Abstract
Laboratory and epidemiological data indicate that high blood sugar levels and/or consuming high glycemia diets are linked to multiple age-related diseases, including age-related macular degeneration, cataract, Parkinson’s disease, Alzheimer’s disease, diabetic retinopathy, and, apparently glaucoma. High concentrations of blood sugar and perturbations of the systems that regulate blood sugar lead to the accumulation of advanced-glycation end products (AGEs). AGEs are toxic compounds that are formed from the combination of sugars and their metabolites with biomolecules in a non-enzymatic biochemical reaction called glycation. In vitro and in vivo data indicate that high sugar consumption is associated with accumulation of AGEs in a variety of human tissues. Hyperglycemia, along with an oxidative environment and limited cell proliferation in many ocular tissues, encourages formation and precludes dilution of AGEs and associated damage by cell division. These circumstances make many eye tissues vulnerable to glycation-derived damage. Here, we summarize research regarding glycation-induced ocular tissue dysfunction and its contribution to the onset and development of eye disorders. We also discuss how management of carbohydrate nutrition may provide a low-cost way to ameliorate the progression of AGEs-related diseases, including age related macular degeneration and some cataracts, as they do for cardiovascular disease and diabetes.
Keywords: Advanced glycation-end products, Age-related macular degeneration, Diabetic retinopathy, Cataracts, Diabetes, Aging, Glycemic index, Cornea, Retina, Trabecular meshwork, Vitreous, Retina, Optic nerve
1. Introduction
The “western” lifestyle is spreading across the globe. Associated with this lifestyle is less activity and increased consumption of processed foods. A majority of processed foods contain added sugars or polysaccharides that are broken down rapidly to sugars which raise blood sugar levels (Popkin and Hawkes, 2016). Coincident with the increased adaptation to a western lifestyle is a rapid increase in the prevalence of metabolic syndrome, diabetes and obesity in both developing and industrialized countries, creating a major worldwide health hazard. The current dietary trend is not sustainable in terms of total cost of healthcare. Consequently, to counter the unhealthy dietary habits, governments are beginning to tax high calorie foods or restrict advertisement of unhealthy foods (NHMRC, 2013).
In order to optimize dietary practices, promote healthy aging, and develop policies that improve dietary practices, it is useful to evaluate and review the scientific literature regarding sugar intake and its effects on health. A growing number of reports indicate that chronic exposure to high glycemia diets contributes to increased risk for multiple human disorders including type II diabetes, cerebrovascular, cardiovascular and eye-related diseases. The latter include cataract, diabetic retinopathy and macular degeneration (Aragno and Mastrocola, 2017; Chiu and Taylor, 2011; Semba et al., 2010; Weikel et al., 2012a, 2012b). Although these diseases may have multiple and different etiologies, all of them have been linked to a common pathogenic factor that accelerates under hyperglycemic conditions: the formation of toxic advanced glycation end products (AGEs) (Weikel et al., 2012a, 2012b). Herein, we summarize the current literature that relates the pathological role of AGEs and the loss of function in ocular tissues. We discuss the advantages of nutritional interventions in AGEs-related diseases, highlighting the usefulness of consumption of low-glycemia diets in the management of blood sugar and its benefits for eye fitness.
2. Too much sugar increases AGEs and causes homeostatic imbalance
Current dietary guidelines for Americans recommend limiting sugar to no more than 10% of daily calories (NAS, 2017). However, these recommendations remain aspirational in America (Marriott et al., 2010). For an average man or woman, with average caloric intake of 2000 or 1600 calories, respectively, this would be 200 or 160 calories of sugar, respectively. For reference, one can of cola has about 39 g of sugar and an ice cream has about 15 g of sugar. On average, consumption is threefold the recommended levels (Marriott et al., 2010). Even in healthy individuals acute ingestion of fructose and glucose has been recently shown to lead to unfavorable metabolic and endocrine responses (Cai et al., 2018).
AGEs are a heterogeneous array of compounds that accumulate in multiple human tissues during normal aging and, especially, under conditions of hyperglycemia (Aragno and Mastrocola, 2017; Chiu and Taylor, 2011; Semba et al., 2010). Laboratory animal and human studies indicate that elevated blood sugar levels are associated with the accumulation of cytotoxic AGEs that are thought to play a role in the aging process as well as to contribute to the onset and exacerbation of age-related diseases (Frimat et al., 2017; Semba et al., 2010; Uchiki et al., 2012; Weikel et al., 2012b). AGEs accumulate particularly rapidly in tissues with a low capacity of regeneration (Semba et al., 2010; Weikel et al., 2012a, 2012b).
Retinal pigmented epithelia cells have the highest proteolytic burden in the body because each RPE cell must digest the outer tips of 30 photoreceptor cells that are shed each morning. Usually, RPE cells do not proliferate. The vast majority of lens cells also do not proliferate. Thus, in these cells, opportunities for dilution of AGEs by replication, or repair of AGEs-associated damage, are limited, making hyperglycemic conditions a major risk factor for eye diseases.
AGEs arise from two main sources: exogenous and endogenous. Exogenous AGEs are ingested and these are found at highest levels in cooked or highly processed foods (Uribarri et al., 2010; Vlassara et al., 2016). Upon digestion, AGEs can be absorbed and released into the blood (Koschinsky et al., 1997a). Nevertheless, only 10% of consumed AGEs are intestinally absorbed and one-third of these AGEs are quickly excreted in the urine within 48 h, thus removed from the circulation (He et al., 1999; Koschinsky et al., 1997b; Rabbani and Thornalley, 2015). In spite of the renal clearance of circulating AGEs, exogenous AGEs contribute around 30% of the total AGEs in our body (Uribarri et al., 2010) and increased levels of serum AGEs are found in patients with renal failure (Uribarri et al., 2003). Among dietary AGEs that are also found in plasma and urine are Nε-(carboxymethyl)lysine (CML), Nε-(1-carboxyethyl)lysine (CEL) and Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine (MG-H1) (Scheijen et al., 2018).
Endogenously formed AGEs create the largest burden of AGEs in the body. These are formed in vivo as result of the exposure of biomolecules to reactive sugars. Thus, it is not surprising that elevated levels of different types of AGEs were described in diabetic conditions more than three decades ago (Ahmed et al., 1986; Miyata and Monnier, 1992; Sell et al., 1991). The formation of these endogenous AGEs requires several reactions between sugars (or their metabolites) with other biomolecules and these reactions are summed up by the term glycation. Glycation is a spontaneous chemical process in which unstable Schiff bases and Amadori products are formed through a reaction between α-amino group of the N-terminal amino acid or the ε-amino groups of lysines and arginines within proteins and the aldehyde or ketone group of the reactive sugar (Stitt, 2001). These intermediates of glycation are reversible and exist at levels that are proportional to the free sugar. However, they are unstable, and due to the complex biochemical processes of condensation, oxidation, re-arrangement, dehydration and degradative reactions, they form a myriad of irreversible, toxic and heterogeneous compounds. Collectively, these are called AGEs (Rabbani and Thornalley, 2015).
Curiously, the molecule of glucose is not a highly reactive sugar in most biological contexts, and AGEs formation derived directly from glucose might take weeks. However, other types of sugars (i.e. fructose) or intermediates derived from glycolysis (i.e. glyceraldehyde-3-phosphate or glucose-6-phosphate) are much more reactive, resulting in an accelerated production of reactive dicarbonyls that culminates in a faster production of AGEs (Hamada et al., 1996; Sadowska-Bartosz et al., 2014; Suarez et al., 1989) (Fig. 1). Of note, the high oxygen concentration and environmental oxidative stress in vascularized parts of the eye contribute to the processes of oxidation that accelerate AGEs formation, making them especially vulnerable to the accumulation of AGEs. Low protein turnover rates in the lens also result in high levels of AGEs in the aged lens, despite the lens being a low oxygen tension environment.
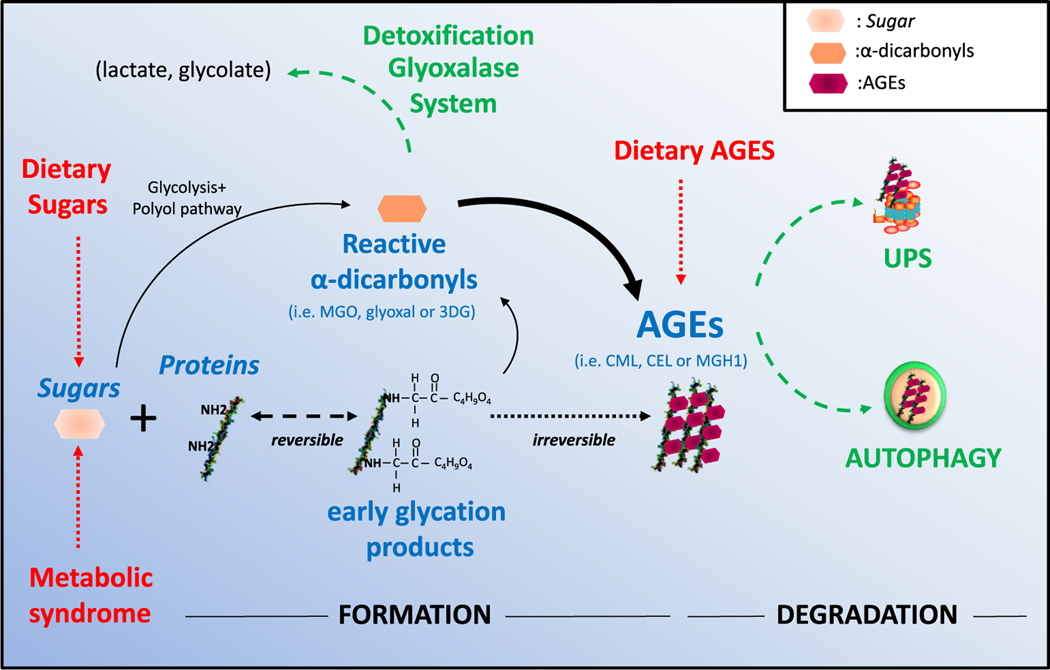
Fig. 1. Formation of AGEs and protective modulatory systems.
