Abstract
BACKGROUND
Renal denervation (RDN) is effective at lowering blood pressure. However, it is unknown if ablative procedures elicit sympathetic denervation of the kidneys in humans. The aim of this investigation was to assess sympathetic innervation of the renal cortex following perivascular chemical RDN, which may be particularly effective at ablating perivascular efferent and afferent nerves.
METHODS
Seven hypertensive patients (4F:3M; 50–65 years) completed PET–CT sympathetic neuroimaging of the renal cortex using 11C-methylreboxetine (11C-MRB, norepinephrine transporter ligand) and 6-[18F]-fluorodopamine (18F-FDA; substrate for the cell membrane norepinephrine transporter) before and 8 weeks after chemical RDN (Peregrine System Infusion Catheter, Ablative Solutions; n = 4; 2F:2M) or control renal angiography (n = 3; 2F:1M). Patients completed physiological phenotyping including 24-hour ambulatory blood pressure, hemodynamics, muscle sympathetic nerve activity, and 24-hour urine collection.
RESULTS
RDN decreased 11C-MRB-derived radioactivity by ~30% (Δ 11C-MRB/chamber: −0.95 a.u. confidence interval (CI): −1.36 to −0.54, P = 0.0002), indicative of efferent RDN. In contrast, 18F-FDA-derived radioactivity increased (Δ 18F-FDA/chamber: 2.72 a.u. CI: 0.73–4.71, P = 0.009), consistent with reduced vesicular turnover. Controls showed no change in either marker. Ambulatory systolic pressure decreased in 3 of 4 patients (−9 mm Hg CI: −27 to 9, P = 0.058), and central systolic pressure decreased in all patients (−23 mm Hg CI: −51 to 5, P = 0.095).
CONCLUSIONS
These results are the first to show efferent sympathetic denervation of the renal cortex following RDN in humans. Further studies of mechanisms underlying variable blood pressure lowering in the setting of documented RDN may provide insights into inconsistencies in clinical trial outcomes.
CLINICAL TRIALS REGISTRATION
Trial Number NCT03465917.
Keywords: blood pressure, hypertension, renal denervation, sympathetic nervous system
Renal denervation (RDN) has emerged as a promising therapeutic approach for the treatment of hypertension based on initial results in preclinical models and more recent clinical trials in humans.1 Early clinical trials (Symplicity HTN-1 and -22–4) established the safety of RDN and demonstrated a blood pressure lowering effect when compared with standard therapy. Some concern emerged though regarding the overall efficacy of RDN after Symplicity HTN-3,5 a prospective, single-blind, randomized, sham-controlled clinical trial, failed to show a greater blood pressure reduction compared with the sham procedure. More recently, a series of clinical trials addressing the experimental limitations of Symplicity HTN-3 showed consistently lowered blood pressure in patients on- and off-medication, which has resurrected enthusiasm for RDN as a treatment for hypertension.6–9 Nevertheless, this enthusiasm is tempered by several important questions regarding the efficacy of different interventions to elicit denervation of the kidneys, and the physiological mechanisms underlying the individual variability in blood pressure response.1
While RDN is effective and reproducible in animal models (~90%–95% efficacy), very little is known regarding the effect of RDN on efferent sympathetic innervation of the kidneys in humans. There are 3 modalities of RDN currently under investigation for the treatment of hypertension: multielectrode endovascular radiofrequency,8,9 endovascular ultrasound,6 and perivascular chemical denervation (alcohol-mediated denervation).10 A significant challenge for these catheter-based modalities is establishing efficacy of the intervention to elicit renal sympathetic denervation. The only study to quantify the efficacy of radiofrequency RDN to reduce efferent renal sympathetic activity (renal norepinephrine “spillover”) found that denervation was incomplete (average decrease in norepinephrine spillover: −47%) and highly variable between subjects (range: 0% to −80%), despite a significant decrease in blood pressure within the cohort.3 Notably, inadequate denervation was a key contributor to the failure of Simplicity HTN-3.11 Therefore, it is important to confirm the relative efficacy of various ablation modalities to elicit denervation in smaller controlled investigations before moving to larger multicenter clinical trials. To date there is no direct evidence demonstrating the ability of any RDN procedure to reduce efferent sympathetic neural innervation of the renal cortex in humans.
The primary aim of this investigation was to establish the efficacy of catheter-based chemical RDN (Peregrine System Infusion Catheter, Ablative Solutions),12 to reduce efferent sympathetic innervation of the renal cortex in hypertensive patients. This approach uses 3 microneedles to deliver small volumes of alcohol, an agent used commonly for clinical neural ablation, directly to the adventitial space of renal arteries where renal sympathetic nerves and afferent nerves are located.10,13 This approach achieves circumferential nerve ablation in preclinical models,13 and first-in-man investigations support the feasibility and safety of the device.10 This investigation presents the first direct assessment of sympathetic innervation of the renal cortex before and after RDN using PET–CT sympathetic neuroimaging of 11C-methylreboxetine (11C-MRB, norepinephrine transporter ligand)14 and 6-[18F]-fluorodopamine (18F-FDA; substrate for cell membrane norepinephrine transporter and intraneuronal vesicular uptake).15 We hypothesized that chemical RDN would reduce both markers of efferent sympathetic innervation to the kidneys.
