Abstract
Normal pressure hydrocephalus (NPH) is a brain disorder caused by disruption of the flow of cerebrospinal fluid (CSF). The dementia-like symptoms of NPH are often mistakenly attributed to Alzheimer’s disease. However, if correctly diagnosed, NPH patients can potentially be treated and their symptoms reversed through surgery. Observing the dilated ventricles through magnetic resonance imaging (MRI) is one element in diagnosing NPH. Diagnostic accuracy therefore benefits from accurate, automatic parcellation of the ventricular system into its sub-compartments. We present an improvement to a whole brain segmentation approach designed for subjects with enlarged and deformed ventricles. Our method incorporates an adaptive ventricle atlas from an NPH-atlas-based segmentation as a prior and uses a more robust relaxation scheme for the multi-atlas label fusion approach that accurately labels the four sub-compartments of the ventricular system. We validated our method on NPH patients, demonstrating improvement over state-of-the-art segmentation techniques.
Keywords: MRI, enlarged brain ventricles, hydrocephalus, segmentation
1. INTRODUCTION
The ventricular system of the human brain comprises four interconnected cavities: the left and right lateral ventricles, and the third and fourth ventricles. Within each ventricle is a network of blood vessels, called choroid plexus, that produce cerebrospinal fluid (CSF). In a healthy brain, CSF flows from the lateral ventricles into the third ventricle via interventricular foramina, and from the third to the fourth ventricle via the cerebral aqueduct. From the fourth ventricle, CSF can pass into the central canal of the spinal cord or into the subarachnoid space.
Normal pressure hydrocephalus (NPH) is a type of brain disorder caused by disruption of CSF flow, leading to the expansion of the ventricles and distortion of the brain shape. Typical symptoms of NPH include urinary incontinence, gait unsteadiness, and dementia.1 The symptoms often overlap with other neurodegenerative diseases, like Alzheimer’s disease (AD), or traumatic vascular diseases of the cerebrum. However, unlike other forms of dementia, NPH is treatable and the symptoms can be reversed to some extent, through shunt surgery or endoscopic third ventriculostomy (ETV) on properly selected patients.1–3 However, it remains a challenge to diagnose those patients who will respond well to treatment.
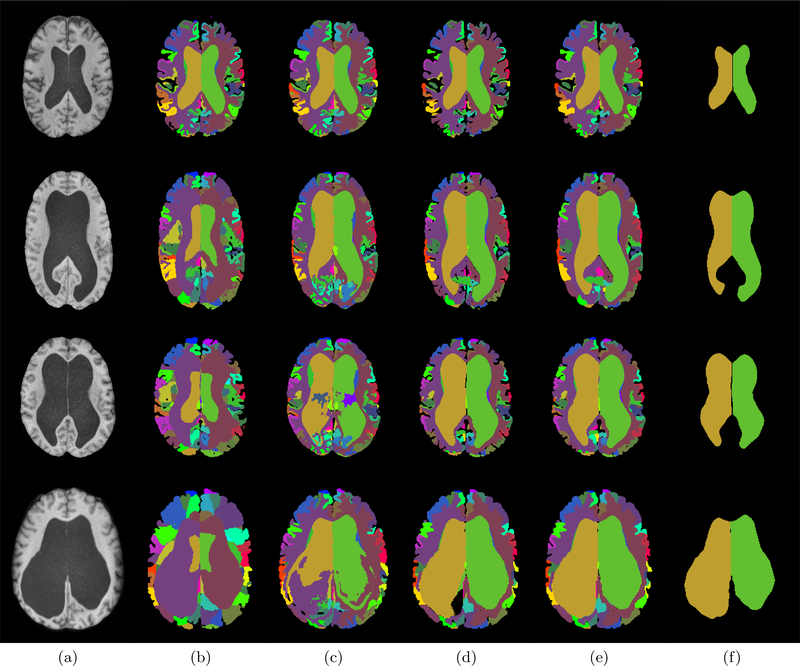
Brain imaging is one method to diagnose NPH. The chronically dilated ventricles can be observed through magnetic resonance imaging (MRI). Examples of T1-weighted (T1-w) Magnetically Prepared Rapidly Acquired Gradient Echo (MPRAGE) images of NPH patients are shown in Fig. 1(a). NPH patients often present with disproportionally dilated ventricular system4 and hence, it would be beneficial to have an accurate parcellation of the ventricular system into its sub-compartments to quantify the relative size of the different compartments. The parcellation could better characterize the ventricular pathology for individual patients and help in the treatment plan. It could also enable researchers to subgroup NPH cases and develop more systematic studies of the disease.
