way for MYC-related in vivo studies [409]. The highest MYC expression levels are seen in LSK-originated myeloerythroid progenitor cells, a continuous proliferating cell population [406]. Compared to LT-HSCs, the MYC expression in MPPs is higher [406]. Also, LSK cells of the mice fetal liver shows increased MYC expression during the proliferation period [410, 411], which all are consistent with the study indicating that MYC hinders differentiation in hematopoiesis and consequently propels proliferation [412].
MYC has a role in maturation and expansion of myeloid and lymphoid cells. Initial studies on lymphoid cells showed that the expression of MYC elevates at the transcriptional level during the maturation of pro-B cells into pre-B cells. Thereafter, MYC level would only be increased upon BCR-mediated activation of mature B cells [413–415]. The expression pattern of MYC during T-cell development and the TCR signaling pathway is similar to B-cells [413, 416, 417]. It has been demonstrated that MYC promotes the proliferation of both T and B lymphocytes, as well as synergizing the prolific effects of IL-7 [409, 418].
Compared to the lymphoid differentiation, the myeloid lineage undergoes a more convoluted path. Although MYC involvement in the development of lymphoid cells has received more attention, recent studies have shown that MYC also plays an important role in myeloid cell maturation [419]. The MYC−/− in mice showed not only a diminished lymphocyte production but also demonstrated dysregulated myeloid proliferation, including thrombocytosis, monocyte and neutrophil reduction, and severe anemia [420]. Unlike other cells, megakaryocytes were increased in the MYC−/− model, and despite their small size, they could produce high number of platelets, causing thrombocytosis. This indicates that megakaryocytes are not affected in the same manner as other myeloid cells [420]. All in all, it is not surprising that deregulation of MYC can contribute to tumorigenesis, particularly in hematological cells. In the subsequent sections, we elaborate on the role of MYC in different types of hematological malignancies.
Role of MYC in lymphocytic leukemia
The first demonstrations of MYC oncogenic capabilities in hematological neoplasms were seen in Eμ-MYC transgenic mice [421–423]. In this model most tumors were developed after 2–5 months, along with mutations in the Arf-Mdm2-p53 pathway [280, 424]. Eμ-MYC transgenic mice models, which overexpress MYC in lymphoid cells mostly develope T-cell lymphoma [425]. Although Burkitt lymphoma is a hallmark of MYC-induced B-cell lymphoma in humans, mice models with induced MYC in their lymphoid lineage could not completely turn to Burkitt lymphoma. To address this, using yeast artificial chromosome (YAC) technology, transgenic mice with 240-kb IgH/MYC translocation region were developed, in which B-cell tumor developed even without Eμ enhancer. This model could mimic B-ALL and Burkitt lymphoma [426–428]. Other mice models were also designed through inserting MYC cDNA into specific regions that transformed mice to Burkitt lymphoma and plasmacytoma model with t(8;14) and t(12;15), respectively [429, 430]. In a more adaptive approach, HSCs derived from fetal liver cells were transduced with either mutant or wild-type MYC-expressing retroviral vectors to produce lymphoma [431]. Although the MYC overexpression has an oncogenic effect, the p53 can counteract it. When the bone marrow (BM) cells with p53wt/wt and null p53−/− phenotypes were transduced with a MYC-encoding retroviral vector, B-cell lymphoma occurred only in p53 null cells [432]. Intriguingly, transduction of BM-obtained p53−/− B-cell lymphoma progenitors by MYC expressing retrovirus, turn the cells into a myeloid lineage in vitro. However, the cells can shift back into B-cell lymphoma upon returning to in vivo environment. This oscillation can be confined via overexpressing Pax5, which maintains the cells in the lymphoid form [433]. Other MYC family members, namely N-MYC, can also contribute to developing AML [434]. Overall, these models have helped to delineate the role of MYC in lymphoid malignancies [435, 436].
Generally, MYC overexpression does not come from mutations in its gene, although some mutations stabilize the MYC protein. Dysregulation of MYC in leukemia and lymphoma, which mostly leads to overexpression, is mainly due to gene amplification, chromosomal translocations, aberrant transcription, and increased stability of mRNA and protein [28]. The high levels of MYC in lymphoid neoplasms mostly confer poor prognosis. It has been revealed that more than 20% of B-ALL patients in different age groups and various demographic backgrounds have MYC overexpression, which implies that routine MYC immunostaining could aid in diagnosing patients at higher risk [437].
B-ALL with t(9;22)
The B-cell receptor–ABL proto-oncogene 1 rearrangement reffered to as t(9;22) (BCR-ABL1) occurs in 20–30% of ALL cases. The resulted shorten chromosome 22 is called the Philadelphia chromosome (Ph) [438]. The product of BCR-ABL1 fusion gene is a tyrosin kinase that can induce MYC activation in mice pre-B cells. However, the BCR-ABL1-mediated activation of MYC does not suffice for tumoriogensis. A second hit by oncoproteins such as c-RAF, c-JUN, or RAS is needed for cells to go through a malignant transformation [439, 440]. The suppression of MYC diminishes BCR-ABL1-mediated transformation, meaning that MYC not only possesses a complementary role but also is essential for ensuring the malignant transformation [439, 441]. Moreover, upon pre-BCR activation in lymphoblasts, MYC would be induced via a spleen tyrosine kinase (SYK)-mediated signaling pathway located in its upstream. Subsequently, MYC induction results in increased transcription of BCR-ABL1 in a positive feedback loop to increase the transcription of MYC oncogene [442, 443]. A study reported that MYC oncogene’s transcription could be diminished if SYK is inhibited by small molecules like PRT318 [443]. Parallel to SYK, Sphingosine kinase 2 (SK2) has the ability of acetylating histone H3 within the MYC gene, which induces a MYC-mediated oncogenicity in ALL-mouse models. Inhibiting SK2 or ablating the SK2 gene in murine models can drastically reduce ALL development through reduction in MYC expression and its downstream target genes [444]. In principle, SK2 inhibitors like ABC294640 may be a potential therapeutic approach toward down-regulating MYC expression in different hematological malignancies [445]. Albeit, some clinical trials with SK2 inhibitors have been conducted on diffuse large B-cell lymphoma (DLBCL) (NCT02229981) and multiple myeloma (MM) (NCT02757326).
A thoroughly studied oncogenic pathway is the Wnt signaling cascade that can promote MYC oncogenicity. The β- and γ-catenins, which are involved in the Wnt signaling pathway [446], can contribute to the pathogenesis of BCR-ABL1-mediated leukemias, including CML and Ph-positive B-ALL [447]. In CML, BCR-ABL1 in HSCs exerts its effects via β-catenin without induction of MYC expression [448]. Contrary to CML, BCR-ABL1 in Ph-positive B-ALL is able to phosphorylate γ-catenin directly and indirectly by SRC family kinase. Subsequently γ-catenin can induce MYC overexpression [447, 448]. On the other hand, following IgM signaling, the MYC mRNA becomes more stable through activation of eIF4 and eIF4GI, supplying higher levels of MYC [449–451]. A positive feedback loop between eIF4 and MYC boosts their activities [452], by which MYC cooperates with MAX, leading to enhanced expression of the BCR-ABL gene [453]. As it was mentioned, BCR-ABL provokes MYC overexpression in a multitude of ways. BCR-ABL1-mediated activation of JAK2 and STAT5 in both CML and Ph-positive B-ALL can sustain elevated MYC levels through promoting its gene expression and guarding MYC against ubiquitination and proteasome-dependent degradation (Fig. 7) [454, 455].
Fig. 7.
The interactions between BCR-ABL and MYC in B-cell lymphocytic leukemia with t(9;22). Dashed arrows represent an indirect pathway of action, and thick arrows show the mediator's direct effect. Both mature and immature BCR signaling cascades are able to increase the MYC expression. sIgM signaling increases the MYC mRNA stability, which results in a higher level of MYC, and increase the BCR-ABL expression. BCR-ABL can induce MYC expression. Pre-BCR signaling can increase MYC stability and expression. Inhibitors of these signaling mediators can significantly reduce the activity and expression of MYC
BCR-ABL tyrosine kinase inhibitors (TKIs), are used in treatment of CML and Ph-positive B-ALL patients. Dasatinib, a second generation of TKI, has a dual function against BCR-ABL positive cells and inhibits BCR-ABL and SRC family kinase [456]. However, in some Ph‐positive cases there are mutations that confer resistance to TKIs. A medium-chain fatty-acid derivative named AIC-47 is capable of suppressing BCR-ABL at the transcriptional level and eliminating the Warburg effect [457, 458]. Further, it was demonstrated that AIC‐47 could act as an anti-leukemic agent via down-regulating the MYC regardless of BCR-ABL mutations [459].
B-ALL with t(12;21)
The ETV6/RUNX1 (TEL/AML1) rearrangement, as a result of t(12;21) has been reported in 20–30% of childhood B-ALL cases [460, 461]. The MYC oncogenic pathway can synergize with ETV6/RUNX1, leading to promoted MYC oncogenic activity. This is due to a slight homology between the N-terminal region of ETV6 and bHLH region of MYC [28]. The ETV6 can fuse with PAX5 as well and mediates the induction of MYC target genes, leading to ALL progression [462, 463]. In ETV6/RUNX1-rearranged ALL, the MYC gene can be overexpressed by the GTP-binding protein RAC1 [464, 465]. RAC1 enhances the phosphorylation of STAT3, and as a result, MYC expression increases. Therefore, STAT3 inhibitors can enhance in B-ALL cells by decreasing MYC expression [464, 466]. An RNA-binding protein named IGF2BP1 can stabilize the ETV6/RUNX1 rearrangement at the post-transcriptional state, leading to an increased level of MYC [467, 468]. In a recent study, ETV6/RUNX1 fusion in a B-ALL model was knocked out using the CRISPR-Cas9 system, causing an enhanced level of apoptosis and reduced proliferation rate. This can eventually diminish the growth of tumor cells [469]. Abrogating the ETV6/RUNX1 seems to decrease the growth of B-ALL cells and this is due to its role in supporting the oncogenic factors like MYC.
B-ALL with the MLL rearrangement
The histone-lysine methyltransferase MLL gene, the so-called KMT2A, is located on chromosome 11 band q23. Chromosomal translocations in this gene frequently occur in both pediatric ALL and AML, representing a considerably poor prognosis in infant leukemia [470–472]. The transloactions involving MYC gene at chromosome 8 are more found in lymphoma comparted to leukemia [471]. It is worth mentioning that the deletion of the CDKN2 gene is frequent in B-ALL patients. It has been shown that t(8;14) is less frequent in cases with wild-type CDKN2 [473]. Furthermore, RS4;11 (KMT2A-AFF1), a B-cell leukemic cell line containing isochromosome i(8q), shows a duplicated MYC gene [474]. The KMT2A-AFF1 rearrangement mostly participates in the leukemic progression as a second hit, and B-ALL patients with this fusion protein possess a significantly promoted MYC gene expression in comparison to patients with AML [475, 476].
Nearly 135 different MLL rearrangements have been found in acute leukemias, which mostly include AF4 (AFF1), AF9, and ELN [477]. The oncogenic activation of the MYC gene mediated by MLL-fusion proteins such as MLL-Fusion/MYC/LIN-28 and MLL-ELN has been reported in B-ALL cases [478, 479]. As mentioned above, p53 can counteract the oncogenic activity of MYC. However, in leukemia cases with MLL rearrangement, MLL protein fusion with USP2 deubiquitinating protein expedites the degradation of p53 by enhancing the promoting USP2 activity. In such cases, MYC activity rises indirectly [480, 481].
The bromodomain and extra-terminal domain (BET) family members are BRD2, BRD3, and BRD4 proteins. These proteins are part of a foundation complex which also includes the super-elongation complex (SEC) and RNA Pol II‑associated factor 1 (PAF1) that all are required for binding of MLL-fused proteins to DNA [482]. The MLL-rearranged proteins bind to a particular complex in the MYC gene's regulatory domains, promoting gene expression at the transcriptional elongation level [483, 484]. Indeed, ChIP and expression analyses have shown that SEC is directly associated with MYC expression in myeloid and lymphoid leukemias [485]. The MLL-SEC rearrangement is stated to be involved in pathogenesis, progression, and metastasis of MLL-rearranged leukemia [486]. Since the BET proteins are therapeutic potential targets, BET inhibitors (iBET) such as iBET-151 and JQ1 (a potent inhibitor of BRD4) were developed to inhibit transcriptional elongation stage of MYC's transcription [483]. 7SK-snRNP complex as the regulator of transcription elongation consists of multiple proteins such as TFEb with kinase activity and HEXIM1, which are positive and negative regulators of transcription elongation, respectively. In order to inhibit TFEb, it is necessary HEXIM1 to interact with other members of 7SK-snRNP complex such as 7SK-RNA. The TEFb is required for initiating the transcriptional elongation of MYC. MYC’s cooperation with BRD4 would be needed if p-TEFb is recruited. This tight interplay between MYC, BRD4, and 7SK-snRNP complex is critical for a fine-tuned transcription elongation [487–490]. The oral iBET Birabresib (OTX015, also known as MK-8628) has been shown to reduce MYC expression and increase HEXIM1 in various types of hematological malignancies [491–493]. The evaluation of OTX015 in AML, ALL, MM, and DLBCL has already passed phase I of a clinical trial (NCT01713582), showcasing promising results [494, 495]. CPI-0610, a BRD4-targeted small molecule can also reduce MYC expression in different types of leukemias [496–498]. It is currently in phase II of a clinical trial (NCT02158858). Additional to agents that indirectly inhibits the MYC activity, there are some direct MYC inhibitors in pre-clinical stage that have been listed in Table 2. For more delineation on clinical trials of MYC inhibitors, a list of clinical trials by these agents in various diseases has also been provided in Table 3.
Table 2.
List of pre-clinical direct MYC inhibitors
| Mechanism | Type | Compound | References |
|---|---|---|---|
| Inhibitor of MYC/Max dimerization | Small Molecule | IIA6B17 | [672] |
| 10058-F4 | [673] | ||
| 10074-G5 | [674] | ||
| JY-3-094 | [675] | ||
| 3jc48–3 | [676] | ||
| MYCro1, MYCro2, MYCro3 | [677] | ||
| MYCMI-6 | [676] | ||
| KJ-Pyr-9 | [678] | ||
| EN4 | [679] | ||
| MYCi361 | [680] | ||
| MYCi975 | [681] | ||
| KI-MS2-008 | [682] | ||
| (Poly)peptide | OmoMYC | [683] | |
| FPPa-OmoMYC | [684] | ||
| Max bHLHZ (OmoMYC) | [685] | ||
| Mxd1 | [686] | ||
| Monoclonal antibody | [687] | ||
| H1 peptide | [688] | ||
| Inhibitor of E-box binding | Small Molecule | JKY-2-169 | [689] |
| (Poly)peptide | OmoMYC | [683] | |
| Max bHLHZ | [685] | ||
| Mxd1 | [686] | ||
| ME47 | [690] |
Table 3.
Clinical trials targeting MYC
| Type | Mechanism | Condition(s) | Compound | Phase | NCT number |
|---|---|---|---|---|---|
| Direct MYC inhibition | siRNA against the MYC | Hepatocellular Carcinoma | DCR-MYC |
Phase 1 Phase 2 |
NCT02314052 |
|
Inhibits MYC/MAX dimerization Inhibition of E-box binding |
Advanced Solid Tumors Non-small-cell lung carcinoma (NSCLC) Triple-negative Breast Cancer |
OMO-103 |
Phase 1 Phase 2 |
NCT04808362 | |
| Inhibits MYC/MAX dimerization | Neoplasms | OMO-1 |
Phase 1 Phase 2 |
NCT03138083 | |
| Downregulation of MYC | Ischemic Stroke | miR-494 | - | NCT03577093 | |
| Interrupts the translation of MYC gene | Neoplasms | AVI-4126 (RESTEN-NG) | Phase 1 | NCT00343148 | |
| Indirect MYC inhibition | Alteration of MYC translation (BET Bromodomain inhibitors) |
Castration-Resistant Prostate Carcinoma Metastatic Prostate Adenocarcinoma Metastatic Prostate Small Cell Carcinoma Stage IV Prostate Cancer AJCC v8 Stage IVA Prostate Cancer AJCC v8 Stage IVB Prostate Cancer AJCC v8 |
ZEN-3694 | Phase 2 | NCT04471974 |
|
Solid Tumor Lymphoma Brain Tumor |
BMS-986158 | Phase 1 | NCT03936465 | ||
| Lymphoma, Non- Hodgkin | CC-95775 (FT-1101) | Phase 1 | NCT04089527 | ||
|
Diffuse Large B-cell Lymphoma (DLBCL) High-Grade B-cell Lymphoma |
RO6870810 | Phase 1 | NCT03255096 | ||
| Neoplasms | GSK525762 | Phase 2 | NCT01943851 | ||
|
Myelofibrosis Primary Myelofibrosis Post-polycythemia Vera Myelofibrosis Post-essential Thrombocythemia Myelofibrosis |
CPI-0610 | Phase 3 | NCT04603495 | ||
|
Metastatic Malignant Solid Neoplasm Recurrent Malignant Solid Neoplasm Recurrent Platinum-Resistant Ovarian Carcinoma Refractory Ovarian Carcinoma |
ZEN-3694 | Phase 1 | NCT04840589 | ||
|
Malignant Solid Tumors Lymphoma Ovarian Cancer Breast Cancer Pancreatic Cancer Prostate Cancer |
AZD5153 | Phase 1 | NCT03205176 | ||
|
Neoplasms NUT Carcinoma |
BI 894999 | Phase 1 | NCT02516553 | ||
|
AML Including AML de Novo and AML Secondary to MDS DLBCL |
Birabresib (MK-8628, OTX015) | Phase 1 | NCT02698189 | ||
| MCL-1 inhibitor | Relapsed or Refractory AML | AZD5991 | Phase 1 Phase 2 | NCT03218683 | |
| AML | S64315 (MIK665) | Phase 1 Phase 2 | NCT04629443 | ||
|
Relapsed or Refractory Multiple Myeloma Relapsed or Refractory AML |
AMG 176 | Phase 1 | NCT02675452 | ||
|
Multiple Myeloma Non-Hodgkins Lymphoma Myelodysplastic Syndrome |
AMG 397 | Phase 1 | NCT03465540 | ||
| Inhibiting BCR-signalling |
Prolymphocytic Leukemia Recurrent Adult Diffuse Large Cell Lymphoma Recurrent Mantle Cell Lymphoma Recurrent Small Lymphocytic Lymphoma Refractory Chronic Lymphocytic Leukemia |
Ibrutinib | Phase 1 | NCT02303392 | |
|
Lymphoma, B-Cell Small Lymphocytic Lymphoma CLL Waldenstrom Macroglobulinemia Mantle Cell Lymphoma Diffuse Large B Cell Lymphoma Richter's Transformation Follicular Lymphoma Marginal Zone Lymphoma |
ARQ 531 |
Phase 1 Phase 2 |
NCT03162536 | ||
|
Epigenetic silencing (HDAC inhibitors) |
Diffuse Large B-cell Lymphoma |
Tucidinostat | Phase 3 | NCT04231448 | |
| Relapsed and refractory lymphoma | Entinostat | Phase 2 | NCT03179930 | ||
| PI3K inhibitor |
B Cells-Tumors B Cell Chronic Lymphocytic Leukemia Follicular Lymphoma Mantle Cell Lymphoma Large B-Cell Diffuse Lymphoma of Bone (Diagnosis) |
Idelalisib | Phase 1 | NCT03151057 | |
| Dual inhibitor of PI3Kδ and CK1ε |
CLL B-cell Non-Hodgkin Lymphoma |
TGR-1202 | Phase 1 | NCT03283137 | |
| Dual inhibitor of PI3Kδ and DNA-PK |
Diffuse Large B Cell Lymphoma Follicular Lymphoma CLL Small Lymphocytic Leukemia B Cell Lymphoma Marginal Zone Lymphoma Waldenstrom Macroglobulinemia Peripheral T Cell Lymphoma |
BR101801 | Phase 1 | NCT04018248 | |
| Dual inhibitor of PI3Kδ and HDACs | Relapsed and/or Refractory DLBCL With MYC Alterations | Fimepinostat (CUDC-907) | Phase 2 | NCT02674750 | |
| Inhibitor of CDK1, CDK2, CDK5 and CDK9 |
Advanced or Metastatic Breast Cancer Triple Negative Breast Cancer |
Dinaciclib | Phase 1 | NCT01676753 | |
| CDK9 inhibitor |
Relapsed Solid Tumors Refractory Solid Tumors Non-Hodgkin Lymphoma |
KB-0742 | Phase 1 | NCT04718675 | |
| Multi kinase inhibitor: inhibits CDKs 1, 2, 7 and 9 together with JAK2 and FLT3 |
AML ALL Blast Crisis MDS Multiple Myeloma |
TG02 | Phase 1 | NCT01204164 | |
| G-quadraplex stabilizer at MYC promoter |
AML High Risk Myelodysplasia |
APTO-253 | Phase 1 | NCT02267863 | |
|
Advanced Solid Tumors Lymphoma |
CX-3543 | Phase 1 | NCT00955786 |
Overexpression of HDACs such as HDAC9 and SIRT1 have an adverse prognosis in MLL-rearranged lymphoblastic leukemia [499], noteworthy, the latter alters the acetylation of critical genes including TP53, MYC, and NF-kβ, causing drug resistance [28, 499–503]. Indeed, a vast spectrum of HDAC inhibitors (iHDACs) are in development, and some have obtained FDA approval [504]. Contrary to other HDACs, HDAC7 which down-regulates MYC is mostly decreased in various types of leukemia including ones with MLL rearrangement [505]. A study showed that ectopic expression of HDAC7 is potentially anti-oncogenic in B-ALL cells [505]. iHDACs have been used individually and in combination with chemotheraputic agents such as cisplatin, etoposide and azacitidine. Moreover, combination of imatinib with iHDACs has shown promising results in MYC-mediated leukemia [506, 507].
The blood enhancer cluster (BENC), a super-enhancer fragment located within MYC gene governs the oncogenic MYC-expression during the proliferation of B-cell precursors. The BENC confers a vast region of accessible chromatin to transcription factors, enhancing gene expression [508–510]. The adverse MLL–AF9 rearrangement has been reported in both childhood myeloid and lymphoid leukemias [511]. Deletion of BENC demonstrated a drastic reduction of leukemic cells as well as improved prognosis of MLL–AF9-rearranged leukemia [512].
Chronic lymphocytic leukemia (CLL)
Chronic lymphocytic leukemia (CLL) is a disease with chronic proliferation of B lymphocytes due to impaired apoptosis and enhanced growth [449, 513]. CLL is a heterogeneous disease with various genetic alterations, such as mutations in immunoglobulin heavy chain variable region (IGHV), MYC translocation, del(11q), del(13q), and del(17p) [514, 515]. MYC translocations are extremely rare in CLL. A comprehensive study evaluated the frequency of MYC translocations in 3405 CLL patients, and showed only a 0.2% (8/3405) occurrence rate [515]. Among them, t(2;8), t(8;22), and t(8;14) were seen in one, two, and five patients, respectively. All CLL patients with MYC rearrangements had poor prognosis, complex cytogenetic abnormalities, and more than 10% prolymphocytes [515]. Some rare CLL cases with MYC translocation have been reported with typical morphology, and proper response to chemotherapy [516, 517]. Another study on 20 patients found that pathogenesis of MYC rearrangements in CLL rely on other genetic abnormalities. For example, complex karyotype is often seen with Richter syndrome transformation, while non-complex karyotype is often associated with proper respons to therapies and achieving remission [518].
Richter syndrome is an aggressive form of CLL and lymphoma associated mostly with molecular aberrations in MYC, CDKN2A/B, NOTCH1, and TP53 [519]. Gain of function mutations of MYC has been shown in 70% of Richter syndrome cases [520]. Despite genetic aberrations in the MYC gene, the MYC hyperactivation in transformed CLL could be as a result of miR-17-92 cluster activity [521], mutations or deletions of MYC regulator MGA [522], and NOTCH1 gene [523], CD40L activation of NF-kβ [524] or BCR (B-cell receptor) signaling [525]. Mutations in CD79 or CARD11 as a part of BCR signaling can provoke chronic BCR signaling in malignant B-cells leading to MYC overexpression [526]. Additionally, the surface immunoglobulin (Ig) of malignant B-cells in some CLL cases is fully glycosylated and mannosylated in the constant and variable regions, respectively. Such an Ig can interact with lectins present in the environment, including DC-SIGN and the mannose receptor [527–529]. Opposite to normal BCR signaling, this Ig can continuously send signals without BCR-antigen endocytosis and upregulates the MYC expression vigorously [527]. Furthermore, the miR-17-92 cluster participates in BCR-mediated upregulation of MYC in which a higher level of MYC leads to induction of miR-17-92, establishing a feed-forward regulatory loop in aggressive forms of CLL [530–532].
The FOXP1 is a transcription factor acting in favor of CLL progression. Although FOXP1 levels should be regulated by miR-150 and miR-34a, the MYC binds to their genes and decreases the gene expression, inducing gain of FOXP1 activity [533, 534]. Fludarabine and doxorubicin are agents capable of swiftly inducing the miR-34a-mediated inhibition of BCR signaling in B-cell neoplasms. These agents are ineffective at hampering BCR signaling in cells with impaired p53 pathway [534, 535].
Unmutated IgHV of BCR, along with heightened surface IgM (sIgM) signaling capability, is associated with high MYC mRNA translation in CLL [450]. Therefore, inhibition of BCR signaling-related factors, BTK and SYK, by Ibrutinib and Tamatinib respectively, can suppress the translational responses [450]. The mutation rate in IgHV depends on the proliferation rate of CLL cells. Cells with accelerated division have less IgHV mutation; in contrast, a higher mutation rate is associated with cells having a slow dividing pattern [536]. Though it is not entirely understood, patients with unmutated IgHV, compared to ones with mutated IgHV, seem to have an inferior survival rate [536, 537]. Mutations in NOTCH1 gene are prominent in CLL cases with unmutated IgHV and intensified sIgM signaling [538]. The gain of function mutations like c.7541_7542delCT in the PEST domain of NOTCH1 gene accumulates and stabilizes the protein, driving an aggressive CLL with high MYC translation [538–540]. Overall, due to cross reaction of NOTCH1 signaling with BCR, use of BCR inhibitor Ibrutinib in CLL could downregulate NOTCH1 activity [541]. This could be a promising approach in downregulation of MYC in CLL.
Various types of BCR signaling inhibitors, such as Ibrutinib, Idelalisib (PI3K inhibitor), Venetoclax (BCL-2 inhibitor), and other novel inhibitors have been developed, and some are approved for standard of care [542, 543]. The BET inhibitors, like the novel GS-5829, target CLL cells, inducing apoptosis via disrupting signaling pathways of MYC, BLK, AKT, and ERK1/2 [544]. Additionally, combinatorial use of BET and BCR inhibitors, have demonstrated further anti-leukemic effects [544, 545]. Despite all the promising results from the standalone targeted- and personalized-therapies, combination chemo-immunotherapy should be used for CLL [537, 546].
Lymphoma
Strong evidence showing MYC’s role in human cancer was first found in Burkitt lymphoma [547]. The role of MYC in causing lymphoma was further demonstrated in B-cell and rarely in T-cell lymphoma. [548].
Burkitt lymphoma invariably shows a translocation of MYC gene to the immunoglobulin gene loci [549]. Eighty percent of these translocations are within the immunoglobulin heavy chain gene locus 14q32. Less frequently the immunoglobulin light chain genes, IGκ or IGλ at 2p12 or 22q11 are involved [550, 551]. The translocations result in MYC hypermutation, creating MYC variants with increased oncogenic activity [552–554]. A study suggested that MYC translocation by itself does not cause Burkitt lymphoma, arguing that inhibitor of DNA binding (ID) proteins are another key factor [555]. MYC and ID3 were identified as the most mutated genes in Burkitt lymphoma [556]. Additionally, there is an interplay between BCR, ID3, and MYC in which, upon BCR signaling, MYC and ID3 would be activated. BCR, ID3, and MYC are all in a positive feedback loop [28, 557]. Robust BCR activation requires a functional PI3K. In Burkitt lymphoma, MYC-induced miR-19 inhibits the PI3K inhibitor PTEN, paving the way for lymphomagenesis [531, 555, 558, 559]. PI3K inhibitors may therefore be effective in treating Burkitt lymphoma (Table 3).
MYC rearrangement occurrence rate in DLBCL as the most common type of non-Hodgkin lymphoma is only 10% [549]. Frequent rearrangements in DLBCL involve BCL6 and/or BCL2 (t(14;18)(q32;q21)), which can be seen nearly in 30% of cases [560]. On the other hand, MYC amplification and gain of functions in DLBCL cases come with high MYC copy number and poor prognosis, which indicates an alternative MYC-dependent lymphomagenesis [561]. MYC gene SNPs in DLBCL cases directly correlate with increased cellular proliferation [28].
MYC stability depends on GSK-3β activity. MYC is degraded following GSK-3β-mediated phosphorylation of MYC's Thr-58 residue [562]. In DLBCL, PI3K activation hampers GSK-3β-mediated downregulation of MYC [525]. Furthermore, PTEN, the natural PI3K inhibitor, is often absent in germinal center B-cell-like DLBCL [563]. In a positive feedback loop, MYC upregulation in DLBCL promotes further BCR signaling via recruiting the MIR17HG cluster [532, 564]. These observations imply that inhibitors of the BCR signaling pathway might be effective in blocking this feedback loop.
Based on WHO classification, cases carrying MYC rearrangement accompanied by either BCL2 or BCL6 translocation are designated as double-hit lymphoma (MYC+/BCL2+ or MYC+/BCL6+) [565]. Lymphomas bearing all three translocations (MYC+/BCL2+/BCL6+) are known as triple-hit lymphomas [566]. These two types are labelled as high-grade B-cell lymphomas [567]. Additionally, double-hit lymphomas with MYC+/BCL2+ translocations carrying a TP53 mutation lead to further inhibition of p53-induced apoptosis [568]. Hence, impaired p53-mediated apoptosis, enhanced BCL2-mediated cell survival by BLC2, and promoted MYC-induced proliferation can together form an aggressive phenotype. The first-line treatment for double-hit lymphoma is R-CHOP chemotherapy [565]; however, a recent study demonstrated that targeted therapy using iBET (JQ1, I-BET, and OTX015) alongside BCL-2 inhibitor (ABT-199) could remarkably minimize proliferation and cell survival [569].
Some low-grade lymphomas like follicular lymphoma (FL) can transform to high-grade lymphomas such as DLBCL. FL is a sluggish type of non-Hodgkin lymphoma characterized by the t(14;18)(q32;q21) translocation [570]. In nearly 30% of cases, FL transforms to DLBCL [571]. The transformation of FL to high-grade lymphoma requires an additional hit [572]. Most transformed FL cases have intensified MYC expression in approximately one-fourth of their cells [573]. In addition to MYC, alterations to TP53, CDKN2A, and c-REL are associated with the proliferative phenotype of transformed FL [572, 574]. Like FL, most mantle cell lymphoma (MCL) cases have heightened MYC expression [573]. Contrary to FL, MCL is considered an aggressive malignancy [575]. The genetic hallmark of MCL is the t(11;14)(q13;q32) translocation [576, 577]. MYC rearrangements are usually seen in double-hit MCL [578, 579].
MCL is a heterogeneous disease in which cell-cycle, DNA damage response, and cell survival genes are mostly altered [580, 581]. MCL has two aggressive variants called the blastoid and pleomorphic [580]. In these subtypes, MYC alterations such as t(8;14), t(2;8) and add(8)(q24) accompanied by TP53 alterations lead to a more aggressive MCL [582–584]. Moreover, MALT1 a key factor in MYC stabilization is constitutively expressed in MCL, promoting disease progression.
Plasma cell neoplasms
Plasmablastic lymphoma (PBL) is another type of aggressive non-Hodgkin lymphoma, and given the presence of CD138+, CD38+ and MUM1+ it originates from plasmablasts rather than B-cells [585]. PBL is usually found in HIV-positive or immunocompromised cases [586], although there are few studies reporting the occurance of PBL in non-immunodificent patients [587, 588]. Here, BLIMP1-mediated gene repression in the plasmablasts, which is required for plasma cells differentiation downregulates several genes, including PAX5, BCL6 and MYC [589, 590]. It has been reported that 50% of PBL cases bear mutations in BLIMP1 gene (PRDM1), affecting MYC regulation [591]. Intriguingly, 80% of cases have co-expression of BLIMP1 and MYC [591], highlighting a synergistic effect between the two molecules. Almost 50% of PBL cases are found to have MYC rearrangements [592]. Gain of function mutations of MYC are also common in PBL [593]. Loss of p53, has also been shown to contribute to PBL’s aggressiveness [594]. BET inhibitors like JQ1 are capable of inducing cell cycle arrest [482] and may be effective in these cases.
Similar to PBL, plasma cells involved in multiple myeloma (MM) also bear IGH-MYC translocation [595]. Hyperdiploidy, MYC structural variants, and mutations in RAS can also induce MM [595, 596]. A recent comprehensive study showed that the progression of MM heavily depends on MYC, RAS, and NF-κB signaling pathways [595]. Another study on 1342 MM patients showed those with MYC rearrangement had a lower survival rate [597]. There are several MYC targeted therapies for MM that are undergoing clinical trials (Table 3) [598]. Among all the agents, Lenalidomide, which can indirectly target MYC by inhibiting its transcription has gained FDA approval [599].
Role of MYC in myelocytic malignancies
MYC dysregulates myeloid differentiation [600], and The deletion of MYC in mice diminishes leukomogenesis. The dysregulated myelopoiesis, leads to notable thrombocytosis, drastic monocytopenia and neutropenia, and severe anemia [419, 420]. Several early studies on myeloid leukemia cell lines, such as MEL, K562, and U937, showed that MYC up-regulation could inhibit cell differentiation [601–603]. Nonetheless, in some leukemic cells like NB4 (promyelocytic leukemia cell line), MYC boosts the retinoic acid-induced differentiation [604]. There have been many in vitro- and in vivo- based studies on the role of MYC in myeloid leukemias [419, 605–607]. Nearly all indicated that in both chronic and acute myeloid leukemia, MYC can impact progression and prognosis of the disease.
