Abstract
BACKGROUND
We evaluated the associations of visit-to-visit blood pressure (BP) variability with incident cardiovascular disease (CVD) and deaths in adults with type 2 diabetes.
METHODS
We analyzed 4,152 participants in Look AHEAD (Action for Health in Diabetes) free of CVD events and deaths during the first 36 months of follow-up. Variability of systolic BP (SBP) and diastolic BP (DBP) across 4 annual visits was assessed using the intraindividual SD, variation independent of the mean, and coefficient of variation. Cox regression was used to generate the adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for CVD (myocardial infarction [MI], stroke, or CVD-related deaths) and mortality.
RESULTS
Over a median of 6.6 years, there were 220 MIs, 105 stroke cases, 62 CVD-related deaths, and 236 deaths. After adjustment for confounders including average BP, the aHRs for the highest (vs. lowest) tertile of SD of SBP were 1.98 (95% CI 1.01–3.92), 1.25 (95% CI 0.90–1.72), 1.26 (95% CI 0.96–1.64), 1.05 (95% CI 0.75–1.46), and 1.64 (95% CI 0.99–2.72) for CVD mortality, all-cause mortality, CVD, MI, and stroke, respectively. The equivalent aHRs for SD of DBP were 1.84 (95% CI 0.98–3.48), 1.43 (95% CI 1.03–1.98), 1.19 (95% CI 0.91–1.56), 1.14 (95% CI 0.82–1.58), and 0.97 (95% CI 0.58–1.60), respectively.
CONCLUSIONS
In a large sample of individuals with type 2 diabetes, a greater variability in SBP was associated with higher cardiovascular mortality and CVD events; a higher variability in DBP was linked to increased overall and cardiovascular mortality.
Keywords: blood pressure, blood pressure variability, cardiovascular diseases, diabetes, hypertension, mortality
Graphical Abstract
Graphical Abstract.
Hypertension and type 2 diabetes are common and tend to coexist in the same individuals.1,2 Among individuals with type 2 diabetes, the presence of hypertension and the degree of its control are major predictors of adverse cardiovascular disease (CVD) events such as coronary artery disease and stroke.3 As such, optimal blood pressure (BP) control remains a top priority in the management of individuals with type 2 diabetes.3 Emerging evidence suggests that visit-to-visit variability of BP may be positively associated with risks of future CVD events independent of average BP and other CVD risk factors.4–11 This is relevant especially for people with diabetes mellitus who may inherently have increased BP variability partly due to their propensity to develop autonomic dysfunction and increased arterial stiffness.12,13
Although studies have evaluated the effect of visit-to-visit variability of BP with CVD events and deaths, the evidence in individuals with type 2 diabetes is overall scant, as these studies were limited in several ways including a retrospective design,14 the lack of diverse study samples,6,14–17 small sample size,18 or short duration of follow-up.6,11,15 Therefore, we used data from the Look AHEAD (Action for Health in Diabetes) study—a large community-based cohort of adults with type 2 diabetes in whom several annual recordings of BP were obtained at the outset.19,20 We hypothesized that a higher variability in systolic BP (SBP) or diastolic BP (DBP) would be associated with greater risks of CVD events and mortality.
METHODS
Study design
We conducted a secondary analysis of the Look AHEAD, a multicenter randomized clinical trial of the effects of intensive lifestyle interventions on CVD outcomes. Details about the rationale and design of Look AHEAD have been reported elsewhere.19,20 Briefly, a total of 5,145 participants were recruited from August 2001 to April 2004 across 16 locations in the United States and randomly assigned to participate in either the intensive lifestyle intervention or to receive diabetes support and education. Eligible participants were aged 45–76 years with a self-report diagnosis of type 2 diabetes confirmed by measured glucose levels, use of antidiabetic medication, or medical records.19,20
For the current study, we used the publicly available Look AHEAD dataset obtained through the NHLBI Biorepository (BioLINCC). We excluded participants with consent restrictions (n = 244), and those who experienced CVD events or died during the first 36 months of follow-up (n = 749). After these exclusions, 4,152 participants were included in our analyses.
The research protocol was approved by the Institutional Review Board at participating centers and each participant gave an informed consent.
Assessment of long-term variability of BP
At each study visit, BP was measured twice from the right arm by certified staff with participants in a seated position using an automated device (Dinamap Monitor Pro 100, Chicago, IL). The first BP was obtained after the participant had rested for 5 minutes, and the second BP was measured after waiting at least 30 seconds. The average of the 2 readings was used as the examination BP.19,20 Long-term variability of BP was defined as the variability of SBP or DBP measured at the 4 visits. Variability was assessed using 3 metrics: (i) the SD of the longitudinal intraindividual BP measurements in each participant; (ii) the variability independent of the mean (VIM) calculated as 100 × SD/meanβ where β is the regression coefficient based on the natural logarithm of SD as a function of the natural logarithm of the mean; (iii) the coefficient of variation (CV) calculated as SD/mean.7 Given that there is no consensus on the ideal measure of variability, we chose to assess several variability indices in an attempt to capture the entire spectrum or various aspects of BP variability.