AGEs can be slowly formed from high concentration of blood sugar through the Maillard reaction (non-enzymatic chemical reaction between amino groups on proteins by reducing sugars) or faster through the reaction with alpha-dicarbonyls. Reactive dicarbonyls include methylglyoxal (MGO), glyoxal, or 3deoxyglucosane (3DG). AGEs include carboxymethyl-lysine (CML), carboxyethyl-lysine (CEL), methylglyoxal-derived hydroimidazolone (MGH1), etc. Protective anti-AGEs mechanisms (highlighted in green) consist of detoxifying routes such as glyoxalase pathway and protein quality control system that include ubiquitin-proteasome system (UPS) and autophagy.
Given the burden of high levels of AGEs, proficient cellular protective mechanisms are required to avoid their deleterious effects. There are multiple capacities to limit the accumulation of AGEs (Fig. 1, highlighted in green). One of these defense mechanisms is the glyoxalase system, a ubiquitous detoxification pathway that converts highly reactive AGEs intermediates (i.e. methylglyoxal or glyoxal) into less reactive biomolecules such as lactate or glycolate (Sousa Silva et al., 2013; Thornalley, 2003). The process involves the sequential activities of two thiol-dependent enzymes, glyoxalase І and glyoxalase ІI, that reduce methylglyoxal to lactate in the presence of glutathione (Thornalley, 2003).
Proteolytic pathways also contribute to protection by searching out and destroying intracellular AGEs. Two major degradative capacities are involved in the clearance of AGEs: the ubiquitin-proteasome system (UPS) and autophagy (Eisermann et al., 2017; Takahashi et al., 2017; Taylor, 2012; Uchiki et al., 2012) (Fig. 1). UPS is a degradative system in which soluble substrates are tagged with ubiquitin and targeted to the proteasome for degradation. Autophagy is a proteolytic route by which substrates are delivered into the lysosomes and degraded by lysosomal proteases. This releases amino acids for recycling. Although these two capacities were thought to be independent degradative routes, recent literature supports a crosstalk and interplay between UPS and autophagy (Bejarano and Cuervo, 2010; Ji and Kwon, 2017). Information about the nature of the AGEs that are targeted for degradation to either proteolytic system or which molecular determinants are involved in the delivery of AGEs is scanty (Uchiki et al., 2012). Recent findings from our group indicate that the UPS and autophagy remove different populations of toxic AGEs, as reported for other pathogenic proteins (Caballero et al., 2018; Ciechanover and Kwon, 2015). The induction of autophagy could also be a compensatory mechanism or complementary capacity when UPS function is insufficient (i.e. after the glycative inhibition of proteasome subunits) (Moheimani et al., 2010; Queisser et al., 2010; Uchiki et al., 2012).
In sum, the human body has defense mechanisms to avoid the burden of AGEs. Nevertheless, consumption of high sugar diets, altered glucose metabolism conditions (i.e. diabetes, insulin resistance or hyperglycemia) or the decline of proteolytic activities with age (Morimoto and Cuervo, 2014) may lead to the accelerated formation and accumulation of these damaging compounds in many tissues.
3. Pathophysiology of AGEs in ocular tissues
Although the exact pathogenic mechanisms remain to be elucidated, emerging evidence suggests a significant role for AGEs in the etiology of multiple age-related disorders, including ocular diseases (Kandarakis et al., 2014; Semba et al., 2010; Shang and Taylor, 2012; Stitt, 2001; Weikel et al., 2012a). AGEs are deposited intracellularly or in the extracellular environments thus participating in the onset of the ocular damage in different ways (Fig. 2). For example, AGEs in circulation may bind at the plasma membrane to cell surface receptors, including scavenger receptors. The best-studied AGE-receptor is called receptor for advanced glycation end-products (RAGE or also AGER), a transmembrane receptor of the immunoglobulin superfamily (Neeper et al., 1992). When AGEs are bound to RAGE, several signaling pathways are activated, including Ras/MAPK/NF-κB, JAK/STAT, and Rac1/Cdc42 leading to oxidative stress and NF-κB activation (Huang et al., 2001; Hudson et al., 2008; Lander et al., 1997) Other receptors capable of binding AGEs are AGE-R1/OST-48/p60, AGE-R2/80K-H/p90, AGE-R3/ galectin-3, SR-A (I/II), SR-B/CD36, SR-BI, SR-E/LOX-1, FEEL-1/FEEL-2 (Araki et al., 1995; Jono et al., 2002; Li et al., 1996; Ohgami et al., 2001a, 2001b, 2002; Tamura et al., 2003; Vlassara et al., 1995).
Fig. 2. Pathophysiology of AGEs in ocular tissues.
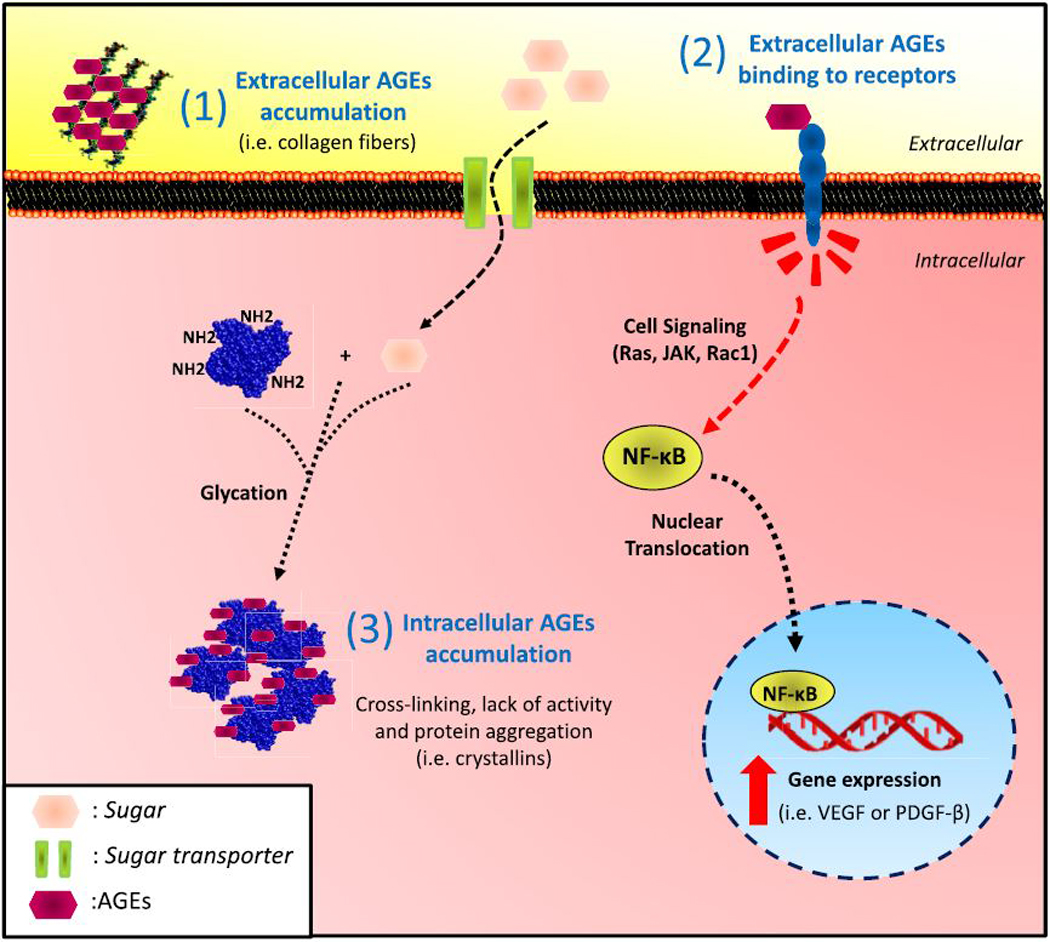
AGEs can 1) accumulate extracellularly and alter the biochemical properties of the extracellular matrix, 2) bind to plasma membrane receptor, triggering nuclear translocation of specific transcription factors or 3) AGEs formed intracellularly induce protein-crosslinking that alter conformational structure of proteins affecting their activities. Accumulation of elevated levels of intracellular aggregates is thought to be cytotoxic.
Alternatively, glycation per se can modify biochemical properties of specific proteins, altering intramolecular function and protein-protein interactions by intermolecular crosslinking and interfering with the functionality of those glycated targets. Typical examples are the glycation of proteins in the extracellular matrix (i.e. collagens fibers) and glycation of intracellular proteins such as crystallins in lens fiber cells (Fig. 2). For example, intracellular protein crosslinking, including glycation associated crosslinking, results in lens stiffening upon aging. Perhaps the trabecular meshwork is subject to the same influences (see below). Extracellular fibers are also modified via glycation-mediated crosslinks resulting in a loss of flexibility and enhanced susceptibility to mechanical stress (Dyer et al., 1993; Taylor et al., 1995).
