Abstract
Bisphenol A (BPA)-free plastic products are widely available. Transient BPA release has been reported in Tritan drinking bottles. This study assessed the effectiveness of common consumer washing methods in removing BPA contamination in Tritan bottles using both ELISA and HPLC-MS/MS assays. BPA release was detected in 2 out of 10 kinds of Tritan drinking bottles tested. Average BPA level was 0.493 μg/L in water samples from a type of Tritan kid drinking bottle following 24-hour incubation at room temperature, corresponding to a release rate of 0.015 ng/cm2/h. Of the common consumer cleaning methods identified in an informal survey, dishwashing was the most effective method that significantly reduced, even eliminated BPA release from the tested BPA-positive Tritan bottles, while rinsing with water and handwashing with soap and water were ineffective. The bioactivity of the leached BPA was confirmed using a rodent cardiac myocyte acute exposure model and an invertebrate 7-day exposure model. The BPA release is possibly the result of surface contamination in the manufacturing process. As a case study, our result may be informative for general consumer practice and for better quality control by the manufactures.
Keywords: Bisphenol A, BPA-free, plastic bottles, contamination, cleaning, bioactivity
1. INTRODUCTION
Bisphenol A (BPA) is a synthetic compound used in the production of polycarbonate plastics and epoxy resins, and a common environmental chemical (Welshons et al., 2006). BPA is classified as an endocrine disrupting chemical (EDC) with potential adverse effects on human health (Diamanti-Kandarakis et al., 2009). Wide-spread human exposure has been documented in various populations (Vandenberg et al., 2007; Calafat et al., 2008; Vandenberg et al., 2010). Studies have linked BPA exposure to diseases and disorders effecting the immune, cardiovascular, reproductive, and neuronal systems (Vandenberg et al., 2007; Diamanti-Kandarakis et al., 2009; Gao and Wang, 2014; Santoro et al., 2019), and BPA has been shown to generate reactive oxygen species in human placenta cells (Perez-Albaladejo et al., 2017). A common exposure route of human exposure is through consumption of food and liquids that have been held in containers with BPA (Geens et al., 2012). Leaching of BPA can occur through residual monomers of BPA from the manufacturing process, or hydrolysis of the polymer (Hoekstra and Simoneau, 2013), and is near ubiquitous in polycarbonate plastic containers (Guart et al., 2014).
With the consumer’s increasing awareness of the potential adverse health effects of BPA exposure, BPA-free consumer products are now widely available that are made of glass, aluminum, or BPA-free plastics. A common BPA-free plastic utilized is the Tritan copolyester plastics composed of three monomers (Osimitz et al., 2012). These plastics are identified as free of bisphenols, including BPA, BPS, and BPF, therefore being favored as a plastic substitute due to their lack of endocrine disrupting properties (Osimitz et al., 2012). Notably, a prior study identified detectable amounts of BPA released from Tritan plastic water bottles, which was attributed to surface residues from casting during the manufacturing process (Guart et al., 2013).
The possible existence of contaminant-residual BPA in BPA-free plastic products poses an issue to the consumer as the individuals may be exposed to BPA without knowledge. Leaching from Tritan bottles has been little studied and needs further assessment. A common example of BPA-free consumer plastic is the reusable plastic water bottles. These bottles are nearly all marked as BPA-free nowadays. In this study, we showed that BPA release occurred in some Tritan water bottle, and assessed the effectiveness of household cleaning methods in removing the BPA contaminant.
2. MATERIALS AND METHODS
2.1. Materials and reagents
Brand new Tritan drinking bottles were randomly selected and purchased from local retailers in Cincinnati, USA and from major online retailers. All plastic bottles were recycled at the conclusion of the study. BPA ELISA kits were purchased from Ecologiena (Japan EnviroChemicals, Ltd). Fluo-4 acetoxymethyl ester (Fluo-4) was from Invitrogen (Thermo Fisher Scientific, Inc). HPLC-graded methanol was from Fisher Scientific (Fairlawn, NJ). Other chemicals were all from Sigma-Aldrich (St Louis, MO) unless otherwise stated. All glassware was rinsed three times with 100% HPLC-grade methanol and air dried in a laminar flow hood prior to use. No polycarbonate plastics were used in the experiments. BPA-free solutions were prepared fresh in glass containers using ultrapure water generated by the Milli-Q IQ7000 water purification system. BPA level in the ultrapure water was found to be 0.014 ± 0.025 μg/L (below detection limit) by ELISA and 0.026 ± 0.007 μg/L by HPLC-MS/MS (n = 3).
2.2. Animals
Animal procedures were done in accordance with protocol approved by the University of Cincinnati Institutional Animal Care and Use Committee and followed recommendations of the Panel on Euthanasia of the American Veterinary Medical Association.
Adult female C57BL6J mice (10-12 weeks of age) were used as nonsurviving sources of ventricular myocytes. Animals were maintained on a 14 h light, 10 h dark light cycle in standard polycarbonate caging with Sani-chip bedding (P.J. Murphy Forest Products Corp. Montville, NJ). Mice were fed with ad libitum Teklad diet 2020 (Harlan Laboratories Inc.) and BPA-free ultrapure water generated by a Milli-Q IQ7000 with EDS-Pak water purification system (resistivity < 18.2 MΩ; <4.5 total oxidizable organics) dispensed from glass water bottles. Lumbriculus variegatus were purchased from Monfort Aquarium and Pets store (Cincinnati, OH) and cultured in a BPA-free artificial fresh water as previously described (Vought and Wang, 2018). The worms were fed with fish food flakes (TetraMin) twice a week, and acclimated for three days prior to exposure studies.