Ascertainment of incident cardiovascular events
Participants free of CVD events or deaths during the first 36 months were followed and queried for incident outcomes through annual visits and semiannual phone calls. These queries were enhanced via searches of relevant records and national databases for deaths. Outcomes were classified by an event adjudication committee.19,20 The outcomes assessed in this study included: (i) all-cause mortality; (ii) cardiovascular mortality; (iii) CVD (composite of myocardial infarction [MI], stroke, and death from cardiovascular causes); (iv) MI events; and (v) stroke cases.
Covariates
At baseline, data on covariates including age, sex, race/ethnicity, duration of diabetes, history of CVD, use of antihypertensive medication (updated at subsequent follow-up visits), current smoking, and alcohol use were collected using standardized questionnaires.19,20 Weight and height were measured certified clinic staff in duplicate using a digital scale and a standard stadiometer, respectively; and the average of the duplicate measures were used for the analyses. Body mass index was calculated as weight in kilograms divided by square of height in meters.19,20 At each of the 4 first annual visits, blood samples were collected from each participant after 12 hours of fasting. Blood assays were performed at the Look AHEAD Central Biochemistry Laboratory.19,20
Plasma total cholesterol was measured using enzymatic methods standardized to the Center for Disease Control and Prevention reference methods.20,21 High-density lipoprotein cholesterol was measured by the treatment of whole plasma with dextran sulfate-magnesium to precipitate all of the apolipoprotein B-containing lipoproteins.22 Glycosylated hemoglobin (HbA1C) was measured using ion exchange high-performance liquid chromatography (Biorad Variant II). Serum creatinine was assayed by the Jaffe method on Hitachi 917 analyzer,20 and the estimated glomerular filtration rate calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.23
Statistical analyses
We created tertiles of intraindividual SD of SBP and DBP and compared participants across those tertiles using the analysis of variance or Kruskal–Wallis test (for continuous variables) or the χ 2 test (for categorical variables).
The follow-up time was calculated from the fourth visit to the earliest of date of outcome, death, or trial’s termination (14 September 2012). Cox proportional hazards models were used to generate hazard ratios and associated 95% confidence intervals for outcomes. Each variability metric was assessed as a continuous variable and tertiles using the lowest tertile as reference group. We constructed nested regression models with the first model (model 1) adjusted for age, sex, race/ethnicity, and treatment arm; the second model (model 2) accounted for covariates in model 1 plus body mass index, current smoking, alcohol drinking, use of antihypertensive medication, history of CVD, estimated glomerular filtration rate, duration of diabetes, mean total to high-density lipoprotein cholesterol ratio, and mean HbA1C; the final model (model 3) adjusted for variables in model 2 with additional adjustment for average SBP when evaluating the variability of SBP or average DBP when evaluating DBP variability. Of note, for models evaluating the VIM of SBP (or DBP), we did not adjust for mean SBP (or DBP) as VIM already accounts for the mean in its calculation.
A 2-sided P value <0.05 was considered statistically significant. All analyses were conducted using STATA 14.2 (Stata, College Station, TX).
RESULTS
Characteristics of study participants
Table 1 displays the characteristics of participants by tertiles of SD of SBP. On average, participants in the top tertile of SD of SBP were older, more likely to be women, and had longer duration of diabetes, lower estimated glomerular filtration rate, as well as higher body mass index, hemoglobin A1C, and BP measures.
Table 1.
