Abstract
Background
More inflammation is associated with greater risk incident hypertension, and Black United States (US) adults have excess burden of hypertension. We investigated whether increased inflammation as quantified by higher C-reactive protein (CRP) explains the excess incidence in hypertension experienced by Black US adults.
Methods
We included 6,548 Black and White REasons for Geographic and Racial Differences in Stroke (REGARDS) participants without hypertension at baseline (2003–2007) who attended a second visit (2013–2016). Sex-stratified risk ratios (RRs) for incident hypertension at the second exam in Black compared to White individuals were estimated using Poisson regression adjusted for groups of factors known to partially explain the Black–White differences in incident hypertension. We calculated the percent mediation by CRP of the racial difference in hypertension.
Results
Baseline CRP was higher in Black participants. The Black–White RR for incident hypertension in the minimally adjusted model was 1.33 (95% confidence interval 1.22, 1.44) for males and 1.15 (1.04, 1.27) for females. CRP mediated 6.6% (95% confidence interval 2.7, 11.3%) of this association in females and 19.7% (9.8, 33.2%) in males. In females, CRP no longer mediated the Black–White RR in a model including waist circumference and body mass index, while in males the Black–White difference was fully attenuated in models including income, education and dietary patterns.
Conclusions
Elevated CRP attenuated a portion of the unadjusted excess risk of hypertension in Black adults, but this excess risk was attenuated when controlling for measures of obesity in females and diet and socioeconomic factors in males. Inflammation related to these risk factors might explain part of the Black–White disparity in hypertension.
Keywords: blood pressure, C-reactive protein, hypertension, inflammation, preventive medicine, racial disparities
Background
Among all diseases, hypertension contributes the greatest increased burden of disability-adjusted life years.1 Black Americans experience a disproportionate burden of hypertension and have among the highest prevalence of any group in the world.2 A recent study from the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort identified several clinical and social factors that contribute to hypertension among Black compared to White participants.3 To reduce the burden of hypertension and its consequences among Black adults, improved understanding of the causes of this difference is needed.
Inflammation may play a role in the development of hypertension since adults with elevated levels of inflammatory markers, including C-reactive protein (CRP), experience a greater incidence of hypertension.4,5 The contribution of inflammation to the Black–White difference in hypertension incidence is unknown, but given the known higher CRP in Blacks compared to Whites, inflammation is a key potential biomarker to explain the difference.6–8 Because inflammation can be reduced with medication and behavioral therapies,9–11 interventions to target inflammation to decrease hypertension risk may lead to potentially larger effects in the Black population and reduce the racial difference in incident hypertension. We studied the role of CRP as a mediator of the racial difference in incident hypertension in the REGARDS cohort and potential reasons for any mediation of the Black–White risk differences seen.
Methods
Cohort
REGARDS enrolled 30,239 adults, aged ≥45 years, between 2003 and 2007 in the contiguous United States (US). The design oversampled Black people (44%) and residents in the stroke belt (56%), a region in the southeastern US including Alabama, Mississippi, Tennessee, Louisiana, Arkansas, Georgia, North Carolina, and South Carolina, where stroke mortality is higher than the rest of the country.12 Potential participants were contacted by mail, followed by a telephone interview to collect demographic, medical history, and risk factor data. Participants were excluded at baseline for active cancer or treatment for cancer in the prior year, medical conditions preventing long-term participation; residency or awaiting placement in a nursing home; inability to communicate in English, Hispanic, or Latino ethnicity; or self-reported race other than Black or White.
A baseline in-home examination included measurement of blood pressure (BP), anthropometrics, collection of blood and urine samples, and review of medications. The telephone and in-person examinations were repeated at a second exam between April 2013 and October 2016. Of the original cohort, 16,150 completed the second in-home exam.
Institutional review boards of each participating institution approved the protocol. Informed consent was obtained initially by telephone and later in writing during in-person exams.
Measures and definitions
BP measurement.
At both in-home exams, seated BP was measured twice. A trained technician used an aneroid sphygmomanometer (American Diagnostic Corporation, Hauppage, NY) to obtain the measurements after the participant was seated for 5 minutes. Measurements were taken in the left arm unless there was a contraindication for measurement on that side. A large cuff was used if the arm circumference was above 13 inches. The cuff was inflated 20 mm Hg beyond the point at which the pulse was obliterated then deflated at 2 mm Hg per second. The average measurement was used to determine systolic BP and diastolic BP. Measurements were monitored for quality control.13
Hypertension definition.
Hypertension was defined using the Joint National Commission 7 (JNC7) BP threshold of ≥140/90 mm Hg or self-report of antihypertensive medication use.14
Inflammation measurement.
Laboratory methods were previously described.15 Phlebotomy was performed in the morning after a 10- to 12-hour fast. Samples were centrifuged locally and shipped overnight on dry ice to the central laboratory at the University of Vermont where they were recentrifuged and stored at −80 °C. CRP measurement used a high-sensitivity particle-enhanced immunonephelometric assay on the BNIII nephelometer (N High Sensitivity CRP, Dade Behring Inc., Deerfield, IL) with an interassay coefficient of variation of 2–6%.
