Abstract
Infected pancreatic necrosis is a postpancreatitis complication that is mainly caused by Enterobacteriaceae and Enterococci. Here, we have reported a very rare case of Lactobacillus paracasei bacteraemia associated with infected pancreatic necrosis and retroperitoneal abscess. In addition to the diagnosis of diabetic ketoacidosis, blood test results revealed a high inflammatory status. CT of the abdomen revealed pancreatic walled-off necrosis. Blood culture and aspiration fluid culture revealed positivity for L. paracasei, leading to the diagnosis of infected pancreatic necrosis. The abscess had spread in the retroperitoneal space later. The patient recovered after receiving antibiotic treatment and endoscopic and percutaneous drainage. L. paracasei can cause invasive infection, including infected pancreatic necrosis and retroperitoneal abscess, which requires aggressive therapy.
Keywords: pancreas and biliary tract, infectious diseases
Background
Lactobacilli are facultatively anaerobic, gram-positive rod bacteria. They constitute the normal flora of the oral cavity, gastrointestinal tract and vagina of humans. However, Lactobacilli have also been reported as a causative organism of serious infections, particularly in immunocompromised patients with malignancy, a history of recent chemotherapy and abdominal surgery, liver cirrhosis and diabetes mellitus.1 Regarding Lactobacillus infections, primary bacteraemia, endocarditis, catheter-related bloodstream infection and intra-abdominal infection including liver abscess have been reported.1–3 The 1-month and 1-year mortality rate of Lactobacillus bacteraemia has been reported to be 26% and 48%, respectively, and appropriate use of antibiotics can significantly lower the mortality rate.4 Our patient developed infected pancreatic necrosis and retroperitoneal abscess due to L. paracasei bacteraemia. It is important to recognise this rare, but serious type of infection.
Case presentation
An 88-year-old Japanese woman presented to our emergency department with acute onset of altered mental status. She had been well until 1 day prior to presentation, when she developed epigastric pain and appetite loss. She became unresponsive the next day. She had a medical history of hypertension, type 2 diabetes mellitus (last glycated haemoglobin level: 9.3%) and dementia. She denied a history of acute pancreatitis. Her regular medications were amlodipine, telmisartan, metformin, vildagliptin, glimepiride, miglitol, pioglitazone, galantamine and memantine. She did not take alcohol.
On physical examination, she appeared tachypnoeic and drowsy. Her body temperature was 35.0°C, blood pressure was 108/73 mm Hg, pulse rate was 88 beats/min, respiratory rate was 24 respirations/min and oxygen saturation was 100% on ambient air. The patient’s consciousness level according to the Glasgow coma scale was E4V3M5. Oral examination revealed dry mouth, and abdominal examination showed periumbilical tenderness. The findings of other examinations were unremarkable.
Blood test results showed leukocytosis (white cell count, 16.7×109/L), markedly elevated C reactive protein level (22.98 mg/L) and hyperglycaemia (blood glucose level, 45.94 mmol/L). Liver function test revealed partially abnormal findings (aspartate aminotransferase level, 20 U/L; alanine aminotransferase level, 26 U/L; lactate dehydrogenase level, 313 U/L; alkaline phosphatase level, 420 U/L; and total bilirubin level, 0.4 mg/dL). Furthermore, she had impaired renal function (urea level, 92 mg/dL; creatinine level, 2.06 mg/dL). Serum amylase and lipase levels were in the normal range. Arterial blood gas analysis showed a pH of 7.236 and wide anion-gap metabolic acidosis with a lactate level of 3.74 mmol/L. Urinalysis revealed strong positivity for ketones. Therefore, the diagnosis of diabetic ketoacidosis was made, and intravenous hydration and insulin therapies were initiated.
