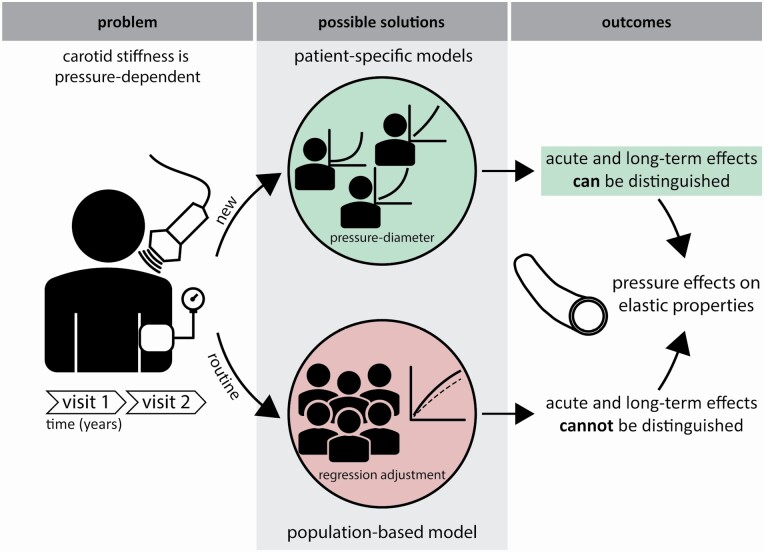
Abstract
Background
Conventional measures for assessing arterial stiffness are inherently pressure dependent. Whereas statistical pressure adjustment is feasible in (larger) populations, it is unsuited for the evaluation of an individual patient. Moreover, statistical “correction” for blood pressure may actually correct for: (i) the acute dependence of arterial stiffness on blood pressure at the time of measurement; and/or (ii) the remodeling effect that blood pressure (hypertension) may have on arterial stiffness, but it cannot distinguish between these processes.
METHODS
We derived—assuming a single-exponential pressure–diameter relationship—3 theoretically pressure-independent carotid stiffness measures suited for individual patient evaluation: (i) stiffness index β0, (ii) pressure-corrected carotid pulse wave velocity (cPWVcorr), and (iii) pressure-corrected Young’s modulus (Ecorr). Using linear regression analysis, we evaluated in a sample of the CATOD study cohort changes in mean arterial pressure (ΔMAP) and comparatively the changes in the novel (Δβ0, ΔcPWVcorr, and ΔEcorr) as well as conventional (ΔcPWV and ΔE) stiffness measures after a 2.9 ± 1.0-year follow-up.
RESULTS
We found no association between ΔMAP and Δβ0, ΔcPWVcorr, or ΔEcorr. In contrast, we did find a significant association between ΔMAP and conventional measures ΔcPWV and ΔE. Additional adjustments for biomechanical confounders and traditional risk factors did neither materially change these associations nor the lack thereof.
Conclusions
Our newly proposed pressure-independent carotid stiffness measures avoid the need for statistical correction. Hence, these measures (β0, cPWVcorr, and Ecorr) can be used in a clinical setting for (i) patient-specific risk assessment and (ii) investigation of potential remodeling effects of (changes in) blood pressure on intrinsic arterial stiffness.
Keywords: arterial remodeling, arterial stiffness, blood pressure, hypertension, pressure dependence
Graphical Abstract
Graphical Abstract.
Arterial stiffness measures are increasingly used in cardiovascular risk management.1–3 However, current measures are inherently pressure dependent, which confounds measured changes in arterial stiffness in a treatment setting.4,5 Usually, this pressure dependence is corrected for by adjusting for mean arterial pressure (MAP) in multivariable regression models. Whereas such statistical adjustment is feasible in (larger) populations, it is unsuited for the evaluation of an individual patient.4,6 Adjustment would be especially relevant in patients receiving antihypertensive drugs, where consequently measured changes in arterial stiffness may not necessarily reflect changes in intrinsic arterial wall properties. Moreover, statistical “correction” for blood pressure may actually correct for 2 processes6: (i) the acute dependence of arterial stiffness on blood pressure at the time of measurement; and/or (ii) the remodeling effect that blood pressure (hypertension) may have on arterial stiffness in the long term, but it cannot distinguish between these processes. Therefore, pressure-independent arterial stiffness measures are much needed to accurately assess progression or regression of arterial stiffening in individual patients in a clinical setting.
