Abstract
Collective cell migration is a widely observed phenomenon during animal development, tissue repair, and cancer metastasis. Considering its broad involvement in biological processes, it is essential to understand the basics behind the collective movement. Based on the topology of migrating populations, tissue-scale kinetics, called the “leader–follower” model, has been proposed for persistent directional collective movement. Extensive in vivo and in vitro studies reveal the characteristics of leader cells, as well as the special mechanisms leader cells employ for maintaining their positions in collective migration. However, follower cells have attracted increasing attention recently due to their important contributions to collective movement. In this Perspective, the current understanding of the molecular mechanisms behind the “leader–follower” model is reviewed with a special focus on the force transmission and diverse roles of leaders and followers during collective cell movement.
INTRODUCTION
Collective cell migration is a key driver for coordinated multicellular movements that has been widely observed in both physiological and pathological processes, such as blood vessel sprouting, neural crest cell migration, tissue regenerationm and cancer metastasis (Wang et al., 2003; Friedl and Gilmour, 2009; Rorth, 2009; Friedl and Wolf, 2010; Haeger et al., 2015; Scarpa and Mayor, 2016; Barriga et al., 2018; Zhang et al., 2019). The most significant feature of collective migration is directional multicellular movement, which distinguishes it from single cell migration (Shellard and Mayor, 2019). In collective cell migration, a tissue-scale polarization among migrating cells, called “leader–follower” kinetics, has been proposed for persistent directional movement (Figure 1; Omelchenko et al., 2003; Gov, 2007; Poujade et al., 2007; Friedl and Gilmour, 2009; Rorth, 2009, 2012). The “leader–follower” model assumes two distinct cell populations in collective cell migration, that is, leaders and followers, which are categorized by their topology within the migrating cell ensemble (Theveneau and Linker, 2017).
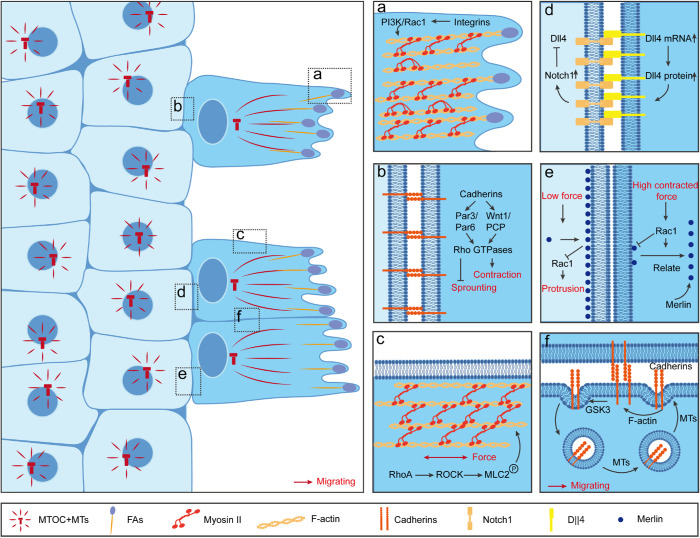
FIGURE 1:
Differentiation and maintenance of leader cells in collective cell migration. Top view of 2D collective sheet migration. Leader cells are front cells in dark blue with polarized centrosome–nucleus axis orientation and distinct “finger-like” protrusion generated from focal adhesions. Follower cells are the major cell population in light blue located in the cell reservoir with random centrosome–nucleus axis orientation and low migratory speed. (A) At the tip of leader cells, large lamellipodia protrusions extend from the cell body and form a “finger-like” morphology. At these sites, strong integrin-based FA connections with ECM activate a downstream PI3K-Rac signaling pathway, which further enhances actomyosin bundle formation and traction force generation. (B) At the rear of the leader cells, transmembrane protein cadherins mediate cell–cell connections with follower cells. At these sites, cadherin-mediated CIL leads to Rho-kinase–dependent myosin light-chain 2 phosphorylation, or Par3/Par6 recruitment at the junctional sites, which result in sprouting inhibition. (C) At the side of leader cells, phosphorylated myosin light chain and F-actin are highly accumulated as thick bundles, which prohibit new protrusion generation from follower cells. (d) Notch1-Dll4 lateral inhibition is reported at the interphase between leader and follower cells, with high Dll4 detected in leader cells, whereas high Notch1 is detected in follower cells. Low cellular stress in leader cells enhances Dll4 mRNA and protein expression, which further determinates the initiation of leader cells and migrating tips. This high Dll4 expression in leader cells enhances the Notch1 expression in follower cells, which in turn inhibits Dll4 expression in these cells. Moreover, high cellular stress also suppresses Dll4 expression in follower cells. (e) Merlin-Rac lateral inhibition is reported at the interphase between leader and follower cells. High contractile forces result in cytoplasmic Merlin through Rac-dependent translocation of Merlin in leader cells, whereas low contractile forces lead to boundary Merlin in follower cells which further inhibit Rac activity and Rac-mediated protrusions in follower cells. (F) At the interphase of lateral membranes between two migrating leader cells, a continuous treadmilling of cadherins is achieved through GSK3-dependent endocytosis processes.