Acute myelocytic leukemia (AML)
AML has various subtypes with a broad spectrum of genetic abnormalities (summarized in Table 1). Unlike lymphoid malignancies, where MYC overexpression is mostly associated with its translocation, the cause of MYC aberrant expression and activity in myeloid malignancies is not thoroughly established [29, 608]. However, a rare recurrent translocation t(3;8) (q26.2;q24), causing MECOM-MYC rearrangement, has been reported to be associated with therapy-related and relapsed AML as well as AML transformed from Ph+ CML [609, 610]. Recently, in a detailed and comprehensive study, hotspot mutations in the MYC gene have been identified in AML patients [611]. Other mutations can coincide with MYC mutation in AML. These include: MYC-FLT3, NPM1-MYC, MYC-DNMT3A, NPM1-MYC-FLT3, NPM1-MYC-DNMT3A, and MYC-FLT3-DNMT3A [611]. MYC acts in favor of the progression and maintenance of AML by participating in promoting transcription and translation of the genes involed in cell growth, self-renewal of leukemia stem cell, and chemoresistance [6, 139, 502, 612, 613]. In all subtypes of AML with cytogenetic abnormanlities, MYC overexpression is mainly a sign of inferior overall survival [614]. Since novel MYC-targeting agents show clinical efficiency in AML, understanding the MYC activity in AML is of critical important [615].
Table 1.
Genetic abnormalities in AML
| Type | Biomarker | Fusion protein/role | References |
|---|---|---|---|
| Rearrangements | t(8;21) (q22;q22.1) | AML1-ETO or RUNX1-CBFA2T1 | [616] |
| inv(16) (p13.1q22) or t(16;16)(p13.1;q22) | CBFB-MYH11 | [617] | |
| t(15;17) (q22;q12) | PML-RARA | [618] | |
| t(6;9) (p23;q24) | DEK-NUp214 | [619] | |
| inv(3) (q21.3q26.2) or t(3;3) (q21.3;q26.2) | GATA2-MECOM | [620] | |
| t(1;22) (p13.3;q13.3) | RBM15/MKL1 | [621] | |
| t(9;22) (q43;q11) | BCR-ABL1 | [622] | |
| t(6;11) (q27;q23) | MLL-AF6 | [623] | |
| t(9;11) (p22;q23) | MLL-AF9 | [624] | |
| t(9;11) (p21.3;q23.3) | MLLT3-KMT2A | [625] | |
| t(6;9) (p23;q34) | DEK-NUp214 | [619] | |
| t(3;8) (q26.2;q24) | MECOM-MYC | [609] | |
| t(5;11) (q35;p15.5) | NUP98-NSD1 | [626] | |
| Mutations | FLT3, KRAS, NRAS, KIT, PTPN11, NF1 | Signaling mediators | [627] |
| DNMT3A, IDH1/2, TET2, ASXL1, EZH2, MLL/KMT2A | Epigenetic mediators | [627] | |
| CEBPA, RUNX1, GATA2 | Transcription factors | [628] | |
| TP53 | Tumor suppressing factor | [629] | |
| SRSF2, U2AF1, SF3B1, ZRSR2, RBM25 | Spliceosome complex | [630] | |
| NPM1 | Nucleophosmin | [631] | |
| RAD21, STAG1, STAG2, SMC1A, SMC3 | Cohesin complex | [632] | |
| MYC | Proto-oncogene | [633] |
Inducing leukemogenesis through overexpressing MYC, is a common approach to study the effects of this proto-oncogene. MYC expression alone does not suffice for the transition of cells to AML; in fact, continuous co-stimulation with IL-3 and GM-CSF are also required, suggesting the vital role of microenvironment and cytokines in the development of MYC-mediated AML [634]. Most recently, leukemic microenvironment-originated exosomes have attracted significant attention. This is due to their ability to carry cargos like MYC between tumor cells, inducing leukemic progression [635]. Investigating leukemia-related exosomes seems beneficial to a better understanding of leukemogenesis, leukemia diagnosis, and efficient therapeutic strategy.
The fine-tunned balance between pro- and anti-apoptotic signals are mostly dysregulated during tumorigenesis. All six members of the anti-apoptotic BCL family, including BCL2, BCLxl, BCLw, BCLb, BFL1, and myeloid cell leukemia 1 (MCL1), have been reported to advance the MYC-induced myeloid leukemogenesis [636]. Results from MYC-induced AML in mice have shown presence of highly expressed anti-apoptotic protein MCL-1 [637]. MCL-1 inhibitors such as AZD5991 (NCT0321868), S64315 (NCT0297936), AMG176 (NCT0267545), and AMG397 (NCT03465540) are under evaluation in phase 1 clinical trials for AML patients.
Aberrant transcription factors encoded by AML-related rearrangements such as AML1-ETO (RUNX1-CBFA2T1), PML-RARα, and ZBTB16 (PLZF)-RARα have been reported to induce Wnt signaling, leading eventually to the upregulation of MYC [638–640]. The Wnt-induced upregulation of MYC in AML with RUNX1-CBFA2T1 fusion protein is associated with a feed-forward loop between RUNX1-CBFA2T1 and γ-catenin. The RUNX1-CBFA2T1 elevates γ-catenin expression and mediates the Wnt-induced MYC upregulation [638]. RUNX1-CBFA2T1 can also induce MYC upregulation by provoking the expression of β-catenin, another Wnt family member [638]. In addition to RUNX1-CBFA2T1, LEO1, a direct and specific substrate for phosphatase of regenerating liver-3 (PRL-3), can bind to β-catenin and increase its activity. This results in transactivation of the MYC gene [641]. Nearly 43% of AML cases are PRL-3+ in which they have exhibited sensitivity to β-catenin inhibition. This shows AML-PRL-3+ is dependent on Wnt signaling [641, 642]. A study found that Kangai 1 (KAI1), also called CD82, is overexpressed in pediatric AML cases. This activates the Wnt/β-catenin pathway and its target MYC, supporting the proliferation of leukemic cells and -chemoresistance to doxorubicin. Remarkably, the knocking-down of CD82 led to apoptosis and repressed growth and reduced chemotherapy resistance in AML cells [643]. Apart from Wnt signaling, proliferation and self-renewal in AML cells with RUNX1-CBFA2T1 significantly depends on TATA-Box binding protein associated Factor 1 (TAF1) [644]. Due to the considerable overlapping of binding sites of TAF1 and RUNX1-CBFA2T1, the knocking down of TAF1 or inhibiting it by Bay-364 can impair the MYC expression and promote differentiation and apoptosis in leukemic cells [644].
Protein phosphatase 2A (PP2A) endogenous inhibitor, ARPP19, might be a potential biomarker for patients with AML as it associated with the induction of MYC overexpression [645]. Based on the ELN risk group classification for AML, in both favorable- and intermediate-risk groups, a high level of ARPP19 increases the necessity of BM transplantation while patients with low levels of ARPP19 can mostly be cured with chemotherapy [645].
Furthermore, FLT3 mutations are found in AML patients. These include internal tandem duplication (ITD) and tyrosine kinase domain (TKD) mutations [646]. These mutations can mediate ligand-independent activation of the canonical Wnt/β-catenin signaling pathway, resulting in the upregulation of MYC and myeloid transformation [647]. Using merely FLT3 tyrosine kinase inhibitors might not be efficient to halt AML progression. This is due to MYC-mediated stabilization of the histone deacetylase SIRT1 which causes treatment resistance in AML [502]. However, strategies such as disrupting Wnt/β-catenin signals or combining Pim-1 kinase inhibitors or PP2A activators with FLT3 inhibitors synergize and promote their anti-leukemic effects in AML [648–650]. Apart from signaling pathways, dysregulation of mRNA splicing is also found in AML, where the splicing regulator RBM25 is reduced [651]. Decrease in RBM25 increases MYC levels followed by enhanced proliferation along with reduced apoptosis in leukemic cells [651].
Cancer cells often utilize double minutes (dmin), homogeneously staining regions (hrs), and ring chromosomes to do extra-chromosomal gene amplification [652]. This mechasnism of gene amplificationoccasionally happens in leukemias [653]. In this manner, two of the most amplified AML-related genes are MYC and MLL [654, 655],which the presence extra-chromosomal gene amplifier implies a poor prognosis although mechanism of action has not been elucidated [656].
Myeloproliferative neoplasms (MPNs)
MPNs are associated with the proliferation of one or more members of the myeloid lineage. There are mainly two categories based on presence of Ph chromose: Ph (BCR-ABL)-positive CML and Ph-negative group including essential thrombocythemia (ET), polycythemia vera (PCV), and primary myelofibrosis (PMF). In the Ph-negative group the involvement of JAK2, MPL, and/or CALR aberrations are frequent [657, 658]. However, the co-occurrence of Ph-positive and other aberrations have been infrequently reported in CML [657].
The aforementioned feed-forward interplay between MYC and BCR-ABL thoroughly describes the BCR-ABL-mediated upregulation of MYC in both CML and Ph-positive ALL. Furthermore, BCR-ABL can mediate the phosphorylation of Ser62 residue of MYC, though dasatinib can induce its dephosphorylation [659]. Progression of CML into blastic crisis is associated with a higher MYC expression level. This correlates with inferior response to imatinib and poorer prognosis [605]. In CML patients who further progress to the blastic crisis phase, endogenous PPA2 inhibitor, CIP2A increases prior blastic phase. CIP2A blocks dephosphorylation of MYC at Ser62 residue [660, 661]. In the blastic phase, the MYC target genes, including the ATP-binding cassette (ABC) transporters, are up-regulated. However, they might play a role in resistance to imatinib [662]. It has been suggested that TKIs can be combined with other therapeutic strategies to overcome ABC-related drug resistance [663].
Fbxw7-mediated ubiquitination of MYC is considered an essential regulatory step in CML [664]. The Fbxw7 mediate decrease in proliferation, survival and maintenance of leukemia-initiating and leukemia stem cells (LIC/LSC). Upon phosphorylation of MB-Box I domain of MYC at Thr-58 and Ser-62, Fbxw7 targets MB-Box I domain and destabilizes the MYC [665]. Phosphorylation of Thr-58 relies on the earlier phosphorylation of Ser-62. Intriguingly, The sole phosphorylation of Ser-62 stabilizes the MYC, whereas further phosphorylation of Thr-58 propels MYC toward ubiquitination and degradation [59]. The BCR-ABL fusion protein is not solely required for LSC survival; thereby, TKIs fail to annihilate LSCs responsible for CML maintenance [666]. To address this impediment, a study recommended an intriguing approach without application of BCR-ABL inhibitors. In this dual approach p53 was activated and MYC was inhibited. This resulted in synergetic extermination of cells, further differentiation, and almost obliteration of transplantable human LSCs in mice,whilie healthy HSCs were spared [667]. This might be a potential strategy in TKI resistance and relapsed patients.
The prevalence of JAK2 V617F mutation in MPNs, including PCV, ET, and PMF, is approximately 90%, 50%, and 50%, respectively [668]. Generally, the signaling cascade initiated by JAK2/STAT5 can mediate MYC expression. In MPNs with JAK2 V617F mutation however, the signaling is independent of any ligand [669]. Hyperactivation of mutated JAK2 needs an intact FERM domain to induce MYC overexpression [670]. Both PIM and JAK2 inhibitors have been used to downregulate MYC and repress MPN cell proliferation; however, combining them can overcome MPN drug resistance and synergistically enhance their suppressing effect upon MYC [671]. The above mentioned strategy implemented by Abraham et al. in which p53 is provoked and MYC is inhibited, has also been suggested as an effective therapeutic approach for JAK2-mediated MPNs [667, 669].
MYC inhibitors
MYC is a well-established oncogene that can be targeted by inhibitors. The table below provides a list of MYC inhibitors in the pre-clinical stage.
Direct MYC inhibition
Studies have pointed that direct MYC inhibition brings about prompt tumor regression, highlighting the importance of this approach [691]. Inhibiting MYC/MAX dimerization and E-box binding by peptides and small molecules, as well as using RNA interferences (miRNA, siRNA) downregulating MYC translation, can directly block MYC activity [691].
Recently, OmoMYC agents as MYC dominant-negative proteins have attracted great attention [683]. Since MYC is a master transcription factor, blocking it by OmoMYC at first seemed to be challenging due to the expected side effects. However, after testing on animal models, side effects have shown to be mild, well-tolerated, and reversible [692]. Intriguingly, OmoMYC could infiltrate cells, inhibiting MYC activity by its spontaneous cell-penetrating ability. Moreover, using OmoMYC through direct tissue delivery and systemic administration in non–small cell lung cancer models showed significant therapeutic potential [692]. OmoMYC can inhibit MYC by two mechanisms; interrupting MYC/MAX dimerization and E-box binding [693]. There are different types of OmoMYC that are in pre-clinical phases. Among them, OMO-103 [NCT04808362] and OMO-1 [NCT03138083] have made it to the clinical trials (Table 3).
In addition to OmoMYC, other compounds can also inhibit MYC/MAX dimerization (Table 2). The MYC/MAX destabilizers, IIA6B17, 10058F4, and 10,074-G5, were extracted from a peptidomimetic library [672, 694]. It seems that IIA6B17 can act against c-Jun, due to the resemblance of its leucine zipper structure [695]. JY-3-094 was found to be able to hinder proliferation in MYC overexpressed cells (HL60 and Daudi cells) via inhibiting MYC/MAX dimerization [696]. If a phenyl ring is added to JY-3-094 the result would be a MYC inhibitor called 3jc48-3 with five times more potential in arresting the cell cycle at G1/G0 [696]. Such significant potential in inhibiting MYC/MAX is thought to be the effect of phenyl ring on F375, I381 and R378 residues in the MYC/MAX dimer [696, 697].
KJ-PYR-9, a compound derived from Kröhnke pyridine library were found to have anti-DNA-binding and anti-MYC/MAX dimerization features [698]. The KJ-PYR-9 effects on inhibiting proliferation in xenografts bearing MYC-amplified human cancer cells seem promising [698]. Other small-molecules with properties like KJ-PYR-9 are MYCro1, MYCro2, and MYCro3 [677, 699]. MYCro3, in combination with Palbociclib, a CDK4/6 inhibitor, has shown great potential in treating HER-2 negative metastatic breast cancer [700], indicating that the direct MYC inhibition can synergically increase the effect of other targeted therapies.
Most recently, based on bimolecular fluorescence complementation, another direct MYC inhibitor, called MYCMI-6 was identified among 2000 agents, which could interrupt the MYC/MAX dimerization [701, 702]. Recent studies have demonstrated that the effect of MYCMI-6 on breast cancer in inducing cell-growth inhibition and apoptosis [678, 703]. EN4 is also a novel covalent small molecule, directly targeting C171 residue of MYC, causing thermal destabilization of MYC and MAX, as well as disrupting MYC transcriptional activity. Overall, the EN4 features enable it to block tumorigenesis [680]. Han et al. discovered two compounds (MYCi361 and MYCi975) capable of phosphorylating Thr-58 residue of MYC, propelling it toward proteasome-mediated MYC degradation [704]. MYCi975 is an enhanced model of MYCi361. Results of in vivo MYCi-induced tumor regression capacity are shown to be promising since it enhances infiltration of immune cells into tumor microenvironment, upregulates PD-L1 on tumor cells, and synergies with anti-PD1 immunotherapy [681, 704].
Stabilization of MAX homodimer by an asymmetric polycyclic lactam termed KI-MS2-008 is another approach toward attenuating MYC/MAX dimerization, resulting in the reduction of MYC protein and the expression of its target genes. Also, in vivo evaluations show that KI-MS2-008 abrogates the ability of tumor cells to grow properly [682]. MAX homodimer stabilizers like KI-MS2-008 could also be utilized alongside monoclonal antibodies against PD-1 or PD-L1 immune checkpoints to synegies the antitumoral effects [705]. Moreover, there is a protein called MXD1 that can couple with MAX, afterwards hijacking E-box of MYC target genes, and antagonizing MYC transcriptional activity [706]. Mad can act similar to MXD1, and it is claimed that Mad is tenfold more potent compared to OmoMYC [686]. A small hybrid protein named ME47 can also inhibit MYC transcriptional activity by seizing the E-box binding site of MYC target genes, resulting in a significant reduction in cell proliferation of tumor xenografts [690]. On the contrary, JKY-2-169 binds to MYC-MAX heterodimer, not allowing it to bind with DNA E-box, without affecting MYC-MAX formation. This JKY-2-169-mediated perturbation of DNA binding has been shown to reduce MYC-induced cell proliferation, cell cycle arrest, and apoptosis [707, 708].
Monoclonal antibodies against MYC can also inhibit its activity. Park et al. showed that these antibodies are capable of targeting MYC and MYC-MAX heterodimer [687]. For antibodies intracellular infiltration remains a challenge. There is a small alpha-helix MYC inhibitor peptide called H1 that can be carried to the nucleus via particular non-toxic carriers and decrease the MYC-MAX dimerization, reducing the expression of MYC target genes [688].
Using siRNAs for in vivo inhibition of MYC translation is another approach, although transporting siRNAs into cells requires reliable carriers. DCR-MYC, an EnCore lipid nanoparticle containing siRNA against MYC, was used in clinical trials to treat solid tumors [NCT02314052] and hematological malignancies[NCT02110563] [691, 709]. Further studies however showed side effects like thrombotic microangiopathy, which terminated the clinical trials [691, 710]. Studies on MYC-targeted siRNAs are still going on.
There are miRNAs such as miR-494, which targets MYC translation. It has been shown that miR-494 is downregulated in ovarian cancer, whereas overexpression of miR-494 hampered the growth of the cancer cells and limited their migration [711]. Additionally, ectopic miR-494 overexpression inhibits the proliferation of pancreatic cancer cells via inducing apoptosis, cell-cycle arrest, and senescence, which remarkably prohibited the invasiveness of the cancer cells [712]. This particular miR has recently undergone clinical trials. A MYC-targeted phosphorodiamidate morpholino oligomer (PMO) called AVI-4126 can prohibit ribosomal assembly, therefore inhibiting MYC translation [677, 713]. AVI-4126 has been extensively applied to various cancers, and results were shown promising, which led this particular PMO to clinical trials.
Indirect MYC inhibition
MYC inhibition indirectly by targeting its regulating factors provides a more flexible approach toward MYC inhibition. MYC regulating factorsinclude BET family, MCL-1, BCR-signaling mediators, HDACs, PI3Kδ, DNA-PK, CDKs, kinases and G-quadraplex (Refer to “The role of MYC in hematopoiesis and hematological malignancies" section). There are various inhibitors for the aforementioned MYC regulators; among them, some have made it to clinical trials (Table 3).
BET family inhibitors
BET family includes BRD2, BRD3, and BRD4 proteins. BET proteins recognize the acetyl-lysine residues of histones, recruiting transcription factors, especially the MYC oncoprotein, to promote gene expression. BET inhibitors (iBETs) were found to be effective in blocking oncoproteins and decreasing tumorigenesis. The first iBET entering clinical trials was Birabresib (MK- 8628, OTX015) [492]. Due to the safety, efficacy, and pharmacokinetics of Birabresib in hematological and solid tumors, phase II clinical trial has been recommended [714].
Among different iBETs, ZEN-3694 is an orally administered pan-BET inhibitor. Currently, ZEN-3694 is under clinical trial in phase I and II. Drug-resistance to some targeted therapies seem to be inevitable, due to the overexpression of master oncogenes like MYC. Using ZEN-3694, alongside Enzalutamide as an inhibitor of androgen receptor for treatment of prostate cancer, synergically enhances the effect of Enzalutamide [715, 716]. FT-1101 (CC-95775) can also act as pan-iBET. A study on the effect of FT-1101 on various human leukemia cell lines exhibited higher inhibition of proliferation in tumor cells compared to JQ1. Monotherapy with FT-1101 displayed tolerance and proper safety [717, 718].
BMS-986158 is another orally bioavailable BET inhibitor, shown to be well-tolerated in treatment of advanced cancers. The sole reported side effect was reversible thrombocytopenia [719, 720]. Among iBETs, BMS-986158 has notable pharmacodynamics profile with a longer half-life.[721]. RO6870810 (also termed RG6146 and TEN-0) is a novel iBET, resembling the JQ1 class. However, it outperforms JQ1 in solubility, metabolic stability, and binding to serotonin receptors. Additionally, alpha assay technology shows the remarkable affinity of RO6870810 toward the acetyl-lysine recognition pocket of the BET family [722, 723].
In (nuclear protein of the testis) NUT carcinoma, the BET family would join the NUT if NUTM1 rearrangements occur. The fused oncoproteins alter MYC regulation. A novel iBET RO6870810 (also known as RG6146 and TEN-0) can dissociate the BRD-NUT oncoproteins from DNA, inhibiting the proliferation of cancer cells. RO6870810 has also been tested on DLBCL where MYC is associated with the aggressiveness of DLBCL [722]. The clinical potential of RO6870810 is now being evaluated in clinical trials.
Recently, Molibresib (GSK525762), an orally administered iBET, has also been tested on NUT carcinoma. Phase I clinical trials assessed safety, tolerance, pharmacokinetics, and pharmacodynamics. The preliminary results recommended moving to phase II [724]. In addition to NUT carcinoma, GSK525762 has also been tested on a vast spectrum of hematological neoplasms in order to indirectly inhibit the master oncoprotein MYC. Minor dose-limiting toxicity related to GSK525762 has been seen in AML patients, including diarrhea and reduced ejection fraction, although both were reversible. Overall, despite seeing a complete response to GSK525762, due to some adverse effects, the phase one clinical trials recommended further investigations [615].
Inhibition of BET family members in hematological neoplasms has shown to be a quite effective treatment approach. Among other iBETs, CPI-0610 has been evaluated in clinical trials for primary myelofibrosis, post-polycythemia vera myelofibrosis and lymphomas [498, 725, 726]. Using CPI-0610 solely or combined with a Janus kinase 1/2 inhibitor (Ruxolitinib) on refractory or intolerant advanced myelofibrosis demonstrated promising efficacy in patients with inadequate responses to Ruxolitinib. This seems to be the effect of MYC inhibition, enhancing Ruxolitinib effects. Following treatment, the patients exhibited improvement of bone marrow function [725]. CPI-0610-mediated MYC inhibition induces G1 arrest and apoptosis in multiple myeloma resulting in tumor regression. Moreover, CPI-0610 alongside immunomodulatory drugs for multiple myeloma such as thalidomide, lenalidomide and pomalidomide can be synergically utilized in multiple myeloma treatment [497].
BRD4 could be targeted by AZD5153, an orally bioavailable iBET. Unlike other monovalent iBETs, the AZD5153 is a bivalent inhibitor that results in further antitumor activity. The effect of AZD5153 on AML, multiple myeloma, and DLBCL xenografts has been reported to be significant. The AZD5153 modulates MYC, E2F, HEXIM1 and mTOR pathway, indicating the remarkable potential of this iBET. Of note, AZD5153-mediated alteration of mTOR positively enhances the effects of AZD5153 on tumor cells [727, 728]. Overexpressed MYC and BCL2 in double-hit lymphoma and double expressing lymphoma has poor prognosis. Utilizing AZD5153 could downregulate several oncogenic factors, including MYC and B-cell development-related factors. Notworthy, AZD5153 neither could downregulate BCL2 family members (anti-apoptotic factors), or induce activation of BH3-only proteins (pro-apoptotic factors). AZD5153 and BCL2 inhibitor (AZD4320) synergistically induced antitumor effects [729]. This potent iBET is now under clinical trials for the treatment of various diseases, including malignant solid tumors [NCT03205176]. Similar to AZD5153, another iBET called BI894999 can also inhibit BRD4 leading to modulation of MYC and HEXIM1 in AML cells. This particular iBET, in combination with CDK9 inhibitor, shows an expedited apoptotic response due to the reduction in global p-Ser2 RNA polymerase II levels [730].
MCL-1 inhibitors
All members of the anti-apoptotic BCL family, especially MCL1, have been reported to advance the MYC-induced myeloid leukemogenesis [636]. Results from MYC-induced AML in a mouse model have shown presence of highly expressed anti-apoptotic protein MCL-1 [637]. High levels of MCL-1 provoke tumorigenesis and drug resistance, indicating the potential of MCL-1 inhibitors as a therapeutic option.
Accordingly, AZD5991, a selective small-molecule targeting MCL-1 could be a potential choice in AML treatment. Due to the significant potential in inducing prompted Bak-dependent apoptosis and high antitumor activity in pre-clinical studies on myeloma and AML, AZD5991 has been chosen to enter clinical trials as a treatment for relapsed or refractory AML. It has been used both as a monotherapy and combined with Bortezomib (inhibitor of the 26S proteasome) or Venetoclax (BCL-2 inhibitor) [731]. S64315 (MIK665), a highly selective MCL-1 inhibitor can act partly similar to AZD5991 by inhibiting MYC activity and inducing Bax/Bak-mediated apoptosis. S64315 is a well-tolerated compound capable of inducing dose-dependent apoptosis, demonstrating potent antitumor activity in hematological malignancies such as AML, multiple myeloma, and lymphoma [732]. Since BCL-2 family members and MYC act cooperatively in cancers [733], inhibiting MCL-1 could be a potential approach for MYC-involved cancers.
AMG class of MCL-1 inhibitors has recently entered phase I clinical trials as a treatment for various hematological malignancies, including relapsed or refractory multiple myeloma. [NCT02675452, NCT03465540]. AMG176 was introduced to be administered intravenously, and AMG397 was developed as the first orally bioavailable MCL1 inhibitor. AMG397 compared to AMG176 has improved potency and pharmacokinetic features [734]. It selectively competes with other BCL family members over the BH3-binding site of MCL1 and elevates the caspase 3/7 activity. It is reported that hematological neoplasms are highly sensitive to this particular compound [734].
BCR-signaling inhibitors
BCR signaling can activate several transcription factors, especially MYC. BCR-signaling mediators, such as BTK are involved in inducing MYC during B-cell development [442, 443]. Ibrutinib, a BCR-signaling inhibitor which is under clinical trials for several hematologic malignancies could be a potential compound for inhibition of MYC induced proliferation in B-cell malignancies [NCT02303392]. It is worth mentioning that in malignancies like MCL, where one-third of cases do not respond to Ibrutinib, MYC is overexpressed, suggesting that MYC can block Ibrutinib activity [735]. The underlying mechanism responsible is MYC-induced BTK overexpression [736].
ARQ531 is a novel BTK inhibitor that can also act against other BCR signaling factors like SRC kinases and ERK-signaling pathway. Targeting a multitude of BCR-signaling related factors in CLL models with ARQ531 showed robust inhibitory potential in treatment of Ibrutinib resistance cells [737]. Due to the great potential of ARQ531 in overcoming resistance to Ibrutinib, it has entered phase I clinical trials for various MYC-involved hematological neoplasms [NCT03162536].
HDAC inhibitors (epigenetic modulators)
Histone deacetylase family members play an essential role in regulating the MYC level [738]. For instance, SIRT1 is a class III histone deacetylase responsible for the acetylation of critical genes, including TP53, MYC, and NF-kβ [28, 499–503]. On the other hand, HDAC7 that is mostly decreased in various types of leukemia can downregulate MYC[505]. Taken together, it seems that HADCs are vastly involved in MYC regulation; thus, inhibition of HDACs provoking MYC activity can be greatly beneficial for the treatment of MYC-involved cancers.
HDAC inhibitor Entinostat (class I iHDAC) is capable of inhibiting HDAC2, a partner of MYC in medulloblastoma. Entinostat-mediated inhibition of HDAC2 reduces MYC transcriptional activity, and hinders MYC-DNA binding, indicating the efficiency of iHDACs [739]. Entinostat has thoroughly been studied on hematological malignancies [740], and now is in phase II clinical trial for relapsed and refractory lymphoma [NCT03179930]. Tucidinostat, an orally bioavailable iHDAC is now in phase III clinical trials [NCT04231448] in combined with R-CHOP regimen,. Administrating this combined treatment, compared to using the R-CHOP regimen alone, displayed a prolonged event‐free survival [741].
PI3K inhibitors
MYC half-life is less than 30 min and its translation significantly depends on the eukaryotic translation initiation factor 4 (eIF4) complex. The eIF4E-binding protein 1 (4E-BP1) sequestrates eIF4E, and following hyperphosphorylation of 4E-BP1, mRNA translation would be initiated as a result of multiple upstream signals. PI3K can independently phosphorylate of 4E-BP1, leading to translation of MYC mRNA [742]. Inhibition of PI3K by compounds such as Idelalisib [743], TGR-1202 [742], Fimepinostat (CUDC-907) [744], and BR101801 [745] can significantly reduce the MYC mRNA translation.
In contrast to Idelalisib, TGR-1202 not only inhibits PI3K but also targets casein kinase-1 ε (CK1ε). The mechanism of action of CK1ε in phosphorylating 4E-BP1 is similar to PI3K. Therefore, the dual inhibitory effect of TGR-1202 makes it clinically more potential than Idelalisib in the treatment of aggressive lymphoma [742]. Both compounds have now entered clinical trials.
The effectiveness of PI3K inhibitors is limited by the parallel activation of other survival-supporting pathways leading to drug resistance [746]. CUDC-907, a dual inhibitor of PI3Kδ and HDACs seems to be able to surpass the limitation of the inhibitors that only targets PI3K [747]. CUDC-907 has been tested in clinical trials on various hematological cancers, including relapsed or refractory lymphoma, multiple myeloma, MYC-altered DLBCL, and CLL. The results showed tolerability, safety, and efficiency [744, 748, 749].
DNA-activated protein kinase (DNA-PK) is a PI3K-related kinase capable of phosphorylating MYC at multiple serine residues, promoting its oncogenic activity in MYC-driven B-cell lymphomas [750, 751]. BR101801 is a first-in-class, orally bioavailable small-molecule capable of targeting both DNA-PK and PI3Kδ. The dual inhibitory mechanism of BR101801 in double-hit lymphoma cells, downregulates the MYC stability regardless of its translocation, amplification, and overexpression. This has led to significant growth inhibition in double-hit and double expressing DLBCL cells. Moreover, the combination of BR101801 with Venetoclax synergically enhances its antitumor effects, highlighting the great potential of this treatment approach [745, 752].
CDK inhibitors
CDK9, part of the positive transcription elongation factor b (p-TEFb) complex is essential for phosphorylation of a serine residue at CTD of RNA polymerase II recruited by MYC [753, 754]. MYC binding to p-TEFb activates RNA polymerase II and initiates transcription. This process has been shown to be critical for maintenance of MYC-driven model of hepatocellular carcinoma [145, 753, 755].
Dinaciclib inhibits kinase ability of CDK1, CDK2, CDK5 and CDK9 in a dose dependent manner. It is now in phase I/II clinical trials for different cancers, including both solid tumors and hematological cancers [756]. Investigation of Dinaciclib-mediated inhibition of CDK9 in aggressive MYC-induced lymphomas demonstrated the remarkable effectiveness of CDK9 inhibitors [757]. Of note, that Dinaciclib reduced the anti-apoptotic factor MCL-1 [757].
TG02 is a CDK inhibitor capable of inhibiting CDK1, CDK2, CDK7, CDK9, JAK2 and FLT3. This multi kinase inhibitor not only inhibits MYC by targeting CDKs but also inhibits BCR-signaling mediators, which could lead to further MYC inhibition and higher antitumor activity [758, 759]. Due to promising results from studies investigating the effects of TG02 on hematological malignancies [758, 759], this agent has entered clinical trials. [NCT01204164].
The most novel ultra-selective CDK9 inhibitor KB-0742, an orally bioavailable inhibitor, has displayed great antitumor potential in pre-clinical investigation [760]. The remarkable results from KB-0742 have led to clinical trials investigating relapsed and refractory solid tumors, and non-Hodgkin lymphoma [NCT04718675].
G-quadraplex stabilizers
The nuclease hypersensitivity element III1 (NHE III1) located at the MYC promoter controls 80–90% of the transcriptional activity of MYC gene. A particular site of NHE III1 creates G-quadruplex acting as a silencer region [761]. It makes this specific region a great target for drugs, stabilizing G-quadruplex.
In general, G-quadruplex stabilization can cause DNA double-strand breaks and promote apoptosis [762]. However, compounds such as CX-3543 can stabilize G-quadruplex of MYC promoter, selectively [763]. This small molecule also interrupts nucleolin/rDNA G-quadruplex formation, subsequently causing apoptosis. CX-3543 was the first G-quadruplex stabilizer to enter clinical trials [764].
APTO-253, a small molecule that regulates CDKN1A (p21), is capable of propelling cell-cycle arrest and triggering apoptosis in AML. Further investigations revealed that APTO-253 decreases the MYC mRNA translation and reduced the MYC levels. The underlying mechanism was found to be the APTO-253-induced G-quadruplex stabilization [765]. AML cells can convert the monomeric APTO-253 into Fe(253)3. Both APTO-253 and its ferrous form are capable of inducing G-quadruplex stabilization at promoters of MYC, and KIT [765]. Due to its promising potential, APTO-253 is now being clinically evaluated in AML and high-risk myelodysplasia patients [NCT02267863].
Conclusion and future perspectives
MYC's twisted biology, particularly in hematopoiesis, has been comprehensively elucidated in recent years. We now realize that MYC facilitates the cancer cells' machinery. Blood malignancies are not an exception. Studies have shown that even temporary inactivation of MYC abrogates tumor progression, implying that MYC regulation could be a potential strategy to treat MYC-involved cancers [766, 767]. However, direct aiming for MYC is challenging. MYC does not possess a specific active site to be targeted by small molecules. This makes it hard to inhibit. Moreover, MYC is mainly found in the nucleus; therefore targeting MYC with antibodies is not feasible [1]. The broad MYC-mediated biological functions, vital to cells, also make it hard to completely eliminate. Future clinical studies will have to evaluate whether MYC should be targeted directly or indirectly in order to achieve a proper therapeutic outcome.