Recently, we demonstrated a method that allows the calculation of a theoretically pressure-independent stiffness index β0.7,8 The β0 method uses an established single-exponential model (fitted to data from ultrasound and blood pressure measurements) to describe the pressure–diameter curve of an artery for an individual patient.9 For the carotid artery, local stiffness can be expressed as (carotid) pulse wave velocity (cPWV) using the Bramwell–Hill equation.5,10 In the present paper, we combine the Bramwell–Hill equation and single-exponential model to derive cPWV for a predefined pressure range (e.g., 80–120 mm Hg). The resultant pressure-corrected measure is (theoretically) fully pressure independent.
Stiffness index β0 and cPWV are structural stiffness measures describing the dynamic elastic property of the artery as a whole. As such, they depend on material stiffness, as well as artery size and geometry. Provided that wall thickness measurements are also available, the above approach may also be used to derive a pressure-corrected incremental Young’s modulus, which is a measure for wall material stiffness.1,11,12
In the present paper, we derive pressure-corrected versions of cPWV (cPWVcorr) and carotid artery Young’s modulus E (Ecorr) and comparatively evaluate them against their conventional counterparts (and β0) in a clinical outpatient setting.13
METHODS
Study population
The present study utilizes data of a cross-sectional study on carotid and aortic stiffness in essential hypertension in relation to classical cardiovascular risk factors and target organ damage (the CATOD study).13 Briefly, hypertensive patients were recruited from the hypertension outpatient clinic of the University Hospital of Pisa, Italy, between September 2007 and January 2011. Main exclusion criteria were known secondary forms of hypertension, end-stage renal disease, any other major comorbidities, and any other disease reducing life expectancy to less than 1 year.13 Patients were followed up after 2.9 ± 1.0 years (mean ± SD). Arterial stiffness assessment was part of their routine follow-up. The total CATOD population comprised 450 patients, of which 147 had follow-up measurements. For the present analysis, we included all consecutive outpatients that had complete vascular assessments at baseline and follow-up (n = 126).
Measurements
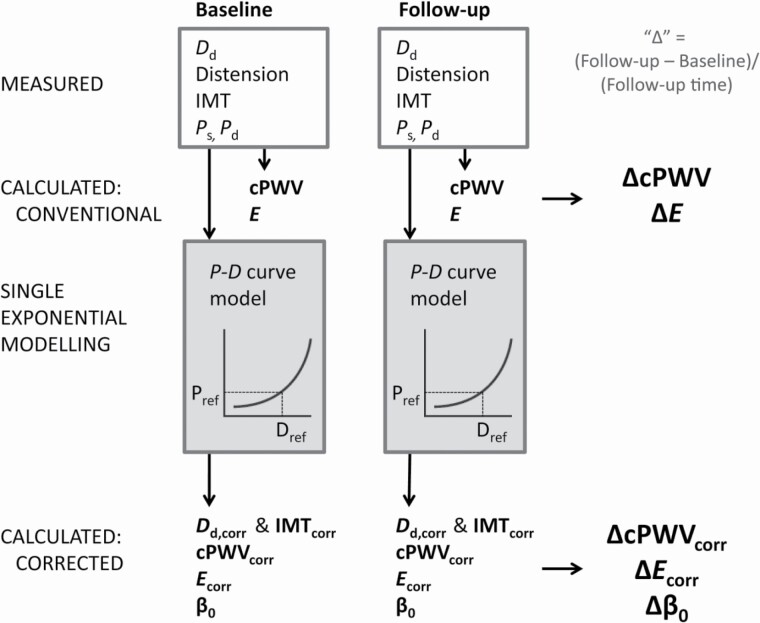
The detailed measurement protocol is described elsewhere.13 In short, brachial blood pressures were measured under quiet conditions after the patient had rested in supine position for at least 10 minutes. Three consecutive measurements were taken at 2-minute intervals using an automatic oscillometric device (OMRON-705IT, Omron Corporation, Kyoto, Japan). Systolic (Ps) and diastolic (Pd) brachial blood pressures were taken as the averages of the respective second and third blood pressure readings. Common carotid artery B-mode scans (at a frame rate of 25 Hz) were obtained by a trained operator using a high-resolution ultrasound scanner with a 10 MHz linear array transducer (MyLab25; ESAOTE, Florence, Italy). Regular B-mode recordings provide reliable estimates of diameter and distension, if averaged over a sufficient number of beats.14,15 Specifically, the left common carotid artery was imaged at least 1 cm proximal to the carotid bulb in longitudinal orientation over a 1-cm-wide region (visually) free of plaques. The recordings were analyzed with commercial software (Carotid Studio, Cardiovascular Suite, Quipu srl, Pisa, Italy) validated for accuracy and precision against the gold-standard radiofrequency approach.14 Briefly, the software automatically detects arterial interfaces and estimates the instantaneous mean diameter as the distance between far and near media–adventitia interfaces. Intima–media thickness (IMT) was estimated at (end-)diastole as the spatial average of the far wall lumen–intima to media–adventitia interface distances, as previously described.14 The (left) common carotid artery systolic (Ds) and diastolic (Dd) diameters and IMT thus obtained were used to calculate carotid stiffness and Young’s modulus. Figure 1 shows how the measurements are used to calculate outcome measures, as further detailed in the next section.