Leader cells are specialized front cells that occupy the leading edge of the collective and assume a “finger-like” structure. In contrast, follower cells, which comprise the majority of the collective, are located in the cell reservoir. Follower cells were long considered to be “passive passengers” that simply moved along with leader cells (Poujade et al., 2007; Rorth, 2012). However, recent studies suggest that in fact follower cells, just like leader cells, become specialized during the polarization process. It is reported that different levels of exposure to extracellular signals stimulate the distinct distributions of adhesion proteins within leader and follower cells (Rorth, 2009, 2012; Khalil and de Rooij, 2019). Specifically, at the leading edge of the collective, leader cells experience asymmetric adherent connections with integrin-based focal adhesions (FA) at their extending fronts (Figure 1A) and cadherin-based adherent junctions (AJ) at their cell–cell connections with follower cells at their trailing edges (Figure 1B; Yamaguchi et al., 2015). However, in the cell reservoir, follower cells experience symmetric AJ adhesions with neighboring cells (Mayor and Carmona-Fontaine, 2010; Khalil and de Rooij, 2019). These differential focal adhesion and adherent protein distributions between leader and follower cells give rise to heterogeneous contractile forces at their cell–cell junctions (Weber, Bjerke, and DeSimone, 2012; Chen et al., 2018; Khalil and de Rooij, 2019), which leads to diverse cellular activities of PI3K-Rac signaling and Rho GTPases in these two populations (Zegers and Friedl, 2014). As a result, leader and follower cells establish a front-to-rear polarity axis during the movement (Capuana, Boström, and Etienne-Manneville, 2020). Following the polarity establishment, cells within the collective employ several different mechanisms, as described below, that help actively maintain their polarization and migration (Venhuizen and Zegers, 2017; Alert and Trepat, 2019).
LEADER CELLS: THE LEADER AND THE RULER
Contact inhibition of locomotion
Contact inhibition of locomotion (CIL) refers to the suppression of cell extension at cell edges that contact neighbors. For the leader cells, the asymmetric adhesion results in a biased CIL, generating large protrusions polarized toward the direction of migration (Desai et al., 2013; Ladoux, Mege, and Trepat, 2016; Mayor and Etienne-Manneville, 2016; George, Bullo, and Campàs, 2017). For the follower cells, the symmetric cell–cell adhesions produce essentially uniform CIL that suppresses the formation of large protrusions around their entire perimeter (Mayor and Carmona-Fontaine, 2010; Desai et al., 2013; Ladoux, Mege, and Trepat, 2016).
At the molecular level, CIL involves cadherin-based AJ, Wnt, and Par signaling (Figure 1B). For example, AJ components (Hidalgo-Carcedo et al., 2011), such as cadherins (Desai et al., 2009; Ozaki et al., 2010; Dumortier et al., 2012; Venhuizen and Zegers, 2017) and catenins (Ozaki et al., 2010; Bazellières et al., 2015), are essential for the maintenance of leader cells polarity. During cancer cell collective migration, cadherins at the rear of leader cells interact with Par3/Par6 protein, which recruit RhoE to cell–cell contacts and thereby promote coherent movement (Hidalgo-Carcedo et al., 2011). In the process of neovascularization, cadherin signals lead to Rho-associated protein kinase (ROCK)–dependent myosin light-chain 2 (MLC2) phosphorylation, which promotes actomyosin contractility and thereby inhibits sprouting (Abraham et al., 2009). Moreover, canonical Wnt signaling induces CIL by activating RhoA at the site of cell contact during neural crest migration (Carmona-Fontaine et al., 2008). As a result, CIL enhances leader cell polarization and prohibits follower extension, maintaining the tissue-scale polarity within the migrating collective (Mayor and Carmona-Fontaine, 2010; Haeger et al., 2015).