2.3. Survey of common consumer drinking bottle cleaning methods
An informal survey was conducted in public spaces (hallway, atrium, etc) at the University of Cincinnati College of Medicine or on social media. Participants were mainly University of Cincinnati medical students, and acquaintance who engage in outdoor recreational activities. Participation in the survey was voluntary, and no personal information was collected. Participants were asked what cleaning method, if any, they used before using a new reusable plastic water bottle. Those who answered that they had never used plastic water bottles were not included in the survey.
2.4. Washing and incubation of plastic water bottles
Based on the above survey result, the following washing protocols were designed to simulate real life common consumer washing practices, and were used to clean brand new plastic drinking bottles: 1) no wash; 2) rinse with water: bottles were filled with 1/3 total volume room temperature BPA-free water and shaken vigorously by hand for 30 s; 3) handwash with water and soap: bottles were filled with 1/3 of total volume hot tap water and 3 drops of dish soap (Dawn Ultra) and shaken vigorously by hand for 30 s. Bottles were then emptied and rinsed twice with cool tap water and once with BPA-free water; 4) dishwasher wash: bottle body and lid were separated and loaded face-down on the top rack of a standard consumer dishwasher (GE Appliance). A single Finish-Powerball Tablet dish detergent (Parsippany, NJ) was added to the dishwasher following instructions. Standard washing cycle of approximately 2 hours was used. Bottles were rinsed once with BPA-free water following dishwashing. For both soap-and-water wash and dishwasher wash, bottles were rinsed thoroughly before water incubation to reduce potential interference of soap or dish detergent with the subsequent analysis, particularly ELISA.
To test BPA migration from bottles, bottles were filled with 200 mL BPA-free ultrapure water and agitated for 24 h on a cell culture rocker (Model 260350, Boekel Scientific) at room temperature (22°C), and were rotated 180 degrees every 8 h to ensure even contact of water to interior of bottles. Glass water bottles were included in each analysis and served as a negative control. Following incubation, water samples were collected into the glass containers or vials. All liquid transfers were performed using polypropylene pipette tips for small volumes, or measured using glass graduated cylinders for larger volumes.
2.5. ELISA measurement of BPA level in water samples
BPA concentration in the collected water extract samples was determined using a supersensitive BPA ELISA kits (Japan EnviroChemicals, Ltd), following manufacture instructions. The kit had a quantitative measuring range of 0.05 to 10 μg/L. For each assay, water sample from a glass water bottle subjected to the same 24 h incubation served as a negative control. A standard curve (four-parameter sigmoidal logistic nonlinear regression) was generated from provided BPA standards. In all assays, BPA concentrations in the negative controls were below the detection limit of the ELISA assay. Triplicate measurements were taken for all standard, control and experimental samples and obtained for the absorption at 450 nm using an EZ Read 400 microplate reader (Biochrom). BPA concentrations of samples were determined based on the standard curve using Excel.
2.6. HPLC-MS/MS measurement of BPA level in water samples
Water samples were shipped to the Senator Frank R. Lautenberg Laboratory at the Icahn School of Medicine at Mount Sinai for BPA analysis. The Lautenberg Laboratory is part of the Human Health Exposure Analysis Resource (HHEAR), a continuation of Children's Health Exposure Analysis Resource (CHEAR), which has participated and qualified in proficiency testing programs for BPA conducted by G-EQUAS (http://www.g-equas.de/) (Goen et al., 2012) and CTQ-OSEQAS (https://www.inspq.qc.ca/en/ctq/eqas/oqesas/description). Sample preparation and quantification of BPA was based on a previously described method for urine and serum (Sasso et al., 2020) with some modifications for water analysis. In brief, 0.025 mL of 13C12-BPA at 4.5 μg/L was added to individual water samples (0.2 mL) for isotope-dilution ultra-high performance liquid chromatography-tandem mass spectrometry (HPLC–MS/MS) analysis. Sample cleanup and concentration was performed by solid phase extraction (SPE) using an Oasis HLB hydrophilic-lipophilic balanced reversed-phase 96-well plate (30 mg sorbent per well, 30 μm particle size; Waters Corporation, Milford, MA). The procedure was automated using a liquid handler (epMotion 5075vtc; Eppendorf, Hauppauge, NY). Water samples were loaded onto the 96-well SPE plate preconditioned with 1 mL each of methanol and water. Each well in the SPE plate was washed with 1 mL water and eluted twice with 0.75 mL methanol for 3 minutes at 300 mbar. The two eluates for each sample were pooled and evaporated to dryness under a gentle nitrogen stream with a SPE Dry 96 evaporator (Biotage, LLC; Charlotte, NC), reconstituted with 0.2 mL of acetonitrile:water (50:50, vol:vol), and a 20 μl injection volume was subjected to analysis. The HPLC-MS/MS (ExionLC AD and Sciex 6500+ triple quadruple MS, AB Sciex; Framingham, MA) was operated in electrospray negative mode for ionization and multiple reaction monitoring (MRM) for data acquisition. Most prominent ion transition was used for quantitation and the most intense second ion transition was used for confirmation (MRM transition of 227 → 212, 133 for BPA and 239 → 224, 139 for 13C12-BPA). Chromatographic separation was achieved on a Betasil C18, 5 μm, 100 × 2.1 mm analytical column with 10 × 2.1 mm guard column (Thermo Scientific, Waltham, MA) using a gradient with LC-MS grade water (mobile phase A) and acetonitrile (mobile phase B). Quality controls (QCs) included in the experimental batch were procedural blanks (n=6), instrumental blanks (n=3), and water spikes of BPA, two each, at low (0.01–0.1 μg/L), medium (0.1–10 μg/L) and upper range (10–100 μg/L) of assay validation. The limit of detection (LOD) was calculated as 3S (three times standard deviation) of ten replicate analyses of LC/MS grade water blank spiked with 0.01 μg/L of BPA. The calculated LOD was 0.02 μg/L. The matrix effect on BPA was assessed by calculating recoveries from the difference between known spiked and measured amounts in water blanks, which were between 80% and 120% for BPA levels above LOD.