Figure 1.
At baseline and follow-up, ultrasonic diastolic diameter (Dd), distension, and intima–media thickness (IMT) and oscillometric systolic and diastolic blood pressures (Ps and Pd, respectively) were measured. From the measurements, the changes at follow-up normalized to follow-up time in years (Δ) were calculated for carotid pulse wave velocity (cPWV), Young’s modulus (E), and the recently introduced stiffness index β0 (at reference pressure of 100 mm Hg). Assuming a single-exponential model of the pressure–diameter (P–D) curve, pressure-corrected diastolic diameter and IMT (Dd,corr and IMTcorr), cPWVcorr, and Ecorr were derived. The Δ’s of corrected and conventional measures are subsequently used as dependent variables in linear regression modeling.
Calculations
The following calculations were performed using SPSS version 26 (IBM Corp, Armonk, NY).
Conventional carotid stiffness measures
Local cPWV (in m/s) was estimated using the Bramwell–Hill equation10:
| (1) |
with Ps and Pd the systolic and diastolic blood pressure; Ds and Dd the systolic and diastolic carotid diameter; and ρ the blood mass density, taken to be 1,050 kg/m3. The Bramwell–Hill equation was derived under the assumption that blood can be considered an incompressible and inviscid liquid, and that the vessel wall is axially constrained.16 The local incremental Young’s modulus (E, in MPa) was subsequently calculated using the rearranged Moens–Korteweg equation11:
| (2) |
with IMT the intima–media thickness of the common carotid artery. The Moens–Korteweg equation assumes, in addition to the aforementioned Bramwell–Hill equation assumptions, the artery wall to be thin (with respect to diameter), and to consist of an incompressible material.16
Pressure-independent stiffness index β0
The calculation of pressure-corrected measures was based on an (assumed) exponential relationship between arterial pressure and diameter9:
| (3) |
with P and D continuous variables describing pressure and diameter; Pref and Dref a reference pressure and diameter; and β0 the pressure-independent stiffness index, as described previously7:
| (4) |
using a reference pressure (Pref) of 100 mm Hg.7
Pressure-corrected cPWV and Young’s modulus
Pressure-corrected versions of conventional cPWV (cPWVcorr) and E (Ecorr) were derived, based on the relationship given in equation (3). Full details on the derivation are given in Supplementary Digital Content 1 online.
Briefly, cPWVcorr and Ecorr were obtained by the following consecutive steps: (i) parameterizing the exponential pressure–diameter (P–D) relationship (finding β0 and Dref) for each individual patient, (ii) calculating the diameters at predefined pressures of 120 and 80 mm Hg using the individualized P–D relationship, (iii) correcting IMT to 80 mm Hg to obtain IMTcorr (assuming incompressibility), and (iv) inputting the obtained values in equations (1) and (2) to calculate cPWVcorr and Ecorr.
Statistical analysis
Values are given as mean ± SD, unless noted otherwise. Because stiffening processes presumably have cumulative effects with progression of time17 and follow-up time varied between individuals, we normalized changes to the follow-up period, with Δ’s defined as: (follow-up value–baseline value)/(follow-up time in years) (Figure 1).
Changes between baseline and follow-up measurements were compared with a paired t-test for continuous variables, and McNemar’s test for categorical variables.