Physical restriction
Physical restriction is another mechanism that leader cells utilize to prohibit protrusion generation and new leader cell formation from follower cells (Figure 1C; Vedula et al., 2013; Reffay et al., 2014). At the side of the “finger-like” structure in leader cells, phosphorylated myosin light chain and F-actin are highly accumulated as thick bundles (Poujade et al., 2007; Reffay et al., 2014), which restrict new protrusion generation from follower cells. Laser ablation of these bundles releases the restriction, and induces new leader cell formation from the ablated site (Reffay et al., 2014). Moreover, this actomyosin structure transmits both mechanical and biochemical signaling inside leader cells as well as between leader cells and their extracellular environment (Pandya, Orgaz, and Sanz-Moreno, 2017). The small RhoA GTPase facilitates finger formation (Omelchenko et al., 2003; Reffay et al., 2014; Friedl, Wolf, and Zegers, 2014). RhoA activates ROCK resulting in MLC2 phosphorylation (Pandya, Orgaz, and Sanz-Moreno, 2017) which, in turn, promotes contraction of actin cables and generation of large forces, which serves as a physical restriction prohibiting new leader formation (Pandya, Orgaz, and Sanz-Moreno, 2017).
Lateral inhibition
During the competition with follower cells, leader cells also use several negative feedback loops to achieve lateral inhibition in order to keep their leading position. One is the Notch1-Dll4 loop between leader cells and follower cells (Figure 1D; Riahi et al., 2015). Dll4 (delta-like ligand 4) is a Notch ligand and a molecular signature of leader cells. During collective cell migration, high Notch1 and low Dll4 signals are detected in follower cells. Inhibiting overall Notch signaling increases Dll4-dependent transcription and translation and the number of leader cells (Riahi et al., 2015). However, inhibiting Dll4 suppresses the formation of leader cells (Riahi et al., 2015). In addition, this Notch1-Dll4 feedback loop is mechanosensitive. Pharmacologically or physically reducing the intercellular tension enhances Dll4 expression and increases the number of leader cells (Riahi et al., 2015; Wang et al., 2017), whereas increasing the intercellular tension by cytoskeleton stabilization reduces Dll4 expression and suppresses leader cell formation (Riahi et al., 2015). Therefore, Notch1 and Dll4 regulate leader cell concentration and separate cell dynamics for leaders and followers.
Merlin-Rac also participates in negative feedback in collective movement (Figure 1E; Das et al., 2015). Merlin is a tumor suppressor that acts upstream of the Hippo pathway (Li et al., 2015). Immunofluorescence revealed differential Merlin subcellular distribution in leader and follower cells: Merlin is mainly accumulated in the cytoplasm of leader cells, but restricted to cell junctions in follower cells (Das et al., 2015). This distribution difference is mediated by the contractile pulling force across the cell–cell boundary. In leader cells, high contractile forces result in cytoplasmic Merlin while low contractile forces in follower cells lead to junctional Merlin. Furthermore, the subcellular Merlin distribution is tightly linked to Rac activity in cell movement. Up-regulation of Rac activity using optogenetic tools induces translocation of Merlin from junctions to cytoplasm, whereas RNAi knock-down of Merlin disrupts Rac polarization. Thus, Merlin and Rac form a negative feedback loop to maintain the proper functions of both leader and follower cells in collective cell migration (Zoch and Morrison, 2015).
Adherent junction remodeling
In addition to the mechanisms discussed above, leader cells also take advantage of active AJ remodeling to achieve higher cadherin cycling rate and migration speed (Figure 1F). During collective astrocyte migration in wound healing, a continuous treadmilling of N-cadherin along the lateral sides of adjacent leader cells is observed (Peglion, Llense, and Etienne-Manneville, 2014). In leader cells, a directional recycling of N-cadherin proteins is observed from the leading edge to the lateral cell–cell contacts. Microtubules deliver endocytic vesicles that contain recycling cadherin components as well as catenins from the leading edge to the lateral edge between two leader cells. These AJ components are then moved to the rear of the cell where they are removed from the cell surface by endocytosis, and then delivered to the leading edge again. In follower cells, N-cadherin is less dynamic and the cells form more stable AJ structures. These results suggest a functional role of AJ remodeling in maintaining the polarity, molecular cycling, and migratory speed of leader cells (Hirata, Park, and Sahai, 2014).
