ABSTRACT
Clostridium perfringens causes necrotic enteritis (NE) in poultry. A chromosomal locus (VR-10B) was previously identified in NE-causing C. perfringens strains that encodes an adhesive pilus (NE pilus), along with a two-component system (TCS) designated here as PilRS. While the NE pilus is important in pathogenesis, the role of PilRS remains to be determined. The current study investigated the function of PilRS, as well as the Agr-like quorum-sensing (QS) system and VirSR TCS in the regulation of pilin production. Isogenic pilR, agrB, and virR null mutants were generated from the parent strain CP1 by insertional inactivation using the ClosTron system, along with the respective complemented strains. Immunoblotting analyses showed no detectable pilus production in the CP1pilR mutant, while production in its complement (CP1pilR+) was greater than wild-type levels. In contrast, pilus production in the agrB and virR mutants was comparable or higher than the wild type but reduced in their respective complemented strains. When examined for collagen-binding activity, the pilR mutant showed significantly lower binding to most collagen types (types I to V) than parental CP1 (P ≤ 0.05), whereas this activity was restored in the complemented strain (P > 0.05). In contrast, binding of agrB and virR mutants to collagen showed no significant differences in collagen-binding activity compared to CP1 (P > 0.05), whereas the complemented strains exhibited significantly reduced binding (P ≤ 0.05). These data suggest the PilRS TCS positively regulates pilus production in C. perfringens, while the Agr-like QS system may serve as a negative regulator of this operon.
IMPORTANCE Clostridium perfringens type G isolates cause necrotic enteritis (NE) in poultry, presenting a major challenge for poultry production in the postantibiotic era. Multiple factors in C. perfringens, including both virulent and nonvirulent, are involved in the development of the disease. We previously discovered a cluster of C. perfringens genes that encode a pilus involved in adherence and NE development, along with a predicted two-component regulatory system (TCS), designated PilRS. In the present study, we have demonstrated the role of PilRS in regulating pilus production and collagen binding of C. perfringens. In addition, the Agr-like quorum sensing signaling pathway was found to be involved in the regulation. These findings have identified additional targets for developing nonantibiotic strategies to control NE disease.
KEYWORDS: pilus, binding, two-component regulatory system, quorum sensing, necrotic enteritis, Clostridium perfringens
INTRODUCTION
Clostridium perfringens is a Gram-positive, spore-forming, anaerobic, rod-shaped bacterium that is widely distributed in the environment, particularly in soil, food, sewage, and the gastrointestinal (GI) tract of both diseased and healthy animals (1). C. perfringens is divided into seven toxinotypes (A through G) according to the production of six toxins, alpha-toxin (CPA), beta-toxin (CPB), epsilon-toxin (ETX), and iota-toxin, enterotoxin (CPE), and necrotic enteritis B-like (NetB) (2).
C. perfringens type G isolates, which produce NetB, cause necrotic enteritis (NE), an important disease of poultry that has been estimated to cost the poultry industry approximately $6 billion (U.S.) in losses per year globally (3). The disease has been historically controlled by prophylactic antibiotic use; however, this application is currently being phased out due to growing concern over the development and spread of antimicrobial-resistant bacteria. The development of strategies to control NE without the use of antibiotics is now a priority to ensure the safe and cost-effective delivery of poultry products to consumers.
The host-pathogen interactions between poultry and C. perfringens are complex and not fully understood, involving the pore-forming toxin NetB along with other virulence factors (4–6). We previously identified a locus prevalent in NE-causing C. perfringens isolates, designated VR-10B (5), that encodes an adhesive sortase-dependent pilus (NE pilus) and is required for NE pathogenesis and binding to collagen (7, 8). Sortase-dependent pili play an important role in the virulence of a number of Gram-positive pathogens by mediating adhesion, host colonization, and biofilm formation (9–11) but have only recently been implicated in C. perfringens pathogenicity. The VR-10B locus consists of seven open-reading frames (ORFs) that encode three pilin subunits (CnaA, FimA, and FimB), a sortase enzyme, and a signal peptidase, as well as a putative two-component regulatory system (TCS) (5) designated here as the PilR response regulator (RR) and PilS sensor histidine kinase (SK), or PilRS. NE pilus production is abrogated in virulent C. perfringens strains following disruption of cnaA, fimA, and fimB, resulting in reduced collagen-binding ability and significant attenuation of virulence (7, 12). In addition, Wade et al. found that cnaA-null mutants had a reduced capacity to colonize chickens during NE development (8). Furthermore, immunization of chickens with recombinant pilins CnaA and FimB partially protected against NE (13), indicating the important role played by this pilus in NE pathogenesis.
A previous report indicated that the VR-10B operon may be positively regulated by the accessory gene regulator (Agr)-like quorum sensing (QS) system (14). The Agr operon, first elucidated in Staphylococcus aureus (15), regulates gene expression in response to changes in local cell densities through secretion of the AgrD-encoded auto-inducing peptide (AIP), which is modified and transported across the cell membrane by AgrB and detected in turn by the cognate AgrA/AgrC TCS (16). C. perfringens carries a homologous Agr-like operon (17), but unlike that of S. aureus, it does not encode a TCS. The expression of several toxin genes, including perfringolysin O (PFO), CPA, CPB, ETX, and NetB, is dependent upon both the Agr-like system and the VirSR TCS (14, 18–22), suggesting that the VirS sensor kinase recognizes the AIP signal. VirSR is a global regulator that controls the transcription of approximately 147 genes, either through direct binding of phosphorylated VirR to upstream “VirR-box” elements or, indirectly, through the regulatory RNA molecule VR-RNA (23). However, the relationship between the Agr-like and VirSR systems is not absolute, as the expression of ETX in type D strains has been shown to depend on the Agr-like system but not VirSR (21).
The PilRS genes are located immediately downstream of the NE pilus structural genes, suggesting they may also be involved in the regulation of pilus expression. The current study objectives were therefore to investigate the function of the PilRS TCS, in addition to the Agr-like QS system and VirSR TCS, in regulating NE pilus production and function as determined by collagen-binding ability.
RESULTS
Identification of novel variant VR-10B1.