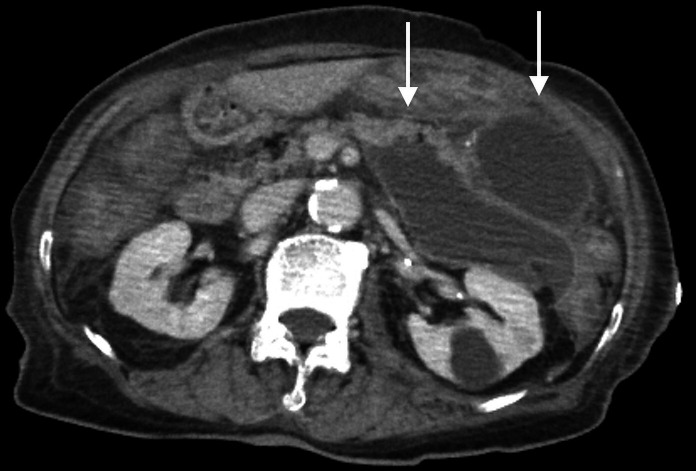
An initial examination could not identify the cause of the elevated inflammatory status. Blood culture was performed. CT of the abdomen revealed encapsulated 10×8×11 cm pancreatic fluid collection limited in the anterior pararenal space, which was consistent with walled-off necrosis (WON) (figure 1). CT severity index was calculated to be as high as 9, as most of the pancreatic parenchyma was replaced by fluid collection.
Figure 1.
CT of the abdomen showing multiple encapsulated fluid collections (white arrows).
Investigations
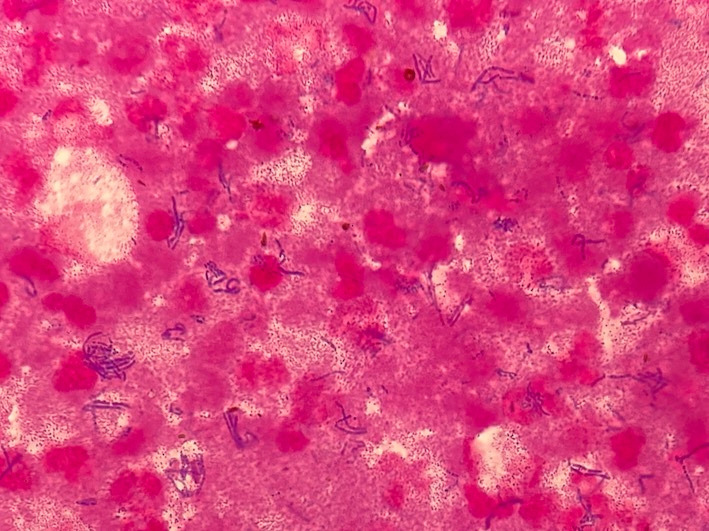
The patient was admitted to the intensive care unit. Neither vasopressor nor respiratory support was demanded. Diabetic ketoacidosis was well treated, and the patient’s mental status became stable without antibiotic therapy. On the fourth day of admission, she was transferred to the medical ward. However, elevated C reactive protein levels persisted. On the fifth day of admission, to clarify whether the fluid collection was infected, endoscopic ultrasound-guided fine-needle aspiration was performed, and intravenous cefmetazole was initiated as empiric therapy. The aspiration fluid was pus. Gram staining was positive only for numerous gram-positive rod bacteria (figure 2). Initial blood culture and aspiration fluid culture subsequently revealed L. paracasei positivity.
Figure 2.
Gram staining of the aspiration fluid showing numerous gram-positive rod bacteria.
Differential diagnosis
Bacteraemia associated with infected pancreatic necrosis caused by L. paracasei was suspected. Although the time when the patient developed acute pancreatitis was unknown, dementia might have blurred her prior history, and preceding pancreatitis led to insulin deficiency, which was the underlying cause of diabetic ketoacidosis. Because none of the common causes of acute pancreatitis including alcohol, gallstones, hypertriglyceridaemia and drugs was pointed out, the aetiology of her pancreatitis was thought to be idiopathic. Unexplained pancreatitis increases with age.5 Follow-up blood culture on the ninth hospital day revealed negative results. As infective endocarditis was not suspected strongly, cardiac ultrasonography was not performed.
Treatment
The patient was initiated on intravenous ampicillin in addition to cefmetazole on the sixth day of hospitalisation, and elevated inflammatory status showed improvement. However, on day 12 of hospitalisation, she developed acute-onset left lower abdominal pain with tenderness. CT of the abdomen was performed again, revealing a new retroperitoneal abscess measuring 5×5×15 cm, spreading beyond the anterior pararenal space (figure 3). Additional percutaneous drainage was performed, and the aspiration fluid culture was positive for L. paracasei. As the pathogen was confirmed to be susceptible to penicillin G, the patient was administered penicillin G intravenously. After treatment with antibiotics and percutaneous drainage, her symptoms and elevated inflammatory status improved, and there was no recurrence of abdominal pain. On day 62 of hospitalisation, follow-up CT showed significant reduction in fluid collection (figure 4).
Figure 3.