FOLLOWER CELLS: UNDERESTIMATED CONTRIBUTORS
To date, it is still incompletely understood how the follower cells actively participate and mechanically contribute to collective cell migration. However, emerging evidence from both in vitro and in vivo studies suggest that these cells play a far more active role than what has been appreciated in the past.
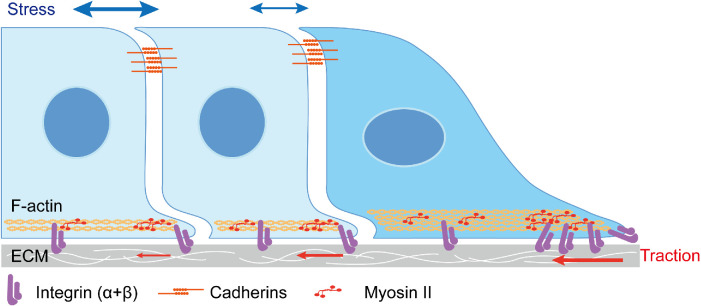
Direct traction force measurement illustrates a large force generated by leader cells and a gradually reduced traction generated by follower cells at the interface between migrating cells and extracellular matrix (ECM; Figure 2; Trepat et al., 2009). However, detailed examination of follower cells revealed that not all followers adhere to the limitations imposed by CIL. That is, cells located in the cell reservoir far away from the leading edge can form “cryptic” protrusions on their basal surface (Farooqui and Fenteany, 2005), which are considered as the source of small traction forces generated in followers (Figure 2). These cryptic protrusions mimic the lamellipodia of leader cells, and actively respond to newly formed wounds with directional orientation toward the migrating margin (Menko, Bleaken, and Walker, 2014). Furthermore, cadherin-based AJ proteins, such as WAVE, Arp2/3 (Ozawa et al. 2020), phosphorylated myosin II (Menko, Bleaken, and Walker, 2014), and Merlin-Rac loop (Das et al., 2015), participate in the formation of cryptic protrusions. In addition to traction forces, follower cells also mechanically contribute stress force for the collective movement. Newton’s third law implies that equal and opposite forces for the basal traction forces in migrating cells accumulate in the form of stress at the AJs of follower cells. Further, this stress, which rises steadily as the distance from the leading edge increases (Figure 2), contributes to collective cell migration (Trepat et al., 2009).
FIGURE 2:
Mechanical force distribution in the “leader–follower” model. Lateral view of 2D collective sheet migration. Leader cells in dark blue exhibit asymmetry exposure of adhesions, which includes integrin-based FA at migratory front and cadherin-mediated AJ at the rear with follower cells. Whereas, follower cells in light blue experience symmetry cadherin-mediated AJ with neighboring cells at their apical surface and small FA formation at their basal surface. From the mechanical force perspective, the collective migrating population participates in a global “tug-of-war.” The highly accumulated integrins at the migrating tip enable leader cells with large traction forces generated with ECM, and small tractions are recorded in follower cells with their “cryptic” protrusions. The balanced forces to traction are the cellular stress, which is mainly transmitted by the cellular cytoskeleton, and the cell–cell junctions. The stress is built up across the entire migrating tissue, and increases steadily as the distance from the leading edge increases.
Besides traction and stress generation, follower cells also contribute to the formation of new leader cells (Vishwakarma et al., 2018). Studies using traction force and monolayer stress microscopy revealed that two to six cell layers behind the prospective leader cells exhibited high local cell-matrix traction. This traction was recorded within 30–45 min after wound generation but before leader cell formation. Importantly, this local force generated in follower cells can be transmitted to and pull on the future leaders and help “elect” them to their fate (Vishwakarma et al., 2018). By modifying the force transduction in the cell populations through substrate confinement and pharmacological treatment, it was found that the distance between different local force centers determined the number of newly formed leader cells as well as the physical separating distance between individual leader cells (Vishwakarma et al., 2018). These results suggest that traction forces in follower cells are essential for leader cell formation.