The PilRS TCS is located approximately 800 bp downstream of the FimB gene within the VR-10B operon found in C. perfringens CP4 and consists of the predicted 238-amino-acid (aa) response regulator PilR and the 432-aa sensor histidine kinase PilS. Amplification of the PilRS region of strain CP1 produced an amplicon of approximately 4 kb, instead of the expected 2.5-kp product predicted from the CP4 genome sequence (Fig. 1A). Sanger sequencing of the CP1 PilRS amplicon revealed a 1,275-bp IS6 family transposase inserted 23 bp upstream of the pilR start codon, which accounts for the larger amplicon size in this strain (Fig. 1B). To determine if this VR-10 variant (designated VR-10B1) is present in other C. perfringens strains, the VR-10B and VR-10B1 sequences encompassing cnaA to pilR were aligned against 200 available C. perfringens RefSeq genome sequences. Among these, 21 genomes carried an intact VR-10B locus (i.e., lacking the transposase gene), whereas one strain (NCTC8081) carried an intact VR-10B1 variant (see Table S2 in the supplemental material). Remarkably, NCTC8081 is a type C strain isolated from the intestines of an individual that died from enteritis necroticans (EN). Additionally, two poultry isolates (JS5388 and Warren) carried the cnaA-fimB and pilR regions of VR-10B on separate contigs. In the CP1 draft genome, these two regions were also initially assembled onto separate contigs but later determined to be misassembled as a result of the intervening repetitive transposase sequence, suggesting that JS5388 and Warren may also carry the VR-10B1 variant.
FIG 1.
Identification of the novel VR-10B1 variant. (A) PCR products amplified from CP1 or CP4 genomic DNA, using primers PilRS-F and PilRS-R targeting the pilR gene and upstream region. (B) Schematic of the VR-10B and VR-10B1 variants, indicating primer locations (small black arrows) and amplicon sizes.
PilRS and VirSR two-component systems are related.
To further elucidate the putative functions of PilR and PilS, amino acid sequences for each were scanned for protein domain signatures against the InterPro databases. PilR contains two domains: an N-terminal “response regulator receiver” domain (IPR001789), responsible for accepting the phosphoryl group from the cognate SK, and a C-terminal LytTr DNA-binding domain (IPR007492), which is the effector domain activated upon phosphorylation. PilS contains a histidine kinase-like HATPase–AgrC-ComD-like ATPase domain (cd16935), also found in the Staphylococcus aureus AgrC and Streptococcus pneumoniae ComD SKs, both of which recognize the Agr AIP. To evaluate how common these domains are within the CP1 genome, all predicted proteins were functionally annotated with InterProScan. Interestingly, the LytTr DNA-binding and HATPase–AgrC-ComD-like domains were detected only in VirR and VirS, respectively, aside from PilR and PilS (Fig. 2A). PilR and VirR share 28% identity (53% similarity), while PilS and VirS share 35% identity (57% similarity), suggesting that the VirSR and PilRS TCSs are evolutionarily related. A phylogenetic tree based on multiple sequence alignment of all predicted SKs and RRs in the CP1 genome depicts this relationship (Fig. 2C). The DNA-binding activity of VirR has been determined to depend on both the FXRXHrS (24) and SKHR (25) motifs located in the C-terminal LytTr domain. Alignment of VirR and PilR demonstrates that while the FXRXHrS motif is conserved in PilR, the SKHR motif is not (Fig. 2B). Searches of the cnaA upstream region, which is a hypothesized target of PilR, did not reveal any putative VirR-boxes, tandem repeats, or palindromic sequences that could potentially serve as a DNA-binding motif (data not shown).
FIG 2.
Sequence comparison of VirSR and PilRS two-component systems. (A) Schematic of PilR, PilS, VirR, and VirS proteins, indicating shared domains. (B) Amino acid sequence alignment of PilR and VirR proteins, indicating consensus sequences. The two key VirR amino acid motifs that are required for DNA binding to VirR-box elements are outlined with boxes. (C and D) Neighbor-joining phylogenetic trees built from multiple protein sequence alignment of all predicted RRs (C) or SKs (D) within the CP1 genome. The VirRS/PilRS branches are outlined with boxes.
Generation of a pilR mutant and complement.
CP1pilR and the complemented strain CP1pilR+ were constructed and confirmed by PCR and sequencing. Specifically, the presence of the ClosTron insert in CP1pilR was verified by PCR amplification of the junction region of the insert and PilR gene, to demonstrate that the intron-targeting region had been inserted into the correct location (Fig. 3A to C). The transformation of the CP1pilR strain with the plasmid expressing PilRS was confirmed by the presence of the plasmid DNA and pilR DNA (Fig. 3D).
FIG 3.
PCR confirmation of CP1 pilR-null mutant and complemented strains. (A) Schematic of PilRS genes showing the ClosTron insert and location of primers used for confirmation: 1, pilR-F1; 2, pilR-R1; 3, CT_erm-F. See Table S1 for primer details. (B) PCR amplification of the pilR flanking ClosTron insert using primers 1 and 2. (C) PCR amplification of pilR/ClosTron junction with primers 1 and 3. (D) PCR amplification of pJIR750-pilRS with primers 2 and pJIR750-F2, located on the pJIR750 shuttle vector.
Immunoblotting analysis of pilus production in C. perfringens strains.
To investigate the regulatory function of the PilRS TCS and Agr-like QS system in pilus expression, an immunoblotting assay was performed using rabbit antisera raised against each of the pilin proteins (CnaA, FimA, and FimB) as primary antibodies. As previously reported, proteins from the cell wall produce a ladder-like pattern, indicating the presence of pili containing variable length polymers. In CP1pilR, little protein was visible and no ladder-like pattern was observed (Fig. 4). The ladder-like banding pattern was restored in the CP1pilR+ strain and the intensity of the bands was greater than that of CP1. The complementation vector contained the native CP1 PilRS genes and upstream region, including the IS6 element, demonstrating that this transposase did not impact PilRS transcription. In contrast, pilus production levels were the same or higher in the CP1virR and CP1agrB mutants, but reduced in their respective complements, compared with CP1. As expected, no pilus production was observed in the three pilin mutants (CP1cnaA, CP1fimA, and CP1fimB).
FIG 4.