Characteristics of study participants by tertiles of SD of systolic blood pressure
| Characteristic | Entire sample | Tertiles of SD of systolic blood pressure, mm Hg | P value | ||
|---|---|---|---|---|---|
| N = 4,152 | T1 (<7.01) | T2 (7.01–10.97) | T3 (>10.97) | ||
| N = 1,384 | N = 1,384 | N = 1,384 | |||
| At baseline | |||||
| Age, years | 58.9 (6.8) | 58.3 (6.8) | 58.8 (6.8) | 59.7 (6.7) | <0.001 |
| Women, % | 58.8 | 55.2 | 59.4 | 61.7 | 0.002 |
| Randomization arm, % | 0.008 | ||||
| Diabetes support and education | 49.2 | 49.9 | 51.7 | 45.9 | |
| Intensive lifestyle intervention | 50.8 | 50.1 | 48.3 | 54.1 | |
| Race/ethnicity, % | 0.356 | ||||
| White | 67.7 | 69.7 | 66.8 | 66.7 | |
| Non-Hispanic Black | 16.7 | 15.8 | 17.4 | 16.8 | |
| Hispanic | 12.0 | 11.6 | 12.1 | 12.3 | |
| Body mass index, kg/m2 | 35.9 (5.9) | 35.2 (5.7) | 35.9 (5.9) | 36.6 (6.0) | <0.001 |
| Current smoking, % | 3.9 | 3.8 | 3.8 | 4.1 | 0.919 |
| Alcohol drinking, % | 34.1 | 34.7 | 34.8 | 32.9 | 0.483 |
| History of cardiovascular disease, % | 13.3 | 11.7 | 12.6 | 15.6 | 0.006 |
| Duration of diabetes, years | 5.0 (2.0–10.0) | 5.0 (2.0–9.0) | 5.0 (2.0–9.0) | 5.0 (2.0–10.0) | 0.004 |
| eGFR, ml/min/1.73 m2 | 89.9 (16.0) | 90.4 (15.4) | 90.5 (16.2) | 88.8 (16.3) | 0.007 |
| During follow-up | |||||
| Average hemoglobin A1C | 7.0 (1.0) | 7.0 (0.9) | 7.0 (1.0) | 7.1 (1.1) | 0.035 |
| Average total-to-HDL cholesterol ratio | 4.2 (1.2) | 4.2 (1.2) | 4.2 (1.1) | 4.3 (1.1) | 0.563 |
| Use of antihypertensive medication, % | 83.1 | 75.4 | 82.9 | 90.9 | <0.001 |
| Average systolic blood pressure, mm Hg | 125.6 (14.1) | 122.6 (13.8) | 124.8 (13.3) | 129.5 (14.3) | <0.001 |
| Average diastolic blood pressure, mm Hg | 68.2 (8.0) | 68.0 (7.9) | 68.1 (8.1) | 68.6 (7.8) | 0.094 |
Data are mean (SD), median (interquartile range), or proportion as appropriate. Abbreviations: eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein.
Participants in the highest tertile of DBP variability were more likely to be Hispanic and to have lower estimated glomerular filtration rate, as well as higher body mass index, total to high-density lipoprotein cholesterol ratio, and BP measurements (Supplementary Table S1 online).
Long-term variability of BP and clinical outcomes
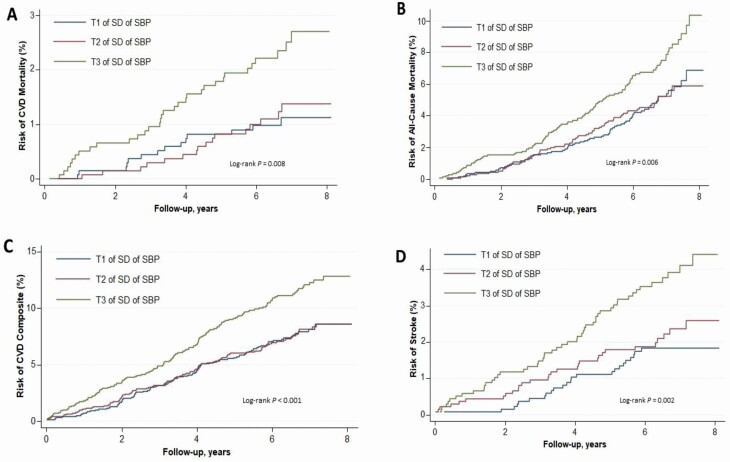
Over a median follow-up period of 6.6 years (interquartile range: 5.9–7.3), there were a total of 62 CVD-related deaths, 236 all-cause deaths, 220 MI events, 105 stroke cases, and 350 experienced the CVD composite. The cumulative Kaplan–Meier curves of clinical outcomes by SD of SBP or DBP are displayed in Figure 1 and Supplementary Figure S1 online, respectively.
Figure 1.
Unadjusted cumulative hazards of cardiovascular mortality (a), all-cause mortality (b), CVD (c), and stroke (d) by tertiles of intraindividual SD of systolic blood pressure. CVD was defined as a composite of myocardial infarction, stroke, and/or death from cardiovascular causes. Abbreviations: CVD, cardiovascular disease; SBP, systolic blood pressure; T, tertile.
Variability of SBP and outcomes
The adjusted hazard ratios by intraindividual SD of SBP are displayed in Table 2. After maximal adjustment including the average SBP, each SD increment in intraindividual SD of SBP was associated with increased hazards of cardiovascular mortality, all-cause mortality, composite CVD as well as stroke, but not MI. When assessed as categories, participants in the highest of SD of SBP (compared with lowest tertile) had statistically significant increased risks of cardiovascular mortality, but not of all-cause mortality, composite CVD, MI, or stroke.
Table 2.
