Abstract
Rationale & Objective:
Preeclampsia, disproportionately affecting Black women, is a leading cause of preterm delivery and risk for future hypertension and chronic kidney disease (CKD). Apolipoprotein L1 (APOL1) kidney risk alleles, common among Blacks, contribute substantially to CKD disparities. Given the strong link between preeclampsia and CKD, we investigated whether maternal and fetal APOL1 risk alleles can jointly influence preeclampsia risk, and explored potential modifiers on the APOL1- preeclampsia association.
Study Design
Nested case-control study.
Setting & Participants
426 Black mother-infant pairs (275 African-Americans; 151 Haitians) from the Boston Birth Cohort.
Exposure
Maternal and fetal APOL1 risk alleles
Outcomes
Preeclampsia
Analytical Approach
Logistic regression models with adjustment for demographic characteristics were applied to analyze associations between fetal and maternal APOL1 risk alleles and risk of preeclampsia, and to investigate effect modification by maternal country-of-origin.
Results
Fetal APOL1 risk alleles tended to be associated with an increased risk of preeclampsia, which was not statistically significant in the total genotyped population. However, this association was modified by maternal country-of-origin (P < 0.05 for interaction tests): fetal APOL1 risk alleles were significantly associated with an increased risk of preeclampsia among African-Americans under recessive (OR=3.6, 95% CI=1.3–9.7, P=0.01) and additive (OR=1.7, 95% CI=1.1–2.6, P=0.01) genetic models, but not in Haitian Americans. Also, maternal-fetal genotype discordance at the APOL1 locus was associated with a 2.6-fold higher risk of preeclampsia (P<0.001) in African-Americans. .
Limitations:
Limited sample size in stratified analyses; self-reported maternal country-of-origin; Pre-pregnancy estimated blomerular filtration rate (eGFR) and proteinuria data in mothers were not collected; unmeasured confounding social and/or environmental factors; no replication study.
Conclusions
This study supports the hypothesis that fetal APOL1 kidney risk alleles are associated with increased risk for preeclampsia in a recessive mode of inheritance in African-Americans, and suggests that maternal-fetal genotype discordance is also associated with this risk. These conclusions underscore the need to better understand maternal-fetal interaction and their genetic and environmental factors as contributors to ethnic disparities in preeclampsia.
Keywords: APOL1 gene, African-Americans, Haitian, Country-of-origin, US-born, non-US-born, Preeclampsia, Fetal genetics
Plain Language Summary
Preeclampsia, characterized by increased blood pressure after 20 weeks of pregnancy, as well as other abnormalities (e.g., protein in the urine), is dangerous to mothers and their infants. Previous studies found that individuals with African ancestry may carry APOL1 genetic variants that increase risk for chronic kidney disease. This study found that fetal high-risk APOL1 genotypes and maternal-fetal APOL1 genotype discordance independently contribute to preeclampsia risk in African-American mothers. This association was not observed in Haitian mother-infant pairs possibly because of different environmental exposures and cultural milieu. Additional studies are required to understand why APOL1 associations with preeclampsia differ by maternal country of origin and to improve management of mothers at risk for preeclampsia.
INTRODUCTION
Preeclampsia, principally characterized by the onset of hypertension after the 20th week of pregnancy, is a leading cause of medically indicated preterm delivery and future risk of cardiovascular diseases1–3 and chronic kidney diseases (CKD)4–6 in mothers and offspring.7 Preeclampsia disproportionally affects Black women worldwide.8 Despite decades of research, the etiology of preeclampsia remains unclear, and there are no established genetic or environmental factors that account for the higher risk of preeclampsia in Blacks. Genetic variants in the apolipoprotein L1 (APOL1) gene account for much of the excess risk of kidney disease in Blacks.9–12 It is estimated that 12–14% of African Americans carry two APOL1 kidney risk alleles. Given that pre-existing CKD increases the risk of developing preeclampsia13, 14 and preeclampsia increases the risk for subsequent CKD,4–6 it is likely that APOL1 variants play a role in preeclampsia susceptibility. Bruggeman et al. reported that pregnant transgenic (Tg) mice expressing the APOL1 Tg-G0 or G2 allele in the placenta developed a preeclampsia-like phenotype. Although the preeclampsia phenotype was observed in both APOL1 Tg-G0 and Tg-G2 mice, Tg-G2 mice exhibited more severe disease.15 Preeclampsia was dependent on the APOL1 genotype of the pup rather than the dam, suggesting transgenic APOL1 expressed by the fetus or placenta triggered this phenotype. Consistently, Reidy et al. found that fetal but not maternal APOL1 high-risk (HR) genotypes were associated with a two-fold increased risk of preeclampsia in two African-American cohorts.16
Previous studies have indicated that both maternal and fetal genetic background influence preeclampsia risk.17 Discordance between maternal and fetal genotypes may lead to altered risk of preeclampsia via the maternal immune system.18 For example, a study of family triads showed that particular combinations of mother-child HLA-G genotypes influenced the risk for preeclampsia.19 We thus planned to test the novel hypothesis that maternal-fetal genotype combinations at the APOL1 locus influence risk of preeclampsia.
Within the U.S. Black population there is a significant variation in country-of-origin (e.g., maternal self-identification country of identity) and nativity (birthplace), which may affect disease profiles via factors such as different African-descent proportions, distinct within-group cultural norms and health behaviors.20–23 Given that the frequencies of APOL1 risk alleles may vary among distinct ethnic populations in Africa and the African diaspora,24, 25 further studies are needed to investigate whether country-of-origin and nativity may modify the associations between APOL1 genotypes and risk of preeclampsia.
We genotyped APOL1 genetic variants in 426 Black mother-infant pairs enrolled in the Boston Birth Cohort (BBC), a predominantly urban, low-income population at high-risk for preeclampsia. We tested maternal and fetal APOL1 kidney risk allele – preeclampsia associations separately, and explored whether these associations were affected by (1) maternal country-of-origin (African-Americans versus Haitians) and (2) maternal birthplace (US-born versus non-US-born). We further tested whether maternal-fetal genotype discordance (or mismatch) for the the APOL1 genotypes affected preeclampsia risk.