Figure 1.
(a) T1-w MPRAGE image of NPH patients. (b)-(f) show the ventricle segmentation generated by (b) MALPEM,5 (c) Joint Label Fusion (JLF),6 (d) RUDOLPH,7,8 (e) MA-LAVA, and (f) Manual delineation (yellow/green is the right/left lateral ventricle).
Ventricle segmentation algorithms can be classified into two main categories. The first category of methods treats the ventricular system as a single unit.9–11 Although, some of them can be used to robustly identify the ventricular system they cannot be used to quantify disproportionate dilation of the ventricular system in NPH. The second category sub-divides the ventricular system into multiple compartments. Multi-atlas label fusion methods have shown they can segment and label the ventricular system into its four cavities5,6 (right and left lateral, 3rd, and 4th ventricles). However, these methods often fail in pathological cases since they rely on good registration results between the atlas and subject images and the enlarged ventricles in NPH patients can cause significant errors in the registration process. In this work, we present an automatic brain segmentation method, providing 138 brain labels in the cerebrum and cerebellum while performing accurate ventricular parcellation even with greatly enlarged ventricles in NPH patients.
The proposed method, referred to as Multi-Atlas Labeling using an Adaptive Ventricle Atlas (MA-LAVA), makes improvements to the RobUst DictiOnary-learning and Label Propagation Hybrid (RUDOLPH) method,7,8 a whole brain segmentation method designed for subjects with enlarged ventricles. RUDOLPH integrates the Subject Specific Sparse Dictionary Learning (S3DL) method12,13 and the Multi-Atlas Label Propagation with Expectation-Maximization (MALPEM) method.5 We have made critical improvements to this method in two ways: 1) We incorporate an adaptive atlas from an NPH-atlas-based segmentation to provide a prior for the multi-atlas label fusion framework; and 2) We compute a weighted distance based on image intensities to correct the anatomical prior where the atlas registration is inaccurate due to large deformations. In Section 2, we provide details on the new method, MA-LAVA, and on all required pre-processing steps. Section 3 includes our experiments on comparing our method with three state-of-the-art brain segmentation approaches, and Section 4 concludes the paper with a discussion of the presented work.
2. METHODS
2.1. Data preprocessing and atlases
The subject’s T1-w MPRAGE image is N4 bias corrected,14 rigidly registered to MNI 152 atlas space, and skull stripped using the method MONSTR.15 The atlas cohort used in the initial multi-atlas registration consists of 15 T1-w MRI atlases with 138 manual labels from Neuromorphometrics Inc*. The NPH atlases used to create the adaptive NPH ventricle atlas were produced by manually delineating 5 T1-w MRIs of NPH subjects, each with 5 labels (the right and left lateral ventricles, third, and fourth ventricles, and a separate label representing all the remaining brain tissue). We classified the NPH patients into mild, moderate, and severe cases according to their ventricular volumes and chose NPH subjects for the atlas to range from mild to severe.
2.2. Multi-Atlas segmentation with an adaptive NPH prior
The first step of the proposed method is to register 15 Neuromorphometrics atlases into the subject’s space, as is done in RUDOLPH. For this we use the SyN deformable registration method16 and generate a probabilistic map Π = {πik, i = 1,...,N, k = 1,...K}, where πik is the probability that a voxel i belongs to the label k. N and K represent the total number of voxels and labels, respectively.
The second step of the method is to create a subject specific adaptive ventricle atlas. This is done by processing the subject’s MR image using the multi-atlas segmentation method MALPEM using 5 NPH images as atlases, each comprising 5 manual labels, as described in Section 2.1. The similarity between these 5 atlases and the subject provides a robust segmentation of the ventricular system for patients with enlarged ventricles. The output of this step is a hard segmentation Z = {zi,i = 1,...,N}, where zi is the label assigned for voxel i, including the left and right lateral ventricles, third ventricle, fourth ventricle, and the remaining brain tissue. This ventricle label map will be incorporated in the next step to correct any mis-registrations of the atlases and the subject image in the multi-atlas registration step.