Incidence and hazard ratios of cardiovascular outcomes by SD of systolic blood pressure
| Outcome | Tertile of SD of systolic blood pressure, mm Hg | P trend | Per SD | ||
|---|---|---|---|---|---|
| T1 (<7.01) | T2 (7.01–10.97) | T3 (>10.97) | |||
| Cardiovascular mortality | |||||
| No events/no at risk | 14/1,384 | 16/1,384 | 32/1,384 | … | 62/4,152 |
| Rate/1,000 person-years | 1.5 (0.9–2.6) | 1.8 (1.1–2.9) | 3.6 (2.5–5.0) | … | 2.3 (1.8–2.9) |
| Model 1 | Reference | 1.14 (0.55–2.33) | 2.26 (1.20–4.25)* | 0.007 | 1.52 (1.23–1.86) ‡ |
| Model 2 | Reference | 1.12 (0.52–2.39) | 2.10 (1.07–4.13)* | 0.018 | 1.46 (1.18–1.81) † |
| Model 3 | Reference | 1.09 (0.51–2.35) | 1.98 (1.01–3.92)* | 0.030 | 1.41 (1.13–1.76) † |
| All-cause mortality | |||||
| No events/no at risk | 68/1,384 | 67/1,384 | 101/1,384 | … | 236/4,152 |
| Rate/1,000 person-years | 7.5 (5.9–9.5) | 7.4 (5.8–9.4) | 11.2 (9.2–13.6) | … | 8.7 (7.6–9.9) |
| Model 1 | Reference | 0.95 (0.68–1.33) | 1.38 (1.01–1.88)* | 0.031 | 1.18 (1.05–1.33) † |
| Model 2 | Reference | 0.90 (0.64–1.28) | 1.28 (0.93–1.76) | 0.099 | 1.14 (1.01–1.29)* |
| Model 3 | Reference | 0.90 (0.63–1.27) | 1.25 (0.90–1.72) | 0.144 | 1.12 (0.99–1.27) |
| CVDa | |||||
| No events/no at risk | 101/1,384 | 100/1,384 | 149/1,384 | … | 350/4,152 |
| Rate/1,000 person-years | 11.4 (9.4–13.9) | 11.4 (9.4–13.9) | 17.3 (14.7–20.3) | … | 13.3 (12.0–14.8) |
| Model 1 | Reference | 0.98 (0.74–1.29) | 1.47 (1.14–1.89) † | 0.002 | 1.23 (1.11–1.35) ‡ |
| Model 2 | Reference | 0.92 (0.69–1.23) | 1.33 (1.02–1.74)* | 0.021 | 1.18 (1.07–1.31) † |
| Model 3 | Reference | 0.90 (0.68–1.20) | 1.26 (0.96–1.64) | 0.066 | 1.14 (1.03–1.26)* |
| Myocardial infarction | |||||
| No events/no at risk | 70/1,384 | 64/1,384 | 86/1,384 | … | 220/4,152 |
| Rate/1,000 person-years | 7.9 (6.2–9.9) | 7.2 (5.7–9.2) | 9.8 (8.0–12.2) | … | 8.3 (7.3–9.5) |
| Model 1 | Reference | 0.91 (0.65–1.27) | 1.24 (0.90–1.70) | 0.178 | 1.13 (0.99–1.28) |
| Model 2 | Reference | 0.86 (0.61–1.22) | 1.12 (0.81–1.56) | 0.448 | 1.09 (0.96–1.24) |
| Model 3 | Reference | 0.84 (0.60–1.19) | 1.05 (0.75–1.46) | 0.722 | 1.05 (0.92–1.19) |
| Stroke | |||||
| No events/no at risk | 24/1,384 | 30/1,384 | 51/1,384 | … | 105/4,152 |
| Rate/1,000 person-years | 2.7 (1.8–4.0) | 3.3 (2.3–4.8) | 5.8 (4.4–7.6) | … | 3.9 (3.2–4.7) |
| Model 1 | Reference | 1.23 (0.72–2.10) | 2.02 (1.24–3.29) † | 0.003 | 1.33 (1.13–1.57) † |
| Model 2 | Reference | 1.14 (0.65–1.98) | 1.80 (1.09–2.99)* | 0.015 | 1.28 (1.08–1.52) † |
| Model 3 | Reference | 1.12 (0.64–1.94) | 1.64 (0.99–2.72) | 0.043 | 1.20 (1.01–1.43)* |
Data are hazard ratios (95% confidence interval) unless otherwise specified. Model 1 adjusted for age, sex, race/ethnicity, and randomization arm; model 2 includes variables in model 1 with further adjustment for body mass index, current smoking, alcohol drinking, use of antihypertensive medications during follow-up, average ratio of total to high-density lipoprotein cholesterol, estimated glomerular filtration rate, duration of diabetes, average HbA1C, and history of cardiovascular disease; model 3 includes variables in model 2 with further adjustment for average systolic blood pressure. Abbreviation: CVD, cardiovascular disease.
aCVD was a composite of myocardial infarction, stroke, and death for cardiovascular causes.