Risk factors for Black-White differences in incident hypertension.
Risk factors measured at baseline were previously described.3 Measures of obesity were measured at the in-home exam. Waist circumference was measured by placing a tape measure halfway between the bottom rib and iliac crest while the participant was standing. Body mass index (BMI) was calculated as mass in kilograms divided by the height in meters squared. Annual household income was self-reported as <$20,000, $20,000 to $34,000, $35,000 to $74,000, ≥$75,000, or refused to answer. Education level was self-reported as less than high school, high school, some college, and college graduate and above. Southern diet score was a dietary pattern identified from factor analysis using data from the Block 98 Food Frequency Questionnaire (FFQ).16 This dietary pattern is characterized by heavily processed meats and sugar-sweetened beverages. This Southern diet score is normally distributed with range from −4.5 to 8.2, with higher scores indicating a diet more consistent with the Southern diet. This FFQ was also used to calculate dietary sodium-to-potassium ratio, a continuous variable ranging from 0.3 to 2.5.17 The Dietary Approaches to Stop Hypertension (DASH) diet score was derived from the FFQ.18 It ranged from 9 to 37, a higher number indicating higher compliance with the DASH diet.
Anti-inflammatory medication use.
Anti-inflammatory medications were defined as statin, metformin, or aspirin use. Use of statin and metformin were based on the medication inventory. Regular aspirin use was self-reported.
Statistical analysis
We excluded participants with baseline hypertension, those missing hypertension status or baseline CRP, or who did not participate in the second in-home exam. We did not exclude participants with any other missing covariates for the primary analysis. We tabulated baseline characteristics by CRP quartiles. The distributions of CRP across race/sex groups were compared using Wilcoxon Rank Sum test. The risk ratio (RR) of hypertension by race and CRP quartile, stratified by sex, was calculated using Poisson regression with robust variance estimation, often called modified Poisson regression.19 The RR of incident hypertension was calculated by race–sex specific CRP quartiles, using the first quartile as the reference group for each race–sex stratum.
Mediation analysis.
After confirming no interactions between either sex and CRP or race and CRP with hypertension incidence (P > 0.10), we proceeded with mediation analysis. We evaluated the percentage mediation of the racial difference in incident hypertension by CRP using 5 models. The base model was minimally adjusted. Subsequent models added baseline risk factors that were previously established as potential mediators of the racial difference in hypertension in REGARDS.3 In addition to these established risk factors, we also considered the use of anti-inflammatory medications at baseline. We chose to cluster related risk factors into specific models, rather than include each risk factor individually. The first model included race, age, sex, and systolic BP. The second model added waist circumference in both males and females and BMI among males. Waist was not included for males because it was not related to the sex-specific race differences in hypertension in REGARDS.3 The third model added education and income. The fourth model added use of anti-inflammatory medications. The final model added Southern diet score, dietary sodium-to-potassium ratio, and DASH diet score.
To estimate percent of the racial difference mediated by CRP, we fit each of the above models with and without log-transformed CRP as a covariate and calculated the percent change in the race parameter. Confidence intervals (CIs) for these percent changes were estimated from the 2.5th and 97.5th percentiles from the distribution of 1,000 bootstrapped samples with replacement.20 For models in which there was substantial attenuation of the Black–White difference in hypertension prior to addition of CRP (i.e., with CIs including the null), we did not estimate mediation.
Sensitivity analyses.
We performed sensitivity analyses. First, we tabulated incident hypertension among those with and without an elevated CRP (threshold of 10 mg/L). Next, we utilized the American Heart Association/American College of Cardiology (AHA/ACC) 2017 hypertension definition of ≥130/80 for all analyses.21 Third, we restricted to participants without any missing covariate data. We did not assess mediation of CRP in sensitivity analyses if they were not also assessed in the primary analysis.
All statistical analyses were performed in SAS 9.4 (SAS Institute, Cary, NC). We used an alpha of 0.05 for our level of significance.
Results
Population
Of 30,239 participants, 17,847 (59%) were excluded for baseline hypertension, 56 (<1%) for data anomalies, 74 (<1%) for missing baseline hypertensive status, and 1,954 (6%) for missing CRP (Supplementary Figure S1 online). Of the remaining 11,536 participants, 1,715 (15%) died and 2,871 (25%) withdrew from study participation before the second exam. Of the remaining 6,950 participants who completed the second exam, 402 (6%) were excluded for missing hypertension status at the second exam, yielding a final analytic sample of 6,548 adults with a median follow-up of 9.2 (SD) years (interquartile range) 8.5, 9.9 years. In contrast to the 44% representation of the entire REGARDS population at baseline, Blacks comprised only 26% (1,696/6,548) of the population in this analysis. This was mostly due to the higher prevalence of baseline hypertension among Blacks.