METHODS
Study design
This is an open-label pilot study describing the first use of the Peregrine System catheter10 for alcohol-mediated perivascular RDN in the United States. Patients were enrolled at the Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital, University of Texas Southwestern Medical Center (UTSW), Dallas, TX where all laboratory testing and procedures took place. Renal PET–CT imaging occurred at the National Institute of Neurological and Stroke Disorders (NINDS; NIH), Bethesda, MD. The protocol was approved by the institutional review board at both institutions, and was conducted in accordance with the Declaration of Helsinki (NCT03465917).
Patients
Seven patients (4F:3M; 50–65 years) were confirmed to have hypertension (systolic >130 mm Hg and diastolic >80 mm Hg) by 24-hour ambulatory blood pressure monitoring.16 Other inclusion criteria include sufficient renal function (estimated glomeruler filtration rate >45 ml/min/1.73 m2), and suitable renal artery anatomy (no clear abnormalities) based on renal angiogram.10 Exclusion criteria included secondary cause of hypertension, or other hemodynamically significant cardiovascular disease, heart disease, renal abnormalities, renal disease, previous RDN, use of SSRI or SNRI medications that could not be withheld for the duration of the study, or fibromuscular dysplasia. Two patients (1M; 1F) with atypical renal artery anatomy (large accessory renal arteries) were excluded from the intervention based on renal angiography but were followed at each time point to serve as controls for the variability of measures (control group). After enrollment, all antihypertension medication was discontinued (4-week washout), except for 2 patients with office systolic blood pressure >180 mm Hg who were confirmed to have stable adherence to medication by pill count for 4 weeks prior to baseline testing and at 1-, 4-, and 8-week postablation. One of the patients that remained on medication (furosemide, clonidine, carvedilol, and amlodipine) was in the control group and had no change in adherence through the 8-week follow-up. The other patient received RDN and remained on antihypertension medication (carvedilol, torsemide, and hydralazine) with no change in adherence through the 8-week follow-up, and thereafter discontinued the use of hydralazine after consulting with their physician. We do not anticipate any impact of these medications on the primary endpoint of sympathetic innervation of the renal cortex. Five patients received RDN; 4 patients (2M; 2F) received bilateral RDN and 1 patient received unilateral RDN due to unanticipated anatomical preclusions encountered during the procedure. Therefore, the patient who received unilateral denervation has a denervated kidney that is represented in the RDN group, and an undenervated kidney represented in the control group for renal sympathetic neuroimaging. This patient, denoted by dashed lines in all figures, is represented in the control group for hemodynamic outcomes because the procedure was incomplete. All patients were followed throughout the study.
Procedures
Patients completed comprehensive assessments of office blood pressure (Welch Allyn, Skaneateles Falls, NY) per established guidelines,17 24-hour ambulatory blood pressure monitoring (Oscar 2, Suntech Medical Instruments, Raleigh, NC), central blood pressure by tonometry (SphygmaCor; AtCor Medical, Sydney, Australia),18 hemodynamics (cardiac output via C2H2 rebreathing,19 total peripheral resistance (TPR), blood volume via carbon monoxide rebreathing,20 autonomic function (muscle sympathetic nerve activity (MSNA) during supine rest and head-up tilt (HUT)), and plasma/urine catecholamine and neurohormone status (blood draw, 24-hour urine collection) as described previously.21 Subsequently, the patients had sympathetic neuroimaging by 11C-MRB and 18F-dopamine (18F-FDA) positron emission tomographic scanning at the NIH Clinical Center in Bethesda, MD.22 Specificity of 11C-MRB for the cell membrane norepinephrine transporter in cardiac sympathetic nerves has been confirmed using desipramine, a norepinephrine reuptake inhibitor, in nonhuman primates,14 humans,23 as well as in cross-sectional analyses of controls and heart transplant recipients.23 After baseline renal imaging at NINDS, RDN procedures were done within 2–3 weeks after returning to Dallas. RDN procedures were done by a single operator (T.A.) using a 3-needle delivery device (Peregrine System Infusion Catheter, Ablative Solutions, Kalamazoo, MI) introduced into the femoral artery and guided into the renal arteries as previously described.10 Patients were monitored for blood pressure, adverse events, and pill compliance where necessary at 24 hours, 1-, 4-, and 8-week post-RDN. Physiological outcome measures (UTSW) and renal sympathetic neuroimaging (NINDS) were repeated at the 8-week time point.