We used multivariable linear regression analysis to assess to what extent the changes-over-time in the pressure-corrected (ΔcPWVcorr, ΔEcorr, and Δβ0) and conventional (ΔcPWV and ΔE) measures were associated with the corresponding change in mean arterial pressure (ΔMAP; crude model). Model 2 was additionally adjusted for biomechanical factors ΔIMT, ΔHR, and drugs. Drugs were defined as a categorical variable for using antihypertensive drugs at follow-up (no drugs, 1 type of antihypertensive drugs, 2, or more types of antihypertensive drugs). Because IMT is mathematically related to E (see above), we did not include ΔIMT and ΔIMTcorr in the models 2 and 3 for ΔE and ΔEcorr, to avoid collinearity. Model 3 was additionally adjusted for age, sex, smoking (yes/no), body mass index (BMI), diabetes (yes/no), and hypercholesterolemia (yes/no). For age, BMI, diabetes, and hypercholesterolemia, the value or status at baseline was taken (as applicable).
All statistical analyses were performed using SPSS (SPSS version 26, IBM Corp, Armonk, NY). A 2-sided P value ≤0.05 was considered statistically significant.
RESULTS
Study population
The total study population consisted of 126 patients, from which 2 were excluded because of missing covariates (n = 1 for BMI, n = 1 for use of antihypertensive drugs) in 1 of the 2 visits. The main regression analysis was ultimately performed with 124 patients. Table 1 shows an overview of the general characteristics of the final study population. The mean follow-up time was 2.9 ± 1.0 years. Diastolic blood pressure and MAP were statistically significantly lower at follow-up compared with baseline. Heart rate and diastolic diameter were statistically significantly higher during follow-up. Although systolic blood pressure was lower during follow-up, this result was not statistically significant.
Table 1.
Study population characteristics and carotid artery dimensions
| Parameter | Baseline | Follow-up |
|---|---|---|
| Sex (# of m/f) | 83/41 | — |
| Age (years) | 57.7 ± 8.6 | 61.8 ± 9.0** |
| Smoking (number, %) | 24 (19.4%) | 24 (19.4%) |
| Diabetes (number, %) | 35 (28.2%) | 35 (28.2%) |
| Hypercholesterolemia (number, %) | 84 (67.7%) | 63 (67.7%)a |
| Antihypertensive drugs (number, %) | 82 (66.1%) | 109 (87.9)** |
| P s (mm Hg) | 142 ± 14 | 141 ± 17 |
| P d (mm Hg) | 82 ± 9 | 79 ± 11** |
| MAP (mm Hg) | 102 ± 9 | 100 ± 11* |
| BMI (kg/m2) | 28.3 ± 4.0 | 28.1 ± 4.2 |
| Heart rate (bpm) | 68 ± 11 | 71 ± 12** |
Data are presented as mean ± SD, n = 124. Abbreviations: BMI, body mass index; Dd, diastolic common carotid diameter; IMT, common carotid intima media thickness (at diastolic pressure); MAP, mean arterial pressure; Pd, diastolic blood pressure; Ps, systolic blood pressure.
a n = 93 due to missing information during follow-up.
Follow-up (2.9 ± 1.0 years) values were tested against baseline values using paired t-tests: *P < 0.05, **P < 0.001.
Group averages and SDs of the pressure-corrected and conventional carotid stiffness measures are shown in Table 2. By definition, the pressure-corrected measures were lower than their conventional counterparts, because the correction normalizes toward a normotensive pressure range of 120/80 mm Hg (systolic/diastolic). All carotid stiffness measures were statistically higher at follow-up compared with baseline.
Table 2.