2.7. Mouse myocyte isolation and Ca2+ spark measurement
Isolation of mice hearts were carried out as previously described (Yan et al., 2011). Mice were anesthetized with an i.p. injection of sodium pentobarbital at a concentration of 80 mg/kg body weight. Upon confirmation of the depth of anesthesia based on the lack of toe pinch reflex and corneal reflex, a cardioectomy was performed. Excised hearts were retrograde-perfused on the Langendorff system with a modified Krebs-Henseleit buffer (KHB), followed by perfusion with KHB plus 0.7mg/mL type II collagenase, 0.1% BSA and 25μM CaCl2 for 10 min. Ventricles of the hearts were then excised, minced and the tissue was dispersed into a cellular suspension. Single myocytes were collected and resuspended for analysis.
Analysis of Ca2+ spark in myocytes was performed as previously described (Liang et al., 2014; Gao et al., 2015; Ma et al., 2017). Quiescent myocytes were loaded with Fluo-4, placed in a plexiglass chamber containing solutions prepared using BPA-free ultrapure water (control) or water samples from plastic bottles, and imaged with a Zeiss LSM 710 inverted confocal microscope at excitation wavelength of 488 nm. Fluorescent signals were obtained by line-scan mode recorded at 3.07- millisecond intervals, with each line comprising 512 pixels spaced at 0.056 mm. Analysis of Ca2+ spark images were carried out by IDL 6.3 software.
2.8. Dorsal blood vessel pulse (DBV) interval measurement
Analysis of the DBV intervals in L. variegatus worms was performed as previously described (Vought and Wang, 2018). Briefly, following 3 days of acclimation in BPA-free solution, the worms were exposed to treatment solutions prepared by either control (BPA-free ultrapure water) or water samples from drinking bottles, in glass containers for 7 day. Following treatments, worms were moved into a homemade shallow glass chamber using polyethylene disposable pipettes, and the pulsing interval of DBV at a specific point of the worm's midbody was measured under a dissecting microscope at room temperature. For each worm, 11 to 12 consecutive intervals were recorded and the average value was regarded as the pulsing interval of a particular worm.
2.9. Data analysis
Statistical analyses were performed using paired Student’s t-test, or unpaired t-test. Differences were considered statistically significant at a value of P < 0.05. Agreement between the HPLC-MS/MS and ELISA measurements were assessed using Lin’s Concordance Correlation Coefficient (ρc) with MedCalc v19.6.4. Linear regression was performed with SigmaPlot 11.0. Data was analyzed with Microsoft Excel and are expressed as mean ± standard deviation (SD).
3. RESULTS
3.1. Common consumer practice in initial cleaning of plastic water bottles
In an informal survey of 114 individuals, consumer cleaning practices of brand-new plastic drinking bottles before first use were assessed. Common answers included use right away without any wash, rinse with tap water, wash with water and soap, and wash with dishwasher (Figure 1).
Figure 1.
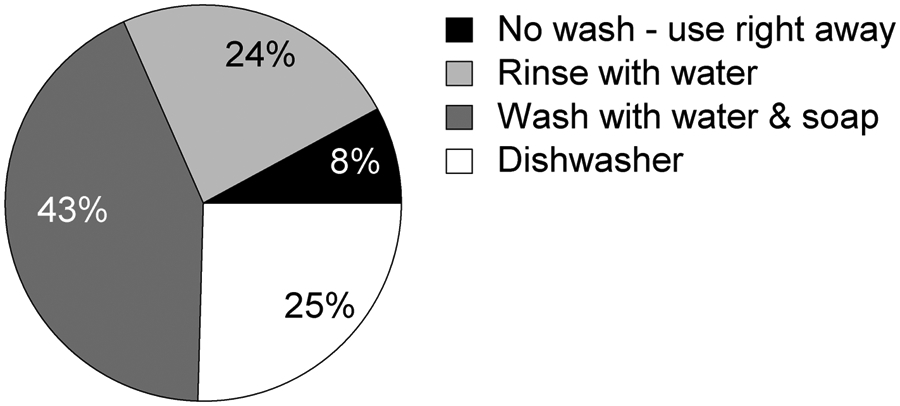
Common consumer practice in cleaning new reusable plastic drinking bottles before first use. N = 114.