Physiological measurements
All autonomic and hemodynamic testing was preceded by 3 days of standardized diet which provided 100 mEq sodium, 100 mEq potassium, and 1,000 mg calcium per day. After arrival and instrumentation, resting hemodynamics and multiunit MSNA of the peroneal nerve24,25 were measured in the supine position followed by a standardized battery of physiological autonomic assessments as previously reported.21
Here, we report the hemodynamic and sympathetic response to HUT initiated at 30° with hemodynamics and MSNA assessed between minutes 3 and 5, and then progressed to 60° with hemodynamics and MSNA assessed between minutes 3–5 and 8–10 as described previously.21
Statistical analysis
The sample size is powered to detect changes in the primary outcome variables 11C-MRB and 18F-FDA-derived radioactivity of the renal cortex. All data were deidentified and analyzed by a research technician blinded to the experimental question and group allocation. Each kidney (left and right) was denervated and analyzed independently and is depicted independently in the figures. To ensure this approach did not bias our results, all statistics were also run using an average value of the left and right kidney for each patient, which did not change the primary outcomes of this manuscript. Absolute activity of 11C-MRB and 18F-FDA-derived radioactivity was normalized to background radioactivity of arterial blood (left ventricular chamber) and compared before and after denervation using 2-way repeated measures ANOVA and corrected for multiple comparisons using Sidak’s multiple comparisons test. This investigation was not powered to test hypotheses related to the effect of chemical RDN on hemodynamics or other physiological parameters. Values pre- and postchemical RDN were compared using 2-way repeated measures ANOVA. Significance was set a priori at P < 0.05. Values are presented as mean ± SD, or change with 95% confidence interval (CI) where applicable (Graphpad Prism 8).
RESULTS
Physical characteristics including body mass index (28 ± 5 kg/m2) did not differ pre- and post-RDN. All procedures were well tolerated with no adverse events reported. There was a significant group × time interaction for both normalized 11C-MRB (P = 0.009) and 18F-FDA (P = 0.031). There was no change in 11C-MRB/chamber activity (P = 0.874) or 18F-FDA/chamber activity (P = 0.882) in control kidneys. RDN significantly decreased normalized 11C-MRB-derived radioactivity (−0.95 a.u. CI: −1.36 to −0.54, P = 0.0002) and increased normalized 18F-FDA-derived radioactivity (2.72 a.u. CI: 0.73 to 4.71, P = 0.009) (Figure 1). The decline in normalized 11C-MRB-derived radioactivity was correlated with the increase in 18F-FDA-derived radioactivity (R2 = 0.50, P = 0.005). There was no difference in myocardial septal 11C-MRB- or 18F-FDA-derived radioactivity supporting the specificity of the findings to the renal cortex (all P > 0.05).
Figure 1.
Effect of chemical renal denervation (RDN) on renal sympathetic innervation in humans. (a) Representative positron emission tomographic scans depicting renal 11C-methylreboxetine (11C-MRB, norepinephrine transporter ligand)-derived radioactivity before, and 8 weeks after chemical RDN in humans. (b) Mean 11C-MRB- and (c) 6-[18F]-fluorodopamine (18F-FDA; substrate for norepinephrine transporter uptake)-derived radioactivity in the renal cortex normalized to radioactivity of arterial blood in the left ventricular chamber (cortex/chamber). Four patients received bilateral renal denervation, 1 patient received unilateral denervation (dashed line), and 2 patients did not undergo denervation (controls). Control kidneys, n = 5; RDN kidneys, n = 9. ANOVA interaction 11C-MRB (P = 0.009) and 18F-FDA (P = 0.031). *P < 0.05 vs. Pre.
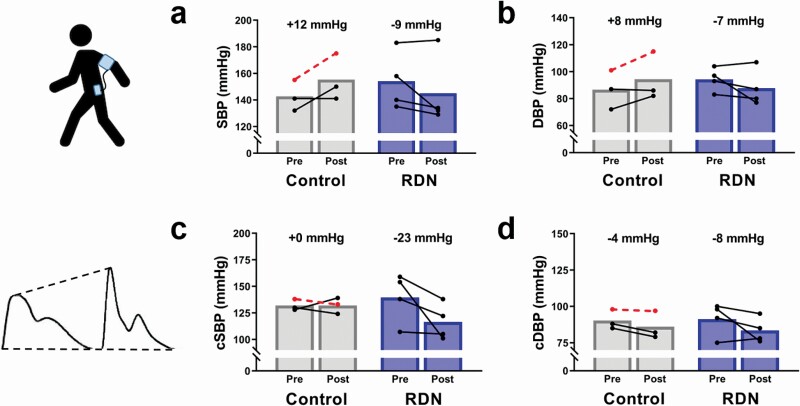
In RDN patients, 24-hour ambulatory blood pressure monitoring showed a decrease in overall systolic blood pressure (−9 mm Hg CI: −27 to 9, P = 0.058) and diastolic pressure (−7 mm Hg CI: −20 to 7, P = 0.095) (Figure 2a,b). Assessment of central blood pressure by radial artery tonometry showed a decrease in central systolic pressure (−23 mm Hg CI: −51 to 5, P = 0.095, Figure 2c) and central diastolic pressure (−8 mm Hg CI: −21 to 5, P = 0.226 Figure 2d) after RDN.