In the era of personalized medicine, the development of gene editing tools such as CRISPR/CAS9, and viral and non-viral based gene therapies have shown to be very promising [768]. As such MYC could be a promising choice for gene manipulation approaches.
Acknowledgements
Thanks to all who helped us to design high-quality figures.
Abbreviations
- BR
basic-region
- HLH
helix-loop-helix
- LZ
leucine-zipper
- MBs
MYC boxes
- DDR
DNA damage response
- BM
bone marrow
- ALL
acute lymphoblastic leukemia
- CLL
chronic lymphocytic leukemia
- NFAT
nuclear factor of activated T cells
- NFAT1-NFAT4
family of transcription factors includes four Ca2+-regulated members
- PTM
post-translational modifications
- TAD
transactivation domain
- ERK
extracellular receptor kinase
- GSK-3β
glycogen synthase kinase
- BRD4
bromodomain protein 4
- HAT
histone acetyltransferase
- MAPK
mitogen-activated protein kinase
- PP2A
protein phosphatase 2A
- wt
wild type
- CKIs
CDK inhibitory proteins
- ORC
origin recognition complex
- PTTG1
securin gene expression
- lncRNA
long-non coding RNA
- MINCR
MYC-induced long noncoding RNA
- YB1
Y box binding protein
- BIN1
bridging integrator 1
- AIF
apoptosis-inducing factor
- TNFR
tumor necrosis factor receptor
- MPPs
multipotent progenitors
- CMPs and CLPs
common myeloid and lymphoid progenitors
- BCR-ABL1
B-cell receptor–ABL proto-oncogene 1
- DLBCL
diffuse large B-cell lymphoma
- MM
multiple myeloma
- MAX
MYC-associated protein X
- TKIs
tyrosine kinase inhibitors
- BENC
blood enhancer cluster
Authors' contributions
M.S. conceived the manuscript; M.S. and R.C. edited the paper; S.E.A., S.R. and B.Z wrote the manuscript and prepared the tables and figures. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and material
Not applicable.
Declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rouzbeh Chegeni, Email: rchegeni@niu.edu.
Majid Safa, Email: safa.m@iums.ac.ir.
References
- 1.Chen H, Liu H, Qing G. Targeting oncogenic Myc as a strategy for cancer treatment. Signal Transduct Target Ther. 2018;3:1–7. doi: 10.1038/s41392-017-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dang CV, O’Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. The c-Myc target gene network. In: Seminars in cancer biology. 2006. [DOI] [PubMed]
- 3.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 4.Adhikary S, Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol. 2005;6:635–645. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- 5.Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nat Rev Cancer. 2002;2:764–776. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- 6.Nie Z, Gangqing H, Wei G, Cui K, Yamane A, Resch W, et al. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell. 2012;151:68–79. doi: 10.1016/j.cell.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas LR, Tansey WP. Proteolytic control of the oncoprotein transcription factor Myc. Adv Cancer Res. 2011;110:77–106. doi: 10.1016/B978-0-12-386469-7.00004-9. [DOI] [PubMed] [Google Scholar]
- 8.Sorrentino V, Drozdoff V, McKinney MD, Zeitz L, Fleissner E. Potentiation of growth factor activity by exogenous c-myc expression. Proc Natl Acad Sci U S A. 1986;83:8167–8171. doi: 10.1073/pnas.83.21.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karn J, Watson JV, Lowe AD, Green SM, Vedeckis W. Regulation of cell cycle duration by c-myc levels. Oncogene. 1989;4(6):773–87. [PubMed]
- 10.Iritani BM, Eisenman RN. c-Myc enhances protein synthesis and cell size during B lymphocyte development. Proc Natl Acad Sci U S A. 1999;96:13180–13185. doi: 10.1073/pnas.96.23.13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armelin HA, Armelin MCS, Kelly K, Stewart T, Leder P, Cochran BH, et al. Functional role for c-myc in mitogenic response to platelet-derived growth factor. Nature. 1984;310:655–660. doi: 10.1038/310655a0. [DOI] [PubMed] [Google Scholar]
- 12.Kaczmarek L, Hyland JK, Watt R, Rosenberg M, Baserga R. Microinjected c-myc as a competence factor. Science (80- ) 1985;228:1313–1315. doi: 10.1126/science.4001943. [DOI] [PubMed] [Google Scholar]
- 13.Amati B, Alevizopoulos K, Vlach J. Myc and the cell cycle. Front Biosci. 1998;22:d250–d268. doi: 10.2741/A239. [DOI] [PubMed] [Google Scholar]
- 14.Britton S, Salles B, Calsou P. c-Myc protein is degraded in response to UV irradiation. Cell Cycle. 2008;7:63–70. doi: 10.4161/cc.7.1.5111. [DOI] [PubMed] [Google Scholar]
- 15.Jiang M, Li Y, Yang Y, Oncogene JW. c-Myc degradation induced by DNA damage results in apoptosis of CHO cells. Oncogene. 2003;22:3252–3259. doi: 10.1038/sj.onc.1206501. [DOI] [PubMed] [Google Scholar]
- 16.Lu H, Meng L, Huang M, Zhu H, et al. DNA damage, c-myc suppression and apoptosis induced by the novel topoisomerase II inhibitor, salvicine, in human breast cancer MCF-7 cells. Cancer Chemother Pharmacol. 2005;55:286–294. doi: 10.1007/s00280-004-0877-z. [DOI] [PubMed] [Google Scholar]
- 17.Herbst A, Hemann MT, Tworkowski KA, Salghetti SE, Lowe SW, Tansey WP. A conserved element in Myc that negatively regulates its proapoptotic activity. EMBO Rep. 2005;6:177–183. doi: 10.1038/sj.embor.7400333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popov N, Herold S, Llamazares M, Schülein C, Eilers M. Fbw7 and Usp28 regulate myc protein stability in response to DNA damage. Cell Cycle. 2007;6:2327–2331. doi: 10.4161/cc.6.19.4804. [DOI] [PubMed] [Google Scholar]
- 19.Herold S, Wanzel M, Beuger V, Frohme C. Negative regulation of the mammalian UV response by Myc through association with Miz-1. Mol Cell. 2002;10:509–521. doi: 10.1016/S1097-2765(02)00633-0. [DOI] [PubMed] [Google Scholar]
- 20.Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-T. [DOI] [PubMed] [Google Scholar]
- 21.Inoue K, Zindy F, Randle DH, Rehg JE, Sherr CJ. Dmp1 is haplo-insufficient for tumor suppression and modifies the frequencies of Arf and p53 mutations in Myc-induced lymphomas. Genes Dev. 2001;15:2934–2939. doi: 10.1101/gad.929901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muthalagu N, Junttila MR, Wiese KE, Wolf E, Morton J, Bauer B, et al. BIM is the primary mediator of MYC-induced apoptosis in multiple solid tissues. Cell Rep. 2014;8:1347–1353. doi: 10.1016/j.celrep.2014.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delgado MD, León J. Myc roles in hematopoiesis and leukemia. Genes Cancer. 2010;1:605–616. doi: 10.1177/1947601910377495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bahram F, Von Der Lehr N, Cetinkaya C, Larsson LG. c-Myc hot spot mutations in lymphomas result in inefficient ubiquitination and decreased proteasome-mediated turnover. Blood. 2000;95:2104–2110. doi: 10.1182/blood.V95.6.2104. [DOI] [PubMed] [Google Scholar]
- 26.Malempati S, Tibbitts D, Cunningham M, Akkari Y, Olson S, Fan G, et al. Aberrant stabilization of c-Myc protein in some lymphoblastic leukemias. Leukemia. 2006;20:1572–1581. doi: 10.1038/sj.leu.2404317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salghetti SE, Kim SY, Tansey WP. Destruction of Myc by ubiquitin-mediated proteolysis: Cancer-associated and transforming mutations stabilize Myc. EMBO J. 1999;18:717–726. doi: 10.1093/emboj/18.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Barrios O, Meler A, Parra M. MYC’s fine line between B cell development and malignancy. Cells. 2020;9:523. doi: 10.3390/cells9020523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schick M, Habringer S, Nilsson JA, Keller U. Pathogenesis and therapeutic targeting of aberrant MYC expression in haematological cancers. Br J Haematol. 2017;179:727–738. doi: 10.1111/bjh.14917. [DOI] [PubMed] [Google Scholar]
- 30.Tansey WP, Mammalian MYC. Proteins and cancer. New J Sci. 2014;2014:757534. doi: 10.1155/2014/757534. [DOI] [Google Scholar]
- 31.Chung HJ, Levens D. c-Myc expression: keep the noise down! Mol Cells. 2005;20:157–166. [PubMed] [Google Scholar]
- 32.Levens D. How the c-myc promoter works and why it sometimes does not. J Natl Cancer Inst Monogr. 2008;2008:41–43. doi: 10.1093/jncimonographs/lgn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macián F, López-Rodríguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20:2476–2489. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- 34.Buchholz M, Schatz A, Wagner M, Michl P, Linhart T, Adler G, et al. Overexpression of c-myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway. EMBO J. 2006;25:3714–3724. doi: 10.1038/sj.emboj.7601246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mognol GP, de Araujo-Souza PS, Robbs BK, Teixeira LK, Viola JP. Transcriptional regulation of the c-Myc promoter by NFAT1 involves negative and positive NFAT-responsive elements. Cell Cycle. 2012;11:1014–1028. doi: 10.4161/cc.11.5.19518. [DOI] [PubMed] [Google Scholar]
- 36.Carabet LA, Rennie PS, Cherkasov A. Therapeutic inhibition of myc in cancer. Structural bases and computer-aided drug discovery approaches. Int J Mol Sci. 2019;20:120. doi: 10.3390/ijms20010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rottmann S, Lüscher B. The Mad side of the Max network: antagonizing the function of Myc and more. Curr Top Microbiol Immunol. 2006;302:63–122. doi: 10.1007/3-540-32952-8_4. [DOI] [PubMed] [Google Scholar]
- 38.Henriksson M, Bakardjiev A, Klein G, Lüscher B. Phosphorylation sites mapping in the N-terminal domain of c-myc modulate its transforming potential. Oncogene. 1993;8:3199–3209. [PubMed] [Google Scholar]
- 39.Lutterbach B, Hann SR. Hierarchical phosphorylation at N-terminal transformation-sensitive sites in c-Myc protein is regulated by mitogens and in mitosis. Mol Cell Biol. 1994;14:5510–5522. doi: 10.1128/mcb.14.8.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000;14:2501–2514. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai WB, Aiba I, Long Y, Lin HK, Feun L, Savaraj N, et al. Activation of Ras/PI3K/ERK pathway induces c-Myc stabilization to upregulate argininosuccinate synthetase, leading to arginine deiminase resistance in melanoma cells. Cancer Res. 2012;72:2622–2633. doi: 10.1158/0008-5472.CAN-11-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sears R, Leone G, DeGregori J, Nevins JR. Ras enhances Myc protein stability. Mol Cell. 1999;3:169–179. doi: 10.1016/S1097-2765(00)80308-1. [DOI] [PubMed] [Google Scholar]
- 43.Devaiah BN, Case-Borden C, Gegonne A, Hsu CH, Chen Q, Meerzaman D, et al. BRD4 is a histone acetyltransferase that evicts nucleosomes from chromatin. Nat Struct Mol Biol. 2016;23:540–548. doi: 10.1038/nsmb.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devaiah BN, Mu J, Akman B, Uppal S, Weissman JD, Cheng D, et al. MYC protein stability is negatively regulated by BRD4. Proc Natl Acad Sci U S A. 2020;117:13457–13467. doi: 10.1073/pnas.1919507117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barone MV, Courtneidge SA. Myc but not Fos rescue of PDGF signalling block caused by kinase-inactive Src. Nature. 1995;378:509–512. doi: 10.1038/378509a0. [DOI] [PubMed] [Google Scholar]
- 46.Chiariello M, Marinissen MJ, Gutkind JS. Regulation of c-myc expression by PDGF through Rho GTPases. Nat Cell Biol. 2001;3:580–586. doi: 10.1038/35078555. [DOI] [PubMed] [Google Scholar]
- 47.Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta. 2009;1795:1–15. doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Sablina AA, Hector M, Colpaert N, Hahn WC. Identification of PP2A complexes and pathways involved in cell transformation. Cancer Res. 2010;70:10474–10484. doi: 10.1158/0008-5472.CAN-10-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruvolo PP. The broken “Off” switch in cancer signaling: PP2A as a regulator of tumorigenesis, drug resistance, and immune surveillance. BBA Clin. 2016;6:87–99. doi: 10.1016/j.bbacli.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnold HK, Sears RC. Protein phosphatase 2A regulatory subunit B56alpha associates with c-myc and negatively regulates c-myc accumulation. Mol Cell Biol. 2006;26:2832–2844. doi: 10.1128/MCB.26.7.2832-2844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin CF, Chen CL, Chiang CW, Jan MS, Huang WC, Lin YS. GSK-3beta acts downstream of PP2A and the PI 3-kinase-Akt pathway, and upstream of caspase-2 in ceramide-induced mitochondrial apoptosis. J Cell Sci. 2007;120:2935–2943. doi: 10.1242/jcs.03473. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi K, Uchida C, Shin RW, Shimazaki K, Uchida T. Prolyl isomerase, Pin1: new findings of post-translational modifications and physiological substrates in cancer, asthma and Alzheimer’s disease. Cell Mol Life Sci. 2008;65:359–375. doi: 10.1007/s00018-007-7270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeh E, Cunningham M, Arnold H, Chasse D, Monteith T, Ivaldi G, et al. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol. 2004;6:308–318. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- 54.Welcker M, Orian A, Jin J, Grim JE, Harper JW, Eisenman RN, et al. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci U S A. 2004;101:9085–9090. doi: 10.1073/pnas.0402770101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arnold HK, Zhang X, Daniel CJ, Tibbitts D, Escamilla-Powers J, Farrell A, et al. The Axin1 scaffold protein promotes formation of a degradation complex for c-Myc. EMBO J. 2009;28:500–512. doi: 10.1038/emboj.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou XZ, Kops O, Werner A, Lu PJ, Shen M, Stoller G, et al. Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol Cell. 2000;6:873–883. doi: 10.1016/S1097-2765(05)00083-3. [DOI] [PubMed] [Google Scholar]
- 57.Farrell AS, Pelz C, Wang X, Daniel CJ, Wang Z, Su Y, et al. Pin1 regulates the dynamics of c-Myc DNA binding to facilitate target gene regulation and oncogenesis. Mol Cell Biol. 2013;33:2930–2949. doi: 10.1128/MCB.01455-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adhikary S, Marinoni F, Hock A, Hulleman E, Popov N, Beier R, et al. The ubiquitin ligase HectH9 regulates transcriptional activation by Myc and is essential for tumor cell proliferation. Cell. 2005;123:409–421. doi: 10.1016/j.cell.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 59.Yada M, Hatakeyama S, Kamura T, Nishiyama M, Tsunematsu R, Imaki H, et al. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 2004;23:2116–2125. doi: 10.1038/sj.emboj.7600217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von der Lehr N, Johansson S, Wu S, Bahram F, Castell A, Cetinkaya C, et al. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol Cell. 2003;11:1189–1200. doi: 10.1016/S1097-2765(03)00193-X. [DOI] [PubMed] [Google Scholar]
- 61.Choi SH, Wright JB, Gerber SA, Cole MD. Myc protein is stabilized by suppression of a novel E3 ligase complex in cancer cells. Genes Dev. 2010;24:1236–1241. doi: 10.1101/gad.1920310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paul I, Ahmed SF, Bhowmik A, Deb S, Ghosh MK. The ubiquitin ligase CHIP regulates c-Myc stability and transcriptional activity. Oncogene. 2013;32:1284–1295. doi: 10.1038/onc.2012.144. [DOI] [PubMed] [Google Scholar]
- 63.Farrell AS, Sears RC. MYC degradation. Cold Spring Harb Perspect Med. 2014;4:a014365. doi: 10.1101/cshperspect.a014365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- 65.van Drogen F, Sangfelt O, Malyukova A, Matskova L, Yeh E, Means AR, et al. Ubiquitylation of cyclin E requires the sequential function of SCF complexes containing distinct hCdc4 isoforms. Mol Cell. 2006;23:37–48. doi: 10.1016/j.molcel.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 66.Grim JE, Gustafson MP, Hirata RK, Hagar AC, Swanger J, Welcker M, et al. Isoform- and cell cycle-dependent substrate degradation by the Fbw7 ubiquitin ligase. J Cell Biol. 2008;181:913–920. doi: 10.1083/jcb.200802076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim SY, Herbst A, Tworkowski KA, Salghetti SE, Tansey WP. Skp2 regulates Myc protein stability and activity. Mol Cell. 2003;11:1177–1188. doi: 10.1016/S1097-2765(03)00173-4. [DOI] [PubMed] [Google Scholar]
- 68.Chung YM, Kim JS, Yoo YD. A novel protein, Romo1, induces ROS production in the mitochondria. Biochem Biophys Res Commun. 2006;347:649–655. doi: 10.1016/j.bbrc.2006.06.140. [DOI] [PubMed] [Google Scholar]
- 69.Chung JS, Lee SB, Park SH, Kang ST, Na AR, Chang TS, et al. Mitochondrial reactive oxygen species originating from Romo1 exert an important role in normal cell cycle progression by regulating p27(Kip1) expression. Free Radic Res. 2009;43:729–737. doi: 10.1080/10715760903038432. [DOI] [PubMed] [Google Scholar]
- 70.Lee SB, Kim JJ, Chung JS, Lee MS, Lee KH, Kim BS, et al. Romo1 is a negative-feedback regulator of Myc. J Cell Sci. 2011;124:1911–1924. doi: 10.1242/jcs.079996. [DOI] [PubMed] [Google Scholar]
- 71.Li S, Jiang C, Pan J, Wang X, Jin J, Zhao L, et al. Regulation of c-Myc protein stability by proteasome activator REGγ. Cell Death Differ. 2015;22:1000–1011. doi: 10.1038/cdd.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mao I, Liu J, Li X, Luo H. REGgamma, a proteasome activator and beyond? Cell Mol Life Sci. 2008;65:3971–3980. doi: 10.1007/s00018-008-8291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jung Y-S, Qian Y, Chen X. Pirh2 RING-finger E3 ubiquitin ligase: its role in tumorigenesis and cancer therapy. FEBS Lett. 2012;586:1397–1402. doi: 10.1016/j.febslet.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hakem A, Bohgaki M, Lemmers B, Tai E, Salmena L, Matysiak-Zablocki E, et al. Role of Pirh2 in mediating the regulation of p53 and c-Myc. PLoS Genet. 2011;7:e1002360. doi: 10.1371/journal.pgen.1002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jin Y, Nenseth HZ, Saatcioglu F. Role of PLZF as a tumor suppressor in prostate cancer. Oncotarget. 2017;8:71317. doi: 10.18632/oncotarget.19813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McConnell MJ, Chevallier N, Berkofsky-Fessler W, Giltnane JM, Malani RB, Staudt LM, et al. Growth suppression by acute promyelocytic leukemia-associated protein PLZF is mediated by repression of c-myc expression. Mol Cell Biol. 2003;23:9375–9388. doi: 10.1128/MCB.23.24.9375-9388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi J, Vogt PK. Posttranslational regulation of Myc by promyelocytic leukemia zinc finger protein. Int J cancer. 2009;125:1558–1565. doi: 10.1002/ijc.24449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cannell IG, Kong YW, Bushell M. How do microRNAs regulate gene expression? Biochem Soc Trans. 2008;36:1224–1231. doi: 10.1042/BST0361224. [DOI] [PubMed] [Google Scholar]
- 79.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 80.Cannell IG, Kong YW, Johnston SJ, Chen ML, Collins HM, Dobbyn HC, et al. p38 MAPK/MK2-mediated induction of miR-34c following DNA damage prevents Myc-dependent DNA replication. Proc Natl Acad Sci U S A. 2010;107:5375–5380. doi: 10.1073/pnas.0910015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cannell I, Bushell M. Regulation of Myc by miR-34c: a mechanism to prevent genomic instability? Cell Cycle. 2010;9:2798–2802. doi: 10.4161/cc.9.14.12182. [DOI] [PubMed] [Google Scholar]
- 82.Mateyak MK, Obaya AJ, Adachi S, Sedivy JM. Phenotypes of c-Myc-deficient rat fibroblasts isolated by targeted homologous recombination. Cell Growth Differ. 1997;8(10):1039–48. [PubMed]
- 83.Sears RC. The life cycle of C-myc: from synthesis to degradation. Cell Cycle. 2004;3(9):1133-37. [PubMed]
- 84.Chanu SI, Sarkar S. The paradox of c-Myc proto-oncogene and its diverse functions. Cell Dev Biol. 2014;3:3. [Google Scholar]
- 85.Eilers M, Schirm S, Bishop JM. The MYC protein activates transcription of the α-prothymosin gene. EMBO J Eur Mol Biol Org. 1991;10:133–141. doi: 10.1002/j.1460-2075.1991.tb07929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schuhmacher M, Staege MS, Pajic A, Polack A, Weidle UH, Bornkamm GW, et al. Control of cell growth by c-Myc in the absence of cell division. Curr Biol. 1999;9:1255–1258. doi: 10.1016/S0960-9822(99)80507-7. [DOI] [PubMed] [Google Scholar]
- 87.Schuhmacher M, Eick D. Dose-dependent regulation of target gene expression and cell proliferation by c-Myc levels. Transcription. 2013;4:192–197. doi: 10.4161/trns.25907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heikkila R, Schwab G, Wickstrom E, Loke SL, Pluznik DH, Watt R, et al. A c-myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. Nature. 1987;328:445–449. doi: 10.1038/328445a0. [DOI] [PubMed] [Google Scholar]
- 89.Wickstrom EL, Bacon TA, Gonzalez A, Freeman DL, Lyman GH, Wickstrom E. Human promyelocytic leukemia HL-60 cell proliferation and c-myc protein expression are inhibited by anantisense pentadecadeoxynucleotide targeted against c-myc mRNA. Proc Natl Acad Sci U S A. 1988;85:1028–1032. doi: 10.1073/pnas.85.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang H, Mannava S, Grachtchouk V, Zhuang D, Soengas MS, Gudkov AV, et al. c-Myc depletion inhibits proliferation of human tumor cells at various stages of the cell cycle. Oncogene. 2008;27:1905–1915. doi: 10.1038/sj.onc.1210823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baudino TA, Cleveland JL. The max network gone mad. Mol Cell Biol. 2001;21:691–702. doi: 10.1128/MCB.21.3.691-702.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hurlin PJ, Huang J. The MAX-interacting transcription factor network. Semin Cancer Biol. 2006;16:265–274. doi: 10.1016/j.semcancer.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 93.Berns K, Hijmans EM, Bernards R. Repression of c-Myc responsive genes in cycling cells causes G1 arrest through reduction of cyclin E/CDK2 kinase activity. Oncogene. 1997;15:1347–1356. doi: 10.1038/sj.onc.1201280. [DOI] [PubMed] [Google Scholar]
- 94.Cerni C, Skrzypek B, Popov N, Sasgary S, Schmidt G, Larsson LG, et al. Repression of in vivo growth of Myc/Ras transformed tumor cells by Mad1. Oncogene. 2002;21:447–459. doi: 10.1038/sj.onc.1205107. [DOI] [PubMed] [Google Scholar]
- 95.Menssen A, Hermeking H. Characterization of the c-MYC-regulated transcriptome by SAGE: identification and analysis of c-MYC target genes. Proc Natl Acad Sci U S A. 2002;99:6274–6279. doi: 10.1073/pnas.082005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2α promotes hypoxic cell proliferation by enhancing c-Myc transcriptional activity. Cancer Cell Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maxwell PH. The HIF pathway in cancer. Semin Cell Dev Biol. 2005;16:523–530. doi: 10.1016/j.semcdb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 98.Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 99.Malumbres M, Harlow E, Hunt T, Hunter T, Lahti JM, Manning G, et al. Cyclin-dependent kinases: a family portrait. Nat Cell Biol. 2009;11:1275–1276. doi: 10.1038/ncb1109-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu H, Wang Z, Jin S, Hao H, Zheng L, Zhou B, et al. Dux4 induces cell cycle arrest at G1 phase through upregulation of p21 expression. Biochem Biophys Res Commun. 2014;446:235–240. doi: 10.1016/j.bbrc.2014.02.105. [DOI] [PubMed] [Google Scholar]
- 101.Schorl C, Sedivy JM. Loss of protooncogene c-Myc function impedes G1 phase progression both before and after the restriction point. Mol Biol Cell. 2003;14:823–835. doi: 10.1091/mbc.e02-10-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–1061. doi: 10.1016/0092-8674(91)90557-F. [DOI] [PubMed] [Google Scholar]
- 103.Weintraub SJ, Chow KNB, Luo RX, Zhang SH, He S, Dean DC. Mechanism of active transcriptional repression by the retinoblastoma protein. Nature. 1995;375:812–816. doi: 10.1038/375812a0. [DOI] [PubMed] [Google Scholar]
- 104.Sherr CJ. D-type cyclins. Trends Biochem Sci. 1995;20:187–190. doi: 10.1016/S0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- 105.Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 106.Nevins JR. The Rb/E2F pathway and cancer. Hum Mol Genet. 2001;10:699–703. doi: 10.1093/hmg/10.7.699. [DOI] [PubMed] [Google Scholar]
- 107.Pavlides SC, Lecanda J, Daubriac J, Pandya UM, Gama P, Blank S, et al. TGF-β activates APC through Cdh1 binding for Cks1 and Skp2 proteasomal destruction stabilizing p27kip1 for normal endometrial growth. Cell Cycle. 2016;15:931–947. doi: 10.1080/15384101.2016.1150393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hydbring P, Malumbres M, Sicinski P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat Rev Mol Cell Biol. 2016;17:280–292. doi: 10.1038/nrm.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 110.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 111.Pavletich NP. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J Mol Biol. 1999;287:821–828. doi: 10.1006/jmbi.1999.2640. [DOI] [PubMed] [Google Scholar]
- 112.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 113.Reynisdóttir I, Massagué J. The subcellular locations of pl5(Ink4b) and p27(Kip1) coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 1997;11:492–503. doi: 10.1101/gad.11.4.492. [DOI] [PubMed] [Google Scholar]
- 114.Ouelle DE, Zindy F, Ashmun RA, Sherr CJ. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell. 1995;83:993–1000. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 115.Weber JD, Jeffers JR, Rehg JE, Randle DH, Lozano G, Roussel MF, et al. p53-independent functions of the p19(ARF) tumor suppressor. Genes Dev. 2000;14:2358–2365. doi: 10.1101/gad.827300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McKeller RN, Fowler JL, Cunningham JJ, Warner N, Smeyne RJ, Zindy F, et al. The Arf tumor suppressor gene promotes hyaloid vascular regression during mouse eye development. Proc Natl Acad Sci U S A. 2002;99:3848–3853. doi: 10.1073/pnas.052484199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weber JD, Taylor LJ, Roussel MF, Sherr CJ, Bar-Sagi D. Nucleolar Arf sequesters Mdm2 and activates p53. Nat Cell Biol. 1999;1:20–26. doi: 10.1038/8991. [DOI] [PubMed] [Google Scholar]
- 118.Datta A, Nag A, Pan W, Hay N, Gartel AL, Colamonici O, et al. Myc-ARF (alternate reading frame) interaction inhibits the functions of Myc. J Biol Chem. 2004;279:36698–36707. doi: 10.1074/jbc.M312305200. [DOI] [PubMed] [Google Scholar]
- 119.Tu WB, Helander S, Pilstål R, Hickman KA, Lourenco C, Jurisica I, et al. Myc and its interactors take shape. Biochim Biophys Acta Gene Regul Mech. 2015;1849:469–483. doi: 10.1016/j.bbagrm.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 120.Gregory MA, Qi Y, Hann SR. The ARF tumor suppressor: keeping Myc on a leash. Cell Cycle. 2005;4(2):249–52. [PubMed]
- 121.Conzen SD, Gottlob K, Kandel ES, Khanduri P, Wagner AJ, O’Leary M, et al. Induction of cell cycle progression and acceleration of apoptosis are two separable functions of c-Myc: transrepression correlates with acceleration of apoptosis. Mol Cell Biol. 2000;20:6008–6018. doi: 10.1128/MCB.20.16.6008-6018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Oster SK, Mao DYL, Kennedy J, Penn LZ. Functional analysis of the n-terminal domain of the myc oncoprotein. Oncogene. 2003;22:1998–2010. doi: 10.1038/sj.onc.1206228. [DOI] [PubMed] [Google Scholar]
- 123.Soucek L, Jucker R, Panacchia L, Ricordy R, Tatò F, Nasi S. Omomyc, a potential Myc dominant negative, enhances Myc-induced apoptosis. Cancer Res. 2002;62(12):3507–10. [PubMed]
- 124.Lee EW, Lee MS, Camus S, Ghim J, Yang MR, Oh W, et al. Differential regulation of p53 and p21 by MKRN1 E3 ligase controls cell cycle arrest and apoptosis. EMBO J. 2009;28:2100–2113. doi: 10.1038/emboj.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yagi A, Hasegawa Y, Xiao H, Haneda M, Kojima E, Nishikimi A, et al. GADD34 Induces p53 Phosphorylation and p21/WAF1 Transcription. J Cell Biochem. 2003;90:1242–1249. doi: 10.1002/jcb.10711. [DOI] [PubMed] [Google Scholar]
- 126.Wu S, Cetinkaya C, Munoz-Alonso MJ, Von Der Lehr N, Bahram F, Beuger V, et al. Myc represses differentiation-induced p21CIP1 expression via Miz-1-dependent interaction with the p21 core promoter. Oncogene. 2003;22:351–360. doi: 10.1038/sj.onc.1206145. [DOI] [PubMed] [Google Scholar]
- 127.García-Gutiérrez L, Delgado MD, León J. Myc oncogene contributions to release of cell cycle brakes. Genes (Basel) 2019;10:244. doi: 10.3390/genes10030244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bretones G, Delgado MD, León J. Myc and cell cycle control. Biochim Biophys Acta Gene Regul Mech. 2015;1849:506–516. doi: 10.1016/j.bbagrm.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 129.Leone G, Sears R, Huang E, Rempel R, Nuckolls F, Park CH, et al. Myc requires distinct E2F activities to induce S phase and apoptosis. Mol Cell. 2001;8:105–113. doi: 10.1016/S1097-2765(01)00275-1. [DOI] [PubMed] [Google Scholar]
- 130.Adams MR, Sears R, Nuckolls F, Leone G, Nevins JR. Complex transcriptional regulatory mechanisms control expression of the E2F3 locus. Mol Cell Biol. 2000;20:3633–3639. doi: 10.1128/MCB.20.10.3633-3639.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sears R, Ohtani K, Nevins JR. Identification of positively and negatively acting elements regulating expression of the E2F2 gene in response to cell growth signals. Mol Cell Biol. 1997;17:5227–5235. doi: 10.1128/MCB.17.9.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rounbehler RJ, Rogers PM, Conti CJ, Johnson DG. Inactivation of E2f1 enhances tumorigenesis in a Myc transgenic model. Cancer Res. 2002;62(11):3276–81. [PubMed]
- 133.Lupini L, Bassi C, Ferracin M, Bartonicek N, D’Abundo L, Zagatti B, et al. miR-221 affects multiple cancer pathways by modulating the level of hundreds messenger RNAs. Front Genet. 2013;4:64. doi: 10.3389/fgene.2013.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim JW, Mori S, Nevins JR. Myc-induced microRNAs integrate Myc-mediated cell proliferation and cell fate. Cancer Res. 2010;70:4820–4828. doi: 10.1158/0008-5472.CAN-10-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hermeking H, Rago C, Schuhmacher M, Li Q, Barrett JF, Obaya AJ, et al. Identification of CDK4 as a target of c-MYC. Proc Natl Acad Sci U S A. 2000;97:2229–2234. doi: 10.1073/pnas.050586197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li Z, Van Calcar S, Qu C, Cavenee WK, Zhang MQ, Ren B. A global transcriptional regulatory role for c-Myc in Burkitt’s lymphoma cells. Proc Natl Acad Sci U S A. 2003;100:8164–8169. doi: 10.1073/pnas.1332764100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yap CS, Peterson AL, Castellani G, Sedivy JM, Neretti N. Kinetic profiling of the c-Myc transcriptome and bioinformatic analysis of repressed gene promoters. Cell Cycle. 2011;10:2184–2196. doi: 10.4161/cc.10.13.16249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hanson KD, Shichiri M, Follansbee MR, Sedivy JM. Effects of c-myc expression on cell cycle progression. Mol Cell Biol. 1994;14:5748–5755. doi: 10.1128/mcb.14.9.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim J, Woo AJ, Chu J, Snow JW, Fujiwara Y, Kim CG, et al. A myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell. 2010;143:313–324. doi: 10.1016/j.cell.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Born TL, Frost JA, Schönthal A, Prendergast GC, Feramisco JR. c-Myc cooperates with activated Ras to induce the cdc2 promoter. Mol Cell Biol. 1994;14:5710–5718. doi: 10.1128/mcb.14.9.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu Z-J, Ueda T, Miyazaki T, Tanaka N, Mine S, Tanaka Y, et al. A critical role for cyclin c in promotion of the hematopoietic cell cycle by cooperation with c-Myc. Mol Cell Biol. 1998;18:3445–3454. doi: 10.1128/MCB.18.6.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 143.Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 144.Lolli G, Johnson LN. CAK-Cyclin-dependent activating kinase: a key kinase in cell cycle control and a target for drugs? Cell Cycle. 2005;4(4):572–77. [PubMed]