Baseline characteristics
Compared to participants in the lower quartiles of CRP, participants in higher quartiles were more likely to be female, Black, and have lower income and education (Table 1). They also had higher levels of hypertension risk factors including increased BMI, higher waist circumference, greater Southern diet pattern, and higher prevalence of smoking. Age was similar across CRP quartiles. Use of statins was higher in lower CRP quartiles. Current alcohol use was lower in higher CRP quartiles.
Table 1.
Baseline characteristics by CRP quartile among those at risk for incident hypertension using JNC7 hypertension definition
| Variable* | N missing covariate | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
|---|---|---|---|---|---|
| N | 1,623 | 1,646 | 1,621 | 1,631 | |
| Age, y | 0 | 61 (8) | 62 (8) | 62 (8) | 61 (8) |
| Female, % | 0 | 43 | 49 | 56 | 67 |
| Black race, % | 0 | 19 | 22 | 26 | 37 |
| Stroke belt, % | 0 | 30 | 36 | 34 | 33 |
| Household income <$25,000, % | 715 | 7 | 9 | 10 | 16 |
| Educational level < HS diploma, % | 0 | 3 | 5 | 6 | 8 |
| BMI, kg/m2 | 16 | 25 (4) | 27 (4) | 28 (5) | 30 (6) |
| Waist circumference, cm | 23 | 86 (14) | 90 (13) | 93 (14) | 97 (15) |
| Current alcohol use, % | 99 | 51 | 49 | 46 | 41 |
| Smoking status | 16 | ||||
| Current, % | 8 | 11 | 12 | 17 | |
| Former, % | 39 | 38 | 38 | 38 | |
| Never, % | 54 | 51 | 49 | 45 | |
| Systolic BP, mm Hg | 0 | 116 (11) | 119 (11) | 120 (11) | 120 (11) |
| Diastolic BP, mm Hg | 0 | 72 (8) | 74 (8) | 74 (7) | 74 (8) |
| Southern diet score | 1,227 | −0.5 (0.9) | −0.3 (0.9) | −0.2 (0.9) | 0.0 (1.0) |
| Dietary sodium-to-potassium ratio | 1,227 | 0.8 (0.2) | 0.9 (0.2) | 0.9 (0.2) | 0.9 (0.3) |
| DASH diet score | 1,227 | 25.7 (4.5) | 24.7 (4.2) | 24.1 (4.2) | 23.6 (4.2) |
| Anti-inflammatory Medications | |||||
| Statin, % | 27 | 25 | 23 | 19 | 18 |
| Aspirin, % | 4 | 36 | 36 | 32 | 31 |
| Metformin, % | 27 | 3 | 3 | 4 | 5 |
Mean (SD) or percentage except where specified. CRP ranges were 0.15–.072 mg/L for quartile 1, 0.73–1.56 mg/L for quartile 2, 1.57–3.65 mg/L for quartile 3, and 3.66–130 mg/L for quartile 4. Abbreviations: BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; DASH, Dietary Approaches to Stop Hypertension; HS, high school; JNC 7, Joint National Commission 7.
CRP by race and sex
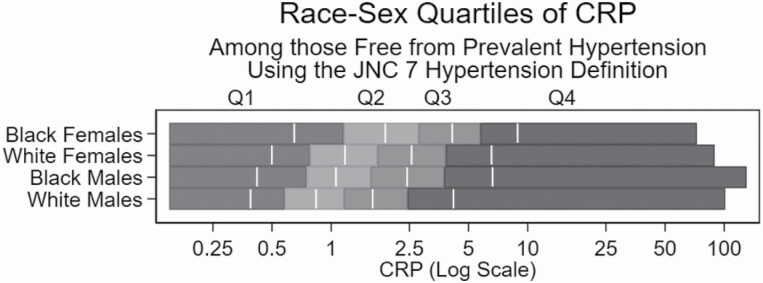
The distribution of CRP into race–sex–based quartiles is shown in Figure 1. The median (interquartile range) CRP was 2.8 (1.2, 5.8) for Black females, 1.7 (0.8, 3.8) for White females, 1.6 (0.8, 3.8) for Black males, and 1.2 (0.6, 2.4) for White males. CRP had different distributions across the race–sex groups (P < 0.0001).
Figure 1.
Race-sex stratified quartiles of CRP using JNC7 hypertension definition. Q1-4 indicates CRP quartile. The white vertical lines indicate the median value of each quartile. CRP is reported in mg/L. Abbreviations: CRP, C-reactive protein; JNC7, Joint National Commission 7.
Incidence of hypertension by race and CRP
Among the 6,548 participants without baseline hypertension, 3,343 (51%) developed incident hypertension. The incidence was 51% (95% CI 49, 53%) among White males and 59% (56, 63%) among Black males, a 12% relative increased risk among Black males (Table 2). Hypertension incidence was 45% (43, 47%) among White females and 62% (59, 65%) among Black females, representing a 26% greater relative adjusted incidence among Black females.