SDS-PAGE and immunoblotting analysis of cell surface fractions from C. perfringens CP1, isogenic null mutants, and the respective complemented strains. Shown are a Coomassie blue-stained SDS-PAGE gel (A) and replicate immunoblots incubated with primary antibodies against CnaA (B), FimA (C), and FimB (D). Each lane was loaded with 5 μg of surface protein sample. Immunoblots were repeated at least three times and a representative image is shown.
Collagen binding assays.
To investigate the role of the proposed regulatory elements of the pili in the binding of C. perfringens to collagen, adhesion assays were utilized to test the binding of wild-type CP1 and isogenic CP1 mutants and complemented strains to collagen types I through V. Similar to the immunoblotting results, binding of the CP1pilR mutant to most collagen types was significantly lower than for CP1 (P ≤ 0.05; Tukey’s test), but its complement was either not significantly different or significantly higher than the parent strain (P ≤ 0.05) (Fig. 5A). In contrast, binding of agrB and virR null mutants to collagen (types I to V) exhibited no significant changes compared with CP1 (P > 0.05), whereas the agrB complemented strain had significantly reduced binding to collagen types I, II, and IV (P ≤ 0.05) (Fig. 5B), and the virR complemented strain showed significantly lower binding to collagen types I, II, III, and IV (P ≤ 0.05) (Fig. 5C). All of the pilin mutants showed negligible binding to collagen (types I to V) relative to parental CP1 (P ≤ 0.05) (Fig. 5D), as reported previously (7).
FIG 5.
Binding of C. perfringens CP1, isogenic null mutants, and the respective complemented strains to collagen types I through V. Letters indicate significantly different groups (P ≤ 0.05; Tukey’s test). Error bars represent standard deviations. (A) Binding of the pilR-null mutant and the respective complemented strain compared with CP1. (B) Binding of the agrB-null mutant and the complemented strain compared with CP1. (C) Binding of the virR-null mutant and the complemented strain compared with CP1. (D) Binding of pilin-null mutant strains compared with CP1.
Blocking of C. perfringens collagen binding by pilin antisera.
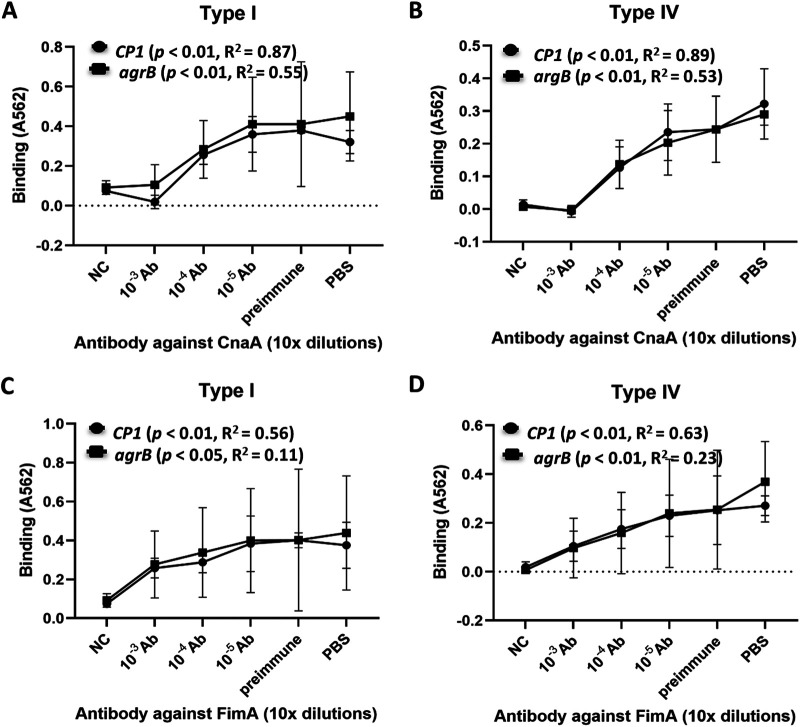
It was previously reported that binding of wild-type CP1 to collagen types I and IV is specifically blocked by CnaA and FimA antisera in a dose-dependent manner (7). To verify that the binding of the agrB null mutant was specifically due to expression of the NE pilus, and not to other unknown proteins controlled by this global regulator, the specificity of adherence to collagen types I and IV was examined in the presence of three dilutions (10−3, 10−4, and 10−5) of rabbit antisera against CnaA or FimA. The binding of CP1 and CP1agrB to collagen types I and IV was blocked by antisera against CnaA (Fig. 6A and B) and FimA (Fig. 6C and D). For both CnaA and FimA antisera, as the concentration decreased, binding of CP1 and CP1agrB to both collagen types increased in a dose-dependent manner (Fig. 6, P values for slopes ≤ 0.05). These observations suggest that the binding of mutant CP1agrB to collagen types I and IV is specifically mediated through the NE pilus.
FIG 6.
Blocking of C. perfringens CP1 and Agr mutant strains binding to collagen by pilin antisera. C. perfringens cultures were incubated with dilutions of rabbit antiserum against CnaA (A and B) or FimA (C and D) for 20 min before being added to wells of a 96-well plate coated with collagen types I (A and C) or IV (B and D). NC, no collagen; PBS, CP1 or CP1agrB incubated with PBS; preimmune, CP1 or CP1agrB incubated with preimmune rabbit serum (10−3 dilution). The analysis of linear regression showed that the dose response of the binding blocked by different concentrations of the antisera was significant, indicated by P values for the slopes and adjusted R2 in the figure.
DISCUSSION
NE caused by C. perfringens remains a major challenge for the poultry industry, particularly in light of recently imposed restrictions on the use of preventative antibiotics, of which NE is the primary target. A thorough understanding of the disease, including the full complement of virulence factors employed by NE-causing C. perfringens strains, is essential for developing novel approaches for therapy and prevention. We recently demonstrated, through visualization with TEM immunogold labeling, that the VR-10B locus encodes a sortase-dependent pilus (7), which mediates collagen binding and is essential for NE pathogenesis (7, 8). The PilRS TCS is located directly downstream from the pilus structural genes, implicating it in the regulation of this operon.