- 145.Cowling VH, Cole MD. The myc transactivation domain promotes global phosphorylation of the RNA polymerase II carboxy-terminal domain independently of direct DNA binding. Mol Cell Biol. 2007;27:2059–2073. doi: 10.1128/MCB.01828-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Parker LL, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science (80- ) 1992;257:1955–1957. doi: 10.1126/science.1384126. [DOI] [PubMed] [Google Scholar]
- 147.Galaktionov K, Chen X, Beach D. Cdc25 cell-cycle phosphatase as a target of c-myc. Nature. 1996;382:511–517. doi: 10.1038/382511a0. [DOI] [PubMed] [Google Scholar]
- 148.Le Sage C, Nagel R, Egan DA, Schrier M, Mesman E, Mangiola A, et al. Regulation of the p27Kip1 tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J. 2007;26:3699–3708. doi: 10.1038/sj.emboj.7601790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Daksis JI, Lu RY, Facchini LM, Marhin WW, Penn LJ. Myc induces cyclin D1 expression in the absence of de novo protein synthesis and links mitogen-stimulated signal transduction to the cell cycle. Oncogene. 1994;9(12):3635–45. [PubMed]
- 150.Jansen-Durr P, Meichle A, Steiner P, Pagano M, Finke K, Botz J, et al. Differential modulation of cyclin gene expression by MYC. Proc Natl Acad Sci U S A. 1993;90:3685–3689. doi: 10.1073/pnas.90.8.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Philipp A, Schneider A, Väsrik I, Finke K, Xiong Y, Beach D, et al. Repression of cyclin D1: a novel function of MYC. Mol Cell Biol. 1994;14:4032–4043. doi: 10.1128/mcb.14.6.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Solomon DL, Philipp A, Land H, Eilers M. Expression of cyclin D1 mRNA is not upregulated by Myc in rat fibroblasts. Oncogene. 1995;11(9):1893–97. [PubMed]
- 153.Bouchard C, Thieke K, Maier A, Saffrich R, Hanley-Hyde J, Ansorge W, et al. Direct induction of cyclin D2 by Myc contributes to cell cycle progression and sequestration of p27. EMBO J. 1999;18:5321–5333. doi: 10.1093/emboj/18.19.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bouchard C, Dittrich O, Kiermaier A, Dohmann K, Menkel A, Eilers M, et al. Regulation of cyclin D2 gene expression by the Myc/Max/Mad network: Myc-dependent TRRAP recruitment and histone acetylation at the cyclin D2 promoter. Genes Dev. 2001;15:2042–2047. doi: 10.1101/gad.907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yu Q, Ciemerych MA, Sicinski P. Ras and Myc can drive oncogenic cell proliferation through individual D-cyclins. Oncogene. 2005;24:7114–7119. doi: 10.1038/sj.onc.1208853. [DOI] [PubMed] [Google Scholar]
- 156.Pérez-Roger I, Solomon DLC, Sewing A, Land H. Myc activation of cyclin E/Cdk2 kinase involves induction of cyclin E gene transcription and inhibition of p27(Kip1) binding to newly formed complexes. Oncogene. 1997;14:2373–2381. doi: 10.1038/sj.onc.1201197. [DOI] [PubMed] [Google Scholar]
- 157.Zeller KI, Zhao XD, Lee CWH, Kuo PC, Yao F, Yustein JT, et al. Global mapping of c-Myc binding sites and target gene networks in human B cells. Proc Natl Acad Sci U S A. 2006;103:17834–17839. doi: 10.1073/pnas.0604129103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Qi Y, Tu Y, Yang D, Chen Q, Xiao J, Chen Y, et al. Cyclin A but not cyclin D1 is essential for c-myc-modulated cell-cycle progression. J Cell Physiol. 2007;210:63–71. doi: 10.1002/jcp.20816. [DOI] [PubMed] [Google Scholar]
- 159.Pusch O, Bernaschek G, Eilers M, Hengstschläger M. Activation of c-Myc uncouples DNA replication from activation of G1-cyclin-dependent kinases. Oncogene. 1997;15:649–656. doi: 10.1038/sj.onc.1201236. [DOI] [PubMed] [Google Scholar]
- 160.Barrett JF, Lewis BC, Hoang AT, Alvarez RJ, Dang CV. Cyclin A links c-Myc to adhesion-independent cell proliferation. J Biol Chem. 1995;270:15923–15925. doi: 10.1074/jbc.270.27.15923. [DOI] [PubMed] [Google Scholar]
- 161.Hoang AT, Cohen KJ, Barrett JF, Bergstrom DA, Dang CV. Participation of cyclin A in Myc-induced apoptosis. Proc Natl Acad Sci U S A. 1994;91:6875–6879. doi: 10.1073/pnas.91.15.6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Haeng RS, Kim J, Bae S, Soh JW, Lee YS. Cdk5-mediated phosphorylation of c-Myc on Ser-62 is essential in transcriptional activation of cyclin B1 by cyclin G1. J Biol Chem. 2008;283:15601–15610. doi: 10.1074/jbc.M800987200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yin XY, Grove L, Datta NS, Katula K, Long MW, Prochownik EV. Inverse regulation of cyclin B1 by c-Myc and p53 and induction of tetraploidy by cyclin B1 overexpression. Cancer Res. 2001;61(17):6487–93. [PubMed]
- 164.Ohtani K, Degregori J, Nevins JR. Regulation of the cyclin E gene by transcription factor E2F1. Proc Natl Acad Sci U S A. 1995;92:12146–12150. doi: 10.1073/pnas.92.26.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jung P, Menssen A, Mayr D, Hermeking H. AP4 encodes a c-MYC-inducible repressor of p21. Proc Natl Acad Sci U S A. 2008;105:15046–15051. doi: 10.1073/pnas.0801773105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Warner BJ, Blain SW, Seoane J, Massagué J. Myc downregulation by transforming growth factor β required for activation of the p15Ink4b G1 arrest pathway. Mol Cell Biol. 1999;19:5913–5922. doi: 10.1128/MCB.19.9.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Staller P, Peukert K, Kiermaier A, Seoane J, Lukas J, Karsunky H, et al. Repression of p15INK4b expression by Myc through association with Miz-1. Nat Cell Biol. 2001;3:392–399. doi: 10.1038/35070076. [DOI] [PubMed] [Google Scholar]
- 168.Wiese KE, Walz S, Von Eyss B, Wolf E, Athineos D, Sansom O, et al. The role of MIZ-1 in MYC-dependent tumorigenesis. Cold Spring Harb Perspect Med. 2013;3:a014290. doi: 10.1101/cshperspect.a014290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jiang G, Espeseth A, Hazuda DJ, Margolis DM. c-Myc and Sp1 contribute to proviral latency by recruiting histone deacetylase 1 to the human immunodeficiency virus type 1 promoter. J Virol. 2007;81:10914–10923. doi: 10.1128/JVI.01208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Feng XH, Liang YY, Liang M, Zhai W, Lin X. Direct interaction of c-Myc with Smad2 and Smad3 to inhibit TGF-β-mediated induction of the CDK inhibitor p15(Ink4B) Mol Cell. 2016;62:152. doi: 10.1016/j.molcel.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 171.Bouchard C, Lee S, Paulus-Hock V, Loddenkemper C, Eilers M, Schmitt CA. FoxO transcription factors suppress Myc-driven lymphomagenesis via direct activation of Arf. Genes Dev. 2007;21:2775–2787. doi: 10.1101/gad.453107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bates S, Phillips AC, Clark PA, Stott F, Peters G, Ludwig RL, et al. p14(ARF) links the tumour suppressors RB and p53. Nature. 1998;395:124–125. doi: 10.1038/25867. [DOI] [PubMed] [Google Scholar]
- 173.Meyer N, Kim SS, Penn LZ. The Oscar-worthy role of Myc in apoptosis. In: Seminars in cancer biology; 2006. p. 275–87. [DOI] [PubMed]
- 174.Chen D, Shan J, Zhu WG, Qin J, Gu W. Transcription-independent ARF regulation in oncogenic stress-mediated p53 responses. Nature. 2010;464:624–627. doi: 10.1038/nature08820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Seoane J, Van LH, Massagué J. Myc suppression of the p21Cip1 Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature. 2002;419:729–734. doi: 10.1038/nature01119. [DOI] [PubMed] [Google Scholar]
- 176.El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-P. [DOI] [PubMed] [Google Scholar]
- 177.Kastan MB, Zhan Q, El-Deiry WS, Carrier F, Jacks T, Walsh WV, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 178.Marhin WW, Chen S, Facchini LM, Fornace AJ, Penn LZ. Myc represses the growth arrest gene gadd45. Oncogene. 1997;14:2825–2834. doi: 10.1038/sj.onc.1201138. [DOI] [PubMed] [Google Scholar]
- 179.Mitchell KO, El-Deiry WS. Overexpression of c-Myc inhibits p21(WAF1/CIP1) expression and induces S-phase entry in 12-O-tetradecanoylphorbol-13-acetate (TPA)-sensitive human cancer cells. Cell Growth Differ. 1999;10:223–230. [PubMed] [Google Scholar]
- 180.Amundson SA, Zhan Q, Penn LZ, Fornace AJ. Myc suppresses induction of the growth arrest genes gadd34, gadd45, and gadd153 by DNA-damaging agents. Oncogene. 1998;17:2149–2154. doi: 10.1038/sj.onc.1202136. [DOI] [PubMed] [Google Scholar]
- 181.Jin S, Tong T, Fan W, Fan F, Antinore MJ, Zhu X, et al. GADD45-induced cell cycle G2-M arrest associates with altered subcellular distribution of cyclin B1 and is independent of p38 kinase activity. Oncogene. 2002;21:8696–8704. doi: 10.1038/sj.onc.1206034. [DOI] [PubMed] [Google Scholar]
- 182.Peukert K, Staller P, Schneider A, Carmichael G, Hänel F, Eilers M. An alternative pathway for gene regulation by Myc. EMBO J. 1997;16:5672–5686. doi: 10.1093/emboj/16.18.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Möröy T, Saba I, Kosan C. The role of the transcription factor Miz-1 in lymphocyte development and lymphomagenesis—binding Myc makes the difference. In: Seminars in immunology; 2011. p. 379–87. [DOI] [PubMed]
- 184.Brenner C, Deplus R, Didelot C, Loriot A, Viré E, De Smet C, et al. Myc represses transcription through recruitment of DNA methyltransferase corepressor. EMBO J. 2005;24:336–346. doi: 10.1038/sj.emboj.7600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wong P-P, Miranda F, Chan KV, Berlato C, Hurst HC, Scibetta AG. Histone demethylase KDM5B collaborates with TFAP2C and myc to repress the cell cycle inhibitor p21cip (CDKN1A) Mol Cell Biol. 2012;32:1633–1644. doi: 10.1128/MCB.06373-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gartel AL, Ye X, Goufman E, Shianov P, Hay N, Najmabadi F, et al. Myc represses the p21 (WAF1/CIP1) promoter and interacts with Sp1/Sp3. Proc Natl Acad Sci U S A. 2001;98:4510–4515. doi: 10.1073/pnas.081074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vaqué JP, Navascues J, Shiio Y, Laiho M, Ajenjo N, Mauleon I, et al. Myc antagonises Ras-mediated growth arrest in leukemia cells through the inhibition of the Ras-ERK-p21Cip1 pathway. J Biol Chem. 2005;280:1112–1122. doi: 10.1074/jbc.M409503200. [DOI] [PubMed] [Google Scholar]
- 188.Jung P, Hermeking H. The c-MYC-AP4-p21 cascade. Cell Cycle. 2009;8:982–929. doi: 10.4161/cc.8.7.7949. [DOI] [PubMed] [Google Scholar]
- 189.Inomata M, Tagawa H, Guo YM, Kameoka Y, Takahashi N, Sawada K. MicroRNA-17-92 down-regulates expression of distinct targets in different B-cell lymphoma subtypes. Blood. 2009;113:396–402. doi: 10.1182/blood-2008-07-163907. [DOI] [PubMed] [Google Scholar]
- 190.Wong P, Iwasaki M, Somervaille TCP, Ficara F, Carico C, Arnold C, et al. The miR-17-92 microRNA polycistron regulates MLL leukemia stem cell potential by modulating p21 expression. Cancer Res. 2010;70:3833–3842. doi: 10.1158/0008-5472.CAN-09-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mateyak MK, Obaya AJ, Sedivy JM. c-Myc regulates cyclin D-Cdk4 and -Cdk6 activity but affects cell cycle progression at multiple independent points. Mol Cell Biol. 1999;19:4672–4683. doi: 10.1128/MCB.19.7.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Martins CP, Berns A. Loss of p27Kip1 but not p21Cip1 decreases survival and synergizes with MYC in murine lymphomagenesis. EMBO J. 2002;21:3739–3748. doi: 10.1093/emboj/cdf364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wu M, Bellas RE, Shen J, Yang W, Sonenshein GE. Increased p27Kip1 cyclin-dependent kinase inhibitor gene expression following anti-IgM treatment promotes apoptosis of WEHI 231 B cells. J Immunol. 1999;163(12):6530–35. [PubMed]
- 194.Wu M, Arsura M, Bellas RE, FitzGerald MJ, Lee H, Schauer SL, et al. Inhibition of c-myc expression induces apoptosis of WEHI 231 murine B cells. Mol Cell Biol. 1996;16:5015–5025. doi: 10.1128/MCB.16.9.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang W, Shen J, Wu M, Arsura M, FitzGerald M, Suldan Z, et al. Repression of transcription of the p27Kip1 cyclin-dependent kinase inhibitor gene by c-Myc. Oncogene. 2001;20:1688–1702. doi: 10.1038/sj.onc.1204245. [DOI] [PubMed] [Google Scholar]
- 196.Chandramohan V, Mineva ND, Burke B, Jeay S, Wu M, Shen J, et al. c-Myc represses FOXO3a-mediated transcription of the gene encoding the p27Kip1 cyclin dependent kinase inhibitor. J Cell Biochem. 2008;104:2091–2106. doi: 10.1002/jcb.21765. [DOI] [PubMed] [Google Scholar]
- 197.Steiner P, Philipp A, Lukas J, Godden-Kent D, Pagano M, Mittnacht S, Bartek J, Eilers M. Identification of a Myc-dependent step during the formation of active G1 cyclin-cdk complexes. EMBO J. 1995;14(19):4814–26. [DOI] [PMC free article] [PubMed]
- 198.Vlach J, Hennecke S, Alevizopoulos K, Conti D, Amati B. Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abrogated by c-Myc. EMBO J. 1996;15(23):6595–604. [PMC free article] [PubMed]
- 199.Soos TJ, Kiyokawa H, Yan JS, Rubin MS, Giordano A, DeBlasio A, Bottega S, Wong B, Mendelsohn J, Koff A. Formation of p27-CDK complexes during the human mitotic cell cycle. Cell Growth Differ. 1996;7(2):135–46. [PubMed]
- 200.Obaya AJ, Sedivy JM. Regulation of cyclin-Cdk activity in mammalian cells. Cell Mol Life Sci. 2002;59:126–142. doi: 10.1007/s00018-002-8410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bretones G, Acosta JC, Caraballo JM, Ferrándiz N, Gómez-Casares MT, Albajar M, et al. SKP2 oncogene is a direct MYC target gene and MYC down-regulates p27 KIP1 through SKP2 in human leukemia cells. J Biol Chem. 2011;286:9815–9825. doi: 10.1074/jbc.M110.165977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Müller D, Bouchard C, Rudolph B, Steiner P, Stuckmann I, Saffrich R, et al. Cdk2-dependent phosphorylation of p27 facilitates its MSc-induced release from cyclin E/cdk2 complexes. Oncogene. 1997;15:2561–2576. doi: 10.1038/sj.onc.1201440. [DOI] [PubMed] [Google Scholar]
- 203.O’Hagan RC, Ohh M, David G, De Alboran IM, Alt FW, Kaelin WG, et al. Myc-enhanced expression of Cul1 promotes ubiquitin-dependent proteolysis and cell cycle progression. Genes Dev. 2000;14:2185–2191. doi: 10.1101/gad.827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Keller UB, Old JB, Dorsey FC, Nilsson JA, Nilsson L, MacLean KH, et al. Myc targets Cks1 to provoke the suppression of p27Kip1, proliferation and lymphomagenesis. EMBO J. 2007;26:2562–2574. doi: 10.1038/sj.emboj.7601691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Montagnoli A, Fiore F, Eytan E, Carrano AC, Draetta GF, Hershko A, et al. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 1999;13:1181–1189. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sutterlüty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Müller U, et al. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999;1:207–214. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- 207.Blanco-Bose WE, Murphy MJ, Ehninger A, Offner S, Dubey C, Huang W, et al. c-Myc and its target foxM1 are critical downstream effectors of constitutive androstane receptor (CAR) mediated direct liver hyperplasia. Hepatology. 2008;48:1302–1311. doi: 10.1002/hep.22475. [DOI] [PubMed] [Google Scholar]
- 208.Valovka T, Schönfeld M, Raffeiner P, Breuker K, Dunzendorfer-Matt T, Hartl M, et al. Transcriptional control of DNA replication licensing by Myc. Sci Rep. 2013;3:1–9. doi: 10.1038/srep03444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Perna D, Fagà G, Verrecchia A, Gorski MM, Barozzi I, Narang V, et al. Genome-wide mapping of Myc binding and gene regulation in serum-stimulated fibroblasts. Oncogene. 2012;31:1695–1709. doi: 10.1038/onc.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fernandez PC, Frank SR, Wang L, Schroeder M, Liu S, Greene J, et al. Genomic targets of the human c-Myc protein. Genes Dev. 2003;17:1115–1129. doi: 10.1101/gad.1067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dominguez-Sola D, Ying CY, Grandori C, Ruggiero L, Chen B, Li M, et al. Non-transcriptional control of DNA replication by c-Myc. Nature. 2007;448:445–451. doi: 10.1038/nature05953. [DOI] [PubMed] [Google Scholar]
- 212.Kops GJPL, Weaver BAA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- 213.Primorac I, Musacchio A. Panta rhei: the APC/C at steady state. J Cell Biol. 2013;201:177–189. doi: 10.1083/jcb.201301130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Menssen A, Epanchintsev A, Rezaei N, Lodygin D, Jung P, Verdoodt B, et al. c-MYC delays prometaphase by direct transactivation of MAD2 and Bub R1: Identification of mechanisms underlying c-MYC-induced DNA damage and chromosomal instability. Cell Cycle. 2007;6:339–352. doi: 10.4161/cc.6.3.3808. [DOI] [PubMed] [Google Scholar]
- 215.Littler S, Sloss O, Geary B, Pierce A, Whetton AD, Taylor SS. Oncogenic MYC amplifies mitotic perturbations. Open Biol. 2019;9:109136. doi: 10.1098/rsob.190136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ciribilli Y, Singh P, Spanel R, Inga A, Borlak J. Decoding c-Myc networks of cell cycle and apoptosis regulated genes in a transgenic mouse model of papillary lung adenocarcinomas. Oncotarget. 2015;6:31569–31592. doi: 10.18632/oncotarget.5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Saito S, Liu X-F, Kamijo K, Raziuddin R, Tatsumoto T, Okamoto I, et al. Deregulation and mislocalization of the cytokinesis regulator ECT2 activate the rho signaling pathways leading to malignant transformation. J Biol Chem. 2004;279:7169–7179. doi: 10.1074/jbc.M306725200. [DOI] [PubMed] [Google Scholar]
- 218.Li Y, Pei J, Xia H, Ke H, Wang H, Tao W. Lats2, a putative tumor suppressor, inhibits G1/S transition. Oncogene. 2003;22:4398–4405. doi: 10.1038/sj.onc.1206603. [DOI] [PubMed] [Google Scholar]
- 219.Yoon HS, Chen X, Yang VW. Krüppel-like factor 4 mediates p53-dependent G1/S cell cycle arrest in response to DNA damage. J Biol Chem. 2003;278:2101–2105. doi: 10.1074/jbc.M211027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Huang Q, Raya A, DeJesus P, Chao SH, Quon KC, Caldwell JS, et al. Identification of p53 regulators by genome-wide functional analysis. Proc Natl Acad Sci U S A. 2004;101:3456–3461. doi: 10.1073/pnas.0308562100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Del Sal G, Ruaro EM, Utrera R, Cole CN, Levine AJ, Schneider C. Gas1-induced growth suppression requires a transactivation-independent p53 function. Mol Cell Biol. 1995;15:7152–7160. doi: 10.1128/MCB.15.12.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wadhwa R, Yaguchi T, Hasan MK, Mitsui Y, Reddel RR, Kaul SC. Hsp70 family member, mot-2/mthsp70/GRP75, binds to the cytoplasmic sequestration domain of the p53 protein. Exp Cell Res. 2002;274:246–253. doi: 10.1006/excr.2002.5468. [DOI] [PubMed] [Google Scholar]
- 223.Mei Y, Wu M. Noncoding RNAs regulating p53 and c-Myc signaling. Adv Exp Med Biol. 2016;927:337–365. doi: 10.1007/978-981-10-1498-7_13. [DOI] [PubMed] [Google Scholar]
- 224.Bueno MJ, Malumbres M. MicroRNAs and the cell cycle. Biochim Biophys Acta Mol Basis Dis. 2011;1812:592–601. doi: 10.1016/j.bbadis.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 225.Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–7722. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 226.Bui TV, Mendell JT. Myc: maestro of microRNAs. Genes Cancer. 2010;1:568–575. doi: 10.1177/1947601910377491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Barsyte-Lovejoy D, Lau SK, Boutros PC, Khosravi F, Jurisica I, Andrulis IL, et al. The c-Myc oncogene directly induces the H19 noncoding RNA by allele-specific binding to potentiate tumorigenesis. Cancer Res. 2006;66:5330–5337. doi: 10.1158/0008-5472.CAN-06-0037. [DOI] [PubMed] [Google Scholar]
- 229.Kitagawa M, Kitagawa K, Kotake Y, Niida H, Ohhata T. Cell cycle regulation by long non-coding RNAs. Cell Mol Life Sci. 2013;70:4785–4794. doi: 10.1007/s00018-013-1423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Berteaux N, Lottin S, Monté D, Pinte S, Quatannens B, Coll J, et al. H19 mRNA-like noncoding RNA promotes breast cancer cell proliferation through positive control by E2F1. J Biol Chem. 2005;280:29625–29636. doi: 10.1074/jbc.M504033200. [DOI] [PubMed] [Google Scholar]
- 231.Izadirad M, Jafari L, James AR, Unfried JP, Wu ZX, Chen ZS. Long noncoding RNAs have pivotal roles in chemoresistance of acute myeloid leukemia. Drug Discov Today. 2021:S1359-6446(21)00153-7. 10.1016/j.drudis.2021.03.017. [DOI] [PubMed]
- 232.Doose G, Haake A, Bernhart SH, López C, Duggimpudi S, Wojciech F, et al. MINCR is a MYC-induced lncRNA able to modulate MYC’s transcriptional network in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 2015;112:E5261–E5270. doi: 10.1073/pnas.1505753112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Popov N, Wanzel M, Madiredjo M, Zhang D, Beijersbergen R, Bernards R, et al. The ubiquitin-specific protease USP28 is required for MYC stability. Nat Cell Biol. 2007;9:765–774. doi: 10.1038/ncb1601. [DOI] [PubMed] [Google Scholar]
- 234.Bunch R, Povirk L, Orr M, et al. Influence of amsacrine (m-AMSA) on bulk and gene-specific DNA damage and c-myc expression in MCF-7 breast tumor cells. Biochem Pharmacol. 1994;47:317–329. doi: 10.1016/0006-2952(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 235.Fornari F, Jr, Jarvis W, Grant S, et al. Growth arrest and non-apoptotic cell death associated with the suppression of c-myc expression in MCF-7 breast tumor cells following acute exposure to doxorubicin. Biochem Pharmacol. 1996;51:931–940. doi: 10.1016/0006-2952(96)00050-0. [DOI] [PubMed] [Google Scholar]
- 236.Orr M, Fornari F, Randolph J, Biophysica DG-B, et al. Transcriptional down-regulation of c-myc expression in the MCF-7 breast tumor cell line by the topoisomerase 11 inhibitor, VM-26. Biochim Biophys Acta Gene Struct Expr. 1995;1262:139–145. doi: 10.1016/0167-4781(95)00064-N. [DOI] [PubMed] [Google Scholar]
- 237.Watson NC, Di YM, Orr MS, Fornari J, Randolph JK, Magnet KJ, et al. Influence of ionizing radiation on proliferation, c-myc expression and the induction of apoptotic cell death in two breast tumour cell lines differing in p53 status. Int J Radiat Biol. 1997;72:547–559. doi: 10.1080/095530097143059. [DOI] [PubMed] [Google Scholar]
- 238.Magnet K, Orr M, et al. Suppression of c-myc expression and c-Myc function in response to sustained DNA damage in MCF-7 breast tumor cells. Biochem Pharmacol. 2001;62:593–602. doi: 10.1016/S0006-2952(01)00699-2. [DOI] [PubMed] [Google Scholar]
- 239.Jain PT, Fornari FA, Randolph JK, Orr MS, Gewirtz DA. Induction of DNA damage, inhibition of DNA synthesis, and suppression of c-myc expression by the topoisomerase I inhibitor, camptothecin, in MCF-7 human breast tumor cells. Biochem Pharmacol. 1998;55:1263–1269. doi: 10.1016/S0006-2952(97)00618-7. [DOI] [PubMed] [Google Scholar]
- 240.Orr M, Watson N, Sundaram S, et al. Ionizing radiation and teniposide increase p21waf1/cip1 and promote Rb dephosphorylation but fail to suppress E2F activity in MCF-7 breast tumor cells. ASPET. 1997;52:373–379. doi: 10.1124/mol.52.3.373. [DOI] [PubMed] [Google Scholar]
- 241.Gorrini C, Squatrito M, Luise C, Syed N. Tip60 is a haplo-insufficient tumour suppressor required for an oncogene-induced DNA damage response. Nature. 2007;448:1063–1067. doi: 10.1038/nature06055. [DOI] [PubMed] [Google Scholar]
- 242.Bassi C, Li YT, Khu K, Mateo F, Baniasadi PS, Elia A, et al. The acetyltransferase Tip60 contributes to mammary tumorigenesis by modulating DNA repair. Cell Death Differ. 2016;23:1198–1208. doi: 10.1038/cdd.2015.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hughes H. Bax Is a Transcriptional Target and Mediator of c-Myc-induced Apoptosis 1. Cancer Res. 2000;60:6318–6325. [PubMed] [Google Scholar]
- 244.Barsyte-Lovejoy D, Mao D. c-Myc represses the proximal promoters of GADD45a and GADD153 by a post-RNA polymerase II recruitment mechanism. Oncogene. 2004;23:3481–3486. doi: 10.1038/sj.onc.1207487. [DOI] [PubMed] [Google Scholar]
- 245.Rogulski K, Li Y, Rothermund K, Pu L, Watkins S, et al. Onzin, a c-Myc-repressed target, promotes survival and transformation by modulating the Akt–Mdm2–p53 pathway. Oncogene. 2005;24:7524–7541. doi: 10.1038/sj.onc.1208897. [DOI] [PubMed] [Google Scholar]
- 246.Pusapati R, et al. ATM promotes apoptosis and suppresses tumorigenesis in response to Myc. Natl Acad Sci. 2006;103:1446–1451. doi: 10.1073/pnas.0507367103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Reimann M, Loddenkemper C, Rudolph C, Schildhauer I, Teichmann B, Stein H, et al. The Myc-evoked DNA damage response accounts for treatment resistance in primary lymphomas in vivo. Blood J Am Soc Hematol. 2007;110:2996–3004. doi: 10.1182/blood-2007-02-075614. [DOI] [PubMed] [Google Scholar]
- 248.Guerra L, Albihn A, Tronnersjö S, Yan Q, Guidi R, Stenerlöw B, et al. Myc is required for activation of the ATM-dependent checkpoints in response to DNA damage. PLoS ONE. 2010;5:e8924. doi: 10.1371/journal.pone.0008924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chiang YC, Teng SC, Su YN, Hsieh FJ, Wu KJ. c-Myc directly regulates the transcription of the NBS1 gene involved in DNA double-strand break repair. J Biol Chem. 2003;278:19286–19291. doi: 10.1074/jbc.M212043200. [DOI] [PubMed] [Google Scholar]
- 250.Lee J. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science. 2004;304:93–96. doi: 10.1126/science.1091496. [DOI] [PubMed] [Google Scholar]
- 251.Lee J-H, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science (80-. ) 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 252.Herold S, Hock A, Herkert B, Berns K, Mullenders J, Beijersbergen R, et al. Miz1 and HectH9 regulate the stability of the checkpoint protein, TopBP1. EMBO J. 2008;27:2851–2861. doi: 10.1038/emboj.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Vafa O, Wade M, Kern S, Beeche M, Pandita TK, Hampton GM, et al. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol Cell. 2002;9:1031–1044. doi: 10.1016/S1097-2765(02)00520-8. [DOI] [PubMed] [Google Scholar]
- 254.Hermeking H, Funk J, Reichert M, Ellwart J. Abrogation of p53-induced cell cycle arrest by c-Myc: evidence for an inhibitor of p21WAF1/CIP1/SDI1. Oncogene. 1995;11:1409–1415. [PubMed] [Google Scholar]
- 255.Seoane J, Le H. Myc suppression of the p21 Cip1 Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature. 2002;419:729–734. doi: 10.1038/nature01119. [DOI] [PubMed] [Google Scholar]
- 256.Sheen JH, Dickson RB. Overexpression of c-Myc alters G(1)/S arrest following ionizing radiation. Mol Cell Biol. 2002;22(6):1819–33. 10.1128/MCB.22.6.1819-1833.2002. [DOI] [PMC free article] [PubMed]
- 257.Sheen J, Woo J. c-Myc alters the DNA damage-induced G2/M arrest in human mammary epithelial cells. Br J Cancer. 2003;89:1479–1485. doi: 10.1038/sj.bjc.6601307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Robinson K, Asawachaicharn N, Galloway DA, Grandori C. c-Myc accelerates S-phase and requires WRN to avoid replication stress. PLoS ONE. 2009;4:e5951. doi: 10.1371/journal.pone.0005951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Karlsson A, Deb-Basu D. Defective double-strand DNA break repair and chromosomal translocations by MYC overexpression. Proc Natl Acad Sci. 2003;100:9974–9979. doi: 10.1073/pnas.1732638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Eischen CM, Woo D, Roussel MF, Cleveland JL. Apoptosis triggered by Myc-induced suppression of Bcl-XL or Bcl-2 Is bypassed during lymphomagenesis. Mol Cell Biol. 2001;21:5063–5070. doi: 10.1128/MCB.21.15.5063-5070.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene Per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006;22:375–382. doi: 10.1016/j.molcel.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 262.Afanasyeva EA, Komarova EY, Larsson L-G, Bahram F, Margulis BA, Guzhova IV. Drug-induced Myc-mediated apoptosis of cancer cells is inhibited by stress protein Hsp70. Int J Cancer. 2007;121:2615–2621. doi: 10.1002/ijc.22974. [DOI] [PubMed] [Google Scholar]
- 263.Kennedy R, Quinn J. The role of BRCA1 in the cellular response to chemotherapy. J Natl Cancer Inst. 2004;96:1659–1668. doi: 10.1093/jnci/djh312. [DOI] [PubMed] [Google Scholar]
- 264.Quinn JE, et al. BRCA1 Functions as a Differential Modulator of Chemotherapy-induced Apoptosis. Cancer Res. 2003;63:6221–6228. [PubMed] [Google Scholar]
- 265.Kennedy RD, Gorski JJ, Quinn JE, Stewart GE, James CR, Moore S, et al. BRCA1 and c-Myc associate to transcriptionally repress psoriasin, a DNA damage-inducible gene. Cancer Res. 2005;65:10265–10272. doi: 10.1158/0008-5472.CAN-05-1841. [DOI] [PubMed] [Google Scholar]
- 266.Albihn A, Lovén J, Ohlsson J, Osorio LM, Henriksson M. c-Myc-dependent etoposide-induced apoptosis involves activation of bax and caspases, and PKCdelta signaling. J Cell Biochem. 2006;98:1597–1614. doi: 10.1002/jcb.20816. [DOI] [PubMed] [Google Scholar]
- 267.Albihn A, Mo H, Yang Y, Henriksson M. Camptothecin-induced apoptosis is enhanced by Myc and involves PKCdelta signaling. Int J Cancer. 2007;121:1821–1829. doi: 10.1002/ijc.22866. [DOI] [PubMed] [Google Scholar]
- 268.Maclean KH, Keller UB, Rodriguez-Galindo C, Nilsson JA, Cleveland JL. c-Myc augments gamma irradiation-induced apoptosis by suppressing Bcl-X L. Mol Cell Biol. 2003;23:7256–7270. doi: 10.1128/MCB.23.20.7256-7270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Arango D, Mariadason J, Wilson A. c-Myc overexpression sensitises colon cancer cells to camptothecin-induced apoptosis. Br J Cancer. 2003;89:1757–1765. doi: 10.1038/sj.bjc.6601338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Supino R, Perego P, Gatti L. A role for c-myc in DNA damage-induced apoptosis in a human TP53-mutant small-cell lung cancer cell line. Eur J Cancer. 2001;37:2247–2256. doi: 10.1016/S0959-8049(01)00268-4. [DOI] [PubMed] [Google Scholar]