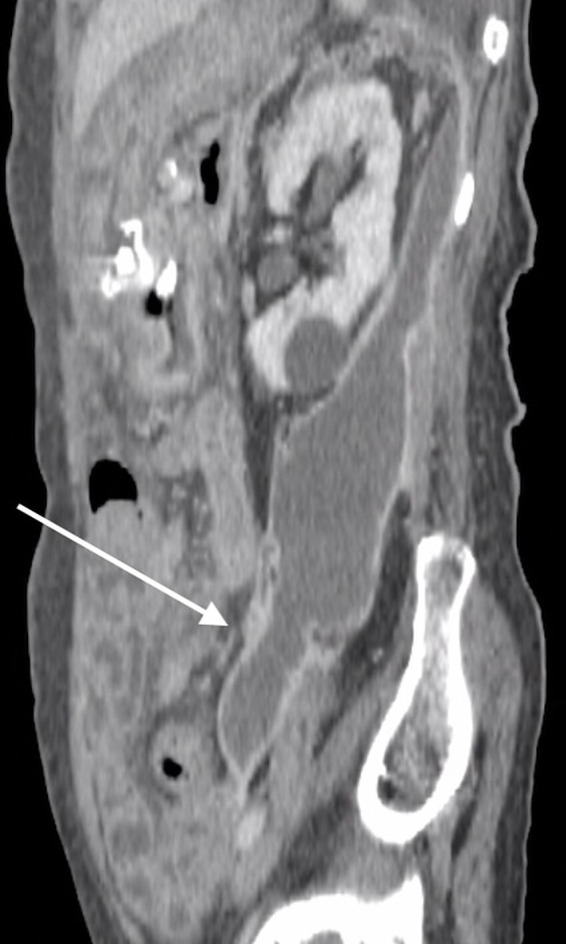
CT of the abdomen on day 12 of hospitalisation showing extension of fluid collection in the pelvic region (white arrow).
Figure 4.
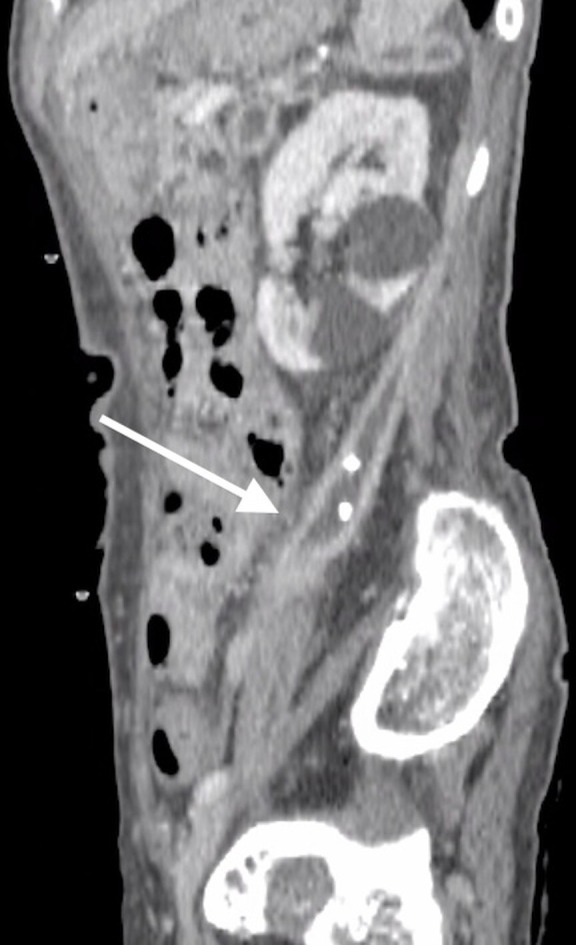
CT of the abdomen on day 62 of hospitalisation showing significant improvement in retroperitoneal fluid collection after percutaneous drainage (white arrow).
Outcome and follow-up
On day 74 of hospitalisation, the patient was transferred to a long-term care facility. She received a 2-week additional course of oral amoxicillin after the last CT. There was no clinical recurrence after the discontinuation of antibiotic therapy in the following 6 months.