3.2. Effectiveness of common consumer cleaning methods in BPA removal from new plastic water bottles
We tested BPA release in 10 different kinds of brand new, unwashed Tritan water bottles. Bottles were filled with BPA-free water and incubated for 24 h at room temperature, and BPA levels in water samples were measured using ELISA. BPA release (> 0.1 μg/L) was detected in water samples from two types of bottles. Out of these two, we randomly selected a 12 ounce BPA-free Tritan kid drinking bottle, and purchased additional bottles of this kind for further testing.
Following incubation, water samples from these kid drinking bottles had an average BPA concentration of 0.445 ± 0.159 μg/L (n = 9). With a bottle internal surface area of 275 cm2, the average BPA migration rate was calculated to be 0.013 ng/cm2/h. These bottles were then equally divided into three groups and subjected to various methods of washing, including rinsing with water, handwashing with soap and water, and washing with a household dishwasher. Neither 1x rinse nor 1x handwashing with soap and water significantly reduced BPA release; BPA concentration in extract water samples was 0.397 and 0.386 μg/L (n = 3), following rinse and 1x handwashing, respectively. The handwashing group was further subjected to an additional 2 rounds and 5 rounds of handwashes (i.e., for a total of 3x and 6x wash). BPA release was not significantly affected by up to 6x handwashing (Figure 2A and C). BPA was not detected in glass water bottles that served as negative control (Figure 2C). By contrast, dishwashing significantly reduced subsequent BPA release from these Tritan bottles. Average BPA concentration in extract water samples was reduced to 0.344 μg/L (n = 3) after 1x dishwashing, and was further reduced to 0.173 and 0.016 μg/L, after a total of 2x and 6x dishwashing, respectively (Figure 2B and C).
Figure 2.
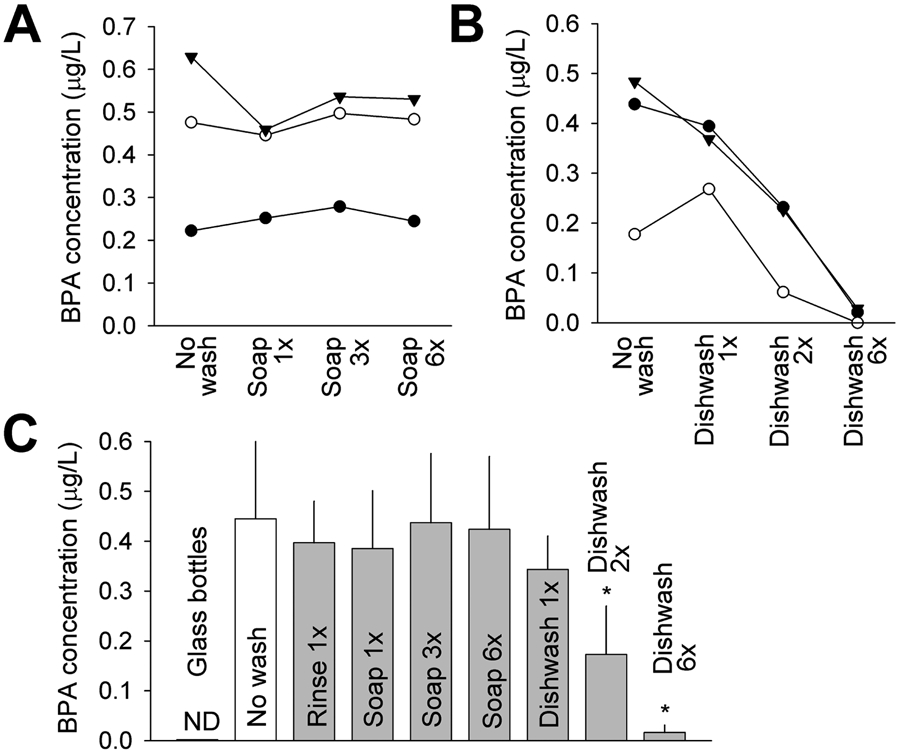
(A) BPA concentration in water samples from 3 individual Tritan water bottles before wash and after 1x, 3x and 6x total rounds of handwashing with soap and water. (B) BPA concentration in water samples from another 3 individual Tritan water bottles before wash and after 1x, 2x and 6x total rounds of dishwashing. (C) Average BPA concentration in water samples in unwashed and various washing groups. N = 9 for the unwashed group and 3 for all other groups. *, P < 0.05 in a paired t-test vs no wash group. ND, not detected. Error bars are S.D.
3.3. Measurement of BPA using HPLC-MS/MS and validation of ELISA
To quantify BPA release and to validate the ELISA results, a HPLC-MS/MS assay was used in parallel with ELISA to analyze the water extracts from another set of 3 bottles of the above kind. To reduce the number of plastic bottles we test (and waste), instead of using naïve bottles for each wash group, these 3 bottles were sequentially subjected to rinse, 2x handwashing, 2x dishwashing, and 6x dishwashing, each step followed by water incubation for 24 hr.