- 271.Ohga T, Koike K, Ono M, Makino Y, Itagaki Y, Tanimoto M, et al. Role of the human Y box-binding protein YB-1 in cellular sensitivity to the DNA-damaging agents cisplatin, mitomycin C, and ultraviolet light. Cancer Res. 1996;56:4224–4228. [PubMed] [Google Scholar]
- 272.Koike K, Uchiumi T, Ohga T, Toh S, Wada M, et al. Nuclear translocation of the Y-box binding protein by ultraviolet irradiation. FEBS Lett. 1997;417:390–394. doi: 10.1016/S0014-5793(97)01296-9. [DOI] [PubMed] [Google Scholar]
- 273.Ise T, Nagatani G, Imamura T, et al. Transcription factor Y-box binding protein 1 binds preferentially to cisplatin-modified DNA and interacts with proliferating cell nuclear antigen. Cancer Res. 1999;59:342–346. [PubMed] [Google Scholar]
- 274.Uramoto H, Izumi H, Ise T, Tada M, Uchiumi T, Kuwano M, et al. p73 interacts with c-Myc to regulate Y-box-binding protein-1 expression*. J Biol Chem. 2002;277:31694–31702. doi: 10.1074/jbc.M200266200. [DOI] [PubMed] [Google Scholar]
- 275.Dubrez L, Goldwasser F, Genne P. The role of cell cycle regulation and apoptosis triggering in determining the sensitivity of leukemic cells to topoisomerase I and II inhibitors. Leukemia. 1995;9:1013–1024. [PubMed] [Google Scholar]
- 276.Adachi S, Obaya AJ, Han Z, Ramos-Desimone N, Wyche JH, Sedivy JM. c-Myc is necessary for DNA damage-induced apoptosis in the G2 phase of the cell cycle. Mol Cell Biol. 2001;21:4929–4937. doi: 10.1128/MCB.21.15.4929-4937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Luoto KR, Meng AX, Wasylishen AR, Zhao H, Coackley CL, Penn LZ, et al. Tumor cell kill by c-MYC depletion: role of MYC-regulated genes that control DNA double-strand break repair. Cancer Res. 2010;70:8748–8759. doi: 10.1158/0008-5472.CAN-10-0944. [DOI] [PubMed] [Google Scholar]
- 278.Cui F, Fan R, Chen Q, He Y, Song M, Shang Z, et al. The involvement of c-Myc in the DNA double-strand break repair via regulating radiation-induced phosphorylation of ATM and DNA-PKcs activity. Mol Cell Biochem. 2015;406:43–51. doi: 10.1007/s11010-015-2422-2. [DOI] [PubMed] [Google Scholar]
- 279.Gordan JD, Lal P, Dondeti VR, Letrero R, Parekh KN, Oquendo CE, et al. HIF-α effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell. 2008;14:435–446. doi: 10.1016/j.ccr.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Schmitt CA, McCurrach ME, De Stanchina E, Wallace-Brodeur RR, Lowe SW. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 1999;13:2670–2677. doi: 10.1101/gad.13.20.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Phesse TJ, Myant KB, Cole AM, Ridgway RA, Pearson H, Muncan V, et al. Endogenous c-Myc is essential for p53-induced apoptosis in response to DNA damage in vivo. Cell Death Differ. 2014;21:956–966. doi: 10.1038/cdd.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Yu Q, He M, Lee NH, Liu ET. Identification of Myc-mediated death response pathways by microarray analysis. J Biol Chem. 2002;277:13059–13066. doi: 10.1074/jbc.M111403200. [DOI] [PubMed] [Google Scholar]
- 284.Ho JSL, Ma W, Mao DYL, Benchimol S. p53-dependent transcriptional repression of c-myc is required for G1 cell cycle arrest. Mol Cell Biol. 2005;25:7423–7431. doi: 10.1128/MCB.25.17.7423-7431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sutcliffe T, Fu L, Abraham J, et al. A functional wild-type p53 gene is expressed in human acute myeloid leukemia cell lines. Am Soc Hematol. 1998;92:2977–2979. [PubMed] [Google Scholar]
- 286.Zhang T, Brazhnik P, Tyson JJ. Exploring mechanisms of the DNA-damage response: p53 pulses and their possible relevance to apoptosis. Cell Cycle. 2007;6:85–94. doi: 10.4161/cc.6.1.3705. [DOI] [PubMed] [Google Scholar]
- 287.Bar-Or RL, Maya R, Segel LA, Alon U, Levine AJ, Oren M. Generation of oscillations by the p53-Mdm2 feedback loop: a theoretical and experimental study. Proc Natl Acad Sci U S A. 2000;97:11250–11255. doi: 10.1073/pnas.210171597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Ma L, Wagner J, Rice JJ, Hu W, Levine AJ, Stolovitzky GA. A plausible model for the digital response of p53 to DNA damage. Proc Natl Acad Sci U S A. 2005;102:14266–14271. doi: 10.1073/pnas.0501352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Porter JR, Fisher BE, Batchelor E. P53 pulses diversify target gene expression dynamics in an mRNA half-life-dependent manner and delineate co-regulated target gene subnetworks. Cell Syst. 2016;2:272–282. doi: 10.1016/j.cels.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Porter JR, Fisher BE, Baranello L, Liu JC, Kambach DM, Nie Z, et al. Global inhibition with specific activation: how p53 and MYC redistribute the transcriptome in the DNA double-strand break response. Mol Cell. 2017;67:1013–1025.e9. doi: 10.1016/j.molcel.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Moberg KH, Tyndall WA, Hall DJ. Wild-type murine p53 represses transcription from the murinec-myc promoter in a human glial cell line. J Cell Biochem. 1992;49:208–215. doi: 10.1002/jcb.240490213. [DOI] [PubMed] [Google Scholar]
- 292.Levy N, Yonish-Rouach E, Oren M, Kimchi A. Complementation by wild-type p53 of interleukin-6 effects on M1 cells: induction of cell cycle exit and cooperativity with c-myc suppression. Mol Cell Biol. 1993;13:7942–7952. doi: 10.1128/mcb.13.12.7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Lin CY, Lovén J, Rahl PB, Paranal RM, Burge CB, Bradner JE, et al. Transcriptional amplification in tumor cells with elevated c-Myc. Cell. 2012;151:56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Hyeon HK, Kuwano Y, Srikantan S, Eun KL, Martindale JL, Gorospe M. HuR recruits let-7/RISC to repress c-Myc expression. Genes Dev. 2009;23:1743–1748. doi: 10.1101/gad.1812509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, et al. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci U S A. 2009;106:3207–3212. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Challagundla KB, Sun X-X, Zhang X, DeVine T, Zhang Q, Sears RC, et al. Ribosomal protein L11 recruits miR-24/miRISC to repress c-Myc expression in response to ribosomal stress. Mol Cell Biol. 2011;31:4007–4021. doi: 10.1128/MCB.05810-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Corney DC, Flesken-Nikitin A, Godwin AK, Wang W, Nikitin AY. MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res. 2007;67:8433–8438. doi: 10.1158/0008-5472.CAN-07-1585. [DOI] [PubMed] [Google Scholar]
- 299.He L, He X, Lim LP, De Stanchina E, Xuan Z, Liang Y, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Kumamoto K, Spillare EA, Fujita K, Horikawa I, Yamashita T, Appella E, et al. Nutlin-3a activates p53 to both down-regulate inhibitor of growth 2 and up-regulate mir-34a, mir-34b, and mir-34c expression, and induce senescence. Cancer Res. 2008;68:3193–3203. doi: 10.1158/0008-5472.CAN-07-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Tarasov V, Jung P, Verdoodt B, Lodygin D, Epanchintsev A, Menssen A, et al. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G 1-arrest. Cell Cycle. 2007;6:1586–1593. doi: 10.4161/cc.6.13.4436. [DOI] [PubMed] [Google Scholar]
- 302.Yi WK, Cannell IG, De Moor CH, Hill K, Garside PG, Hamilton TL, et al. The mechanism of micro-RNA-mediated translation repression is determined by the promoter of the target gene. Proc Natl Acad Sci U S A. 2008;105:8866–8871. doi: 10.1073/pnas.0800650105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lujambio A, Calin GA, Villanueva A, Ropero S, Sánchez-Céspedes M, Blanco D, et al. A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci U S A. 2008;105:13556–13561. doi: 10.1073/pnas.0803055105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Li Y, Challagundla KB, Sun XX, Zhang Q, Dai MS. MicroRNA-130a associates with ribosomal protein L11 to suppress c-Myc expression in response to UV irradiation. Oncotarget. 2015;6:1101–1114. doi: 10.18632/oncotarget.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Sakamuro D, Elliott KJ, Wechsler-Reya R, Prendergast GC. BIN1 is a novel MYC-interacting protein with features of a tumour suppressor. Nat Genet. 1996;14:69–77. doi: 10.1038/ng0996-69. [DOI] [PubMed] [Google Scholar]
- 306.Cassimere EK, Pyndiah S, Sakamuro D. The c-MYC-interacting proapoptotic tumor suppressor BIN1 is a transcriptional target for E2F1 in response to DNA damage. Cell Death Differ. 2009;16:1641–1653. doi: 10.1038/cdd.2009.98. [DOI] [PubMed] [Google Scholar]
- 307.Elliott K, Sakamuro D, Basu A, Du W, Wunner W, Staller P, et al. Bin1 functionally interacts with Myc and inhibits cell proliferation via multiple mechanisms. Oncogene. 1999;18:3564–3573. doi: 10.1038/sj.onc.1202670. [DOI] [PubMed] [Google Scholar]
- 308.Kinney EL, Tanida S, Rodrigue AA, Johnson JK, Tompkins VS, Sakamuro D. Adenovirus E1A oncoprotein liberates c-Myc activity to promote cell proliferation through abating Bin1 expression via an Rb/E2F1-dependent mechanism. J Cell Physiol. 2008;216:621–631. doi: 10.1002/jcp.21437. [DOI] [PubMed] [Google Scholar]
- 309.Pyndiah S, Tanida S, Ahmed KM, Cassimere EK, Choe C, Sakamuro D. c-MYC suppresses BIN1 to release poly(ADP-ribose) polymerase 1: a mechanism by which cancer cells acquire cisplatin resistance. Sci Signal. 2011;4:r19. doi: 10.1126/scisignal.2001556. [DOI] [PubMed] [Google Scholar]
- 310.Meyer-Ficca ML, Meyer RG, Jacobson EL, Jacobson MK. Poly(ADP-ribose) polymerases: managing genome stability. Int J Biochem Cell Biol. 2005;37:920–926. doi: 10.1016/j.biocel.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 311.El-Khamisy SF, Masutani M, Suzuki H, Caldecott KW. A requirement for PARP-1 for the assembly or stability of XRCC1 nuclear foci at sites of oxidative DNA damage. Nucleic Acids Res. 2003;31:5526–5533. doi: 10.1093/nar/gkg761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Masson M, Niedergang C, Schreiber V, Muller S, Menissier-de Murcia J, de Murcia G. XRCC1 is specifically associated with poly(ADP-ribose) polymerase and negatively regulates its activity following DNA damage. Mol Cell Biol. 1998;18:3563–3571. doi: 10.1128/MCB.18.6.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Prendergast GC. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene. 2008;27:3889–3900. doi: 10.1038/onc.2008.35. [DOI] [PubMed] [Google Scholar]
- 314.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 315.Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: “PAR-laying” NAD+ into a nuclear signal. Genes Dev. 2005;19:1951–1967. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- 316.Di Fagagna FDA, Hande MP, Tong WM, Lansdorp PM, Wang ZQ, Jackson SP. Functions of poly(ADP-ribose) polymerase in controlling telomere length and chromosomal stability. Nat Genet. 1999;23:76–80. doi: 10.1038/12680. [DOI] [PubMed] [Google Scholar]
- 317.Ramalingam A, Farmer GE, Stamato TD, Prendergast GC. Bin1 interacts with and restrains the DNA end-binding protein complex Ku. Cell Cycle. 2007;6:1914–1918. doi: 10.4161/cc.6.15.4514. [DOI] [PubMed] [Google Scholar]
- 318.Chen LL, Lin HP, Zhou WJ, He CX, Zhang ZY, Cheng ZL, et al. SNIP1 recruits TET2 to regulate c-MYC target genes and cellular DNA damage response. Cell Rep. 2018;25:1485.e4–1500.e4. doi: 10.1016/j.celrep.2018.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Roche KC, Wiechens N, Owen-Hughes T, Perkins ND. The FHA domain protein SNIP1 is a regulator of the cell cycle and cyclin D1 expression. Oncogene. 2004;23:8185–8195. doi: 10.1038/sj.onc.1208025. [DOI] [PubMed] [Google Scholar]
- 320.Roche KC, Rocha S, Bracken CP, Perkins ND. Regulation of ATR-dependent pathways by the FHA domain containing protein SNIP1. Oncogene. 2007;26:4523–4530. doi: 10.1038/sj.onc.1210233. [DOI] [PubMed] [Google Scholar]
- 321.Felix K, Polack A, Pretsch W, Jackson SH, Feigenbaum L, Bornkamm GW, et al. Moderate hypermutability of a transgenic lacZ reporter gene in Myc-dependent inflammation-induced plasma cell tumors in mice. Cancer Res. 2004;64:530–537. doi: 10.1158/0008-5472.CAN-03-2602. [DOI] [PubMed] [Google Scholar]
- 322.Taylor C, Mai S. c-Myc-associated genomic instability of the dihydrofolate reductase locus in vivo. Cancer Detect Prev. 1998;22:350–356. doi: 10.1046/j.1525-1500.1998.CDOA36.x. [DOI] [PubMed] [Google Scholar]
- 323.Felsher DW, Bishop JM. Transient excess of MYC activity can elicit genomic instability and tumorigenesis. Proc Natl Acad Sci U S A. 1999;96:3940–3944. doi: 10.1073/pnas.96.7.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ray S, Atkuri KR, Deb-Basu D, Adler AS, Chang HY, Herzenberg LA, et al. MYC can induce DNA breaks in vivo and in vitro independent of reactive oxygen species. Cancer Res. 2006;66:6598–6605. doi: 10.1158/0008-5472.CAN-05-3115. [DOI] [PubMed] [Google Scholar]
- 325.Dang CV, Li F, Lee LA. Could MYC induction of mitochondrial biogenesis be linked to ROS production and genomic instability? Cell Cycle. 2005;4:1465–1466. doi: 10.4161/cc.4.11.2121. [DOI] [PubMed] [Google Scholar]
- 326.Wade M, Wahl GM. c-Myc, genome instability, and tumorigenesis: the devil is in the details. Curr Top Microbiol Immunol. 2006;302:169–203. doi: 10.1007/3-540-32952-8_7. [DOI] [PubMed] [Google Scholar]
- 327.Gorgoulis VG, Vassiliou LVF, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 328.Classon M, Henriksson M, Sümegi J, Klein G, Hammaskjöld ML. Elevated c-myc expression facilitates the replication of SV40 DNA in human lymphoma cells. Nature. 1987;330:272–274. doi: 10.1038/330272a0. [DOI] [PubMed] [Google Scholar]
- 329.Campaner S, Amati B. Two sides of the Myc-induced DNA damage response: from tumor suppression to tumor maintenance. Cell Div. 2012;7:6. doi: 10.1186/1747-1028-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Nishitani H, Lygerou Z, Nishimoto T, Nurse P. The Cdt1 protein is required to license DNA for replication in fission yeast. Nature. 2000;404:625–628. doi: 10.1038/35007110. [DOI] [PubMed] [Google Scholar]
- 331.Hofmann JF, Beach D. cdt1 is an essential target of the Cdc10/Sct1 transcription factor: requirement for DNA replication and inhibition of mitosis. EMBO J. 1994;13:425–434. doi: 10.1002/j.1460-2075.1994.tb06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Maiorano D, Moreau J, Méchali M. XCDT1 is required for the assembly of pre-replicative complexes in Xenopus laevis. Nature. 2000;404:622–625. doi: 10.1038/35007104. [DOI] [PubMed] [Google Scholar]
- 333.Kurashima K, Sekimoto T, Oda T, Kawabata T, Hanaoka F, Yamashita T. Polη, a Y-family translesion synthesis polymerase, promotes cellular tolerance of Myc-induced replication stress. J Cell Sci. 2018;131:jcs212183. doi: 10.1242/jcs.212183. [DOI] [PubMed] [Google Scholar]
- 334.Kolli S, Buchmann AM, Williams J, Weitzman S, Thimmapaya B. Antisense-mediated depletion of p300 in human cells leads to premature exit and up-regulation of c-MYC. Proc Natl Acad Sci U S A. 2001;98:4646–4651. doi: 10.1073/pnas.081141998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Rajabi HN, Baluchamy S, Kolli S, Nag A, Srinivas R, Raychaudhuri P, et al. Effects of depletion of CREB-binding protein on c-Myc regulation and cell cycle G1-S transition. J Biol Chem. 2005;280:361–374. doi: 10.1074/jbc.M408633200. [DOI] [PubMed] [Google Scholar]
- 336.Sankar N, Kadeppagari RK, Thimmapaya B. c-Myc-induced aberrant DNA synthesis and activation of DNA damage response in p300 knockdown cells. J Biol Chem. 2009;284:15193–15205. doi: 10.1074/jbc.M900776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Song L, Dai T, Xie Y, Wang C, Lin C, Wu Z, et al. Up-regulation of miR-1245 by c-myc targets BRCA2 and impairs DNA repair. J Mol Cell Biol. 2012;4:108–117. doi: 10.1093/jmcb/mjr046. [DOI] [PubMed] [Google Scholar]
- 338.Li Z, Owonikoko TK, Sun SY, Ramalingam SS, Doetsch PW, Xiao ZQ, et al. c-Myc suppression of DNA double-strand break repair. Neoplasia (United States) 2012;14:1190–1202. doi: 10.1593/neo.121258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Mao DYL, Watson JD, Yan PS, Barsyte-Lovejoy D, Khosravi F, Wei-Lynn Wong W, et al. Analysis of MYC bound loci identified by CpG island arrays shows that Max is essential for MYC-dependent repression. Curr Biol. 2003;13:882–886. doi: 10.1016/S0960-9822(03)00297-5. [DOI] [PubMed] [Google Scholar]
- 340.Sallmyr A, Fan J, Rassool FV. Genomic instability in myeloid malignancies: increased reactive oxygen species (ROS), DNA double strand breaks (DSBs) and error-prone repair. Cancer Lett. 2008;270:1–9. doi: 10.1016/j.canlet.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 341.Muvarak N, Nagaria P, Rassool FV. Genomic instability in chronic myeloid leukemia: targets for therapy? Curr Hematol Malig Rep. 2012;7:94–102. doi: 10.1007/s11899-012-0119-0. [DOI] [PubMed] [Google Scholar]
- 342.Muvarak N, Kelley S, Robert C, Baer MR, Perrotti D, Gambacorti-Passerini C, et al. C-MYC generates repair errors via increased transcription of alternative-NHEJ factors, LIG3 and PARP1, in tyrosine kinase-activated leukemias. Mol Cancer Res. 2015;13:699–712. doi: 10.1158/1541-7786.MCR-14-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Fan J, Li L, Small D, Rassool F. Cells expressing FLT3/ITD mutations exhibit elevated repair errors generated through alternative NHEJ pathways: implications for genomic instability and therapy. Blood J Am Soc Hematol. 2010;116:5298–5305. doi: 10.1182/blood-2010-03-272591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Li L, Robert C, Rassool VF. The role of error-prone alternative non-homologous end-joining in genomic instability in cancer. Rijeka: InTech; 2011. [Google Scholar]
- 345.Jin Z, May WS, Gao F, Flagg T, Deng X. Bcl2 suppresses DNA repair by enhancing c-Myc transcriptional activity. J Biol Chem. 2006;281:14446–14456. doi: 10.1074/jbc.M511914200. [DOI] [PubMed] [Google Scholar]
- 346.Partlin MM, Homer E, Robinson H, McCormick CJ, Crouch DH, Durant ST, et al. Interactions of the DNA mismatch repair proteins MLH1 and MSH2 with c-MYC and MAX. Oncogene. 2003;22:819–825. doi: 10.1038/sj.onc.1206252. [DOI] [PubMed] [Google Scholar]
- 347.Bucci B, D’Agnano I, Amendola D, Citti A, Raza GH, Miceli R, et al. Myc down-regulation sensitizes melanoma cells to radiotherapy by inhibiting MLH1 and MSH2 mismatch repair proteins. Clin Cancer Res. 2005;11:2756–2767. doi: 10.1158/1078-0432.CCR-04-1582. [DOI] [PubMed] [Google Scholar]
- 348.Harrington EA, Bennett MR, Fanidi A, Evan GI. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 1994;13:3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 350.Candé C, Cecconi F, Dessen P, Kroemer G. Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci. 2002;115:4727–4734. doi: 10.1242/jcs.00210. [DOI] [PubMed] [Google Scholar]
- 351.Brentnall M, Rodriguez-Menocal L, De Guevara RL, Cepero E, Boise LH. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013;14:32. doi: 10.1186/1471-2121-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018;25:65–80. doi: 10.1038/cdd.2017.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.O’Neill KL, Huang K, Zhang J, Chen Y, Luo X. Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane. Genes Dev. 2016;30:973–988. doi: 10.1101/gad.276725.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 355.Lavrik I, Golks A, Krammer PH. Death receptor signaling. J Cell Sci. 2005;118:265–267. doi: 10.1242/jcs.01610. [DOI] [PubMed] [Google Scholar]
- 356.Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 357.Chang DW, Xing Z, Capacio VL, Peter ME, Yang X. Interdimer processing mechanism of procaspase-8 activation. EMBO J. 2003;22:4132–4142. doi: 10.1093/emboj/cdg414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Schleich K, Buchbinder JH, Pietkiewicz S, Kähne T, Warnken U, Öztürk S, et al. Molecular architecture of the DED chains at the DISC: regulation of procaspase-8 activation by short DED proteins c-FLIP and procaspase-8 prodomain. Cell Death Differ. 2016;23:681–694. doi: 10.1038/cdd.2015.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/S0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 360.Gross A, Yin XM, Wang K, Wei MC, Jockel J, Milliman C, et al. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 361.Hughes MA, Powley IR, Jukes-Jones R, Horn S, Feoktistova M, Fairall L, et al. Co-operative and hierarchical binding of c-FLIP and caspase-8: a unified model defines how c-FLIP isoforms differentially control cell fate. Mol Cell. 2016;61:834–849. doi: 10.1016/j.molcel.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Juin P, Hueber AO, Littlewood T, Evan G. c-Myc-induced sensitization to apoptosis is mediated through cytochrome c release. Genes Dev. 1999;13:1367–1381. doi: 10.1101/gad.13.11.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Mitchell KO, Ricci MS, Miyashita T, Dicker DT, Jin Z, Reed JC, et al. Bax is a transcriptional target and mediator of c-myc-induced apoptosis. Cancer Res. 2000;60:6318–6325. [PubMed] [Google Scholar]
- 364.Cao X, Bennett RL, May WS. c-Myc and caspase-2 are involved in activating Bax during cytotoxic drug-induced apoptosis. J Biol Chem. 2008;283:14490–14496. doi: 10.1074/jbc.M801107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Juin P, Hunt A, Littlewood T, Griffiths B, Swigart LB, Korsmeyer S, et al. c-Myc functionally cooperates with Bax to induce apoptosis. Mol Cell Biol. 2002;22:6158–6169. doi: 10.1128/MCB.22.17.6158-6169.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Nieminen AI, Eskelinen VM, Haikala HM, Tervonen TA, Yan Y, Partanen JI, et al. Myc-induced AMPK-phospho p53 pathway activates Bak to sensitize mitochondrial apoptosis. Proc Natl Acad Sci U S A. 2013;110:E1839–E1848. doi: 10.1073/pnas.1208530110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Eischen CM, Woo D, Roussel MF, Cleveland JL. Apoptosis triggered by Myc-induced suppression of Bcl-X(L) or Bcl-2 is bypassed during lymphomagenesis. Mol Cell Biol. 2001;21:5063–5070. doi: 10.1128/MCB.21.15.5063-5070.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Hueber AO, Zörnig M, Lyon D, Suda T, Nagata S, Evan GI. Requirement for the CD95 receptor-ligand pathway in c-Myc-induced apoptosis. Science (80- ) 1997;278:1305–1309. doi: 10.1126/science.278.5341.1305. [DOI] [PubMed] [Google Scholar]
- 369.Klefstrom J, Västrik I, Saksela E, Valle J, Eilers M, Alitalo K. c-Myc induces cellular susceptibility to the cytotoxic action of TNF-alpha. EMBO J. 1994;13:5442–5450. doi: 10.1002/j.1460-2075.1994.tb06879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Klefstrom J, Arighi E, Littlewood T, Jäättelä M, Saksela E, Evan GI, et al. Induction of TNF-sensitive cellular phenotype by c-Myc involves p53 and impaired NF-kappaB activation. EMBO J. 1997;16:7382–7392. doi: 10.1093/emboj/16.24.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Nilsson JA, Cleveland JL. Myc pathways provoking cell suicide and cancer. Oncogene. 2003;22:9007–9021. doi: 10.1038/sj.onc.1207261. [DOI] [PubMed] [Google Scholar]
- 372.Sayyadi M, Safaroghli-Azar A, Safa M, Abolghasemi H, Momeny M, Bashash D. NF-κB-dependent mechanism of action of c-Myc inhibitor 10058–F4: highlighting a promising effect of c-Myc inhibition in Leukemia cells, irrespective of p53 status. Iran J Pharm Res. 2020;19:153. doi: 10.22037/ijpr.2020.112926.14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Klefstrom J, Verschuren EW, Evan G. c-Myc augments the apoptotic activity of cytosolic death receptor signaling proteins by engaging the mitochondrial apoptotic pathway. J Biol Chem. 2002;277:43224–43232. doi: 10.1074/jbc.M206967200. [DOI] [PubMed] [Google Scholar]
- 374.Järvinen K, Hotti A, Santos L, Nummela P, Hölttä E. Caspase-8, c-FLIP, and caspase-9 in c-Myc-induced apoptosis of fibroblasts. Exp Cell Res. 2011;317:2602–2615. doi: 10.1016/j.yexcr.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 375.Brunner T, Kasibhatla S, Pinkoski MJ, Frutschi C, Yoo NJ, Echeverri F, et al. Expression of Fas ligand in activated T cells is regulated by c-Myc. J Biol Chem. 2000;275:9767–9772. doi: 10.1074/jbc.275.13.9767. [DOI] [PubMed] [Google Scholar]
- 376.Haupt S, Berger M, Goldberg Z, Haupt Y. Apoptosis: the p53 network. J Cell Sci. 2003;116:4077–4085. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- 377.Zhao R, Gish K, Murphy M, Yin Y, Notterman D, Hoffman WH, et al. The transcriptional program following p53 activation. In: Cold spring harbor symposia quantitative biology. 2000; vol. 65, p. 475–82. [DOI] [PubMed]
- 378.Bossi G, Lapi E, Strano S, Rinaldo C, Blandino G, Sacchi A. Mutant p53 gain of function: reduction of tumor malignancy of human cancer cell lines through abrogation of mutant p53 expression. Oncogene. 2006;25:304–309. doi: 10.1038/sj.onc.1209026. [DOI] [PubMed] [Google Scholar]
- 379.Wawryk-Gawda E, Chylińska-Wrzos P, Lis-Sochocka M, Chłapek K, Bulak K, Jędrych M, et al. P53 protein in proliferation, repair and apoptosis of cells. Protoplasma. 2014;251:525–533. doi: 10.1007/s00709-013-0548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Geyer RK, Yu ZK, Maki CG. The MDM2 RING-finger domain is required to promote p53 nuclear export. Nat Cell Biol. 2000;2:569–573. doi: 10.1038/35023507. [DOI] [PubMed] [Google Scholar]
- 381.Zindy F, Eischen CM, Randle DH, Kamijo T, Cleveland JL, Sherr CJ, et al. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science (80- ) 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 383.Leu JI, Dumont P, Hafey M, Murphy ME, George DL. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol. 2004;6:443–450. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- 384.Blyth K, Stewart M, Bell M, James C, Evan G, Neil JC, et al. Sensitivity to myc-induced apoptosis is retained in spontaneous and transplanted lymphomas of CD2-mycERTM mice. Oncogene. 2000;19:773–782. doi: 10.1038/sj.onc.1203321. [DOI] [PubMed] [Google Scholar]
- 385.Raveh T, Droguett G, Horwitz MS, DePinho RA, Kimchi A. DAP kinase activates a p19ARF/p53-mediated apoptotic checkpoint to suppress oncogenic transformation. Nat Cell Biol. 2001;3:1–7. doi: 10.1038/35050500. [DOI] [PubMed] [Google Scholar]
- 386.Raveh T, Kimchi A. DAP kinase-a proapoptotic gene that functions as a tumor suppressor. Exp Cell Res. 2001;264:185–192. doi: 10.1006/excr.2000.5134. [DOI] [PubMed] [Google Scholar]
- 387.Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta Mol Cell Res. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 388.Tanaka H, Matsumura I, Ezoe S, Satoh Y, Sakamaki T, Albanese C, et al. E2F1 and c-Myc potentiate apoptosis through inhibition of NF-kappaB activity that facilitates MnSOD-mediated ROS elimination. Mol Cell. 2002;9:1017–1029. doi: 10.1016/S1097-2765(02)00522-1. [DOI] [PubMed] [Google Scholar]
- 389.Packham G, Cleveland JL. Ornithine decarboxylase is a mediator of c-Myc-induced apoptosis. Mol Cell Biol. 1994;14:5741–5747. doi: 10.1128/mcb.14.9.5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Chandramohan V, Jeay S, Pianetti S, Sonenshein GE. Reciprocal control of forkhead box O 3a and c-Myc via the phosphatidylinositol 3-kinase pathway coordinately regulates p27 Kip1 levels. J Immunol. 2004;172:5522–5527. doi: 10.4049/jimmunol.172.9.5522. [DOI] [PubMed] [Google Scholar]
- 391.Peck B, Ferber EC, Schulze A. Antagonism between FOXO and MYC regulates cellular powerhouse. Front Oncol. 2013;3:96. doi: 10.3389/fonc.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Delpuech O, Griffiths B, East P, Essafi A, Lam EW-F, Burgering B, et al. Induction of Mxi1-SRα by FOXO3a contributes to repression of Myc-dependent gene expression. Mol Cell Biol. 2007;27:4917–4930. doi: 10.1128/MCB.01789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Gan B, Lim C, Chu G, Hua S, Ding Z, Collins M, et al. FoxOs enforce a progression checkpoint to constrain mTORC1-activated renal tumorigenesis. Cancer Cell. 2010;18:472–484. doi: 10.1016/j.ccr.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Amente S, Zhang J, Lubrano Lavadera M, Lania L, Avvedimento EV, Majello B. Myc and PI3K/AKT signaling cooperatively repress FOXO3a-dependent PUMA and GADD45a gene expression. Nucleic Acids Res. 2011;39:9498–9507. doi: 10.1093/nar/gkr638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Jensen KS, Binderup T, Jensen KT, Therkelsen I, Borup R, Nilsson E, et al. FoxO3A promotes metabolic adaptation to hypoxia by antagonizing Myc function. EMBO J. 2011;30:4554–4570. doi: 10.1038/emboj.2011.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Ferber EC, Peck B, Delpuech O, Bell GP, East P, Schulze A. FOXO3a regulates reactive oxygen metabolism by inhibiting mitochondrial gene expression. Cell Death Differ. 2012;19:968–979. doi: 10.1038/cdd.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Fernandez-Vidal A, Mazars A, Manenti S. CDC25A: a rebel within the CDC25 phosphatases family? Anticancer Agents Med Chem. 2008;8:825–831. doi: 10.2174/187152008786847684. [DOI] [PubMed] [Google Scholar]
- 399.Kagaya S, Kitanaka C, Noguchi K, Mochizuki T, Sugiyama A, Asai A, et al. A functional role for death proteases in s-Myc- and c-Myc-mediated apoptosis. Mol Cell Biol. 1997;17:6736–6745. doi: 10.1128/MCB.17.11.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Hoffman B, Liebermann DA. The proto-oncogene c-myc and apoptosis. Oncogene. 1998;17:3351–3357. doi: 10.1038/sj.onc.1202592. [DOI] [PubMed] [Google Scholar]
- 401.Zhang Y, Wang Z, Li X, Magnuson NS. Pim kinase-dependent inhibition of c-Myc degradation. Oncogene. 2008;27:4809–4570. doi: 10.1038/onc.2008.123. [DOI] [PubMed] [Google Scholar]
- 402.Mochizuki T, Kitanaka C, Noguchi K, Muramatsu T, Asai A, Kuchino Y. Physical and functional interactions between Pim-1 kinase and Cdc25A phosphatase. Implications for the Pim-1-mediated activation of the c-Myc signaling pathway. J Biol Chem. 1999;274:18659–18666. doi: 10.1074/jbc.274.26.18659. [DOI] [PubMed] [Google Scholar]
- 403.Jagannathan-Bogdan M, Zon LI. Hematopoiesis. Development. 2013;12:2463–2467. doi: 10.1242/dev.083147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 405.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 406.Reavie L, Della GG, Crusio K, Aranda-Orgilles B, Buckley SM, Thompson B, et al. Regulation of hematopoietic stem cell differentiation by a single ubiquitin ligase-substrate complex. Nat Immunol. 2010;11:207–215. doi: 10.1038/ni.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Wilson A, Murphy MJ, Oskarsson T, Kaloulis K, Bettess MD, Oser GM, et al. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 2004;18:2747–2763. doi: 10.1101/gad.313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Varmus HE. The molecular genetics of cellular oncogenes. Annu Rev Genet. 1984;18:553–612. doi: 10.1146/annurev.ge.18.120184.003005. [DOI] [PubMed] [Google Scholar]