METHODS
Study Design, Setting and Participants
Using a nested case-control study design, we studied 213 Black mother-infant pairs with preeclampsia (Cases) and 213 Black pairs without preeclampsia (Controls) from the BBC. The BBC began in 1998 at the Boston Medical Center, as previously reported elsewhere.26, 27 The BBC study population mirrors the patient population of the Boston Medical Center and contains a relatively high prevalence of preterm births. Mothers who delivered singleton live births were invited to participate in the BBC study within 24 to 72 hours after delivery. Pregnancies that involved multiple gestations, fetal chromosomal abnormalities, major birth defects, or were the result of in vitro fertilization were excluded. After providing written informed consent, each mother was interviewed using a standardized questionnaire to gather socio-demographic and other epidemiologic data. Their electronic medical records (EMR) at delivery were abstracted. The study protocol was approved by the Institutional Review Boards of Boston University Medical Center and of the Johns Hopkins Bloomberg School of Public Health.
Selection of cases and controls for the present study is presented in Figure 1. Briefly, we included all available Black mother-infant pairs with preeclampsia (cases, n=213), and we sought to enroll the same number of Black controls. For control selection (Figure 1), we first performed individual matching on three variables including maternal age (+/− 5 years), parity (defined as the number of times that the mother has given birth to a fetus with a gestational age of 24 weeks or more) and infant’s sex. For those cases without matching controls, we selected controls that matched with the cases on either two of the three variables. After selection, cases and controls were largely comparable on the three matching variables (Table 1).
Figure 1.
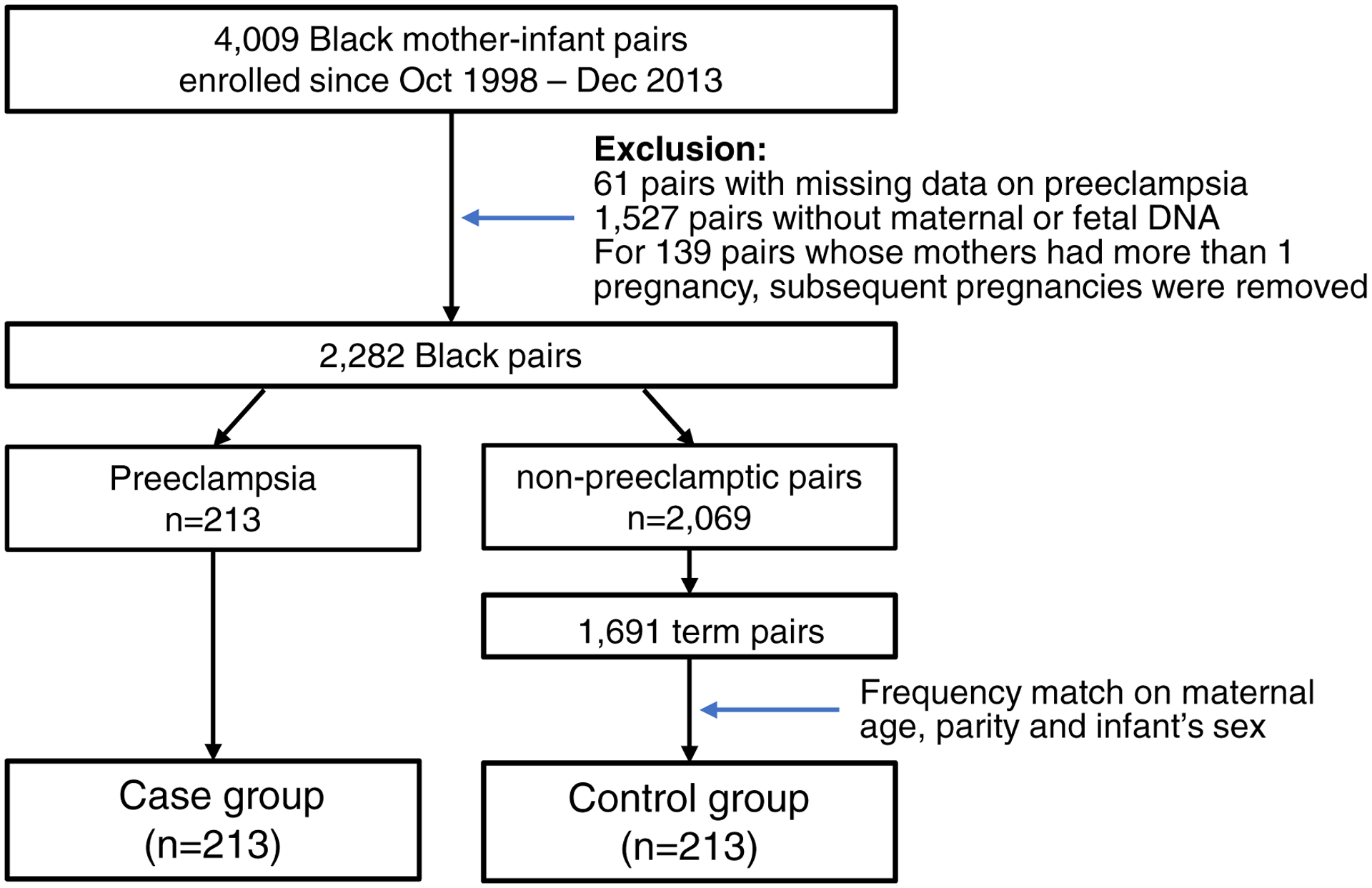
Flow charts of the study participant
Table 1.