Published results also support that follower cells are more than auxiliary players after the leader–follower polarity is established. Mathematical modeling suggests that cell-to-cell variation and noisiness of individual cell motion in follower cells increase the overall sensory ability and the collective accuracy of migrating groups (Camley and Rappel, 2017). Moreover, during the collective migration of the zebrafish posterior lateral line primordium, both leaders and followers are required to express sufficient chemokine receptor Cxcr4 in responding to the chemokine Cxc12a, which is essential for coupling and efficient migration (Colak-Champollion et al., 2019). The cadherin expressed in follower cells not only mediates adhesion between leader and follower cells, but also is required for follower cells to pull attractant-blind neighboring cells (Colak-Champollion et al., 2019). Furthermore, the leaders and followers can switch their positions and roles based on their cellular status (Inaki et al., 2012). In the Drosophila egg chamber, a cluster of six to eight epithelial cells together with two captured polar cells migrate forward in a tumbling way (Montell, 2003; Bianco et al., 2007). During this process, all epithelial cells in the border cell cluster continuously exchange their spatial position, and keep the cell with highest signal state at the leading front (Inaki et al., 2012). Experiments based on genetic manipulations in Drosophila showed that when followers were enhanced with high Rac expression or PDGF/VEGF receptor (PVR) receptor expression, follower cells can swap their position from back to front, become new leader cells, and maintain their position at leading front, controlling the overall cluster migration (Inaki et al., 2012).
In short, the activity and contributions of follower cells are more important than is often recognized: experimental studies show mechanical contributions (Trepat et al., 2009), sensory guidance (Colak-Champollion et al., 2019), and switchable roles (Inaki et al., 2012) for follower cells in collective migration. These results suggest a modified “leader–follower” model for collective cell migration, in which each cell in a collective migrating tissue, whether a leader or a follower, participates in a global “tug-of-war” and contributes to a global tensile stress (Trepat et al., 2009; Ladoux, Mege, and Trepat, 2016; Ladoux and Mège, 2017). We would also argue that as the majority of the cells within migrating collectives, follower cells are deserving of more attention. Specific avenues of potential research are suggested below.
PERSPECTIVES
Perhaps the most promising approach for learning more about collective cell migration and the relative contributions of leader and follower cells is to take advantage of high spatiotemporal imaging of live cells in 2D and 3D cell culture systems (Liang, Park, and Guan, 2007; Yamada and Sixt, 2019) and in intact animals (Wang et al., 2006; Aman and Piotrowski, 2010; Schumacher, 2019). For example, it was recently found that distinct propagation waves of extracellular signal-related kinase (ERK) were observed in the opposite direction of collective migration (Aoki et al., 2017). This ERK wave tightly links both the leader cells and the follower cells. The initial wave of ERK activation advances in the edge cells (one cell row behind the leaders) where mechanical stretch is generated from polarized leader cells (Hino et al., 2020). Then the ERK activation triggers edge cell contraction, which leads to a pulling force that activates another round of ERK signaling in neighboring cells. As a result, a tissue-scale ERK propagation is generated from leader cells to follower cells (Hino et al., 2020). This stress-polarity coupling between migrating cells may be essential for long-distance transmission of guidance cues and efficient collective migration. Moreover, live cell recordings also reveal that collective cell migration takes place with multiple additional cellular dynamic events (Aman and Piotrowski, 2010; Martin, 2010), such as cell oscillation (Martin, Kaschube, and Wieschaus, 2009; Solon et al., 2009) and active cell intercalation (Bertet, Sulak, and Lecuit, 2004; Caussinus, Colombelli, and Affolter, 2008).
Considering the importance of collective cell migration in development and metastatic invasion, a more comprehensive “leader–follower” model with detailed molecular regulations and diverse contributions from leader cells and follower cells is of great importance for potential strategies for developmental defects, the prevention and treatment for cancers, and advances in tissue engineering.
Acknowledgments
We thank Bill Bement for editing this article, Yusuke Toyama and Xiang Teng for discussion on follower cell’s contributions, and Benoît Ladoux and Pernille Rørth for discussion on the leader–follower model in collective cell migration. This work was supported by The National Key Research and Development Program of China (Grant no. 2019YFA0906004), the National Natural Science Foundation of China (Grants no. 81991513, no. 82022047, no. 81630066, and no. 81870532), and the Guangdong Provincial Science and Technology Innovation Council (Grant no. 2017B030301018).