To correct the anatomical prior from step 1, RUDOLPH adopts a relaxation framework from MALPEM and modifies it by accounting for the ventricular CSF label from S3DL.5 This step in RUDOLPH is briefly described next (see details in Ref. 7). Assuming a Gaussian distribution, a parameter set (μk, σk) for each label k is estimated based on Π. The eight CSF-like labels within Neuromorphometrics are grouped together and build a common parameter set (μCSF, σCSF). In RUDOLPH, Π is relaxed to ΠR as follows. At voxel i, where πik for at least one k is CSF-like, a fraction αik of the prior probability, πik, is redistributed from label k to one of the eight CSF-like labels kCSF,i, where kCSF,i is the CSF-like label with the highest prior probability or the CSF-like label of a voxel with the shortest Euclidean distance to i and that is marked as CSF in S3DL. In NPH patients with extremely large ventricles, both of these conditions sometimes fail in RUDOLPH when the prior from S3DL is inaccurate at the boundary of the enlarged ventricles. That is because at the boundary of the severely dilated ventricles the highest prior probability is often cortical CSF leading to the closest CSF-like label to become cortical CSF instead of the desired ventricular CSF.
In MA-LAVA we address this in two ways: 1) we look at the ventricular system segmentation Z from our adaptive NPH atlas; and 2) based on Z, we determine the CSF-like label by computing a weighted distance based on image intensities instead of the Euclidean distance. We identify the appropriate CSF-like label kCSF,i as
| (1) |
where represents the CSF-like labels from the multi-atlas registration, and is the weighted distance from the voxel i to the nearest voxel j with label k, which is defined as
| (2) |
Here n is the voxel on the line segment that connects voxels i and j, Ii is the intensity of voxel i, and λ is a penalty factor. The weighted distance is large if a ventricle CSF voxel goes through white matter voxels when searching for the closest CSF voxel. We follow the framework in RUDOLPH and MALPEM to calculate fraction αik based on the probability that the voxel with intensity Ii comes from either the intensity distribution estimated by label k or estimated by label kCSF,i,
| (3) |
The relaxed prior ΠR is computed as
| (4) |
The relaxed prior ΠR is the initial input to an intensity-refined Expectation-Maximization (EM)17 and is updated through an adaptive atlas framework.10 The adaptive prior in iteration t + 1 is computed as
| (5) |
where G is a Gaussian kernel, κ is the adaption factor, is the original relaxed prior probability, and is the posterior probability estimated in iteration t.
Smoothness is enforced with a global and stationary Markov Random Field.18 The fusion-based (i.e., Π) and EM-based segmentations are eventually combined through a weighted scheme to estimate the final segmentation, as in RUDOLPH and MALPEM.
3. EXPERIMENTS AND RESULTS
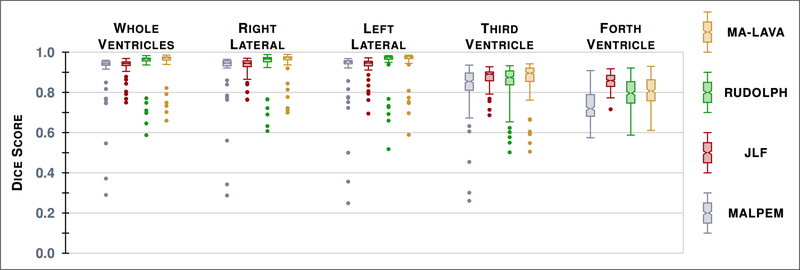
To validate MA-LAVA, the parcellation of the ventricular system was done by following a manual delineation protocol8 that uses the subject’s T1-w MRI to identify the four sub-compartments of the ventricular system. In this work, a total of 41 subjects from mild to severe cases of NPH were delineated. We processed the 41 NPH subjects using MA-LAVA and three multi-atlas whole brain segmentation methods, MALPEM, Joint Label Fusion (JLF),6 and RUDOLPH. Visual comparison (Fig. 1) of the three methods demonstrates that MA-LAVA provides a more robust segmentation, particularly on the boundary of the ventricles in NPH cases with extremely enlarged ventricles. We computed the Dice score19 for the 41 NPH patients on the ventricular system segmentation. The results are reported in Table 1 and a visual comparison is shown in Figure 2. A paired Wilcoxon Signed-Rank Test20 comparing JLF and MA-LAVA on the whole ventricular system has p-value < 0.005. We get a p-value < 0.0005 when comparing MALPEM and RUDOLPH to MA-LAVA on the whole ventricular system. MA-LAVA produces more accurate segmentation of the whole ventricular system and right and left lateral ventricles than MALPEM, JLF, and RUDOLPH. These results also show statistical significance (See detailed p-values in Table 2). We note that JLF gives better Dice scores of the third and fourth ventricles than MA-LAVA.