Characteristics of the 426 Black mother-infant pairs with and without preeclampsia
| Variables | Enrolled pairsa | Eligible controls in the BBCb | |
|---|---|---|---|
| Controls | |||
| n | 213 | 1691 | |
| Maternal age (year) mean±SD | 29.2±6.9 | 28.2±6.6 | |
| Marital status, n (%) | |||
| Married | 70 (32.9) | 584 (34.5) | |
| Others | 137 (64.3) | 1076 (63.6) | |
| Missing | 6 (2.8) | 31 (1.8) | |
| Maternal pre-pregnancy BMI, n (%) | |||
| 18.5–24.9 kg/m2 | 106 (49.8) | 783 (46.3) | |
| 25.0–29.9 kg/m2 | 49 (23.0) | 455 (26.9) | |
| ≥ 30 kg/m2 | 40 (18.8) | 351 (20.8) | |
| Missing | 18 (8.5) | 102 (6.0) | |
| Maternal country-of-origin, | |||
| Haitian | 76 (35.7) | 538 (31.8) | |
| Maternal nativity or birthplace, n (%) | |||
| Non-US-born | 138 (64.8) | 1008 (59.6) | |
| US-born | 75 (35.2) | 674 (39.9) | |
| Missing | 0 | 9 (0.5) | |
| Years stayed in the U.S. | |||
| <5.0 years | 53 (24.9) | 390 (23.1) | |
| 5.0–9.9 years | 31 (14.6) | 245 (14.5) | |
| ≥10.0 years | 36 (16.9) | 225 (13.3) | |
| Born in the U.S. | 75 (35.2) | 674 (39.9) | |
| Missing | 18 (8.4) | 157 (9.3) | |
| Maternal highest education level, n (%) | |||
| ≤ High school | 127 (59.6) | 1005 (59.5) | |
| College or above | 83 (39.0) | 662 (39.1) | |
| Missing | 3 (1.4) | 24 (1.4) | |
| Nulliparity, n (%) | 105 (49.3) | 723 (42.8) | |
| Maternal smoking during pregnancy, n (%) | |||
| Never | 187 (87.8) | 1402(82.9) | |
| Quitter | 12 (5.6) | 127 (7.5) | |
| Current smoker | 10 (4.7) | 144 (8.5) | |
| Missing | 4 (1.9) | 18 (1.1) | |
| Alcohol drinking during pregnancy, n (%) | |||
| No | 192 (90.1) | 1488 (88.0) | |
| Yes | 13 (6.1) | 126 (7.5) | |
| Missing | 8 (3.8) | 77 (4.5) | |
| Chronic hypertension, n (%) | |||
| No | 209 (98.1) | 1630 (96.4) | |
| Yes | 4 (1.9) | 55 (3.3) | |
| Missing | 0 | 6 (0.4) | |
| Preterm birth, n (%) | 0 | 0 | |
| Type of delivery, C-section, n (%) | 47 (22.1) | 487 (28.9)b* | |
| Newborn sex, Female, n (%) | 109 (51.2) | 841 (49.7) | |
| Small for gestational age (SGA), n (%) | 18 (8.5) | 193 (11.4) | |
Shown are characteristics of Black mother-infant pairs with preeclampsia (cases) and matched mother-infant pairs without pre-eclampsia (controls), as well as all the eligible Black mother-infant pairs without preeclampsia in the parent Boston Birth cohort.
The difference of population characteristics between preeclampsia cases and controls was tested based on univariate logistic regression models.
denotes P < 0.05;
denotes P <0.01;
denotes P < 0.001.
The difference of population characteristics between the enrolled 213 mother-infant pairs without preeclampsia and all the eligible Black mother-infant pairs without preeclampsia in the BBC, by t-test for continuous variables and chi-square test for categorical variables.
Definition of Outcome
Physician diagnoses of preeclampsia were extracted from the maternal EMR.28 Preeclampsia was defined according to the report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy, as systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg on at least two occasions, and proteinuria ≥1+, after 20 weeks of gestation. For women with preexisting hypertension, evidence of worsening hypertension (a SBP ≥160 mmHg, or a DBP of ≥110 mmHg) was required for a diagnosis of preeclampsia. Hemolysis, elevated liver enzymes, and low platelets developing during pregnancy (HELLP) syndrome was considered present when a physician made this diagnosis contemporaneously; the 213 cases included 210 mothers with preeclampsia and 3 with HELLP.
Data Measurement
Using a standard questionnaire interview, maternal epidemiological factors were collected, including self-reported ethnicity that was defined by maternal country-of-origin (African-Americans vs. Haitian), birthplace (US-born vs. non-US-born), age at delivery, highest education level, parity, smoking and alcohol consumption during pregnancy. Non-US-born mothers were asked about how long they resided in the U.S., which was converted into a categorical variable (<5.0 years, 5.0–9.9 years, ≥10.0 years). Maternal pre-pregnancy body mass index (BMI), calculated as self-reported weight (kg) divided by height squared (m2), was categorized into four groups: normal (<25.0 kg/m2), overweight (25–29.9 kg/m2), obesity (≥30 kg/m2), and unknown. Clinical complications before and during pregnancy, including chronic hypertension and pre-existing or gestational diabetes were extracted from EMR. The missing rate of each covariate is presented in Table 1. In data analyses, we replaced missing data with the most frequent values (for categorical variables with missingness in ≤5 subjects) or by using a missing indicator/category (for categorical variables with missingness in >5 subjects).
APOL1 Genotyping, and Genetic Ancestry Proportion
Maternal DNA was isolated from blood within 1–3 days of delivery, and fetal DNA was isolated from umbilical cord blood. Three APOL1 variants (G1 allele: rs73885319 A>G and rs60910145 T>G, and G2 allele: rs71785313 6-bp deletion) were genotyped using custom TaqMan assays (Thermo Fisher Scientific, Waltham, MA) in maternal and fetal DNA samples.29 The APOL1 alleles, including G1, G2 and G0 (the latter being neither the G1 nor G2 allele), were coded accordingly. The number of APOL1 kidney risk alleles carried by each subject was coded as 0 for the G0/G0 genotype, 1 for the G0G1 or G0G2 genotype, or 2 for the G1G1, G1G2, or G2G2 genotypes. The APOL1 high-risk (HR) genotype was defined as carriage of any combination of 2 G1 and/or G2 risk alleles and the low-risk (LR) genotype was defined as carriage of 0 or 1 risk alleles. The APOL1 risk alleles were analyzed for associations with preeclampsia under three genetic modes of inheritance: recessive (2 vs. 1 or 0 risk alleles; or HR vs. LR genotypes); dominant (1 or 2 risk alleles vs. 0 risk alleles); and additive (coded as 0, 1, or 2 risk alleles as a continuous variable).
A random subset of 161 mother-infant pairs (82 with preeclampsia and 79 without preeclampsia) were genotyped using the Infinium Quality Control array (Infinium QC Array-24), containing 15,949 ancestry informative markers for ancestry estimation. A principal component analysis (“EIGENSTRAT” function in R “AssocTests” package) was performed to calculate eigenvectors for each subject. The first two eigenvectors, PC1 and PC2, representing the estimated genetic ancestry for each subject, were then plotted by maternal country-of-origin and birthplace, to indicate whether genetic ancestry varied by these two variables.
Statistical Analyses
Maternal and infant characteristics were compared between the case and controls using univariate logistic regression models. Maternal and fetal APOL1 genotypes in the controls were examined for agreement with Hardy-Weinberg equilibrium (HWE) expectations, seperately, using χ2 tests. Logistic regression models were applied to analyze the association of each maternal or fetal APOL1 risk allele with risk of preeclampsia, adjusting for conventional factors associated with preeclampsia (pre-pregnancy BMI, smoking status, alcohol drinking, chronic hypertension), country-of-origin and factors varied between cases and controls in our cohort (years the mothers resided in the U.S.). Point estimates and 95% confidence intervals (CI) of odds ratios (OR) were calculated. Recessive, additive, and dominant modes of inheritance were tested. Interaction effects between APOL1 genotypes and maternal country-of-origin (African-Americans versus Haitians) and maternal birthplace (US-born versus non-US-born) were tested by adding these two variables and their interaction term into the same logistic models. Stratified analyses by maternal country-of-origin and birthplace were also performed.