In the following sections, we summarize the literature about the detection of AGEs in ocular tissues and their role in initiation and progression of sight threatening disorders.
3.1. Cornea
AGEs accumulate in the corneal stroma and lamina during normal aging. A specific example is glycation and crosslinking of corneal collagen (Malik et al., 1992). Some AGEs fluoresce. Higher values of corneal AGE fluorescence are found in patients with proliferative diabetic retinopathy compared to control subjects (Sato et al., 2001). Furthermore, enhanced levels of AGEs are detected in diabetic cornea with a more prominent reactivity in stroma, basal laminae and Bowman’s membrane. Importantly, collagen crosslinking appears to be involved in the central corneal thickening, which alters corneal biomechanics properties (Brummer et al., 2011; Kaji et al., 2000; Sady et al., 1995). In vitro data showed that AGEs can delay corneal epithelial wound healing and also induce apoptosis in human corneal epithelial cells through increased generation of intracellular ROS via NADPH oxidase activation and upregulation of JNK and p38 MAPK pathways (Shi et al., 2013a, 2013b). Glycation in the stromal matrix is thought to increase aggregation of collagen fibrils in the cornea contributing to diabetic keratopathy (Kim et al., 2011; Zou et al., 2012). High concentrations of AGEs are also found in vivo in the corneal epithelium, basement membrane and stromal keratocytes of long-term diabetic monkeys and in streptozotocin-injected Sprague-Dawley rats (Kim et al., 2011; Zou et al., 2012).
3.2. Lens
The lens is probably the eye tissue in which the pathogenic role of AGEs has been best characterized at the molecular level both in normal aging and in a diabetic context (Abiko et al., 1999; Ahmed et al., 1997; Chellan and Nagaraj, 1999; Frye et al., 1998; Lyons et al., 1991; Matsumoto et al., 1997; Nagaraj et al., 2012; Pokupec et al., 2003; Stevens et al., 1978; Tessier et al., 1999; Zarina et al., 2000). The role of sugar in diabetic cataract has been extensively studied and several reports established a plasma glycemic threshold above which incidence of diabetic cataract increases exponentially (Nagaraj et al., 1996; Swamy-Mruthinti et al., 1999). Higher levels of AGEs are found in diabetic patients (Hashim and Zarina, 2011; Pokupec et al., 2003).
As might be expected, protein glycation increases dramatically with age (Raghavan et al., 2015; Tessier et al., 1999). Associated with this glycation are covalent crosslinking of crystallins, resulting in altered tertiary structure, and aggregation. Chaperone activity is also lost and lens fiber cell integrity is compromised (Chellan and Nagaraj, 1999; Shamsi et al., 2000). For example, αA-crystallin and γB-crystallin have been shown to be thus modified, resulting in aggregation, insolubility, and lens opacity (Beswick and Harding, 1987; Casey et al., 1995; Kumar et al., 2007; Nahomi et al., 2013; Perry et al., 1987).
The lens capsule is also a target for glycation. Recently, different AGEs were found accumulated in human lens capsules in an age-dependent manner, with higher levels observed in cataractous lens capsules compared to normal lens capsules (Raghavan et al., 2015). It was suggested that AGEs in the lens capsule could promote the TGFβ2-mediated fibrosis of lens epithelial cells during posterior capsule opacification (Raghavan et al., 2015).
In addition, in vitro and in vivo data support a cytotoxic effect of AGEs on the vitality of lens epithelial cells through the induction of apoptosis in a NF-κB-dependent manner, suggesting that AGEs accumulation might be a causative factor of injury to the lens epithelium (Hashim and Zarina, 2011; Kim et al., 2012). We also demonstrated that lens proteolytic potential is compromised upon aging (Jahngen-Hodge et al., 1992; Jahngen et al., 1986a, 1986b, 1990; Shang and Taylor, 1995). This would have the effect of accelerating accumulation of AGEs, and associated pathology.
Both carbohydrate restriction and calorie restriction, and compounds inhibiting the accumulation of AGEs have shown efficacy to delay cataract progress in humans and mice (Chiu et al., 2010; Nagaraj et al., 2002; Swamy-Mruthinti et al., 1996; Taylor et al., 1995). Several other studies report anti-cataract activity of dietary flavonoids through the reduction of glycation-induced protein aggregation (Du et al., 2017; Muthenna et al., 2012; Patil et al., 2016). Interestingly, treatment with an AGE cross-link breaker has been shown to be sufficient in disrupting aggregates from diabetic human lenses (Hollenbach et al., 2003).
3.3. Vitreous humor
Structural changes in the vitreous humor are associated with normal aging, but are detected earlier in diabetic conditions. These include posterior vitreous detachment and liquefaction (Gehl et al., 2016; Stitt et al., 1998; van Deemter et al., 2009). Glycation seems to play a pathologic role in vitreous degeneration in diabetic vitreopathy, and is associated with aberrant crosslinks in collagenous fibrils (Sebag et al., 1992). Glycated collagen dissociates from hyaluronan, a hydrophilic glycosaminoglycan, destabilizing the vitreous gel structure and causing morphological changes within the cortical gel, leading to vitreous dysfunction (Sebag et al., 1992; Stitt et al., 1998). From a chemical perspective, it is likely that the hyaluronan is also subject to glycative modification, but this remains to be fully investigated. Vitreous AGE-associated fluorescence increases in a glucose-concentration dependent manner in streptozotocin-induced diabetic rats (Villa et al., 2017). Data from ex vivo porcine vitreous show that AGEs accumulation compromises the vitreous permeability, thus impeding the diffusion of molecules (Lee et al., 2015). Changes in vitreous permeability might increase the retention of cytokines or drugs, with implications in the pathogenesis of ocular diseases such as diabetic retinopathy. High glucose concentrations in bovine vitreous recapitulates the phenotype of crosslinked-collagen fibers and, interestingly, the process is inhibited by aminoguanidine, an inhibitor of AGE formation (Stitt et al., 1998).
3.4. Retina
Similar to cornea, lens and vitreous, retinal AGEs increase with age and diabetes, especially in the outer retina. This insult has been proposed as a major pathological factor in age-related macular degeneration (AMD) (Glenn and Stitt, 2009; Karachalias et al., 2003; Nagaraj et al., 2012; Uchiki et al., 2012). Modification due to glycation is thought to lead to thickening and changes in the physical properties of Bruch’s membrane and extracellular matrix, causing a dysfunctional outer retina. Extracellular deposits, called drusen, and changes in biomechanical properties of Bruch’s membrane are two of the major early features of AMD. Interestingly, Bruch’s membrane and drusen deposits display high AGE-immunoreactivity in an age-dependent manner (Farboud et al., 1999; Handa et al., 1998, 1999). Additionally, higher AGEs levels are found in AMD-patients compared to control subjects as well as in AMD-mouse models (Hammes et al., 1999; Handa et al., 1999; Rowan et al., 2014, 2017; Uchiki et al., 2012; Weikel et al., 2012b). In addition, thickening of Bruch’s membrane and breakdown of the blood retinal barrier takes place even in non-diabetic animals when infused with pre-formed AGEs (Clements et al., 1998; Stitt et al., 2000).
Another complication is that high concentrations of AGEs result in aberrant levels of platelet derived growth factor B (PDGF-B) and vascular endothelial growth factor (VEGF). This is thought to contribute to the early vascular damage in diabetic retinopathy (Handa et al., 1998; Kandarakis et al., 2015; Lu et al., 1998; Murata et al., 1997). In addition, exposure to glycative stress compromises the vitality of cells within the retinal pigment epithelium (Reber et al., 2002; Roehlecke et al., 2016; Wang et al., 2016; Yamagishi et al., 2002). As indicated above, these cells are ultimately responsible for the maintenance of the functionality of photoreceptors and the integrity of choriocapillaris.
Hyperglycemia is also associated with compromises to inner vasculature, particularly in diabetic patients. This is associated with accumulation of AGEs in Müller glia (Chiarelli et al., 1999; Curtis et al., 2011; Gardiner et al., 2003; Hammes et al., 1998; Stitt et al., 1997). High levels of diet-induced AGEs have been associated with Müller glial malfunction in diabetic retinopathy (Curtis et al., 2011; Zong et al., 2010).
As with lens and cataract, compounds that inhibit AGEs formation delay the onset of diabetic retinopathy, at least in animal models (Gardiner et al., 2003; Hammes et al., 1991, 1994, 1995). Also, consistent with a role for AGEs in the initiation of diabetic retinopathy, the overexpression of glyoxalase 1, the limiting enzyme in the methylglyoxal-detoxifying glyoxalase system, reduces retinal AGEs in diabetic rats (Berner et al., 2012).
Recently it has been shown that specific loss of autophagy, one of the proteolytic activities involved in the clearance of AGEs, in the retinal pigment epithelium also induces AMD-features in an age-dependent manner with a concomitant accumulation of AGEs (Zhang et al., 2017). Compromised redox status of glycatively/oxidatively stressed RPE can also lead to cytotoxicity due to limited UPS activity (Jahngen-Hodge et al., 1997).