- 409.Huang CY, Bredemeyer AL, Walker LM, Bassing CH, Sleckman BP. Dynamic regulation of c-Myc proto-oncogene expression during lymphocyte development revealed by a GFP-c-Myc knock-in mouse. Eur J Immunol. 2008;38:342–349. doi: 10.1002/eji.200737972. [DOI] [PubMed] [Google Scholar]
- 410.Sanders JA, Schorl C, Patel A, Sedivy JM, Gruppuso PA. Postnatal liver growth and regeneration are independent of c-myc in a mouse model of conditional hepatic c-myc deletion. BMC Physiol. 2012;12:1–15. doi: 10.1186/1472-6793-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci U S A. 1995;92:10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Acosta JC, Ferrándiz N, Bretones G, Torrano V, Blanco R, Richard C, et al. Myc inhibits p27-induced erythroid differentiation of leukemia cells by repressing erythroid master genes without reversing p27-mediated cell cycle arrest. Mol Cell Biol. 2008;28:7286–7295. doi: 10.1128/MCB.00752-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Dose M, Khan I, Guo Z, Kovalovsky D, Krueger A, Von Boehmer H, et al. c-Myc mediates pre-TCR-induced proliferation but not developmental progression. Blood. 2006;108:2669–2677. doi: 10.1182/blood-2006-02-005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Klemsz MJ, Justement LB, Palmer E, Cambier JC. Induction of c-fos and c-myc expression during B cell activation by IL-4 and immunoglobulin binding ligands. J Immunol. 1989;143:1032–1039. [PubMed] [Google Scholar]
- 415.Larsson LG, Schena M, Carlsson M, Sallstrom J, Nilsson K. Expression of the c-myc protein is down-regulated at the terminal stages during in vitro differentiation of B-type chronic lymphocytic leukemia cells. Blood. 1991;77:1025–1032. doi: 10.1182/blood.V77.5.1025.1025. [DOI] [PubMed] [Google Scholar]
- 416.Lindsten T, June CH, Thompson CB. Multiple mechanisms regulate c-myc gene expression during normal T cell activation. EMBO J. 1988;7:2787–2794. doi: 10.1002/j.1460-2075.1988.tb03133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Preston GC, Sinclair LV, Kaskar A, Hukelmann JL, Navarro MN, Ferrero I, et al. Single cell tuning of Myc expression by antigen receptor signal strength and interleukin-2 in T lymphocytes. EMBO J. 2015;34:2008–2024. doi: 10.15252/embj.201490252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Habib T, Park H, Tsang M, De Alborán IM, Nicks A, Wilson L, et al. Myc stimulates B lymphocyte differentiation and amplifies calcium signaling. J Cell Biol. 2007;179:717–731. doi: 10.1083/jcb.200704173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Delgado MD, Albajar M, Gomez-Casares MT, Batlle A, León J. MYC oncogene in myeloid neoplasias. Clin Transl Oncol. 2013;15:87–94. doi: 10.1007/s12094-012-0926-8. [DOI] [PubMed] [Google Scholar]
- 420.Guo Y, Niu C, Breslin P, Tang M, Zhang S, Wei W, et al. c-Myc-mediated control of cell fate in megakaryocyte-erythrocyte progenitors. Blood. 2009;114:2097–2106. doi: 10.1182/blood-2009-01-197947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- 422.Schmidt EV, Pattengale PK, Weir L, Leder P. Transgenic mice bearing the human c-myc gene activated by an immunoglobulin enhancer: a pre-B-cell lymphoma model. Proc Natl Acad Sci U S A. 1988;85:6047–6051. doi: 10.1073/pnas.85.16.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Langdon WY, Harris AW, Cory S, Adams JM. The c-myc oncogene perturbs B lymphocyte development in Eμ-myc transgenic mice. Cell. 1986;47:11–18. doi: 10.1016/0092-8674(86)90361-2. [DOI] [PubMed] [Google Scholar]
- 424.Schmitt CA, Fridman JS, Yang M, Baranov E, Hoffman RM, Lowe SW. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell. 2002;1:289–298. doi: 10.1016/S1535-6108(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 425.Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell. 1999;4:199–207. doi: 10.1016/S1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- 426.Palomo C, Zou X, Nicholson IC, Bützler C, Brüggemann M. B-cell tumorigenesis in mice carrying a yeast artificial chromosome-based immunoglobulin heavy/c-myc translocus is independent of the heavy chain intron enhancer (Eμ) Cancer Res. 1999;59:5625–5628. [PubMed] [Google Scholar]
- 427.Hecht JL, Aster JC. Molecular biology of Burkitt’s lymphoma. J Clin Oncol. 2000;18:3707–3721. doi: 10.1200/JCO.2000.18.21.3707. [DOI] [PubMed] [Google Scholar]
- 428.Magrath I. The pathogenesis of burkitt’s lymphoma. Adv Cancer Res. 1990;55:133–270. doi: 10.1016/S0065-230X(08)60470-4. [DOI] [PubMed] [Google Scholar]
- 429.Sung SP, Joong SK, Tessarollo L, Owens JD, Peng L, Seong SH, et al. Insertion of c-Myc into Igh induces B-cell and plasma-cell neoplasms in mice. Cancer Res. 2005;65:1306–1315. doi: 10.1158/0008-5472.CAN-05-1015. [DOI] [PubMed] [Google Scholar]
- 430.Janz S. Myc translocations in B cell and plasma cell neoplasms. DNA Repair (Amst) 2006;5:9–10. doi: 10.1016/j.dnarep.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 431.Hemann MT, Bric A, Teruya-Feldstein J, Herbst A, Nilsson JA, Cordon-Cardo C, et al. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants. Nature. 2005;436:807–811. doi: 10.1038/nature03845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Yu D, Thomas-Tikhonenko A. A non-transgenic mouse model for B-cell lymphoma: in vivo infection of p53-null bone marrow progenitors by a Myc retrovirus is sufficient for tumorigenesis. Oncogene. 2002;21:1922–1927. doi: 10.1038/sj.onc.1205244. [DOI] [PubMed] [Google Scholar]
- 433.Yu D, Allman D, Goldschmidt MH, Atchison ML, Monroe JG, Thomas-Tikhonenko A. Oscillation between B-lymphoid and myeloid lineages in Myc-induced hematopoietic tumors following spontaneous silencing/reactivation of the EBF/Pax5 pathway. Blood. 2003;101:1950–1955. doi: 10.1182/blood-2002-06-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Kawagoe H, Kandilci A, Kranenburg TA, Grosveld GC. Overexpression of N-Myc rapidly causes acute myeloid leukemia in mice. Cancer Res. 2007;67:10677–10685. doi: 10.1158/0008-5472.CAN-07-1118. [DOI] [PubMed] [Google Scholar]
- 435.Kohnken R, Porcu P, Mishra A. Overview of the use of murine models in leukemia and lymphoma research. Front Oncol. 2017;7:22. doi: 10.3389/fonc.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Sheikh-Zeineddini N, Bashash D, Safaroghli-Azar A, Riyahi N, Shabestari RM, Janzamin E, et al. Suppression of c-Myc using 10058–F4 exerts caspase-3-dependent apoptosis and intensifies the antileukemic effect of vincristine in pre-B acute lymphoblastic leukemia cells. J Cell Biochem. 2019;120:14004–14016. doi: 10.1002/jcb.28675. [DOI] [PubMed] [Google Scholar]
- 437.Allen A, Gill K, Hoehn D, Sulis M, Bhagat G, Alobeid B. C-myc protein expression in B-cell acute lymphoblastic leukemia, prognostic significance? Leuk Res. 2014;36:1061–1066. doi: 10.1016/j.leukres.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 438.Moorman AV, Harrison CJ, Buck GAN, Richards SM, Secker-Walker LM, Martineau M, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109:3189–3197. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- 439.Sawyers CL, Callahan W, Witte ON. Dominant negative MYC blocks transformation by ABL oncogenes. Cell. 1992;70:901–910. doi: 10.1016/0092-8674(92)90241-4. [DOI] [PubMed] [Google Scholar]
- 440.Advani AS, Pendergast AM. Bcr-Abl variants: Biological and clinical aspects. Leuk Res. 2002;26:713–720. doi: 10.1016/S0145-2126(01)00197-7. [DOI] [PubMed] [Google Scholar]
- 441.Afar DEH, Goga A, McLaughlin J, Witte ON, Sawyers CL. Differential complementation of Bcr-Abl point mutants with c-Myc. Science (80- ) 1994;264:424–426. doi: 10.1126/science.8153630. [DOI] [PubMed] [Google Scholar]
- 442.Eswaran J, Sinclair P, Heidenreich O, Irving J, Russell LJ, Hall A, et al. The pre-B-cell receptor checkpoint in acute lymphoblastic leukaemia. Leukemia. 2015;29:1623–1631. doi: 10.1038/leu.2015.113. [DOI] [PubMed] [Google Scholar]
- 443.Köhrer S, Havranek O, Seyfried F, Hurtz C, Coffey GP, Kim E, et al. Pre-BCR signaling in precursor B-cell acute lymphoblastic leukemia regulates PI3K/AKT, FOXO1 and MYC, and can be targeted by SYK inhibition. Leukemia. 2016;30:1246–1254. doi: 10.1038/leu.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Wallington-Beddoe CT, Powell JA, Tong D, Pitson SM, Bradstock KF, Bendall LJ. Sphingosine kinase 2 promotes acute lymphoblastic leukemia by enhancing myc expression. Cancer Res. 2014;74:2803–2815. doi: 10.1158/0008-5472.CAN-13-2732. [DOI] [PubMed] [Google Scholar]
- 445.Britten CD, Garrett-Mayer E, Chin SH, Shirai K, Ogretmen B, Bentz TA, et al. A phase I study of ABC294640, a first-in-class sphingosine kinase-2 inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2017;23:4642–4650. doi: 10.1158/1078-0432.CCR-16-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Wickline ED, Du Y, Stolz DB, Kahn M, Monga SPS. γ-Catenin at adherens junctions: mechanism and biologic implications in hepatocellular cancer after β-catenin knockdown. Neoplasia (United States) 2013;15:421. doi: 10.1593/neo.122098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Luong-Gardiol N, Siddiqui I, Pizzitola I, Jeevan-Raj B, Charmoy M, Huang Y, et al. γ-Catenin-dependent signals maintain BCR-ABL1 + B cell acute lymphoblastic leukemia. Cancer Cell. 2019;35:649–663. doi: 10.1016/j.ccell.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 448.O’hare T, Zabriskie MS, Eiring AM, Deininger MW. Pushing the limits of targeted therapy in chronic myeloid leukaemia. Nat Rev Cancer. 2012;12:513–526. doi: 10.1038/nrc3317. [DOI] [PubMed] [Google Scholar]
- 449.Krysov S, Dias S, Paterson A, Mockridge CI, Potter KN, Smith KA, et al. Surface IgM stimulation induces MEK1/2-dependent MYC expression in chronic lymphocytic leukemia cells. Blood. 2012;119:170–179. doi: 10.1182/blood-2011-07-370403. [DOI] [PubMed] [Google Scholar]
- 450.Yeomans A, Thirdborough SM, Valle-Argos B, Linley A, Krysov S, Hidalgo MS, et al. Engagement of the B-cell receptor of chronic lymphocytic leukemia cells drives global and MYC-specific mRNA translation. Blood. 2016;127:449–457. doi: 10.1182/blood-2015-07-660969. [DOI] [PubMed] [Google Scholar]
- 451.Pelletier J, Graff J, Ruggero D, Sonenberg N. Targeting the eIF4F translation initiation complex: a critical nexus for cancer development. Cancer Res. 2015;75:250–263. doi: 10.1158/0008-5472.CAN-14-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Lin CJ, Cencic R, Mills JR, Robert F, Pelletier J. c-Myc and eIF4F are components of a feedforward loop that links transcription and translation. Cancer Res. 2008;68:5326–5334. doi: 10.1158/0008-5472.CAN-07-5876. [DOI] [PubMed] [Google Scholar]
- 453.Sharma N, Magistroni V, Piazza R, Citterio S, Mezzatesta C, Khandelwal P, et al. BCR/ABL1 and BCR are under the transcriptional control of the MYC oncogene. Mol Cancer. 2015;14:1–11. doi: 10.1186/s12943-015-0407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Xie S, Lin H, Sun T, Arlinghaus RB. Jak2 is involved in c-Myc induction by Bcr-Abl. Oncogene. 2002;21:7137–7146. doi: 10.1038/sj.onc.1205942. [DOI] [PubMed] [Google Scholar]
- 455.Wu SC, Li LS, Kopp N, Montero J, Chapuy B, Yoda A, et al. Activity of the type II JAK2 inhibitor CHZ868 in B cell acute lymphoblastic leukemia. Cancer Cell. 2015;28(29):41. doi: 10.1016/j.ccell.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Rix U, Hantschel O, Dürnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110:4055–4063. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 457.Shinohara H, Taniguchi K, Kumazaki M, Yamada N, Ito Y, Otsuki Y, et al. Anti-cancer fatty-acid derivative induces autophagic cell death through modulation of PKM isoform expression profile mediated by bcr-abl in chronic myeloid leukemia. Cancer Lett. 2015;360:28–38. doi: 10.1016/j.canlet.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 458.Shinohara H, Kumazaki M, Minami Y, Ito Y, Sugito N, Kuranaga Y, et al. Perturbation of energy metabolism by fatty-acid derivative AIC-47 and imatinib in BCR-ABL-harboring leukemic cells. Cancer Lett. 2016;371:1–11. doi: 10.1016/j.canlet.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 459.Shinohara H, Sugito N, Kuranaga Y, Heishima K, Minami Y, Naoe T, et al. Potent antiproliferative effect of fatty-acid derivative AIC-47 on leukemic mice harboring BCR-ABL mutation. Cancer Sci. 2019;110:751–760. doi: 10.1111/cas.13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Borkhardt A, Cazzaniga G, Viehmann S, Valsecchi MG, Ludwig WD, Burci L, et al. Incidence and clinical relevance of TEL/AML1 fusion genes in children with acute lymphoblastic leukemia enrolled in the German and Italian multicenter therapy trials. Blood. 1997;90:571–577. doi: 10.1182/blood.V90.2.571. [DOI] [PubMed] [Google Scholar]
- 461.Schäfer D, Olsen M, Lähnemann D, Stanulla M, Slany R, Schmiegelow K, et al. Five percent of healthy newborns have an ETV6-RUNX1 fusion as revealed by DNA-based GIPFEL screening. Blood. 2018;131:821–826. doi: 10.1182/blood-2017-09-808402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Cazzaniga G, Daniotti M, Tosi S, Giudici G, Aloisi A, Pogliani E, et al. The paired box domain gene PAX5 is fused to ETV6/TEL in an acute lymphoblastic leukemia case. Cancer Res. 2001;61:4666–4670. [PubMed] [Google Scholar]
- 463.Smeenk L, Fischer M, Jurado S, Jaritz M, Azaryan A, Werner B, et al. Molecular role of the PAX 5- ETV 6 oncoprotein in promoting B-cell acute lymphoblastic leukemia. EMBO J. 2017;36:718–735. doi: 10.15252/embj.201695495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Raptis L, Arulanandam R, Geletu M, Turkson J. The R(h)oads to Stat3: Stat3 activation by the Rho GTPases. Exp Cell Res. 2011;317:1787–1795. doi: 10.1016/j.yexcr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Mulloy JC, Cancelas JA, Filippi MD, Kalfa TA, Guo F, Zheng Y. Rho GTPases in hematopoiesis and hemopathies. Blood. 2010;115:936–947. doi: 10.1182/blood-2009-09-198127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Mangolini M, De Boer J, Walf-Vorderwülbecke V, Pieters R, Den Boer ML, Williams O. STAT3 mediates oncogenic addiction to TEL-AML1 in t(12;21) acute lymphoblastic leukemia. Blood. 2013;122:542–549. doi: 10.1182/blood-2012-11-465252. [DOI] [PubMed] [Google Scholar]
- 467.Stoskus M, Vaitkeviciene G, Eidukaite A, Griskevicius L. ETV6/RUNX1 transcript is a target of RNA-binding protein IGF2BP1 in t(12;21)(p13;q22)-positive acute lymphoblastic leukemia. Blood Cells Mol Dis. 2016;57:30–34. doi: 10.1016/j.bcmd.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 468.Stöhr N, Hüttelmaier S. IGF2BP1: a post-transcriptional “driver” of tumor cell migration. Cell Adhes Migr. 2012;6:312–318. doi: 10.4161/cam.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Montaño A, Ordoñez JL, Alonso-Pérez V, Hernández-Sánchez J, Santos S, González T, et al. ETV6/RUNX1 fusion gene abrogation decreases the oncogenicity of tumour cells in a preclinical model of acute lymphoblastic leukaemia. Cells. 2020;9:215. doi: 10.3390/cells9010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Sanjuan-Pla A, Bueno C, Prieto C, Acha P, Stam RW, Marschalek R, et al. Revisiting the biology of infant t(4;11)/MLL-AF4+ B-cell acute lymphoblastic leukemia. Blood. 2015;126:2676–2685. doi: 10.1182/blood-2015-09-667378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Meeker ND, Cherry AM, Bangs CD, Frazer JK. A pediatric B lineage leukemia with coincident MYC and MLL translocations. J Pediatr Hematol Oncol. 2011;33:158–160. doi: 10.1097/MPH.0b013e3181e65c39. [DOI] [PubMed] [Google Scholar]
- 472.Chowdhury T, Brady HJM. Insights from clinical studies into the role of the MLL gene in infant and childhood leukemia. Blood Cells Mol Dis. 2008;40:192–199. doi: 10.1016/j.bcmd.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 473.Xu N, Li YL, Zhou X, Cao R, Li H, Lu QS, et al. CDKN2 gene deletion as poor prognosis predictor involved in the progression of adult B-lineage acute lymphoblastic leukemia patients. J Cancer. 2015;6:1114. doi: 10.7150/jca.11959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Ragusa D, Makarov EM, Britten O, Moralli D, Green CM, Tosi S. The RS4;11 cell line as a model for leukaemia with t(4;11)(q21;q23): revised characterisation of cytogenetic features. Cancer Rep. 2019;2:e1207. doi: 10.1002/cnr2.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Hyrenius-Wittsten A, Pilheden M, Sturesson H, Hansson J, Walsh MP, Song G, et al. De novo activating mutations drive clonal evolution and enhance clonal fitness in KMT2A-rearranged leukemia. Nat Commun. 2018;9:1–13. doi: 10.1038/s41467-018-04180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Neff T, Sinha AU, Kluk MJ, Zhu N, Khattab MH, Stein L, et al. Polycomb repressive complex 2 is required for MLL-AF9 leukemia. Proc Natl Acad Sci U S A. 2012;109:5028–5033. doi: 10.1073/pnas.1202258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Meyer C, Burmeister T, Gröger D, Tsaur G, Fechina L, Renneville A, et al. The MLL recombinome of acute leukemias in 2017. Leukemia. 2018;32:273–284. doi: 10.1038/leu.2017.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Schreiner S, Birke M, García-Cuéllar MP, Zilles O, Greil J, Slany RK. MLL-ENL causes a reversible and myc-dependent block of myelomonocytic cell differentiation. Cancer Res. 2001;61:6480–6486. [PubMed] [Google Scholar]
- 479.Jiang X, Huang H, Li Z, Li Y, Wang X, Gurbuxani S, et al. Blockade of miR-150 maturation by MLL-fusion/MYC/LIN-28 is required for MLL-associated leukemia. Cancer Cell. 2012;22:524–535. doi: 10.1016/j.ccr.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Sacco JJ, Coulson JM, Clague MJ, Urbé S. Emerging roles of deubiquitinases in cancer-associated pathways. IUBMB Life. 2010;62:140–157. doi: 10.1002/iub.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Meyer C, Lopes BA, Caye-Eude A, Cavé H, Arfeuille C, Cuccuini W, et al. Human MLL/KMT2A gene exhibits a second breakpoint cluster region for recurrent MLL–USP2 fusions. Leukemia. 2019;33:2306–2340. doi: 10.1038/s41375-019-0451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478:529–533. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Ott CJ, Kopp N, Bird L, Paranal RM, Qi J, Bowman T, et al. BET bromodomain inhibition targets both c-Myc and IL7R in high-risk acute lymphoblastic leukemia. Blood. 2012;120:2843–2852. doi: 10.1182/blood-2012-02-413021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Luo Z, Lin C, Guest E, Garrett AS, Mohaghegh N, Swanson S, et al. The super elongation complex family of RNA polymerase II elongation factors: gene target specificity and transcriptional output. Mol Cell Biol. 2012;32:2608–2617. doi: 10.1128/MCB.00182-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Luo Z, Lin C, Shilatifard A. The super elongation complex (SEC) family in transcriptional control. Nat Rev Mol Cell Biol. 2012;13:543–547. doi: 10.1038/nrm3417. [DOI] [PubMed] [Google Scholar]
- 487.Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, et al. C-Myc regulates transcriptional pause release. Cell. 2010;141:432–445. doi: 10.1016/j.cell.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Bisgrove DA, Mahmoudi T, Henklein P, Verdin E. Conserved P-TEFb-interacting domain of BRD4 inhibits HIV transcription. Proc Natl Acad Sci U S A. 2007;104:13690–13695. doi: 10.1073/pnas.0705053104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Bowry A, Piberger AL, Rojas P, Saponaro M, Petermann E. BET inhibition induces HEXIM1- and RAD51-dependent conflicts between transcription and replication. Cell Rep. 2018;25:2061–2069. doi: 10.1016/j.celrep.2018.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Dey A, Chao SH, Lane DP. HEXIM1 and the control of transcription elongation: from cancer and inflammation to AIDS and cardiac hypertrophy. Cell Cycle. 2007;6:1856–1863. doi: 10.4161/cc.6.15.4556. [DOI] [PubMed] [Google Scholar]
- 491.Roulin L, Ali A, Masse A, Coudé M-M, Bluteau D, Braun T, et al. Activity of OTX015 (MK-8628), a BET-bromodomain inhibitor, in acute myeloid leukemia (AML) progenitor cells. Blood. 2015;126:2588. doi: 10.1182/blood.V126.23.2588.2588. [DOI] [Google Scholar]
- 492.Astorgues-Xerri L, Vázquez R, Odore E, Rezai K, Kahatt C, Mackenzie S, et al. Insights into the cellular pharmacological properties of the BET-inhibitor OTX015/MK-8628 (birabresib), alone and in combination, in leukemia models. Leuk Lymphoma. 2019;60:3067–3070. doi: 10.1080/10428194.2019.1617860. [DOI] [PubMed] [Google Scholar]
- 493.Coudé MM, Braun T, Berrou J, Dupont M, Bertrand S, Masse A, et al. BET inhibitor OTX015 targets BRD2 and BRD4 and decreases c-MYC in acute leukemia cells. Oncotarget. 2015;6:17698. doi: 10.18632/oncotarget.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Berthon C, Raffoux E, Thomas X, Vey N, Gomez-Roca C, Yee K, et al. Bromodomain inhibitor OTX015 in patients with acute leukaemia: A dose-escalation, phase 1 study. Lancet Haematol. 2016;3:e186–e195. doi: 10.1016/S2352-3026(15)00247-1. [DOI] [PubMed] [Google Scholar]
- 495.Odore E, Lokiec F, Cvitkovic E, Bekradda M, Herait P, Bourdel F, et al. Phase I population pharmacokinetic assessment of the oral bromodomain inhibitor OTX015 in patients with haematologic malignancies. Clin Pharmacokinet. 2016;55:397–405. doi: 10.1007/s40262-015-0327-6. [DOI] [PubMed] [Google Scholar]
- 496.Alqahtani A, Choucair K, Ashraf M, Hammouda DM, Alloghbi A, Khan T, et al. Bromodomain and extra-terminal motif inhibitors: a review of preclinical and clinical advances in cancer therapy. Future Sci OA. 2019;5:FSO372. doi: 10.4155/fsoa-2018-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Siu KT, Ramachandran J, Yee AJ, Eda H, Santo L, Panaroni C, et al. Preclinical activity of CPI-0610, a novel small-molecule bromodomain and extra-terminal protein inhibitor in the therapy of multiple myeloma. Leukemia. 2017;31:1760–1769. doi: 10.1038/leu.2016.355. [DOI] [PubMed] [Google Scholar]
- 498.Kremyanskaya M, Hoffman R, Mascarenhas J, Verstovsek S, Mertz J, Garner F, et al. A Phase 2 study of Cpi-0610, a bromodomain and extraterminal (BET) inhibitor, in patients with myelofibrosis (MF) Blood. 2018;132:5481. doi: 10.1182/blood-2018-99-119157. [DOI] [Google Scholar]
- 499.Vega-García N, Malatesta R, Estella C, Pérez-Jaume S, Esperanza-Cebollada E, Torrebadell M, et al. Paediatric patients with acute leukaemia and KMT2A (MLL) rearrangement show a distinctive expression pattern of histone deacetylases. Br J Haematol. 2018;182:542–553. doi: 10.1111/bjh.15436. [DOI] [PubMed] [Google Scholar]
- 500.José-Enériz ES, Gimenez-Camino N, Agirre X, Prosper F. HDAC inhibitors in acute myeloid leukemia. Cancers (Basel) 2019;11:1794. doi: 10.3390/cancers11111794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Mummery A, Narendran A, Lee K-Y. Targeting epigenetics through histone deacetylase inhibitors in acute lymphoblastic leukemia. Curr Cancer Drug Targets. 2011;11:882–893. doi: 10.2174/156800911796798922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Li L, Osdal T, Ho Y, Chun S, McDonald T, Agarwal P, et al. SIRT1 activation by a c-MYC oncogenic network promotes the maintenance and drug resistance of human FLT3-ITD acute myeloid leukemia stem cells. Cell Stem Cell. 2014;15:431–446. doi: 10.1016/j.stem.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Zehtabcheh S, Yousefi AM, Salari S, Safa M, Momeny M, Ghaffari SH, et al. Abrogation of histone deacetylases (HDACs) decreases survival of chronic myeloid leukemia cells: new insight into attenuating effects of the PI3K/c-Myc axis on panobinostat cytotoxicity. Cell Biol Int. 2021;45:1111–1121. doi: 10.1002/cbin.11557. [DOI] [PubMed] [Google Scholar]
- 504.Suraweera A, O’Byrne KJ, Richard DJ. Combination therapy with histone deacetylase inhibitors (HDACi) for the treatment of cancer: Achieving the full therapeutic potential of HDACi. Front Oncol. 2018;8:92. doi: 10.3389/fonc.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Barneda-Zahonero B, Collazo O, Azagra A, Fernández-Duran I, Serra-Musach J, Islam ABMMK, et al. The transcriptional repressor HDAC7 promotes apoptosis and c-Myc downregulation in particular types of leukemia and lymphoma. Cell Death Dis. 2015;6:e1635. doi: 10.1038/cddis.2014.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Haery L, Thompson RC, Gilmore TD. Histone acetyltransferases and histone deacetylases in B- and T-cell development, physiology and malignancy. Genes Cancer. 2015;6:184. doi: 10.18632/genesandcancer.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Verza FA, Das U, Fachin AL, Dimmock JR, Marins M. Roles of histone deacetylases and inhibitors in anticancer therapy. Cancers (Basel) 2020;12:1664. doi: 10.3390/cancers12061664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Shi J, Whyte WA, Zepeda-Mendoza CJ, Milazzo JP, Shen C, Roe JS, et al. Role of SWI/SNF in acute leukemia maintenance and enhancer-mediated Myc regulation. Genes Dev. 2013;27:2648–2662. doi: 10.1101/gad.232710.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Benetatos L, Benetatou A, Vartholomatos G. Enhancers and MYC interplay in hematopoiesis. J Mol Med. 2020;98:471–481. doi: 10.1007/s00109-020-01891-1. [DOI] [PubMed] [Google Scholar]
- 510.Bahr C, von Palekse L, Uslu V, Remeseiro S, Takayama N, Ng S, et al. A cluster of enhancer modules directs differential MYC expression along the normal and leukemic haematopoietic stem cell hierarchies. Blood. 2017;130:1150. [Google Scholar]
- 511.Horton SJ, Jaques J, Woolthuis C, Van Dijk J, Mesuraca M, Huls G, et al. MLL-AF9-mediated immortalization of human hematopoietic cells along different lineages changes during ontogeny. Leukemia. 2013;27:1116–1126. doi: 10.1038/leu.2012.343. [DOI] [PubMed] [Google Scholar]
- 512.Bahr C, Von Paleske L, Uslu VV, Remeseiro S, Takayama N, Ng SW, et al. A Myc enhancer cluster regulates normal and leukaemic haematopoietic stem cell hierarchies. Nature. 2018;553:515–520. doi: 10.1038/nature25193. [DOI] [PubMed] [Google Scholar]
- 513.Speedy HE, Beekman R, Chapaprieta V, Orlando G, Law PJ, Martín-García D, et al. Insight into genetic predisposition to chronic lymphocytic leukemia from integrative epigenomics. Nat Commun. 2019;10:1–9. doi: 10.1038/s41467-019-11582-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Shuai W, Lin P, Strati P, Patel KP, Routbort MJ, Hu S, et al. Clinicopathological characterization of chronic lymphocytic leukemia with MYD88 mutations: L265P and non-L265P mutations are associated with different features. Blood Cancer J. 2020;10:1–11. doi: 10.1038/s41408-020-00351-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Huh YO, Lin KIC, Vega F, Schlette E, Yin CC, Keating MJ, et al. MYC translocation in chronic lymphocytic leukaemia is associated with increased prolymphocytes and a poor prognosis. Br J Haematol. 2008;142:36–44. doi: 10.1111/j.1365-2141.2008.07152.x. [DOI] [PubMed] [Google Scholar]
- 516.Kuriakose P, Perveen N, Maeda K, Wiktor A, Van Dyke DL. Translocation (8;14)(q24;q32) as the sole cytogenetic abnormality in B-cell prolymphocytic leukemia. Cancer Genet Cytogenet. 2004;150:156–158. doi: 10.1016/j.cancergencyto.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 517.Dai HP, Xue YQ, Zhang J, Wu YF, Pan JL, Wang Y, et al. Translocation t(2;8)(p12;q24) in two patients with B cell chronic lymphocytic leukemia. Acta Haematol. 2009;120:232–236. doi: 10.1159/000203402. [DOI] [PubMed] [Google Scholar]
- 518.Li Y, Hu S, Wang SA, Li S, Huh YO, Tang Z, et al. The clinical significance of 8q24/MYC rearrangement in chronic lymphocytic leukemia. Mod Pathol. 2016;29:444–451. doi: 10.1038/modpathol.2016.35. [DOI] [PubMed] [Google Scholar]
- 519.Rossi D, Spina V, Gaidano G. Biology and treatment of Richter syndrome. Blood. 2018;131:2761–2772. doi: 10.1182/blood-2018-01-791376. [DOI] [PubMed] [Google Scholar]
- 520.Filip D, Mraz M. The role of MYC in the transformation and aggressiveness of ‘indolent’ B-cell malignancies. Leuk Lymphoma. 2020;61:510–524. doi: 10.1080/10428194.2019.1675877. [DOI] [PubMed] [Google Scholar]
- 521.Mihailovich M, Bremang M, Spadotto V, Musiani D, Vitale E, Varano G, et al. MiR-17-92 fine-tunes MYC expression and function to ensure optimal B cell lymphoma growth. Nat Commun. 2015;6:1–15. doi: 10.1038/ncomms9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.De Paoli L, Cerri M, Monti S, Rasi S, Spina V, Bruscaggin A, et al. MGA, a suppressor of MYC, is recurrently inactivated in high risk chronic lymphocytic leukemia. Leuk Lymphoma. 2013;54:1087–1090. doi: 10.3109/10428194.2012.723706. [DOI] [PubMed] [Google Scholar]
- 523.Fabbri G, Holmes AB, Viganotti M, Scuoppo C, Belver L, Herranz D, et al. Common nonmutational NOTCH1 activation in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2017;114:E2911–E2919. doi: 10.1073/pnas.1702564114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Zhang W, Kater AP, Widhopf GF, Chuang HY, Enzler T, James DF, et al. B-cell activating factor and v-Myc myelocytomatosis viral oncogene homolog (c-Myc) influence progression of chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2010;107:18956–18960. doi: 10.1073/pnas.1013420107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Wang WG, Liu ZB, Jiang XN, Lee J, Zhou XY, Li XQ. MYC protein dysregulation is driven by BCR-PI3K signalling in diffuse large B-cell lymphoma. Histopathology. 2017;71:778–785. doi: 10.1111/his.13287. [DOI] [PubMed] [Google Scholar]
- 526.Seda V, Mraz M. B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells. Eur J Haematol. 2015;94:193–205. doi: 10.1111/ejh.12427. [DOI] [PubMed] [Google Scholar]
- 527.Coelho V, Krysov S, Ghaemmaghami AM, Emara M, Potter KN, Johnson P, et al. Glycosylation of surface Ig creates a functional bridge between human follicular lymphoma and microenvironmental lectins. Proc Natl Acad Sci U S A. 2010;107:18587–15892. doi: 10.1073/pnas.1009388107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Radcliffe CM, Arnold JN, Suter DM, Wormald MR, Harvey DJ, Royle L, et al. Human follicular lymphoma cells contain oligomannose glycans in the antigen-binding site of the B-cell receptor. J Biol Chem. 2007;282:7405–7415. doi: 10.1074/jbc.M602690200. [DOI] [PubMed] [Google Scholar]
- 529.Krysov S, Potter KN, Mockridge CI, Coelho V, Wheatley I, Packham G, et al. Surface IgM of CLL cells displays unusual glycans indicative of engagement of antigen in vivo. Blood. 2010;115:4198–4205. doi: 10.1182/blood-2009-12-254847. [DOI] [PubMed] [Google Scholar]