Figure 2.
Effect of chemical renal denervation (RDN) on 24-hour ambulatory blood pressure and central blood pressure. 24-Hour ambulatory monitoring of (a) systolic blood pressure (SBP) and (b) diastolic blood pressure (DBP), and assessment of (c) central systolic blood pressure (cSBP) and (d) central diastolic blood pressure (cDBP) by applanation tonometry before (Pre), and 8 weeks after (Post) chemical RDN in humans. Control patients, n = 3; RDN patients, n = 4. Dashed lines indicate a patient that received unilateral RDN. All P > 0.05.
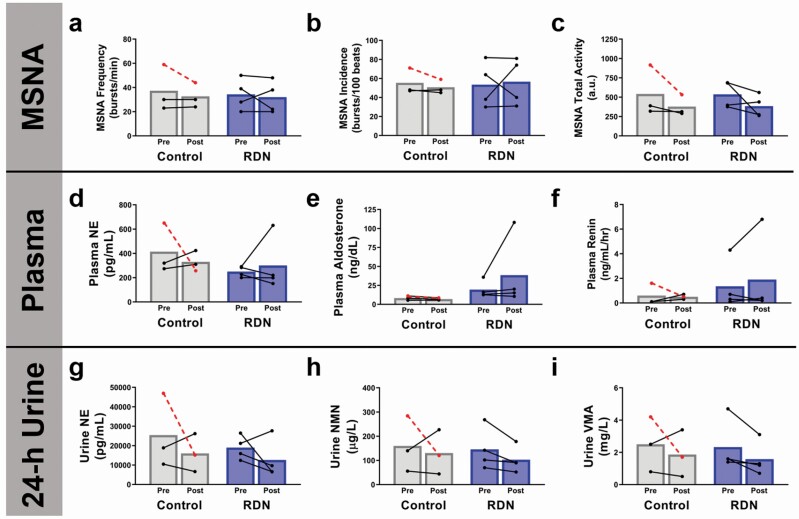
There was no consistent change in supine MSNA burst frequency, burst incidence, or total activity measured in the peroneal nerve in RDN patients (Figure 3a–c). Similarly, there was no consistent group level change in plasma markers of neurohormonal activation (Figure 3d–f). However, 3 out of 4 patients had decreased urine norepinephrine (−6,389 pg/ml CI: −30,408 to 17,631; P = 0.805), and all 4 patients showed a decrease in urine-derived markers of catecholamine metabolism including normetanephrine (−43 µg/l CI: −176 to 90; P = 0.843) and vanillylmandelic acid (−0.75 mg/l CI: −2.65 to 1.15; P = 0.904).
Figure 3.
Effect of chemical renal denervation (RDN) on muscle sympathetic nervous system activity (MSNA), and 24-hour urine, and plasma markers of neurohormonal activation. Direct measurement of MSNA (a) burst frequency, (b) burst incidence, and (c) total activity, as well as markers of neurohormonal activation including plasma-derived (d) norepinephrine (NE), (e) aldosterone, (f) renin, and 24-hour urine-derived (g) NE, (h) normetanephrine (NMN), (i) vanillylmandelic acid (VMA) before (Pre), and 8 weeks after (Post) chemical RDN in humans. Control patients, n = 3; RDN patients, n = 4. Dashed lines indicate a patient that received unilateral RDN. All P > 0.05.
Supine cardiac output was unchanged (−0.06 l/min CI: −0.60 to 0.71, P = 0. 719), while TPR tended to be lower (−267 dyn s/cm5 CI: −738 to 204, P = 0.376) (Figure 4a–c). All patients mounted an appropriate hemodynamic response to HUT before and after RDN (Figure 4d–f). The mechanisms of blood pressure reduction for each individual are summarized in Table 1.
Figure 4.
Effect of chemical renal denervation (RDN) on supine hemodynamics and autonomic regulation of blood pressure during head-up tilt (HUT). Supine (a) mean arterial pressure (MAP; electrosphygmomanometry), (b) cardiac output (Qc; acetylene rebreathe), and (c) calculated total peripheral resistance (TPR), and the change in (d) MAP, (e) Qc, and (f) TPR in response to 30° HUT during minutes 2–5, 60° HUT during minutes 2–5, and 60° HUT during minutes 8–10 before (Pre), and 8 weeks after (Post) chemical RDN in humans. Control patients, n = 3; RDN patients, n = 4. Dashed lines indicate a patient that received unilateral RDN. All P > 0.05.
Table 1.