3.5. Optic nerve
Several reports suggest pathophysiological roles for AGEs in the development of optic neuropathy (Amano et al., 2001; Terai et al., 2012; Tezel et al., 2007; Wang et al., 2009). AGEs are detected in the lamina cribrosa, the collagenous matrix that supports the optic nerve axonal structure, and these levels increase with age (Albon et al., 2000). It is thought that glycation of matrix proteins might decrease the flexibility of the lamina cribrosa, negatively influencing the function of the optic nerve. Immunoreactivity of AGEs and the receptor RAGE in the optic nerve head is more prominent in eyes from glaucoma donors that in age-matched individuals (Tezel et al., 2007). AGE accumulations in the optic nerve, around vessel and in cribriform plates, are also described in streptozotocin-induced diabetic rats, as well as in diabetic patients (Amano et al., 2001; Terai et al., 2012). AGE inhibitors partially reduced the level of glycation in the optic nerve in aged rhesus monkeys and in streptozotocin-induced diabetic rats (Ino-ue et al., 1998; Kiland et al., 2009).
3.6. Trabecular meshwork
Degeneration of the trabecular meshwork leads to pathogenic elevated eye pressure in glaucoma, although the causes remain unknown. Hints about mechanism may be gleaned from in vitro experiments. Exposure to glycative stress enhances cellular senescence of cultured human trabecular meshwork cells and decreased survival (Park and Kim, 2012). However, to date it is unclear whether AGEs might affect the physiological function of the trabecular meshwork in vivo or if glycation might influence the intraocular pressure by altering the aqueous outflow pathway. By analogy to the other tissues discussed above, it seems reasonable to anticipate that protein crosslinking and accumulation of debris within the trabecular meshwork would exacerbate outflow and related to glaucoma. However, no association was found between primary open-angle glaucoma and RAGE polymorphisms (Moschos et al., 2017).
4. Glycemic index: nutritional intervention to fight AGEs-derived toxicity in the eye
Different approaches have been proposed to prevent the accumulation of AGEs and associated pathologies. While there have been some successes in reducing AGEs accumulation by inhibiting Amadori product formation, breaking AGEs crosslinks, or by preventing the interaction of AGEs with receptors at the plasma membrane (Engelen et al., 2013), delaying the formation of these moieties seems promise the most practical and simplest approach. We proposed that limiting dietary glycemia would optimize blood glucose levels, and limit formation of AGEs and the associated pathology in ocular tissues (Ludwig, 2002; Rowan et al., 2017; Uchiki et al., 2012; Weikel et al., 2012a; Whitcomb et al., 2015).
The recent, nascent literature is unanimous in suggesting that the consumption of lower glycemic index foods is salutary (Chiu and Taylor, 2011). For the same caloric content, lower glycemic index foods release glucose into the blood stream more gradually. For example, vegetables and whole grains are slowly digested, and release glucose gradually into the bloodstream, while white bread or refined sugar are foods that rapidly break down during digestion, and are easily converted into glucose that is quickly released into the blood. Crucially, every study published to date indicates that consuming lower glycemia diets diminishes risk for onset and progress of AMD and cortical cataract (Chiu et al., 2011; Chiu et al., 2006a, 2006b, 2007b, 2007a; Weikel and Taylor, 2011; Weikel et al., 2012a). Interestingly, a recent study reported that low-GI diets could exert a protective effect in retinal microvasculature (Sanchez-Aguadero et al., 2016). Together, this literature implies that changing diets from high to lower glycemia may provide a simple, low cost, readily achievable way to delay progress of AMD or even reverse it (Rowan et al., 2017). We calculate that by changing five slices of white bread to five slices of whole grain bread one can achieve healthier dietary glycemia. This would save about 100,000 people from AMD in 5 years (Chiu et al., 2006a, 2007a, 2007b; Kaushik et al., 2008). This data is consistent with findings that animals fed high-GI diets show an accumulation of AGEs and increased retinal lesions and AMD-like phenotypes (Rowan et al., 2017; Uchiki et al., 2012; Weikel et al., 2012b). Unfortunately, the typical American diet is a high-GI diet (Chiu and Taylor, 2011).
5. Conclusions and considerations for future research
Aging and hyperglycemia induce the accumulation of AGEs and a myriad of sequelle that compromise cellular and organ function. This includes compromises to cellular proteins and their functions, cellular protein editing capacities, limited flexibility, perhaps enhanced compression, limited outflow and increased pressure. Aging is associated with a decline of anti-AGE pathway capacities. A significant body of data suggests that AGEs play a pathological role in cataracts, AMD or diabetic retinopathy. Accumulation may also be involved in glaucoma or diabetic keratopathy as well as diabetic optic neuropathy. Intervention trials could be appropriate, but these are costly and time-consuming. Since they appear to be without risk for users, diets that emphasize whole grains would appear to confer benefits with regard to these diseases (Chiu et al., 2011; Chiu et al., 2007a, 2017; Hogg et al., 2017; Nunes et al., 2018; Weikel et al., 2012a). More elucidation of mechanisms would justify the required blinded control human studies that would confirm the laboratory and epidemiologic findings and pave the way for new dietary guidelines for Americans for preserving vision for the burgeoning elderly population.
Acknowledgments
This work was funded by NIH RO1 EY 13250, RO1 EY21212, RO1 EY26979, USDA contract 1950–510000-060–03A U.S. Department of Agriculture-Agriculture Research Service (ARS) and USDA AFRI Grant 12212122. The authors declare no competing financial interests. We are grateful to Elizabeth Whitcomb, Sheldon Rowan, Jonathan Volkin and Opeoluwa Olukorede for critical reading and editorial help.
References
- Abiko T, Abiko A, Ishiko S, Takeda M, Horiuchi S, Yoshida A, 1999. Relationship between autofluorescence and advanced glycation end products in diabetic lenses. Exp. Eye Res. 68, 361–366. [DOI] [PubMed] [Google Scholar]
- Ahmed MU, Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW, 1997. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem. J. 324 (Pt 2), 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed MU, Thorpe SR, Baynes JW, 1986. Identification of N-Carboxymethyllysine as a degradation product of fructoselysine in glycatedprotein. J. Biol. Chem. 261, 4889–4894. [PubMed] [Google Scholar]
- Albon J, Karwatowski WS, Easty DL, Sims TJ, Duance VC, 2000. Age related changes in the non-collagenous components of the extracellular matrix of the human lamina cribrosa. Br. J. Ophthalmol. 84, 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano S, Kaji Y, Oshika T, Oka T, Machinami R, Nagai R, Horiuchi S, 2001. Advanced glycation end products in human optic nerve head. Br. J. Ophthalmol. 85, 52–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragno M, Mastrocola R, 2017. Dietary sugars and endogenous formation of advanced glycation endproducts: emerging mechanisms of disease. Nutrients 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki N, Higashi T, Mori T, Shibayama R, Kawabe Y, Kodama T, Takahashi K, Shichiri M, Horiuchi S, 1995. Macrophage scavenger receptor mediates the endocytic uptake and degradation of advanced glycation end products of the Maillard reaction. Eur. J. Biochem. 230, 408–415. [DOI] [PubMed] [Google Scholar]
- Bejarano E, Cuervo AM, 2010. Chaperone-mediated autophagy. Proc. Am. Thorac. Soc. 7, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner AK, Brouwers O, Pringle R, Klaassen I, Colhoun L, McVicar C, Brockbank S, Curry JW, Miyata T, Brownlee M, Schlingemann RO, Schalkwijk C, Stitt AW, 2012. Protection against methylglyoxal-derived AGEs by regulation of glyoxalase 1 prevents retinal neuroglial and vasodegenerative pathology. Diabetologia 55, 845–854. [DOI] [PubMed] [Google Scholar]
- Beswick HT, Harding JJ, 1987. Conformational changes induced in lens α - and γ-crystallins by modification with glucose 6-phosphate. Biochemistry Journal 246, 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer G, Littlechild S, McCall S, Zhang Y, Conrad GW, 2011. The role of nonenzymatic glycation and carbonyls in collagen cross-linking for the treatment of keratoconus. Invest. Ophthalmol. Vis. Sci. 52, 6363–6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero B, Wang Y, Diaz A, Tasset I, Juste YR, Stiller B, Mandelkow EM, Mandelkow E, Cuervo AM, 2018. Interplay of pathogenic forms of human tau with different autophagic pathways. Aging Cell 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Li J, Shi J, Yang B, Tang J, Truby H, Li D, 2018. Acute metabolic and endocrine responses induced by glucose and fructose in healthy young subjects: a double-blinded, randomized, crossover trial. Clin. Nutr. 37, 459–470 Edinburgh, Scotland. [DOI] [PubMed] [Google Scholar]