*P < 0.05.
† P < 0.01.
‡ P < 0.001.
Variability of DBP and outcomes
Table 3 displays the associations of long-term variability of DBP (assessed as intraindividual SD) of DBP and clinical outcomes. After multivariable adjustment, each SD increase in the intraindividual SD of DBP was associated with statistically significant higher risks of cardiovascular mortality, all-cause mortality, but not composite CVD, MI, and stroke. Likewise, individuals in the highest (vs. lowest) tertile of SD of DBP had increased hazards of cardiovascular mortality and all-cause mortality, but not composite CVD, MI, and stroke, respectively.
Table 3.
Incidence and hazard ratios of cardiovascular outcomes by SD of diastolic blood pressure
| Outcome | Tertile of SD of diastolic blood pressure, mm Hg | P trend | Per SD | ||
|---|---|---|---|---|---|
| T1 (< 3.58) | T2 (3.58–5.54) | T3 (> 5.54) | |||
| Cardiovascular mortality | |||||
| No events/no at risk | 16/1,384 | 16/1,384 | 30/1,384 | … | 62/4,152 |
| Rate/1,000 person-years | 1.8 (1.1–2.9) | 1.8 (1.1–2.9) | 3.3 (2.3–4.7) | … | 2.3 (1.8–2.9) |
| Model 1 | Reference | 1.07 (0.53–2.14) | 1.93 (1.05–3.54)* | 0.027 | 1.36 (1.12–1.66) † |
| Model 2 | Reference | 1.02 (0.50–2.09) | 1.89 (1.01–3.57)* | 0.035 | 1.34 (1.08–1.66) † |
| Model 3 | Reference | 1.04 (0.50–2.13) | 1.84 (0.98–3.48) | 0.046 | 1.29 (1.05–1.59)* |
| All-cause mortality | |||||
| No events/no at risk | 65/1,384 | 74/1,384 | 97/1,384 | … | 236/4,152 |
| Rate/1,000 person-years | 7.2 (5.6–9.2) | 8.2 (6.5–10.2) | 10.7 (8.8–13.1) | … | 8.7 (7.6–9.9) |
| Model 1 | Reference | 1.20 (0.86–1.67) | 1.49 (1.09–2.04)* | 0.012 | 1.19 (1.06–1.34) † |
| Model 2 | Reference | 1.18 (0.84–1.65) | 1.44 (1.05–2.00)* | 0.024 | 1.17 (1.04–1.32)* |
| Model 3 | Reference | 1.18 (0.84–1.66) | 1.43 (1.03–1.98)* | 0.029 | 1.16 (1.03–1.30)* |
| CVDa | |||||
| No events/no at risk | 104/1,384 | 118/1,384 | 128/1,384 | … | 350/4,152 |
| Rate/1,000 person-years | 11.9 (9.8–14.4) | 13.5 (11.2–16.1) | 14.7 (12.3–17.4) | … | 13.3 (12.0–14.8) |
| Model 1 | Reference | 1.19 (0.91–1.54) | 1.25 (0.97–1.63) | 0.088 | 1.12 (1.01–1.24)* |
| Model 2 | Reference | 1.13 (0.86–1.47) | 1.20 (0.92–1.57) | 0.172 | 1.09 (0.98–1.21) |
| Model 3 | Reference | 1.13 (0.86–1.48) | 1.19 (0.91–1.56) | 0.196 | 1.08 (0.98–1.20) |
| Myocardial infarction | |||||
| No events/no at risk | 69/1,384 | 72/1,384 | 79/1,384 | … | 220/4,152 |
| Rate/1,000 person-years | 7.8 (6.2–9.9) | 8.1 (6.4–10.2) | 9.0 (7.2–11.2) | … | 8.3 (7.3–9.5) |
| Model 1 | Reference | 1.09 (0.79–1.52) | 1.18 (0.85–1.63) | 0.321 | 1.06 (0.93–1.21) |
| Model 2 | Reference | 1.01 (0.72–1.42) | 1.15 (0.83–1.59) | 0.409 | 1.04 (0.91–1.19) |
| Model 3 | Reference | 1.01 (0.72–1.42) | 1.14 (0.82–1.58) | 0.432 | 1.04 (0.91–1.18) |
| Stroke | |||||
| No events/no at risk | 31/1,384 | 40/1,384 | 34/1,384 | … | 105/4,152 |
| Rate/1,000 person-years | 3.5 (2.4–4.9) | 4.5 (3.3–6.1) | 3.8 (2.7–5.3) | … | 3.9 (3.2–4.7) |
| Model 1 | Reference | 1.32 (0.83–2.11) | 1.10 (0.67–1.79) | 0.721 | 1.09 (0.91–1.31) |
| Model 2 | Reference | 1.27 (0.79–2.05) | 0.99 (0.60–1.64) | 0.942 | 1.03 (0.85–1.25) |
| Model 3 | Reference | 1.27 (0.79–2.05) | 0.97 (0.58–1.60) | 0.870 | 1.01 (0.83–1.23) |
Data are hazard ratios (95% confidence interval) unless otherwise specified. Model 1 adjusted for age, sex, race/ethnicity, and randomization arm; model 2 includes variables in model 1 with further adjustment for body mass index, current smoking, alcohol drinking, use of antihypertensive medications during follow-up, average ratio of total to high-density lipoprotein cholesterol, estimated glomerular filtration rate, duration of diabetes, average HbA1C, and history of cardiovascular disease; model 3 includes variables in model 2 with further adjustment for average diastolic blood pressure. Abbreviation: CVD, cardiovascular disease.