To investigate whether maternal and fetal APOL1 genotype discordance affected the risk of preeclampsia, we generated a binary variable (“MFG_index”) to represent the APOL1 maternal-fetal genotype discordance state, which was coded as “yes” if there were any differences between maternal and fetal APOL1 genotypes, or “no” if maternal and fetal APOL1 genotypes were the same. The association between the APOL1 maternal-fetal genotype discordance and risk of preeclampsia was analysed using the logistic regression model, with adjustment of covariates as described above, as well as with additional adjustment for the main effect of maternal and/or fetal APOL1 genotypes.
RESULTS
Participant Characteristics
As presented in Table 1, the prevalence of pre-pregnancy overweight / obesity, chronic hypertension, and US-born nativity was higher in mothers with preeclampsia (n=213, cases) than in mothers without (n=213, controls); all are well-established risk factors for preeclampsia. Preeclamptic mothers were more likely to have Caesarean delivery and have a small-for-gestational age (SGA) infant for the preeclamptic pregnancy (all P < 0.05). Participant characteristics between the 213 controls enrolled in this study and all the available Black controls (n=1,691) in the BBC (Fig 1) are shown in Table 1. These variables were comparable between these two control groups, except that Caesarean delivery was less frequent in the 213 controls (Table 1).
Distribution of APOL1 Kidney Risk Alleles and Associations with Preeclampsia
In mothers without preeclampsia (Controls), the minor allele frequencies (MAFs) for rs73885319, rs60910145 and rs71785313 were approximately 19%, 19% and 9%, respectively, which were comparable to MAFs in their infants (Table S1). Genotypic distribution of rs71785313 obeyed HWE expectations in both mothers and infants. Genotypic distribution of rs73885319 and rs60910145 (in near-complete linkage disequilibrium with each other, R2 ~ 0.99) in the mothers slightly deviated from HWE (P <0.05), with an excess of the rs73885319-GG genotype and the rs60910145-GG genotype (Table S1). This deviation was no longer significant when the HWE test was performed separately in Haitian and African-American mothers.
Of the 421 successfully genotyped mothers, 214 (51%), 155 (37%) and 52 (12%) carried 0, 1 or 2 APOL1 risk alleles, respectively. Among the 417 genotyped infants, 201 (48%), 169 (41%) and 47 (11%) carried 0, 1 and 2 risk alleles, respectively. Among all genotyped subjects, neither fetal (Table 2) nor maternal (Table 3) APOL1 risk alleles, under the three genetic models, had significant associations with risk of preeclampsia, although fetal APOL1 HR genotypes showed a trend toward an increased risk of preeclampsia.
Table 2.
Association of fetal APOL1 risk alleles with risk of preeclampsia
| Group | APOL1 risk allele | Cont., n(%) | Cases, n(%) | ORa | 95%CIa | Pa |
|---|---|---|---|---|---|---|
| Total | 0 | 110 (52.9) | 91 (43.5) | Ref | 1.00–1.00 | |
| 1 | 79 (38.0) | 90 (43.1) | 1.17 | 0.73–1.87 | 0.5 | |
| 2b | 19 (9.1) | 28 (13.4) | 1.79 | 0.87–3.70 | 0.1 | |
| Recessive | 19 (9.1) | 28 (13.4) | 1.67 | 0.83–3.34 | 0.2 | |
| Dominant | 98 (47.1) | 118 (56.5) | 1.28 | 0.83–1.99 | 0.3 | |
| Additive | - | - | 1.28 | 0.93–1.77 | 0.1 | |
| APOL1 × maternal country-of-origin Interaction† | ||||||
| APOL1 risk allele in a recessive model | 0.04 | |||||
| APOL1 risk allele in an dominant model | 0.2 | |||||
| APOL1 risk allele in a additive model | 0.05 | |||||
| Stratified by maternal origin of country | ||||||
| African-Americans | 0 | 74 (55.6) | 54 (40.3) | Ref | 1.00–1.00 | |
| 1 | 52 (39.1) | 59 (44.0) | 1.35 | 0.75–2.44 | 0.3 | |
| 2c | 7 (5.3) | 21 (15.7) | 4.06 | 1.43–11.6 | 0.009 | |
| Recessive | 7 (5.3) | 21 (15.7) | 3.55 | 1.29–9.74 | 0.01 | |
| Dominant | 59 (44.4) | 80 (59.7) | 1.64 | 0.94–2.87 | 0.08 | |
| Additive | - | - | 1.72 | 1.11–2.64 | 0.01 | |
| Haitian† | 0 | 36 (48.0) | 37 (49.3) | Ref | 1.00–1.00 | |
| 1 | 27 (36.0) | 31 (41.3) | 0.93 | 0.42–2.07 | 0.9 | |
| 2d | 12 (16.0) | 7 (9.3) | 0.55 | 0.17–1.78 | 0.3 | |
| Recessive | 12 (16.0) | 7 (9.3) | 0.57 | 0.18–1.74 | 0.3 | |
| Dominant | 39 (52.0) | 38 (50.6) | 0.81 | 0.39–1.71 | 0.6 | |
| Additive | 0.79 | 0.46–1.35 | 0.4 | |||
Shown are the association of fetal APOL1 risk alleles with risk of preeclampsia, stratified by maternal country-of-origin.
Adjusted for pre-pregnancy BMI category, smoking status during pregnancy, alcohol drinking during pregnancy, chronic hypertension, country-of-origin (for the analyses in the total sample only) and years the mothers stayed in the US;
The 2-degree of freedom test was performed for APOL1 risk allele, with bP=0.3 in the total sample, cP= 0.02 in African Americans, and dP=0.6 in Haitian subset.
In the interaction test, maternal country-of-origin was traited as a binary variable with the “African-American” category as the reference group.
Table 3.