Abbreviations used:
- AJ
adherent junction
- CIL
contract inhibition of locomotion
- Dll4
delta-like ligand4
- ECM
extracellular matrix
- ERK
extracellular signal-related kinase
- FA
focal adhesion
- MLC2
myosin light chain 2
- MT
microtubue
- N-cadherin
neural cadherin
- PVR
PDGF/VEGF receptor
- ROCK
Rho-associated protein kinase.
Footnotes
REFERENCES
- Abraham S, Yeo M, Montero-Balaguer M, Paterson H, Dejana E, Marshall CJ, Mavria G (2009). VE-cadherin-mediated cell-cell interaction suppresses sprouting via signaling to MLC2 phosphorylation. Curr Biol 19, 668–674. [DOI] [PubMed] [Google Scholar]
- Alert R, Trepat X (2019). Physical models of collective cell migration. Ann Rev Condens Matter Phys 11, 77–101. [Google Scholar]
- Aman A, Piotrowski T (2010). Cell migration during morphogenesis. Dev Biol 341, 20–33. [DOI] [PubMed] [Google Scholar]
- Aoki K, Kondo Y, Naoki H, Hiratsuka T, Itoh RE, Matsuda M (2017). Propagating wave of ERK activation orients collective cell migration. Dev Cell 43, 305–317.e5. [DOI] [PubMed] [Google Scholar]
- Barriga EH, Franze K, Charras G, Mayor R (2018). Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature 554, 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazellières E, Conte V, Elosegui-Artola A, Serra-Picamal X, Bintanel-Morcillo M, Roca-Cusachs P, Muñoz JJ, Sales-Pardo M, Guimerà R, Trepat X (2015). Control of cell-cell forces and collective cell dynamics by the intercellular adhesome. Nat Cell Biol 17, 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T (2004). Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429, 667–671. [DOI] [PubMed] [Google Scholar]
- Bianco A, Poukkula M, Cliffe A, Mathieu J, Luque CM, Fulga TA, Rorth P (2007). Two distinct modes of guidance signalling during collective migration of border cells. Nature 448, 362–365. [DOI] [PubMed] [Google Scholar]
- Camley BA, Rappel W-J (2017). Cell-to-cell variation sets a tissue-rheology–dependent bound on collective gradient sensing. Proc Natl Acad Sci USA 114, E10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuana L, Boström A, Etienne-Manneville S (2020). Multicellular scale front-to-rear polarity in collective migration. Curr Opin Cell Biol 62, 114–122. [DOI] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Matthews HK, Kuriyama S, Moreno M, Dunn GA, Parsons M, Stern CD, Mayor R (2008). Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature 456, 957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caussinus E, Colombelli J, Affolter M (2008). Tip-cell migration controls stalk-cell intercalation during Drosophila tracheal tube elongation. Curr Biol 18, 1727–1734. [DOI] [PubMed] [Google Scholar]
- Chen T, Beng Saw T, Mège R-M, Ladoux B (2018). Mechanical forces in cell monolayers. J Cell Sci 131, jcs218156. [DOI] [PubMed] [Google Scholar]
- Colak-Champollion T, Lan L, Jadhav AR, Yamaguchi N, Venkiteswaran G, Patel H, Cammer M, Meier-Schellersheim M, Knaut H (2019). Cadherin-mediated cell coupling coordinates chemokine sensing across collectively migrating cells. Curr Biol 29, 2570–2579.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T, Safferling K, Rausch S, Grabe N, Boehm H, Spatz JP. (2015). A molecular mechanotransduction pathway regulates collective migration of epithelial cells. Nat Cell Biol 17, 276–287. [DOI] [PubMed] [Google Scholar]
- Desai RA, Gao L, Raghavan S, Liu WF, Chen CS (2009). Cell polarity triggered by cell-cell adhesion via E-cadherin. J Cell Sci 122, 905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RA, Gopal SB, Chen S, Chen CS (2013). Contact inhibition of locomotion probabilities drive solitary versus collective cell migration. J R Soc Interface 10, 20130717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumortier JG, Martin S, Meyer D, Rosa FM, David NB (2012). Collective mesendoderm migration relies on an intrinsic directionality signal transmitted through cell contacts. Proc Natl Acad Sci USA 109, 16945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui R, Fenteany G (2005). Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J Cell Sci 118, 51–63. [DOI] [PubMed] [Google Scholar]
- Friedl P, Gilmour D (2009). Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 10, 445–457. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K (2010). Plasticity of cell migration: a multiscale tuning model. J Cell Biol 188, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Wolf K, Zegers MM (2014). Rho-directed forces in collective migration. Nat Cell Biol 16, 208–210. [DOI] [PubMed] [Google Scholar]