Individual change in primary determinants of supine blood pressure 8 weeks after chemical renal denervation
| Subject | Δ MAP (mm Hg) | Δ Qc (l/min) | Δ TPR (dyn s/cm5) | Δ MSNA (bursts/min) | Δ Plasma Vol. (ml) | Δ Renin (ng/ml/hr) | Δ Aldosterone (ng/dl) |
|---|---|---|---|---|---|---|---|
| 1 | −41 | −0.10 | −717 | +10 | −69 | +0.3 | +2.0 |
| 2 | 2 | −0.41 | +171 | 0 | +680 | +2.5 | +72.2 |
| 3 | −5 | +0.12 | −201 | −16 | −672 | −0.5 | −2.1 |
| 4 | −8 | +0.61 | −321 | −3 | −907 | −0.1 | −4.1 |
Abbreviations: MAP, mean arterial pressure; MSNA, muscle sympathetic nervous system activity; Qc, cardiac output; TPR, total peripheral resistance.
DISCUSSION
Using state of the art renal sympathetic neuroimaging, this study is the first to demonstrate significant efferent sympathetic denervation of the renal cortex after catheter-based RDN in humans. 11C-MRB-derived radioactivity, a ligand of the norepinephrine transporter and marker of sympathetic noradrenergic innervation, decreased in every kidney that received chemical RDN. On average the decrease in 11C-MRB activity was approximately 30% indicating successful denervation, but also significant residual innervation. This is in line with findings using catheter-based radiofrequency RDN and renal norepinephrine spillover.3 In contrast, 18F-FDA-derived radioactivity increased after chemical RDN. 18F-FDA is a substrate for norepinephrine transporter uptake and reflects sympathetic noradrenergic uptake, vesicular storage, and clearance. Lower renal sympathetic activity may explain the increase in 18F-FDA due to reduced sympathetically mediated vesicular exocytosis and accumulation of 18F-FDA within residual neurons. Alternatively, this finding may represent a compensatory increase in norepinephrine transporter activity by residual sympathetic neurons. Despite the plasticity of efferent neurons, blood pressure remained depressed indicating that afferent neural signaling may also contribute to the blood pressure lowering effect of RDN in humans.
Efficacy of catheter-based denervation in humans
Many animal models of RDN use invasive procedures that achieve efferent RDN with greater than 90% efficacy, however, clinical trials of catheter-based RDN often have no indication or quantification of effective denervation of the kidney.26 A single study in humans using catecholamine spillover technique showed that the efficacy of catheter-based radiofrequency denervation to reduce renal specific catecholamine spillover is ~45%, with large intraindividual variability.3 With expanding options for catheter-based denervation (radiofrequency, ultrasound, and chemical), it is increasingly important to document the efficacy of new approaches to achieve sympathetic denervation of the kidney.
RDN procedures are likely to impact renal sympathetic innervation in 2 primary ways. The first is a reduction of viable neurons innervating the kidney, while the second is a loss of sympathetic neural transmission to renal sympathetic nerve terminals. This investigation utilized 2 sympathetic neuroimaging agents that provide distinct information regarding the presence of noradrenergic neurons (11C-MRB) and noradrenergic vesicular uptake, storage, and clearance (18F-FDA). 11C-MRB is a ligand for the norepinephrine transporter that does not enter neurons and therefore serves as a cell surface marker indicating the presence of noradrenergic neurons.2311C-MRB activity is not expected to be directly influenced by intraneuronal storage dynamics or vesicular exocytosis due to changes in sympathetic activity. In this investigation 11C-MRB-derived radioactivity was decreased by ~30% following denervation (Figure 1a,b). Whether a 30% decrease in 11C-MRB-derived radioactivity is directly proportional to a 30% loss of neural tissue is not known. However, it is clear that this degree of denervation is physiologically significant and similar to what is observed in cardiac tissues of patients with pure autonomic failure and heart transplant recipients.23 However, like other catheter-based modalities of RDN, there is likely significant residual sympathetic innervation of the kidney after the procedure.3
In contrast to 11C-MRB, 18F-FDA is taken up into sympathetic neurons and is primarily stored in vesicles. Changes in vesicular storage, metabolism, and sympathetic nerve activity (induces vesicular exocytosis) have been shown to impact the observed levels of 18F-FDA. RDN increased 18F-FDA-derived radioactivity of the renal cortex. This finding is similar to previous studies in healthy adults using pharmacological ganglionic inhibition of sympathetic nervous system activity via trimethaphan (nicotinic acetylcholine receptor antagonist). Reduced signaling to sympathetic nerve terminals will slow the clearance rate of 18F-FDA via vesicular exocytosis.27 Therefore, postganglionic denervation of sympathetic neurons upstream of the renal cortex by RDN would be expected to display the same accumulation of 18F-FDA produced by ganglionic inhibition. This accumulation may be sufficient to outweigh the reduction in 18F-FDA accumulation expected due to a loss of norepinephrine transporter availability. In support of this interpretation, the degree of denervation (i.e., the decrease in 11C-MRB-derived radioactivity) was significantly correlated with the increase in 18F-FDA. Alternatively, a compensatory increase in 18F-FDA uptake by residual neurons may be possible and related to observations made in humans and animal models that document functional and anatomical compensation and reinnervation of previously denervated tissues.28–30
In the individual that received unilateral denervation, 11C-MRB-derived radioactivity did not change in the undenervated kidney, whereas the kidney that was successfully denervated showed a decrease in 11C-MRB-derived radioactivity. This procedural circumstance provides a unique comparison of a denervated kidney to a “control kidney” within the same individual and further supports the efficacy of chemical RDN to induce denervation (Figure 1b, dashed lines). However, blood pressure remained elevated in this individual after the procedure (Figure 2a,b) indicating that the kidneys may not contribute equally to hypertension in certain individuals and that a single kidney may compensate for the other in its ability to maintain hypertensive signaling around a high set point (Figure 1c). Together, the results of the current investigation support the efficacy of chemical RDN to elicit efferent denervation of the renal cortex in humans. However, like other catheter-based interventions, the effect is variable and incomplete compared with animal models with comprehensive surgical denervation. Larger, multicenter investigations are needed to establish similar efficacy and to implement strategies aimed at improving procedure quality.