Conventional and corrected carotid dimensions and stiffness measures
| Parameter | Baseline | Follow-up | Δ (unit/year) |
|---|---|---|---|
| IMT (mm) | 0.75 ± 0.15 | 0.78 ± 0.18 | 0.01 ± 0.08 |
| IMTcorr (mm) | 0.76 ± 0.15 | 0.78 ± 0.18 | 0.01 ± 0.08 |
| D d (mm) | 7.24 ± 0.85 | 7.55 ± 0.87 | 0.12 ± 0.30** |
| D d,corr (mm) | 7.20 ± 0.87 | 7.56 ± 0.88 | 0.13 ± 0.30** |
| cPWV (m/s) | 7.5 ± 1.3 | 7.7 ± 1.2 | 0.1 ± 0.6* |
| cPWVcorr (m/s) | 7.1 ± 1.2 | 7.5 ± 1.2 | 0.1 ± 0.6* |
| E (MPa) | 0.59 ± 0.23 | 0.65 ± 0.23 | 0.02 ± 0.11* |
| E corr (MPa) | 0.53 ± 0.20 | 0.61 ± 0.22 | 0.03 ± 0.10** |
| β 0 | 8.5 ± 2.9 | 9.3 ± 2.9 | 0.3 ± 1.3* |
Data are presented as mean ± SD, n = 124. . Abbreviations: β0, pressure-independent stiffness index; cPWV, carotid pulse wave velocity; cPWVcorr, pressure-corrected cPWV; Dd, diastolic diameter; Dd,corr, diameter corresponding to a pressure of 80 mm Hg; E, Young’s modulus; Ecorr, pressure-corrected Young’s modulus; IMT, intima media thickness; IMTcorr, IMT corresponding to a pressure of 80 mm Hg.
Follow-up (2.9 ± 1.0 years) values were tested against baseline values using paired t-tests: *P < 0.05, **P < 0.001.
Carotid stiffness
Table 3 shows the regression models for ΔcPWV and ΔcPWVcorr. There was a significant positive relationship between ΔMAP and ΔcPWV (β = 0.023, confidence interval, CI: [0.000; 0.045] m/s/mm Hg, P = 0.047). For ΔcPWVcorr, there was no significant relation with ΔMAP (β = −0.018, CI: [−0.040; 0.003] m/s/mm Hg, P = 0.091). Further adjustments in models 2 and 3 did not materially change these associations (Table 3).
Table 3.
Final regression models for the associations of ΔcPWV and ΔcPWVcorr, as well as ΔE and ΔEcorr with ΔMAP
| ΔcPWV | ΔcPWVcorr | |||
|---|---|---|---|---|
|
β (95% CI) (m/s/mm Hg) |
P value |
β (95% CI) (m/s/mm Hg) |
P value | |
| Crude | 0.023 (0.000; 0.045) | 0.047 | −0.018 (−0.040; 0.003) | 0.091 |
| Model 2 | 0.027 (0.005; 0.050) | 0.019 | −0.018 (−0.039; 0.004) | 0.105 |
| Model 3 | 0.026 (0.002; 0.050) | 0.035 | −0.019 (−0.042; 0.004) | 0.104 |
| ΔE | ΔEcorr | |||
| Crude | 0.007 (0.003; 0.012) | 0.001 | −0.001 (−0.005; 0.002) | 0.446 |
| Model 2 | 0.007 (0.003; 0.011) | 0.001 | −0.002 (−0.005; 0.002) | 0.398 |
| Model 3 | 0.007 (0.002; 0.012) | 0.003 | −0.002 (−0.006; 0.002) | 0.350 |
Abbreviations: Δ denotes β, unstandardized regression coefficient; BMI, body mass index; CI, 95% confidence interval; cPWV, carotid pulse wave velocity; cPWVcorr, pressure-corrected cPWV according to equation (S1); E, Young’s modulus; Ecorr, pressure-corrected Young’s modulus according to equation (S6); MAP, mean arterial pressure. For ΔcPWV model 2: crude model + ΔIMT, ΔHR, use of antihypertensive drugs at the first visit. Model 3: model 2 + age, sex, smoking status, body mass index, diabetes status, and hypercholesterolemia. For ΔE model 2: crude model + ΔHR and use of antihypertensive drugs at the first visit. Model 3: model 2 + age, sex, smoking status, BMI, diabetes status, and hypercholesterolemia. In models 2 and 3 for ΔcPWV the change in intima–media thickness ΔIMT was used. Correspondingly, in the models for ΔcPWVcorr the pressure-corrected ΔIMTcorr was used.
Notably, ΔIMT was the only other significant determinant of ΔcPWV in model 2 (β = 1.859, CI: [0.494; 3.224] m/s/mm, P = 0.008; data not shown in Table 3). In the final model (model 3), ΔIMT remained a significant determinant of ΔcPWV (β = 1.967, CI: [0.554; 3.380] m/s/mm, P = 0.007). These findings were corroborated by the fact that in model 2, ΔIMTcorr was the only significant determinant of ΔcPWVcorr (β = 1.573, CI: [0.253; 2.894] m/s/mm, P = 0.020) which persisted in model 3 (β = 1.694, CI: [0.328; 3.060] m/s/mm, P = 0.016).