Associations of maternal APOL1 risk alleles with preeclampsia
| Group | APOL1 risk allele | Cont., n (%) | Cases, n (%) | ORa | 95% CIa | Pa |
|---|---|---|---|---|---|---|
| Total | 0 | 115 (55.0) | 98 (46.4) | Ref | 1.00–1.00 | |
| 1 | 69 (33.0) | 86 (40.8) | 1.50 | 0.93–2.40 | 0.09 | |
| 2b | 25 (12.0) | 27 (12.8) | 1.11 | 0.55–2.23 | 0.8 | |
| Recessive | 25 (12.0) | 27 (12.8) | 0.95 | 0.49–1.86 | 0.9 | |
| Dominant | 94 (45.0) | 113 (53.6) | 1.39 | 0.90–2.14 | 0.1 | |
| Additive | - | - | 1.17 | 0.86–1.60 | 0.3 | |
| APOL1 × maternal country-of-origin Interaction† | ||||||
| APOL1 risk allele in a recessive model | 0.02 | |||||
| APOL1 risk allele in a dominant model | 0.1 | |||||
| APOL1 risk allele in an additive model | 0.03 | |||||
| Stratified by maternal country-of-origin | ||||||
| African-Americans | 0 | 75 (56.4) | 56 (40.9) | Ref | 1.00–1.00 | |
| 1 | 45 (33.8) | 60 (43.8) | 1.63 | 0.89–2.98 | 0.1 | |
| 2c | 13 (9.8) | 21 (15.3) | 2.20 | 0.91–5.34 | 0.08 | |
| Recessive | 13 (9.8) | 21 (15.3) | 1.81 | 0.77–4.23 | 0.2 | |
| Dominant | 58 (43.6) | 81 (59.1) | 1.76 | 1.01–3.06 | 0.05 | |
| Additive | 1.53 | 1.02–2.28 | 0.04 | |||
| Haitian | 0 | 40 (52.6) | 42 (56.8) | Ref | 1.00–1.00 | |
| 1 | 24 (31.6) | 26 (35.1) | 1.15 | 0.51–2.56 | 0.7 | |
| 2d | 12 (15.8) | 6 (8.1) | 0.31 | 0.08–1.17 | 0.08 | |
| Recessive | 12 (15.8) | 6 (8.1) | 0.30 | 0.08–1.09 | 0.07 | |
| Dominant | 36 (47.4) | 32 (43.2) | 0.83 | 0.40–1.74 | 0.6 | |
| Additive | 0.72 | 0.42–1.23 | 0.2 | |||
Shown are associations of maternal APOL1 risk alleles with preeclampsia, stratified by maternal country-of-origin .
Adjusted for pre-pregnancy BMI category, smoking status during pregnancy, Alcohol drinking during pregnancy, chronic hypertension, country-of-origin (for the analyses in the total sample only) and years the mothers stayed in the US.
The 2-degree of freedom test was performed for maternal APOL1 risk allele, bP=0.2 in the total sample, cP= 0.1 in African-Americans, and dP=0.1 in Haitian subset.
In the interaction test, maternal country-of-origin is traited as a binary variable with the “African-American” category as the reference group.
Modification Effects by Maternal country-of-origin and Birthplace
To test whether the associations between APOL1 genotypes and risk of preeclampsia varied by maternal country-of-origin (Haitian vs African-Americans), we performed interaction tests, and found a borderline interaction between fetal APOL1 risk alleles and maternal country-of-origin on risk of preeclampsia, with P< 0.05 for the interaction term when APOL1 alleles were analyzed using the recessive or additive mode of inheritance (Table 2). In stratified analyses, we found that, among the African-American subset, fetal APOL1 risk alleles were significantly associated with an increased risk of preeclampsia, under recessive (OR=3.6, 95%CI=1.3–9.7, P=0.01) and additive modes of inheritance (OR=1.7, 95%CI=1.1–2.6, P=0.01). No such associations were observed in Haitians (Table 2). In comparison, no significant interactions were observed between fetal APOL1 risk alleles and maternal birthplace, although fetal APOL1 risk alleles were significantly associated with a higher risk of preeclampsia under an additive (OR=2.2, 95%CI=1.2–4.0, P=0.01) and a dominant model (OR=2.1, 95%CI=1.0–4.4, P=0.04) in US-born but not in non-US-born subsets (Table S2).
Similar interactions were also observed between maternal APOL1 risk alleles and country-of-origin on risk of preeclampsia, in recessive (P=0.02) and additive (P=0.03) modes of inheritance (Table 3), respectively. With stratified analyses and in an additive mode of inheritance, maternal APOL1 risk alleles were borderline-significantly associated with a higher risk of preeclampsia (OR=1.5, 95%CI=1.0–2.3, P=0.04) only in the African-American subset rather than in Haitian subset. A similar borderline association was observed when maternal APOL1 risk alleles were analyzed in a dominant mode (Table 3). No significant effect modification of maternal birthplace was observed on the relationships between maternal APOL1 risk alleles and risk of preeclampsia.
In a subset of the mother-infant pairs, we performed ancestry principal component analyses, which did not reveal significant differences in population substructure by maternal country-of-origin or birthplace (Fig S1).
APOL1 Mother-fetal Genotype Discordance and Risk of Preeclampsia
Among the 411 pairs with available genotypic data in both mothers and infants, 198 pairs had a discordance between maternal and fetal APOL1 genotypes. Preeclampsia risk was significantly higher in pairs with APOL1 maternal-fetal genotype discordance, compared with those without such discordance (P=0.04). Again, we found that this association was significantly modified by maternal origin-of-country (with P= 0.006 for interaction, Table 4): APOL1 maternal-fetal genotype discordance was associated with ~ 2.6 fold higher risk of preeclampsia (95%CI=1.5–4.6, P <0.001) in African-Americans but not in Haitians. These associations remained largely unchanged after adjusting for the main effect of maternal and/or fetal APOL1 risk alleles (Table 4).
Table 4.
The associations between APOL1 maternal-fetal genotype discordance and risk of preeclampsia.
| MFG discordancea | Cont., n(%) | Cases, n(%) | Model 1b | Model 2c | Model 3d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Orb | 95% CI | P | ORb | 95% CI | P | ORb | 95% CI | P | |||
| Total | |||||||||||
| No | 113 (55.4) | 100 (48.3) | Ref | 1.00–1.00 | Ref | 1.00–1.00 | Ref | 1.00–1.00 | |||
| Yes | 91 (44.6) | 107 (51.7) | 1.58 | 1.02–2.44 | 0.04 | 1.53 | 0.95–2.45 | 0.08 | 1.46 | 0.89–2.38 | 0.1 |
| P for MFG discordance – maternal country-of-origin interaction | 0.006 | 0.006 | 0.004 | ||||||||
| African-American mother-infant pairs | |||||||||||
| No | 80 (62.0) | 59 (44.4) | Ref | 1.00–1.00 | Ref | 1.00–1.00 | Ref | 1.00–1.00 | |||
| Yes | 49 (38.0) | 74 (55.6) | 2.61 | 1.48–4.60 | <0.001 | 2.34 | 1.29–4.26 | 0.005 | 2.19 | 1.19–4.03 | 0.01 |
| Haitian mother-infant pairs | |||||||||||
| No | 33 (44.0) | 41 (55.4) | Ref | 1.00–1.00 | Ref | Ref | 1.00–1.00 | ||||
| Yes | 42 (56.0) | 33 (44.6) | 0.60 | 0.29–1.27 | 0.2 | 0.68 | 0.30–1.57 | 0.4 | 0.68 | 0.27–1.68 | 0.4 |
Shown are the associations between APOL1 maternal-fetal genotype discordance and risk of preeclampsia, both as a total group and stratified by maternal country-of-origin.