Physiological mechanisms of RDN-mediated blood pressure reduction
The detailed physiological phenotyping employed in this investigation illustrates the varied mechanisms by which RDN may elicit changes in blood pressure and the heterogeneity of responses between individuals. TPR fell markedly in 3 of 4 patients (Figure 3a–c), however the mechanism remains relatively unclear. Reduced renal vascular resistance may contribute in part to this reduction, but is not likely to explain the full effect on TPR. Numerous investigations have reported lower multiunit and single unit MSNA after RDN when blood pressure reductions are observed.31–34 However, the fall in MSNA after RDN is often dissociated from the fall in blood pressure.31,33 In this investigation there was a consistent decrease in MSNA quantified as total activity but no convincing change in either burst frequency or incidence (Figure 3a–c). The disconnect between changes in MSNA, TPR, and blood pressure is not unique to RDN.35 It is well established that standard quantification of MSNA burst frequency or incidence does not always correlate well with prevailing blood pressure.35–37 Further, changes in MSNA (increases or decreases) must be considered in the wider context of the baroreflex operating point. Future investigations are needed to unravel the complex contribution of action potential outflow patterns38 and postjunctional regulation of vasoconstrictor signaling39 to blood pressure control in humans.
Finally, it is worth noting that a single subject (subject 2, Table 1) displayed strikingly higher baseline levels of plasma aldosterone and renin, with large increases during HUT stress (data not shown). Despite a 9 mm Hg decrease in 24-hour ambulatory systolic blood pressure, baseline plasma renin and aldosterone remained elevated after RDN This was also the only subject to increase plasma volume after RDN (Δ +680 ml vs. Δ −549 ml range: −70 to −907). While we are unable to make any definitive mechanistic conclusions, this case provides a compelling example where physiological phenotyping could provide insight into compensatory responses that reduce efficacy of RDN within an individual, but that may be amenable to targeted adjuvant therapy (e.g., specific renin-angiotensin-aldosterone-system inhibitors). Additionally, the exaggerated plasma aldosterone and renin responses observed during HUT may provide insight into a group of patients that may or may not be suitable candidates for RDN. In the future, larger investigations using high resolution physiological phenotyping are necessary to better understand why blood pressure falls in response to RDN, and why some individuals do not respond.
Limitations and considerations
While this investigation was appropriately powered to detect changes in PET–CT derived sympathetic innervation before and after chemical RDN, future studies are necessary to establish the efficacy of chemical RDN in a larger multioperator investigation. This study was not powered to make conclusions regarding the physiological mechanisms of blood pressure regulation, however we hope the discussion provides insight regarding the potential utility of high resolution physiological phenotyping in clinical trials moving forward.
We show that catheter-based chemical RDN reduces sympathetic innervation of the renal cortex in humans, but that residual sympathetic innervation may compensate by altering catecholamine uptake or clearance and may be a source of functional compensation. Additionally, we provide rationale for the inclusion of physiological testing in future investigations of RDN.
ACKNOWLEDGMENTS
The authors would like to acknowledge Courtney Holmes, CMT for her technical assistance in the preparation of this manuscript.
FUNDING
The research reported here was funded by a High Risk-High Impact pilot grant program, UT Southwestern to B.D.L., and supported (in part) by the Division of Intramural Research, NINDS, NIH. C.M.H. was supported by NHLBI, NIH award number F32HL137285.
DISCLOSURE
Although catheters and training on the procedure were provided by Ablative Solutions, the company had no role in the design or interpretation of this study.