aCVD was a composite of myocardial infarction, stroke, and death for cardiovascular causes.
*P < 0.05.
† P < 0.01.
Supplementary analyses
We tested the robustness of our results by performing additional analyses assessing BP variability using the VIM and CV. Consistent with our main analyses, each SD increase in the VIM of SBP was associated with higher risks of cardiovascular mortality, all-cause mortality, composite CVD, and stroke, but not MI (Supplementary Table S2 online). After adjusting for relevant confounders, each SD increment in the VIM of DBP was associated with statistically significant increased hazards of cardiovascular mortality as well as all-cause mortality, but not composite CVD, MI, or stroke (Supplementary Table S3 online). When variability was measured using the CV, each SD increase in CV of SBP led to increased risks of cardiovascular mortality, all-cause mortality, CVD composite, and stroke (Supplementary Table S4 online). Moreover, each SD increment in CV of DBP was related to increased hazards of cardiovascular mortality and all-cause mortality; but not CVD composite, MI, or stroke (Supplementary Table S5 online).
Discussion
We evaluated the associations of long-term variability in BP with cardiovascular outcomes and mortality among individuals with type 2 diabetes. We made several observations. First, higher levels of variability in SBP were associated with greater cardiovascular mortality and CVD events. Second, a higher variability in DBP was associated with increased overall and cardiovascular mortality. These associations were independent of average BP levels. Our findings confirm the importance of BP variability in the assessment of CVD risk in adults with type 2 diabetes and underscore the necessity of consistent BP control in this high-risk population.
Our study complements the available body of evidence by performing a comprehensive assessment of the relations of long-term variability in BP with CVD outcomes and deaths in a large and racially diverse sample of adults with type 2 diabetes. Individuals with type 2 diabetes have greater rates of autonomic dysfunction and arterial stiffness, which might result in high BP variability,12,13 yet there is a dearth of epidemiological data exploring BP variability and CVD outcomes in this population. Indeed, a recent systematic review showed that prior epidemiologic studies exploring these associations in type 2 diabetes are scarce and have several limitations including the lack of diverse sample, smaller sample sizes, and shorter duration of follow-up.11 Additionally, our study explored multiple outcomes and attempted to capture the full spectrum of BP variability by assessing multiple variability indices. Our findings of a positive association between visit-to-visit variability with CVD and deaths are agreement with prior studies conducted in both the general population,4,5,7–9,24 and the few reports of individuals with type 2 diabetes.6,11,14–18 Additionally, the positive association between variability of SBP and cerebrovascular accidents is consistent with prior studies from the general population although these reports were not specific to people with diabetes.5,8,9
A number of mechanisms may explain the positive relationship between higher visit-to-visit variability in BP and CVD events and mortality among people with type 2 diabetes. First, type 2 diabetes is positively related to poor arterial compliance. Indeed, BP variability is increased with arterial stiffness which may reduce the ability to adjust for greater fluctuations in stroke volume (due to autonomic dysfunction), leading to amplified variations of SBP and therefore increasing the rates of adverse vascular events.25,26 Second, the rate of autonomic dysfunction is elevated in type 2 diabetes which increases BP variability.27 The resulting heightened sympathetic response has been shown to increase the rates of CVD events and mortality.27,28 Third, mechanistic studies have shown that high BP variability is associated with several end-organ complications including aortic hypertrophy, myocardial damage (inflammation and apoptosis of cardiac myocytes), direct endothelial damage, and activation of renin–angiotensin system.29 Finally, the stronger association between BP variability and mortality in our study is potentially related to the higher rates of microvascular disease which are known to be associated with greater mortality rates in individuals with diabetes.30,31
A few limitations to our study should be acknowledged. First, this was an observational study, hence there is a possibility of residual confounding. Second, our study sample was limited to people with type 2 diabetes, hence our results are not generalizable to other hyperglycemic states including type 1 diabetes. Third, our study lacked data on adherence to antihypertensive medication, which may affect BP variability over time. Finally, given that our study relied on only 4 time points to assess BP variability, we may have underestimated BP variability and consequently the magnitude of our effect estimates. Indeed, Levitan et al. have previously established that visit-to-visit variability of BP increases with the number of visits used to calculate it.32 Despite these few limitations, strengths of this study include a large and diverse prospective cohort, the recording of BP values at regular preset intervals spread over a 36-month period for the entire cohort, the long duration of follow-up, the standardized assessment of BP and other covariates, as well the blinded adjudication of outcomes.