- George M, Bullo F, Campàs O (2017). Connecting individual to collective cell migration. Sci Rep 7, 9720–9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gov NS (2007). Collective cell migration patterns: follow the leader. Proc Natl Acad Sci USA 104, 15970–15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeger A, Wolf K, Zegers MM, Friedl P (2015). Collective cell migration: guidance principles and hierarchies. Trends Cell Biol 25, 556–566. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, Leitinger B, Sahai E (2011). Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol 13, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino N, Rossetti L, Marín-Llauradó A, Aoki K, Trepat X, Matsuda M, Hirashima T (2020). ERK-mediated mechanochemical waves direct collective cell polarization. Dev Cell 53, 646-660.e8. [DOI] [PubMed] [Google Scholar]
- Hirata E, Park D, Sahai E (2014). Retrograde flow of cadherins in collective cell migration. Nat Cell Biol 16, 621–623. [DOI] [PubMed] [Google Scholar]
- Inaki M, Vishnu S, Cliffe A, Rørth P (2012). Effective guidance of collective migration based on differences in cell states. Proc Natl Acad Sci USA 109, 2027–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil AA, de Rooij J (2019). Cadherin mechanotransduction in leader-follower cell specification during collective migration. Exp Cell Res 376, 86–91. [DOI] [PubMed] [Google Scholar]
- Ladoux B, Mege RM, Trepat X (2016). Front–rear polarization by mechanical cues: from single cells to tissues. Trends Cell Biol 26, 420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladoux B, Mège R-M (2017). Mechanobiology of collective cell behaviours. Nat Rev Mol Cell Biol 18, 743–757. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhou H, Li F, Chan SW, Lin Z, Wei Z, Yang Z, Guo F, Lim CJ, Xing W, et al. (2015). Angiomotin binding-induced activation of Merlin/NF2 in the Hippo pathway. Cell Res 25, 801–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CC, Park AY, Guan JL (2007). In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2, 329–333. [DOI] [PubMed] [Google Scholar]
- Martin AC (2010). Pulsation and stabilization: contractile forces that underlie morphogenesis. Dev Biol 341, 114–125. [DOI] [PubMed] [Google Scholar]
- Martin AC, Kaschube M, Wieschaus EF (2009). Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457, 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor R, Carmona-Fontaine C (2010). Keeping in touch with contact inhibition of locomotion. Trends Cell Biol 20, 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor R, Etienne-Manneville S (2016). The front and rear of collective cell migration. Nat Rev Mol Cell Biol 17, 97–109. [DOI] [PubMed] [Google Scholar]
- Menko AS, Bleaken BM, Walker JL (2014). Regional-specific alterations in cell-cell junctions, cytoskeletal networks and myosin-mediated mechanical cues coordinate collectivity of movement of epithelial cells in response to injury. Exp Cell Res 322, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell DJ (2003). Border-cell migration: the race is on. Nat Rev Mol Cell Biol 4, 13–24. [DOI] [PubMed] [Google Scholar]
- Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM (2003). Rho-dependent formation of epithelial “leader” cells during wound healing. Proc Natl Acad Sci USA 100, 10788–10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki C, Yoshioka M, Tominaga S, Osaka Y, Obata S, Suzuki ST (2010). p120-catenin is essential for N-cadherin-mediated formation of proper junctional structure, thereby establishing cell polarity in epithelial cells. Cell Struct Funct 35, 81–94. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Hiver S, Yamamoto T, Shibata T, Upadhyayula S, Mimori-Kiyosue Y, Takeichi M (2020). Adherens junction regulates cryptic lamellipodia formation for epithelial cell migration. J Cell Biol 219, e202006196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya P, Orgaz JL, Sanz-Moreno V (2017). Actomyosin contractility and collective migration: may the force be with you. Curr Opin Cell Biol 48, 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peglion F, Llense F, Etienne-Manneville S (2014). Adherens junction treadmilling during collective migration. Nat Cell Biol 16, 639–651. [DOI] [PubMed] [Google Scholar]