MFG (maternal-fetal genotype) discordance was coded as “yes” if there were any differences between maternal and fetal APOL1 genotypes and “no” if there were no differences between maternal and fetal APOL1 genotypes.
Model 1: The associations were tested using the logistic regression model, adjusted for pre-pregnancy BMI category, smoking status during pregnancy, alcohol drinking during pregnancy, chronic hypertension, country-of-origin (for the analyses in the total sample only) and years the mothers stayed in the US.
Model 2= Model 1 + maternal APOL1 allele status
Model 3 = Model 1+ maternal APOL1 allele status + fetal APOL1 allele status
DISCUSSION
This study confirmed the adverse impact of fetal APOL1 risk alleles on preeclampsia risk among African-Americans but not among Haitians, which suggests that other environmental or genetic factors may modify APOL1 risk allele penetrance for preeclampsia. Importantly, we found that APOL1 maternal-fetal genotype discordance was associated with a 2.6-fold higher risk of preeclampsia in African-Americans, which was not confounded by maternal or fetal APOL1 genotypes. This finding suggests that both maternal and fetal APOL1 high-risk genotypes contribute to the pathogenesis of preeclampsia.
Although the pathophysiological mechanisms by which APOL1 risk variants damage the kidney are not yet fully defined, current evidence suggests multiple mechanisms may contribute, including plasma membrane cation channel activity, endosomal trafficking, mitochondrial function, pro-inflammatory cytokine expression, activation of inflammasomes and protein kinase R, and interactions with APOL3.30–34 In the placenta, APOL1 is expressed in trophoblasts, Hoffbauer cells, and endothelial cells. Previously, we showed very low levels of placental growth factor expression that, together with high APOL1 expression in trophoblasts, raise the possibility that an APOL1-mediated defect in placentation contributes to preeclampsia. Consistently, circulating antibodies against APOL1 are found in women with preeclampsia, and serum APOL1 levels are higher in women with preeclampsia.35, 36
Very few studies have investigated the role of APOL1 risk alleles in preeclampsia.16, 37 Reidy and colleagues, in two US study cohorts, report that fetal but not maternal APOL1 HR genotype was associated with about 2-fold higher risk of preeclampsia.16 Miller et al, in 395 preeclamptic cases and 282 controls, confirmed that infant APOL1 genotype was associated with preeclampsia, but in a dominant inheritance model (Supplementary Fig 2).37 Neither study investigated potential factors that might modify APOL1-preeclampsia associations, nor did they test the impact of maternal-fetal genotype discordance. Robertson et al.38 analyzed data from two genome-wide association studies (GWAS) of prematurity, including 960 mothers from our BBC study and 519 mothers and 867 infants from the GENEVA study. They found no significant associations between APOL1 HR genotypes and preeclampsia. The two GWAS were originally designed for genetic studies of prematurity, which may lead to subject selection bias when preeclampsia was analyzed as the outcome of interest, as preeclampsia is a cause of prematurity. Also, fetal GWAs were not available from the BBC, and the determination of APOL1 G2 variant in the mothers was based on imputation. Finally, in the GENEVA study, preeclampsia and chronic hypertension were assessed as a pooled binary trait. Using chronic hypertension to define whether the mother had preeclampsia could lead to phenotype misclassification. Also, only ~50% of the study population had data for this binary trait, limiting statistical power. In the African-American subset from this study, we replicated the association between fetal APOL1 risk alleles and preeclampsia under the recessive mode of inheritance as reported by Reidy et al in Blacks from NYC and Tennessee.16 Although the present results indicated that this association remained significant in an additive mode of inheritance, we note that the effect size for carrying 1 risk allele (OR=1.4) was modest when compared to the effect size of carrying 2 risk alleles (OR=4.1). Therefore, we propose that the impact of fetal APOL1 genotypes on the risk of preeclampsia is best explained by the recessive model, but this awaits further validation in a larger cohort.
The present study suggests that maternal country-of-origin modifies the relationship between fetal APOL1 HR genotypes and preeclampsia risk. One possible explanation is that there are differences in regional African or European genetic contributions between Haitians and African Americans. Although our admixture analyses indicate that African-American and Haitian subsets had comparable genetic ancestry, the ancestry informative markers genotyped in this study were designed to differentiate between European and African ancestry, and may not be able to distinguish regional African or European contributions that might differ between Haitians and African-Americans. Another possibility is that there are distinct environmental/social exposures or other unmeasured genetic variants between these two subsets that modify the APOL1-preeclampsia associations.
We observed a borderline-significant, positive relationship between maternal APOL1 risk allele and preeclampsia risk in African-Americans under both the additive and dominant modes of inheritance, compared to null association as reported by Reidy et al. In comparison, the study by Thakoordeen-Reddy e al reported that the maternal APOL1-G1 risk allele was associated with 2.2 times higher risk of having early-onset preeclampsia in 428 South African women of African ancestry.39 Maternal APOL1 risk alleles may affect the risk of preeclampsia directly or through transmission of the risk allele to the infant, since mothers would be obligate carriers of at least one risk allele for a fetus carrying two risk alleles and have a 50% chance of being the source of the one risk alleles in the heterozygous fetus. We further demonstrated that, APOL1 maternal-fetal genotype discordance was a risk factor for preeclampsia in African Americans. This finding, if validated in other studies, suggests a potential joint mother-fetal genetic contribution to preeclampsia risk, highlighting the need to evaluate combined maternal-fetal genotypes when assessing pregnancy complications as the outcomes. Although the underlying biological mechanism for the impact of maternal-fetal genotype combination is unknown, a possible explanation is suggested by the immunological maladaptation theory which proposes that maternal immune system does not adapt fully to the semi-allogenic fetus. While the mother is usually tolerant of discordant fetal antigens, there are exceptions. These include paternal antigens that are discordant from those of the mother and are absent from semen. Further studies are needed to validate our findings on the joint effects of mother-fetal APOL1 genotypes.