- Casey EB, Zhao HR, Abraham EC, 1995. Role of glycine 1 and lysine 2 in the glycation of bovine gamma B-crystallin. Site-directed mutagenesis of lysine to threonine. J. Biol. Chem. 270, 20781–20786. [DOI] [PubMed] [Google Scholar]
- Chellan P, Nagaraj RH, 1999. Protein crosslinking by the Maillard reaction: dicarbonyl-derived imidazolium crosslinks in aging and diabetes. Arch. Biochem. Biophys. 368, 98–104. [DOI] [PubMed] [Google Scholar]
- Chiarelli F, de Martino M, Mezzetti A, Catino M, Morgese G, Cuccurullo F, Verrotti A, 1999. Advanced glycation end products in children and adolescents with diabetes: relation to glycemic control and early microvascular complications. J. Pediatr. 134, 486–491. [DOI] [PubMed] [Google Scholar]
- Chiu C-J, Liu S, Willett WC, Wolever TMS, Brand-Miller JC, Barclay AC, Taylor A, 2011. Informing food choices and health outcomes by use of the dietary glycemic index. Nutr. Rev. 69, 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CJ, Chang ML, Li T, Gensler G, Taylor A, 2017. Visualization of dietary patterns and their associations with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 58, 1404–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CJ, Hubbard LD, Armstrong J, Rogers G, Jacques PF, Chylack J,L. T, Hankinson SE, Willett WC, Taylor A, 2006a. Dietary glycemic index and carbohydrate in relation to early age-related macular degeneration. Am. J. Clin. Nutr. 83, 880–886. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Milton RC, Gensler G, Taylor A, 2006b. Dietary carbohydrate intake and glycemic index in relation to cortical and nuclear lens opacities in the Age-Related Eye Disease Study. Am. J. Clin. Nutr. 83, 1177–1184. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Milton RC, Gensler G, Taylor A, 2007a. Association between dietary glycemic index and age-related macular degeneration in nondiabetic participants in the Age-Related Eye Disease Study. Am. J. Clin. Nutr. 86, 180–188. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Milton RC, Klein R, Gensler G, Taylor A, 2007b. Dietary carbohydrate and the progression of age-related macular degeneration: a prospective study from the Age-Related Eye Disease Study. Am. J. Clin. Nutr. 86, 1210–1218. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Robman L, McCarty CA, Mukesh BN, Hodge A, Taylor HR, Taylor A, 2010. Dietary carbohydrate in relation to cortical and nuclear lens opacities in the melbourne visual impairment project. Invest. Ophthalmol. Vis. Sci. 51, 2897–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CJ, Taylor A, 2011. Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Prog. Retin. Eye Res. 30, 18–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A, Kwon YT, 2015. Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Exp. Mol. Med. 47, e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements RS Jr., Robison WG Jr., Cohen MP, 1998. Anti-glycated albumin therapy ameliorates early retinal microvascular pathology in db/db mice. J. Diabetes Complicat. 12, 28–33. [DOI] [PubMed] [Google Scholar]
- Curtis TM, Hamilton R, Yong PH, McVicar CM, Berner A, Pringle R, Uchida K, Nagai R, Brockbank S, Stitt AW, 2011. Muller glial dysfunction during diabetic retinopathy in rats is linked to accumulation of advanced glycation end-products and advanced lipoxidation end-products. Diabetologia 54, 690–698. [DOI] [PubMed] [Google Scholar]
- Du L, Hao M, Li C, Wu W, Wang W, Ma Z, Yang T, Zhang N, Isaac AT, Zhu X, Sun Y, Lu Q, Yin X, 2017. Quercetin inhibited epithelial mesenchymal transition in diabetic rats, high-glucose-cultured lens, and SRA01/04 cells through transforming growth factor-beta2/phosphoinositide 3-kinase/Akt pathway. Mol. Cell. Endocrinol. 452, 44–56. [DOI] [PubMed] [Google Scholar]
- Dyer DG, Dunn JA, Thorpe SR, Bailie KE, Lyons TJ, McCance DR, Baynes JW, 1993. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J. Clin. Invest. 91, 2463–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisermann DJ, Wenzel U, Fitzenberger E, 2017. Inhibition of chaperone-mediated autophagy prevents glucotoxicity in the Caenorhabditis elegans mev-1 mutant by activation of the proteasome. Biochem. Biophys. Res. Commun. 484, 171–175. [DOI] [PubMed] [Google Scholar]
- Engelen L, Stehouwer CD, Schalkwijk CG, 2013. Current therapeutic interventions in the glycation pathway: evidence from clinical studies. Diabetes Obes. Metabol. 15, 677–689. [DOI] [PubMed] [Google Scholar]
- Farboud B, Aotaki-Keen A, Miyata T, Hjelmeland LM, Handa JT, 1999. Development of a polyclonal antibody with broad epitope specificity for advanced glycation end-products and localization of these epitopes in Bruch’s membrane of the aging eye. Mol. Vis. 5, 11. [PubMed] [Google Scholar]
- Frimat M, Daroux M, Litke R, Neviere R, Tessier FJ, Boulanger E, 2017. Kidney, heart and brain: three organs targeted by ageing and glycation. Clin. Sci. 131, 1069–1092 London, England: 1979. [DOI] [PubMed] [Google Scholar]
- Frye EB, Degenhardt TP, Thorpe SR, Baynes JW, 1998. Role of the Maillard reaction in aging of tissue proteins. Advanced glycation end product-dependent increase in imidazolium cross-links in human lens proteins. J. Biol. Chem. 273, 18714–18719. [DOI] [PubMed] [Google Scholar]
- Gardiner TA, Anderson HR, Stitt AW, 2003. Inhibition of advanced glycation endproducts protects against retinal capillary basement membrane expansion during long-term diabetes. J. Pathol. 201, 328–333. [DOI] [PubMed] [Google Scholar]
- Gehl Z, Bakondi E, Resch MD, Hegedus C, Kovacs K, Lakatos P, Szabo A, Nagy Z, Virag L, 2016. Diabetes-induced oxidative stress in the vitreous humor. Redox biology 9, 100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn JV, Stitt AW, 2009. The role of advanced glycation end products in retinal ageing and disease. Biochim. Biophys. Acta 1790, 1109–1116. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Araki N, Koh N, Nakamura J, Horiuchi S, Hotta N, 1996. Rapid formation of advanced glycation end products by intermediate metabolites of glycolytic pathway and polyol pathway. Biochem. Biophys. Res. Commun. 228, 539–543. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Brownlee M, Edelstein D, Saleck M, Martin S, Federlin K, 1994. Aminoguanidine inhibits the development of accelerated diabetic retinopathy in the spontaneous hypertensive rat. Diabetologia 37, 32–35. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Hoerauf H, Alt A, Schleicher E, Clausen JT, Bretzel RG, Laqua H, 1999. N(epsilon)(carboxymethyl)lysin and the AGE receptor RAGE colocalize in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 40, 1855–1859. [PubMed] [Google Scholar]
- Hammes HP, Martin S, Federlin K, Geisen K, Brownlee M, 1991. Aminoguanidine treatment inhibits the development of experimental diabetic retinopathy. Proc. Natl. Acad. Sci. U. S. A. 88, 11555–11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes HP, Strodter D, Weiss A, Bretzel RG, Federlin K, Brownlee M, 1995. Secondary intervention with aminoguanidine retards the progression of diabetic retinopathy in the rat model. Diabetologia 38, 656–660. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Wellensiek B, Kloting I, Sickel E, Bretzel RG, Brownlee M, 1998. The relationship of glycaemic level to advanced glycation end-product (AGE) accumulation and retinal pathology in the spontaneous diabetic hamster. Diabetologia 41, 165–170. [DOI] [PubMed] [Google Scholar]
- Handa JT, Reiser KM, Matsunaga H, Hjelmeland LM, 1998. The advanced glycation endproduct pentosidine induces the expression of PDGF-B in human retinal pigment epithelial cells. Exp. Eye Res. 66, 411–419. [DOI] [PubMed] [Google Scholar]
- Handa JT, Verzijl N, Matsunaga H, Aotaki-Keen A, Lutty GA, te Koppele JM, Miyata T, Hjelmeland LM, 1999. Increase in the advanced glycation end product pentosidine in Bruch’s membrane with age. Invest. Ophthalmol. Vis. Sci. 40, 775–779. [PubMed] [Google Scholar]
- Hashim Z, Zarina S, 2011. Advanced glycation end products in diabetic and non-diabetic human subjects suffering from cataract. Age 33, 377–384 Dordrecht, Netherlands. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Sabol J, Mitsuhashi T, Vlassara H, 1999. Dietary glycotoxins: inhibition of reactive products by aminoguanidine facilitates renal clearance and reduces tissue sequestration. Diabetes 48, 1308–1315. [DOI] [PubMed] [Google Scholar]
- Hogg RE, Woodside JV, McGrath A, Young IS, Vioque JL, Chakravarthy U, de Jong PT, Rahu M, Seland J, Soubrane G, Tomazzoli L, Topouzis F, Fletcher AE, 2017. Mediterranean diet score and its association with age-related macular degeneration: the European eye study. Ophthalmology 124, 82–89. [DOI] [PubMed] [Google Scholar]