Table 2.
Sex-race stratified hypertension incidence and adjusted RRs, JNC7 hypertension definition
| Group | n Cases/N at risk | Hypertension incidence (95% CI) | Adjusted RR |
|---|---|---|---|
| (95% CI) | |||
| Males | |||
| White | 1,190/2344 | 51% (49, 53) | Ref |
| Black | 395/665 | 59% (56, 63) | 1.12 (1.04, 1.20) |
| Females | |||
| White | 1,121/2508 | 45% (43, 47) | Ref |
| Black | 637/1031 | 62% (59, 65) | 1.26 (1.18, 1.34) |
Adjusted for age, baseline systolic blood pressure, and region. The CI estimated through modified Poisson model. Abbreviations: CI, confidence interval; RR, risk ratios; JNC 7, Joint National Commission 7.
Higher CRP quartiles were associated with a greater incidence of hypertension, with an adjusted RR of 1.19 (95% CI 1.12, 1.28) for those in the 4th compared to 1st quartile of CRP (Table 3); P for trend across quartiles <0.001). There was no significant difference in the association of CRP with incident hypertension by race (Pinteraction = 0.46) or sex (Pinteraction = 0.65).
Table 3.
Overall and sex-race stratified hypertension incidence and adjusted RRs by quartile of CRP, JNC7 hypertension definition
| CRP quartile by group | n Cases/N at risk | Hypertension incidence (95%) CI) | Adjusted RR (95% CI) |
|---|---|---|---|
| All | |||
| Q1 | 713/1629 | 44% (41, 46%) | Ref |
| Q2 | 817/1652 | 49% (47, 52%) | 1.06 (0.99, 1.14) |
| Q3 | 859/1631 | 53% (50, 55%) | 1.10 (1.02, 1.18) |
| Q4 | 954/1636 | 58% (56, 61%) | 1.19 (1.12, 1.28) |
| Males | |||
| White | |||
| Q1 | 261/588 | 44% (41, 49%) | Ref |
| Q2 | 287/586 | 49% (45, 53%) | 1.06 (0.94, 1.19) |
| Q3 | 317/585 | 54% (50, 58%) | 1.13 (1.01, 1.27) |
| Q4 | 325/585 | 56% (52, 60%) | 1.14 (1.02, 1.28) |
| Black | |||
| Q1 | 90/166 | 54% (47, 62%) | Ref |
| Q2 | 101/166 | 61% (54, 69%) | 1.08 (0.90, 1.28) |
| Q3 | 95/167 | 57% (50, 65%) | 1.03 (0.86, 1.24) |
| Q4 | 109/166 | 66% (59, 73%) | 1.16 (0.98, 1.37) |
| Females | |||
| White | |||
| Q1 | 228/624 | 37% (33, 41%) | Ref |
| Q2 | 273/628 | 43% (40, 48%) | 1.07 (0.94, 1.22) |
| Q3 | 308/628 | 49% (45, 53%) | 1.16 (1.02, 1.31) |
| Q4 | 312/628 | 50% (46, 54%) | 1.14 (1.01, 1.30) |
| Black | |||
| Q1 | 145/257 | 56% (51, 63%) | Ref |
| Q2 | 146/258 | 57% (51, 63%) | 0.96 (0.83, 1.11) |
| Q3 | 168/259 | 65% (59, 71%) | 1.12 (0.98, 1.28) |
| Q4 | 178/257 | 69% (64, 75%) | 1.14 (0.99, 1.30) |
Q1–4 indicates each quartile. Adjusted for age, baseline systolic BP, and region. The CI estimated through modified Poisson model. Abbreviations: BP, blood pressure; CI, confidence interval; CRP, C-reactive protein; RR, risk ratios; JNC 7, Joint National Commission 7.
Race–sex–specific quartiles of CRP had similar associations with hypertension incidence as the overall analysis (Table 3). The trend across CRP quartiles was P = 0.02 White males, P = 0.02 for White females, P = 0.18 for Black males, and P = 0.10 for Black females. The point estimates for each sex–race strata were similar.
Mediation analysis
Females.
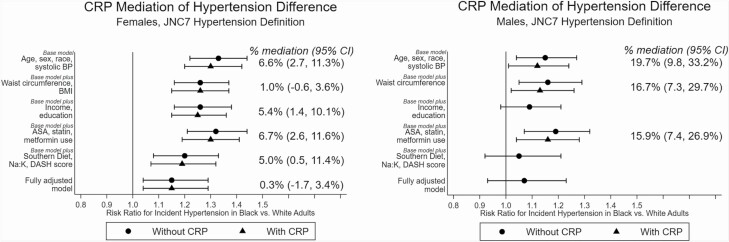
The Black–White RR for incident hypertension in females was 1.33 (95% CI 1.22, 1.44) without CRP in the base model (Figure 2). This RR was attenuated in models that added waist circumference and BMI (RR 1.26; 1.16, 1.37), income and education (RR 1.26; 1.16, 1.38), and dietary patterns (RR 1.20; 1.08, 1.33). However, the RR was essentially unchanged in the model adding anti-inflammatory medications (RR 1.32; 1.21, 1.44). The fully adjusted model incorporating all covariates was the most attenuated (RR 1.15; 1.04, 1.29).