In the current study, we demonstrate that disruption of the PilR gene completely abolishes NE pilus production, while complementation restores expression to greater than wild-type levels. These results clearly show that the PilRS TCS plays an integral role as the primary positive regulator of NE pilus production. The mechanisms by which the PilR RR regulates pilus production are unknown, whether by directly controlling transcription of the VR-10B operon through binding to upstream promoter elements, or indirectly through secondary regulators. Sequence analysis of the PilRS and VirSR TCSs reveals they are related, both in terms of sequence similarity and shared protein domains involved in signal recognition and DNA-binding specificity. VirR activates the transcription of several genes directly through binding to upstream “VirR-boxes” (26–28), but most genes within the VirSR regulon are modulated through the secondary regulatory RNA molecule, VR-RNA (23). The binding of VirR to VirR-box promoter elements is dependent upon at least two amino acid motifs, FXRXHrS and SKHR (25, 29). The fact that the SKHR motif is absent from PilR suggests that the promoter element recognized by PilR likely differs from that of VirR. Preliminary searches to identify candidate PilR-binding sequences upstream of the VR-10B operon were unsuccessful. The PilS SK, like VirS, contains a histidine kinase-like ATPase domain similar to that of S. aureus AgrC, which is part of the Agr QS operon. This family also includes the S. pneumoniae ComD SK of the ComD-ComE TCS, involved in quorum sensing and genetic competence (30). These observations warrant further investigations to (i) better understand the respective regulons controlled by VirSR and PilRS, which may involve cross talk with Agr-like QS, and (ii) define the environmental signal(s) sensed by PilS.
We previously reported that the Agr-like QS system positively regulates pilin gene transcription (14). The Agr-like QS signaling pathway typically proceeds via the VirSR TCS to affect transcription, presumably through detection of the agrD-encoded AIP by the VirS SK (18–20). In the current study, isogenic virR and agrB mutants did not display reduced pilus protein production as expected but instead appeared to produce equal or greater amounts than wild-type CP1. In contrast, pilus production in both of the complemented strains was reduced, likely due to higher than wild-type expression levels of the complemented gene. This suggests the Agr-like QS system, via the VirSR TCS, is in fact a negative regulator of NE pilus production. It is unlikely that the observed difference in pilus production between strains was due to unequal protein loading, as the same amount of quantified protein was loaded for each, and similar results were obtained from several replicate experiments. Furthermore, the collagen-binding activity of the mutant and complemented strains supports the same conclusion. In general, the collagen-binding ability of both CP1agrB and CP1virR was similar to wild-type CP1 but significantly reduced in the complemented strains. This was in contrast to the PilR mutant, where the opposite trend was observed. The Agr-like QS system is a global regulator that controls a large set of genes, and it is therefore possible that other collagen-binding proteins within this regulon have increased in expression in CP1agrB, and thereby influence the collagen-binding results. The ability of CnaA and FimA antisera to block collagen binding of CP1agrB rules out this possibility and indicates that collagen binding in this mutant strain is specifically mediated by the NE pilus. The reason for the difference between the current and previous studies is unclear, although they vary in the level at which expression was examined (RNA versus protein). It is therefore possible that regulation of the NE pilus by the Agr-like QS system also occurs through posttranscriptional mechanisms, resulting in a net decrease in pilus production, despite increasing transcription. Another explanation may be differences in the bacterial growth conditions used, as the current study evaluated plate-grown cultures while the previous study examined liquid cultures. Recently, Soncini et al. reported that C. perfringens could sense and respond to growth on different surfaces, leading to changes in the expression of type IV pili at both transcriptional and posttranscriptional levels between plate-grown and liquid-grown strains (31).
It is not immediately clear what benefit might be gained from repressing pilus production during periods of high cell density. The expression of other virulence factors, including NetB, is upregulated by the Agr-like QS system, presumably to coordinate an effective attack on host tissues once a sufficient number of cells have accumulated (14). Several other Gram-positive pilus islets have been found to encode regulatory proteins that modulate their expression, though no TCSs have thus far been identified to the best of our knowledge. The S. pneumoniae PI-1 pilus islet encodes the RlrA regulator, which controls pilus production through a positive feedback loop, resulting in a biphasic expression pattern that produces populations expressing high or low numbers of pili (32, 33). The PI-1 pilus was found to be preferentially expressed during early colonization stages, where adhesion is important, and reduced during later stages, presumably to evade the immune response induced against the pilus antigen (34). Similarly, the S. pyogenes FCT pilus-encoding region carries genes for two regulatory proteins, Nra/RofA and MsrR, which have contradictory effects on pilus expression, resulting in bistable expression (35, 36). In this case, the pilus is expressed at lower temperatures, corresponding to the environment of superficial skin infections, to mediate binding to keratinocytes (37). It is therefore possible that, similar to the biphasic expression of the PI-1 pilus, NE pilus production is downregulated during later stages of infection, when it is no longer required, in order to evade the induced immune response. Further studies are required to define the in vivo expression pattern of the NE pilus, both during various stages of infection and at different host sites.
Previous investigations of the VR-10 locus have found that VR-10B is carried exclusively by poultry isolates, of which most are associated with NE (5, 8). A recent comparative genomic analysis of 67 C. perfringens strains revealed that VR-10B is highly conserved, while a novel third variant (VR-10C), containing only the flanking genes, was identified in nonpathogenic strains (38). We define here a fourth variant, designated VR-10B1, that contains a transposase gene in the region between the pilus structural genes and PilRS TCS. Transposable elements are found in a number of Streptococci and Enterococci pilus operons and point toward the likely horizontal acquisition of these islets (39). While carriage of this variant is atypical of poultry NE strains, the transposase insertion does not appear to impact the expression of pilR, as demonstrated by the mutagenesis and complementation experiments shown here. In addition to CP1, VR-10B1 was found in at least one other strain (NCTC8081), which, interestingly, is not a poultry type A strain but is a human type C strain isolated in 1946 from a lethal case of enteritis necroticans (EN) (40). This represents the first known nonpoultry isolate that carries the NE pilus-encoding genes. EN is a rare but often fatal disease that was prevalent in Germany for several years following World War II. It reemerged in the 1960s and 1970s in Papua New Guinea, where it was referred to as Pigbel (41). While CPB, a pore-forming toxin related to NetB (4), is required for the development of EN, it is not known if additional virulence factors are involved. It would be informative to survey additional EN-associated strains to determine if VR-10B1 is limited to NCTC8081.