- Hollenbach S, Thampi P, Viswanathan T, Abraham EC, 2003. Cleavage of in vitro and in vivo formed lens protein cross-links by a novel cross-link breaker. Mol. Cell. Biochem. 243, 73–80. [DOI] [PubMed] [Google Scholar]
- Huang JS, Guh JY, Chen HC, Hung WC, Lai YH, Chuang LY, 2001. Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. J. Cell. Biochem. 81, 102–113. [DOI] [PubMed] [Google Scholar]
- Hudson BI, Kalea AZ, Del Mar Arriero M, Harja E, Boulanger E, D’Agati V, Schmidt AM, 2008. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J. Biol. Chem. 283, 34457–34468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino-ue M, Ohgiya N, Yamamoto M, 1998. Effect of aminoguanidine on optic nerve involvement in experimental diabetic rats. Brain Res. 800, 319–322. [DOI] [PubMed] [Google Scholar]
- Jahngen-Hodge J, Cyr D, Laxman E, Taylor A, 1992. Ubiquitin and ubiquitin conjugates in human lens. Exp. Eye Res. 55, 897–902. [DOI] [PubMed] [Google Scholar]
- Jahngen-Hodge J, Obin MS, Gong X, Shang F, Nowell TR Jr., Gong J, Abasi H, Blumberg J, Taylor A, 1997. Regulation of ubiquitin-conjugating enzymes by glutathione following oxidative stress. J. Biol. Chem. 272, 28218–28226. [DOI] [PubMed] [Google Scholar]
- Jahngen JH, Haas AL, Ciechanover A, Blondin J, Eisenhauer D, Taylor A, 1986a. The eye lens has an active ubiquitin-protein conjugation system. J. Biol. Chem. 261, 13760–13767. [PubMed] [Google Scholar]
- Jahngen JH, Lipman RD, Eisenhauer DA, Jahngen EG Jr., Taylor A, 1990. Aging and cellular maturation cause changes in ubiquitin-eye lens protein conjugates. Arch. Biochem. Biophys. 276, 32–37. [DOI] [PubMed] [Google Scholar]
- Jahngen JJ, Eisenhauer D, Taylor A, 1986b. Lens proteins are substrates for the reticulocyte ubiquitin conjugation system. Curr. Eye Res. 5, 725–733. [DOI] [PubMed] [Google Scholar]
- Ji CH, Kwon YT, 2017. Crosstalk and interplay between the ubiquitin-proteasome system and autophagy. Mol. Cell. 40, 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jono T, Miyazaki A, Nagai R, Sawamura T, Kitamura T, Horiuchi S, 2002. Lectinlike oxidized low density lipoprotein receptor-1 (LOX-1) serves as an endothelial receptor for advanced glycation end products (AGE). FEBS Lett. 511, 170–174. [DOI] [PubMed] [Google Scholar]
- Kaji Y, Usui T, Oshika T, Matsubara M, Yamashita H, Araie M, Murata T, Ishibashi T, Nagai R, Horiuchi S, Amano S, 2000. Advanced glycation end products in diabetic corneas. Invest. Ophthalmol. Vis. Sci. 41, 362–368. [PubMed] [Google Scholar]
- Kandarakis SA, Piperi C, Moschonas DP, Korkolopoulou P, Papalois A, Papavassiliou AG, 2015. Dietary glycotoxins induce RAGE and VEGF up-regulation in the retina of normal rats. Exp. Eye Res. 137, 1–10. [DOI] [PubMed] [Google Scholar]
- Kandarakis SA, Piperi C, Topouzis F, Papavassiliou AG, 2014. Emerging role of advanced glycation-end products (AGEs) in the pathobiology of eye diseases. Prog. Retin. Eye Res. 42, 85–102. [DOI] [PubMed] [Google Scholar]
- Karachalias N, Babaei-Jadidi R, Ahmed N, Thornalley PJ, 2003. Accumulation of fructosyl-lysine and advanced glycation end products in the kidney, retina and peripheral nerve of streptozotocin-induced diabetic rats. Biochem. Soc. Trans. 31, 1423–1425. [DOI] [PubMed] [Google Scholar]
- Kaushik S, Wang JJ, Flood V, Tan JS, Barclay AW, Wong TY, Brand-Miller J, Mitchell P, 2008. Dietary glycemic index and the risk of age-related macular degeneration. Am. J. Clin. Nutr. 88, 1104–1110. [DOI] [PubMed] [Google Scholar]
- Kiland JA, Gabelt BT, Tezel G, Lutjen-Drecoll E, Kaufman PL, 2009. Effect of the age cross-link breaker alagebrium on anterior segment physiology, morphology, and ocular age and rage. Trans. Am. Ophthalmol. Soc. 107, 146–158. [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim CS, Sohn E, Jeong IH, Kim H, Kim JS, 2011. Involvement of advanced glycation end products, oxidative stress and nuclear factor-kappaB in the development of diabetic keratopathy. In: Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 249. pp. 529–536. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim OS, Kim CS, Sohn E, Jo K, Kim JS, 2012. Accumulation of argpyrimidine, a methylglyoxal-derived advanced glycation end product, increases apoptosis of lens epithelial cells both in vitro and in vivo. Exp. Mol. Med. 44, 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschinsky T, He C-J, Mitsuhashi T, Bucala R, Liu C, Buenting C, Heitmann K, Vlassara H, 1997a. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. Unit. States Am. 94, 6474–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschinsky T, He CJ, Mitsuhashi T, Bucala R, Liu C, Buenting C, Heitmann K, Vlassara H, 1997b. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. U. S. A. 94, 6474–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PA, Kumar MS, Reddy GB, 2007. Effect of glycation on alpha-crystallin structure and chaperone-like function. Biochem. J. 408, 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM, 1997. Activation of the receptor for advanced glycation end products triggers a p21ras-dependent Mitogen-activated protein kinase pathway regulated by oxidant stress. J. Biol. Chem. 272 1780–17814. [DOI] [PubMed] [Google Scholar]
- Lee OT, Good SD, Lamy R, Kudisch M, Stewart JM, 2015. Advanced glycation end-product accumulation reduces vitreous permeability. Invest. Ophthalmol. Vis. Sci. 56, 2892–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Mitsuhashi T, Wojciechowicz D, Shimizu N, Li J, Stitt A, He C, Banerjee D, Vlassara H, 1996. Molecular identity and cellular distribution of advanced glycation end-product receptors: relationship of p60 to OST-48 and p90 to 80K-H membrane proteins. Proc. Natl. Acad. Sci. U. S. A. 93, 11047–11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Kuroki M, Amano S, Tolentino M, Keough K, Kim I, Bucala R, Adamis AP, 1998. Advanced glycation end products increase retinal vascular endothelial growth factor expression. J. Clin. Invest. 101, 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DS, 2002. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. Jama 287, 2414–2423. [DOI] [PubMed] [Google Scholar]
- Lyons TJ, Silvestri G, Dunn JA, Dyer DG, Baynes JW, 1991. Role of glycation in modification of lens crystallins in diabetic and nondiabetic senile cataracts. Diabetes 40, 1010–1015. [DOI] [PubMed] [Google Scholar]
- Malik NS, Moss SJ, Ahmed N, Furth AJ, Wall RS, Meek KM, 1992. Ageing of the human corneal stroma: structural and biochemical changes. Biochim. Biophys. Acta 20, 222–228. [DOI] [PubMed] [Google Scholar]
- Marriott BP, Olsho L, Hadden L, Connor P, 2010. Intake of added sugars and selected nutrients in the United States, national health and nutrition examination survey (NHANES) 2003–2006. Crit. Rev. Food Sci. Nutr. 50, 228–258. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Ikeda K, Horiuchi S, Zhao H, Abraham EC, 1997. Immunochemical evidence for increased formation of advanced glycation end products and inhibition by aminoguanidine in diabetic rat lenses. Biochem. Biophys. Res. Commun. 241, 352–354. [DOI] [PubMed] [Google Scholar]
- Miyata S, Monnier V, 1992. Immunohistochemical detection of advanced glycosylation end products in diabetic tissues using monoclonal antibody to pyrraline. J. Clin. Invest. 89, 1102–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moheimani F, Morgan PE, van Reyk DM, Davies MJ, 2010. Deleterious effects of reactive aldehydes and glycated proteins on macrophage proteasomal function: possible links between diabetes and atherosclerosis. Biochim. Biophys. Acta 1802, 561–571. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Cuervo AM, 2014. Proteostasis and the aging proteome in health and disease. The journals of gerontology. Series A, Biological sciences and medical sciences 69 (Suppl. 1), S33–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschos MM, Chatziralli I, Sioziou A, Gazouli M, 2017. Receptor of advanced glycation end products gene polymorphism and primary open-angle glaucoma. Ophthalmic Res. 58, 81–84. [DOI] [PubMed] [Google Scholar]
- Murata T, Nagai R, Ishibashi T, Inomuta H, Ikeda K, Horiuchi S, 1997. The relationship between accumulation of advanced glycation end products and expression of vascular endothelial growth factor in human diabetic retinas. Diabetologia 40, 764–769. [DOI] [PubMed] [Google Scholar]
- Muthenna P, Akileshwari C, Reddy GB, 2012. Ellagic acid, a new antiglycating agent: its inhibition of N-(carboxymethyl)lysine. Biochem. J. 442, 221–230. [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Kern TS, Sell DR, Fogarty J, Engerman RL, Monnier VM, 1996. Evidence of a glycemic threshold for the formation of pentosidine in diabetic dog lens but not in collagen. Diabetes 45, 587–594. [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Linetsky M, Stitt AW, 2012. The pathogenic role of Maillard reaction in the aging eye. Amino Acids 42, 1205–1220. [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Sarkar P, Mally A, Biemel KM, Lederer MO, Padayatti PS, 2002. Effect of pyridoxamine on chemical modification of proteins by carbonyls in diabetic rats: characterization of a major product from the reaction of pyridoxamine and methylglyoxal. Arch. Biochem. Biophys. 402, 110–119. [DOI] [PubMed] [Google Scholar]