Figure 2.
Mediation analysis of the Black–White difference in incident hypertension, JNC7 definition of hypertension.CRP is log-transformed. 95% CI is estimated by bootstrapping with 1,000 models. BMI was not included for males because it was not related to the sex-specific race differences in hypertension in REGARDS.3 The analytical subpopulations were 3,539 (F) and 3,009 (M) for the base model, 3,509 (F) and 3,007 (M) for the model adding waist circumference ± BMI, 3,539 (F) and 3,009 (M) for the model adding income and education, 3,520 (F) and 2,997 (M) for the model adding ASA, statin, and metformin use, 2,921 (F) and 2,400 (M) for the model adding dietary patterns, and 2,883 (F) and 2,389 (M) for the fully adjusted model. Abbreviations: ASA, aspirin; BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; DASH, Dietary Approaches to Stop Hypertension; JNC 7, Joint National Commission 7; Na:K, sodium-to-potassium dietary ratio; PA, physical activity; SDS, Southern diet score; Waist, waist circumference.
When CRP was added to models, it mediated the female Black–White RR for incident hypertension in several models. The estimated mediation was similar in the base model (6.6%; 2.7, 11.3%) and models that added income and education (5.4%; 1.4, 10.1%), anti-inflammatory medications (6.7%; 2.6, 11.6%), and diet factors (5.0%; 0.5, 11.4%). We did not observe mediation in the model with waist circumference and BMI (1.0%; −0.6, 3.6%) or in the fully adjusted model (0.3%; −1.7, 3.4%).
Males.
For males, the Black–White RR for incident hypertension was 1.15 (1.04, 1.27) in the base model without CRP. This RR was attenuated in models incorporating income and education (1.09; 0.98, 1.21) and dietary patterns (1.05; 0.92, 1.21). As in females, the attenuation was greatest for the model incorporating dietary patterns. We did not observe attenuation in the models incorporating waist circumference (1.16; 1.05, 1.29) or anti-inflammatory medications (1.19; 1.07, 1.32). The attenuation of the fully adjusted model (1.07; 0.93, 1.23) was similar to the model incorporating dietary patterns.
Because of the full attenuation in the models assessing income and education, dietary patterns, and the fully adjusted model, we did not assess mediation by CRP in these models. The percent mediation by adding CRP to the models was similar for the base model (19.7%, 9.8, 33.2%) and the models incorporating waist circumference (16.7%; 7.3, 29.7%), and anti-inflammatory medication use (15.9%; 7.4%, 26.9%).
Sensitivity analyses
Incident hypertension by CRP thresholds of 10 mg/L appear in Supplementary Table S4 online. A larger proportion of adults with CRP ≥10 mg/dL developed hypertension when compared to those with CRP <10 mg/dL (P = 0.03).
In sensitivity analyses comparing the incidence of hypertension and mediation analysis using the 2017 AHA/ACC hypertension threshold of 130/80 mm Hg, the findings were similar for males and females as they were for the primary analysis and JNC7 threshold of 140/90 mm Hg. The wider confidence bounds reflected the smaller analytical population (Supplementary Figure S4 online). In sensitivity analyses including participants without any missing covariate data, findings were similar for the 2 definitions of hypertension (Supplementary Figures S5 and S6 online).
Discussion
In this large prospective cohort study of Black and White males and females across the contiguous US, Blacks had higher CRP levels and more incident hypertension than Whites and the racial difference in hypertension incidence was more pronounced for females than males. Among females, CRP attenuated 5 to 7% of the racial difference in hypertension, even after adjusting for baseline BP, income and education, anti-inflammatory medication use, and dietary patterns. However, CRP was no longer a mediator after accounting for measures of obesity. This suggests that differences in BMI and waist circumference influence hypertension partly through inflammation. Among males, CRP attenuated 15 to 20% of the racial disparity, regardless of other factors accounted for. We could not assess income and education or dietary patterns in males as they attenuated too large of a component of the racial difference to allow addition of CRP to the model.