REFERENCES
- 1. Schlaich MP, Kiuchi MG, Esler MD. Renal denervation—ready for prime time!? The steep SPYRAL stairs to RADIANCE in hypertension treatment. Hypertension 2018; 72:287–290. [DOI] [PubMed] [Google Scholar]
- 2. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators . Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376:1903–1909. [DOI] [PubMed] [Google Scholar]
- 3. Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281. [DOI] [PubMed] [Google Scholar]
- 4. Krum H, Schlaich MP, Sobotka PA, Böhm M, Mahfoud F, Rocha-Singh K, Katholi R, Esler MD. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet 2014; 383:622–629. [DOI] [PubMed] [Google Scholar]
- 5. Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL; SYMPLICITY HTN-3 Investigators . A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401. [DOI] [PubMed] [Google Scholar]
- 6. Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, Basile J, Kirtane AJ, Wang Y, Lobo MD, Saxena M, Feyz L, Rader F, Lurz P, Sayer J, Sapoval M, Levy T, Sanghvi K, Abraham J, Sharp ASP, Fisher NDL, Bloch MJ, Reeve-Stoffer H, Coleman L, Mullin C, Mauri L; RADIANCE-HTN Investigators . Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018; 391:2335–2345. [DOI] [PubMed] [Google Scholar]
- 7. Weber MA, Mahfoud F, Schmieder RE, Kandzari DE, Tsioufis KP, Townsend RR, Kario K, Böhm M, Sharp ASP, Davies JE, Osborn JW, Fink GD, Euler DE, Cohen DL, Schlaich MP, Esler MD. Renal denervation for treating hypertension: current scientific and clinical evidence. JACC Cardiovasc Interv 2019; 12:1095–1105. [DOI] [PubMed] [Google Scholar]
- 8. Böhm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, Tsioufis K, Pocock S, Konstantinidis D, Choi JW, East C, Lee DP, Ma A, Ewen S, Cohen DL, Wilensky R, Devireddy CM, Lea J, Schmid A, Weil J, Agdirlioglu T, Reedus D, Jefferson BK, Reyes D, D’Souza R, Sharp ASP, Sharif F, Fahy M, DeBruin V, Cohen SA, Brar S, Townsend RR; SPYRAL HTN-OFF MED Pivotal Investigators . Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet 2020; 395:1444–1451. [DOI] [PubMed] [Google Scholar]
- 9. Kandzari DE, Böhm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, Brar S, Cohen SA, Fahy M, Pilcher G, Kario K; SPYRAL HTN-ON MED Trial Investigators . Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018; 391:2346–2355. [DOI] [PubMed] [Google Scholar]
- 10. Fischell TA, Ebner A, Gallo S, Ikeno F, Minarsch L, Vega F, Haratani N, Ghazarossian VE. Transcatheter alcohol-mediated perivascular renal denervation with the peregrine system: first-in-human experience. JACC Cardiovasc Interv 2016; 9:589–598. [DOI] [PubMed] [Google Scholar]
- 11. Kandzari DE, Bhatt DL, Brar S, Devireddy CM, Esler M, Fahy M, Flack JM, Katzen BT, Lea J, Lee DP, Leon MB, Ma A, Massaro J, Mauri L, Oparil S, O’Neill WW, Patel MR, Rocha-Singh K, Sobotka PA, Svetkey L, Townsend RR, Bakris GL. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J 2015; 36:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahfoud F, Renkin J, Sievert H, Bertog S, Ewen S, Böhm M, Lengelé JP, Wojakowski W, Schmieder R, van der Giet M, Parise H, Haratani N, Pathak A, Persu A. Alcohol-mediated renal denervation using the peregrine system infusion catheter for treatment of hypertension. JACC Cardiovasc Interv 2020; 13:471–484. [DOI] [PubMed] [Google Scholar]
- 13. Fischell TA, Vega F, Raju N, Johnson ET, Kent DJ, Ragland RR, Fischell DR, Almany SL, Ghazarossian VE. Ethanol-mediated perivascular renal sympathetic denervation: preclinical validation of safety and efficacy in a porcine model. EuroIntervention 2013; 9:140–147. [DOI] [PubMed] [Google Scholar]
- 14. Ding YS, Lin KS, Garza V, Carter P, Alexoff D, Logan J, Shea C, Xu Y, King P. Evaluation of a new norepinephrine transporter PET ligand in baboons, both in brain and peripheral organs. Synapse 2003; 50:345–352. [DOI] [PubMed] [Google Scholar]
- 15. Goldstein DS, Cheshire WP Jr. Roles of cardiac sympathetic neuroimaging in autonomic medicine. Clin Auton Res 2018; 28:397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018; 138:e484–e594. [DOI] [PubMed] [Google Scholar]
- 17. Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111:697–716. [DOI] [PubMed] [Google Scholar]
- 18. Ding FH, Fan WX, Zhang RY, Zhang Q, Li Y, Wang JG. Validation of the noninvasive assessment of central blood pressure by the SphygmoCor and Omron devices against the invasive catheter measurement. Am J Hypertens 2011; 24:1306–1311. [DOI] [PubMed] [Google Scholar]