- Nahomi RB, Wang B, Raghavan CT, Voss O, Doseff AI, Santhoshkumar P, Nagaraj RH, 2013. Chaperone peptides of alpha-crystallin inhibit epithelial cell apoptosis, protein insolubilization, and opacification in experimental cataracts. J. Biol. Chem. 288, 13022–13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAS, 2017. Redesigning the process for establishing the dietary guidelines for Americans. In: Redesigning the Process for Establishing the Dietary Guidelines for Americans. 2017. National Academy of Sciences, Washington DC. [Google Scholar]
- Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A, 1992. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J. Biol. Chem. 267, 14998–15004. [PubMed] [Google Scholar]
- NHMRC, 2013. National health and Medical research Council. Australian Dietary Guidelines, Canberra (AUST). [Google Scholar]
- Nunes S, Alves D, Barreto P, Raimundo M, da Luz Cachulo M, Farinha C, Lains I, Rodrigues J, Almeida C, Ribeiro L, Figueira J, Santos L, Silva R, 2018. Adherence to a Mediterranean diet and its association with age-related macular degeneration. The Coimbra Eye Study-Report 4. Nutrition 51–52, 6–12 Burbank, Los Angeles County, Calif. [DOI] [PubMed] [Google Scholar]
- Ohgami N, Nagai R, Ikemoto M, Arai H, Kuniyasu A, Horiuchi S, Nakayama H, 2001a. CD36, a member of class B scavenger receptor family, is a receptor for advanced glycation end products. Ann. N. Y. Acad. Sci. 947, 350–355. [DOI] [PubMed] [Google Scholar]
- Ohgami N, Nagai R, Ikemoto M, Arai H, Miyazaki A, Hakamata H, Horiuchi S, Nakayama H, 2002. CD36, serves as a receptor for advanced glycation endproducts (AGE). J. Diabetes Complicat. 16, 56–59. [DOI] [PubMed] [Google Scholar]
- Ohgami N, Nagai R, Miyazaki A, Ikemoto M, Arai H, Horiuchi S, Nakayama H, 2001b. Scavenger receptor class B type I-mediated reverse cholesterol transport is inhibited by advanced glycation end products. J. Biol. Chem. 276, 13348–13355. [DOI] [PubMed] [Google Scholar]
- Park CH, Kim JW, 2012. Effect of advanced glycation end products on oxidative stress and senescence of trabecular meshwork cells. Kor. J. Ophthalmol.: Kor. J. Ophthalmol. 26, 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil KK, Meshram RJ, Dhole NA, Gacche RN, 2016. Role of dietary flavonoids in amelioration of sugar induced cataractogenesis. Arch. Biochem. Biophys. 593, 1–11. [DOI] [PubMed] [Google Scholar]
- Perry RE, Swamy MS, Abraham EC, 1987. Progressive changes in LensCrystallin glycation and high-molecular-weight aggregate formation leading to cararact development in streptozotocin-diabetic rats. Exp. Eye Res. 44, 269–282. [DOI] [PubMed] [Google Scholar]
- Pokupec R, Kalauz M, Turk N, Turk Z, 2003. Advanced glycation endproducts in human diabetic and non-diabetic cataractous lenses. In: Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 241. pp. 378–384. [DOI] [PubMed] [Google Scholar]
- Popkin BM, Hawkes C, 2016. Sweetening of the global diet, particularly beverages: patterns, trends, and policy responses. The lancet. Diabetes & endocrinology 4, 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queisser MA, Yao D, Geisler S, Hammes HP, Lochnit G, Schleicher ED, Brownlee M, Preissner KT, 2010. Hyperglycemia impairs proteasome function by methylglyoxal. Diabetes 59, 670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani N, Thornalley PJ, 2015. Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease. Biochem. Biophys. Res. Commun. 458, 221–226. [DOI] [PubMed] [Google Scholar]
- Raghavan C, Smuda M, Smith A, Howell S, Smith D, Singh A, Gupta P, Glomb M, Wormstone M, Nagaraj RH, 2015. Advanced Glycation Endproductts in Human Lens Capsule Promote the TGFβ2-mediated Epithelial to Mesenchymal Transition of Lens Epithelial Cells: Implications for Posterior Capsule Opacification. [Google Scholar]
- Reber F, Kasper M, Siegner A, Kniep E, Seigel G, Funk RH, 2002. Alteration of the intracellular pH and apoptosis induction in a retinal cell line by the AGE-inducing agent glyoxal. Graefes Arch. Clin. Exp. Ophthalmol. 240, 1022–1032. [DOI] [PubMed] [Google Scholar]
- Roehlecke C, Valtink M, Frenzel A, Goetze D, Knels L, Morawietz H, Funk RH, 2016. Stress responses of human retinal pigment epithelial cells to glyoxal. In: Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 254. pp. 2361–2372. [DOI] [PubMed] [Google Scholar]
- Rowan S, Jiang S, Korem T, Szymanski J, Chang ML, Szelog J, Cassalman C, Dasuri K, McGuire C, Nagai R, Du XL, Brownlee M, Rabbani N, Thornalley PJ, Baleja JD, Deik AA, Pierce KA, Scott JM, Clish CB, Smith DE, Weinberger A, Avnit-Sagi T, Lotan-Pompan M, Segal E, Taylor A, 2017. Involvement of a gut-retina axis in protection against dietary glycemia-induced agerelated macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 114, E4472–E4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan S, Weikel K, Chang ML, Nagel BA, Thinschmidt JS, Carey A, Grant MB, Fliesler SJ, Smith D, Taylor A, 2014. Cfh genotype interacts with dietary glycemic index to modulate age-related macular degeneration-like features in mice. Invest. Ophthalmol. Vis. Sci. 55, 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowska-Bartosz I, Galiniak S, Bartosz G, 2014. Kinetics of glycoxidation of bovine serum albumin by glucose, fructose and ribose and its prevention by food components. Molecules 19, 18828–18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sady C, Khosrof S, Nagaraj R, 1995. Advanced Maillard reaction and crosslinking of corneal collagen in diabetes. Biochem. Biophys. Res. Commun. 214, 793–797. [DOI] [PubMed] [Google Scholar]
- Sanchez-Aguadero N, Alonso-Dominguez R, Recio-Rodriguez JI, Patino-Alonso MC, Gomez-Marcos MA, Martin-Cantera C, Schmolling-Guinovart Y, Garcia-Ortiz L, Group EI, 2016. Dietary glycemic index and retinal microvasculature in adults: a cross-sectional study. Nutr. J. 15, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato E, Mori F, Igarashi S, Abiko T, Takeda M, Ishiko S, Yoshida A, 2001. Corneal advanced glycation end products increase in patients with proliferative diabetic retinopathy. Diabetes Care 24, 479–482. [DOI] [PubMed] [Google Scholar]
- Scheijen J, Hanssen NMJ, van Greevenbroek MM, Van der Kallen CJ, Feskens EJM, Stehouwer CDA, Schalkwijk CG, 2018. Dietary intake of advanced glycation endproducts is associated with higher levels of advanced glycation endproducts in plasma and urine: the CODAM study. Clin. Nutr. 37, 919–925. [DOI] [PubMed] [Google Scholar]
- Sebag J, Buckingham B, Charles MA, Reiser K, 1992. Biochemical abnormalities in vitreous of humans with proliferative diabetic retinopathy. Arch. Ophthalmol. 110, 1472–1476 Chicago, Ill.: 1960. [DOI] [PubMed] [Google Scholar]
- Sell DR, Nagaraj RH, Grandhee SK, Odetti P, Lapolla A, Fogarty J, Monnier VM, 1991. Pentosidine: a molecular marker for the cumulative damage to proteins in diabetes, aging and uremia. Diabetes Met. Rev. 7, 239–251. [DOI] [PubMed] [Google Scholar]
- Semba RD, Nicklett EJ, Ferrucci L, 2010. Does accumulation of advanced glycation end products contribute to the aging phenotype? The journals of gerontology. Series A, Biological sciences and medical sciences 65, 963–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsi FA, Sharkey E, Creighton D, Nagaraj RH, 2000. Maillard reactions in lens proteins: methylglyoxal-mediated modifications in the rat lens. Exp. Eye Res. 70, 369–380. [DOI] [PubMed] [Google Scholar]
- Shang F, Taylor A, 1995. Oxidative stress and recovery from oxidative stress are associated with altered ubiquitin conjugating and proteolytic activities in bovine lens epithelial cells. Biochem. J. 307 (Pt 1), 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang F, Taylor A, 2012. Roles for the ubiquitin-proteasome pathway in protein quality control and signaling in the retina: implications in the pathogenesis of age-related macular degeneration. Mol. Aspect. Med. 33, 446–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Chen H, Yu X, Wu X, 2013a. Advanced glycation end products delay corneal epithelial wound healing through reactive oxygen species generation. Mol. Cell. Biochem. 383, 253–259. [DOI] [PubMed] [Google Scholar]
- Shi L, Yu X, Yang H, Wu X, 2013b. Advanced glycation end products induce human corneal epithelial cells apoptosis through generation of reactive oxygen species and activation of JNK and p38 MAPK pathways. PLoS One 8, e66781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa Silva M, Gomes RA, Ferreira AE, Ponces Freire A, Cordeiro C, 2013. The glyoxalase pathway: the first hundred years... and beyond. Biochem. J. 453, 1–15. [DOI] [PubMed] [Google Scholar]