Discussion
Lactobacilli are a relatively rare pathogen causing serious infections, especially in immunocompromised patients. Regarding Lactobacillus bacteraemia, a case series study indicated that one of the most prevalent foci was intra-abdominal infection, including liver abscess and cholecystitis.1 2 6 A previous study indicated that Lactobacillus, which belongs to the normal gut flora, might pass through the intestinal wall, enter the bloodstream and cause intra-abdominal infection in an immunocompromised condition.1 Other studies have reported that Lactobacillus has the ability to adhere to the intestinal wall through the extracellular matrix and degrade the wall using glycosidase and protease, and these microbiological characteristics may explain the mechanism of bacterial translocation.7–9
In our case, L. paracasei caused unique infected pancreatic necrosis with a quite invasive feature, spreading and forming giant retroperitoneal abscess later. Such an invasive infection similar to our case has not been reported thus far. Infected pancreatic necrosis is predominantly caused by microbes constituting the normal gut flora, including Escherichia coli, Klebsiella pneumoniae, Enterococcus and Candida.10 Microbes reach the WON through either the bloodstream or the biliary tract, especially in patients with biliary tract disorders.11 A previous case report showed a middle-aged otherwise healthy man who developed Lactobacillus-infected pancreatic necrosis after gallstone pancreatitis who could not undergo endoscopic retrograde cholangiopancreatography due to oedematous ampulla.12 Although the results of blood culture and the pathogenesis were not considered in the report, Lactobacillus might have migrated to the pancreas through the biliary tract due to cholestasis related to cholelithiasis. In our case, the patient was immunocompromised because she had a history of poorly controlled diabetes mellitus. Bacteraemia was confirmed, and no remarkable biliary tract disorder was detected, which might be consistent with bacterial translocation.
Although the patient received effective antibiotic treatment and endoscopic drainage, fluid collection spread to the retroperitoneal space. The fluid spread along the posterior perirenal fascia, sparing the perirenal space. This unique expansion might be explained by the theory of interfascial plane, in which fasciae are composed of multiple connective tissue layers and can serve as a potential space for fluid or gas.13 14 The fluid reached the pelvis through the combined interfascial plane. Delay in initiating effective antibiotic treatment might be a reason for the aggressive spread of the infection. A previous study reported the refractoriness of Lactobacillus infections. In that study, patients with Lactobacillus liver abscess required a longer hospital stay than those with other pyogenic liver abscesses.2 It is unclear whether this refractoriness was due to microbiological characteristics or underlying conditions of patients.
Although fine-needle aspiration had been performed for definitive diagnosis, a recent retrospective study revealed that 20% of infected pancreatic necrosis could be overlooked.15 It is no longer recommended as routine practice in the recent guidelines published in 2019 by World Society of Emergency Surgery.16 However, it may be useful if the diagnosis cannot be made clinically as in our case.17
Classically, the standard therapy for infected pancreatic necrosis is open necrosectomy in addition to antibiotic therapy. However, in recent years, the surgical step-up approach, percutaneous drainage first followed by video-assisted retroperitoneal debridement if necessary, has been used to reduce complications associated with the classical approach.18 The concept of the endoscopic step-up approach, comprising transluminal drainage followed by endoscopic debridement, is also emerging. A recent randomised controlled study revealed that the endoscopic step-up approach was not superior to the surgical step-up approach in terms of mortality and major complications, but it reduced the risk of pancreatic fistula and length of hospital stay.19 The step-up approach is supported in the previously cited guidelines.16 Our patient was treated based on these evidence, and clinical improvement was confirmed without necrosectomy.
We encountered an invasive case of L. paracasei-infected pancreatic necrosis and retroperitoneal abscess. To the best of our knowledge, invasive Lactobacillus pancreatic necrosis, similar to our case, has not been reported thus far. We should be careful about the potential infectiousness of these usually less virulent bacteria, especially in immunocompromised patients.
Learning points.
Lactobacillus paracasei is a potential pathogen causing invasive infection mainly in immunocompromised patients.
Infected pancreatic necrosis is caused by organisms constituting the gut flora, and L. paracasei can be a causative organism.
Although L. paracasei is believed to be less virulent, aggressive drainage should be performed to treat the invasive infection.
Footnotes
Contributors: TM and TS conceived the idea of reporting our case. TM and HT contributed to the acquisition and analysis of data. TM wrote the manuscript. HT and TS revised the report critically. All authors approved the final version of the report. TS supervised the project.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Next of kin consent obtained.