In summary, this work describes the previously uncharacterized PilRS TCS, which appears to be the primary positive regulator of NE pilus production. We also provide evidence that the Agr-like QS system acts as a negative regulator in this system, likely by signaling through the VirSR TCS, to which PilRS is related. These findings provide new insights into the regulation of this key NE virulence factor, as well as offering clues toward the possible evolutionary origins of this pilus islet.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
C. perfringens CP1, a field isolate from an NE case in ON, Canada (42), was used as the parent strain for all of the mutants and complemented strains used in this study. The generation of agrB and virR null mutant strains CP1agrB and CP1virR, and complemented strains CP1agrB+ and CP1virR+, was described by Yu et al. (14) and the generation of pilin-null mutants CP1cnaA, CP1fimA, and CP1fimB was described by Lepp et al. (7).
All C. perfringens strains were grown overnight at 37°C under anaerobic conditions on blood agar or brain heart infusion (BHI) (Fisher) plates or in BHI broth supplemented with 34 μg ml−1 chloramphenicol (Sigma-Aldrich, St. Louis, MO, USA) and 10 μg ml−1 erythromycin (Thermo Fisher, Burlington, ON, Canada), as necessary. The mutant strains were supplemented with 10 μg ml−1 erythromycin, and the complemented strains with 10 μg ml−1 erythromycin and 34 μg ml−1 chloramphenicol. Escherichia coli Stellar (TaKaRa Bio, Mountain View, CA, USA) competent cells were used for cloning in Luria-Bertani (LB) broth or agar (Difco) supplemented with 34 μg ml−1 chloramphenicol (Sigma-Aldrich), as required.
PilRS sequence analysis.
The PilRS region of the VR-10B locus was amplified from CP1 and CP4 genomic DNA using primers pilRS-F and pilRS-R (see Table S1 in the supplemental material), and Sanger sequencing was carried out on both amplicons. All predicted proteins from the CP1 genome were annotated for protein domain signatures with InterProScan v5.48-83.0. Genes were predicted to encode RRs if they contained Pfam domain PF00072, and SKs if they contained PF00512, PF07568, PF07730, PF07536, PF06580, PF01627, PF02895, PF05384, PF10090, PTHR42878, PTHR45528, PTHR43547, PTHR43711, or PTHR40448. Multiple alignments of the amino acid sequences of all predicted C. perfringens CP1 RRs and SKs were performed separately with Clustal Omega and neighbor-joining trees were generated. To identify putative PilR DNA-binding motifs within the VR-10B promoter, the approximately 700-bp noncoding sequence upstream of cnaA to the preceding gene were scanned for VirR box consensus sequence CCANTTN24CCANTT (43) using the EMBOSS fuzzynuc tool (https://www.bioinformatics.nl/cgi-bin/emboss/), as well as the etandem, palindrome, and einverted tools. To identify the VR-10B1 variant in other C. perfringens genomes, 200 currently available RefSeq genomes were downloaded from NCBI and local BLAST searches were performed against each using both the VR-10B and VR-10B1 sequences.
Construction and confirmation of pilR mutant and complemented strains.
The ClosTron mutagenesis system was used to insertionally inactivate pilR in wild-type CP1, as described by Heap et al. (2010) (44, 45), to generate CP1 pilR43::ermB (referred to here as CP1pilR). The ClosTron intron-targeting region was designed to insert at 43 bp of the pilR open-reading frame sense strand using the Perutka algorithm implemented at www.clostron.com. The intron-targeting region was synthesized and cloned into the ClosTron plasmid pMTL007C-E2 by DNA 2.0 (Menlo Park, CA, USA) to generate pMTL007C-E2::pilR43s. The resultant plasmid was electroporated into CP1 as described previously (14, 46). To verify that the intron had been inserted into the expected location, PCR was performed to amplify the region spanning the insertion site with the primer pair pilR-F1/R1 (Table S1). Additionally, the region spanning the junction between the ClosTron insert and the PilR gene was amplified using primers CT_Erm-F and pilR-F1, and verified by Sanger sequencing.
To construct the pilR complemented strain CP1 pilR43::ermB (pilR+) (referred to here as CP1pilR+), pilRS was amplified from CP1 genomic DNA using primers pilR-comp-F1 and pilR-comp-R1 (Table S1) to generate a 3.8-kb fragment that included pilRS, the IS6 transposase element, and an ∼500-bp upstream region, which was digested with restriction enzymes BamHI and HindIII. The purified fragment was ligated into the E. coli-C. perfringens shuttle vector pJIR750 to generate pJIR750-pilR, which was transformed into E. coli Stellar competent cells. The plasmid insert was confirmed by sequencing and electroporated into CP1pilR, as previously described (14). Transformants were selected on BHI agar supplemented with 15 μg ml−1 thiamphenicol and 10 μg ml−1 erythromycin.
Isolation of cell surface protein from C. perfringens strains.
Total cell surface proteins were extracted from C. perfringens cultures essentially as described previously (47). Strains were grown overnight anaerobically at 37°C on blood agar plates and subcultured in BHI broth to an optical density at 600 nm (OD600) of approximately 0.8 to 1.0. An aliquot (100 μl) of the subculture was spread onto a BHI plate and incubated overnight to produce a confluent lawn. The overnight culture was harvested from the surface of plates with phosphate-buffered saline (PBS) and the suspension was centrifuged at 5,000 × g for 2 min, washed once with PBS, and adjusted to an OD600 of 1.0 in PBS. Cells (10 ml) were pelleted by centrifugation at 6,000 × g for 5 min. Bacterial cells were washed once with 1 ml SMM buffer (0.5 M sucrose, 10 mM MgCl2, 10 mM maleate), pH 6.8, and resuspended in 1 ml SMM buffer, to which 60 μl of 5 U/μl of mutanolysin (Sigma) in muramidase buffer (2 mM acetic acid, 48 mM sodium acetate) and 10 μl of 0.1 M phenylmethylsulfonyl fluoride (PMSF) (Sigma) were added. After approximately 6 h of incubation at 37°C with constant rotation, the resultant protoplasts were centrifuged at 20,000 × g for 5 min and the supernatant of cell wall proteins was removed. The protein content of the cell wall fraction was quantitated by Pierce BCA protein assay kit (Thermo Fisher Scientific), mixed with 4× NuPAGE LDS sample buffer (Thermo Fisher Scientific), and boiled for 5 min prior to immunoblotting assays.