HPLC-MS/MS confirmed the presence of BPA in extract water samples and the results closely matched the measurements of ELISA (Figure 3A). There is remarkably strong agreement between HPLC-MS/MS and ELISA results, with a Lin’s concordance correlation coefficient (ρc) of 0.98 (95% CI = 0.96 to 0.99) (Figure 3B). The correlation can be best fitted with a line of [BPA](ELISA) = 0.902 [BPA](HPLC-MS/MS) – 0.016, giving the impression of a small (10%) underestimate by ELISA, although the small number of data points (n = 18) does not warrant such a conclusion. Average data (Figure 3C) generally agreed with those shown in Figure 2. Average BPA was 0.636 μg/L (LC-MS/MS) and 0.563 μg/L (ELISA) after rinse only (n = 3), and soap and water handwash had a notable but statistically insignificant effect in reducing BPA release, while repeated dishwashing completely abolished BPA release.
Figure 3.
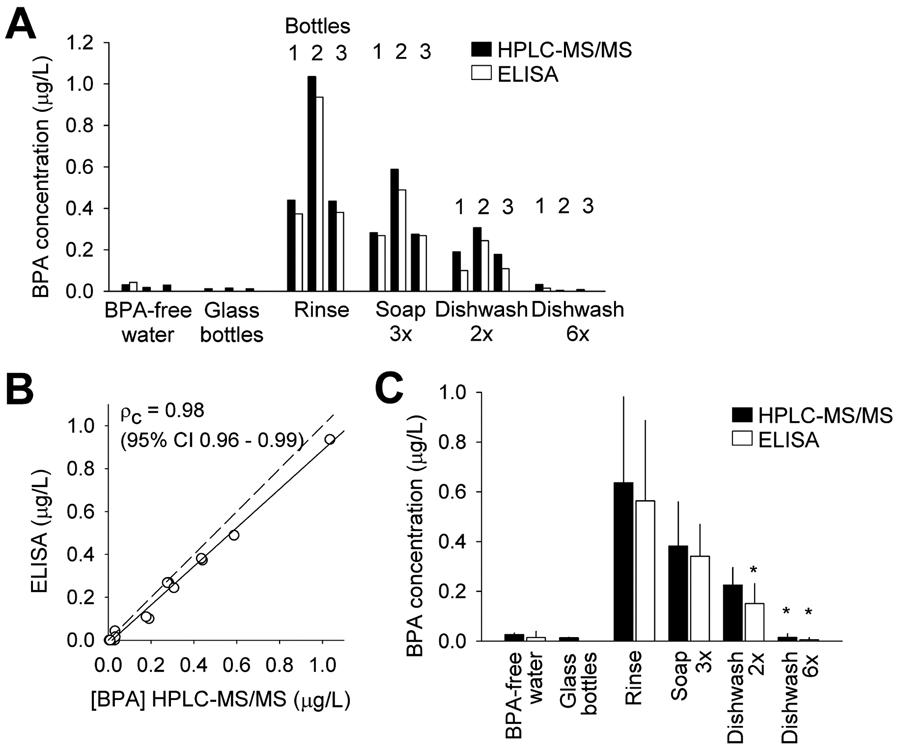
(A) BPA concentration in BPA-free water, water samples from 3 glass bottles after 24 h incubation, and water samples after 24 h incubation in 3 Tritan water bottles (bottles 1, 2 and 3) following indicated washes, measured using both HPLC-MS/MS and ELISA. (B) Plot of BPA concentrations measured using HPLC-MS/MS vs BPA concentrations in the same samples measured using ELISA (18 sample total). Lin’s concordance correlation coefficient (ρc) between the two measurements is indicated. Dash line indicates perfect concordance. Data points were fitted with a line (solid) [BPA](ELISA) = 0.902 [BPA](HPLC-MS/MS) – 0.016 (r = 0.996). (C) Average BPA concentration, measured by HPLC-MS/MS and ELISA, in the indicated sample groups (n = 3). *, P < 0.05 in a paired t-test vs rinse group. Error bars are S.D.
3.4. Biological activity of BPA in water samples from drinking bottles
BPA has a wide range of effects on biological organisms (Flint et al., 2012; Murata and Kang, 2018). We used two model systems, rodent cardiac myocytes and the annelid Lumbriculus variegatus, to test whether BPA released from the water bottles was bioactive.
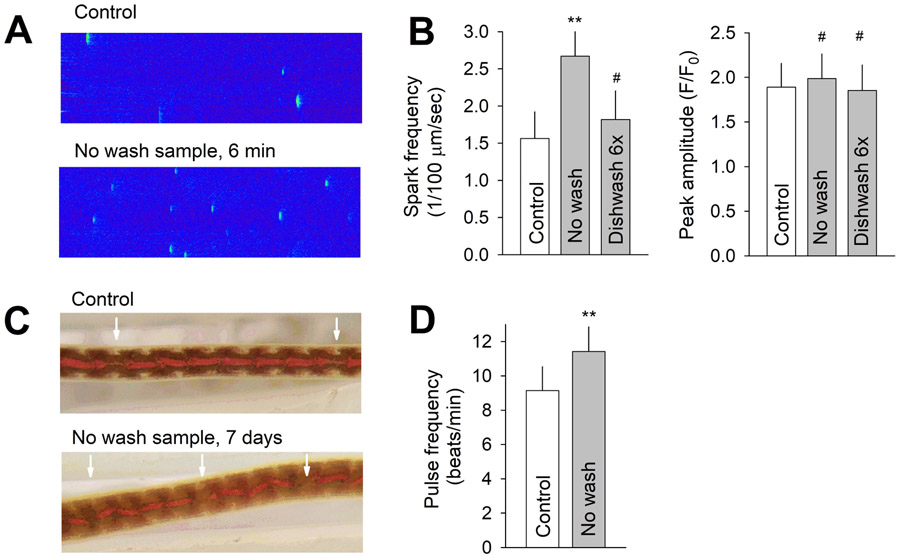
Ca2+ sparks are spontaneous Ca2+ release from sarcoplasmic reticulum in cardiac myocytes (Cheng et al., 1993). Acute exposure to water sample from unwashed bottles, but not from bottles dishwashed six times, significantly increased the average frequency of Ca2+ spark from 1.56 to 2.67 sparks/100 μm/s in female mouse ventricular myocytes (Figure 4A and B). No significant differences were observed in spark peak amplitude. Seven-day exposure to water sample from unwashed bottles significantly increased the pulse frequency of the dorsal blood vessel (DBV) in L. variegatus from 8.9 to 11.2 beats/min (Figure 4C and D).