Despite its high prevalence, the underlying factors contributing to hypertension remain elusive. Inflammation likely plays an etiologic role as numerous studies have documented an association between higher levels of inflammatory markers and increased risk of incident hypertension.4,5,22–24 For example, participants with CRP levels >3.5 mg/L in the Women’s Health Study had a 52% increased risk of incident hypertension at 8 years of follow-up when compared to those with levels <0.43 mg/L.4 A similar association was seen in the more diverse Multi-Ethnic Study of Atherosclerosis.24 Several risk factors for hypertension are also pro-inflammatory, including obesity and specific dietary patterns.4,5,22–27 Weight loss and dietary modifications reduce inflammation and may prevent hypertension.27–33
To our knowledge, this is the first study of the role of inflammation on the racial disparity of hypertension. As was shown previously in REGARDS, the racial disparity of hypertension is greater among females than males and risk factors of income and education, measures of adiposity, and dietary patterns attenuated the Black–White disparity.3 Here, inflammation was a potent mediating factor for the excess risk of hypertension among both Black males and females. Among females, CRP mediated the excess risk of hypertension when accounting for income and education, dietary patterns, and anti-inflammatory use. However, there was a loss of mediation by CRP when incorporating measures of adiposity. Therefore, the association between excess weight and excess hypertension among Black females may act through inflammation. Among males, CRP mediated the excess risk of hypertension even when accounting for waist circumference and anti-inflammatory medication use. However, the racial disparity was fully attenuated in the non-CRP models adding income and education and in the model adding dietary patterns, so we were unable to assess the potential role of CRP for these models. Similar patterns were observed when using the lower 130/80 mm Hg hypertension definition and among those with complete ascertainment of covariates.
There are several clinical implications for these findings. National guidelines encourage optimization of health factors and behaviors across the spectrum of cardiovascular risk.34 Hypertension is a key condition that dramatically increases the risk of related conditions like cardiovascular disease and cognitive decline, especially among Blacks.35,36 Primordial prevention efforts focusing on normotensive adults with elevated CRP may augment current population-level risk reduction strategies. To specifically address differences in hypertension incidence among Blacks, future research may assess the role of weight loss and healthy dietary interventions among overweight or obese females with elevated CRP and dietary modifications among males with elevated CRP. Further, elevated levels of inflammatory markers are associated with excess risk for CVD when accounting for other traditional risk factors, i.e., smoking, hypertension, and dyslipidemia. Large clinical trials have found a benefit for the use of anti-inflammatory medications like statins and aspirin in reducing the risk of CVD events in primary prevention populations with elevated CRP.11,37 Statin use is associated with a modest reduction in BP.38 Whether anti-inflammatory medications play a role in prevention of hypertension in normotensive adults with elevated inflammatory markers is unknown.
This analysis has limitations. First, there were fewer Black adults at risk for hypertension due to their higher prevalence of hypertension at baseline, which led to lower power in the race-sex specific analyses. Second, ascertainment of hypertensive status depended on self-report of antihypertensive use and a single-time measure of BP, which may have led to misclassification of hypertension status. Third, CRP was only measured once. As it is an acute phase reactant, inclusion of participants who had recent acute illness may have led to misclassification. We suspect that this misclassification was rare and occurred at random in this population. Finally, 25% of participants with normal BP at baseline withdrew or died prior to the second exam. However, one prior evaluation of attrition in REGARDS found no evidence of selection bias that might contribute to estimation of risk factor effects on racial disparities in cardiovascular disease events.39 Attendance at visit 2 was incomplete, though a prior REGARDS analysis did not find evidence of attrition bias in this population when assessing using inverse probability weighting.3 Genetic variants also alter CRP levels, these data are unavailable in this population so we were unable to assess the contribution of these genetic variants to incident hypertension.40 We based the models off of a previous REGARDS analysis3 and did not include all hypertension risk factors, including levels of uric acid, hormone replacement therapy, and sleep deprivation.41–43
This analysis has several strengths. This cohort has a high level of geographic diversity across the US, included a large number of Blacks and Whites, and has carefully measured phenotypic data collected by trained technicians. Assessment of BP measurement used a rigorous protocol in the home, which may have reduced misclassification from white-coat hypertension that might occur in a study using field centers.
In conclusion, inflammation is a risk factor for hypertension and attenuates the racial disparity in hypertension affecting Black Americans. Multimodal interventions to address this disparity could target normotensive adults with elevated CRP and suboptimal risk factors.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the other investigators, staff, and participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.uab.edu/soph/regardsstudy/. The authors dedicate this manuscript to our colleague and friend, Dr Nancy Jenny, who passed away unexpectedly during its drafting. She was a highly respected and accomplished scientist and mentor. She will be remembered for her generous, collaborative nature, sharp wit, and inquisitive spirit.
FUNDING
This research project is supported by cooperative agreement U01 NS041588 co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA. Representatives of the NINDS were involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data.
Data Availability
The data from the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort study. Deidentified participant data and a data dictionary are available by contacting Ms Margaret Stewart (megstewart@uab.edu). Data are provided to those where the use of the data has been approved and where a data use agreement is in place. Alternatively, the study will support the analysis at the study coordinating center for approved manuscripts led by scientific investigators not directly affiliated with the study.
Disclosures
APC is the recipient of a research grant from Amgen, Inc. The authors declared no conflict of interest.