Carotid Young’s modulus
The change in MAP was a significant determinant of ΔE (β = 0.007, CI: [0.003; 0.012] MPa/mm Hg, P = 0.001) but not of ΔEcorr (β = −0.001, CI: [−0.005; 0.002] MPa/mm Hg, P = 0.446). Further adjustments in models 2 and 3 did not materially change these associations (Table 3).
Stiffness index β0
Table 4 shows that, already in the crude model, ΔMAP was not a significant determinant of Δβ0 (β = −0.042, CI: [−0.092; 0.009] mm Hg−1, P = 0.103). Further adjustments in models 2 and 3 did not materially change the association (Table 4). ΔIMT was a significant determinant of Δβ0 in models 2 and 3 (respectively: β = 3.422, CI: [0.321; 6.522] mm−1, P = 0.031; and β = 3.654, CI: [0.442; 6.866] mm−1, P = 0.026).
Table 4.
Final regression models for the association of Δβ0 with ΔMAP
| Δβ0 | ||
|---|---|---|
|
β (95% CI) (mm Hg−1) |
P value | |
| Crude | −0.042 (−0.092; 0.009) | 0.103 |
| Model 2 | −0.040 (−0.090; 0.010) | 0.119 |
| Model 3 | −0.043 (−0.097; 0.011) | 0.119 |
Abbreviations: Δ denotes (follow-up value–baseline value)/(follow-up time in years) β, unstandardized regression coefficient; β0, pressure-independent stiffness index according to equation (4); BMI, body mass index; CI, 95% confidence interval; MAP, mean arterial pressure. Model 2: crude model + corrected ΔIMT, ΔHR, and use of antihypertensive drugs at the first visit. Model 3: model 2 + age, sex, smoking status, BMI, diabetes status, and hypercholesterolemia.
Discussion
In the present study, we tested 3 novel pressure-corrected carotid artery stiffness measures (β0, cPWVcorr, and Ecorr) in a hypertensive outpatient population followed up at 2.9 ± 1.0 years. We evaluated the association of changes in these novel measures and their conventional counterparts with changes in MAP as observed over the follow-up period. The changes in the conventional stiffness measures (cPWV and E) were significantly related to the changes in MAP, while the changes in the pressure-corrected measures were not. This finding is essential, as it illustrates the (known) pressure dependence of conventional stiffness measures. More importantly, the pressure-independent indices allow discrimination of potential remodeling effects due to blood pressure (hypertension), from acute confounding of blood pressure during vascular measurements.
An advantage of the presented pressure corrections is that they do not require any additional measurements. Therefore, reanalysis of existing datasets is possible. Both Δβ0 and ΔcPWVcorr can be readily calculated when transverse carotid (or aortic) cyclic dimensions are available.14,15,18,19 In addition, regional (i.e., transit time) pulse wave velocity measurements can be similarly pressure corrected.20 When IMT data are also recorded during such measurements (as done in the present study), it is possible to gain additional information on material stiffness of the carotid wall, using the pressure-corrected Young’s modulus, Ecorr. This provides information on the material properties of the wall at a normalized pressure (of 80 mm Hg in this case). In our calculations, we assumed the artery wall to consist of a single, homogeneous material. Therefore, Ecorr represents the “average” material stiffness of the wall material.
In our statistical analyses, ΔIMT was shown to be positively correlated to ΔcPWV and, similarly, ΔIMTcorr was associated with ΔcPWVcorr. Such associations are consistent with the Moens–Korteweg relationship (equation (2)), in which an isolated increase in vessel wall thickness will increase local pulse wave velocity.11 Changes in carotid wall thickness have been described in relation to wall stress adaptation, with an increased IMT reflecting compensatory action to normalize wall stress.1,21
In the present study, we did not find statistically significant associations between the change in stiffness measures and known risk factors (model 3). It is important to note that we assessed associations of “single-point accounts” of risk factors with changes in carotid stiffness over time and that this population showed a globally well controlled cardiovascular profile, achieved by an increase in use of cardiovascular medication over time. The present results indicate that there may not be a difference in the change of carotid stiffness or Young’s modulus related to age, sex, diabetes, smoking, or hypercholesterolemic status. Using actual blood glucose and total cholesterol levels (normalized to follow-up time) instead of the categorical variables diabetes and hypercholesterolemia did not lead to different results. Presumed there may be such differences, the follow-up time in the present study may have been insufficient to reveal them.