Figure 4.
(A) Representative recordings of Ca2+ sparks in a quiescent mouse ventricular myocyte under BPA-free control and 6 min after exposure to solution prepared using water extract from unwashed tested bottles (No wash). (B) Average Ca2+ spark frequency (left) and peak spark amplitude (right) under control and upon exposure to indicated solutions. N = 15, 8, and 7 myocytes for control, no wash, and dishwashing 6x group, respectively. (C) Representative images of L. variegatus showing the DBV and pulses propagating along the vessel (arrow), after 7-day exposure to control BPA-free solution and solution prepared using water extract from no wash bottles. (D) Average DBV pulsing rate in control and treated groups. N = 21 and 22. ** P < 0.001, # P > 0.1 vs control in unpaired t-test. Error bars are S.D.
4. DISCUSSION
Chemical migration from consumer plastics, particularly water bottles, has been a much-studied topic (Hoekstra and Simoneau, 2013; Cwiek-Ludwicka and Ludwicki, 2014). In this study, we assessed, from a consumer’s point of view, which household cleaning methods are effective in removing possible BPA contamination in BPA-free plastics. Consistent with previous report (Guart et al., 2013), we detected BPA release in some (2 out of 10 kinds) of the Tritan water bottles, and selected a Tritan kid drinking bottle for further testing. Of the common household washing methods we tested, dishwashing reduced, and even eliminated, BPA leaching from the sample bottles. BPA concentration in water samples decreased significantly following two dishwasher washes, and was reduced to 0.016 μg/L (below detection limit) after 6 rounds of washes. Other methods, including rinsing with water and handwashing with soap and water, did not appear to remove the BPA contaminant in the bottles that we tested. The presence of BPA in the water extracts was confirmed and further quantified using a HPLC-MS/MS assay, which validated the ELISA results. BPA in the water samples collected from the test bottles was found to be bioactive, readily eliciting biological responses in both a vertebrate and an invertebrate testing model.
The level of BPA release in the BPA positive kid drinking bottle (0.493 μg/L in 24 h, n = 12) was similar to the range of BPA migration found in polycarbonate bottles. BPA migration from polycarbonate is influenced by various factors, including temperature, time, and pH, and varies significantly depending on the experimental condition (Le et al., 2008; Li et al., 2010; Cooper et al., 2011; Hoekstra and Simoneau, 2013). When tested at room temperature and using unheated water, BPA migration from polycarbonate bottles after 24 incubation was reported to be 0.48~1.11 μg/L (Li et al., 2010) and 0.08-0.36 μg/L (Le et al., 2008). The release rate in our study was calculated to be 0.015 ng/cm2/h. Comparable studies of polycarbonate bottles at room temperature reported BPA release rate of 0.0012 to 0.03 ng/cm2/h (Tan and Mustafa, 2003).
Tritan plastics are free of bisphenols (Osimitz et al., 2012). The transient BPA release from the sample Tritan bottles in our study were distinct from those described from polycarbonate bottles. Dishwashing and handwashing with detergent markedly increase BPA release by up to 500-fold in polycarbonate bottles, likely due to detergent degradation of the polycarbonate polymer (Maia et al., 2009). By contrast, dish detergent washing had little effect (handwashing) or reduced (dishwashing) BPA release in the tested Tritan bottles, suggesting that release of residual BPA, rather than PC polymer degradation, was likely involved. Further, repeated dishwashing reduced, eventually eliminated BPA release, suggesting that the BPA release from Tritan bottle was likely the result of superficial BPA contamination, possibly introduced during the manufacturing process. A study of BPA release from Tritan bottles by Guart A et al reached a similar conclusion (Guart et al., 2013).
We can only speculate the possible source of BPA contamination of Tritan bottles. We toured a plastic manufacturing factory in Ohio (unrelated to any of the products we tested) and consulted with experts at the factory. Plastic bottles are manufactured though injection molding (Marcus, 1973) or extrusion molding (Chou et al., 2000); both of which utilize heat, molds, and air pressure to form the plastic into the desired shape (Marcus, 1973; Koch et al., 2012). In both molding methods, air is used to expand heated plastic into the desired shape (Halden, 2010). High level of airborne BPA has been reported in plastic injection molding factory (Kouidhi et al., 2017). It is possible that the manufacturing space for Tritan plastics was not properly separated from other manufacturing processes that involved BPA. Under such condition, the surface of casting plastics could come in contact with BPA contaminants such as airborne BPA monomers during the air expansion process. In addition, it is a possibility that BPA contamination may be introduced by the manufacturing apparatus that had prior contact with BPA or polycarbonate plastics. Our study may help the plastic manufactures to identify and eliminate any contamination problems.