Table 1.
The mean Dice score (and standard deviation) over 41 NPH patients measuring similarity between manual labels and automatically generated labels from the four methods. We compare four cavities of the ventricular system: right lateral ventricle (RLV), left lateral ventricle (LLV), third ventricle (3rd), and fourth ventricle (4th).
| MALPEM | JLF | RUDOLPH | MA-LAVA | |
|---|---|---|---|---|
| Whole | 0.8797(±0.1512) | 0.9198(±0.0539) | 0.9240(±0.1002) | 0.9343(±0.0837) |
| RLV | 0.8835(±0.1523) | 0.9199(±0.0543) | 0.9232(±0.0988) | 0.9363(±0.0777) |
| LLV | 0.8823(±0.1614) | 0.9213(±0.0601) | 0.9275(±0.1078) | 0.9363(±0.0920) |
| 3rd | 0.7970(±0.1541) | 0.8651(±0.0560) | 0.8242(±0.1150) | 0.8340(±0.1200) |
| 4th | 0.7227(±0.0846) | 0.8503(±0.0438) | 0.7904(±0.0769) | 0.8079(±0.0753) |
Figure 2.
Box plots of the Dice score19 with our manual delineations over 41 NPH patients for the segmentation by the four methods, evaluated on the whole ventricular system and its four cavities.
Table 2.
p-values for a paired two-sided Wilcoxon Signed-Rank Test20 between the listed methods for the whole ventricular system and its four ventricle cavities across the 41 patients.
| Comparison | Whole | RLV | LLV | 3rd | 4th |
|---|---|---|---|---|---|
| MALPEM vs. MA-LAVA | 0.0003 | 0.0003 | 0.0013 | 0.2258 | 1.728e-11 |
| JLF vs. MA-LAVA | 0.0036 | 0.0015 | 0.0056 | 0.0616 | 0.0002 |
| RUDOLPH vs. MA-LAVA | 5.002e-11 | 9.056e-9 | 5.83e-8 | 0.0039 | 9.397e-7 |
4. CONCLUSION AND DISCUSSION
We present refinements to a whole brain segmentation method that provides robust parcellation of the ventricular system in patients with enlarged ventricles. The new method, MA-LAVA, incorporates an adaptive NPH ventricle atlas as prior information for a multi-atlas label fusion framework and provides a new relaxation scheme to correct anatomical priors where registration-based segmentation fails due to large deformation. We validated our method on 41 NPH subjects and compared with three brain segmentation approaches. We have shown that MA-LAVA is more robust on identifying the boundary of the ventricular system across 41 NPH patients than MALPEM, JLF and RUDOLPH. We also demonstrated improvement in terms of overlap with manual delineations in lateral ventricles measured by Dice score. The significant improvements occur in severe cases of NPH patients, where the other algorithms fail to capture the boundary of the enlarged ventricular system.
Future work includes improvements when segmenting the third and fourth ventricles. This could be done by combining the lateral ventricle segmentations from MA-LAVA with the third and fourth ventricle segmentations from JLF. We also plan to subdivide the lateral ventricles into subchambers (anterior, posterior, and inferior horns) to analyze the relative volumetric ratios within the ventricular system of NPH patients in greater details. The ultimate goal is to better understand the correlation between the volumetrics of these structures and the surgical outcomes for NPH patients.
ACKNOWLEDGMENTS
This work was supported by the NIH/NINDS under grant R21-NS096497, the National Multiple Sclerosis Society grant RG-1507-05243, the Department of Defense in the Center for Neuroscience and Regenerative Medicine, and the Icelandic Centre for Research (RANNIS) under grant 173942051.