This study is the first to systematically investigate the impact of maternal APOL1 genotype, fetal APOL1 genotype and their combination on preeclampsia in Blacks, as well as to explore the modification effects by maternal country-of-origin. Several limitations should be acknowledged. First, statistical power was relatively limited, especially for stratified analyses. Second, maternal country-of-origin was self-reported, and may be subject to reporting bias. Other population characteristics were collected (within 1–3 days after delivery) after the diagnosis of presence or absence of preeclampsia, and thus may lead to recall bias. However, none of the participants knew their APOL1 genotypes during their interview, so recall bias, if it exists, will not substantially influence the identified APOL1-- preeclampsia associations. Third, pre-pregnancy estimated blomerular filtration rate (eGFR) and proteinuria data in mothers were not collected in this study, and thus, their impact on the APOL1 – preeclampsia associations can not be analyzed. Fourth, we acknowledge the possibility that the associations between fetal APOL1 risk allele (or maternal-fetal genotype discordance) and risk of preeclampsia in African-Americans may be driven by some unmeasured genetic/environmental variables, and that the APOL1 risk allele may actually serves as the surrogate for the unmeasured variable. Fifth, in the analyses for APOL1 maternal-fetal genotype discordance, we applied the most robust coding scheme for the genotypic discordance between the mothers and the infants, as reported.18 However, it is likely that other discordance models may be more predictive, and further studies with case-parent triads are needed to validate and extend our findings. Sixth, the data reported here have been collected since 1998, well before the introduction of the 2019 definition of preeclampsia. The new definition, in which proteinuria is no longer necessary for diagnosis, will not significantly change our classification of the case vs control group, and thus, will not significantly change the associations direction as we reported.
In conclusion, this study suggests that the effects of both maternal and fetal APOL1 risk alleles are risk factors for preeclampsia but APOL1 penetrance may be modified by maternal country-of-origin, which may reflect differences in as yet undetected environmental and/or genetic exposures. Future research addressing these knowledge gaps would advance understanding of preeclampsia, enable more effective prevention and treatment of preeclampsia and might have implications for the prevention and treatment of APOL1-mediated CKD across generations.
Supplementary Material
Table S1. Minor allele frequency and Hardy-Weinberg tests of the three genotyped variants in the APOL1 gene in Black mother and their infants from the BBC.
Table S2. Characteristics of the non-Haitian Black mothers stratified by maternal birthplace
Figure S1. Plot for the principal component analysis of ancestry informative genotypes in 161 mother-infant pairs, colored by country-of-origin and birthplace. Figure S1A is the plot in the mothers and Figure S1B is the plot in the infants. In each plot, X axis and Y axis represent the first and the second principal component or eigenvector (PC1 and PC2) from the principal analyses, respectively.
Figure S2. The forest plot with the odds ratio and confidence intervals as reported previously on associations between fetal APOL1 risk alleles and risk of preeclampsia by Reidy et al (2018) and by miller et al (2020). Einstein Montefiore Center (EMC) and University of Tennessee Health Science Center (UTHSC) are the two study cohorts reported by Reidy et al (2018).
Acknowledgements:
We thank all of the study participants in the BBC for supporting this study. We are also grateful for the dedication and hard work of the field team at the Department of Pediatrics, Boston University School of Medicine, and for the support of the obstetric nursing staff at Boston Medical Center. The authors thank Linda Rosen of the Boston University Clinical Data Warehouse for assistance in obtaining relevant clinical information; the Clinical Data Warehouse service is supported by Boston University Clinical and Translational Institute and the National Institutes of Health Clinical and Translational Science Award (grant U54-TR001012).
Support:
The Boston Birth Cohort (the parent study) is supported in part by the National Institute of Health (NIH) grants (R03HD096136, R21HD085556, 2R01HD041702, R01HD086013, R01HD098232, and R21AI154233). This project was supported in part by the NIDDK Intramural Research Program (ZO1 DK-043308, JBK), an NIH Bench to Bedside grant award (supplementing ZO1 DK-043308 and 3R01HD098232-02S1), and by the National Cancer Institute of NIH Intramural Research Program (CAW) and under contract HHSN26120080001E. Hong X is also supported by the Johns Hopkins Population Center (NICHD R24HD042854). Chen TK was supported by the Extramural Grant Program by Satellite Healthcare, a not-for-profit renal care provider. KR receives support from a catalytic seed grant from the NIH CTSA Grant Number 1 UL1 TR001073 (Einstein/Montefiore), NIH NIDDK and the Preeclampsia Foundation. The funders were not involved in study design, data collection, analysis, reporting, or the decision to submit for publication.
Disclaimer:
Research reported in this publication was supported through the NIH Bench-to-Bedside award made possible by Office of Research on Women’s Health (ORWH). The content of this publication does not necessarily reflect the view or policy of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
REFERENCES
- 1.Bergen NE, Schalekamp-Timmermans S, Roos-Hesselink J, Roeters van Lennep JE, Jaddoe VVW, Steegers EAP. Hypertensive disorders of pregnancy and subsequent maternal cardiovascular health. Eur J Epidemiol. 2018;33 (8): 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veerbeek JH, Hermes W, Breimer AY, et al. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension. 2015;65(3): 600–606. [DOI] [PubMed] [Google Scholar]
- 3.Hammad IA, Meeks H, Fraser A, et al. Risks of cause-specific mortality in offspring of pregnancies complicated by hypertensive disease of pregnancy. Am J Obstet Gynecol. 2020;222(1): 75 e71–75 e79. [DOI] [PubMed] [Google Scholar]
- 4.Kristensen JH, Basit S, Wohlfahrt J, Damholt MB, Boyd HA. Pre-eclampsia and risk of later kidney disease: nationwide cohort study. BMJ. 2019;365: l1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khashan AS, Evans M, Kublickas M, et al. Preeclampsia and risk of end stage kidney disease: A Swedish nationwide cohort study. PLoS Med. 2019;16(7): e1002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett PM, McCarthy FP, Kublickiene K, et al. Adverse Pregnancy Outcomes and Long-term Maternal Kidney Disease: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3(2): e1920964. [DOI] [PubMed] [Google Scholar]
- 7.Bokslag A, van Weissenbruch M, Mol BW, de Groot CJ. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum Dev. 2016;102: 47–50. [DOI] [PubMed] [Google Scholar]