REFERENCES
- 1. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT-A, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FGR, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang Y-H, Khatibzadeh S, Khoo J-P, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CDH, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJC, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJL, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet Lond Engl. 2012; 380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 2018; 137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 3. Howard G, Cushman M, Moy CS, Oparil S, Muntner P, Lackland DT, Manly JJ, Flaherty ML, Judd SE, Wadley VG, Long DL, Howard VJ. Association of clinical and social factors with excess hypertension risk in Black compared with white US adults. JAMA 2018; 320:1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA 2003; 290:2945–2951. [DOI] [PubMed] [Google Scholar]
- 5. Niskanen L, Laaksonen DE, Nyyssönen K, Punnonen K, Valkonen VP, Fuentes R, Tuomainen TP, Salonen R, Salonen JT. Inflammation, abdominal obesity, and smoking as predictors of hypertension. Hypertension 2004; 44:859–865. [DOI] [PubMed] [Google Scholar]
- 6. Howard G, Cushman M, Kissela BM, Kleindorfer DO, McClure LA, Safford MM, Rhodes JD, Soliman EZ, Moy CS, Judd SE, Howard VJ; REasons for Geographic And Racial Differences in Stroke (REGARDS) Investigators . Traditional risk factors as the underlying cause of racial disparities in stroke: lessons from the half-full (empty?) glass. Stroke 2011; 42:3369–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cushman M, McClure LA, Howard VJ, Jenny NS, Lakoski SG, Howard G. Implications of increased C-reactive protein for cardiovascular risk stratification in black and white men and women in the US. Clin Chem 2009; 55:1627–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howard G, Prineas R, Moy C, Cushman M, Kellum M, Temple E, Graham A, Howard V. Racial and geographic differences in awareness, treatment, and control of hypertension: the reasons for geographic and racial differences in stroke study. Stroke 2006; 37:1171–1178. [DOI] [PubMed] [Google Scholar]
- 9. Albert MA, Danielson E, Rifai N, Ridker PM; PRINCE Investigators . Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 2001; 286:64–70. [DOI] [PubMed] [Google Scholar]
- 10. Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1999; 100:230–235. [DOI] [PubMed] [Google Scholar]
- 11. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ; JUPITER Study Group . Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 12. Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005; 25:135–143. [DOI] [PubMed] [Google Scholar]
- 13. Howard VJ, Woolson RF, Egan BM, Nicholas JS, Adams RJ, Howard G, Lackland DT. Prevalence of hypertension by duration and age at exposure to the stroke belt. J Am Soc Hypertens 2010; 4:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee . The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003; 289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 15. Gillett SR, Boyle RH, Zakai NA, McClure LA, Jenny NS, Cushman M. Validating laboratory results in a national observational cohort study without field centers: the reasons for geographic and racial differences in stroke cohort. Clin Biochem 2014; 47:243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Judd SE, Gutiérrez OM, Newby PK, Howard G, Howard VJ, Locher JL, Kissela BM, Shikany JM. Dietary patterns are associated with incident stroke and contribute to excess risk of stroke in black Americans. Stroke 2013; 44:3305–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Judd SE, Aaron KJ, Letter AJ, Muntner P, Jenny NS, Campbell RC, Kabagambe EK, Levitan EB, Levine DA, Shikany JM, Safford M, Lackland DT. High sodium:potassium intake ratio increases the risk for all-cause mortality: the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Nutr Sci 2013; 2:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008; 168:713–720. [DOI] [PubMed] [Google Scholar]
- 19. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–706. [DOI] [PubMed] [Google Scholar]
- 20. MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol 2007; 58:593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension 2018; 71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 22. Lakoski SG, Cushman M, Palmas W, Blumenthal R, D’Agostino RB Jr, Herrington DM. The relationship between blood pressure and C-reactive protein in the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol 2005; 46:1869–1874. [DOI] [PubMed] [Google Scholar]
- 23. Wang TJ, Gona P, Larson MG, Levy D, Benjamin EJ, Tofler GH, Jacques PF, Meigs JB, Rifai N, Selhub J, Robins SJ, Newton-Cheh C, Vasan RS. Multiple biomarkers and the risk of incident hypertension. Hypertension 2007; 49:432–438. [DOI] [PubMed] [Google Scholar]