References
- 1.Lee M-R, Tsai C-J, Liang S-K, et al. Clinical characteristics of bacteraemia caused by Lactobacillus spp. and antimicrobial susceptibilities of the isolates at a medical centre in Taiwan, 2000-2014. Int J Antimicrob Agents 2015;46:439–45. 10.1016/j.ijantimicag.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 2.Sherid M, Samo S, Sulaiman S, et al. Liver abscess and bacteremia caused by Lactobacillus: role of probiotics? case report and review of the literature. BMC Gastroenterol 2016;16:138. 10.1186/s12876-016-0552-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon JP, Lee TA, Bolanos JT, et al. Pathogenic relevance of Lactobacillus: a retrospective review of over 200 cases. Eur J Clin Microbiol Infect Dis 2005;24:31–40. 10.1007/s10096-004-1253-y [DOI] [PubMed] [Google Scholar]
- 4.Salminen MK, Rautelin H, Tynkkynen S, et al. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L. rhamnosus GG. Clin Infect Dis 2004;38:62–9. 10.1086/380455 [DOI] [PubMed] [Google Scholar]
- 5.Forsmark CE, Swaroop Vege S, Wilcox CM, Vege SS, Mel Wilcox C. Acute pancreatitis. N Engl J Med Overseas Ed 2016;375:1972–81. 10.1056/NEJMra1505202 [DOI] [PubMed] [Google Scholar]
- 6.Chery J, Dvoskin D, Morato FP, et al. Lactobacillus fermentum, a pathogen in documented cholecystitis. Int J Surg Case Rep 2013;4:662–4. 10.1016/j.ijscr.2013.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oakey HJ, Harty DW, Knox KW. Enzyme production by lactobacilli and the potential link with infective endocarditis. J Appl Bacteriol 1995;78:142–8. 10.1111/j.1365-2672.1995.tb02834.x [DOI] [PubMed] [Google Scholar]
- 8.Harty DW, Oakey HJ, Patrikakis M, et al. Pathogenic potential of lactobacilli. Int J Food Microbiol 1994;24:179–89. 10.1016/0168-1605(94)90117-1 [DOI] [PubMed] [Google Scholar]
- 9.Apostolou E, Kirjavainen PV, Saxelin M, et al. Good adhesion properties of probiotics: a potential risk for bacteremia? FEMS Immunol Med Microbiol 2001;31:35–9. 10.1111/j.1574-695X.2001.tb01583.x [DOI] [PubMed] [Google Scholar]
- 10.Schmidt PN, Roug S, Hansen EF, et al. Spectrum of microorganisms in infected walled-off pancreatic necrosis - impact on organ failure and mortality. Pancreatology 2014;14:444–9. 10.1016/j.pan.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 11.Räty S, Sand J, Nordback I. Difference in microbes contaminating pancreatic necrosis in biliary and alcoholic pancreatitis. Int J Pancreatol 1998;24:187–91. 10.1007/BF02788421 [DOI] [PubMed] [Google Scholar]
- 12.Z'Graggen WJ, Fankhauser H, Lammer F, et al. Pancreatic necrosis infection due to Lactobacillus paracasei in an immunocompetent patient. Pancreatology 2005;5:108–9. 10.1159/000084786 [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa K, Idoguchi K, Tanaka H, et al. Classification of acute pancreatitis based on retroperitoneal extension: application of the concept of interfascial planes. Eur J Radiol 2006;60:445–52. 10.1016/j.ejrad.2006.06.014 [DOI] [PubMed] [Google Scholar]
- 14.Kalia S, Gupta R, Shenvi SD, et al. Inguinoscrotal region as an unusual site of extra-pancreatic collections in infected pancreatic necrosis. Gastroenterol Rep 2016;4:246–50. 10.1093/gastro/gou090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madenci AL, Michailidou M, Chiou G, et al. A contemporary series of patients undergoing open debridement for necrotizing pancreatitis. Am J Surg 2014;208:324–31. 10.1016/j.amjsurg.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 16.Leppäniemi A, Tolonen M, Tarasconi A, et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg 2019;14:27. 10.1186/s13017-019-0247-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Baal MC, Bollen TL, Bakker OJ, et al. The role of routine fine-needle aspiration in the diagnosis of infected necrotizing pancreatitis. Surgery 2014;155:442–8. 10.1016/j.surg.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 18.van Santvoort HC, Besselink MG, Bakker OJ, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010;362:1491–502. 10.1056/NEJMoa0908821 [DOI] [PubMed] [Google Scholar]
- 19.van Brunschot S, van Grinsven J, van Santvoort HC, et al. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet 2018;391:51–8. 10.1016/S0140-6736(17)32404-2 [DOI] [PubMed] [Google Scholar]