- 8.Urquia ML, Glazier RH, Gagnon AJ, et al. Disparities in pre-eclampsia and eclampsia among immigrant women giving birth in six industrialised countries. BJOG. 2014;121(12): 1492–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reidy KJ, Hjorten R, Parekh RS. Genetic risk of APOL1 and kidney disease in children and young adults of African ancestry. Curr Opin Pediatr. 2018;30(2): 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993): 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369(23): 2183–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams WW, Pollak MR. Health disparities in kidney disease--emerging data from the human genome. N Engl J Med. 2013;369(23): 2260–2261. [DOI] [PubMed] [Google Scholar]
- 13.Masuyama H, Nobumoto E, Okimoto N, Inoue S, Segawa T, Hiramatsu Y. Superimposed preeclampsia in women with chronic kidney disease. Gynecol Obstet Invest. 2012;74(4): 274–281. [DOI] [PubMed] [Google Scholar]
- 14.Bramham K, Briley AL, Seed PT, Poston L, Shennan AH, Chappell LC. Pregnancy outcome in women with chronic kidney disease: a prospective cohort study. Reprod Sci. 2011;18(7): 623–630. [DOI] [PubMed] [Google Scholar]
- 15.Bruggeman LA, Wu Z, Luo L, et al. APOL1-G0 or APOL1-G2 Transgenic Models Develop Preeclampsia but Not Kidney Disease. J Am Soc Nephrol. 2016;27(12): 3600–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reidy KJ, Hjorten RC, Simpson CL, et al. Fetal-Not Maternal-APOL1 Genotype Associated with Risk for Preeclampsia in Those with African Ancestry. Am J Hum Genet. 2018;103(3): 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray KJ, Saxena R, Karumanchi SA. Genetic predisposition to preeclampsia is conferred by fetal DNA variants near FLT1, a gene involved in the regulation of angiogenesis. Am J Obstet Gynecol. 2018;218(2): 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parimi N, Tromp G, Kuivaniemi H, et al. Analytical approaches to detect maternal/fetal genotype incompatibilities that increase risk of preeclampsia. BMC Med Genet. 2008;9: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hylenius S, Andersen AM, Melbye M, Hviid TV. Association between HLA-G genotype and risk of pre-eclampsia: a case-control study using family triads. Mol Hum Reprod. 2004;10(4): 237–246. [DOI] [PubMed] [Google Scholar]
- 20.Wartko PD, Wong EY, Enquobahrie DA. Maternal Birthplace is Associated with Low Birth Weight Within Racial/Ethnic Groups. Matern Child Health J. 2017;21(6): 1358–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammond WP, Mohottige D, Chantala K, Hastings JF, Neighbors HW, Snowden L. Determinants of usual source of care disparities among African American and Caribbean Black men: findings from the National Survey of American Life. J Health Care Poor Underserved. 2011;22(1): 157–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huffman FG, De La Cera M, Vaccaro JA, et al. Healthy Eating Index and Alternate Healthy Eating Index among Haitian Americans and African Americans with and without Type 2 Diabetes. J Nutr Metab. 2011;2011: 398324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messiah SE, Atem F, Lebron C, et al. Comparison of Early Life Obesity-Related Risk and Protective Factors in Non-Hispanic Black Subgroups. Matern Child Health J. 2020;24(9): 1130–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadkarni GN, Gignoux CR, Sorokin EP, et al. Worldwide Frequencies of APOL1 Renal Risk Variants. N Engl J Med. 2018;379(26): 2571–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limou S, Nelson GW, Kopp JB, Winkler CA. APOL1 kidney risk alleles: population genetics and disease associations. Adv Chronic Kidney Dis. 2014;21(5): 426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang G, Divall S, Radovick S, et al. Preterm birth and random plasma insulin levels at birth and in early childhood. Jama. 2014;311(6): 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Zuckerman B, Pearson C, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287(2): 195–202. [DOI] [PubMed] [Google Scholar]
- 28.Bustamante Helfrich B, Chilukuri N, He H, et al. Maternal vascular malperfusion of the placental bed associated with hypertensive disorders in the Boston Birth Cohort. Placenta. 2017;52: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.David VA, Binns-Roemer EA, Winkler CA. Taqman Assay for Genotyping CKD-Associated APOL1 SNP rs60910145: A Cautionary Note. Kidney Int Rep. 2019;4(1): 184–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma L, Chou JW, Snipes JA, et al. APOL1 Renal-Risk Variants Induce Mitochondrial Dysfunction. J Am Soc Nephrol. 2017;28(4): 1093–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah SS, Lannon H, Dias L, et al. APOL1 Kidney Risk Variants Induce Cell Death via Mitochondrial Translocation and Opening of the Mitochondrial Permeability Transition Pore. J Am Soc Nephrol. 2019;30(12): 2355–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jha A, Kumar V, Haque S, et al. Alterations in plasma membrane ion channel structures stimulate NLRP3 inflammasome activation in APOL1 risk milieu. FEBS J. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Toole JF, Schilling W, Kunze D, et al. ApoL1 Overexpression Drives Variant-Independent Cytotoxicity. J Am Soc Nephrol. 2018;29(3): 869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okamoto K, Rausch JW, Wakashin H, et al. APOL1 risk allele RNA contributes to renal toxicity by activating protein kinase R. Commun Biol. 2018;1: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen Q, Liu LY, Yang T, et al. Peptidomic Identification of Serum Peptides Diagnosing Preeclampsia. PLoS One. 2013;8(6): e65571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elliott SE, Parchim NF, Liu C, et al. Characterization of antibody specificities associated with preeclampsia. Hypertension. 2014;63(5): 1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller AK, Azhibekov T, O’Toole JF, et al. Association of preeclampsia with infant APOL1 genotype in African Americans. BMC Med Genet. 2020;21(1): 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson CC, Gillies CE, Putler RKB, et al. An investigation of APOL1 risk genotypes and preterm birth in African American population cohorts. Nephrol Dial Transplant. 2017;32(12): 2051–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thakoordeen-Reddy S, Winkler C, Moodley J, et al. Maternal variants within the apolipoprotein L1 gene are associated with preeclampsia in a South African cohort of African ancestry. Eur J Obstet Gynecol Reprod Biol. 2020;246: 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Minor allele frequency and Hardy-Weinberg tests of the three genotyped variants in the APOL1 gene in Black mother and their infants from the BBC.
Table S2. Characteristics of the non-Haitian Black mothers stratified by maternal birthplace
Figure S1. Plot for the principal component analysis of ancestry informative genotypes in 161 mother-infant pairs, colored by country-of-origin and birthplace. Figure S1A is the plot in the mothers and Figure S1B is the plot in the infants. In each plot, X axis and Y axis represent the first and the second principal component or eigenvector (PC1 and PC2) from the principal analyses, respectively.
Figure S2. The forest plot with the odds ratio and confidence intervals as reported previously on associations between fetal APOL1 risk alleles and risk of preeclampsia by Reidy et al (2018) and by miller et al (2020). Einstein Montefiore Center (EMC) and University of Tennessee Health Science Center (UTHSC) are the two study cohorts reported by Reidy et al (2018).