- 24. Lakoski SG, Cushman M, Siscovick DS, Blumenthal RS, Palmas W, Burke G, Herrington DM. The relationship between inflammation, obesity and risk for hypertension in the Multi-Ethnic Study of Atherosclerosis (MESA). J Hum Hypertens 2011; 25:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shihab HM, Meoni LA, Chu AY, Wang NY, Ford DE, Liang KY, Gallo JJ, Klag MJ. Body mass index and risk of incident hypertension over the life course: the Johns Hopkins Precursors Study. Circulation 2012; 126:2983–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jayalath VH, de Souza RJ, Ha V, Mirrahimi A, Blanco-Mejia S, Di Buono M, Jenkins AL, Leiter LA, Wolever TM, Beyene J, Kendall CW, Jenkins DJ, Sievenpiper JL. Sugar-sweetened beverage consumption and incident hypertension: a systematic review and meta-analysis of prospective cohorts. Am J Clin Nutr 2015; 102:914–921. [DOI] [PubMed] [Google Scholar]
- 27. Gay HC, Rao SG, Vaccarino V, Ali MK. Effects of different dietary interventions on blood pressure: systematic review and meta-analysis of randomized controlled trials. Hypertension 2016; 67:733–739. [DOI] [PubMed] [Google Scholar]
- 28. Ryan AS, Ge S, Blumenthal JB, Serra MC, Prior SJ, Goldberg AP. Aerobic exercise and weight loss reduce vascular markers of inflammation and improve insulin sensitivity in obese women. J Am Geriatr Soc 2014; 62:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sams VG, Blackledge C, Wijayatunga N, Barlow P, Mancini M, Mancini G, Moustaid-Moussa N. Effect of bariatric surgery on systemic and adipose tissue inflammation. Surg Endosc 2016; 30:3499–3504. [DOI] [PubMed] [Google Scholar]
- 30. Nicklas JM, Sacks FM, Smith SR, LeBoff MS, Rood JC, Bray GA, Ridker PM. Effect of dietary composition of weight loss diets on high-sensitivity c-reactive protein: the Randomized POUNDS LOST trial. Obesity (Silver Spring) 2013; 21:681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dickinson HO, Mason JM, Nicolson DJ, Campbell F, Beyer FR, Cook JV, Williams B, Ford GA. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens 2006; 24:215–233. [DOI] [PubMed] [Google Scholar]
- 32. Nissensohn M, Román-Viñas B, Sánchez-Villegas A, Piscopo S, Serra-Majem L. The effect of the mediterranean diet on hypertension: a systematic review and meta-analysis. J Nutr Educ Behav 2016; 48:42–53.e1. [DOI] [PubMed] [Google Scholar]
- 33. Soltani S, Chitsazi MJ, Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) on serum inflammatory markers: a systematic review and meta-analysis of randomized trials. Clin Nutr 2018; 37:542–550. [DOI] [PubMed] [Google Scholar]
- 34. Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Sorlie P, Stone NJ, Wilson PWF, American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014; 63:2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Howard G, Banach M, Cushman M, Goff DC, Howard VJ, Lackland DT, McVay J, Meschia JF, Muntner P, Oparil S, Rightmyer M, Taylor HA. Is blood pressure control for stroke prevention the correct goal? The lost opportunity of preventing hypertension. Stroke 2015; 46:1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gorelick PB, Furie KL, Iadecola C, Smith EE, Waddy SP, Lloyd-Jones DM, Bae HJ, Bauman MA, Dichgans M, Duncan PW, Girgus M, Howard VJ, Lazar RM, Seshadri S, Testai FD, van Gaal S, Yaffe K, Wasiak H, Zerna C; American Heart Association/American Stroke Association . Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke 2017; 48:e284–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997; 336:973–979. [DOI] [PubMed] [Google Scholar]
- 38. Golomb BA, Dimsdale JE, White HL, Ritchie JB, Criqui MH. Reduction in blood pressure with statins: results from the UCSD Statin Study, a randomized trial. Arch Intern Med 2008; 168:721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Long DL, Howard G, Long DM, Judd S, Manly JJ, McClure LA, Wadley VG, Safford MM, Katz R, Glymour MM. An investigation of selection bias in estimating racial disparity in stroke risk factors. Am J Epidemiol 2019; 188:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ellis J, Lange EM, Li J, Dupuis J, Baumert J, Walston JD, Keating BJ, Durda P, Fox ER, Palmer CD, Meng YA, Young T, Farlow DN, Schnabel RB, Marzi CS, Larkin E, Martin LW, Bis JC, Auer P, Ramachandran VS, Gabriel SB, Willis MS, Pankow JS, Papanicolaou GJ, Rotter JI, Ballantyne CM, Gross MD, Lettre G, Wilson JG, Peters U, Koenig W, Tracy RP, Redline S, Reiner AP, Benjamin EJ, Lange LA. Large multiethnic Candidate Gene Study for C-reactive protein levels: identification of a novel association at CD36 in African Americans. Hum Genet 2014; 133:985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2011; 63:102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Swica Y, Warren MP, Manson JE, Aragaki AK, Bassuk SS, Shimbo D, Kaunitz A, Rossouw J, Stefanick ML, Womack CR. Effects of oral conjugated equine estrogens with or without medroxyprogesterone acetate on incident hypertension in the Women’s Health Initiative hormone therapy trials. Menopause 2018; 25:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Calhoun DA, Harding SM. Sleep and hypertension. Chest. 2010; 138:434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort study. Deidentified participant data and a data dictionary are available by contacting Ms Margaret Stewart (megstewart@uab.edu). Data are provided to those where the use of the data has been approved and where a data use agreement is in place. Alternatively, the study will support the analysis at the study coordinating center for approved manuscripts led by scientific investigators not directly affiliated with the study.
Disclosures
APC is the recipient of a research grant from Amgen, Inc. The authors declared no conflict of interest.