The toxicity of BPA is mediated by its endocrine disrupting biological effects (Welshons et al., 2006; Diamanti-Kandarakis et al., 2009; Murata and Kang, 2018). We tested the bioactivity of the presumed BPA in water samples from the Tritan kid drinking bottles using a mouse ventricular cardiomyocyte acute exposure assay and a L. variegatus chronic exposure assay. Previously, we reported that BPA, at nanomolar concentration, promoted arrhythmogenesis through alteration of myocyte Ca2+ handling in female rodent ventricular myocytes (Yan et al., 2011). A key mechanism that underlies BPA’s pro-arrhythmic action was an increase of sarcoplasmic reticulum Ca2+ leak, manifested as increased Ca2+ spark frequency. We found that acute exposure to water sample from unwashed bottles mimicked the effect of BPA, and significant increase of Ca2+ spark frequency in mouse cardiac myocytes. Dishwashing of the bottles for 6 times abolished such effect. We recently showed that BPA exposure for five days affected the physiology, including pulsing rate of the DBV, of the annelid L. variegatus (Vought and Wang, 2018). Similarly, we found that a 7-day exposure to water sample from unwashed bottles significantly increased the DBV pulse frequency in L. variegatus, mimicking the effect of nanomolar BPA. These results, using both a vertebrate and an invertebrate model and acute and seven-day exposure paradigms, suggest that BPA released from the tested Tritan bottles was bioactive, and thus had the potential of eliciting the endocrine disrupting toxicities that have been attributed to BPA exposure.
Our study was intended neither as a comprehensive quality control survey of BPA-free water bottles on the market, nor to identify the particular bottles that may have BPA contamination. For these reasons, we tested only a limited number of bottles (10 different kinds) and omitted the brand information in this report. Due to the small sample size, our results should not be interpreted as an indication that BPA contamination is common in BPA-free Tritan bottles. There are likely significant variations in the exact washing method and products that consumers use, such as dish detergent, washing duration, brand and condition of dishwasher, and loading position on the dishwasher rack, all of which may influence the outcome. In addition, BPA contamination, if any, likely differs in its exact nature among different bottles depending on the manufacturing process. In contrast to our study, Guart A et al found that BPA release from Tritan bottles was not detectable in the second test, without further washing (Guart et al., 2013). For these reasons, our result should not be extrapolated to other bottles, and should not be interpreted as a specific guideline of consumer practice. Instead, as a case study, our result may be informative for the general practice of consumers and for better quality control on the manufacture’s part.
HIGHLIGHTS.
Transient BPA release from some Tritan drinking bottles was detected
BPA release was likely due to surface contamination in the manufacturing process
The released BPA was bioactive as assessed using two independent bioassays
Effect of common consumer cleaning methods in removing BPA contamination was assessed
Dishwashing, but not rinsing or handwashing, was effective in elimination BPA release
5. ACKNOWLEDGEMENT
This work was supported by the National Institutes of Health R01ES027855, University of Cincinnati Center for Environmental Genetics through the NIEHS award P30ES006096, and NIH/NIEHS funding from NIH/NIEHS U2C ES026561 and P30ES023515. The Mount Sinai CHEAR/HHEAR laboratory hub acknowledges Mr. Ravi Jagani who performed the measurements of bisphenol A in water samples. We thank Dr. Bryan Mackenzie in the Department of Pharmacology and Systems Physiology at the University of Cincinnati for expert advice on statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL, 2008. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environmental health perspectives 116, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB, 1993. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science (New York, N.Y.) 262, 740–744. [DOI] [PubMed] [Google Scholar]
- Chou ST, Ko LE, Lim PS, Huang JL, Yang CS, 2000. Effect of age and sex on plasma total homocysteine in Taiwanese subjects. Chin J Physiol 43, 159–164. [PubMed] [Google Scholar]
- Cooper JE, Kendig EL, Belcher SM, 2011. Assessment of bisphenol A released from reusable plastic, aluminium and stainless steel water bottles. Chemosphere 85, 943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwiek-Ludwicka K, Ludwicki JK, 2014. Endocrine disruptors in food contact materials; is there a health threat? Roczniki Panstwowego Zakladu Higieny 65, 169–177. [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC, 2009. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 30, 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S, Markle T, Thompson S, Wallace E, 2012. Bisphenol A exposure, effects, and policy: a wildlife perspective. Journal of environmental management 104, 19–34. [DOI] [PubMed] [Google Scholar]