Footnotes
REFERENCES
- [1].Williams MA and Relkin NR, “Diagnosis and management of idiopathic normal-pressure hydrocephalus,” Neurology: Clinical Practice 3(5), 375–385 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hakim S and Adams RD, “The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure: Observations on cerebrospinal fluid hydrodynamics,” Journal of the neurological sciences 2(4), 307–327 (1965). [DOI] [PubMed] [Google Scholar]
- [3].Hopf NJ, Grunert P, Fries G, Resch KD, and Perneczky A, “Endoscopic third ventriculostomy: Outcome analysis of 100 consecutive procedures,” Neurosurgery 44(4), 795–804 (1999). [DOI] [PubMed] [Google Scholar]
- [4].Yamada S, Ishikawa M, and Yamamoto K, “Optimal diagnostic indices for idiopathic normal pressure hydrocephalus based on the 3D quantitative volumetric analysis for the cerebral ventricle and subarachnoid space,” American Journal of Neuroradiology 36(12), 2262–2269 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ledig C, Heckemann RA, Hammers A, Lopez JC, Newcombe VF, Makropoulos A, Lötjönen J, Menon DK, and Rueckert D, “Robust whole-brain segmentation: Application to traumatic brain injury,” Medical image analysis 21(1), 40–58 (2015). [DOI] [PubMed] [Google Scholar]
- [6].Landman B and Warfield S, “MICCAI 2012 workshop on multi-atlas labeling,” in [Medical Image Computing and Computer Assisted Intervention Conference], (2012). [Google Scholar]
- [7].Ellingsen LM, Roy S, Carass A, Blitz AM, Pham DL, and Prince JL, “Segmentation and labeling of the ventricular system in normal pressure hydrocephalus using patch-based tissue classification and multi-atlas labeling,” in [Proceedings of SPIE–the International Society for Optical Engineering], 9784, NIH Public Access (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Carass A, Shao M, Li X, Dewey BE, Blitz AM, Roy S, Pham DL, Prince JL, and Ellingsen LM, “Whole Brain Parcellation with Pathology: Validation on Ventriculomegaly Patients,” in [International Workshop on Patch-Based Techniques in Medical Imaging], 20–28, Springer; (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bazin P-L and Pham DL, “Topology-preserving tissue classification of magnetic resonance brain images,” IEEE transactions on medical imaging 26(4), 487–496 (2007). [DOI] [PubMed] [Google Scholar]
- [10].Shiee N, Bazin P-L, Cuzzocreo JL, Blitz A, and Pham DL, “Segmentation of brain images using adaptive atlases with application to ventriculomegaly,” in [Biennial International Conference on Information Processing in Medical Imaging], 1–12, Springer; (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Patenaude B, Smith SM, Kennedy DN, and Jenkinson M, “A Bayesian model of shape and appearance for subcortical brain segmentation,” Neuroimage 56(3), 907–922 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Roy S, Carass A, Prince JL, and Pham DL, “Subject specific sparse dictionary learning for atlas based brain MRI segmentation,” in [International Workshop on Machine Learning in Medical Imaging], 248–255, Springer; (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Roy S, He Q, Sweeney E, Carass A, Reich DS, Prince JL, and Pham DL, “Subject-specific sparse dictionary learning for atlas-based brain MRI segmentation,” IEEE journal of biomedical and health informatics 19(5), 1598–1609 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, and Gee JC, “N4ITK: Improved N3 bias correction,” IEEE transactions on medical imaging 29(6), 1310–1320 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Roy S, Butman JA, Pham DL, Initiative ADN, and others, “Robust skull stripping using multiple MR image contrasts insensitive to pathology,” NeuroImage 146, 132–147 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Avants BB, Epstein CL, Grossman M, and Gee JC, “Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain,” Medical image analysis 12(1), 26–41 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Van Leemput K, Maes F, Vandermeulen D, and Suetens P, “Automated model-based tissue classification of MR images of the brain,” IEEE transactions on medical imaging 18(10), 897–908 (1999). [DOI] [PubMed] [Google Scholar]
- [18].Zhang J, “The mean field theory in EM procedures for Markov random fields,” IEEE Transactions on signal processing 40(10), 2570–2583 (1992). [DOI] [PubMed] [Google Scholar]
- [19].Dice LR, “Measures of the amount of ecologic association between species,” Ecology 26(3), 297–302 (1945). [Google Scholar]
- [20].Wilcoxon F, “Individual comparisons by ranking methods,” Biometrics bulletin 1(6), 80–83 (1945). [Google Scholar]