Immunoblotting assays for examination for pilus production in C. perfringens strains.
Extracts of cell surface protein were separated on NuPAGE 3 to 8% Tris-acetate protein gels (Thermo Fisher Scientific) by electrophoresis at 150 V for 1 h. One gel was loaded with 10 μg of extracts of cell surface protein and used for staining with Biosafe Coomassie stain (Bio-Rad), while identical gels loaded with 5 μg cell surface protein were transferred onto individual polyvinylidene difluoride (PVDF) membranes (Invitrogen) by electroblotting at 30V overnight at 4°C in 1× transfer buffer (48 mM Tris, 39 mM glycine, 20% methanol, 0.1% SDS). Membranes were washed twice for 5 min in 20 ml of Milli-Q water after removal from 1× transfer buffer and then incubated in Tris-buffered saline (TBS) blocking solution (20 mM Tris, 150 mM NaCl) containing 3% (wt/vol) bovine serum albumin (BSA) and 0.05% (vol/vol) Tween 20 for 2 h at 4°C. After washing 3 times with TBST (1× TBS, 0.05% Tween 20) for 5 min each, the membranes were incubated with rabbit antiserum (1:1,000) raised against CnaA, FimA, or FimB (7) at 22°C for 1 h, washed 3 times with TBST for 5 min each, and then incubated with a goat anti-rabbit IgG alkaline phosphatase (AP)-conjugated secondary antibody (1:2,000) (Cedarlane) at 22°C for 0.5 h. Membranes were washed 3 times with TBST for 5 min each and then washed 3 times with Milli-Q water for 2 min each. Alkaline phosphatase activity was detected by adding 2.5 ml of a CDP-Star chemiluminescent substrate (Thermo Fisher Scientific) to each membrane for 5 min. Membranes were imaged on a Bio-Rad GelDoc XR system using Image Lab software.
Adhesion assay.
Bacterial adhesion to collagen types I through V (Sigma) was assayed as described by Xiao et al. (48) and Wade et al. (8), with modifications. Collagen types assayed were: type I from rat tail, type II from chicken sternal cartilage, type III from human placenta, type IV from human placenta, and type V from human placenta. Wells of Nunclon Delta Surface 96-well plates (Thermo Fisher) were coated with 50 μl collagen (1 mg/ml in PBS) per well overnight at 4°C and blocked in 200 μl of PBS containing 0.5% (wt/vol) BSA for 2 h at 4°C, and then rinsed 3 times with 100 μl PBS. C. perfringens strains were grown overnight anaerobically at 37°C on blood agar plates, subcultured in BHI broth, and grown to an OD600 of approximately 0.8 to 1.0. An 100-μl aliquot of the subculture was spread onto a BHI plate and incubated overnight to produce a confluent lawn. The overnight culture was harvested from the surface of plates in PBS and the suspension was centrifuged at 5,000 × g for 2 min, washed once with PBS, and adjusted to an OD600 of 1 in PBS. Bacterial cells were added to the wells of 96-well plates in 50-μl aliquots and incubated at 22°C for 2.5 h with gentle shaking. Wells were rinsed 3 times with 100 μl of PBS and air dried. Cells were stained with 0.5% (wt/vol) crystal violet for 5 min, rinsed 3 times with 100 μl PBS, and then air dried. A 1:1 ethanol-acetone (vol/vol) solution (50 μl) was added to each well to destain adherent cells and absorbance was measured at 562 nm. Wells incubated with bacteria but without collagen, and wells coated with collagen but without added bacteria, were used as blank and negative controls, respectively. Blank values were subtracted from all test sample absorbance values. All assays were repeated three times with each sample having triplicate wells, except for assays with collagen V, which were repeated twice.
Antibody-blocking adhesion assays.
The wild type (CP1) and mutant CP1agrB were used to test whether their binding to collagens I and IV could be blocked by rabbit antisera against pilins CnaA, FimA, and FimB. Specifically, bacterial cells (50 μl) resuspended to an OD600 of 2 were incubated for 20 min at 22°C with three dilutions (10−3, 10−4, and 10−5) of antibodies (50 μl) against CnaA, FimA, or FimB before being added to wells coated with collagen I or collagen IV. Wells incubated with bacterial cells but without collagen served as blank controls; wells coated with collagen but without added bacterial cells were used as negative controls. Cultures of CP1 or CP1agrB (50 μl) plus PBS (50 μl), and 10−3 dilution of preimmune rabbit serum obtained from the rabbits from which antisera against CnaA, FimA, or FimB were prepared, were also used as controls. Other steps in this assay remained the same as those of regular adhesion assays described above, including that each assay was repeated three times.
Statistical analyses.
The data on binding of C. perfringens CP1, isogenic null mutants, and respective complemented strains to collagen (types I through V) were analyzed by one-way ANOVA using the GLM procedure of SAS (SAS Release 9.4, SAS Institute Inc., Cary, NC, USA). Significant differences (P ≤ 0.05) among the strains on each collagen type were identified by the Tukey-Kramer test. The dose response in binding of CP1 and CP1agrB to collagen (types I and IV) blocked by different dilutions of antiserum was analyzed by the linear regression using the REG procedure of SAS.
ACKNOWLEDGMENTS
This work was supported by Agriculture and Agri-Food Canada and the Canadian Poultry Research Council through Poultry Cluster Program III (AAFC Project ID: J-002040).
Plasmid pJIR750 was a gift from J. I. Rood at Monash University, Australia.
Footnotes
Supplemental material is available online only.
Contributor Information
Joshua Gong, Email: Joshua.gong@canada.ca.