- Gao X, Ma J, Chen Y, Wang HS, 2015. Rapid responses and mechanism of action for low-dose bisphenol S on ex vivo rat hearts and isolated myocytes: evidence of female-specific proarrhythmic effects. Environmental health perspectives 123, 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Wang HS, 2014. Impact of bisphenol a on the cardiovascular system - epidemiological and experimental evidence and molecular mechanisms. International journal of environmental research and public health 11, 8399–8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geens T, Aerts D, Berthot C, Bourguignon JP, Goeyens L, Lecomte P, Maghuin-Rogister G, Pironnet AM, Pussemier L, Scippo ML, Van Loco J, Covaci A, 2012. A review of dietary and non-dietary exposure to bisphenol-A. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 50, 3725–3740. [DOI] [PubMed] [Google Scholar]
- Goen T, Schaller KH, Drexler H, 2012. External quality assessment of human biomonitoring in the range of environmental exposure levels. Int. J. Hyg. Environ. Health 215, 229–232. [DOI] [PubMed] [Google Scholar]
- Guart A, Bono-Blay F, Borrell A, Lacorte S, 2014. Effect of bottling and storage on the migration of plastic constituents in Spanish bottled waters. Food Chem. 156, 73–80. [DOI] [PubMed] [Google Scholar]
- Guart A, Wagner M, Mezquida A, Lacorte S, Oehlmann J, Borrell A, 2013. Migration of plasticisers from Tritan and polycarbonate bottles and toxicological evaluation. Food chemistry 141, 373–380. [DOI] [PubMed] [Google Scholar]
- Halden RU, 2010. Plastics and health risks. Annual review of public health 31, 179–194. [DOI] [PubMed] [Google Scholar]
- Hoekstra EJ, Simoneau C, 2013. Release of bisphenol A from polycarbonate: a review. Critical reviews in food science and nutrition 53, 386–402. [DOI] [PubMed] [Google Scholar]
- Koch SE, Gao X, Haar L, Jiang M, Lasko VM, Robbins N, Cai W, Brokamp C, Varma P, Tranter M, Liu Y, Ren X, Lorenz JN, Wang HS, Jones WK, Rubinstein J, 2012. Probenecid: novel use as a non-injurious positive inotrope acting via cardiac TRPV2 stimulation. Journal of molecular and cellular cardiology 53, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouidhi W, Thannimalay L, Soon CS, Ali Mohd M, 2017. Occupational exposure to bisphenol A (BPA) in a plastic injection molding factory in Malaysia. International journal of occupational medicine and environmental health 30, 743–750. [DOI] [PubMed] [Google Scholar]
- Le HH, Carlson EM, Chua JP, Belcher SM, 2008. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicology letters 176, 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ying GG, Su HC, Yang XB, Wang L, 2010. Simultaneous determination and assessment of 4-nonylphenol, bisphenol A and triclosan in tap water, bottled water and baby bottles. Environ Int 36, 557–562. [DOI] [PubMed] [Google Scholar]
- Liang Q, Gao X, Chen Y, Hong K, Wang HS, 2014. Cellular mechanism of the nonmonotonic dose response of bisphenol A in rat cardiac myocytes. Environmental health perspectives 122, 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Hong K, Wang HS, 2017. Progesterone Protects Against Bisphenol A-Induced Arrhythmias in Female Rat Cardiac Myocytes via Rapid Signaling. Endocrinology 158, 778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia J, Cruz J, Sendón R, Bustos J, Sanchez J, Paseiro P, 2009. Effect of detergents in the release of bisphenol A from polycarbonate baby bottles. Food Research International 42, 1410–1414. [Google Scholar]
- Marcus P, 1973. Injection blow molding method. US Patent US3776991A.
- Murata M, Kang JH, 2018. Bisphenol A (BPA) and cell signaling pathways. Biotechnology advances 36, 311–327. [DOI] [PubMed] [Google Scholar]
- Osimitz TG, Eldridge ML, Sloter E, Welsh W, Ai N, Sayler GS, Menn F, Toole C, 2012. Lack of androgenicity and estrogenicity of the three monomers used in Eastman's Tritan copolyesters. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 50, 2196–2205. [DOI] [PubMed] [Google Scholar]
- Perez-Albaladejo E, Fernandes D, Lacorte S, Porte C, 2017. Comparative toxicity, oxidative stress and endocrine disruption potential of plasticizers in JEG-3 human placental cells. Toxicology in vitro : an international journal published in association with BIBRA 38, 41–48. [DOI] [PubMed] [Google Scholar]
- Santoro A, Chianese R, Troisi J, Richards S, Nori SL, Fasano S, Guida M, Plunck E, Viggiano A, Pierantoni R, Meccariello R, 2019. Neuro-toxic and reproductive effects of BPA. Current neuropharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasso AF, Pirow R, Andra SS, Church R, Nachman RM, Linke S, Kapraun DF, Schurman SH, Arora M, Thayer KA, Bucher JR, Birnbaum LS, 2020. Pharmacokinetics of bisphenol A in humans following dermal administration. Environ Int 144, 106031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BL, Mustafa AM, 2003. Leaching of bisphenol A from new and old babies' bottles, and new babies' feeding teats. Asia-Pacific journal of public health 15, 118–123. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G, 2010. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environmental health perspectives 118, 1055–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV, 2007. Human exposure to bisphenol A (BPA). Reproductive toxicology 24, 139–177. [DOI] [PubMed] [Google Scholar]
- Vought V, Wang HS, 2018. Impact of common environmental chemicals bisphenol A and bisphenol S on the physiology of Lumbriculus variegatus. Environ. Toxicol. Pharmacol 60, 225–229. [DOI] [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, vom Saal FS, 2006. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 147, S56–69. [DOI] [PubMed] [Google Scholar]
- Yan S, Chen Y, Dong M, Song W, Belcher SM, Wang HS, 2011. Bisphenol A and 17beta-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS One 6, e25455. [DOI] [PMC free article] [PubMed] [Google Scholar]