- Poujade M, Grasland-Mongrain E, Hertzog A, Jouanneau J, Chavrier P, Ladoux B, Buguin A, Silberzan P (2007). Collective migration of an epithelial monolayer in response to a model wound. Proc Natl Acad Sci USA 104, 15988–15993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reffay M, Parrini MC, Cochet-Escartin O, Ladoux B, Buguin A, Coscoy S, Amblard F, Camonis J, Silberzan P (2014). Interplay of RhoA and mechanical forces in collective cell migration driven by leader cells. Nat Cell Biol 16, 217–223. [DOI] [PubMed] [Google Scholar]
- Riahi R, Sun J, Wang S, Long M, Zhang DD, Wong PK (2015). Notch1-Dll4 signalling and mechanical force regulate leader cell formation during collective cell migration. Nat Commun 6, 6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorth P (2009). Collective cell migration. Annu Rev Cell Dev Biol. 25, 407–429. [DOI] [PubMed] [Google Scholar]
- Rorth P (2012). Fellow travellers: emergent properties of collective cell migration. EMBO Rep 13, 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa E, Mayor R (2016). Collective cell migration in development. J Cell Biol 212, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher L (2019). Collective cell migration in development. Adv Exp Med Biol 1146, 105–116. [DOI] [PubMed] [Google Scholar]
- Shellard A, Mayor R (2019). Supracellular migration—beyond collective cell migration. J Cell Sci 132. [DOI] [PubMed] [Google Scholar]
- Solon J, Kaya-Copur A, Colombelli J, Brunner D (2009). Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell 137, 1331–1342. [DOI] [PubMed] [Google Scholar]
- Theveneau E, Linker C (2017). Leaders in collective migration: are front cells really endowed with a particular set of skills? [version 1; peer review: 2 approved]. F1000Res 6, 1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepat X, Wasserman MR, Angelini TE, Millet E, Weitz DA, Butler JP, Fredberg JJ (2009). Physical forces during collective cell migration. Nat Phys 5, 426–430. [Google Scholar]
- Vedula SR, Ravasio A, Lim CT, Ladoux B (2013). Collective cell migration: a mechanistic perspective. Physiology (Bethesda) 28, 370–379. [DOI] [PubMed] [Google Scholar]
- Venhuizen J-H, Zegers MM (2017). Making heads or tails of it: cell-cell adhesion in cellular and supracellular polarity in collective migration. Cold Spring Harb Perspect Biol 9, a027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwakarma M, Di Russo J, Probst D, Schwarz US, Das T, Spatz JP (2018). Mechanical interactions among followers determine the emergence of leaders in migrating epithelial cell collectives. Nat Commun 9, 3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Bo J, Bridges T, Dugan KD, Pan TC, Chodosh LA, Montell DJ (2006). Analysis of cell migration using whole-genome expression profiling of migratory cells in the Drosophila ovary. Dev Cell 10, 483–495. [DOI] [PubMed] [Google Scholar]
- Wang S, Sun J, Xiao Y, Lu Y, Zhang DD, Wong PK (2017). Intercellular tension negatively regulates angiogenic sprouting of endothelial tip cells via Notch1-Dll4 signaling. Adv Biosyst 1, 1600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Zhao M, Forrester JV, McCaig CD (2003). Electric fields and MAP kinase signaling can regulate early wound healing in lens epithelium. Invest Ophthalmol Vis Sci 44, 244–249. [DOI] [PubMed] [Google Scholar]
- Weber GF, Bjerke MA, DeSimone DW (2012). A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev Cell 22, 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM, Sixt M (2019). Mechanisms of 3D cell migration. Nat Rev Mol Cell Biol 20, 738–752. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, Mizutani T, Kawabata K, Haga H (2015). Leader cells regulate collective cell migration via Rac activation in the downstream signaling of integrin beta1 and PI3K. Sci Rep 5, 7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers MM, Friedl P (2014). Rho GTPases in collective cell migration. Small GTPases 5, e28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Goliwas KF, Wang W, Taufalele PV, Bordeleau F, Reinhart-King CA (2019). Energetic regulation of coordinated leader–follower dynamics during collective invasion of breast cancer cells. Proc Natl Acad Sci USA 116, 7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoch A, Morrison H (2015). Merlin’s wizardry guides cohesive migration. Nat Cell Biol 17, 212–213. [DOI] [PubMed] [Google Scholar]