The clinical and research implications of our findings are manifold for patients with type 2 diabetes. Visit-to-visit fluctuations of BP appear as an independent predictor of adverse outcomes in this population. More research is needed to establish practical and reliable ways of assessing long-term variability of BP in clinical practice. Additionally, considerable debate remains about the ideal therapeutic strategies for individuals with type 2 diabetes known to have elevated BP variability. Although antihypertensive classes such as calcium-channel blockers and non-loop diuretics have been suggested to be partially effective at controlling BP variability, optimal approaches remain to be determined. Indeed, reduction of BP variability may contribute to the end-organ protective effects of certain BP-lowering medications.29
In conclusion, in a large community-based sample of adults with type 2 diabetes, a higher long-term variability of SBP was independently associated with a greater risk of CVD events and cardiovascular mortality; whereas a higher variability in DBP was associated with greater overall and cardiovascular mortality. Our findings highlight the relevance of visit-to-visit variability of BP in the prediction of CVD outcomes and deaths in people type 2 diabetes and underscore the necessity of stable and consistent BP control in this population.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank the staff and participants of the Look AHEAD Study for their valuable contributions. Look AHEAD was conducted by the Look AHEAD Research Group and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); the National Heart, Lung, and Blood Institute (NHLBI); the National Institute of Nursing Research (NINR); the National Institute of Minority Health and Health Disparities (NIMHD); the Office of Research on Women’s Health (ORWH); and the Centers for Disease Control and Prevention (CDC). The data [and samples] from Look AHEAD were supplied by the NIDDK Central Repositories. This manuscript was not prepared under the auspices of the Look AHEAD and does not represent analyses or conclusions of the Look AHEAD Research Group, the NIDDK Central Repositories, or the NIH.
FUNDING
Dr Echouffo Tcheugui was supported by NIH/NHLBI grant K23 HL153774.
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 2020; 141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 2. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 2015; 314:1021–1029. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020; 43:S111–S134. [DOI] [PubMed] [Google Scholar]
- 4. Gosmanova EO, Mikkelsen MK, Molnar MZ, Lu JL, Yessayan LT, Kalantar-Zadeh K, Kovesdy CP. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. J Am Coll Cardiol 2016; 68:1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehlum MH, Liestøl K, Kjeldsen SE, Julius S, Hua TA, Rothwell PM, Mancia G, Parati G, Weber MA, Berge E. Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. Eur Heart J 2018; 39:2243–2251. [DOI] [PubMed] [Google Scholar]
- 6. Hata J, Arima H, Rothwell PM, Woodward M, Zoungas S, Anderson C, Patel A, Neal B, Glasziou P, Hamet P, Mancia G, Poulter N, Williams B, Macmahon S, Chalmers J; ADVANCE Collaborative Group . Effects of visit-to-visit variability in systolic blood pressure on macrovascular and microvascular complications in patients with type 2 diabetes mellitus: the ADVANCE trial. Circulation 2013; 128:1325–1334. [DOI] [PubMed] [Google Scholar]
- 7. Muntner P, Whittle J, Lynch AI, Colantonio LD, Simpson LM, Einhorn PT, Levitan EB, Whelton PK, Cushman WC, Louis GT, Davis BR, Oparil S. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: a cohort study. Ann Intern Med 2015; 163:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Havenon A, Fino NF, Johnson B, Wong KH, Majersik JJ, Tirschwell D, Rost N. Blood pressure variability and cardiovascular outcomes in patients with prior stroke: a secondary analysis of PRoFESS. Stroke 2019; 50:3170–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shimbo D, Newman JD, Aragaki AK, LaMonte MJ, Bavry AA, Allison M, Manson JE, Wassertheil-Smoller S. Association between annual visit-to-visit blood pressure variability and stroke in postmenopausal women: data from the Women’s Health Initiative. Hypertension 2012; 60:625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, McManus RJ. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ 2016; 354:i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiriacò M, Pateras K, Virdis A, Charakida M, Kyriakopoulou D, Nannipieri M, Emdin M, Tsioufis K, Taddei S, Masi S, Georgiopoulos G. Association between blood pressure variability, cardiovascular disease and mortality in type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 2019; 21:2587–2598. [DOI] [PubMed] [Google Scholar]