- 19. Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG, Pawelczyk JA. Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. J Appl Physiol (1985) 2007; 103:867–874. [DOI] [PubMed] [Google Scholar]
- 20. Dias KA, Lawley JS, Gatterer H, Howden EJ, Sarma S, Cornwell WK III, Hearon CM Jr, Samels M, Everding B, Liang AS, Hendrix M, Piper T, Thevis M, Bruick RK, Levine BD. Effect of acute and chronic xenon inhalation on erythropoietin, hematological parameters, and athletic performance. J Appl Physiol (1985) 2019; 127:1503–1510. [DOI] [PubMed] [Google Scholar]
- 21. Howden EJ, East C, Lawley JS, Stickford ASL, Verhees M, Fu Q, Levine BD. Integrative blood pressure response to upright tilt post renal denervation. Am J Hypertens 2017; 30:632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li ST, Tack CJ, Fananapazir L, Goldstein DS. Myocardial perfusion and sympathetic innervation in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2000; 35:1867–1873. [DOI] [PubMed] [Google Scholar]
- 23. Goldstein DS, Isonaka R, Holmes C, Ding YS, Sharabi Y. Cardiac sympathetic innervation and vesicular storage in pure autonomic failure. Ann Clin Transl Neurol 2020; 7:1908–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vallbo AB, Hagbarth KE, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 1979; 59:919–957. [DOI] [PubMed] [Google Scholar]
- 25. Hart EC, Head GA, Carter JR, Wallin BG, May CN, Hamza SM, Hall JE, Charkoudian N, Osborn JW. Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. Am J Physiol Heart Circ Physiol 2017; 312:H1031–H1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Esler M. Illusions of truths in the Symplicity HTN-3 trial: generic design strengths but neuroscience failings. J Am Soc Hypertens 2014; 8:593–598. [DOI] [PubMed] [Google Scholar]
- 27. Goldstein DS, Holmes C, Stuhlmuller JE, Lenders JW, Kopin IJ. 6-[18F]fluorodopamine positron emission tomographic scanning in the assessment of cardiac sympathoneural function—studies in normal humans. Clin Auton Res 1997; 7:17–29. [DOI] [PubMed] [Google Scholar]
- 28. Booth LC, Nishi EE, Yao ST, Ramchandra R, Lambert GW, Schlaich MP, May CN. Reinnervation of renal afferent and efferent nerves at 5.5 and 11 months after catheter-based radiofrequency renal denervation in sheep. Hypertension 2015; 65:393–400. [DOI] [PubMed] [Google Scholar]
- 29. Mulder J, Hökfelt T, Knuepfer MM, Kopp UC. Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am J Physiol Regul Integr Comp Physiol 2013; 304:R675–R682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coates G, Chirakal R, Fallen EL, Firnau G, Garnett ES, Kamath MV, Scheffel A, Nahmias C. Regional distribution and kinetics of [18F]6-flurodopamine as a measure of cardiac sympathetic activity in humans. Heart 1996; 75:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grassi G, Seravalle G, Brambilla G, Trabattoni D, Cuspidi C, Corso R, Pieruzzi F, Genovesi S, Stella A, Facchetti R, Spaziani D, Bartorelli A, Mancia G. Blood pressure responses to renal denervation precede and are independent of the sympathetic and baroreflex effects. Hypertension 2015; 65:1209–1216. [DOI] [PubMed] [Google Scholar]
- 32. Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension 2013; 61:457–464. [DOI] [PubMed] [Google Scholar]
- 33. Hering D, Marusic P, Walton AS, Lambert EA, Krum H, Narkiewicz K, Lambert GW, Esler MD, Schlaich MP. Sustained sympathetic and blood pressure reduction 1 year after renal denervation in patients with resistant hypertension. Hypertension 2014; 64:118–124. [DOI] [PubMed] [Google Scholar]
- 34. Seravalle G, D’Arrigo G, Tripepi G, Mallamaci F, Brambilla G, Mancia G, Grassi G, Zoccali C. Sympathetic nerve traffic and blood pressure changes after bilateral renal denervation in resistant hypertension: a time-integrated analysis. Nephrol Dial Transplant 2017; 32:1351–1356. [DOI] [PubMed] [Google Scholar]
- 35. Joyner MJ, Charkoudian N, Wallin BG. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp Physiol 2008; 93:715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension 1989; 14:177–183. [DOI] [PubMed] [Google Scholar]
- 37. Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 2005; 45:522–525. [DOI] [PubMed] [Google Scholar]
- 38. Shoemaker JK, Klassen SA, Badrov MB, Fadel PJ. Fifty years of microneurography: learning the language of the peripheral sympathetic nervous system in humans. J Neurophysiol 2018; 119:1731–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hearon CM Jr, Dinenno FA. Escape, lysis, and feedback: endothelial modulation of sympathetic vasoconstriction. Curr Opin Pharmacol 2019; 45:81–86. [DOI] [PubMed] [Google Scholar]