- 530.Ntoufa S, Papakonstantinou N, Apollonio B, Gounari M, Galigalidou C, Fonte E, et al. B cell anergy modulated by TLR1/2 and the miR-17∼92 cluster underlies the indolent clinical course of chronic lymphocytic leukemia stereotyped subset #4. J Immunol. 2016;196:4410–4417. doi: 10.4049/jimmunol.1502297. [DOI] [PubMed] [Google Scholar]
- 531.Olive V, Bennett MJ, Walker JC, Ma C, Jiang I, Cordon-Cardo C, et al. miR-19 is a key oncogenic component of mir-17-92. Genes Dev. 2009;23:2839–2849. doi: 10.1101/gad.1861409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Psathas JN, Doonan PJ, Raman P, Freedman BD, Minn AJ, Thomas-Tikhonenko A. Lymphoid neoplasia: the Myc-miR-17-92 axis amplifies B-cell receptor signaling via inhibition of ITIM proteins—a novel lymphomagenic feed-forward loop. Blood. 2013;122:4220–4229. doi: 10.1182/blood-2012-12-473090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Mraz M, Chen L, Rassenti LZ, Ghia EM, Li H, Jepsen K, et al. MiR-150 influences B-cell receptor signaling in chronic lymphocytic leukemia by regulating expression of GAB1 and FOXP1. Blood. 2014;124:84–95. doi: 10.1182/blood-2013-09-527234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Cerna K, Oppelt J, Chochola V, Musilova K, Seda V, Pavlasova G, et al. MicroRNA miR-34a downregulates FOXP1 during DNA damage response to limit BCR signalling in chronic lymphocytic leukaemia B cells. Leukemia. 2019;33:403–414. doi: 10.1038/s41375-018-0230-x. [DOI] [PubMed] [Google Scholar]
- 535.Mraz M, Malinova K, Kotaskova J, Pavlova S, Tichy B, Malcikova J, et al. miR-34a, miR-29c and miR-17-5p are downregulated in CLL patients with TP53 abnormalities. Leukemia. 2009;23:1159–1163. doi: 10.1038/leu.2008.377. [DOI] [PubMed] [Google Scholar]
- 536.Rozovski U, Keating MJ, Estrov Z. Why Is the immunoglobulin heavy chain gene mutation status a prognostic indicator in chronic lymphocytic leukemia? Acta Haematol. 2018;140:51–54. doi: 10.1159/000491382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Rotbain EC, Frederiksen H, Hjalgrim H, Rostgaard K, Egholm GJ, Zahedi B, et al. IGHV mutational status and outcome for patients with chronic lymphocytic leukemia upon treatment: a danish nationwide population-based study. Haematologica. 2020;105:1621. doi: 10.3324/haematol.2019.220194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.D’Avola A, Drennan S, Tracy I, Henderson I, Chiecchio L, Larrayoz M, et al. Surface IgM expression and function are associated with clinical behavior, genetic abnormalities, and DNA methylation in CLL. Blood. 2016;128:816–826. doi: 10.1182/blood-2016-03-707786. [DOI] [PubMed] [Google Scholar]
- 539.Arruga F, Bracciamà V, Yeomans A, D’Avola A, Coscia M, D’Arena GF, et al. NOTCH1 stabilization By PEST mutations enhances IgM-mediated activity in chronic lymphocytic leukemia. Blood. 2018;132:1832. doi: 10.1182/blood-2018-99-118064. [DOI] [Google Scholar]
- 540.D’Avola A, Yeomans A, Drennan S, Rose-Zerilli M, Strefford JC, Stevenson FK, et al. Global and MYC-specific translation is enhanced in activated chronic lymphocytic leukemia cells carrying NOTCH1 C7541_7542delct mutations. Blood. 2016;128:970. doi: 10.1182/blood.V128.22.970.970. [DOI] [Google Scholar]
- 541.DelPapa B, Baldoni S, Dorillo E, DeFalco F, Rompietti C, Cecchini D, et al. Decreased NOTch1 activation correlates with response to ibrutinib in chronic lymphocytic leukemia. Clin Cancer Res. 2019;25:7540–7553. doi: 10.1158/1078-0432.CCR-19-1009. [DOI] [PubMed] [Google Scholar]
- 542.Jain N, O’Brien S. BCR inhibitor failure in CLL: an unmet need. Blood. 2016;128:2193–2194. doi: 10.1182/blood-2016-09-739664. [DOI] [PubMed] [Google Scholar]
- 543.Mato AR, Nabhan C, Barr PM, Ujjani CS, Hill BT, Lamanna N, et al. Outcomes of CLL patients treated with sequential kinase inhibitor therapy: a real world experience. Blood. 2016;128:2199–2205. doi: 10.1182/blood-2016-05-716977. [DOI] [PubMed] [Google Scholar]
- 544.Kim E, ten Hacken E, Sivina M, Clarke A, Thompson PA, Jain N, et al. The BET inhibitor GS-5829 targets chronic lymphocytic leukemia cells and their supportive microenvironment. Leukemia. 2020;34:1588–1598. doi: 10.1038/s41375-019-0682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Carrà G, Panuzzo C, Morena D, Lingua MF, Fantino C, Brancaccio M, et al. BET inhibitors in chronic lymphocytic leukemia: JQ1 synergizes with venetoclax in promoting apoptosis. Blood. 2017;130:2542. [Google Scholar]
- 546.Burger JA, O’Brien S. Evolution of CLL treatment—from chemoimmunotherapy to targeted and individualized therapy. Nat Rev Clin Oncol. 2018;15:510–527. doi: 10.1038/s41571-018-0037-8. [DOI] [PubMed] [Google Scholar]
- 547.Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982;79:7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Nguyen L, Papenhausen P, Shao H. The role of c-MYC in B-cell lymphomas: diagnostic and molecular aspects. Genes (Basel) 2017;8:116. doi: 10.3390/genes8040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 550.Seegmiller AC, Garcia R, Huang R, Maleki A, Karandikar NJ, Chen W. Simple karyotype and bcl-6 expression predict a diagnosis of Burkitt lymphoma and better survival in IG-MYC rearranged high-grade B-cell lymphomas. Mod Pathol. 2010;23:909–920. doi: 10.1038/modpathol.2010.76. [DOI] [PubMed] [Google Scholar]
- 551.Einerson RR, Law ME, Blair HE, Kurtin PJ, McClure RF, Ketterling RP, et al. Novel FISH probes designed to detect IGK-MYC and IGL-MYC rearrangements in B-cell lineage malignancy identify a new breakpoint cluster region designated BVR2. Leukemia. 2006;20:1790–1799. doi: 10.1038/sj.leu.2404340. [DOI] [PubMed] [Google Scholar]
- 552.Bemark M, Neuberger MS. The c-MYC allele that is translocated into the IgH locus undergoes constitutive hypermutation in a Burkitt’s lymphoma line. Oncogene. 2000;19:3404–3410. doi: 10.1038/sj.onc.1203686. [DOI] [PubMed] [Google Scholar]
- 553.Cesarman E, Dalla-Favera R, Bentley D, Groudine M. Mutations in the first exon are associated with altered transcription of c-myc in Burkitt lymphoma. Science (80- ) 1987;238:1272–1275. doi: 10.1126/science.3685977. [DOI] [PubMed] [Google Scholar]
- 554.Rabbitts TH, Forster A, Hamlyn P, Baer R. Effect of somatic mutation within translocated c-myc genes in Burkitt’s lymphoma. Nature. 1984;309:592–597. doi: 10.1038/309592a0. [DOI] [PubMed] [Google Scholar]
- 555.Schmitz R, Young RM, Ceribelli M, Jhavar S, Xiao W, Zhang M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490:116–120. doi: 10.1038/nature11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Love C, Sun Z, Jima D, Li G, Zhang J, Miles R, et al. The genetic landscape of mutations in Burkitt lymphoma. Nat Genet. 2012;44:1321. doi: 10.1038/ng.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Pan L, Sato S, Frederick JP, Sun X-H, Zhuang Y. Impaired immune responses and B-cell proliferation in mice lacking the Id3 gene. Mol Cell Biol. 1999;19:5969–5980. doi: 10.1128/MCB.19.9.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Schiffman JD, Lorimer PD, Rodic V, Jahromi MS, Downie JM, Bayerl MG, et al. Genome wide copy number analysis of paediatric Burkitt lymphoma using formalin-fixed tissues reveals a subset with gain of chromosome 13q and corresponding miRNA over expression. Br J Haematol. 2011;155:477–486. doi: 10.1111/j.1365-2141.2011.08883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Dinneen K, Timlin DM, O’Hare K, Walker J, Castriciano G, Connolly Y, et al. Incidence of single hit Bcl-2 and Bcl-6 rearrangements in DLBCL: the Irish experience. J Clin Pathol. 2020;73:689–690. doi: 10.1136/jclinpath-2020-206725. [DOI] [PubMed] [Google Scholar]
- 561.Valera A, López-Guillermo A, Cardesa-Salzmann T, Climent F, González-Barca E, Mercadal S, et al. MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica. 2013;98:1554. doi: 10.3324/haematol.2013.086173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Martelli AM, Evangelisti C, Paganelli F, Chiarini F, McCubrey JA. GSK-3: a multifaceted player in acute leukemias. Leukemia. 2021. 10.1038/s41375-021-01243-z. [DOI] [PubMed]
- 563.Pfeifer M, Grau M, Lenze D, Wenzel SS, Wolf A, Wollert-Wulf B, et al. PTEN loss defines a PI3K/AKT pathway-dependent germinal center subtype of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2013;110:12420–12425. doi: 10.1073/pnas.1305656110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Tagawa H, Karube K, Tsuzuki S, Ohshima K, Seto M. Synergistic action of the microRNA-17 polycistron and Myc in aggressive cancer development. Cancer Sci. 2007;98:1482–1490. doi: 10.1111/j.1349-7006.2007.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Friedberg JW. How I treat double-hit lymphoma. Blood. 2017;130:590–596. doi: 10.1182/blood-2017-04-737320. [DOI] [PubMed] [Google Scholar]
- 566.Ziemba JB, Wolf Z, Weinstock M, Asakrah S. Double-hit and triple-hit follicular lymphoma. Am J Clin Pathol. 2020;153:672–685. doi: 10.1093/ajcp/aqz208. [DOI] [PubMed] [Google Scholar]
- 567.Grimm KE, O’Malley DP. Aggressive B cell lymphomas in the 2017 revised WHO classification of tumors of hematopoietic and lymphoid tissues. Ann Diagn Pathol. 2019;38:6–10. doi: 10.1016/j.anndiagpath.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 568.Gebauer N, Bernard V, Gebauer W, Thorns C, Feller AC, Merz H. TP53 mutations are frequent events in double-hit B-cell lymphomas with MYC and BCL2 but not MYC and BCL6 translocations. Leuk Lymphoma. 2015;56:179–185. doi: 10.3109/10428194.2014.907896. [DOI] [PubMed] [Google Scholar]
- 569.Li W, Gupta SK, Han W, Kundson RA, Nelson S, Knutson D, et al. Targeting MYC activity in double-hit lymphoma with MYC and BCL2 and/or BCL6 rearrangements with epigenetic bromodomain inhibitors. J Hematol Oncol. 2019;12:1–13. doi: 10.1186/s13045-018-0686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Freedman A, Jacobsen E. Follicular lymphoma: 2020 update on diagnosis and management. Am J Hematol. 2020;95:316–327. doi: 10.1002/ajh.25696. [DOI] [PubMed] [Google Scholar]
- 571.Al-Tourah AJ, Gill KK, Chhanabhai M, Hoskins PJ, Klasa RJ, Savage KJ, et al. Population-based analysis of incidence and outcome of transformed non-hodgkin’s lymphoma. J Clin Oncol. 2008;26:5165–5169. doi: 10.1200/JCO.2008.16.0283. [DOI] [PubMed] [Google Scholar]
- 572.Aukema SM, van Pel R, Nagel I, Bens S, Siebert R, Rosati S, et al. MYC expression and translocation analyses in low-grade and transformed follicular lymphoma. Histopathology. 2017;71:960–971. doi: 10.1111/his.13316. [DOI] [PubMed] [Google Scholar]
- 573.Chisholm KM, Bangs CD, Bacchi CE, Molina-Kirsch H, Cherry A, Natkunam Y. Expression profiles of MYC protein and MYC gene rearrangement in lymphomas. Am J Surg Pathol. 2015;39:294–303. doi: 10.1097/PAS.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 574.Tadros S, Green MR. Genomic drivers in follicular lymphoma. In: Fowler NH, editor. Follicular lymphoma curr manag nov approaches [Internet]. Cham: Springer International Publishing; 2020. p. 47–64. Available from: 10.1007/978-3-030-26211-2_3.
- 575.Hanel W, Epperla N. Emerging therapies in mantle cell lymphoma. J Hematol Oncol. 2020;13:1–18. doi: 10.1186/s13045-020-00914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Yatabe Y, Suzuki R, Tobinai K, Matsuno Y, Ichinohasama R, Okamoto M, et al. Significance of cyclin D1 overexpression for the diagnosis of mantle cell lymphoma: a clinicopathologic comparison of cyclin D1-positive MCL and cyclin D1-negative MCL-like B-cell lymphoma. Blood. 2000;95:2253–2261. [PubMed] [Google Scholar]
- 577.Wang M, Sun L, Qian J, Han X, Zhang L, Lin P, et al. Cyclin D1 as a universally expressed mantle cell lymphoma-associated tumor antigen for immunotherapy. Leukemia. 2009;23:1320–1328. doi: 10.1038/leu.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Zhou J, Hu L, Zuo M, Zhou Y, Li G, Zhang X. An uncommon case of double-hit mantle cell lymphoma that demonstrates a transformation process. Am J Clin Pathol. 2020;153:49–57. doi: 10.1093/ajcp/aqz133. [DOI] [PubMed] [Google Scholar]
- 579.Setoodeh R, Schwartz S, Papenhausen P, Zhang L, Sagatys EM, Moscinski LC, et al. Double-hit mantle cell lymphoma with MYC gene rearrangement or amplification: a report of four cases and review of the literature. Int J Clin Exp Pathol. 2013;6:155. [PMC free article] [PubMed] [Google Scholar]
- 580.Kolodziej M, Jesionek-Kupnicka D, Braun M, Atamanyuk V, Sloniec S, Cebulski J, et al. Classification of aggressive and classic mantle cell lymphomas using synchrotron Fourier Transform Infrared microspectroscopy. Sci Rep. 2019;9:1–8. doi: 10.1038/s41598-019-49326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Royo C, Salaverria I, Hartmann EM, Rosenwald A, Campo E, Beà S. The complex landscape of genetic alterations in mantle cell lymphoma. In: Seminars in cancer biology. 2011. [DOI] [PubMed]
- 582.Hao S, Sanger W, Onciu M, Lai R, Schlette EJ, Medeiros LJ. Mantle cell lymphoma with 8q24 chromosomal abnormalities: a report of 5 cases with blastoid features. Mod Pathol. 2002;15:1266–1272. doi: 10.1097/01.MP.0000037310.82136.99. [DOI] [PubMed] [Google Scholar]
- 583.Hu Z, Medeiros LJ, Chen Z, Chen W, Li S, Konoplev SN, et al. Mantle cell lymphoma with MYC rearrangement: a report of 17 patients. Am J Surg Pathol. 2017;41:216–224. doi: 10.1097/PAS.0000000000000758. [DOI] [PubMed] [Google Scholar]
- 584.Sander B, Quintanilla-Martinez L, Ott G, Xerri L, Kuzu I, Chan JKC, et al. Mantle cell lymphoma—a spectrum from indolent to aggressive disease. Virchows Arch. 2016;468:245–257. doi: 10.1007/s00428-015-1840-6. [DOI] [PubMed] [Google Scholar]
- 585.Swerdlow SH, Campo E, Pileri SA, Lee Harris N, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125:2323–2330. doi: 10.1182/blood-2014-10-567479. [DOI] [PubMed] [Google Scholar]
- 587.Li YJ, Li JW, Chen KL, Li J, Zhong MZ, Liu XL, et al. HIV-negative plasmablastic lymphoma: report of 8 cases and a comprehensive review of 394 published cases. Blood Res. 2020;55:49. doi: 10.5045/br.2020.55.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Yamada T, Kitamura N, Sasabe E, Yamamoto T. Plasmablastic lymphoma of the upper gingiva in an HIV-negative elderly patient. Oral Maxillofac Surg Cases. 2015;1:19–24. doi: 10.1016/j.omsc.2015.05.002. [DOI] [Google Scholar]
- 589.Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–62. doi: 10.1016/S1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 590.Sciammas R, Davis MM. Modular nature of blimp-1 in the regulation of gene expression during b cell maturation. J Immunol. 2004;172:5427–5440. doi: 10.4049/jimmunol.172.9.5427. [DOI] [PubMed] [Google Scholar]
- 591.Montes-Moreno S, Martinez-Magunacelaya N, Zecchini-Barrese T, De Villambrosía SG, Linares E, Ranchal T, et al. Plasmablastic lymphoma phenotype is determined by genetic alterations in MYC and PRDM1. Mod Pathol. 2017;30:85–94. doi: 10.1038/modpathol.2016.162. [DOI] [PubMed] [Google Scholar]
- 592.Valera A, Balagué O, Colomo L, Martínez A, Delabie J, Taddesse-Heath L, et al. IG/MYC rearrangements are the main cytogenetic alteration in plasmablastic lymphomas. Am J Surg Pathol. 2010;34:1686. doi: 10.1097/PAS.0b013e3181f3e29f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Taddesse-Heath L, Meloni-Ehrig A, Scheerle J, Kelly JC, Jaffe ES. Plasmablastic lymphoma with MYC translocation: evidence for a common pathway in the generation of plasmablastic features. Mod Pathol. 2010;23:991–999. doi: 10.1038/modpathol.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Simonitsch-Klupp I, Hauser I, Ott G, Drach J, Ackermann J, Kaufmann J, et al. Diffuse large B-cell lymphomas with plasmablastic/plasmacytoid features are associated with TP53 deletions and poor clinical outcome. Leukemia. 2004;18:146–155. doi: 10.1038/sj.leu.2403206. [DOI] [PubMed] [Google Scholar]
- 595.Misund K, Keane N, Stein CK, Asmann YW, Day G, Welsh S, et al. MYC dysregulation in the progression of multiple myeloma. Leukemia. 2020;34:322–326. doi: 10.1038/s41375-019-0543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Kuehl WM, Bergsagel PL. Multiple myeloma: evolving genetic events and host interactions. Nat Rev Cancer. 2002;2:175–187. doi: 10.1038/nrc746. [DOI] [PubMed] [Google Scholar]
- 597.Abdallah N, Baughn LB, Vincent Rajkumar S, Kapoor P, Gertz MA, Dispenzieri A, et al. Implications of MYC rearrangements in newly diagnosed multiple myeloma. Clin Cancer Res. 2020;26:6581–6588. doi: 10.1158/1078-0432.CCR-20-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Jovanović KK, Roche-Lestienne C, Ghobrial IM, Facon T, Quesnel B, Manier S. Targeting MYC in multiple myeloma. Leukemia. 2018;32:1295–1306. doi: 10.1038/s41375-018-0036-x. [DOI] [PubMed] [Google Scholar]
- 599.Manier S, Huynh D, Shen YJ, Zhou J, Yusufzai T, Salem KZ, et al. Inhibiting the oncogenic translation program is an effective therapeutic strategy in multiple myeloma. Sci Transl Med. 2017;9:eaal2668. doi: 10.1126/scitranslmed.aal2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Leon J, Ferrandiz N, Acosta JC, Delgado MD. Inhibition of cell differentiation: a critical mechanism for MYC-mediated carcinogenesis? Cell Cycle. 2009;8:1148–1157. doi: 10.4161/cc.8.8.8126. [DOI] [PubMed] [Google Scholar]
- 601.Dmitrovsky E, Kuehl WM, Hollis GF, Kirsch IR, Bender TP, Segal S. Expression of a transfected human c-myconcogene inhibits differentiation of a mouse erythroleukaemia cell line. Nature. 1986;322:748–750. doi: 10.1038/322748a0. [DOI] [PubMed] [Google Scholar]
- 602.Delgado MD, Lerga A, Cañelles M, Gómez-Casares MT, León J. Differential regulation of Max and role of c-Myc during erythroid and myelomonocytic differentiation of K562 cells. Oncogene. 1995;10:1659–1666. [PubMed] [Google Scholar]
- 603.Bahram F, Wu S, Öberg F, Lüscher B, Larsson L-G. Regulation posttranslational of Myc function in response to phorbol ester/interferon-γ–induced differentiation of v-Myc–transformed U-937 monoblasts. Blood. 1999;93:3900–3912. doi: 10.1182/blood.V93.11.3900. [DOI] [PubMed] [Google Scholar]
- 604.Uribesalgo I, Buschbeck M, Gutiérrez A, Teichmann S, Demajo S, Kuebler B, et al. E-box-independent regulation of transcription and differentiation by MYC. Nat Cell Biol. 2011;13:1443–1449. doi: 10.1038/ncb2355. [DOI] [PubMed] [Google Scholar]
- 605.Albajar M, Gómez-Casares MT, Llorca J, Mauleon I, Vaqué JP, Acosta JC, et al. MYC in chronic myeloid leukemia: Induction of aberrant DNA synthesis and association with poor response to imatinib. Mol Cancer Res. 2011;9:564–576. doi: 10.1158/1541-7786.MCR-10-0356. [DOI] [PubMed] [Google Scholar]
- 606.Skoda RC, Tsai SF, Orkin SH, Leder P. Expression of c-MYC under the control of GATA-1 regulatory sequences causes erythroleukemia in transgenic mice. J Exp Med. 1995;181:1603–1613. doi: 10.1084/jem.181.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Luo H, Li Q, O’Neal J, Kreisel F, Le Beau MM, Tomasson MH. c-Myc rapidly induces acute myeloid leukemia in mice without evidence of lymphoma-associated antiapoptotic mutations. Blood. 2005;106:2452–2461. doi: 10.1182/blood-2005-02-0734. [DOI] [PubMed] [Google Scholar]
- 608.Farhadi E, Safa M, Sharifi AM, Bashash D. PRIMA-1 induces caspase-mediated apoptosis in acute promyelocytic leukemia NB4 cells by inhibition of nuclear factor-κB and downregulation of Bcl-2, XIAP, and c-Myc. Anticancer Drugs. 2017;28:51–58. doi: 10.1097/CAD.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 609.Tang G, Hu S, Wang SA, Xie W, Lin P, Xu J, et al. t(3;8)(q26.2;q24) often leads to MECOM/MYC rearrangement and is commonly associated with therapy-related myeloid neoplasms and/or disease progression. J Mol Diagn. 2019;21:343–351. doi: 10.1016/j.jmoldx.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 610.Smith SC, Qdaisat TZS, Althof PA, Dave BJ, Sanmann JN. MECOM rearrangement involving the MYC locus: two additional patients with the rare translocation, t(3;8)(q26.2;q24), and molecular review. Leuk Res. 2020;95:106387. doi: 10.1016/j.leukres.2020.106387. [DOI] [PubMed] [Google Scholar]
- 611.Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–2221. doi: 10.1056/NEJMoa1516192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Nanbakhsh A, Pochon C, Mallavialle A, Amsellem S, Bourhis JH, Chouaib S. C-Myc regulates expression of NKG2D ligands ULBP1/2/3 in AML and modulates their susceptibility to NK-mediated lysis. Blood. 2014;123:3585–3595. doi: 10.1182/blood-2013-11-536219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Fauriat C, Olive D. AML drug resistance: C-Myc comes into play. Blood. 2014;123:3528–3530. doi: 10.1182/blood-2014-04-566711. [DOI] [PubMed] [Google Scholar]
- 614.Brondfield S, Umesh S, Corella A, Zuber J, Rappaport AR, Gaillard C, et al. Direct and indirect targeting of MYC to treat acute myeloid leukemia. Cancer Chemother Pharmacol. 2015;76:35–46. doi: 10.1007/s00280-015-2766-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Dickinson M, Kamdar M, Huntly BJ, Fernández De Larrea C, Cordoba R, Mateos M-V, et al. A phase i study of molibresib (GSK525762), a selective bromodomain (BRD) and extra terminal protein (BET) inhibitor: results from part 1 of a phase I/II open label single agent study in subjects with non-Hodgkin’s lymphoma (NHL) Blood. 2018;132:1682. doi: 10.1182/blood-2018-99-117089. [DOI] [Google Scholar]
- 616.Wang Z, Guan W, Wang M, Chen J, Zhang L, Xiao Y, et al. AML1-ETO inhibits acute myeloid leukemia immune escape by CD48. Leuk Lymphoma. 2021;62:937–943. doi: 10.1080/10428194.2020.1849680. [DOI] [PubMed] [Google Scholar]
- 617.Biernacki MA, Foster KA, Woodward KB, Coon ME, Cummings C, Cunningham TM, et al. CBFB-MYH11 fusion neoantigen enables T cell recognition and killing of acute myeloid leukemia. J Clin Invest. 2020;130:5127–5141. doi: 10.1172/JCI137723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Liquori A, Ibañez M, Sargas C, Sanz MÁ, Barragán E, Cervera J. Acute promyelocytic leukemia: a constellation of molecular events around a single PML-RARA fusion gene. Cancers (Basel) 2020;12:624. doi: 10.3390/cancers12030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Mendes A, Fahrenkrog B. NUP214 in leukemia: it’s more than transport. Cells. 2019;8:76. doi: 10.3390/cells8010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Yamaoka A, Suzuki M, Katayama S, Orihara D, Engel JD, Yamamoto M. EVI1 and GATA2 misexpression induced by inv(3)(q21q26) contribute to megakaryocyte-lineage skewing and leukemogenesis. Blood Adv. 2020;4:1722–1736. doi: 10.1182/bloodadvances.2019000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Nakano Y, Yamasaki K, Otsuka Y, et al. Acute myeloid leukemia with RBM15-MKL1 presenting as severe hepatic failure. Glob Pediatr Health. 2017;4:2333794X16689011. 10.1177/2333794X16689011. [DOI] [PMC free article] [PubMed]
- 622.Kulemina O, Siordia N, Bogdanov K, Alexeeva J, Girshova L, Lomaia E, et al. BCR-ABL1+ AML de novo and CBF-Leukemia At Relapse: Game Of Clones. Blood. 2019;134:5138. doi: 10.1182/blood-2019-129815. [DOI] [Google Scholar]
- 623.Numata A, Kwok HS, Kawasaki A, Li J, Zhou QL, Kerry J, et al. The basic helix-loop-helix transcription factor SHARP1 is an oncogenic driver in MLL-AF6 acute myelogenous leukemia. Nat Commun. 2018;9:1–16. doi: 10.1038/s41467-018-03854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Anstee NS, Bilardi RA, Ng AP, Xu Z, Robati M, Vandenberg CJ, et al. Impact of elevated anti-apoptotic MCL-1 and BCL-2 on the development and treatment of MLL-AF9 AML in mice. Cell Death Differ. 2019;26:1316–1331. doi: 10.1038/s41418-018-0209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Kingsley MC, Riedel SS, Xie HM, Stabler SP, Pastuer T, Bernt KM. Tight regulation of H3K79 methylation levels in KMT2A-rearranged AML. Blood. 2018;132:3884. doi: 10.1182/blood-2018-99-114784. [DOI] [Google Scholar]
- 626.Mohanty S, Jyotsana N, Sharma A, Kloos A, Gabdoulline R, Othman B, et al. Targeted inhibition of the nup98-nsd1 fusion oncogene in acute myeloid leukemia. Cancers (Basel) 2020;12:2766. doi: 10.3390/cancers12102766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Di Nardo CD, Cortes JE. Mutations in AML: prognostic and therapeutic implications. Hematology. 2016;1:348–355. doi: 10.1182/asheducation-2016.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Brown AL, Hahn CN, Scott HS. Secondary leukemia in patients with germline transcription factor mutations (RUNX1, GATA2, CEBPA) Blood. 2020;136:24–35. doi: 10.1182/blood.2019000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Welch JS. Patterns of mutations in TP53 mutated AML. Best Pract Res Clin Haematol. 2018;31:379–383. doi: 10.1016/j.beha.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Visconte V, Nakashima MO, Rogers HJ. Mutations in splicing factor genes in myeloid malignancies: significance and impact on clinical features. Cancers (Basel) 2019;11:1844. doi: 10.3390/cancers11121844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Falini B, Brunetti L, Martelli MP. How I diagnose and treat NPM1-mutated AML. Blood. 2021;137:589–599. doi: 10.1182/blood.2020008211. [DOI] [PubMed] [Google Scholar]
- 632.Fisher JB, McNulty M, Burke MJ, Crispino JD, Rao S. Cohesin mutations in myeloid malignancies. Trends Cancer. 2017;3:282–293. doi: 10.1016/j.trecan.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Lavallée VP, Lemieux S, Boucher G, Gendron P, Boivin I, Girard S, et al. Identification of MYC mutations in acute myeloid leukemias with NUP98-NSD1 translocations. Leukemia. 2016;30:1621–1624. doi: 10.1038/leu.2016.19. [DOI] [PubMed] [Google Scholar]
- 634.Bulaeva E, Pellacani D, Nakamichi N, Hammond CA, Beer P, Lorzadeh A, et al. MYC-induced human acute myeloid leukemia requires a continuing IL3/GM-CSF co-stimulus. Blood. 2020;136:2764–2773. doi: 10.1182/blood.2020006374. [DOI] [PubMed] [Google Scholar]
- 635.Mudgapalli N, Nallasamy P, Chava H, Chava S, Pathania AS, Gunda V, et al. The role of exosomes and MYC in therapy resistance of acute myeloid leukemia: challenges and opportunities. Mol Aspects Med. 2019;70:21–32. doi: 10.1016/j.mam.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Beverly LJ, Varmus HE. MYC-induced myeloid leukemogenesis is accelerated by all six members of the antiapoptotic BCL family. Oncogene. 2009;28:1274–1279. doi: 10.1038/onc.2008.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Xiang Z, Luo H, Payton JE, Cain J, Ley TJ, Opferman JT, et al. Mcl1 haploinsufficiency protects mice from Myc-induced acute myeloid leukemia. J Clin Invest. 2010;120:2109–2118. doi: 10.1172/JCI39964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Müller-Tidow C, Steffen B, Cauvet T, Tickenbrock L, Ji P, Diederichs S, et al. Translocation products in acute myeloid leukemia activate the wnt signaling pathway in hematopoietic cells. Mol Cell Biol. 2004;24:2890–2904. doi: 10.1128/MCB.24.7.2890-2904.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Puccetti E, Ruthardt M. Acute promyelocytic leukemia: PML/RARα and the leukemic stem cell. Leukemia. 2004;185:1169–1175. doi: 10.1038/sj.leu.2403367. [DOI] [PubMed] [Google Scholar]
- 640.Gruszka AM, Valli D, Alcalay M. Wnt signalling in acute myeloid leukaemia. Cells. 2019;8:1403. doi: 10.3390/cells8111403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Chong PSY, Zhou J, Chooi JY, Chan ZL, Toh SHM, Tan TZ, et al. Non-canonical activation of β-catenin by PRL-3 phosphatase in acute myeloid leukemia. Oncogene. 2019;38:1508–1519. doi: 10.1038/s41388-018-0526-3. [DOI] [PubMed] [Google Scholar]
- 642.Zhou J, Bi C, Chng WJ, Cheong LL, Liu SC, Mahara S, et al. PRL-3, a metastasis associated tyrosine phosphatase, is involved in FLT3-ITD signaling and implicated in anti-aml therapy. PLoS ONE. 2011;6:e19798. doi: 10.1371/journal.pone.0019798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Ji H, Chen L, Xing Y, Li S, Dai J, Zhao P, et al. CD82 supports survival of childhood acute myeloid leukemia cells via activation of Wnt/β-catenin signaling pathway. Pediatr Res. 2019;85:1024–1031. doi: 10.1038/s41390-019-0370-3. [DOI] [PubMed] [Google Scholar]
- 644.Xu Y, Man N, Karl D, Martinez C, Liu F, Sun J, et al. TAF1 plays a critical role in AML1-ETO driven leukemogenesis. Nat Commun. 2019;10:1–15. doi: 10.1038/s41467-018-07882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Mäkelä E, Löyttyniemi E, Salmenniemi U, Kauko O, Varila T, Kairisto V, et al. Arpp19 promotes Myc and Cip2a expression and associates with patient relapse in acute myeloid leukemia. Cancers (Basel) 2019;11:1774. doi: 10.3390/cancers11111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.V100.1.59. [DOI] [PubMed] [Google Scholar]
- 647.Kajiguchi T, Chung EJ, Lee S, Stine A, Kiyoi H, Naoe T, et al. FLT3 regulates β-catenin tyrosine phosphorylation, nuclear localization, and transcriptional activity in acute myeloid leukemia cells. Leukemia. 2007;21:2476–2484. doi: 10.1038/sj.leu.2404923. [DOI] [PubMed] [Google Scholar]
- 648.Jiang X, Mak PY, Mu H, Tao W, Mak DH, Kornblau S, et al. Disruption of wnt/b-catenin exerts antileukemia activity and synergizes with flt3 inhibition in flt3-mutant acute myeloid leukemia. Clin Cancer Res. 2018;24:2417–2429. doi: 10.1158/1078-0432.CCR-17-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Lee JK, Scarpa M, Kapoor S, Baer MR. Abstract 2056: Combined FLT3 and Pim kinase inhibitor treatment downregulates c-Myc early in apoptosis induction in acute myeloid leukemia with FLT3-ITD. Cancer Res. 2019;79:2056–2056. [Google Scholar]
- 650.Scarpa M, Singh P, Kapoor S, Lee JK, Niyongere S, Narla G, et al. PP2A activators enhance efficacy of FLT3 inhibitors in FLT3-ITD acute myeloid leukemia cells through AKT Inactivation-dependent Pim-1 and c-Myc proteasomal degradation. Blood. 2019;134:1276. doi: 10.1182/blood-2019-129011. [DOI] [Google Scholar]
- 651.Ge Y, Schuster MB, Pundhir S, Rapin N, Bagger FO, Sidiropoulos N, et al. The splicing factor RBM25 controls MYC activity in acute myeloid leukemia. Nat Commun. 2019;10:1–14. doi: 10.1038/s41467-018-07882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Verhaak RGW, Bafna V, Mischel PS. Extrachromosomal oncogene amplification in tumour pathogenesis and evolution. Nat Rev Cancer. 2019;19:283–288. doi: 10.1038/s41568-019-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Mitelman F, Johansson BMF. Mitelman database of chromosome aberrations and gene fusions in cancer. Ann Arbor: University Library; 2020. [Google Scholar]
- 654.Huh YO, Tang G, Talwalkar SS, Khoury JD, Ohanian M, Bueso-Ramos CE, et al. Double minute chromosomes in acute myeloid leukemia, myelodysplastic syndromes, and chronic myelomonocytic leukemia are associated with micronuclei, MYC or MLL amplification, and complex karyotype. Cancer Genet. 2016;209:313–320. doi: 10.1016/j.cancergen.2016.05.072. [DOI] [PubMed] [Google Scholar]