- 12. Zhou TL, Henry RMA, Stehouwer CDA, van Sloten TT, Reesink KD, Kroon AA. Blood pressure variability, arterial stiffness, and arterial remodeling. Hypertension 2018; 72:1002–1010. [DOI] [PubMed] [Google Scholar]
- 13. Spallone V. Blood pressure variability and autonomic dysfunction. Curr Diab Rep 2018; 18:137. [DOI] [PubMed] [Google Scholar]
- 14. Wan EY, Fung CS, Yu EY, Fong DY, Chen JY, Lam CL. Association of visit-to-visit variability of systolic blood pressure with cardiovascular disease and mortality in primary care Chinese patients with type 2 diabetes—a retrospective population-based cohort study. Diabetes Care 2017; 40:270–279. [DOI] [PubMed] [Google Scholar]
- 15. Ohkuma T, Woodward M, Jun M, Muntner P, Hata J, Colagiuri S, Harrap S, Mancia G, Poulter N, Williams B, Rothwell P, Chalmers J; ADVANCE Collaborative Group . Prognostic value of variability in systolic blood pressure related to vascular events and premature death in type 2 diabetes mellitus: the ADVANCE-ON Study. Hypertension 2017; 70:461–468. [DOI] [PubMed] [Google Scholar]
- 16. McMullan CJ, Lambers Heerspink HJ, Parving HH, Dwyer JP, Forman JP, de Zeeuw D. Visit-to-visit variability in blood pressure and kidney and cardiovascular outcomes in patients with type 2 diabetes and nephropathy: a post hoc analysis from the RENAAL study and the Irbesartan Diabetic Nephropathy Trial. Am J Kidney Dis 2014; 64:714–722. [DOI] [PubMed] [Google Scholar]
- 17. Hsieh YT, Tu ST, Cho TJ, Chang SJ, Chen JF, Hsieh MC. Visit-to-visit variability in blood pressure strongly predicts all-cause mortality in patients with type 2 diabetes: a 5.5-year prospective analysis. Eur J Clin Invest 2012; 42:245–253. [DOI] [PubMed] [Google Scholar]
- 18. Cardoso CRL, Leite NC, Salles GF. Prognostic importance of visit-to-visit blood pressure variability for micro- and macrovascular outcomes in patients with type 2 diabetes: the Rio de Janeiro Type 2 Diabetes Cohort Study. Cardiovasc Diabetol 2020; 19:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ; Look AHEAD Research Group . Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003; 24:610–628. [DOI] [PubMed] [Google Scholar]
- 20. Bray G, Gregg E, Haffner S, Pi-Sunyer XF, WagenKnecht LE, Walkup M, Wing R; Look Ahead Research Group . Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diab Vasc Dis Res 2006; 3:202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol 1986; 129:101–123. [DOI] [PubMed] [Google Scholar]
- 22. Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem 1982; 28:1379–1388. [PubMed] [Google Scholar]
- 23. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diaz KM, Tanner RM, Falzon L, Levitan EB, Reynolds K, Shimbo D, Muntner P. Visit-to-visit variability of blood pressure and cardiovascular disease and all-cause mortality: a systematic review and meta-analysis. Hypertension 2014; 64:965–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nichols WW, Nicolini FA, Pepine CJ. Determinants of isolated systolic hypertension in the elderly. J Hypertens Suppl 1992; 10:S73–S77. [PubMed] [Google Scholar]
- 26. Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014; 63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care 2003; 26:1553–1579. [DOI] [PubMed] [Google Scholar]
- 28. Palatini P, Julius S. The role of cardiac autonomic function in hypertension and cardiovascular disease. Curr Hypertens Rep 2009; 11:199–205. [DOI] [PubMed] [Google Scholar]
- 29. Su DF. Treatment of hypertension based on measurement of blood pressure variability: lessons from animal studies. Curr Opin Cardiol 2006; 21:486–491. [DOI] [PubMed] [Google Scholar]
- 30. Brownrigg JR, Hughes CO, Burleigh D, Karthikesalingam A, Patterson BO, Holt PJ, Thompson MM, de Lusignan S, Ray KK, Hinchliffe RJ. Microvascular disease and risk of cardiovascular events among individuals with type 2 diabetes: a population-level cohort study. Lancet Diabetes Endocrinol 2016; 4:588–597. [DOI] [PubMed] [Google Scholar]
- 31. Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Retinopathy predicts cardiovascular mortality in type 2 diabetic men and women. Diabetes Care 2007; 30:292–299. [DOI] [PubMed] [Google Scholar]
- 32. Levitan EB, Kaciroti N, Oparil S, Julius S, Muntner P. Blood pressure measurement device, number and timing of visits, and intra-individual visit-to-visit variability of blood pressure. J Clin Hypertens (Greenwich) 2012; 14:744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.