- Stevens VJ, Rouzer CA, Monnier VM, Cerami A, 1978. Diabetic cataract formation: potential role of glycosylation of lens crystallins. Proceedings of the National Academy of Science USA 75, 2918–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt AW, 2001. Advanced glycation: an important pathological event in diabetic and age related ocular disease. Br. J. Ophthalmol. 85, 746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt AW, Bhaduri T, McMullen CB, Gardiner TA, Archer DB, 2000. Advanced glycation end products induce blood-retinal barrier dysfunction in normoglycemic rats. Mol. Cell Biol. Res. Commun. 3, 380–388. [DOI] [PubMed] [Google Scholar]
- Stitt AW, Li YM, Gardiner TA, Bucala R, Archer DB, Vlassara H, 1997. Advanced glycation end products (AGEs) co-localize with AGE receptors in the retinal vasculature of diabetic and of AGE-infused rats. Am. J. Pathol. 150, 523–531. [PMC free article] [PubMed] [Google Scholar]
- Stitt AW, Moore JE, Sharkey JA, Murphy G, Simpson DA, Bucala R, Vlassara H, Archer DB, 1998. Advanced glycation end products in vitreous: structural and functional implications for diabetic vitreopathy. Invest. Ophthalmol. Vis. Sci. 39, 2517–2523. [PubMed] [Google Scholar]
- Suarez G, Rajaram R, Oronsky AL, Gawinowicz MA, 1989. Nonenzymatic glycation of bovine serum albumin by fructose (fructation). Comparison with the Maillard reaction initiated by glucose. J. Biol. Chem. 264, 3674–3679. [PubMed] [Google Scholar]
- Swamy-Mruthinti S, Green K, Abraham EC, 1996. Inhibition of cataracts in moderately diabetic rats by aminoguanidine. Exp. Eye Res. 62, 505–510. [DOI] [PubMed] [Google Scholar]
- Swamy-Mruthinti S, Shaw SM, Zhao HR, Green K, Abraham EC, 1999. Evidence of a glycemic threshold for the development of cataracts in diabetic rats. Curr. Eye Res. 18, 423–429. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Takabatake Y, Kimura T, Maejima I, Namba T, Yamamoto T, Matsuda J, Minami S, Kaimori JY, Matsui I, Matsusaka T, Niimura F, Yoshimori T, Isaka Y, 2017 May. Autophagy inhibits the accumulation of advanced glycation end products by promoting lysosomal biogenesis and function in the kidney proximal tubules. Diabetes 66 (5), 1359–1372. 10.2337/db16-0397. Epub 2017 Feb 28. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Adachi H, Osuga J, Ohashi K, Yahagi N, Sekiya M, Okazaki H, Tomita S, Iizuka Y, Shimano H, Nagai R, Kimura S, Tsujimoto M, Ishibashi S, 2003. FEEL-1 and FEEL-2 are endocytic receptors for advanced glycation end products. J. Biol. Chem. 278, 12613–12617. [DOI] [PubMed] [Google Scholar]
- Taylor A, 2012. Mechanistically linking age-related diseases and dietary carbohydrate via autophagy and the ubiquitin proteolytic systems. Autophagy 8, 1404–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Lipman RD, Jahngen-Hodge J, Palmer V, Smith D, Padhye N, Dallal GE, Cyr DE, Laxman E, Shepard D, et al. , 1995. Dietary calorie restriction in the Emory mouse: effects on lifespan, eye lens cataract prevalence and progression, levels of ascorbate, glutathione, glucose, and glycohemoglobin, tail collagen breaktime, DNA and RNA oxidation, skin integrity, fecundity, and cancer. Mechanisms of ageing and development 79, 33–57. [DOI] [PubMed] [Google Scholar]
- Terai N, Spoerl E, Haustein M, Hornykewycz K, Haentzschel J, Pillunat LE, 2012. Diabetes mellitus affects biomechanical properties of the optic nerve head in the rat. Ophthalmic Res. 47, 189–194. [DOI] [PubMed] [Google Scholar]
- Tessier F, Obrenovich M, Monnier VM, 1999. Structure and mechanism of formation of human lens fluorophore LM-1. Relationship to vesperlysine A and the advanced Maillard reaction in aging, diabetes, and cataractogenesis. J. Biol. Chem. 274, 20796–20804. [DOI] [PubMed] [Google Scholar]
- Tezel G, Luo C, Yang X, 2007. Accelerated aging in glaucoma: immunohistochemical assessment of advanced glycation end products in the human retina and optic nerve head. Invest. Ophthalmol. Vis. Sci. 48, 1201–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornalley PJ, 2003. Glyoxalase I–structure, function and a critical role in the enzymatic defence against glycation. Biochem. Soc. Trans. 31, 1343–1348. [DOI] [PubMed] [Google Scholar]
- Uchiki T, Weikel KA, Jiao W, Shang F, Caceres A, Pawlak DB, Handa JT, Brownlee M, Nagaraj R, Taylor A, 2012. Glycation-altered proteolysis as a pathobiologic mechanism that links dietary glycemic index, aging, and age-related disease (in non diabetics). Aging Cell 11, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribarri J, Peppa M, Cai W, Goldberg T, Lu M, Baliga S, Vassalotti JA, Vlassara H, 2003. Dietary glycotoxins correlate with circulating advanced glycation end product levels in renal failure patients. Am. J. Kidney Dis.: the official journal of the National Kidney Foundation 42, 532–538. [DOI] [PubMed] [Google Scholar]
- Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, Yong A, Striker GE, Vlassara H, 2010. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet Assoc. 110, 911–916 e912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deemter M, Ponsioen TL, Bank RA, Snabel JM, van der Worp RJ, Hooymans JM, Los LI, 2009. Pentosidine accumulates in the aging vitreous body: a gender effect. Exp. Eye Res. 88, 1043–1050. [DOI] [PubMed] [Google Scholar]
- Villa M, Parravano M, Micheli A, Gaddini L, Matteucci A, Mallozzi C, Facchiano F, Malchiodi-Albedi F, Pricci F, 2017. A quick, simple method for detecting circulating fluorescent advanced glycation end-products: correlation with in vitro and in vivo non-enzymatic glycation. Metab., Clin. Exp. 71, 64–69. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Cai W, Tripp E, Pyzik R, Yee K, Goldberg L, Tansman L, Chen X, Mani V, Fayad ZA, Nadkarni GN, Striker GE, He JC, Uribarri J, 2016. Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: a randomised controlled trial. Diabetologia 59, 2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H, Li YM, Imani F, Wojciechowicz D, Yang Z, Liu FT, Cerami A, 1995. Identification of galectin-3 as a high-affinity binding protein for advanced glycation end products (AGE): a new member of the AGE-receptor complex. Mol. Med. 1, 634–646. [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Ross-Cisneros FN, Aggarwal D, Liang CY, Sadun AA, 2009. Receptor for advanced glycation end products is upregulated in optic neuropathy of Alzheimer’s disease. Acta Neuropathol. 118, 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Xing Y, Chen C, Chen Z, Qian Z, 2016. Advanced glycation end-product (AGE) induces apoptosis in human retinal ARPE-19 cells via promoting mitochondrial dysfunction and activating the Fas-FasL signaling. Biosci. Biotechnol. Biochem. 80, 250–256. [DOI] [PubMed] [Google Scholar]
- Weikel K, Taylor A, 2011. Nutritional modulation of cataract and age-related macular degeneration. Mol. Aspect. Med. 33, 318–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weikel KA, Chiu CJ, Taylor A, 2012a. Nutritional modulation of age-related macular degeneration. Mol. Aspect. Med. 33, 318–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weikel KA, Fitzgerald P, Shang F, Caceres MA, Bian Q, Handa JT, Stitt AW, Taylor A, 2012b. Natural history of age-related retinal lesions that precede AMD in mice fed high or low glycemic index diets. Invest. Ophthalmol. Vis. Sci. 53, 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb EA, Chiu CJ, Taylor A, 2015. Dietary glycemia as a determinant of health and longevity. Mol. Aspect. Med. 46, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S, Amano S, Inagaki Y, Okamoto T, Koga K, Sasaki N, Yamamoto H, Takeuchi M, Makita Z, 2002. Advanced glycation end products-induced apoptosis and overexpression of vascular endothelial growth factor in bovine retinal pericytes. Biochem. Biophys. Res. Commun. 290, 973–978. [DOI] [PubMed] [Google Scholar]
- Zarina S, Zhao HR, Abraham EC, 2000. Advanced glycation end products in human senile and diabetic cataractous lenses. Mol. Cell. Biochem. 210, 29–34. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cross SD, Stanton JB, Marmorstein AD, Le YZ, Marmorstein LY, 2017. Early AMD-like defects in the RPE and retinal degeneration in aged mice with RPEspecific deletion of Atg5 or Atg7. Mol. Vis. 23, 228–241. [PMC free article] [PubMed] [Google Scholar]
- Zong H, Ward M, Madden A, Yong PH, Limb GA, Curtis TM, Stitt AW, 2010. Hyperglycaemia-induced pro-inflammatory responses by retinal Muller glia are regulated by the receptor for advanced glycation end-products (RAGE). Diabetologia 53, 2656–2666. [DOI] [PubMed] [Google Scholar]
- Zou C, Wang S, Huang F, Zhang YA, 2012. Advanced glycation end products and ultrastructural changes in corneas of long-term streptozotocin-induced diabetic monkeys. Cornea 31, 1455–1459. [DOI] [PubMed] [Google Scholar]