In our analysis, we chose to only use antihypertensive drug use as an explanatory variable, and not to stratify for different classes and dosages of drugs. This choice was made to avoid overfitting of our models. In the 10-year follow-up MESA study cohort, Gepner et al. found no consistent association of stiffness progression with specific antihypertensive drug classes, whereas plain blood pressure control as such was a clear determinant.22 In future studies targeting arterial wall destiffening by specific drugs in outpatients, the pressure-corrected measures could be especially useful. Because the proposed measures are theoretically pressure-independent, statistical blood pressure correction can be avoided, potentially allowing smaller sample sizes in such studies.
The pressure correction as presented here is subject to an assumed single-exponential relationship between pressure and diameter (equation (3)).9,23 Consequently, the correction will leave residual pressure dependence in case the pressure–diameter relation has a higher-order curvilinearity. This may potentially be the case in hypotensive and/or younger subjects, where elastin dominates arterial mechanics.7,24
Finally, the total CATOD cohort comprised 450 patients.13 Compared with patients that did not have a follow-up measurement, the patients included in this study had a less favorable cardiovascular risk profile and higher blood pressure (Supplementary Table S1 online). A possible explanation is that patients with better controlled hypertension and/or with a relatively favorable cardiovascular risk profile are often referred back to the GP. Therefore, our follow-up cohort might not be representative of the general hypertensive population.
In conclusion, our newly proposed pressure-independent carotid stiffness measures do not require statistical correction. Hence, these measures (β0, cPWVcorr, and Ecorr) can be used in a clinical setting for (i) patient-specific risk assessment and (ii) investigation of potential remodeling effects of (changes in) blood pressure. Moreover, these measures may allow smaller sample sizes in intervention studies targeting arterial wall destiffening.
Supplementary Material
FUNDING
B.S. was supported by Endeavour Research Fellowship ERF_PDR_142613_2015 awarded by the Australian Government, by Rubicon grant 452172006 awarded by the Netherlands Organisation for Scientific Research (NWO), and by the European Union’s Horizon 2020 research and innovation program (no. 793805).
AUTHORS’ CONTRIBUTION
S.T., L.G., and R.M.B. contributed to data acquisition; M.B., B.S., S.B., and K.D.R. contributed to data curation, formal analysis, and writing of the original manuscript; M.H.G.H., S.T., L.G., T.D., and R.M.B. critically edited and revised the manuscript.
DISCLOSURE
L.G. is a cofounder of QUIPU srl, a spin-off company of the National Research Council and the University of Pisa, Italy.
DATA AVAILABILITY
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. Humphrey JD, Harrison DG, Figueroa CA, Lacolley P, Laurent S. Central artery stiffness in hypertension and aging: a problem with cause and consequence. Circ Res 2016; 118:379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F; Task Force Members . 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 3. Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T, Artery S; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries . Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012; 30:445–448. [DOI] [PubMed] [Google Scholar]
- 4. Spronck B, Delhaas T, De Lepper AG, Giroux J, Goldwasser F, Boutouyrie P, Alivon M, Reesink KD. Patient-specific blood pressure correction technique for arterial stiffness: evaluation in a cohort on anti-angiogenic medication. Hypertens Res 2017; 40:752–757. [DOI] [PubMed] [Google Scholar]
- 5. Spronck B, Heusinkveld MH, Vanmolkot FH, Roodt JO, Hermeling E, Delhaas T, Kroon AA, Reesink KD. Pressure-dependence of arterial stiffness: potential clinical implications. J Hypertens 2015; 33:330–338. [DOI] [PubMed] [Google Scholar]
- 6. Spronck B, Delhaas T, Butlin M, Reesink KD, Avolio AP. Options for dealing with pressure dependence of pulse wave velocity as a measure of arterial stiffness: an update of cardio-ankle vascular index (CAVI) and CAVI0. Pulse (Basel) 2018; 5:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spronck B, Avolio AP, Tan I, Butlin M, Reesink KD, Delhaas T. Arterial stiffness index beta and cardio-ankle vascular index inherently depend on blood pressure but can be readily corrected. J Hypertens 2017; 35:98–104. [DOI] [PubMed] [Google Scholar]