- 655.Amin AJ, Shaw M, Tadros J, Benn H, Maroules M. Double minute chromosome in acute myeloid leukemia. Blood Am Soc Hematol. 2006;108:4428–4428. [Google Scholar]
- 656.L’Abbate A, Tolomeo D, Cifola I, Severgnini M, Turchiano A, Augello B, et al. MYC-containing amplicons in acute myeloid leukemia: genomic structures, evolution, and transcriptional consequences. Leukemia. 2018;32:2152–2166. doi: 10.1038/s41375-018-0033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Boddu P, Chihara D, Masarova L, Pemmaraju N, Patel KP, Verstovsek S. The co-occurrence of driver mutations in chronic myeloproliferative neoplasms. Ann Hematol. 2018;97:2071–2080. doi: 10.1007/s00277-018-3402-x. [DOI] [PubMed] [Google Scholar]
- 658.Ghalesardi OK, Khosravi A, Azizi E, Ahmadi SE, Hajifathali A, Bonakchi H, et al. The prognostic importance of BCR-ABL transcripts in chronic myeloid leukemia: a systematic review and meta-analysis. Leuk Res. 2021;101:106512. doi: 10.1016/j.leukres.2021.106512. [DOI] [PubMed] [Google Scholar]
- 659.Pan C, Olsen JV, Daub H, Mann M. Global effects of kinase inhibitors on signaling networks revealed by quantitative phosphoproteomics. Mol Cell Proteomics. 2009;8:2796–2808. doi: 10.1074/mcp.M900285-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Lucas CM, Harris RJ, Giannoudis A, Copland M, Slupsky JR, Clark RE. Cancerous inhibitor of PP2A (CIP2A) at diagnosis of chronic myeloid leukemia is a critical determinant of disease progression. Blood. 2011;117:6660–6668. doi: 10.1182/blood-2010-08-304477. [DOI] [PubMed] [Google Scholar]
- 661.Porro A, Iraci N, Soverini S, Diolaiti D, Gherardi S, Terragna C, et al. c-MYC oncoprotein dictates transcriptional profiles of ATP-binding cassette transporter genes in chronic myelogenous leukemia CD34 + hematopoietic progenitor cells. Mol Cancer Res. 2011;9:1054–1066. doi: 10.1158/1541-7786.MCR-10-0510. [DOI] [PubMed] [Google Scholar]
- 662.Giannoudis A, Davies A, Harris RJ, Lucas CM, Pirmohamed M, Clark RE. The clinical significance of ABCC3 as an imatinib transporter in chronic myeloid leukaemia. Leukemia. 2014;28:1360–1363. doi: 10.1038/leu.2014.38. [DOI] [PubMed] [Google Scholar]
- 663.Beretta GL, Cassinelli G, Pennati M, Zuco V, Gatti L. Overcoming ABC transporter-mediated multidrug resistance: the dual role of tyrosine kinase inhibitors as multitargeting agents. Eur J Med Chem. 2017;142:271–289. doi: 10.1016/j.ejmech.2017.07.062. [DOI] [PubMed] [Google Scholar]
- 664.Reavie L, Buckley SM, Loizou E, Takeishi S, Aranda-Orgilles B, Ndiaye-Lobry D, et al. Regulation of c-Myc ubiquitination controls chronic myelogenous leukemia initiation and progression. Cancer Cell. 2013;23:362–375. doi: 10.1016/j.ccr.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Yeh CH, Bellon M, Nicot C. FBXW7: A critical tumor suppressor of human cancers. Mol Cancer. 2018;17:1–19. doi: 10.1186/s12943-018-0857-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Graham SM, Jørgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.V99.1.319. [DOI] [PubMed] [Google Scholar]
- 667.Abraham SA, Hopcroft LEM, Carrick E, Drotar ME, Dunn K, Williamson AJK, et al. Dual targeting of p53 and c-MYC selectively eliminates leukaemic stem cells. Nature. 2016;534:341–346. doi: 10.1038/nature18288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7:673–683. doi: 10.1038/nrc2210. [DOI] [PubMed] [Google Scholar]
- 669.Pearson S, Williamson AJK, Blance R, Somervaille TCP, Taylor S, Azadbakht N, et al. Proteomic analysis of JAK2V617F-induced changes identifies potential new combinatorial therapeutic approaches. Leukemia. 2017;31:2717–2725. doi: 10.1038/leu.2017.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Wernig G, Gonneville JR, Crowley BJ, Rodrigues MS, Reddy MM, Hudon HE, et al. The Jak2V617F oncogene associated with myeloproliferative diseases requires a functional FERM domain for transformation and for expression of the Myc and Pim proto-oncogenes. Blood. 2008;111:3751–3759. doi: 10.1182/blood-2007-07-102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Huang SMA, Wang A, Greco R, Li Z, Barberis C, Tabart M, et al. Combination of PIM and JAK2 inhibitors synergistically suppresses MPN cell proliferation and overcomes drug resistance. Oncotarget. 2014;5:3362. doi: 10.18632/oncotarget.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Berg T, Cohen SB, Desharnais J, Sonderegger C, Maslyar DJ, Goldberg J, et al. Small-molecule antagonists of Myc/Max dimerization inhibit Myc-induced transformation of chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 2002;99:3830–3835. doi: 10.1073/pnas.062036999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Xu Y, Shi J, Yamamoto N, Moss JA, Vogt PK, Janda KD. A credit-card library approach for disrupting protein-protein interactions. Bioorganic Med Chem. 2006;14:2660–2673675. doi: 10.1016/j.bmc.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 674.Sayyadi M, Safaroghli-Azar A, Pourbagheri-Sigaroodi A, Abolghasemi H, Anoushirvani AA, Bashash D. c-Myc inhibition using 10058–F4 increased the sensitivity of acute promyelocytic leukemia cells to arsenic trioxide via blunting PI3K/NF-κB axis. Arch Med Res. 2020;51:636–644. doi: 10.1016/j.arcmed.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 675.Lao-On U, Rojvirat P, Chansongkrow P, Phannasil P, Siritutsoontorn S, Charoensawan V, et al. c-Myc directly targets an over-expression of pyruvate carboxylase in highly invasive breast cancer. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165656. doi: 10.1016/j.bbadis.2019.165656. [DOI] [PubMed] [Google Scholar]
- 676.Wang XN, Su XX, Cheng SQ, Sun ZY, Huang ZS, Ou TM. MYC modulators in cancer: a patent review. Expert Opin Ther Pat. 2019;29:353–367. doi: 10.1080/13543776.2019.1612878. [DOI] [PubMed] [Google Scholar]
- 677.Whitfield JR, Beaulieu ME, Soucek L. Strategies to inhibit Myc and their clinical applicability. Front Cell Dev Biol. 2017;5:10. doi: 10.3389/fcell.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.AlSultan D, Kavanagh E, O’Grady S, Eustace AJ, Castell A, Larsson LG, et al. The novel low molecular weight MYC antagonist MYCMI-6 inhibits proliferation and induces apoptosis in breast cancer cells. Invest New Drugs. 2021;39:587–594. doi: 10.1007/s10637-020-01018-w. [DOI] [PubMed] [Google Scholar]
- 679.Rihawi K, Alfieri R, Fiorentino M, Fontana F, Capizzi E, Cavazzoni A, et al. MYC amplification as a potential mechanism of primary resistance to crizotinib in ALK-rearranged non-small cell lung cancer: a brief report. Transl Oncol. 2019;12:116–121. doi: 10.1016/j.tranon.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Boike L, Cioffi AG, Majewski FC, Co J, Henning NJ, Jones MD, et al. Discovery of a functional covalent ligand targeting an intrinsically disordered cysteine within MYC. Cell Chem Biol. 2021;28:4–13. doi: 10.1016/j.chembiol.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Han H, Jain AD, Truica MI, Izquierdo-Ferrer J, Anker JF, Lysy B, et al. Small-molecule MYC inhibitors suppress tumor growth and enhance immunotherapy. Cancer Cell. 2019;36:483–497. doi: 10.1016/j.ccell.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Struntz NB, Chen A, Deutzmann A, Wilson RM, Stefan E, Evans HL, et al. Stabilization of the max homodimer with a small molecule attenuates Myc-driven transcription. Cell Chem Biol. 2019;26:711–723. doi: 10.1016/j.chembiol.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 683.Massó-Vallés D, Soucek L. Blocking Myc to treat cancer: reflecting on two decades of omomyc. Cells. 2020;9:883. doi: 10.3390/cells9040883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Wang E, Sorolla A, Cunningham PT, Bogdawa HM, Beck S, Golden E, et al. Tumor penetrating peptides inhibiting MYC as a potent targeted therapeutic strategy for triple-negative breast cancers. Oncogene. 2019;38:140–150. doi: 10.1038/s41388-018-0421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Montagne M, Beaudoin N, Fortin D, Lavoie CL, Klinck R, Lavigne P. The max b-HLH-LZ can transduce into cells and inhibit c-Myc transcriptional activities. PLoS ONE. 2012;7:e32172. doi: 10.1371/journal.pone.0032172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Demma MJ, Hohn MJ, Sun A, Mapelli C, Hall B, Walji A, et al. Inhibition of Myc transcriptional activity by a mini-protein based upon Mxd1. FEBS Lett. 2020;594:1467–1476. doi: 10.1002/1873-3468.13759. [DOI] [PubMed] [Google Scholar]
- 687.Park BK, Gautam A, Maharjan S, Lee SI, Lee Y, Kwon HJ. Production of Anti-c-Myc monoclonal antibody inhibiting DNA binding of c-Myc and Max dimer by epitope peptide–CpG-DNA–liposome complex without carriers. Int J Pept Res Ther. 2019;25:75–82. doi: 10.1007/s10989-017-9649-6. [DOI] [Google Scholar]
- 688.Ting TA, Chaumet A, Bard FA. Targeting c-Myc with a novel peptide nuclear delivery device. Sci Rep. 2020;10:1–13. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Wang H, Ramakrishnan A, Fletcher S, Prochownik EV. A quantitative, surface plasmon resonance-based approach to evaluating DNA binding by the c-Myc oncoprotein and its disruption by small molecule inhibitors. J Biol Methods. 2015;2:e18. doi: 10.14440/jbm.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Lustig LC, Dingar D, Tu WB, Lourenco C, Kalkat M, Inamoto I, et al. Inhibiting MYC binding to the E-box DNA motif by ME47 decreases tumour xenograft growth. Oncogene. 2017;36:6830–6837. doi: 10.1038/onc.2017.275. [DOI] [PubMed] [Google Scholar]
- 691.Madden SK, de Araujo AD, Gerhardt M, Fairlie DP, Mason JM. Taking the Myc out of cancer: toward therapeutic strategies to directly inhibit c-Myc. Mol Cancer. 2021;20:1–18. doi: 10.1186/s12943-020-01291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Beaulieu ME, Jauset T, Massó-Vallés D, Martínez-Martín S, Rahl P, Maltais L, et al. Intrinsic cell-penetrating activity propels omomyc from proof of concept to viable anti-myc therapy. Sci Transl Med. 2019;11:eaar5012. doi: 10.1126/scitranslmed.aar5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Allen-Petersen BL, Sears RC. Mission possible: advances in MYC therapeutic targeting in cancer. BioDrugs. 2019;33:539–553. doi: 10.1007/s40259-019-00370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Yin X, Giap C, Lazo JS, Prochownik EV. Low molecular weight inhibitors of Myc–Max interaction and function. Oncogene. 2003;22:6151–6159. doi: 10.1038/sj.onc.1206641. [DOI] [PubMed] [Google Scholar]
- 695.Fletcher S, Prochownik EV. Small-molecule inhibitors of the Myc oncoprotein. Biochim Biophys Acta Gene Regul Mech. 2015;1849:525–543. doi: 10.1016/j.bbagrm.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Chauhan J, Wang H, Yap JL, Sabato PE, Hu A, Prochownik EV, et al. Discovery of methyl 4’-methyl-5-(7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)-[1,1’-biphenyl]-3-carboxylate, an improved small-molecule inhibitor of c-Myc-max dimerization. ChemMedChem. 2014;9:2274–2285. doi: 10.1002/cmdc.201402189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Lu H, Zhou Q, He J, Jiang Z, Peng C, Tong R, et al. Recent advances in the development of protein–protein interactions modulators: mechanisms and clinical trials. Signal Transduct Target Ther. 2020;5:1–23. doi: 10.1038/s41392-019-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Hart JR, Garner AL, Yu J, Ito Y, Sun M, Ueno L, et al. Inhibitor of MYC identified in a Kröhnke pyridine library. Proc Natl Acad Sci U S A. 2014;111:12556–12561. doi: 10.1073/pnas.1319488111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Stellas D, Szabolcs M, Koul S, Li Z, Polyzos A, Anagnostopoulos C, et al. Therapeutic effects of an anti-Myc drug on mouse pancreatic cancer. J Natl Cancer Inst. 2014;106:dju320. doi: 10.1093/jnci/dju320. [DOI] [PubMed] [Google Scholar]
- 700.Ji W, Zhang W, Wang X, Shi Y, Yang F, Xie H, et al. c-myc regulates the sensitivity of breast cancer cells to palbociclib via c-myc/miR-29b-3p/CDK6 axis. Cell Death Dis. 2020;11:1–3. doi: 10.1038/s41419-019-2182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Chen A, Koehler AN. Transcription factor inhibition: lessons learned and emerging targets. Trends Mol Med. 2020;26:508–518. doi: 10.1016/j.molmed.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Chen L, Cheng B, Sun Q, Lai L. Ligand-based optimization and biological evaluation of N-(2,2,2-trichloro-1-(3-phenylthioureido)ethyl)acetamide derivatives as potent intrinsically disordered protein c-Myc inhibitors. Bioorg Med Chem Lett. 2021;31:127711. doi: 10.1016/j.bmcl.2020.127711. [DOI] [PubMed] [Google Scholar]
- 703.Foley SA, Castell A, Kavanagh E, Synnott NC, Crown J, Larsson L-G, et al. MYC as a therapeutic target for the treatment of triple-negative breast cancer. J Clin Oncol. 2019;37:e12550. doi: 10.1200/JCO.2019.37.15_suppl.e12550. [DOI] [Google Scholar]
- 704.Scafuro M, Capasso L, Carafa V, Altucci L, Nebbioso A. Gene transactivation and transrepression in myc-driven cancers. Int J Mol Sci. 2021;22:3458. doi: 10.3390/ijms22073458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Bailly C, Vergoten G. Protein homodimer sequestration with small molecules: focus on PD-L1. Biochem Pharmacol. 2020;174:113821. doi: 10.1016/j.bcp.2020.113821. [DOI] [PubMed] [Google Scholar]
- 706.Lafita-Navarro MC, Blanco R, Mata-Garrido J, Liaño-Pons J, Tapia O, García-Gutiérrez L, et al. MXD1 localizes in the nucleolus, binds UBF and impairs rRNA synthesis. Oncotarget. 2016;7:69536. doi: 10.18632/oncotarget.11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Jung KY, Wang H, Teriete P, Yap JL, Chen L, Lanning ME, et al. Perturbation of the c-Myc-Max protein-protein interaction via synthetic α-helix mimetics. J Med Chem. 2015;58:3002–3024. doi: 10.1021/jm501440q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Kim H, Yoo M, Jung K-Y. A promising but challenging strategy for cancer treatment: disruption of Myc-Max heterodimerization. Int J Clin Pharmacol Pharmacother. 2016;1:2. [Google Scholar]
- 709.Tolcher AW, Papadopoulos KP, Patnaik A, Rasco DW, Martinez D, Wood DL, et al. Safety and activity of DCR-MYC, a first-in-class Dicer-substrate small interfering RNA (DsiRNA) targeting MYC, in a phase I study in patients with advanced solid tumors. J Clin Oncol. 2015;33(15_suppl):11006–11006. 10.1200/jco.2015.33.15_suppl.11006.
- 710.Miller AJ, Chang A, Cunningham PN. Chronic microangiopathy due to DCR-MYC, a Myc-targeted short interfering RNA. Am J Kidney Dis. 2020;75:513–516. doi: 10.1053/j.ajkd.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 711.Yuan J, Wang K, Xi M. Mir-494 inhibits epithelial ovarian cancer growth by targeting c-myc. Med Sci Monit. 2016;22:617. doi: 10.12659/MSM.897288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Liu Y, Li X, Zhu S, Zhang JG, Yang M, Qin Q, et al. Ectopic expression of MIR-494 inhibited the proliferation, invasion and chemoresistance of pancreatic cancer by regulating SIRT1 and c-Myc. Gene Ther. 2015;22:729–738. doi: 10.1038/gt.2015.39. [DOI] [PubMed] [Google Scholar]
- 713.Devi GR, Beer TM, Corless CL, Arora V, Weller DL, Iversen PL. In vivo bioavailability and pharmacokinetics of a c-MYC antisense phosphorodiamidate morpholino oligomer, AVI-4126, in solid tumors. Clin Cancer Res. 2005;11:3930–3938. doi: 10.1158/1078-0432.CCR-04-2091. [DOI] [PubMed] [Google Scholar]
- 714.Lewin J, Soria JC, Stathis A, Delord JP, Peters S, Awada A, et al. Phase Ib trial with birabresib, a small-molecule inhibitor of bromodomain and extraterminal proteins, in patients with selected advanced solid tumors. J Clin Oncol. 2018;36:3007–3014. doi: 10.1200/JCO.2018.78.2292. [DOI] [PubMed] [Google Scholar]
- 715.Csizmarik A, Hadaschik B, Kramer G, Nyirady P, Szarvas T. Mechanisms and markers of resistance to androgen signaling inhibitors in patients with metastatic castration-resistant prostate cancer. In: Urologic oncology: seminars and original investigations; 2021. [DOI] [PubMed]
- 716.Aggarwal RR, Schweizer MT, Nanus DM, Pantuck AJ, Heath EI, Campeau E, et al. A phase Ib/IIa study of the Pan-BET inhibitor ZEN-3694 in combination with enzalutamide in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2020;26:5338–5347. doi: 10.1158/1078-0432.CCR-20-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Patel MR, Garcia-Manero G, Paquette R, Dinner S, Donnellan WB, Grunwald MR, et al. Phase 1 dose escalation and expansion study to determine safety, tolerability, pharmacokinetics, and pharmacodynamics of the BET inhibitor FT-1101 as a single agent in patients with relapsed or refractory hematologic malignancies. Blood. 2019;134(Supplement_1):3907. 10.1182/blood-2019-124741.
- 718.Millan DS, Alvarez Morales MA, Barr KJ, Cardillo D, Collis A, Dinsmore CJ, et al. FT-1101: a structurally distinct pan-bet bromodomain inhibitor with activity in preclinical models of hematologic malignancies. Blood. 2015;126(23): 1367. 10.1182/blood.V126.23.1367.1367.
- 719.Hilton J, Cristea MC, Voskoboynik M, Postel-Vinay S, Edenfield W, Gavai A, et al. Initial results from a phase I/IIa trial evaluating BMS-986158, an inhibitor of the bromodomain and extra-terminal (BET) proteins, in patients (pts) with advanced cancer. Ann Oncol. 2018;29:viii134. doi: 10.1093/annonc/mdy279.399. [DOI] [Google Scholar]
- 720.Gavai AV, Norris D, Tortolani D, O’Malley D, Zhao Y, Quesnelle C, et al. Abstract 5789: discovery of clinical candidate BMS-986158, an oral BET inhibitor, for the treatment of cancer. Cancer Res. 2018;78(13 Supplement):5789. 10.1158/1538-7445.AM2018-5789.
- 721.Sun Y, Han J, Wang Z, Li X, Sun Y, Hu Z. Safety and efficacy of bromodomain and extra-terminal inhibitors for the treatment of hematological malignancies and solid tumors: a systematic study of clinical trials. Front Pharmacol. 2021;11:2440. doi: 10.3389/fphar.2020.621093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Shapiro GI, LoRusso P, Dowlati A, Do KT, Jacobson CA, Vaishampayan U, et al. A phase 1 study of RO6870810, a novel bromodomain and extra-terminal protein inhibitor, in patients with NUT carcinoma, other solid tumours, or diffuse large B-cell lymphoma. Br J Cancer. 2021;124:744–753. doi: 10.1038/s41416-020-01180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.Piha-Paul SA, Hann CL, French CA, Cousin S, Braña I, Cassier PA, et al. Phase 1 study of molibresib (GSK525762), a bromodomain and extra-terminal domain protein inhibitor, in NUT carcinoma and other solid tumors. JNCI Cancer Spectr. 2020;4:pkz093. doi: 10.1093/jncics/pkz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725.Mascarenhas J, Harrison C, Luptakova K, Christo J, Wang J, Mertz JA, et al. MANIFEST-2, a global, phase 3, Randomized, Double-Blind, Active-Control Study of CPI-0610 and Ruxolitinib Vs. Placebo and Ruxolitinib in JAK-Inhibitor-Naive Myelofibrosis Patients. Blood 2020;136(Supplement 1):43. 10.1182/blood-2020-140901.
- 726.Blum KA, Abramson J, Maris M, Flinn I, Goy A, Mertz J, et al. A phase I study of CPI-0610, a bromodomain and extra terminal protein (BET) inhibitor in patients with relapsed or refractory lymphoma. Ann Oncol. 2018;29:ii7. doi: 10.1093/annonc/mdy048. [DOI] [Google Scholar]
- 727.Rhyasen GW, Hattersley MM, Yao Y, Dulak A, Wang W, Petteruti P, et al. AZD5153: a novel bivalent BET bromodomain inhibitor highly active against hematologic malignancies. Mol Cancer Ther. 2016;15:2563–2574. doi: 10.1158/1535-7163.MCT-16-0141. [DOI] [PubMed] [Google Scholar]
- 728.Bradbury RH, Callis R, Carr GR, Chen H, Clark E, Feron L, et al. Optimization of a series of bivalent triazolopyridazine based bromodomain and extraterminal inhibitors: the discovery of (3R)-4-[2-[4-[1-(3-methoxy-[1,2,4]triazolo[4,3-b]pyridazin-6-yl)-4-piperidyl]phenoxy]ethyl]-1,3-dimethyl-piperazin-2-one (AZD5153) J Med Chem. 2016;59:7801–7817. doi: 10.1021/acs.jmedchem.6b00070. [DOI] [PubMed] [Google Scholar]
- 729.Takimoto-Shimomura T, Tsukamoto T, Maegawa S, Fujibayashi Y, Matsumura-Kimoto Y, Mizuno Y, et al. Dual targeting of bromodomain-containing 4 by AZD5153 and BCL2 by AZD4320 against B-cell lymphomas concomitantly overexpressing c-MYC and BCL2. Invest New Drugs. 2019;37:210–222. doi: 10.1007/s10637-018-0623-8. [DOI] [PubMed] [Google Scholar]
- 730.Gerlach D, Tontsch-Grunt U, Baum A, Popow J, Scharn D, Hofmann MH, et al. The novel BETi BI 894999 represses super-enhancer associated transcription and synergizes with CDK9 inhibition in AML by induction of apoptosis. Oncogene. 2018;37:2687–2701. doi: 10.1038/s41388-018-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Tron AE, Belmonte MA, Adam A, Aquila BM, Boise LH, Chiarparin E, et al. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat Commun. 2018;9:1–14. doi: 10.1038/s41467-018-07551-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732.Maragno AL, Mistry P, Kotschy A, Szlavik Z, Murray J, Davidson J, et al. Abstract 4482: S64315 (MIK665) is a potent and selective Mcl1 inhibitor with strong antitumor activity across a diverse range of hematologic tumor models. Cancers. 2019;12:574. [Google Scholar]
- 733.Fairlie WD, Lee EF. Co-operativity between myc and bcl-2 pro-survival proteins in cancer. Int J Mol Sci. 2021;22:2841. doi: 10.3390/ijms22062841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Caenepeel S, Karen R, Belmontes B, Verlinsky A, Tan H, Yang Y, et al. Abstract 6218: discovery and preclinical evaluation of AMG 397, a potent, selective and orally bioavailable MCL1 inhibitor. Cancer Res. 2020;80(16 Supplement):6218. 10.1158/1538-7445.AM2020-6218.
- 735.Lee J, Zhang LL, Wu W, Guo H, Li Y, Sukhanova M, et al. Activation of MYC, a bona fide client of HSP90, contributes to intrinsic ibrutinib resistance in mantle cell lymphoma. Blood Adv. 2018;2:2039–2051. doi: 10.1182/bloodadvances.2018016048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736.Moyo TK, Wilson CS, Moore DJ, Eischen CM. Myc enhances B-cell receptor signaling in precancerous B cells and confers resistance to Btk inhibition. Oncogene. 2017;36:4653–4661. doi: 10.1038/onc.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Reiff SD, Mantel R, Smith LL, Greene JT, Muhowski EM, Fabian CA, et al. The btk inhibitor arq 531 targets ibrutinib-resistant cll and richter transformation. Cancer Discov. 2018;8:1300–1315. doi: 10.1158/2159-8290.CD-17-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Rifaï K, Judes G, Idrissou M, Daures M, Bignon YJ, Penault-Llorca F, et al. SIRT1-dependent epigenetic regulation of H3 and H4 histone acetylation in human breast cancer. Oncotarget. 2018;9:30661. doi: 10.18632/oncotarget.25771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 739.Ecker J, Thatikonda V, Sigismondo G, Selt F, Valinciute G, Oehme I, et al. Reduced chromatin binding of MYC is a key effect of HDAC inhibition in MYC amplified medulloblastoma. Neuro Oncol. 2021;22:226–239. doi: 10.1093/neuonc/noaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Knipstein J, Gore L. Entinostat for treatment of solid tumors and hematologic malignancies. Expert Opin Investig Drugs. 2011;20:1455–1467. doi: 10.1517/13543784.2011.613822. [DOI] [PubMed] [Google Scholar]
- 741.Zhao W, Dai K. Phase III Study of Tucidinostat in Combination With R-CHOP in Patients With Newly Diagnosed Double-Expressor DLBCL. Case Med Res. 2020. 10.31525/ct1-nct04231448.
- 742.Deng C, Lipstein MR, Scotto L, Jirau Serrano XO, Mangone MA, Li S, et al. Silencing c-Myc translation as a therapeutic strategy through targeting PI3Kδ and CK1ε in hematological malignancies. Blood. 2017;129:88–99. doi: 10.1182/blood-2016-08-731240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743.Kahl BS, Spurgeon SE, Furman RR, Flinn IW, Coutre SE, Brown JR, et al. Results of a phase I study of idelalisib, a PI3Kδ inhibitor, in patients with relapsed or refractory mantle cell lymphoma (MCL) Blood. 2014;123:3398–3405. doi: 10.1182/blood-2013-11-537555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Oki Y, Kelly KR, Flinn I, Patel MR, Gharavi R, Ma A, et al. CUDC-907 in relapsed/refractory diffuse large B-cell lymphoma, including patients with MYC-alterations: results from an expanded phase I trial. Haematologica. 2017;102:1923. doi: 10.3324/haematol.2017.172882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 745.Jung H-S, Kim NH, Wang J, Son MK, Kim B-K, Jeon B, et al. Combination of BR101801 and venetoclax demonstrates synergistic activity in DLBCL cell lines harboring double hit and double expressor alterations. Blood. 2017;130:4114. doi: 10.1182/blood.V130.Suppl_1.4114.4114. [DOI] [Google Scholar]
- 746.Dyer MJS, Vogler M, Samuel J, Jayne S, Wagner S, Pritchard C, et al. Precision medicines for B-cell leukaemias and lymphomas; progress and potential pitfalls. Br J Haematol. 2013;6:725–733. doi: 10.1111/bjh.12219. [DOI] [PubMed] [Google Scholar]
- 747.Qian C, Lai CJ, Bao R, Wang DG, Wang J, Xu GX, et al. Cancer network disruption by a single molecule inhibitor targeting both histone deacetylase activity and phosphatidylinositol 3-kinase signaling. Clin Cancer Res. 2012;18:4104–4113. doi: 10.1158/1078-0432.CCR-12-0055. [DOI] [PubMed] [Google Scholar]
- 748.Younes A, Berdeja JG, Patel MR, Flinn I, Gerecitano JF, Neelapu SS, et al. Safety, tolerability, and preliminary activity of CUDC-907, a first-in-class, oral, dual inhibitor of HDAC and PI3K, in patients with relapsed or refractory lymphoma or multiple myeloma: An open-label, dose-escalation, phase 1 trial. Lancet Oncols. 2016;17:622–631. doi: 10.1016/S1470-2045(15)00584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 749.Chen Y, Peubez C, Smith V, Xiong S, Kocsis-Fodor G, Kennedy B, et al. CUDC-907 blocks multiple pro-survival signals and abrogates microenvironment protection in CLL. J Cell Mol Med. 2019;23:340–348. doi: 10.1111/jcmm.13935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 750.Iijima S, Teraoka H, Date T, Tsukada K. DNA-activated protein kinase in Raji Burkitt’s lymphoma cells. Eur J Biochem. 1992;206:595–603. doi: 10.1111/j.1432-1033.1992.tb16964.x. [DOI] [PubMed] [Google Scholar]
- 751.Shortt J, Martin BP, Newbold A, Hannan KM, Devlin JR, Baker AJ, et al. Combined inhibition of PI3K-related DNA damage response kinases and mTORC1 induces apoptosis in MYC-driven B-cell lymphomas. Blood. 2013;121:2964–2974. doi: 10.1182/blood-2012-08-446096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Wang S, Kwon SM, Lee BR, Jeon B, Kim SJ, Yang E, et al. Abstract 4439: BR101801 triggers anti-tumor immunity and enhances efficacy of immune checkpoint antibodies in syngeneic model. Cancer Res. 2020;80(16 Supplement):4439. 10.1158/1538-7445.AM2020-4439.
- 753.Kanazawa S, Soucek L, Evan G, Okamoto T, Peterlin BM. c-Myc recruits P-TEFb for transcription, cellular proliferation and apoptosis. Oncogene. 2003;22:5707–5711. doi: 10.1038/sj.onc.1206800. [DOI] [PubMed] [Google Scholar]
- 754.Marshall NF, Peng J, Xie Z, Price DH. Control of RNA polymerase II elongation potential by a novel carboxyl- terminal domain kinase. J Biol Chem. 1996;271:27176–27183. doi: 10.1074/jbc.271.43.27176. [DOI] [PubMed] [Google Scholar]
- 755.Gargano B, Amente S, Majello B, Lania L. P-TEFb is a crucial co-factor for Myc transactivation. Cell Cycle. 2007;6:2031–2037. doi: 10.4161/cc.6.16.4554. [DOI] [PubMed] [Google Scholar]
- 756.Parry D, Guzi T, Shanahan F, Davis N, Prabhavalkar D, Wiswell D, et al. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol Cancer Ther. 2010;9:2344–2353. doi: 10.1158/1535-7163.MCT-10-0324. [DOI] [PubMed] [Google Scholar]
- 757.Gregory GP, Hogg SJ, Kats LM, Vidacs E, Baker AJ, Gilan O, et al. CDK9 inhibition by dinaciclib potently suppresses Mcl-1 to induce durable apoptotic responses in aggressive MYC-driven B-cell lymphoma in vivo. Leukemia. 2015;29:1437–1441. doi: 10.1038/leu.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758.Goh KC, Novotny-Diermayr V, Hart S, Ong LC, Loh YK, Cheong A, et al. TG02, a novel oral multi-kinase inhibitor of CDKs, JAK2 and FLT3 with potent anti-leukemic properties. Leukemia. 2012;26:236–243. doi: 10.1038/leu.2011.218. [DOI] [PubMed] [Google Scholar]
- 759.Chen R, Tsai J, Thompson PA, Chen Y, Xiong P, Liu C, et al. The multi-kinase inhibitor TG02 induces apoptosis and blocks B-cell receptor signaling in chronic lymphocytic leukemia through dual mechanisms of action. Blood Cancer J. 2021;11:1–15. doi: 10.1038/s41408-020-00390-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 760.Richters A, Doyle SK, Freeman DB, Lee C, Leifer BS, Jagannathan S, et al. Modulating androgen receptor-driven transcription in prostate cancer with selective CDK9 inhibitors. Cell Chem Biol. 2021;28:134–147. doi: 10.1016/j.chembiol.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 761.Yang D, Hurley L. Structure of the biologically relevant g-quadruplex in the c-MYC promoter. Nucleosides Nucleotides Nucleic Acids. 2006;25:951–968. doi: 10.1080/15257770600809913. [DOI] [PubMed] [Google Scholar]
- 762.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Paul R, Das T, Debnath M, Chauhan A, Dash J. G-quadruplex-binding small molecule induces synthetic lethality in breast cancer cells by inhibiting c-MYC and BCL2 expression. ChemBioChem. 2020;21:963–970. doi: 10.1002/cbic.201900534. [DOI] [PubMed] [Google Scholar]
- 764.Drygin D, Siddiqui-Jain A, O’Brien S, Schwaebe M, Lin A, Bliesath J, et al. Anticancer activity of CX-3543: a direct inhibitor of rRNA biogenesis. Cancer Res. 2009;69:7653–7661. doi: 10.1158/0008-5472.CAN-09-1304. [DOI] [PubMed] [Google Scholar]
- 765.Local A, Zhang H, Benbatoul KD, Folger P, Sheng X, Tsai CY, et al. APTO-253 stabilizes G-quadruplex DNA, inhibits MYC expression, and induces DNA damage in acute myeloid leukemia cells. Mol Cancer Ther. 2018;17:1177–1186. doi: 10.1158/1535-7163.MCT-17-1209. [DOI] [PubMed] [Google Scholar]
- 766.Shachaf CM, Felsher DW. Tumor dormancy and MYC inactivation: pushing cancer to the brink of normalcy. Cancer Res. 2005;65:4471–4474. doi: 10.1158/0008-5472.CAN-05-1172. [DOI] [PubMed] [Google Scholar]
- 767.Soucek L, Whitfield J, Martins CP, Finch AJ, Murphy DJ, Sodir NM, et al. Modelling Myc inhibition as a cancer therapy. Nature. 2008;455:679–683. doi: 10.1038/nature07260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 768.Dorgalaleh A, Bahraini M, Ahmadi SE. Personalized anesthesia in hematology. In: Dabbagh A, editor. Personalized medicine in anesthesia, pain and perioperative medicine. Cham: Springer; 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.