Michael J. Federle, University of Illinois at Chicago
REFERENCES
- 1.Hassan KA, Elbourne LD, Tetu SG, Melville SB, Rood JI, Paulsen IT. 2015. Genomic analyses of Clostridium perfringens isolates from five toxinotypes. Res Microbiol 166:255–263. 10.1016/j.resmic.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Rood JI, Adams V, Lacey J, Lyras D, McClane BA, Melville SB, Moore RJ, Popoff MR, Sarker MR, Songer JG, Uzal FA, Van Immerseel F. 2018. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe 53:5–10. 10.1016/j.anaerobe.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wade B, Keyburn A. 2015. The true cost of necrotic enteritis. World Poult 31:16–17. [Google Scholar]
- 4.Keyburn AL, Boyce JD, Vaz P, Bannam TL, Ford ME, Parker D, Di Rubbo A, Rood JI, Moore RJ. 2008. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog 4:e26. 10.1371/journal.ppat.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lepp D, Gong J, Songer JG, Boerlin P, Parreira VR, Prescott JF. 2013. Identification of accessory genome regions in poultry Clostridium perfringens isolates carrying the netB plasmid. J Bacteriol 195:1152–1166. 10.1128/JB.01032-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prescott JF, Parreira VR, Mehdizadeh Gohari I, Lepp D, Gong J. 2016. The pathogenesis of necrotic enteritis in chickens: what we know and what we need to know: a review. Avian Pathol 45:288–294. 10.1080/03079457.2016.1139688. [DOI] [PubMed] [Google Scholar]
- 7.Lepp D, Zhou Y, Ojha S, Mehdizadeh Gohari I, Carere J, Yang C, Prescott JF, Gong J. 2021. Clostridium perfringens produces an adhesive pilus required for the pathogenesis of necrotic enteritis in poultry. J Bacteriol 203:e00578-20. 10.1128/JB.00578-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wade B, Keyburn AL, Haring V, Ford M, Rood JI, Moore RJ. 2016. The adherent abilities of Clostridium perfringens strains are critical for the pathogenesis of avian necrotic enteritis. Vet Microbiol 197:53–61. 10.1016/j.vetmic.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Telford JL, Barocchi MA, Margarit I, Rappuoli R, Grandi G. 2006. Pili in Gram-positive pathogens. Nat Rev Microbiol 4:509–519. 10.1038/nrmicro1443. [DOI] [PubMed] [Google Scholar]
- 10.Danne C, Dramsi S. 2012. Pili of Gram-positive bacteria: roles in host colonization. Res Microbiol 163:645–658. 10.1016/j.resmic.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Khare B, V L Narayana S. 2017. Pilus biogenesis of Gram-positive bacteria: roles of sortases and implications for assembly. Protein Sci 26:1458–1473. 10.1002/pro.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wade B, Keyburn AL, Seemann T, Rood JI, Moore RJ. 2015. Binding of Clostridium perfringens to collagen correlates with the ability to cause necrotic enteritis in chickens. Vet Microbiol 180:299–303. 10.1016/j.vetmic.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Lepp D, Ojha S, Mehdizadeh Gohari I, Chakravarty B, Prescott JF, Gong J. 2019. Immunization with subunits of a novel pilus produced by virulent Clostridium perfringens strains confers partial protection against necrotic enteritis in chickens. Vet Microbiol 230:7–13. 10.1016/j.vetmic.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Yu Q, Lepp D, Mehdizadeh Gohari I, Wu T, Zhou H, Yin X, Yu H, Prescott JF, Nie SP, Xie MY, Gong J. 2017. The Agr-like quorum sensing system is required for pathogenesis of necrotic enteritis caused by Clostridium perfringens in poultry. Infect Immun 85:e00975-16. 10.1128/IAI.00975-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng HL, Novick RP, Kreiswirth B, Kornblum J, Schlievert P. 1988. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol 170:4365–4372. 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bassler BL, Losick R. 2006. Bacterially speaking. Cell 125:237–246. 10.1016/j.cell.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Ohtani K, Yuan Y, Hassan S, Wang R, Wang Y, Shimizu T. 2009. Virulence gene regulation by the agr system in Clostridium perfringens. J Bacteriol 191:3919–3927. 10.1128/JB.01455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Chen J, Vidal JE, McClane BA. 2011. The Agr-like quorum-sensing system regulates sporulation and production of enterotoxin and beta2 toxin by Clostridium perfringens type A non-food-borne human gastrointestinal disease strain F5603. Infect Immun 79:2451–2459. 10.1128/IAI.00169-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidal JE, Ma M, Saputo J, Garcia J, Uzal FA, McClane BA. 2012. Evidence that the Agr-like quorum sensing system regulates the toxin production, cytotoxicity and pathogenicity of Clostridium perfringens type C isolate CN3685. Mol Microbiol 83:179–194. 10.1111/j.1365-2958.2011.07925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, McClane BA. 2012. Role of the Agr-like quorum-sensing system in regulating toxin production by Clostridium perfringens type B strains CN1793 and CN1795. Infect Immun 80:3008–3017. 10.1128/IAI.00438-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Rood JI, McClane BA. 2011. Epsilon-toxin production by Clostridium perfringens type D strain CN3718 is dependent upon the agr operon but not the VirS/VirR two-component regulatory system. mBio 2:e00275-11. 10.1128/mBio.00275-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Cheung JK, Keyburn AL, Carter GP, Lanckriet AL, Van Immerseel F, Moore RJ, Rood JI. 2010. The VirSR two-component signal transduction system regulates NetB toxin production in Clostridium perfringens. Infect Immun 78:3064–3072. 10.1128/IAI.00123-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohtani K, Hirakawa H, Tashiro K, Yoshizawa S, Kuhara S, Shimizu T. 2010. Identification of a two-component VirR/VirS regulon in Clostridium perfringens. Anaerobe 16:258–264. 10.1016/j.anaerobe.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Ohtani K. 2016. Gene regulation by the VirS/VirR system in Clostridium perfringens. Anaerobe 41:5–9. 10.1016/j.anaerobe.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 25.McGowan S, Lucet IS, Cheung JK, Awad MM, Whisstock JC, Rood JI. 2002. The FxRxHrS motif: a conserved region essential for DNA binding of the VirR response regulator from Clostridium perfringens. J Mol Biol 322:997–1011. 10.1016/s0022-2836(02)00850-1. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu T, Ba-Thein W, Tamaki M, Hayashi H. 1994. The virR gene, a member of a class of two-component response regulators, regulates the production of perfringolysin O, collagenase, and hemagglutinin in Clostridium perfringens. J Bacteriol 176:1616–1623. 10.1128/jb.176.6.1616-1623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okumura K, Ohtani K, Hayashi H, Shimizu T. 2008. Characterization of genes regulated directly by the VirR/VirS system in Clostridium perfringens. J Bacteriol 190:7719–7727. 10.1128/JB.01573-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung JK, Rood JI. 2000. The VirR response regulator from Clostridium perfringens binds independently to two imperfect direct repeats located upstream of the pfoA promoter. J Bacteriol 182:57–66. 10.1128/JB.182.1.57-66.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGowan S, O'Connor JR, Cheung JK, Rood JI. 2003. The SKHR motif is required for biological function of the VirR response regulator from Clostridium perfringens. J Bacteriol 185:6205–6208. 10.1128/JB.185.20.6205-6208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li YH, Tang N, Aspiras MB, Lau PC, Lee JH, Ellen RP, Cvitkovitch DG. 2002. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J Bacteriol 184:2699–2708. 10.1128/JB.184.10.2699-2708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soncini SR, Hartman AH, Gallagher TM, Camper GJ, Jensen RV, Melville SB. 2020. Changes in the expression of genes encoding type IV pili-associated proteins are seen when Clostridium perfringens is grown in liquid or on surfaces. BMC Genomics 21:45. 10.1186/s12864-020-6453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Angelis G, Moschioni M, Muzzi A, Pezzicoli A, Censini S, Delany I, Lo Sapio M, Sinisi A, Donati C, Masignani V, Barocchi MA. 2011. The Streptococcus pneumoniae pilus-1 displays a biphasic expression pattern. PLoS One 6:e21269. 10.1371/journal.pone.0021269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hava DL, Hemsley CJ, Camilli A. 2003. Transcriptional regulation in the Streptococcus pneumoniae rlrA pathogenicity islet by RlrA. J Bacteriol 185:413–421. 10.1128/JB.185.2.413-421.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pancotto L, De Angelis G, Bizzarri E, Barocchi MA, Del Giudice G, Moschioni M, Ruggiero P. 2013. Expression of the Streptococcus pneumoniae pilus-1 undergoes on and off switching during colonization in mice. Sci Rep 3:2040. 10.1038/srep02040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreikemeyer B, Nakata M, Koller T, Hildisch H, Kourakos V, Standar K, Kawabata S, Glocker MO, Podbielski A. 2007. The Streptococcus pyogenes serotype M49 Nra-Ralp3 transcriptional regulatory network and its control of virulence factor expression from the novel eno ralp3 epf sagA pathogenicity region. Infect Immun 75:5698–5710. 10.1128/IAI.00175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakata M, Podbielski A, Kreikemeyer B. 2005. MsmR, a specific positive regulator of the Streptococcus pyogenes FCT pathogenicity region and cytolysin-mediated translocation system genes. Mol Microbiol 57:786–803. 10.1111/j.1365-2958.2005.04730.x. [DOI] [PubMed] [Google Scholar]
- 37.Abbot EL, Smith WD, Siou GP, Chiriboga C, Smith RJ, Wilson JA, Hirst BH, Kehoe MA. 2007. Pili mediate specific adhesion of Streptococcus pyogenes to human tonsil and skin. Cell Microbiol 9:1822–1833. 10.1111/j.1462-5822.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 38.Lacey JA, Allnutt TR, Vezina B, Van TTH, Stent T, Han X, Rood JI, Wade B, Keyburn AL, Seemann T, Chen H, Haring V, Johanesen PA, Lyras D, Moore RJ. 2018. Whole genome analysis reveals the diversity and evolutionary relationships between necrotic enteritis-causing strains of Clostridium perfringens. BMC Genomics 19:379. 10.1186/s12864-018-4771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kreikemeyer B, Gamez G, Margarit I, Giard JC, Hammerschmidt S, Hartke A, Podbielski A. 2011. Genomic organization, structure, regulation and pathogenic role of pilus constituents in major pathogenic Streptococci and Enterococci. Int J Med Microbiol 301:240–251. 10.1016/j.ijmm.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Zeissler J, Rassfeld-Sternberg L. 1949. Enteritis necroticans due to Clostridium welchii type F. Br Med J 1:267–269. 10.1136/bmj.1.4597.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shrestha A, Uzal FA, McClane BA. 2019. Enterotoxic clostridia: Clostridium perfringens enteric diseases. Microbiol Spectr 6:977–990. 10.1128/microbiolspec.GPP3-0003-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson DR, Parreira VR, Kulkarni RR, Prescott JF. 2006. Live attenuated vaccine-based control of necrotic enteritis of broiler chickens. Vet Microbiol 113:25–34. 10.1016/j.vetmic.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Lepp D, Roxas B, Parreira VR, Marri PR, Rosey EL, Gong J, Songer JG, Vedantam G, Prescott JF. 2010. Identification of novel pathogenicity loci in Clostridium perfringens strains that cause avian necrotic enteritis. PLoS One 5:e10795. 10.1371/journal.pone.0010795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heap JT, Cartman ST, Kuehne SA, Cooksley C, Minton NP. 2010. ClosTron-targeted mutagenesis. Methods Mol Biol 646:165–182. 10.1007/978-1-60327-365-7_11. [DOI] [PubMed] [Google Scholar]
- 45.Heap JT, Kuehne SA, Ehsaan M, Cartman ST, Cooksley CM, Scott JC, Minton NP. 2010. The ClosTron: mutagenesis in Clostridium refined and streamlined. J Microbiol Methods 80:49–55. 10.1016/j.mimet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 46.Jiraskova A, Vitek L, Fevery J, Ruml T, Branny P. 2005. Rapid protocol for electroporation of Clostridium perfringens. J Microbiol Methods 62:125–127. 10.1016/j.mimet.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Chang C, Huang I-H, Hendrickx APA, Ton-That H. 2013. Visualization of Gram-positive bacterial pili, p 77–95. In Delcour HA (ed), Bacterial cell surfaces: methods and protocols. Humana Press, Totowa, NJ. 10.1007/978-1-62703-245-2_5. [DOI] [PubMed] [Google Scholar]
- 48.Xiao J, Höök M, Weinstock GM, Murray BE. 1998. Conditional adherence of Enterococcus faecalis to extracellular matrix proteins. FEMS Immunol Med Microbiol 21:287–295. 10.1016/S0928-8244(98)00083-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2. Download JB.00096-21-s0001.xlsx, XLSX file, 0.01 MB (15.9KB, xlsx)