- 8. Spronck B. Stiff vessels approached in a flexible way: advancing quantification and interpretation of arterial stiffness. Artery Res 2018; 21:63–68. [Google Scholar]
- 9. Hayashi K, Handa H, Nagasawa S, Okumura A, Moritake K. Stiffness and elastic behavior of human intracranial and extracranial arteries. J Biomech 1980; 13:175–184. [DOI] [PubMed] [Google Scholar]
- 10. Bramwell J, Hill A. The velocity of the pulse wave in man. Proc R Soc Lond B 1922; 93:298–306. [Google Scholar]
- 11. O’Rourke M. Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertension 1990; 15:339–347. [DOI] [PubMed] [Google Scholar]
- 12. Spronck B, Humphrey J. Arterial stiffness: different metrics, different meanings. J Biomech Eng 2019; 141: 0910041–09100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bruno RM, Cartoni G, Stea F, Armenia S, Bianchini E, Buralli S, Giannarelli C, Taddei S, Ghiadoni L. Carotid and aortic stiffness in essential hypertension and their relation with target organ damage: the CATOD study. J Hypertens 2017; 35:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bianchini E, Bozec E, Gemignani V, Faita F, Giannarelli C, Ghiadoni L, Demi M, Boutouyrie P, Laurent S. Assessment of carotid stiffness and intima-media thickness from ultrasound data: comparison between two methods. J Ultrasound Med 2010; 29:1169–1175. [DOI] [PubMed] [Google Scholar]
- 15. Steinbuch J, Hoeks AP, Hermeling E, Truijman MT, Schreuder FH, Mess WH. Standard B-mode ultrasound measures local carotid artery characteristics as reliably as radiofrequency phase tracking in symptomatic carotid artery patients. Ultrasound Med Biol 2016; 42:586–595. [DOI] [PubMed] [Google Scholar]
- 16. Nichols WW, O’Rourke MF, Vlachopoulos C.. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. CRC Press: London, UK, 2011. [Google Scholar]
- 17. Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, Damasceno A, Delles C, Gimenez-Roqueplo AP, Hering D, López-Jaramillo P, Martinez F, Perkovic V, Rietzschel ER, Schillaci G, Schutte AE, Scuteri A, Sharman JE, Wachtell K, Wang JG. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet 2016; 388:2665–2712. [DOI] [PubMed] [Google Scholar]
- 18. Dogui A, Kachenoura N, Frouin F, Lefort M, De Cesare A, Mousseaux E, Herment A. Consistency of aortic distensibility and pulse wave velocity estimates with respect to the Bramwell-Hill theoretical model: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2011; 13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang C, Guo D, Lan F, Zhang H, Luo J. Noninvasive measurement of regional pulse wave velocity in human ascending aorta with ultrasound imaging: an in-vivo feasibility study. J Hypertens 2016; 34:2026–2037. [DOI] [PubMed] [Google Scholar]
- 20. Spronck B, Mestanik M, Tonhajzerova I, Jurko A, Jurko T, Avolio AP, Butlin M. Direct means of obtaining CAVI0—a corrected cardio-ankle vascular stiffness index (CAVI)—from conventional CAVI measurements or their underlying variables. Physiol Meas 2017; 38:N128–N137. [DOI] [PubMed] [Google Scholar]
- 21. Spronck B, Heusinkveld MH, Donders WP, de Lepper AG, Op’t Roodt J, Kroon AA, Delhaas T, Reesink KD. A constitutive modeling interpretation of the relationship among carotid artery stiffness, blood pressure, and age in hypertensive subjects. Am J Physiol Heart Circ Physiol 2015; 308:H568–H582. [DOI] [PubMed] [Google Scholar]
- 22. Gepner AD, Tedla Y, Colangelo LA, Tattersall MC, Korcarz CE, Kaufman JD, Liu K, Burke GL, Shea S, Greenland P, Stein JH. Progression of carotid arterial stiffness with treatment of hypertension over 10 years: the Multi-Ethnic Study of Atherosclerosis. Hypertension 2017; 69:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meinders JM, Hoeks AP. Simultaneous assessment of diameter and pressure waveforms in the carotid artery. Ultrasound Med Biol 2004; 30:147–154. [DOI] [PubMed] [Google Scholar]
- 24. Reesink KD, Spronck B. Constitutive interpretation of arterial stiffness in clinical studies: a methodological review. Am J Physiol Heart Circ Physiol 2019; 316:H693–H709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.